1. Introduction
Xylopia aethiopica is a tree of more than 20 m of height and 60–75 cm of diameter which grows in the forest zone and especially along the rivers in arid areas. The fruit is a slightly hooked cylindrical pod reaching 2–3 mm in width. The mature fruits of green colour take a brown-black coloration after drying and are used as spices (often instead of pepper), in traditional medicine (against the flu, the bronchitis and the dysentery).
Several studies are reported on biological activity of X. aethiopica. Fruit powder, its essential oil (Okonkwo, Okoye, 1996; Ngamo et al., 2001; Kouninki et al., 2005) or leave essential oil (Asawalam et al., 2006) can be used against cowpea bruchid Callosobruchus maculatus (Fab.) (Coleoptera: bruchidae) or maize weevil Sithophylus zeamais Motsch. (Coleoptera: curculionidae). X. aethiopica is also active against the termites and other bugs who tackle wood (Ladjide et al., 1995). The microbiological activity of X. aethiopica essential oil against Escherichia coli, Staphylococcus aureus or Aspergillus flavus, among other microorganisms, has been well established (Tatsadjieu et al., 2003; Asekun, Adeniyi 2004; Konnings et al., 2004).
Among the compounds that confer to X. aethiopica its biologic properties one can mention, the diterpenes belonging to the kauranes, the trachylobanes and the kolovanes families (Hasan et al., 1982; Harrigan et al., 1994). It is also noticed that the features of the ether extract of X. aethiopica are favorable to its incorporation in the resins used for the manufacture of the paintings (Ajiwe et al., 1998).
The composition of fruit essential oil of X. aethiopica given in the literature, shows that it is constituted of monoterpenes hydrocarbon. These compounds are represented mainly by b-pinene 37.0–40.5% (Tomi et al., 1996), 12.0–42.0% (Ayedoun et al., 1996), 18.3% (Jirovetz et al., 1997) 9.9–19.1% (Keita et al., 2003) or by sabinene 36.0% according to Poitou et al. (1996). Germacrene D is the most important sesquiterpene and the oxygenated compounds are mainly the 1,8-cineole and the terpinen-4-ol. A survey undertaken on X. aethiopica essential oil from Egypt showed very particular composition with more than two third of oxygenated compounds 23.4% of terpinen-4-ol, 16.3% of 1,8-cineole and 11.1% of a-terpineol (Karawya et al., 1979). A similar composition with oxygenated monoterpenes (15.1% of 1,8-cineole, 6.6% of terpinen-4-ol) has been reported in essential oil from Nigeria (Asekun, Adeniyi, 2004).
The aim of the present work is to study the composition of X. aethiopica essential oil from four localities in Cameroon, and to emphasize the occurrence of a diterpene never mentioned in X. aethiopica essential oil which appears to be ent-13-epi manoyl oxide.
2. Experimental
2.1. Essential oil extraction
Dried fruits of X. aethiopica were obtained from four localities: Bafoussam, Douala, Ngaoundere and Yaounde.
They were ground and subjected to hydrodistillation during four hours using a Clevenger-type (Figure 1) apparatus. Yellowish essential oils were then obtained.
2.2. Extraction and purification of a natural diterpene
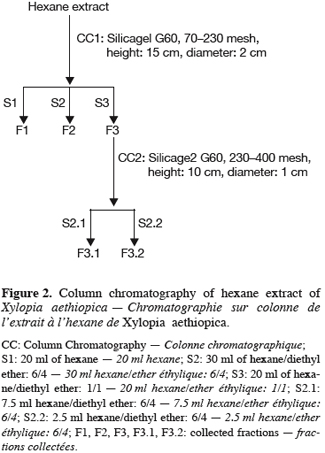
The isolation of enriched fractions containing a natural diterpene were carried out on n-hexane extracts from ground fruits since its content in the essential oil is too low (<1%). Ground fruits (30 g) were mixed with 200 ml of n-hexane and the raw extract (300 mg) was fractionated by successive column chromatography purifications with silica gel G60 (5% water content). Several preliminary trials have been undertaken and the optimized protocol is summarized in figure 2.
2.3. Gas chromatography and gc-ms analyses
GC analyses were performed on an Agilent 6890 series apparatus fitted with a split/splitless injector (splitless mode). The operating conditions were as follows: 30 m*0.25 mm HP 5MS (crosslinked 5% phenyl dimethylsiloxane), film thickness: 0.25 µm, temperature programme: from 40°C–230°C at 5°C/min with a final hold of 5 min. at 280°C. Helium at 49.9 KPa was used as carrier gas and the FID detector was maintained at 250°C.
The oil constituents were identified on the basis of their retention and fragmentation data by using GC/MS analytical conditions similar to that of GC-FID. The mass spectra were recorded on a Agilent 5973 mass spectrometer coupled to an Agilent gas chromatograph (EI mode 70eV, source temperature 230°C, scanned mass ranged 35 to 350 amu). The characteristic fragmentation patterns have been analysed and compared to those of Wiley 275.L database. The retention data (retention indices) were compared to those of Adams (2001) and Joulain and König (1998).
2.4. NMR analysis
Fraction F3.2, collected during column chromatography, was analysed by NMR (CDCl3, 500MHz) in the Unit of Structural Chemistry and Reaction Mechanisms (CSTR) of the Catholic University of Louvain-la-Neuve (Belgium).
3. Results and discussion
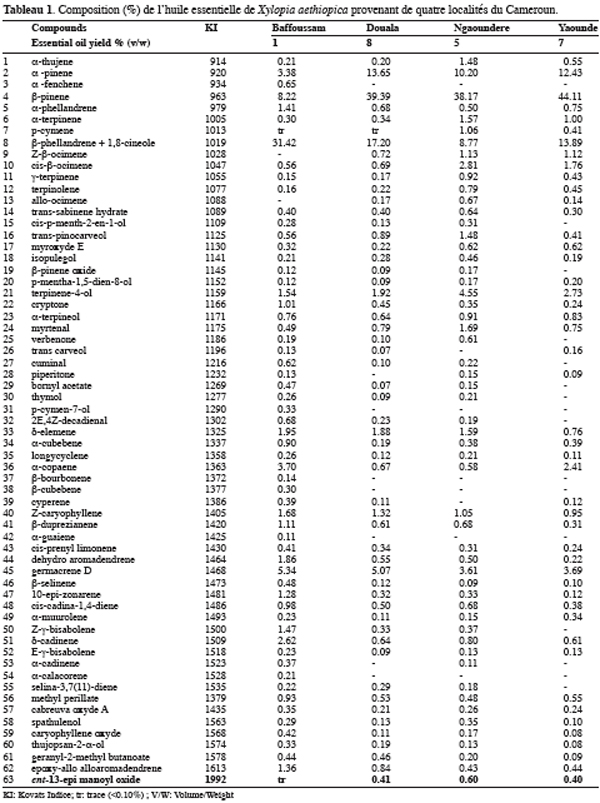
The four analyzed samples contained mainly monoterpenes hydrocarbons (41.76–77.04%), in particular a-pinene (3.38–13.65%), b-pinene (8.22–44.11%). The b-pinene appears like an important compound in the essential oil of X. aethiopica since it is also the major compound in the oil of Guinea with 37.00 to 40.50% (Tomi et al., 1996), of Mali with 9.90% (Keita et al., 2003) and of Cameroon with 18.30% (Jirovetz et al., 1997). However the sample from Bafoussam is particular with 31.42% of b-phellandrene+1,8-cineole against 8.77 to 17.20% for the other samples. With the forementioned analytical conditions, b-phellandrene and 1,8-cineole coeluted, they were therefore summed in table 1. It is noteworthy that the essential oil originating from Bafoussam contained 14.56% of unidentified compounds each representing less than 1%. The origin of the fruits could explain the differences observed, but also the treatments that the fruits undergo after the harvest. Indeed, according to Ayedoum et al. (1996), the a-pinene and the sabinene can vary respectively from 4 to 16% and 3 to 35% according to whether the fruits are boiled or smoked before the drying.
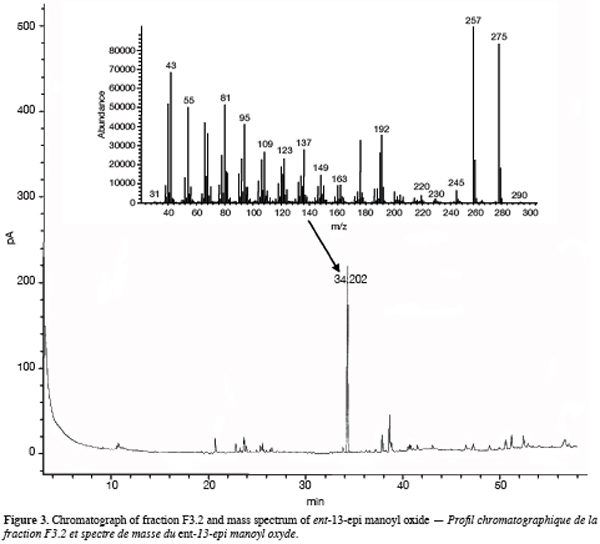
One particular compound appeared in small amount (less than 1%) at a retention time of 34.2 min. Its molecular ion suggests a diterpene. The molecule, of moderate volatility, was systematically observed after long distillation times and only Jirovetz et al. (1997) mentioned the occurrence of such a compound in the essential oil of X. aethiopica without having proposed any identification. Successive column chromatography purifications (Figure 2) led to the recovery of 58.50 mg of an enriched fraction called F3.2 (Figure 3) which contained exclusively diterpenes, notably a derivative of manoyl oxide type. In agreement with Angelopoulou et al. (2001), the recorded mass spectra and the m/z (mass to charge ratio)=275 and 257 intensity ratio (ion m/z=257 higher than ion m/z=275), oriented identification toward the ent-13-epi manoyl oxide (Figure 4) rather than manoyl oxide where the two ions are almost equal. Comparing to the 1H NMR spectrum of pure ent-13-epi manoyl oxide (Demetzos et al., 2002), the recorded 1H NMR spectrum (Figure 5) showed the occurrence of the doublets H-15 cis and H-15 trans. A selective irradiation at 6 ppm confirmed the presence of H-14 since the doublets H-15 were reduced to two singlets by suppression of H-14/H-15 coupling. This confirmed well that the molecule of interest is the ent-13-epi manoyl oxide. Successive injections of the essential oil and the purified product revealed the same kovats index (KI=1992).
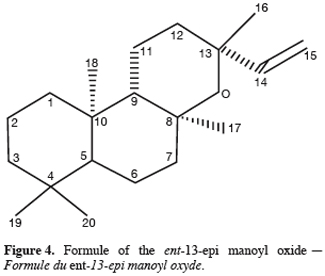
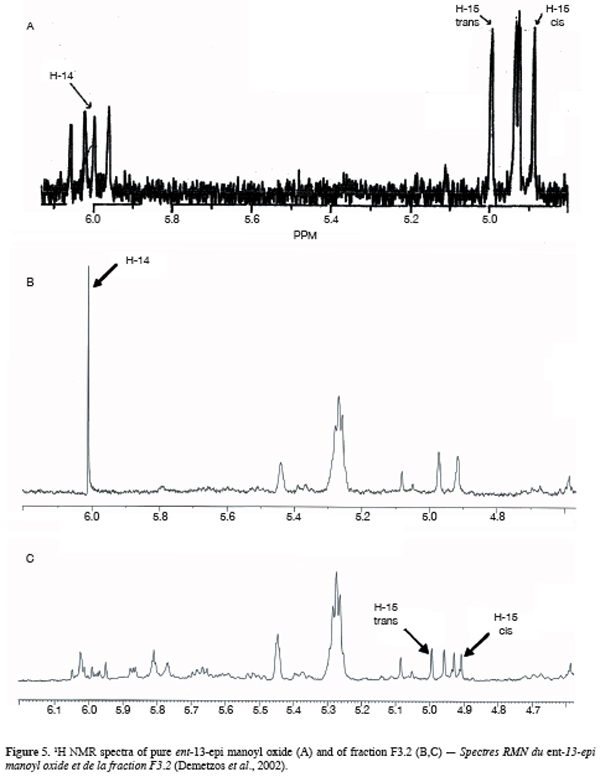
4. Conclusion
More than 60 compounds were identified in the four samples of X. aethiopica essential oils which show the complexity of this natural extract with insecticide activity (Ngamo et al., 2001; Kouninki et al., 2005). The main chemical compounds are: b-pinene, b-phellandrene+1,8-cineole, a-pinene, terpinen-4-ol and germacrene D with different content in each sample. With GC-MS and NMR investigations, it was possible to unambiguously identify the ent-13-epi manoyl oxide, a diterpene which is reported for the first time in X. aethiopica essential oils. Nevertheless due to its very low proportion in all analysed hydrodistillates it is not established that this molecule could play a significant role in the essential oil activity.
Acknowledgments
The authors gratefully acknowledge Prof A. Schanck (Unité de Chimie structurale et des Mécanismes réactionnels) of the Catholic University of Louvain (UCL, Belgium) for NMR analyses; and Belgian University Cooperation to the Development (CUD) for financial support of the project “STOREPROTECT”.
