- Startpagina tijdschrift
- Volume 73 (2020)
- La descente d'insectes diptères sur les bovins est associée à la transmission contaminante de la fièvre aphteuse lors d'épidémies à Ngaoundéré-Cameroun
Weergave(s): 1544 (9 ULiège)
Download(s): 203 (0 ULiège)
La descente d'insectes diptères sur les bovins est associée à la transmission contaminante de la fièvre aphteuse lors d'épidémies à Ngaoundéré-Cameroun

Documenten bij dit artikel
Version PDF originaleRésumé
Cette étude visait à identifier les sites de prédilection d'atterrissage des Diptères hématophages communs et à les associer aux sites d'excrétion de fièvre aphteuse. Trois groupes de zébu Goudali (appartenant au troupeau du projet DFG-COBE) de différentes couleurs (noir, marron et blanc) du même âge ont été retenus. Les observations ont été faites en Octobre/Novembre 2016 (sept jours consécutifs) et en Janvier 2017 (sept jours consécutifs). Les données de la littérature sur les zones infectées par les doses infectieuses du virus de la fièvre aphteuse (TCID50 / ml) dans les cas cliniques ont été utilisées pour cartographier ces sites sur des bovins et les associer aux sites de prédilection pour l’atterrissage des insectes diptères hématophages. Le nombre total d'insectes piqueurs observés sur le bétail était de 26779 et les groupes de mouches suivants ont été identifiés par ordre de grandeur: Stomoxys (17453), Culicids (8925), Simulium (293), Chrysops (74) et Tabanus (34). Chrysops a préféré se poser sur les pattes antérieures. Les moustiques préféraient se poser autour des pattes antérieures. Tabanus préférait se poser autour de la tête. Stomoxys préférait se poser autour des pattes antérieures. Simulium préférait se poser au niveau du ventre. Les pattes étaient les parties du corps présentant la fréquence la plus élevée d'insectes vecteurs. La prédilection d'atterrissage de chaque groupe d'insectes différait statistiquement (p <0,05). La couleur brune des animaux était particulièrement attrayante pour la plupart des groupes d'insectes. D'après le test d'association, un nombre important de chaque groupe d'insectes était associé à au moins un des sites de contamination par le virus de la fièvre aphteuse, mais Stomoxys et les moustiques avaient une propension plus élevée à être contaminés que les autres, en raison de leurs nombres élevés d'atterrissage sur les animaux exposés.
Abstract
This study was designed to identify the landing preference sites of common hematophagous symbovine Dipterans and relate it to the Foot-&-Mouth Disease Virus (FMDV) shedding sites. Three sets of zebu Goudali (from the DFG-COBE project herd) of different colors (black, brown and white) of same ages were restrained to sticks. Observations were made in October/November 2016 (seven days consecutively) and January 2017 (seven days consecutively). Data from literature on FMDV infectious doses (TCID50/ml) shedding areas in clinical cases was used to map such sites on cattle and associate it to the landing predilection sites of hematophagous dipterous insects.
The total number of observed landing insects on cattle was 26779 and the following fly groups were identified in order of magnitude: Stomoxys (17453), culicids (8925), Simulium (293), Chrysops (74) and Tabanus (34). Chrysops preferred landing around front legs. Culicids preferred landing around front legs. Tabanus preferred landing around the head. Stomoxys preferred landing around front legs. Simulium preferred to alight around the belly region. Legs represented the highest insect-vector frequency spots. The alighting predilection of each insect-group differed statistically (P<0.05). The brown color of animals was most attractive to most insect groups. From the association test, an important number of each insect group observed was associated to at least one of the FMD contamination spots on cattle, but Stomoxys and culicids had a higher propensity of being contaminated as compared to others based on their high landing numbers on the exposed animals.
Inhoudstafel
1. INTRODUCTION
1Foot-&-Mouth Disease (FMD) is an Apthovirus of the family Picornaviridae and is a highly contagious virus disease of even-toed domestic and wild ungulates. It is caused by seven serotypes notably O, A, C, Asia 1, SAT 1, SAT 2 and SAT3 (Kasanga et al., 2014). Bertram et al. (2018) identified sequences of three serotypes (topotype O Africa/lineage East Africa, A/Africa and SAT2 topotype and sub-lineage Lib-12) from the Oropharyngeal Fluids (OPFs) and epithelial tissues of clinical and subclinical cattle from Ngaoundere during the 2015 FMD epidemic. Transmission pathways include contact with infected animals, fomites, soil, air and animal secretions (Hess, 1971), but the role of invertebrates in the spread of FMD is not clearly defined. USDA: APHIS: VS (1994) categorized invertebrates especially biting insects as high hazards in the spread of FMDV. This can be confirmed by the reports of Carn (1996), Ferris et al. (1955) and Hyslop (1970) who reported that viruses of the family Picornaviridae as well as vesicle-forming viruses (like the vesicular stomatitis virus-VSV and FMD) can be mechanically transmitted by tabanids, Stomoxys and mosquitoes. FMD is an economically important disease because it causes high morbidity and mortality rate in calves but low in adults as well as precludes international trade between endemic and non-endemic countries.
2Based on the abundant nature of tabanids and muscids especially Stomoxys in the absence of tsetse flies, it has been reported that such biting flies are responsible for the spread of dangerous diseases such as FMD in cattle herds in Ngaoundere (Sevidzem et al., 2019a). The recovery of the FMDV RNA from Stomoxys niger niger Macquart 1851 (Diptera: Muscidae) body parts (Sevidzem et al., 2019b) around the cattle market in Ngaoundere raised an alarm on the implication of biting flies in the spread of dangerous pathogens. The experiment of Arzt et al. (2018) revealed the transmission of FMDV from persistently infected cattle to naïve cattle recipients via mechanical transfer of unprocessed OPFs and Vesicular Epithelial Tissues (VETs). The occurrence of high anti-FMD antibodies in brown cattle as compared to other color coat of cattle has been reported by Dickmu et al. (in press), but there is no link between FMD infection and color of host. However, high cases in brown animals can be related to the contaminative transmission caused by insect vectors which are mostly attracted to color of host and preferably brown and black colors (Krčmar et al., 2014). However, if cattle were to be used as live attractive targets or traps (Gimonneau et al., 2016) to reduce biting flies and other blood-feeding arthropods of cattle, there is need to know the landing dynamics of these fly groups on live cattle and associate it to the risk of picking infective agents like FMDV as baseline information for their control in pasture areas. The present objective was to associate the landing dynamics data from live cattle exposed to blood sucking insects and associate it with an FMD risk cattle map to show the implication of the different fly groups in the contaminative transmission of the disease in Ngaoundere.
2. MATERIAL AND METHODS
2.1 Description of the study area
3The cattle paddock where the experiment was carried out falls between Latitude 7° 11’N and Longitude 13° 34’E. It was elevated at about 1000 m a.s.l. The site was a cattle breeding zone of the Adamawa plateau in Cameroon. The dominant cattle breed of this area was Goudali, but others like White Fulani, Red Fulani, Bokolodji, Charolais and their cross breeds (metis) prevailed in low numbers in some herds. Cattle breeds of this region had different color coats ranging from brown, white, black and a mixture of colors (red+brown, brown+white, black+white etc.). Greater than 90 % of herds in this region are sedentary. The climate is of the Soudan Guinean type with landscape dominated by gallery forest and open grass savanna. The main water body of this area consisted of river Vina du Sud (Sevidzem et al., 2019c) (Figure 1).
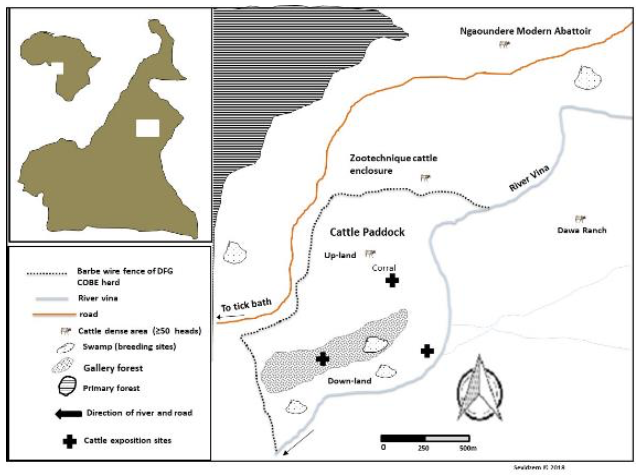
Figure 1 : M p of the DFG-COBE cattle paddock showing cattle exposition sites
2.2 Field exposure experiment to observe boophilic flies
4Three animals from the DFG-COBE cattle herd were used for the experiment, i.e. animal type 1 (red color), animal type 2 (white color) and animal type 3 (black color) with minimum 80 % color coverage. The age of the animals was between two and six years. The animals were restrained on fixed wood poles and kept at equi-distances of 10 m (Barros & Foil, 2007). The animals were changed daily with a fresh set and were fed with fresh grass and water ad libitum. Cattle exposition was carried out in the potential breeding sites (i.e. in the marshy low land beside river Vina du Sud, in the gallery forest and around the cattle overnight park) of flies. The animal body-parts destined to indicate the fly predilections sites were mapped on a bovine model (Figure 2). Three well trained observers (to distinguish the flies up to the genus level) were close as 50 cm to the animal to identify and count the flies per lateral side of each animal. Fly-counts on the exposed animals were made daily for 14 days (i.e. seven days at the end of the rainy season (October to November 2016) and seven days at the beginning of the dry season (January 2017)) with daily rotation scheme (i.e. 3×3 Latin square experimental design with rotation). Landing was observed on one side of the animal and only during 6 h (morning (8 h to 10 h), afternoon (13 h to 15 h), evening (16 h to 18 h)) during the 12 h day and 2 h (18 h to 20 h) during the 12 h night. A fly was confirmed landed when it alights and remains in position for 20 seconds. Observation was carried out following that of Hansens (2015).
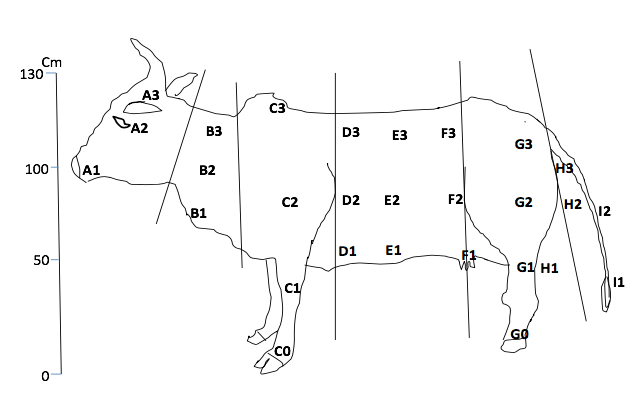
Figure 2 : Map of cattle showing potential predilection sites of flies
2.3 Fly identification
5Identification was carried out on the spot up to genus level for Tabanidae, Muscidae and Simuliidae by well-trained observers by strictly applying the criteria found in already published taxonomic keys. For tabanids, the key of Oldroyd (1957) was used. Stomoxys were identified using the identification key of Zumpt (1973). For Simulium, the key of Freeman & Meillon (1953) was used. For mosquitoes, it was difficult to separate them at genus level directly on animal so they were simply identified as culicids. Culicids were identified using the key of Gilles & De Meillon (1968) and Jupp (1996).
2.4 Data on FMDV excretion or secretion dose
6Data from 32 published scientific articles conceringing FMDV infection experiments were exploited by consulting the published document of Bravo de Rueda (2015). Data on FMDV in secretions and excretions were collected from 32 scientific articles published between 1965 and 2007 (Bravo de Rueda, 2015) found in internal databases and through the electronic (external) databases Scopus and PubMed in 2010, all reporting experimental trials involving FMDV infection. The quantify of the FMDV excreted or secreted was maintained in Tissue Culture Infectious Dose 50 per milliliter (TCID50/ml) unit. The infectious dose range from 0.95 to 10.15 TCID50/ml was considered. The data was from cattle, sheep and pigs.
2.5 Data analysis
7Data analysis was carried out using the R-software (R version 3.4.0). The Principal Component Analysis (PCA) test was used to associate the biting sites of various fly groups to the FMD shedding sites on cattle. The Kruskal Wallis rank sum test was used to compare the number of alighting biting insects with respect to predilection sites as well as skin color. The significant level of all tests was kept at p<0.05.
2.6 Ethical Statement
8Animal use protocols were reviewed and approved by the Ohio State University Institutional Animal Care and Use Committee (Protocol Number: 2012A00000154). Restrained cattle were supplied with water and fresh grass ad libitum. Animals were changed and a fresh set recruited to avoid stressing the animals. Experimental herders were present to carefully restrain the animals. Written authorization was received from the project Director of the DFG-COBE project to use the animals.
3. RESULTS
3.1 Insect group composition and their predilection sites on the exposed animals
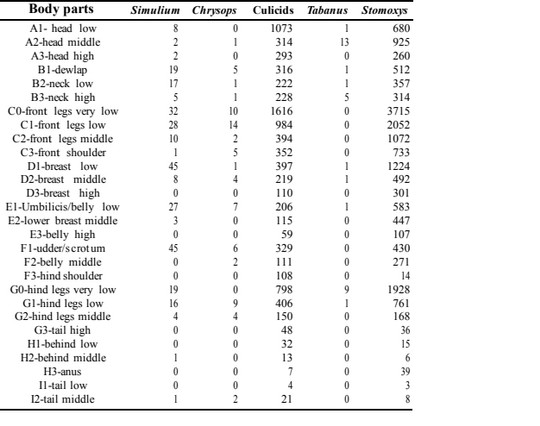
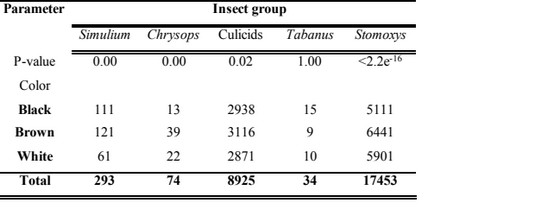
9The total number of observations of the various fly groups on cattle was 26779. The following taxonomic taxa were identified: Stomoxys (17453), culicids (8925), Simulium (293), Chrysops (74) and Tabanus (34) (Table 1). There was a statistically significant difference (P<0.05) in the alighting predilection sites on cattle for each insect group. The predilection sites of Simulium was around the belly region (D1 and F1) and there was a statistically significant difference (P<0.05) in their alighting predilection sites on cattle. The predilection sites of Chrysops was the front legs (C0 and C1) and there was a statistically significant difference (P<0.05) in their alighting preference on exposed cattle. Culicids were most frequent around the front legs (C0), but there was a statistically significant difference (P<0.05) in their alighting sites on cattle. Tabanus were most frequent around the head (A2) with a statistically significant difference (P<0.05) in their alighting sites. Stomoxys were most frequent around the front legs (C0) with a statistically significant difference (P<0.05) in their landing sites. It occurred that most insect groups had preference for the legs and there was an overlap of some insect groups for some body parts like legs (Table 1).
Table 1 : Number of fly counts with respected to the body part of cattle
3.2 The color preference of different insect groups
10The animal color with the highest insect group frequency was brown. Simulium mostly preferred landing on brown than their black and white counterparts with a statistically significant difference (Kruskal Wallis Chi-squared test=10.961, df=2, P=0.00). Chrysops highly preferred landing on brown animals than other colors with a statistically significant difference (Kruskal Wallis Chi-squared test=15.989, df=2, P=0.00). Culicids recorded highest landing preference for brown than other colors with a statistically significant difference (Kruskal Wallis Chi-squared test=7.068, df=2, P=0.02). The individuals of the genus Tabanus preferred landing on black cattle than other colors with no statistically significant difference (Kruskal Wallis Chi-squared test=2.254 x 10-6, df=2, P=1.00). Stomoxys preferred landing on brown cattle than on those with other colors with a statistically significant difference (Kruskal Wallis Chi-squared test=73.601, df=2, P<2.2x10-16).
Table 2 : Color preference of the different insect groups
3.3 The different FMDV shedding sites on cattle
11The FMD discharge doses (TCID50/ml) from the different body spots of animals was gotten from already published experimental works (Bravo de Rueda, 2015)
Table 3 : FMDV discharge doses with respect to the source and route of contamination
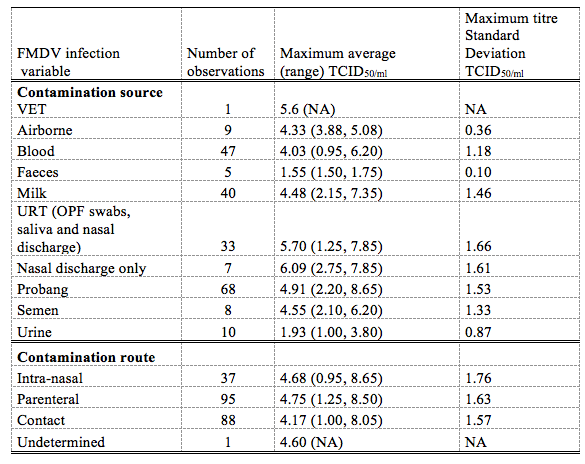
Total refers to all the maximum titres observations that where encountered. VET, vesicular eptihelia tissue NA (not available), *TCID50 per animal per day for airborne excretion; dose of infection and days post infection were divided as above and belox themedian of the maximum titer calculated using the maximum when either the dose of infection or the days post infection was available (Bravo de Rueda, 2015)
12According to Bravo de Rueda (2015), excretion of FMD occurred in unequal concentration in excretions from the different body parts of domestic animals (cattle, sheep and pigs). For the Upper Respiratory Tract (URT) consisting of Oropharyngeal Fluid (OPF) swabs, salivary and nasal discharges had mean infectious dose of 5.70±1.66, Vesicular Epithelia Tissues (VETs) around the mouth, tongue, udder, scrotum and inter-digital spaces had mean infectious dose of 5.6, milk with infectious dose of 4.48±1.46, semen with infectious dose of 4.55±1.33, urine with infectious dose of 1.93±0.87 and faeces with infectious dose of 1.55±0.10.
3.4 Association of predilection sites of haematophagous flies and FMD discharge spots on cattle
13The multiple analyses of variance [Principal Component Analysis (PCA)], where body parts constituted the rows and species constituted the columns was used to associate alighting predilection sites of insect groups with sites of contamination with FMD. The letters in red stood for low FMDV shedding sites while those in blue stood for high FMDV shedding sites (Figure 3). From the association test, all the insect groups had a high probability of landing on FMDV high risk spots [A1, C0, E1, F1, G0 and H3] due to their high alighting propensity for such zones (Figure 3).
Figure 3. Analysis of correspondence showing the association of FMD risk grading and fly groups preferred body-parts of cattle.
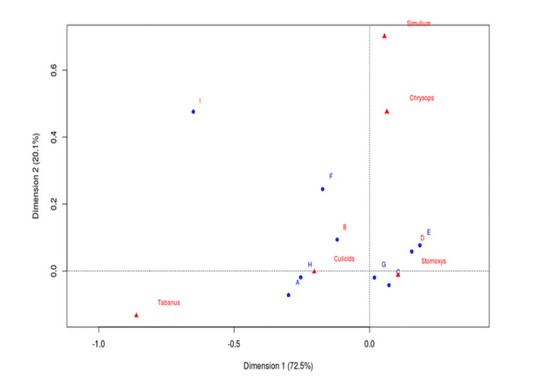
* letters in blue signify body part regions with high FMDV excretion potentiel while those in red signifies body regions with low FMDV excretion. Letters A to I represent biting preference sites of the various fly-groups as annotated on the cattle model where : A-head regions (high FMD risk region), B-neck region (low FMD risk), Co/Go-lower-leg regions (high risk of FMD), D-the trunk region is a low FMD excretion/secretion zone (highly close to urine or faecal discharge hence high risk of contamination with FMDV) and I-tail region is a low FMDV risk region
14The sketch of FMDV excretion/secretion spots on cattle using the published data of Bravo de Rueda (2015) can be seen in figure 4. The nasal area is a high source for FMDV contamination, followed by VET around the mouth, legs and testicular/mammary region, milk, semen, faeces and urine. Such spots are FMDV contamination risk areas for alighting boophilic hematophagous insects because of the high infectious dose (1.55 to 5.60 TCID50/ml) discharged from those sites .
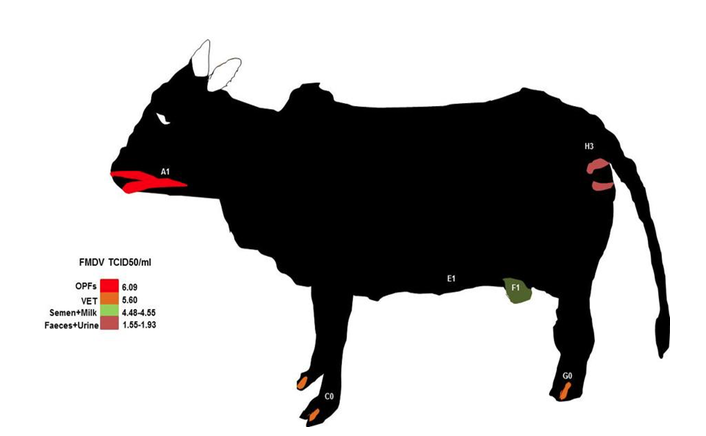
Figure 4 : bovine model showing FMDV contamination risk grading based on the excreted doses from the various body parts (data grom Bravo de Rueda, 2015). OPFs : oropharyngeal fluids, VET: vesicular eptihelia tissue. The letters from A to H show the alighting prefenrece sites of some haematophagous flies.
4. DISCUSSION
15The total number of alighting insects on cattle in the present study was alarming and was dominated by culicids and Stomoxys. Fly groups that were likely to be observed or caught on cattle included surface feeders, blood-sucking and myiasis causing flies (Lloyd & Dipeolu, 1974). The most dominant group observed on cattle constituted of muscids. Such an observation was like that of Lloyd & Dipeolu (1974). The abundant nature of Stomoxys in the present study was in line with the report of Mihok & Clausen (1996). Stomoxys have been reported to adapt in several environments (Zumpt, 1973; Mavoungou et al., 2017). The second most dominant group constituted of culicids and was like the observation made by Muenworn et al. (2009) who showed that culicids were more attractive to cattle than humans. However, the landing predilection for cattle body parts by the different fly groups was different and most of the times overlapped. This landing site overlap resulted in scramble feeding which was a possible risk factor for their contamination when feeding on open sores or lesions in the case of vesicle forming diseases like Vesicular Stomatitis Virus (VSV) and FMDV. Landing preferences recorded in the present study revealed alighting preference discrepancies by the insect groups identified where Stomoxys preferred the front legs, culicids preferred front legs, Simulium preferred belly region, Chrysops preferred front legs and Tabanus preferred the head region. An overlap in the different fly groups predilection sites in our study has been observed for some groups like muscids and tabanids, scrambling for landing sites around the lower leg (Mihok & Clausen, 1996; Baldacchino et al., 2013; Lendzele et al., 2019). The probable reason for the choice of legs by most insect groups was that the skin there was thinner and blood capillaries were closer to the surface of the skin (Todd, 1964; Lendzele et al., 2019). Those that preferred the head region like Tabanus might be because they were orientated to CO2 emissions from this part of the body (Mullens & Gerhardt, 1979).
16The implication of color coat in the prevalence of FMD in Cameroon by Dickmu et al. (in press), showed that brown cattle were the most infected and this was in line with the present finding that brown cattle were preferably attacked by three out of the four fly groups identified. Among the three vector groups associated with brown color coat was Stomoxys where S. n. niger was recently shown to be contaminated with infective doses of the FMDV RNA during the 2016 epidemic in the environs of the Ngaoundere cattle market (Sevidzem et al., 2019b). Arzt et al. (2018) showed the mechanical transmission of FMD from persistently infected carrier cattle to naïve counterparts via the transfer of OPFs. Based on the association of biting preference and FMD risk graded spots, Stomoxys were strongly-positively correlated with front legs, Tabanus (head), culicids (front legs), Simulium (belly region) and Chrysops (front legs). From the association of cattle fly-frequencies/bionomics with respect to different annotated parts and the FMD risk graded maps, it occurred that all the fly groups had a high probability of landing on FMDV high risk spots due to their high landing propensity for FMD risk zones on cattle. This present result is in line with the report of Carn (1996), Ferris et al. (1955) and Hyslop (1970) who reported that tabanids, Stomoxys and mosquitoes can transmit viruses of the family Picornaviridae through horizontal transmission. However, since all the fly groups showed equal chances of being contaminated, Stomoxys and culicids will have a higher probability of being contaminated as compared to others based on their high alighting densities on the exposed animals.
5. CONCLUSIONS
17The number of insect groups landing on exposed cattle was high and were identified in order of magnitude: Stomoxys, culicids, Simulium, Chrysops and Tabanus. Based on landing predilection site with insect group, it was observed that Chrysops preferred landing around front legs. Culicids preferred landing around front legs. Tabanus preferred the head region and Stomoxys preferred front legs. Brown colored cattle were most attractive to most insect groups. From the association test, all the landing insect groups had a high probability of landing on FMDV high risk spots due to their high landing propensity for such zones.
ACKNOWLEDGEMENTS
18We are grateful to PD. Dr. Renz for giving us the permission to use the experimental animals of the DFG-COBE project. We equally thank the herders and Dr Eisenbarth for assisting during fly observations.
Bibliographie
Arzt J. et al., 2018. Transmission of Foot-and-Mouth Disease from persistently infected carrier cattle to naïve cattle via transfer of Oropharyngeal fluid. Clinical Science and Epidemiology, 3.
Baldacchino F. et al., 2013. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): a review. Parasite, 20, 26.
Barros A.T.M. & Foil L.D., 2007. The influence of distance on movement of tabanids (Diptera: Tabanidae) between horses. Veterinary Parasitology, 144, 380–384.
Bertram M. et al., 2018. Effect of vaccination on cattle sub-clinically infected with Foot-and-Mouth disease virus in Cameroon. Preventive Veterinary Medicine, 155, 1-10.
Bravo de Rueda C., 2015. The role of infection routes and species differences in the transmission of FMDV. PhD thesis, Wageningen University, Wageningen, NL, 138 p.
Carn V.M., 1996. The role of dipterous insects in the mechanical transmission of animal viruses. Brazilian Veterinary Journal, 152, 377-93.
Dickmu J. et al., in press. Molecular Epidemiology of Foot-and-Mouth Disease Virus in North Region of Cameroon.
Ferris D.H., Hanson R.P., Dicke R.J. & Roberts R.H., 1955. Experimental transmission of vesicular stomatitis virus by Diptera. Journal of Infectious Diseases, 96, 184-192.
Freeman P. & Meillon B., 1953. Simuliidae of the Ethiopian Region. British Museum (National History) London, 1-224 p.
Gilles M.T. & De Meillon B., 1968. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). 2nd Ed. South African Institute for Medical Research, Johannesburg, 1-343 p.
Gimonneau G. et al., 2016. Insecticide and Repellent Mixture Pour-On Protects Cattle against Animal Trypanosomosis. PLOS Neglected Tropical Diseases, 1-16.
Hansens C.A., 2015. Stable Flies, Winter Bedding, and Summer Dairy Cow Comfort. Thesis submitted to the faculty of the University of Minnesota in partial fulfillment of the requirements for the degree of Master of Science, 133 p.
Hess W.R., 1971. African swine fever. Virology Monographs, 9, 1-33.
Hyslop N.G., 1970. The epizootiology and epidemiology of foot-and-mouth disease. Advances in Veterinary, Science and Comparative Medicine, 14, 261-307.
Jupp P.G., 1996. Mosquitoes of Southern Africa: Culicinae and Toxorhynchinae. Ekogilde Publishers, Hartebeespoort, South Africa, 156 p.
Kasanga C. et al., 2014. Rapid, sensitive and effective diagnostic tools for foot-and-mouth disease virus in Africa. Onderstepoort Journal of Veterinary Research, 81, 5.
Krčmar S., Adolić R.V., Lajoš P. & Lukačević I., 2014. Efficiency of colored modified box traps for sampling of tabanids. Parasite, 21, 67.
Lendzele SS. et al., 2019. Aspects of the bionomics of hematophagous symbovine dipterans in a hyperinfested rangeland of Ngaoundere (Adamawa-Cameroon). Journal of Asia-Pacific Entomology, 22, 1019-1030.
Lloyd D.H. & Dipeolu O.O., 1974. Seasonal Prevalence of flies feeding on cattle in Northern Nigeria. Tropical Animal Health and Production, 6, 231-236.
Mavoungou JF. et al., 2017. Breeding Sites of Stomoxys spp. (Diptera: Muscidae), a Preliminary Study in the Makokou Region (North-East-Gabon). Vector Biology Journal, 2, 1.
Mihok S. & Clausen P.H., 1996. Feeding habits of Stomoxys spp. stable flies in a Kenyan forest. Medical and Veterinary Entomology, 10, 392-394.
Muenworn V. et al., 2009. Biting activity and host preference of the malaria vectors Anopheles maculatus and Anopheles sawadwongporni (Diptera: Culicidae) in Thailand. Journal of Vector Ecology, 34, 62-69.
Mullens B.A. & Gerhardt R.R., 1979. Feeding-behavior of some Tennessee Tabanidae. Environmental Entomology, 8, 1047-1051.
Oldroyd H., 1957. The horse-flies (Diptera: Tabanidae) of the Ethiopian region. III. Subfamilies Chrysopinae, Scepsidinae and Pangoniinae and a revised classification. London, UK, British Museum (Natural History), 489 p.
Sevidzem S.L., Mavoungou J.F. & Mintsa N.R., 2019a. Veterinary Pharmaceuticals Sold in Cattle Markets for the Management of Foot-and-Mouth Disease and Flies in Vina Division (Adamawa-Cameroon). Dairy and Veterinary Science Journal, 10, 001-0013.
Sevidzem S.L. et al., 2019b. Risk Factors for the Contamination of Wild Stomoxys niger niger Macquart 1851 (Diptera: Muscidae) with the Foot-and-Mouth Disease Virus. Current Research in Agricultural Sciences, 6, 95-108.
Sevidzem S.L. et al., 2019c. Insecticide coated screen models reduce insect-vector population in a pasture area in Ngaoundere, Cameroon. Trends in Applied Sciences Research, 14, 80-89.
Todd D.H., 1964. The biting fly Stomoxys calcitrans (L.) in dairy herds in Newzealand. Journal of Agriculture Research, 7, 60-79.
USDA: APHIS: VS, 1994. Foot-and-Mouth Disease: Sources of Outbreaks and Hazard Categorization of Modes of Virus Transmission Centers for Epidemiology and Animal Health. South Howes, Suite 200 Fort Collins, Colorado, 80521.
Zumpt F., 1973. The Stomoxyine biting flies of the world. Diptera: Muscidae. Taxonomy, biology, economic importance and control measures. Stuttgart: Gustav Fischer Verlag, 175 p.