- Home
- Volume 73 (2020)
- Thrips diversity and Frankliniella occidentalis trends on three melon cultivars at Biskra, Algeria
View(s): 2309 (20 ULiège)
Download(s): 93 (0 ULiège)
Thrips diversity and Frankliniella occidentalis trends on three melon cultivars at Biskra, Algeria

Attached document(s)
original pdf fileEditor's Notes
Reçu le 19 décembre 2019, accepté le 31 août 2020
Cet article est distribué suivant les termes et les conditions de la licence CC-BY (http://creativecommons.org/licenses/by/4.0/deed.fr)
Résumé
Dans le monde, les thrips ravageurs des agro-écosystèmes sont bien étudiés. Cependant, en Algérie ce groupe d’insecte reste mal connu. Le but de l’étude est de déterminer la composition des thrips et la dynamique de population de F. occidentalis sur trois cultivars de melon (Star plus, DRM et Mimosa). Six espèces de thrips dont deux sont considérées nuisibles d’importance économique ont été identifiées : Frankliniella occidentalis et Thrips tabaci. Les autres espèces sont Odontothrips loti, Aeolothrips intermedius, T. minutissimus et Melanthrips fuscus. Frankliniella occidentalis (thrips californien) est une espèce eudominante, tandis que T. tabaci est subrésidente. Les pièges bleus adhésifs permettent de détecter les thrips tôt avant la croissance des cultures. Une relation positive et significative a été observée pour l’abondance de thrips sur les fleurs et feuilles de tous les cultivars. Le thrips californien est le principal thrips nuisible. Il est présent sur tous les cultivars à des taux différents. Les nombres les plus élevés du thrips californien ont été enregistrés sur les fleurs de DRM et les feuilles Star plus. Les abondances les plus faibles ont été notées sur les fleurs et les feuilles du cultivar Mimosa. L’activité de vol de F. occidentalis a été observée au début de la saison de croissance du melon. La dynamique de population des thrips sur les trois variétés présente la même tendance. Un à deux pics ont été enregistrés sur les fleurs et feuilles de tous les cultivars. Ils sont observés en avril où les températures sont élevées et l’humidité faible, leur nombre n’était pas considérable. Aucun dommage aux fruits n’a été observé in situ durant l’étude. Ce travail a permis d’améliorer les connaissances des thrips liés à quelques variétés de melon les plus utilisées sous serre dans la région de Biskra. La présence de ces ravageurs peut constituer une source de préoccupation pour les agriculteurs. Etendre le suivi à d’autres cultures et localités pour connaitre leur statut et distribution reste une priorité à cause des dégâts directs qu’ils peuvent occasionner et le risque de transmission de virus.
Abstract
Globally, thrips pest of agro-ecosystems are well studied. Nevertheless, in Algeria this insect group remains poorly known. The research was conducted to determine thrips composition and population changes of F. occidentalis on three melon cultivar. The study highlighted six thrips species on three melon cultivars (Star plus, DRM and Mimosa), including two of economic importance pests, namely Frankliniella occidentalis and Thrips tabaci. The remaining species are: Odontothrips loti, Aeolothrips intermedius, T. minutissimus and Melanthrips fuscus. The western flowers thrips (WFT) F. occidentalis was eudominant species, while T. tabaci was subrecedent. Blue sticky traps allow detecting thrips early for monitoring and from crop development starting. A positive and significant relationship was observed between thrips abundance in traps and WFT recovered from flowers and leaves on all cultivars. The highest numbers of WFT were recorded on DRM flowers and Star plus leaves while the least numbers were noticed on Mimosa flowers and leaves. F. occidentalis flight activity was observed early in the growing season. Similar trend of thrips population changes was recorded during crop season. Nevertheless, one to two peaks of F. occidentalis population were registered on flowers and leaves on all cultivars. They were mainly observed in April when temperatures were high and humidity low, their numbers were not considerable. No fruits damage was observed in situ on all cultivars. This work allowed to improve knowledge about thrips linked to some melon cultivars the most used in greenhouse in the region of Biskra. The presence of these pests can be a source of concern for farmers. Extending monitoring to other crops and localities to know their status and distribution remains a priority due to direct damage caused and virus transmission hazard.
Table of content
1 INTRODUCTION
1Cucurbits crops such melons are cultivated for their fruits throughout the world (Romay et al., 2014). In Algeria, melon cultivation is grown in open fields or in greenhouses. They are harvested and consumed essentially mature as fruits. Melon cucurbit is a crop of high economic importance in Biskra (Algeria) given cultivated areas dedicated each year to this crop (about 1,292 ha). The production was estimated to more than 52203 tons/ ha at Biskra (M. Gouaned, pers. comm.).
2Melon crop faces various viral, fungal diseases and other pests of economic importance including nematodes, mites, whiteflies, aphids and thrips (Romay et al., 2014; Abdel-Rahman et al., 2016; Sharma et al., 2016). The latter are among the most important melon pests at Briska as worldwide, corresponding nearly 6000 species (Reynaud, 2010). Only 1% have been reported as serious pests of crops (Mound & Teulon 1995) and 0.15% are known to be vectors of tospoviruses (Mound, 2001), comprising the western flowers thrips (WFT) Frankliniella occidentalis. Larvae and adults of the latter are considered very harmful species on plants (Reitz, 2009; Mouden et al., 2017), causing direct damage on different plant parts (stems, leaves, flowers, fruits...etc.) and transmitting viral diseases such as Tomato spotted wilt virus (TSWV) and other tospoviruses (Reitz, 2009). Most thrips have cryptic behavior (Mound, 1983; Morse & Hoddle, 2006). Their small size makes them difficult to detect on plants due to delayed damage appearance, which may defer pest control start (Reitz, 2009). Various methods are used to control thrips and protect crops, such as chemical and biological control (Demirozer et al., 2012). Plants display various traits and strategies of defense for their protection against pests (Peterson et al., 2016). Selection of suitable melon varieties correspond to potential undesirable plants for thrips and one preventive control strategy (Papadaki et al., 2008).
3The main objective of this study was to investigate thrips species diversity and abundance on three cultivars of melon under greenhouse environment considering the both thrips species diversity and population changes in Biskra conditions.
2 MATERIAL AND METHODS
2.1 Crops sets
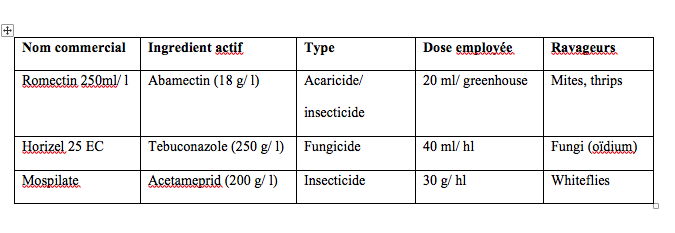
4The study was conducted in three plastic tunnel greenhouse of 400 m² surface areas, sited at Oued Beraze (east of Biskra) (N34°42.096’, E006°00.167’). Greenhouses were oriented east-west. Melons were cultivated after two years of tomato crop growing. Each greenhouse was planted with one melon cultivar. The first greenhouse (G1) was cultivated with Star plus cultivar, the second (G2) with DRM (pineapple melon) and the third (G3) with Mimosa cultivar. The seeds were sown in nursery before transplanting. The greenhouses contained about 300 plants arranged in six rows. The space between rows was 1 m and 0.9 m between plants. Melons were conducted horizontally on the ground surface without plastic mulch. The crops were irrigated with drip system three times a week according to crop needs. The soil is poor in organic matter, strongly calcareous and very salty with alkaline pH. The most encountered pests were whiteflies, aphids and mites. The pesticides used during cultivation were summarized in table 1. Predatory bugs like Orius sp. and Chrysopa vulgaris Schneider were frequently observed.
Table 1 : Pesticides used during melon cultivation at Oued Beraze (Biskra)
2.2 Thrips sampling
2.2.1 Blue sticky traps
5Sticky traps were commonly used for detecting thrips occurrence and adult flight starting (Cloyd, 2009; Ugine et al., 2011). Two blue traps (26 x 21 cm in size) were set in each greenhouse entrances. To avoid the edge effect, the traps were positioned a few meters (approximately 8 m) away from greenhouse doors. The traps were hung at 30 cm above plants and replaced regularly. The thrips were weekly counted in situ using a pocket magnifier glass (x10).
2.2.2 Blue water traps
6Different colors preferences and attractiveness among many thrips species have been studied (Hoddle et al., 2002; Rodriguez-Saona et al., 2010). However, most thrips were captured and attracted by blue water traps. These help in determining thrips diversity and providing information on thrips activity (Andjus et al., 2001). Three blue water traps (20 cm diameter and 12 cm in deep) were set per greenhouse on the ground and spaced from each other by few meters (approximately 10 m). Two of them were positioned at each greenhouse entrance and one at the middle. The containers were filled to with water and few drops of detergent. Trap contents were weekly collected and brought back to laboratory until crop end. Thrips were carefully recovered in the strainer under binocular magnifier and then placed in tubes containing 70% ethanol to be further slide mounted and identified with identification keys (Mound, 1974; Mound et al., 1976; Mound & Walker, 1982, 1986).
2.2.3 Flowers recovery
7Ten fully opened melon flowers (one flower per plant) were collected randomly each week and immediately placed in plastic bags to bring back to the laboratory. The flowers were carefully examined under a binocular magnifying glass for thrips extracting using a moistened brush. Recovered thrips were put in tubes containing 70% ethanol. Bloom sampling continued until plants stop producing flowers. Adult thrips were identified and counted (Manandhar et al., 2017).
2.2.4 Leaves shaking
8Shaking method allows to recover 80% of adults and 18% of larvae from leaves (Gonzalez-Zamora & Garcia-Mari, 2003). Foliage shaking started about three weeks after melon transplanting due to plant vigor. Every week, thirty leaves taken randomly were shaken 15 times. The leaves were gently and vigorously shaken to dislodge thrips and avoid damaging the plants on a white plateau of 30 cm diameter and 1.5 cm depth. Thrips dropped and were gathered with a moistened brush and placed in tubes containing 70% ethanol. Thrips were brought back to the laboratory, slide mounted for identification and counting.
2.3 Statistical analyses
Domination coefficient was calculated informing about the amount of collected species participating among sampled specimens in a given area using the formula from Kasprzak & Niedbała (1981): Di (%) = ni / N 100
Di – domination coefficient of particular species in percent,
ni – number of particular species,
9N − total number of all thrips species collected
10There are six classes of domination coefficient, they are as follows: eudominant (32.0-100.0%); dominant (10.0-31.9%); subdominant (3.2-9.9%); recedent (1.0–3.1%), subrecedent (0.3-0.9%) and sporadic (< 0.3%).
11Recorded data from sampling were transformed using natural logarithm ln (x + 1) before analysis. One-way ANOVA analysis was performed for significance, and then LSD test was used to separate means at P< 0.05. Relationship between thrips collected on flowers and caught on blue sticky traps on the different cultivars was performed with Pearson’s correlation; P and t values were calculated using t- test of student. Data analyses were carried out using Statistica 6 StatSoft, Inc. 1984-2003.
3 RESULTS
3.1 Thrips composition
12A total of 2664 thrips individuals were collected from the two sampling methods. The terebrantian thrips were estimated to be 98%. Six thrips species on melon crop were determined: Frankliniella occidentalis Pergande, Thrips tabaci Lindeman, Odontothrips loti Haliday, Aeolothrips intermedius Bagnall, T. minutissimus L., Melanthrips fuscus Sulzer. Some unidentified tubulifers for different reasons were collected during the study (< 2%). The population of F. occidentalis (eudominant species) was the commonest species and very significant on the three melon cultivars. The calculated domination coefficient revealed a percentage of 84.9% on Star plus, 83.2% on DRM and 87.1% on Mimosa cultivar. Followed by T. minutissimus as dominant species on Star plus (13.6%), DRM (13.9%) and as subdominant on Mimosa cultivar (6.6%). On Star plus, the population was subrecedent (0.8%), recedent on DRM (2.3%) and subdominant on Mimosa (4.1%). However for O. loti, it was subrecedent on all cultivars (0.7% on Star plus, 0.5% on DRM and 0.3% on Mimosa). Only one specimen of M. fuscus was collected on Star plus and DRM cultivar. Calculated coefficients of dominance were respectively two times sporadic and one time recedent on Mimosa. T. tabaci was only recovered on Mimosa cultivar as subrecedent species (0.8%). In overall, thrips composition of melon cultivars was similar and varied in numbers of specimen.
3.2 Seasonal trends in thrips abundance
13DRM cultivar hosted larger thrips populations in blue sticky traps (69.1 ± 88.5), followed by Mimosa cultivar (208.8 ± 196.1) while the lowest thrips captures was noticed on Star plus cultivar (157.5 ± 125.8). The difference between thrips average number caught in blue sticky traps in the different greenhouses was not significant (F = 2.90, P = 0.065). However, the least significant difference test revealed that the difference in thrips abundance was significant between Star plus and DRM cultivar (P = 0.035).
14Flight activity was low and increased progressively from March to mid April for DRM cultivar, beginning of May for Star plus and for Mimosa cultivar. Catches continued until plants grubbing-up by farmer on mid May (Figure 1).
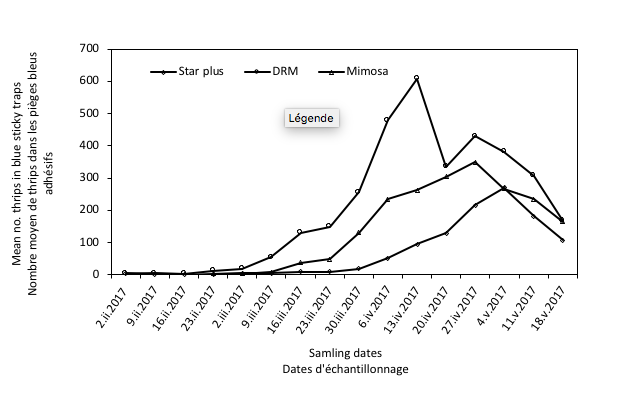 Figure 1: Mean numbers of thrips caught in blue sticky traps coming from three melon cultivars at Oued Beraze (Biskra).
Figure 1: Mean numbers of thrips caught in blue sticky traps coming from three melon cultivars at Oued Beraze (Biskra).
Letters in lowercase indicate that there are no differences between mean numbers of F. occidentalis on flowers and leaves of the different cultivars
Letters in uppercase indicate that there are no differences between mean numbers of F. occidentalis on flowers and leaves of the same cultivar
15Mean numbers of F. occidentalis on flowers and leaves of the different cultivars are presented (Figure 2).
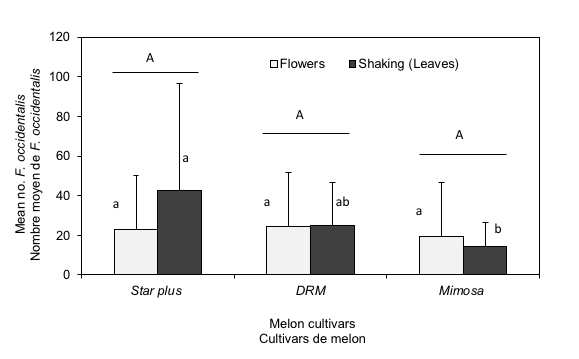 Figure 2: Comparison between seasonal mean numbers of Frankliniella occidentalis collected on flowers and obtained from shaking leaves on the different melon cultivars.
Figure 2: Comparison between seasonal mean numbers of Frankliniella occidentalis collected on flowers and obtained from shaking leaves on the different melon cultivars.
16No statistical significance was observed between abundance of western flower thrips population among flowers (F = 1.12, P = 0.343) and leaves (F = 0.43, P = 0.656) of all cultivars. Excepting for mean numbers of F. occidentalis recorded on leaves of Star plus in relation to mean thrips collected on Mimosa cultivar which was significant (P = 0.049).
3.3 Comparison of trapping and thrips collection on plants
17The flowers number of melon crop was low at first and then increased during the growing season. The relationship observed between number of thrips captured in blue sticky traps and the number of F. occidentalis sampled on flowers of Star plus and DRM cultivars was strong (Figure 3a, b).
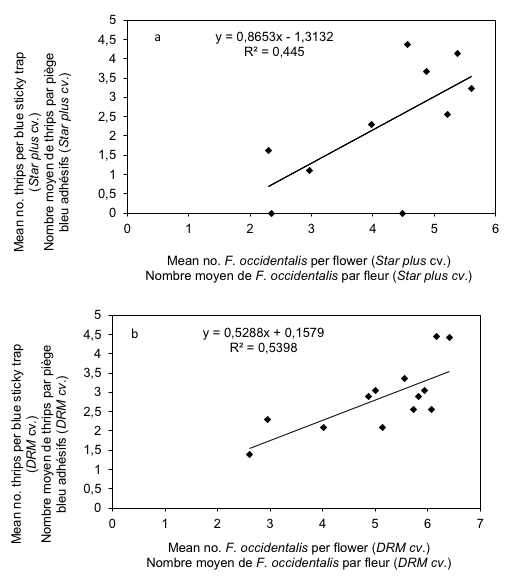
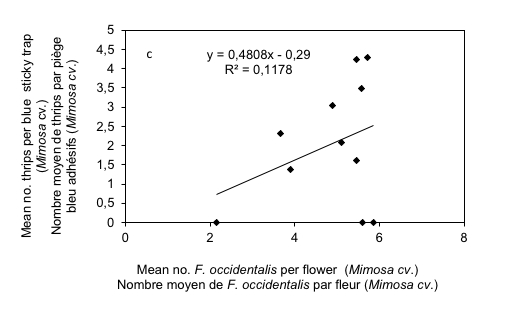
18The correlation coefficients recorded for these two cultivars were respectively R = 0.67 and R = 0.74 for Star plus and DRM respectively. This relationship was positive, and significantly correlated for both cultivars (t = 4.94, P < 0.001 and t = 9.79, P < 0.001 for Star plus and DRM respectively). For Mimosa cultivar, a medium intensity correlation was observed (R = 0.34) and this relationship was positive, and significantly correlated (t = 5.92, P < 0.001) (Figure 3c).
19Regarding WFT sampled on leaves by shaking method, the relationship with total number of thrips caught in blue sticky traps was very strong for Star plus (R = 0.80) (Figure 3d) and Mimosa cultivars (R = 0.89) (Figure 3f); whereas it was strong for DRM cultivar (R = 0.76) (Figure 3e). All relationships were positive, and significantly correlated (t = 3.76 and P = 0.004; t = 9.39 and P < 0.001; t = 11.67 and P < 0.001 for Star plus, DRM and Mimosa respectively).
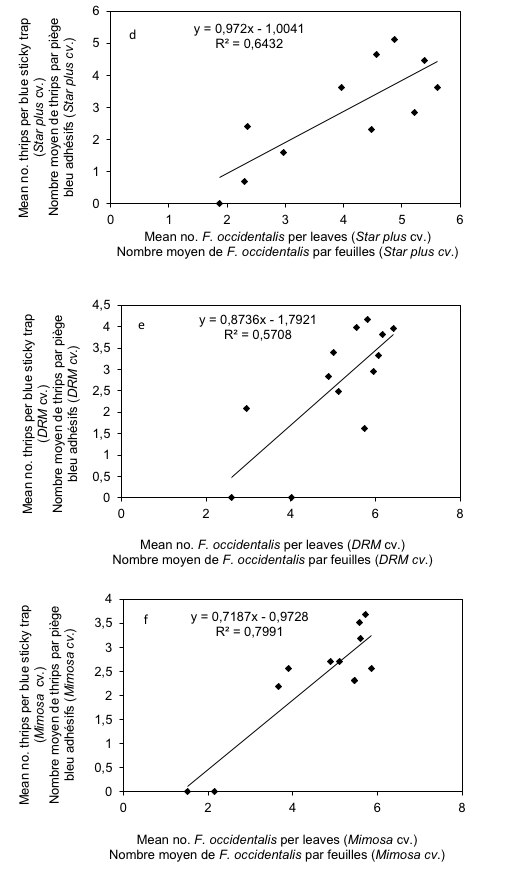 Figure 3: Relationship between mean numbers of thrips per flowers and leaves with mean thrips number caught in blue sticky traps on melon cultivars (Star plus, DRM and Mimosa)
Figure 3: Relationship between mean numbers of thrips per flowers and leaves with mean thrips number caught in blue sticky traps on melon cultivars (Star plus, DRM and Mimosa)
20Globally, there was a positive correlation between WFT issued from flowers samples and leaves shaking with captured thrips in blue sticky traps.
3.4 Population changes of Frankliniella occidentalis
21Adults of F. occidentalis on leaves and flowers were observed from February to March (Figure 4).
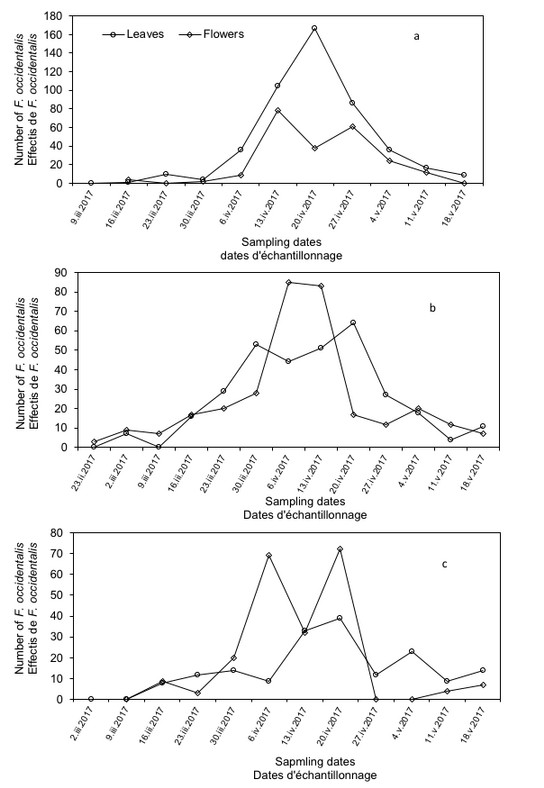 Figure 4: Population changes of Frankliniella occidentalis on leaves and flowers of melon cultivars, (a) Star plus, (b) DRM and (c) Mimosa.
Figure 4: Population changes of Frankliniella occidentalis on leaves and flowers of melon cultivars, (a) Star plus, (b) DRM and (c) Mimosa.
22On Star plus cultivar leaves (Figure 4a), an increase trend of F. occidentalis was registered from the beginning to mid April corresponding to peak numbers of western flower thrips. The temperature recorded during this period was between 21.7-29.2°C and 15-49% relative humidity. This number is decreasing until the end season, corresponding to crop yellowing and drying. The temperature recorded at this time was quite high (35.7-37.2°C) with a decrease in relative humidity (26-35%).Similar trend was observed on DRM and Mimosa cultivars (Figure 4b, c), with an optimum in mid April. However, F. occidentalis number on Star plus cultivar noted at this peak was greater than that on the other cultivars. Regarding temperatures and relative humidity recorded during this period, they were respectively 21.2-34.7°C and 12-32% before and 38.2-40.2°C / 10-28% after this date in greenhouse with DRM cultivar. In Mimosa greenhouse, 30.0-33.7°C / 10-39% were recorded before and 40-43°C / 10-45% after this date.
23About F. occidentalis population changes on melon flowers, the number of western flower thrips was low on the cultivar Star plus at the beginning of cultivation then reached maximum numbers in mid April. However, after this date the number of thrips has decreased until crop season end. Temperature and relative humidity registered before and after this date was between 21.7-41.5°C/ 15-31%; 29.2-37.2°C/ 26-49%, respectively. On flowers of DRM cultivar, F. occidentalis numbers were slightly higher at the beginning of cultivation, continued to increase until they reached its maximum beginning of April then decreased up to the season end (Figure 4b). Recorded temperature and relative humidity were situated between 21.2-32.7°C/ 12-26% before this date and 38.2-41.7°C/ 10-32% after this date. For Mimosa cultivar (Figure 4c), the number of western flower thrips increased until a mid April peak, then declined towards the crop end. The recorded temperature and humidity were respectively 30.0-33.7°C/ 11-39% before mid April and 40.0-53.2°C/ 10-45% after this date.
4 DISCUSSION
24Knowing thrips species occurring an agro-ecosystem is fundamental in building pest control programs (Mirab-balou et al., 2017). Here, six thrips species were determined on melon crop, including two species of economic concern, namely F. occidentalis and T. tabaci. They cause significant direct damages to plants in addition to tospovirus transmission (Reitz, 2009; Gill et al., 2015) and they were recorded on several plant species (Lee et al., 2001).
25The WFT abundance was more important compared to onion thrips (T. tabaci) and was predominant on all melon cultivars while T. tabaci was observed on Mimosa cultivar only. F. occidentalis was dominant on french bean in Kenya (Kasina et al., 2009), cucumber in Korea (Park et al., 2009), on cucumber and pepper crops at Bejaia (east coast of Algeria) (Oudjiane et al., 2018) and on tomato and pepper/ chili pepper at Biskra (Laamari & Houamel, 2015). Five thrips species were found in coastal and sub-coastal part of Algiers, including F. occidentalis, O. loti (Benmessaoud-Boukhalfa et al., 2010). Also, Razi and colleagues (2013) listed seven thrips species on broad bean in different region of Biskra. According to these studies, four species are in common, namely F. occidentalis, O. loti, A. intermedius and M. fuscus. However at Zeralda (west coast of Algiers), the two economically important pests F. occidentalis and T. tabaci were observed in tomato greenhouse (Djebara et al., 2018).
26The thrips abundance differed according to melon cultivars: higher on DRM and weaker on Star plus cultivars possibly related to the preceeding tomato crop. The latter was removed in December and replaced by melon since January. Also, the thrips number may be increased with flower occurrence (Elimem & Chermiti, 2009). In Biskra, farmers usually allowed 1 to 3 melon fruits per plant in maximum to develop. It would be interesting to remove excess of flowers to reduce thrips number. In addition, the WFT also used flowering weed species to infest crops. Katayama (2006) suggested that suppressing flowering weeds in spring should be an effective thrips control approach.
27Blue sticky traps allowed early detection of thrips in greenhouses, even before first captures by direct sampling methods. According to Natwick et al. (2007), blue sticky traps were already found to be sensitive in detecting thrips presence prematurely on crops such as lettuce and onion. Rodriguez-Saona et al. (2010) found white sticky traps more effective than blue traps in New Jersey blueberries due to higher attractiveness corresponding to white blueberries flowers coinciding with thrips flight activity. However, Abdul Alim et al. (2018) showed that monitoring thrips in persimmon orchards with yellow sticky traps was more appropriate. Changes in air temperature also influenced T. tabaci density on sticky traps (Rodriguez-Saona et al., 2010; Szostek & Schwartz, 2015). In this study, the low number of arthropods in early season may be due to low temperatures and host plants lack in the field (Summers et al., 2010).
28On flowers, the numbers of F. occidentalis were not significant on all cultivars. The difference was significant between leaves of Star plus and Mimosa cultivars while Mimosa and DRM cultivars attracted less F. occidentalis than Star plus. Similarly, Messelink & de Kogel (2005) recorded a significant difference in F. occidentalis populations among chrysanthemum cultivars. On cotton, Khan et al. (2014) revealed that genotypes differed in their response to thrips population. Likewise, T. tabaci densities on onion were similar on all cultivars at the beginning and then vary according to the cultivars (Malik et al., 2010). The number of thrips on plants depends not only on cultivars but also with fertilization rate and water (Schuch et al., 1998).
29According to Broughton & Harrison (2012), the monitoring effectiveness of trapping as tool was dependent on number of thrips caught in traps with the number of thrips collected on crops.
30In this study, significant relationship between number of all thrips catches on blue sticky traps and number of WFT recovered from blossom and issued from melon leaves occurred. Then, a strong and positive correlation was reported between thrips caught in sticky traps and collected on onion crop (MacIntyre-Allen et al., 2005) and was higher in dry season on Mango orchard panicles (Aliakbarpour & Rawi, 2011). Likewise, the same relation was revealed by Muvea et al. (2014) with thrips captured on colored sticky traps and thrips infesting French bean leaves and flowers (destructive methods) and tapping (non destructive methods). In Western Australia on deciduous fruit trees, Broughton & Harrison (2012) recorded poor correlation between thrips catches in traps and tapping samples. In tomato crops, Covaci et al. (2012) indicated no correlation between sticky traps and tapping method of leaves.
31According to statistical analysis, the difference between thrips density on the three cultivars was not significant, but it seems that changes were not similar on the leaves unlike the flowers. Essentially one to two peaks were recorded during the cropping season. These numbers were registered in April, where temperature and humidity did not exceed 37.5°C and 35.7% respectively. Arif et al. (2006) and Malik et al. (2010) pointed out that T. tabaci population got their peak in August on onion cultivars. In Tunisia, Elimem & Chermiti (2009, 2013) recorded the maximum number of F. occidentalis in June on roses and during April and May for citrus orchard. No injury due to thrips was observed in situ on all melon cultivars assuming that economic threshold has not been reached.
5 CONCLUSIONS
32The melons cultivar used in this study (Star plus, DRM and Mimosa) were the most cultivated cucurbits at Biskra. There was no real program of thrips control in vegetable crops and cucurbits particularly. This should be among the priorities due to the presence of insect virus vectors such as thrips transmitted tospoviruses. This study allowed the reporting of six thrips species including two of economic importance. The blue sticky trap is important tool in greenhouses allowing early detection of thrips presence which confirmed earlier studies. A positive and strong relationship between thrips abundance was determined on blue sticky traps and thrips recovered from flowers and leaves shaking. The general trend of thrips on different cultivars was weak at first and important towards crop end when temperatures increased from spring. This allowed planning thrips control on melon crops at Biskra without insecticide treatments abuse in an integrated pest management program.
6 ACKNOWLEDGEMENTS
33The authors are very grateful to M. Benazrine M. agricultural engineer and the farmer of Oued Beraze (Biskra) for their help; Maatalah N. for providing us blue sticky traps. The study was supported by the PRFU project under identification number D04N01UN070120190001 and by the Algerian Ministry of Higher Education and Scientific Research (DGRSDT/M.E.S.R.S.).
Bibliographie
Abdel-Rahman M.A.A., Ali M.M.A., Awad A.M.A., Shafea A.M.H. & Abdel-Rahem G.H., 2016. Co-existence of pests and their associated predators inhabiting cantaloupe plants, Cucumis melo L. in Assiut, Egypt. Assiut University Bulletin for Environmental Researches, 19, 1-9.
Abdul Alim M.D., Song J., Seo H.J. & Choi J.J., 2018. Monitoring thrips species with yellow sticky traps in astringent persimmon orchards in Korea. Applied Entomology Zoology, 53, 75-84.
Aliakbarpour H. & Rawi C.S.M.D., 2011. Evaluation of yellow sticky traps for monitoring the population of thrips (Thysanoptera) in a Mango orchard. Environmental Entomology, 40(4), 873-879.
Andjus L., Spasic R. & Dopudja M., 2001. Thrips from coloured water traps in Serbian wheat fields. In: Marullo R.& Mound L.A.,eds. Thrips and tospoviruses : Book of proceedings, 2-7 July, 2001, Calabria, Italy, 345–350.
Arif M.J., Gogi M.D. & Ahmad G., 2006. Role of morpho-physical plant factors imparting resistance in cotton against thrips, Thrips tabaci Lind. (Thripidae: Thysanoptera). Arab Journal of Plant Protection, 24, 57-60.
Benmessaoud-Boukhalfa H., Mouhouche F. & Belmazouzi F.Z., 2010. Inventory and identification of some Thrips species in coastal and sub-coastal regions of Algeria. Agriculture and Biology journal of North America, 1(5), 755-761.
Broughton S. & Harrison J., 2012. Evaluation of monitoring methods for thrips and the effect of trap colour and semiochemicals on sticky trap capture of thrips (Thysanoptera) and beneficial insects (Syrphidae, Hemerobiidae) in deciduous fruit trees in Western Australia. Crop Protection, 42, 156-163.
Cloyd R.A., 2009. Western flower thrips (Frankliniella occidentalis) management on ornamental crops grown in greenhouses: Have we reached an impasse? Pest Technology 3(1), 1-9.
Covaci A.D., Oltean I. Raica P.A. & Mitre V., 2012. Monitoring of western flower thrips population in a greenhouse tomato crop. Bulletin UASVM Agriculture, 69(1), 214-220.
Demirozer O., Tyler-Julian K., Funderburk J., Leppla N. & Reitz S., 2012. Frankliniella occidentalis (Pergande) integrated pest management programs for fruiting vegetables in Florida. Pest Management Science, 68, 1537-1545.
Djebara F., Benzahra A., Mimeche F. & Saharaoui L., 2018. Diversity of entomofauna associated with greenhouse-grown tomatoes in Algiers (North Algeria). Studia UBB Biologia, 63(2), 139-151.
Elimem M. & Chermiti B., 2009. Population dynamics of Frankliniella occidentalis Pergande (1895) (Thysanoptera : Thripidae) and evaluation of its different ecotypes and their evolution in a rose (Rosa hybrida) greenhouse in the Sahline Region, Tunisia. African Journal of Plant Science and Biotechnology, 3, 53-62.
Elimem M. & Chermiti B., 2013. Thrips species composition and seasonal dynamic populations in an organic citrus orchard in the central eastern coast of Tunisia. IOBC-WPRS Bulletin, 95, 77-82.
Gill H.K., Garg H., Gill A.K., Gillett-Kaufman J.L. & Nault B.A., 2015. Onion thrips (Thysanoptera: Thripidae) biology, ecology, and management in onion production systems. Journal of Integrated Pest Management, 6(1), 6. doi: https://doi.org/10.1093/jipm/pmv006.
Gonzalez-Zamora J.E. & Garcia-Mari F., 2003. The efficiency of several sampling methods for Frankliniella occidentalis (Thysan., Thripidae) in strawberry flowers. Journal of Applied Entomology, 127, 516-521.
Hoddle M.S., Robinson L. & Morgan D., 2002. Attraction of thrips (Thysanoptera: Thripidae and Aeolothripidae) to colored sticky cards in a California avocado orchard. Crop Protection, 21(5), 383-388.
Kasina M., Nderitu J., Nyamasyo G., Waturu C., Olubayo F., Obudho E., 2009. Within-plant distribution and seasonal population dynamics of flower thrips (Thysanoptera: Thripidae) infesting French beans (Phaseolus vulgaris L.) in Kenya. Spanish Journal of Agricultural Research, 7(3), 652-659.
Kasprzak K. & Niedbała W., 1981. Wskaźniki biocenotyczne stoso- wane przy porządkowaniu i analizie danych w badaniach ilościowych. In: Górny M., Grüm L., eds. Metody Stosowane w Zoologii Gleby. PWN, Warszawa, 379-416.
Katayama H., 2006. Seasonal prevalence of the occurrence of western flower thrips Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) on weed hosts growing around ornamental fields. Applied Entomolology and Zoology, 41(1), 93-98.
Khan M.A., Ali A., Aslam M., Tahir Z., Khan M.M. & Nadeem I., 2014. The role of morphological and chemical plant traits imparting resistance in Bt cotton genotypes against thrips, Thrips tabaci (Lind.). Pakistan Journal of Agricultural Sciences, 51, 725-731.
Laamari M. & Houamel S., 2015. Première observation de Thrips tabaci et de Frankliniella occidentalis sur les cultures sous serre en Algérie. EPPO Bulletin, 45(2), 205-206.
Lee G.S., Lee J.H., Kang S.H. & Woo K.S., 2001. Thrips species (Thysanoptera: Thripidae) in winter season and their vernal activities on Jeju Island, Korea. Journal of Asia Pacific Entomology, 4(2), 115-122.
Macintyre-Allen J.K., Scott-Dupree C.D., Tolman J.H. & Harris C.R., 2005. Evaluation of sampling methodology for determining the population dynamics of onion thrips (Thysanoptera: Thripidae) in Ontario onion fields. Journal of Economic Entomology, 98(6), 2272-2281.
Malik M.F., Rashid M., Iqbal J. & Ahmad A., 2010. Resistance determination against thrips of promising onion varieties in the agro-ecosystem of Balochistan, Pakistan. Punjab University Journal of Zoology, 25, 1-11.
Manandhar R., Wang K.H., Hooks C.R.R. & Wright M.G., 2017. Effects of strip-tilled cover cropping on the population density of thrips and predatory insects in a cucurbit agroecosystem. Journal of Asia Pacific of Entomology, 20(4), 1254-1259.
Messelink G.J. & De Kogel W.J., 2005. Impact of chrysanthemum cultivar, fertilization and soil-dwelling predatory mites on Frankliniella occidentalis. Proceedings of the Netherlands Entomological Society Meeting, 16, 101-107.
Mirab-Balou M., Mahmoudi M. & Tong X. 2017. Diversity of thrips species (Thysanoptera) in fruit orchards in Qazvin province, northwestern Iran. Journal of Crop Protection, 6(3), 363-375.
Morse J.G. & Hoddle M.S., 2006. Invasion biology of thrips. Annual Review of Entomology, 51, 67-89.
Mouden S., Sarmiento K.F., Klinkhamer P.G. & Leiss K.A., 2017. Integrated pest management in western flower thrips: past, present and future. Pest Management Science, 73, 813-822.
Mound L.A., 1974. The complex of spore feeding Thysanoptera (Phlaeothripidae : Idolothripinae). Bulletin of the British Museum (Natural History). Entomology, 31(5), 109-188.
Mound L.A., 1983. Natural and disrupted patterns of geographical distribution in Thysanoptera (Insecta). Journal of Biogeography, 10, 119-133.
Mound L.A., 2001. So many thrips-so few tospovirus. In: Marullo R. & Mound L.A., eds. Thrips and tospoviruses : Book of proceedings, 2-7 July, 2001, Calabria, Italy, 15-18.
Mound L.A., Morison G.D., Pitkin B.R. & Palmer J.M., 1976. Thysanoptera. Handbooks for the Identification of British Insects. London: Royal Entomological Society of London (RES), 1-79.
Mound L.A. & Teulon D.A.J., 1995. Thysanoptera as phytophagous opportunists. In : Parker B.L., Skinner M. & Lewis T., eds. Thrips biology and management. Boston: Springer, 3-19.
Mound L.A. & Walker A.K., 1982. Terebrantia (Insecta: Thysanoptera). Fauna of New Zealand 1.
Mound L.A. & Walker A.K., 1986. Tubulifera (Insecta: Thysanoptera). Fauna of New Zealand 10.
Muvea A.M., Waiganjo M.M., Kutima H.L., Osiemo Z., Nyasani J.O. & Subramanian S., 2014. Attraction of pest thrips (Thysanoptera: Thripidae) infesting French beans to coloured sticky traps with Lurem-TR and its utility for monitoring thrips populations. International Journal of Tropical Insect Science, 34(3), 197-206.
Natwick, E.T., Byers J.A., Chu C., Lopez, M. & Tomas J.H., 2007. Early detection and mass trapping of Frankliniella occidentalis and Thrips tabaci in vegetable crops. Southwestern Entomologist, 32(4), 229-238.
Oudjiane A., Razi S., Bounaceur F., Boussad F. & Benrima A., 2018. Fluctuations saisonnières et dégâts de Frankliniella occidentalis (Pergande, 1895) (Thysanoptera: Thripidae) sur cultures maraichères sous serre dans la région de Bejaia. Revue Agrobiologia, 8(1), 948-957.
Papadaki M., Harizanova V. & Bournazakis A., 2008. Influence of host plant on the population density of Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on different vegetable cultures in greenhouses. Bulgarian Journal of Agricultural Science, 14(5), 454-459.
Park J.J., Lee D.H., Shin K.I., Lee J.H. & Cho K., 2009. Analysis of spatial and temporal associations of adult and immature Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) in cucumber greenhouses. Applied Entomology and Zoology, 44(4), 569-577.
Peterson J.A., Ode P.J., Oliveira-Hofman C. & Harwood J.D., 2016. Integration of plant defense traits with biological control of arthropod pests: challenges and opportunities. Frontiers in Plant Science, 7, 1794. doi: https://doi.org/10.3389/fpls.2016.01794
Razi S., Laamari M., Ouamen S. & Bernard E.C., 2013. Thysanoptera survey on Vicia faba (Broad bean) in the arid Biskra region of Algeria. Agriculture and Biology Journal of North America, 4(3), 268-274.
Reitz S.R., 2009. Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): the making of a pest. Florida Entomologist, 92(1), 7-13.
Reynaud P., 2010. Thrips (Thysanoptera). BioRisk, 4, 767-791.
Rodriguez-Saona C.R., Polavarapu S., Barry J.D., Polk D., Jörnsten R., Oudemans P.V. & Liburd O.E., 2010. Color preference, seasonality, spatial distribution and species composition of thrips (Thysanoptera: Thripidae) in northern highbush blueberries. Crop Protection, 29(11), 1331-1340.
Romay G., Lecoq H. & Desbiez C., 2014. Cucurbit crops and their viral diseases in Latin America and the Caribbean Islands: A review. Journal of Plant Pathology, 96, 227-242.
Schuch U.K., Redak R.A. & Bethke J.A., 1998. Cultivar, fertilizer, and irrigation affect vegetative growth and susceptibility of chrysanthemum to western flower thrips. Journal of American Society of Horticultural Science, 123(4), 727-733.
Sharma A., Rana C. & Shiwani K., 2016. Important insect pests of cucurbits and their management. In : Pessarakli M., eds. Handbook of cucurbits: growth, cultural practices and physiology. Boca Raton: CRC Press, 327-359.
Summers C.G., Newton, A.S., Mitchell J.P. & Stapleton J.J., 2010. Population dynamics of arthropods associated with early-season tomato plants as influenced by soil surface microenvironment. Crop Protection, 29(3), 249-254.
Szostek S. & Schwartz H.F., 2015. Overwintering Sites of Iris yellow spot virus and Thrips tabaci (Thysanoptera: Thripidae) in Colorado. Southwestern Entomologist, 40(2), 273-290.
Ugine T.A., Sanderson J.P., Wraight S.P., Shipp L., Wang K. & Nyrop J.P., 2011. Binomial sampling of western flower thrips infesting flowering greenhouse crops using incidence-mean models. Environmental Entomology, 40(2), 381-390.