Fragmentation and insects: theory and application to calcareous grasslands
Résumé
Fragmentation et insectes : théorie et application aux pelouses calcaires. La perte d’habitat constitue la plus importante menace à long terme pour la survie des espèces et découle de trois processus principaux : la destruction de l’habitat, l’augmentation de la fragmentation et l’altération de la qualité de l’habitat. La fragmentation de l’habitat, qui se traduit par la création de plusieurs petits fragments d’habitat spatialement isolés à partir d’un seul fragment continu, a pour conséquence la diminution de l’abondance, de la densité et de la diversité spécifiques ; l’augmentation des effets de lisière et de l’isolement des fragments d’habitat restants. Quelques résultats préliminaires sur les effets de la fragmentation sur les communautés (diversité et abondance spécifiques) de papillons de jour des pelouses calcaires de la vallée du Viroin (Belgique) seront présentés.
Abstract
Habitat loss poses the greatest threat to the long-term survival of species on earth and has three major components: straightforward destruction of habitat, increasing fragmentation and deterioration of habitat quality. Habitat fragmentation, i.e. the reduction of continuous habitat into several smaller spatially isolated remnants, decreases species richness, increases edge effects, decreases density and abundance of species, alters interspecific interactions and ecological processes, and decreases connectivity. Some preliminary results of the effects of fragmentation on butterfly communities (species diversity and abundance) of the calcareous grasslands of the Viroin valley (Belgium) will be presented.
1. Introduction
1Habitat loss poses the greatest threat to the long-term survival of species on earth and has three major components: straightforward destruction of habitat, increasing fragmentation and deterioration of habitat quality. Habitat fragmentation, i.e. the reduction of continuous habitat into several smaller spatially isolated remnants, decreases area, increases edge effects, alters ecological processes, and decreases connectivity (Debinski, Holt, 2000). Following the theory of island biogeography, species richness in habitat fragments is expected to be a function of island size and degree of isolation. Smaller, more isolated fragments are expected to retain fewer species than larger, less isolated patches. Decreases in species richness, in density and in species abundance, and alterations of interspecific interactions are some possible biotic effect of habitat loss and fragmentation. Consequently, habitat loss and fragmentation are recognized as the major causes of the current biodiversity crisis (Fahrig, Merriam, 1984; Wilcox, Murphy, 1985; Saunders et al., 1991; Sih et al., 2000; Baguette, 2001).
2Ehrlich (1989) observed that butterflies are ideal organisms for the study in conservation biology since they are well understood taxonomically, and easily recognized and marked in the field. Lepidoptera can also be used as umbrella species (sustaining habitat to conserve this species will also conserve many other taxa) for biodiversity conservation (New, 1997). Finally, an exceptionally large fraction of butterflies, in Northern Europe in particular, have declined, become endangered or gone extinct, creating an urgent need for efficient conservation measures.
3Previous studies have shown that changes in landscape patterns and in habitat quality due to habitat loss and fragmentation modify the structure and composition of butterfly communities (Tscharntke et al., 2002; Collinge et al., 2003). Specialist species appear to be the most affected (Kruess, Tscharntke, 1994; Tscharntke et al., 2002; Krauss et al., 2003).
4This study is part of the BIOCORE research program funded by the Belgian Scientific Policy (BELSPO). The main aims of this research program include the study of the effects of fragmentation on species number, community composition and performance (i.e. population dynamics and genetics, and phenotypic fitness) on one of Europe’s most species rich habitats, calcareous grasslands. Due to the abandonment of agropastoral techniques, these habitats have disappeared or greatly decreased in size. Both plant and animal (Lepidoptera) species are used as models in this program. Lepidoptera are particularly threatened on calcareous grasslands, i.e. 52% of the threatened Lepidoptera in Europe are found on this habitat (Van Swaay, 2002). Ultimately, BIOCORE aims to provide clear guidelines to conserve the present biodiversity of the calcareous grasslands of the Viroin Valley, especially with respect to minimal population sizes and the connectivity between habitat fragments. Belgian calcareous grasslands have severely declined in surface and quality over the past century due to the abandonment of traditional agropastoral practices (such as extensive grazing), and the subsequent re-colonization by forest species, and land use intensification (particularly coniferous plantations).
5Here we present preliminary results of the effect of calcareous grassland fragmentation (decrease in total habitat area and increase in isolation) on butterfly species richness and community composition with analyses on past and present data. We also evaluate current species abundance and diversity, in a selected number of calcareous grassland fragments, in relation to butterfly species diversity and abundance. These analyses were carried out separately for both specialist and generalist species in an attempt to detect different reactions to the fragmentation process.
2. Material and methods
6First, the evolution of total original habitat area and its connectivity from 1905 to 2000 in a part of the Viroin Valley was estimated using maps from Bruynseels and Vermander (1984) and aerial photographs of the Institut Geographique National (2000). These maps were analyzed using Arcview© to obtain fragment areas and an indication of connectivity, or average distance between sites.
7The evolution of the butterfly communities over the last century was evaluated by means of past publications concerning Lepidopteran diversity in the Calestienne region. The number of common, rare and extinct species in the Calestienne from 1930 to 2000 was based on data from Lhomme (1923), Van Schepdael (1963), Fontaine et al. (1983), Goffart and Baguette (1991) and Lafranchis (2000). Those species that were mentioned were considered either as rare or as common according to the frequency at which they were observed by the above-mentioned authors.
8Secondly, to evaluate current species abundance and diversity, sixteen calcareous grasslands were selected at the extreme western part of the Viroin Valley (Figure 1). These sites differed in their size, degree of isolation, vegetation, topographic particularities, and thermal exposition. With the exception of three sites, all sites are subject to restoration efforts (extensive grazing and mowing). During the butterfly’s flight season of 2003 (April to September), all selected calcareous grasslands were visited every two weeks to estimate the species diversity and abundance by standardized transect counts. Study locations were visited under similar climatic conditions, i.e. dry weather with minimal temperatures of 18°C. Transects were carried out in a similar manner in all study sites, i.e. a crisscross type of path (with ten meters between the loops) walked at a regular pace until the entire area of the site was covered (Pollard, 1977). If necessary, butterflies were netted for identification and then released.
9Habitat specialists were distinguished from generalists using two criteria. First of all, their distribution in Belgium is entirely limited to calcareous grasslands (Goffart, De Bast, 2000) and secondly calcareous grasslands must be the preferential or exclusive habitat of the species based on information from Goffart and Baguette (1991).
3. Results and discussion
10Due to urbanization and abandonment of traditional agropastoral methods the coverage of calcareous grasslands has greatly diminished over the past century. Indeed, in 1905, 41.95% (i.e. 972 hectares, 22 fragments) of the total habitat in the studied part of the Viroin Valley was occupied by calcareous grasslands. In 1948, this coverage has already diminished by half (21.21%). In 1982, calcareous grasslands accounted for only 5.68%. in 2000, 22 fragments remained representing about 1% (i.e. 9.35 hectares) of the total habitat coverage. This severe habitat loss was accompanied by a decrease in butterfly diversity. Indeed, while in 1930 a total of 92 Lepidopteran species were present, only 77 species were still present in the 2000’s. The number of rare or endangered species also increased drastically during this time period, passing from 15 in the 1930’s to 38 in the 2000’s. Specialist species were much more affected by habitat loss and fragmentation than were generalist species. In fact, while 25.00% of the specialist species went extinct between the 1930’s and 2000’s, only 10.53% of the generalist species went extinct over the same time period. These results confirm studies by Kruess and Tscharntke (1994) and Tscharntke et al. (2002) that specialist species are most affected by habitat loss and fragmentation.
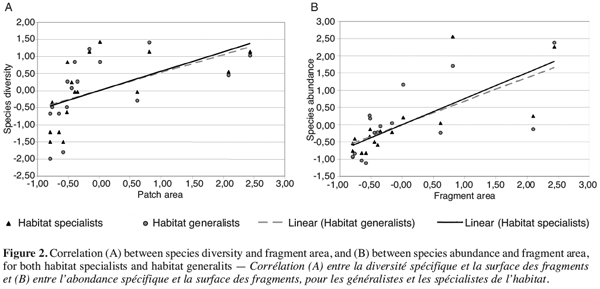
11During the 2003 field season, a total of 62 butterfly species were identified. Using a Bravais-Pearson’s correlation test, a significant effect of habitat area on species diversity and abundance was found for both specialist and generalist butterflies (Figure 2a, b). Indeed butterfly diversity increased with increasing habitat size for both specialist species (r = 0.57, p <0.05) and generalist species (r = 0.52, p<0.05). The number of individuals per species, or species abundance, was also highly and significantly correlated with habitat area (specialist species: r = 0.75, p<0.001; generalist species: r = 0.68, p<0.01). Numerous other studies have also detected this pattern (Steffan-Dewenter, Tscharntke, 2000; Koh et al., 2002; Krauss et al., 2003). On the other hand, connectivity, or the average distance between sites, was neither significantly correlated with species diversity nor with species abundance and this for both generalist (respectively, r = 0.10, p = 0.706, and r = 0.13, p = 0.624) and specialist species (respectively, r = 0.07, p = 0.807, and r = 0.01, p = 0.960). We believe that isolation has already had its effects on species diversity and abundance in the past. The isolation threshold was most likely surpassed in the 1980’s when calcareous habitat coverage in this region fell below 20% of its initial area.
4. Conclusions
12This study has clearly demonstrated and confirmed theoretical predictions that a decrease in habitat area leads to a decrease in species diversity and abundance and to an alteration of butterfly community composition. Indeed, based on data gathered from historical documents, it is clear that butterfly communities have evolved during the past century with a significant decrease in species diversity. Habitat loss and fragmentation appear to have a particularly strong impact on specialist species. Current Lepidopteran species diversity and abundance in the Viroin Valley is highly correlated to habitat size but not to connectivity.
13Acknowledgements
14We would like to thank Julie Choutt for help with the field work and bibliographical research, Jan Butaye (Laboratory for Forest, Nature and Landscape Research, University of Leuven) for help with GIS maps, and Louis-Marie Delescaille for giving informative information about the study region. Site access was provided by the Ministère de la Région Wallonne. We would also like to thank Politique Scientifique (BELSPO) for funding this project (PADDII EV10/26A, 2003-2006).
Bibliographie
Baguette M. (2001). La biodiversité en crise. Probio 4, p. 185–199.
Bruynseels G., Vermander J. (1984). L’évolution de la végétation calcicole de Nismes à Vaucelles. Parcs Nat. Ardenne Gaume 34, p. 71–80.
Collinge SK., Prudic KL., Oliver JC. (2003). Effects of local habitat characteristics and landscape context on grassland butterfly diversity. Conserv. Biol. 17, p. 178–187.
Colmant L., Decocq O., Delescaille L.M., Dewitte Th., Duvigneaud J., Henry A., Hofmans K., Saintenoy-Simon J. (2004). Les pelouses calcicoles en Région Wallonne. Entente nationale pour la protection de la nature. L. Woué (Ed). 2. éd.
Debinski DM., Holt RD. (2000). A survey and overview of habitat fragmentation experiments. Conserv. Biol. 14, p. 342–355.
Ehrlich PR. (1989) The structure and dynamics of Butterfly populations. In Vane-Wright RI., Ackery PR. (Eds). The biology of butterflies. Princeton: Princeton University Press.
Fahrig L., Merriam G. (1984). Conservation of fragmented populations. Conserv. Biol. 8, p. 50–59.
Fontaine M., Leestmans R., Duvigneaud J. (1983). Les Lépidoptères de la partie méridionale de l’Entre-Sambre- et-Meuse et de la pointe de Givet. Linn. Belg. 9, p. 3–62.
Goffart P., Baguette M. (1991). Enquête sur les Lépidoptères Rhopalocères menacés de Wallonie. Rapport final Région Wallonne.
Goffart P., De Bast B. (2000). Atlas préliminaire des papillons de jour de Wallonie. Publication du Groupe de Travail Lépidoptères. Jambes, Belgique: Ministère de la Région wallonne, 80 p.
Koh LP., Sodhi NS., Tan HTW., Peh KSH. (2002). Factors affecting the distribution of vascular plants, springtails, butterflies and birds on small tropical islands. J. Biogeogr. 29, p. 93–108.
Krauss J., Steffan-Dewenter I., Tscharntke T. (2003). How does landscape context contribute to effects of habitat fragmentation on diversity and population density of butterflies ? J. Biogeogr. 30, p. 889–900.
Kruess A., Tscharntke T. (1994). Habitat fragmentation, species loss, and biological control. Science 264, p. 1581–1584.
Lafranchis T. (2000). Les papillons de jour de France, Belgique et Luxembourg et leurs chenilles. Mèze, France : Coll. Parthénope, Biotope.
Lhomme L. (1923). Catalogue des Lépidoptères de France et de Belgique : Volume I. Paris : Léon Lhomme, 800 p.
New TR. (1997). Are Lepidoptera an effective 'umbrella group' for biodiversity conservation? J. Insect Conserv. 1, p. 5–12.
Pollard E. (1977). A method for assessing changes in the abundance of butterflies. Biol. Conserv. 12, p. 115–134.
Saunders DA., Hobbs RJ., Margules CR. (1991). Biological consequences of ecosystem fragmentation - A Review. Conserv. Biol. 5, p. 18–32.
Sih A., Jonsson BG., Luikart G. (2000). Habitat loss: ecological, evolutionary and genetic consequences. Trends Ecol. Evol. 15, p. 132–134.
Steffan Dewenter I., Tscharntke T. (2000). Butterfly community structure in fragmented habitats. Ecol. Lett. 3 (5), p. 449–456.
Tscharntke T., Steffan-Dewenter I., Kruess A., Thies C. (2002). Characteristics of insect populations on habitat fragments: a mini review. Ecol. Res. 17, p. 229–239.
Van Schepdael J. (1963). Nismes ou le bonheur de l’entomologiste. Parcs Nat. Ardenne Gaume 18, p. 51–55.
van Swaay CAM. (2002) The importance of calcareous grasslands for butterflies in Europe. Biol. Conserv. 104, p. 315–318.
Wilcox BA., Murphy DD. (1985). Conservation strategy: the effects of fragmentation on extinction. Am. Nat. 125, p. 879–887.
