- Startpagina tijdschrift
- Volume 75 (2022)
- Influence of cocoa cultivation on the diversity and density of termites in the Azaguié zone (South of Côte d’Ivoire)
Weergave(s): 1748 (12 ULiège)
Download(s): 98 (0 ULiège)
Influence of cocoa cultivation on the diversity and density of termites in the Azaguié zone (South of Côte d’Ivoire)

Documenten bij dit artikel
Version PDF originaleRésumé
Les termites jouent un rôle primordial dans l'aération et la fertilisation des sols où ils abondent. Cependant les pratiques culturales influence leur écologie. Cette étude qui s’est déroulée à Azaguié au Sud de la Côte d’Ivoire avait pour objectif de connaître la diversité de termites qui colonisent les plantations de cacaoyers en comparaison avec le milieu naturel. Trois classes d’âge de plantations ont été définies : PC1 (de 0 à 5 ans), PC2 (de 6 à 10 ans) et PC3 (plus de 30 ans). Dans chaque classe de plantations, trois transects de 100 m de long chacun, subdivisés en 20 sections de 10 m2 (10 m x 2 m) ont été délimités. Trois (3) monolithes ont été également creusés par transect afin de connaître la densité des termites hypogés. Les vieilles plantations avec 18 espèces sont plus diversifiées comparativement aux parcelles PC1 (14 espèces) et PC2 (16 espèces). Le milieu naturel enregistre la plus forte richesse spécifique avec 21 espèces échantillonnées. L’abondance relative des termites champignonnistes dans les transects montre une grande capacité d’adaptation de ces insectes (18 occurrences dans les parcelles PC1 à 26 occurrences dans le milieu naturel). Pour ce qui est des termites hypogés, seize espèces ont été récoltées dans tous les milieux confondus. L’indice de Similarité de Jaccard a montré une grande différence entre les espèces de termites hypogés des différents milieux. Aucune différence significative n’a été observée entre les densités des termites des milieux observés. Cependant, la distribution des termites dans la profondeur du sol est inégalement répartie et la strate superficielle de 0 – 10 cm concentre la majorité des termites dans les parcelles PC1, PC3 et le Milieu Naturel. Dans les parcelles de 6 à 10 ans (PC2), les termites semblent occuper de manière uniforme les horizons de sols échantillonnés. La cacaoculture influe sur la diversité et la densité des termites et la cessation des activités agricoles entrainent la recomposition de la biodiversité des termites.
Abstract
Termites play an important role in the aeration and fertilization of soils where they abound. However, cultural practices influence their ecology. This study, which took place in Azaguié in the south of Côte d'Ivoire, aimed to know the diversity of termites that colonize cocoa plantations in comparison with the natural area. Three age classes of plantations have been defined: PC1 (from 0 to 5 years), PC2 (6 to 10 years old) and PC3 (over 30 years old). In each class of plantations, three transects 100 m long each, subdivided into 20 sections of 10 m2 (10 m x 2 m) have been demarcated. Three (3) monoliths were also dug by transect in order to know the density of hypogeal termites. Old plantations were more diversified with 18 species compared to plots PC1 (14 species) and PC2 (16 species). The natural area recorded the highest specific richness with 21 species sampled. The relative abundance of fungal termites in transects showed a high adaptability of these insects (18 occurrences in plots PC1 to 26 occurrences in natural area). With hypogeal termites, 16 species have been collected in all habitat types. The Jaccard Similarity Index showed large differences between species of hypogenous termites according to habitat types. No significant difference was observed between termite densities in selected plots. However, the distribution of termites in the depth of the soil was unevenly distributed and the surface layer of 0 - 10 cm concentrated the majority of termites. Cocoa production influences termite diversity and density, but the cessation of agricultural activities results in the recomposition of termite biodiversity.
Inhoudstafel
Reçu le 05 juillet 2021, accepté le 13 janvier 2022
Cet article est distribué suivant les termes et les conditions de la licence CC-BY (http://creativecommons.org/licenses/by/4.0/deed.fr).
INTRODUCTION
1Termites, from Isoptera order represent one of the most abundant invertebrate groups in tropical ecosystems (Collins, 1983). They represent more than 10% and 95% of all animal and soil insect biomasses respectively in tropical regions (Kouassi, 1987; Eggleton & Bignell, 1995). To date, 3106 species were documented (Krishna et al., 2013). Social insects play a major ecological role in the decomposition of litter (Mando et al., 1996; Dawes, 2010). They also participate in the improvement of physico-chemical properties including soil aeration (Lavelle, 1997; Jouquet, 2002; Tano et al., 2005). As termites are very mobile insects, they are involved in food webs as litter decomposers or as prey for certain organisms (Konaté et al., 2005; Yeo, 2006). Certain species of termites can serve as biological indicators to estimate the state of degradation of soils and ecosystems (Boga, 2007; Dosso et al., 2013). Indeed, many studies have shown that termites are very sensitive to modification of their environment. Although most of the works on termite diversity and assemblage have been carried out in natural ecosystems (Sangaré & Bodot, 1980; Kouassi, 1987), increasing interest in the impact of transformed ecosystems (agriculture) on the latter is booming (Coulibaly et al., 2016; Akpesse et al., 2018; Diahuissié et al., 2021) given their importance. In the Ivory Coast, large areas of forests, guaranteeing the stability of biodiversity, are decimated each year for the benefit of the establishment of cocoa plantations (Assiri et al., 2009). Also, crop protection using pesticides implemented in these agrosystems can be a brake on the proliferation of insects in general and termites particularly (Ano et al., 2018). Given the high sensitivity of termites to changes in their environments and their importance in soil fertilization, it is important to know the assembly of termites in this monoculture. Works related to the impact of plantations of cocoa trees on termites occurred in west-central Côte d'Ivoire (Tra Bi, 2013). However, no data is available on the impact of this culture on termites. This study was carried out with the general objective to study the impact of cocoa farming on the abundance, diversity and density of termites in the Azaguié area in the south of Ivory Coast.
MATERIAL AND METHODS
Study site
2This study was carried out in Azaguié in the Agneby-Tiassa region (5 ° 35 ’North latitude and 3 ° 55 ’West longitude) south of the Ivory Coast. The city of Azaguié benefits from a subequatorial climate which includes 4 seasons (two rainy and two dry seasons). The great dry season runs from December to March. It is followed by the great season rainy which begins in March and ends in July. The short dry season runs from August to September and the short rainy season runs from September to November (Kouamé et al., 2014). The recorded monthly average values of rainfall show a very large variation ranging from 30 to 320 mm (Kouamé et al., 2014). Humidity relative annual average in Azaguié varies between 70.5 and 83.2%. Soils of this region can be classified into three major groups (Voko Bi et al., 2013) hydromorphic, weakly or moderately desaturated ferralitic soils according to their mineral contents (Yoboué et al., 2019).
METHODS
Plot selection
3Nursery plants less than 2 years old, plantations of 3 to 10 years and old plantations of more than 30 years were available. Cocoa farming, long abandoned in favor of other cultures is gaining momentum today in Azaguié. This explains the absence of plantations between 10 and 30 years old. For this study, three classes of plantations were defined according to the age of the cocoa trees in the city. Young plots (PC1) made up of 0 to 5-year-old cocoa trees; plots in production (PC2) made up of cocoa trees 6 to 10 years old and old plantations (PC3) made up of cocoa trees over 30 years old. Three plots of 1 hectare were sampled by age class with the forest as a reference area.
Sampling
4Firstly, a transect method was applied for sampling of litter termite species based on a standardized rapid biodiversity assessment recommended by Jones & Eggleton (2000). It was related to delimitation of 100 m long and 2 m wide transects in each studied plantation. The demarcated transect was subdivided into 20 sections of 10 m2 each (5 m x 2 m). The different sections were searched for termites in the litter, structures biogenic and on the feet of cocoa trees or trees that were found in prospected sections. Also, 12 earth monoliths (10 cm wide and 12 cm deep) were made in each section. Each section was searched by 2 times of 30 min i.e. a sampling effort of one hour per person (Coulibaly et al., 2016; Diahuissié et al., 2021). All termites collected were stored in pill boxes containing 70% ethanol.
Methods of excavation squares
5The excavation squares method of Anderson & Ingram (1989) makes possible to estimate the density of termite populations in surveyed areas. To know hypogenous termite density and abundance, three TSBF (Tropical Soil Biology and Fertility) monoliths were carried out in each prospected plot (25 cm side x 30 cm deep soil depth using a pickax and a hoe). The monolith obtained was cut into three layers of soil 10 cm thick (0-10, 10-20 and 20-30 cm). All termites contained in the sorted soil were collected and stored in pill boxes containing 70% alcohol (Kouassi, 1987; Akpesse, 2004; Tra Bi et al., 2014)
Identification of collected termites
6Based on the morphological criteria of the soldiers, identification keys were used with soldier termites (Emerson (1928); Bouillon & Mathot (1965); Harris (1966, 1968) and Sands (1965, 1992)). Workers were identified using the Sands Key (1972). After identification, each species was classified in feeding groups, namely fungus-growers, soil- and wood-feeders.
Data analysis
7The efficiency of the sampling method was measured from the non-parametric test of Jacknife 1 with 500 randomizations. From EstimateS software (Version 9.1.0), the number of expected species has been identified (Colwell & Elsensohn, 2014). For each habitat, the specific richness (S) which was the total number of species sampled was determined. Other clues have made it possible to assess the settlement of termites, this was the Shannon index (H’); equity (E) and the Simpson index (IS). These indices were calculated using the PAST software (version 2.17c). One-way variance analysis (ANOVA), Kruskal-Wallis and Newman Keuls tests were performed with the XLSTAT software (version 2016.02.28451). Abundance relative termites (A), based on the occurrence of the considered species was calculated: A = ni / N, with ni = number of sections where species i was collected; N = number section total of the type of plantation or primary forest. Termites have also been classified according to their trophic groups and the relative abundance of each group has been calculated. The similarity between the different backgrounds has been highlighted from the Jaccard index S = A Ո B / A U B. (Magurran, 1988). This index is equal to 1 when the 2 media are identical and 0 when the two media have no species in common.
RESULTS
Effectiveness of the sampling method
8The accumulation curves obtained from the Jacknife 1 estimator of wealth specific observed showed an average coverage rate of 87.19% compared to that expected. This rate was indicative of the high efficiency of the sampling method (Figure 1). The lowest coverage rate was recorded in class PC1 with a value of more than 78%.
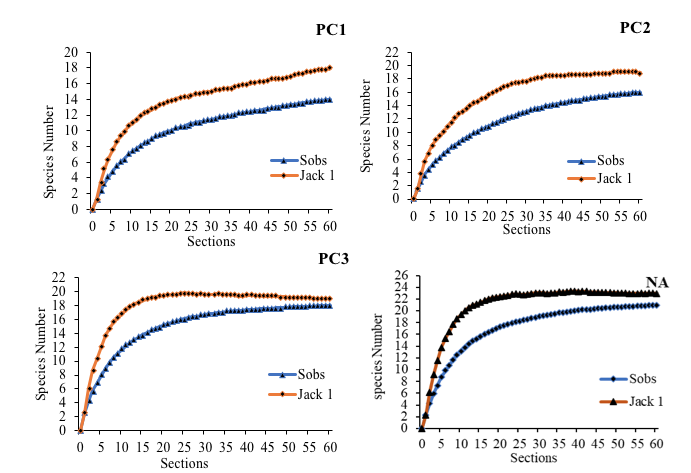
PC1: Plots of 0 to 5 years PC2: Plots of 5 to 10 years PC3: Plots over 30 years NA: Natural Area
Figure 1: Accumulation curves of observed specific richness (Sobs) and expected specific richness (Jack 1) according to the environments studied.
Diversity of termites in transects
9In total, 27 species were collected from all sampled plots and were divided into 2 families, namely Rhinotermitidae and Termitidae. They belonged to 6 subfamilies and 18 genera. The subfamilies of Coptotermitinae and that of Rhinotermitinae were the least represented, each with only one species. The sub-families of Apicotermitinae and that of Nasutitermitinae were represented by 2 species each. The Macrotermitinae subfamily was the most represented with 11 species and was closely followed by Termitinae with 10 species (Table 1). The natural area (NA) corresponded to highest specific richness with 21 species. It was followed by plots over 30 years old with 18 species. During the sampling in the transects, 3 species were collected only in the natural habitat, they are Shedorinotermes lamanianus, Protermes minutus and Procubitermes sjöstedti. Allognatotermes sp and Termes sp have been specific to plots over 30 years old.
Table 1: Specific diversity and abundance of termites according to age gradient of investigated plots using a transect method

TG : Trophic group, W : wood-feeders, F : fungus-growers, S : soil-feeders, PC1-2-3 : plots of 0 to 5 years, 5 to 10 years, more than 30 years respectively, NA : Natural Area.
10The author's name followed by the year of publication are in parenthesis
Termite Diversity Indices
11The Shannon index varied little between different habitats studied with an average index of 2.44. It reached its highest value in the natural habitat (H’= 2.63) while lowest index was recorded in the young plots (H’= 2.28). With values between 0.88 and 0.90, Simpson index followed similar trends as Shannon index. Highest value was recorded in the Natural habitat (IS = 0.9) while young plots and 5 to 10-year-old plots recorded the same value of Simpson index (0.88). Fairness was highest in young plots with a value of 0.87. Although fairness varied little from one class to another, lowest value was recorded in plots of 5 to 10 years (E = 0.85).
Similarity between habitat types
12The similarity assessment between the different habitat types shows that the natural area and plots over 30 years old show good similarity (0.63). Likewise, the similarity was strong between the 0 to 5-year-old plots and the 5 to 10-year-old plots (0.67). The lowest similarity was recorded between plots 0-5 years old and those over 30 years old (0.45).
13
Relative abundance of termites in transects
14The study of the relative abundance (occurrences) of termites showed a great variability in the distribution of termites in surveyed areas (Table 1). The mean abundances of the most important termites were recorded in old cocoa trees (48.7 ± 2.1 occurrences) followed by natural area (46.0 occurrences ± 30.5). Lowest termite abundances were recorded in young cocoa trees (25.3 ± 1.5 occurrences) while no significant variation between habitats was found (p = 0.210) (Table 2). According to trophic groups, fungus-growers largely dominated the stands in prospected areas with the exception of old plots. The relative abundance of this group varied significantly from one habitat to another (p = 0.028). Wood-boring group also varied significantly from one habitat type to another (p < 0,001). However, the relative abundance of soil-feeders did not show any significant variation from one habitat to another (p = 0,232).
Table 2: Relative abundance of termite different trophic groups in the transects
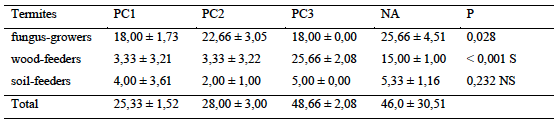
P: probability; NS and S for not significant and significant effect, PC1-2-3: Plots of 0 to 5 years, 5 to 10 years, more than 30 years respectively, NA: Natural Area
Diversity of hypogeal termites
15Sampling of hypogeal termites yielded a total of 16 species divided into two families and five subfamilies (Table 3). Fungus-growers were the most represented with 6 species, followed by soil-feeders with 5 species. The natural area was the most diverse with 10 sampled species, followed by plots over 30 years old with 8 species. Three species were common to the 4 habitat types, they were all fungus-growers namely Ancistrotermes guineensis, Microtermes subhyalinus and Protermes properens. Acantotermes acantothorax, Basidentitermes potens and Trinervitermes trinervus were collected only in the natural area. Astalotermes sp was collected only in class PC1. The Jaccard Similarity Index showed a very large difference between habitats (Table 4). Only the similarity between young plots and those in production showed an index higher than 0.5 (IS = 0.55). The lowest similarity was recorded between young plots and plots over 30 (SI = 0.25).
Table 3: Inventory of hypogeal termites in Azaguié cocoa farms
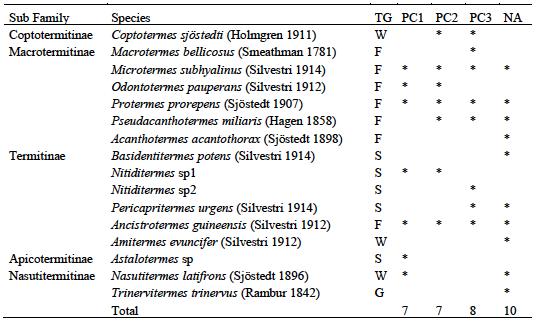
TG: Trophic Group, W: wood-feeders, F: fungus-growers, S: soil-feeders, PC1-2-3 : Plots of 0 to 5 years, 5 to 10 years, more than 30 years respectively, NA: Natural Area.
16The author's name followed by the year of publication are in parenthesis
Table 4: Jaccard Diversity Index of hypogenous termites between kinds of environments
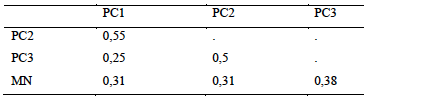
PC1-2-3: Plots of 0 to 5 years, 5 to 10 years, more than 30 years respectively, NA: Natural Area
Average density of hypogeal termites
17Analysis of hypogeal termite density varied in the number of individuals in habitat types sampled. Also, termites were present in all slices of monoliths. The natural area recorded the highest number of individuals with more than 21.000 individuals /m2 while only ten times less abundance were observed in plots over 30 years old. Regarding the distribution of termites in soil layers, the young plots (PC1) followed similar variations as in natural area. In these two habitats, the density of termites decreased with soil depth. Although there was no significant difference between termite densities as a function of the soil strata sampled. PC2 and PC3 plots showed fewer individual abundances in layer 2 compared to layers 1 and 3
Table 5 : Variation in the vertical distribution of termites by habitat types (Number of individuals/m2
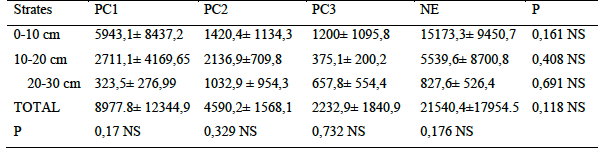
P: probability; NS: not significant (Kruskal-Wallis test; p <0.05) PC1: Plots of 0 to 5 years PC2: Plots of 5 to 10 years PC3: Plots over 30 years NA: Natural Area
Variation in trophic group densities
18Regarding the different trophic groups, foragers were only found in the natural area (Figure 2). The layer horizons taken separately showed difference in the colonization of termite trophic groups (Figure 2). The first stratum (0-10 cm) recorded the highest frequencies by trophic group (37 to 100% of individuals). However, plots of 5 to 10 years old showed a more or less equitable distribution between fungus growers and wood-feeders in the first 2 soil layers. Also, in class PC2, soil-feeders were collected only from the deep soil layers. As for the other orchards, the intermediate strata (10-20 cm) were richer in terms of individuals than the deepest strata (20-30 cm).
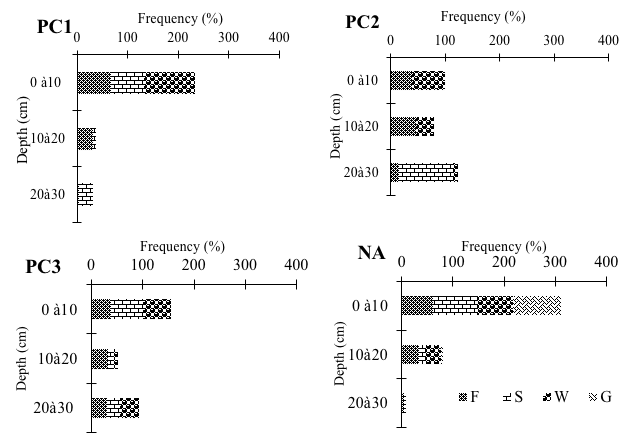
F: fungus-growers, S: soil-feeders, W: wood-feeders, G: grass-feeders, PC1-2-3: Plots of 0 to 5 years, 5 to 10 years and over 30 years respectively, NA: Natural Area
Figure 2: Density frequencies of different trophic groups as a function of the depth of the soil in each habitat types
DISCUSSION
19Sampling termites by transect method allowed 27 species to be collected from all the Azaguié cocoa trees and the adjacent natural habitat. The coverage rate for this sampling varies from 78 to 94% for an average of 87% of expected species actually observed. This rate showed a high efficiency of the sampling method. These results were much closed to those obtained by Tra Bi et al. (2012) in cocoa farms in the Oumé region. However, these authors worked at all stages of cocoa tree evolution. As for sampling of hypogeal termite fauna in cocoa plantations, it allowed us to collect 16 species in total. The natural habitat had the highest diversity with 10 species. These results indicated that natural area was more stable than the plots used for cocoa. Indeed, several authors have shown that the specific richness of a particular habitat was strongly linked to its stability (Inoue et al., 2001; Eggleton et al., 2002). For these authors, the more stable habitat, the more its specific richness increases. The high diversity of termites collected during this study would be related to the period of sampling. Termites were sampled from August to September just after the heavy rainy season. During this period the shallow soil horizons provide more food and are therefore more attractive to termites. They tend to be found in the shallow horizons of the soil making it easy to sample (Sarr, 1999; Coulibaly et al., 2016). The young plots recorded the lowest specific richness. These plantations, which predate the natural habitat, have undergone several anthropogenic pressures such as deforestation, plowing and the use of chemical inputs for the establishment of monoculture. These practices would therefore influence soil fauna and particularly on termites. These observations are consistent with those of Eggleton et al., 2002; Donovan et al., 2007; Tra Bi et al., 2012. These authors have shown that soil exploitation affects the trophic structure and the specific richness of termites. In the middle-aged plots (5-10 years), 16 species were collected in the transects. Indeed, in these plots, the branches of the cocoa trees develop to form the crown of plants which will shelter the most pods, making the canopy more and denser. Also, the branches and dried leaves that fall are sources of food for termites, which would promote the species diversity increase. These results are like those obtained in class 2 and 3 cocoa plantations by Tra Bi et al. in 2012. Also, the high occurrence of termites of the genus Nasutitermes in these plots could be explained by the cessation of agricultural activities in these plots. These termites, building tree nests, are particularly sensitive to insecticides. They also need large, robust trees for building their nests (Gathorne-Hardy et al., 2001; Eggleton et al., 2002; Tra Bi et al., 2012). Old plantations which do not undergo any agricultural activity (no weeding, no chemical treatments) constitute an environment conducive to the proliferation of arboreal termites but globally also to all termites. Indeed, the cessation of agricultural activities in cocoa farms over 30 years old induces the colonization of rapidly growing plants, thus increasing the litter biomass and the proliferation of woody plants (Baar et al., 2000; Serpantié & Ouattara, 2001). They are therefore suitable micro habitats and food sources for termites. In terms of density, although there is no significant difference between habitat types, the frequency of individuals encountered differed between soil slices. The first layer was the most colonized by termites. Several studies have shown that this layer was the most colonized because of the resource viability (Kouassi, 1987; Decaëns et al., 1994; Mathieu, 2004, Tsukamoto & Sabang, 2005, Tra Bi et al., 2014). The distribution of hypogeal termites in the class PC2 practically homogeneous between the different layers could be explained by the pressures related to agricultural activities. Although they were present in the PC2 class, soil-feeders were found only in the last stratum (20-30 cm). Termites therefore tended to seek stability in the soil depth (Coulibaly, 2014). The Ancistrotermes and Microtermes geni were found in significant proportion in all the habitat sampled. This could be explained by their ability to adapt. According to Tahiri et al. (2008), these termites can adapt to all environments and to colonize the nests of other species. Also, they are the least bothered by anthropogenic actions.
CONCLUSIONS
20This study showed the impact of cocoa farming on termite communities. The natural area due to its richness and its abundance represented the most diverse habitat type. In cocoa farms, plots over 30 years old recorded the highest specific wealth both along transects and monoliths. Although they colonized all soil strata, termite activity was most intensive in the 0-10 cm stratum. The assembly of hypogeal termites differed from one habitat type to another. The establishment of cocoa culture influences the distribution of termites. However, the assembly of termites in old plantations tends to stabilize as in the natural habitat.
Bibliographie
Akpesse A. A. M 2004. Impact des termites dans les cultures vivrières (Riz, Maïs) en zone soudanienne (Boro-Borotou, Côte-d’Ivoire) : étude expérimentale de traitements insecticides. Thèse de Doctorat, Université de Cocody-Abidjan, Côte d’Ivoire, 163 p.
Akpesse A. A., Kissi T. A., Diby Y. K., Coulibaly T. & Koua K. H 2018. Diversity and damages of termites on papaya trees (Carica papaya) in M'brimbo (south of Côte d’Ivoire) International Journal of Entomology Research 3 (6), 60-64.
Anderson J. M. & Ingram J.1989. Tropical soil biology and fertility program. Methods handbook. C.A.B. Oxford, 171 p.
Ano E., Tahiri A., Diby Y. & Siapo Y.2018. Évaluation des pratiques phytosanitaires paysannes dans les cacaoyères : Cas du département d’Abengourou (Est, Côte d’Ivoire). Journal of Animal & Plant Sciences, 38 (1), 6159-6174.
Assiri A. A., Yoro G. R., Deheuvels O. Kebe B. I., Adiko A & Assa A.2009. Les caractéristiques agronomiques des vergers de cacaoyer (Theobroma cacao L.) en Côte d’Ivoire. Journal of Animal and Plant Sciences, 1, 55-66.
Baar R., Cordeiro M. D., Denich M. & Fölster H.2000. Floristic inventory of secondary vegetation in agricultural systems of East-Amazonia. Biodiversity and Conservation, 13, 501-528.
Boga J.P. 2007. Étude expérimentale de l’impact de matériaux de termitières et de la paille sur la levée, la croissance, le rendement du maïs et du riz et la fertilité des sols cultivés en savane sub-soudanienne : Booro-Borotou, Côte d’Ivoire. Thèse de Doctorat, Université de Cocody-Abidjan, Côte d’Ivoire. 231 p.
Bouillon A & Mathot G. 1965. Quel est ce termite Africain ? Zooleon°1, Leopoldville Univ, Leopoldville. 115 p.
Collins N.M. 1983. Populations, age, structure and survivorship of colonies of Macrotermes bellicosus (Isoptera: Macrotemtitinae). Journal of Animal and Ecology, 50, 293-311.
Colwell R. K. & Elsensohn J.2014. EstimateS turns 20: statistical estimation of species richness and shared species from samples, with non-parametric extrapolation. Ecography 37 (6), 609–613.
Coulibaly T. 2014. Diversité et dégâts des termites dans les vergers de manguiers (Mangifera indica L, Anacardiaceae) de la région de Korhogo (Côte d‘Ivoire) : Essai de lutte par utilisation d‘extraits aqueux de trois plantes locales. Thèse de Doctorat d‘Université, Université Felix Houphouët Boigny, Côte d‘Ivoire, 158 p.
Coulibaly T., Akpesse A. A. M., Boga J.P., Yapi A., Kouassi K.P. & Roisin Y 2016. Change in termite communities along a chronosequence of mango tree orchards in the north of Côte d’Ivoire. J Insect Conserv 20 (6), 1011–1019
Dawes TZ. 2010. Restablishment of ecological functionning by mulching and termites invasion in a degraded soil in an Autralian savanna. Soil Biology and Biochemestry, 42 (10), 1825-1834.
Decaëns T., Lavelle P., Jiineiiez J. J., Escobar G. & Rippstein G. 1994. Impact of land management on soil macrofauna in the Oriental Llanos of Colombia, European Journal Soil Biology, 30 (4), 157-168.
Diahuissié F., Coulibaly T., Akpesse A. & Kouassi Ph. 2021. Influence of cashew Anacardium occidentale L. Cultivation on termite diversity in the Korhogo savannah zone (Northern Côte d’Ivoire). Int J Trop Insect Sci 41, 1709–1715
Donovan S. E., Griffiths G. J. K., Homathevi R &Winder L. 2007. The spatial pattern of soil-dwelling termites in primary and logged forest in Sabah, Malaysia. Ecology Entomology, 32, 1-10.
Dosso K., Deligne J., Yéo K., Konaté S. & Linsenmair K.E. 2013. Changes in the termite assemblage across a sequence of land-use systems in the rural area around Lamto Reserve in central Côte d’Ivoire. Journal of Insectes Conservation 17, 1047-1057.
Eggleton P. & Bignell D. E. 1995. Monitoring the response of tropical insect to changes in the environment: troubles with termites, In: Insects in a changing environment. (Eds.), HARRINGTON, R. and STORK, N. E. Academic press, London, 473-497.
Eggleton P. P., Bignell D. E., Hauser S., Dibog L., Norgrove L. & Madong B. 2002. Termite diversity across an anthropogenic disturbance gradient in the humid forest zone of West Africa. Agriculture, Ecosystems and Environment, 90, 189–202.
Emerson A. E. 1928. Termites of the Belgian Congo and the Cameroon. Bulletin of the American Museum of Natural History, (Entomology), 95 (7), 401-574.
Gathorne-Hardy F., Syaukani & Eggleton P. 2001. The effects of altitude and rainfall on the composition of the termites (Isoptera) of the Leuser Ecosystem (Sumatra, Indonesia), Journal of Tropical Ecology, 17, 379–393.
Harris W. V. 1966. The genus Ancistrotermes (Isoptera). Bulletin of the British Museum (Natural History), (Entomology), 18 (1), 1-20.
Harris W. V. 1968. On the genus Coptotermes in Africa (Isoptera: Rhinotermitidae). Proceedings of the Royal Entomological Society of London (B), 35 (11-12), 161-171.
Inoue T., Takematsu Y., Hyodo F., Sugimoto A., Yamada A., Klangkaew C., Kirtibutr N. & Abe T. 2001. The abundance and Biomass and Subterrenean Termites (Isoptera) in a Dry Evergreen Forest of North east Thailand, Sociobiology, 37 (1), 41-52.
Jones D. T. & Eggleton P .2000. Sampling termite assemblage in tropical forest: testing a rapid biodiversity assessment protocol. Journal of applied Ecology, 37, 191- 203.
Jouquet P. 2002. De la structure biogénique au phénotype étendu. Les termites champignonnistes comme ingénieurs de l'écosystème. Thèse de Doctorat, Université Paris VI, 141 p.
Konaté S., Tondoh J. E. & Yeo K. 2005. Diversité et rôle écologiques des fourmis terricoles de Côte d’Ivoire. Bioterre, 65-88.
Kouamé N., Dick A, Assidjo N. & Anno A. 2014. Étude de la croissance du bananier plantain (Musa sp., AAB, cultivar Corne 1) dans les régions de Yamoussoukro et Azaguié (Cote d’Ivoire). Journal of Applied Biosciences 76, 6411– 6424.
Kouassi K. PH. 1987. Étude comparative de la macrofaune endogée d’écosystèmes naturels et transformés de Côte d’Ivoire. Thèse 3ème cycle: Université d’Abidjan, 129 p.
Krishna K., Grimaldi D. A., Krishna V. & Engel M. S. 2013. Treatise on the isoptera of the world. Bulletin of the American museum of natural history, 377, 2704 p.
Lavelle P., Bignell D. E. & Lepage M. 1997. Soil function in a changing world: the role of invertebrate ecosystem engineers. Eur J Soil Resto 33, 159–193.
Mando A., Stroosnijder L. & Brussaar L. 1996. Effects of termites on infiltration into crusted soil. Geoderma, 74 (1-2), 107-113.
Mathieu J. 2004. Étude de la macrofaune du sol dans une zone de déforestation en Amazonie du Sud- Est, au Brésil, dans le contexte de l’agriculture familiale. Thèse de doctorat, Université Pierre et Marie Curie, Paris 6, 236 p.
Magurran A. E. 2004. Measuring Biological Diversity. Blackwell publishing, Publishing: Oxford, UK. 256 p.
Sands W. A. 1965. A revision of the termite subfamily Nasutitermitinae (Isoptera, Termitidae) from the Ethiopian region. Bulletin of the British Museum (Natural History), (Entomology), Supplement 4, 1-172.
Sands W. A. 1972. The soldier less termites of Africa (Isoptera: Termitidae). Bulletin of the British Museum (Natural History), (Entomology), Supplement. 18: 244 p. +Annexes.
Sands W. A. 1992. The termite genus Amitermes in Africa and the Middle East. Natural Research Institute Bulletin 51. Chatham, United Kingdom, 140 p.
Sangaré Y & Bodot P. 1980. Données préliminaires sur la faune des termites en forêt tropicale humide (Région de Taï, sud-ouest de la Côte d’Ivoire) Inventaire, classification éthologique et biologique des genres et espèces répertoriés. Annales de l’Université d’Abidjan, Série E, 13, 123-141.
Sarr M. 1999. Étude écologique des peuplements de termites dans les jachères et dans les cultures en zone soudano-sahélienne, au Sénégal. Thèse de Doctorat de 3ème cycle de Biologie Animale. Université Cheikh Anta Diop de Dakar, 117 p.
Serpantié G. & Ouattara B. 2001. Fertilité et jachères en Afrique de l’Ouest. In : FLORET C. & Pontanier R., John Libbey Euro text : La jachère en Afrique tropicale, p. 21-83.
Tahiri A. Y., Tano Y. S. & Foua-BI K. 2008. Effet toxique et mode d’action du chlorpyrifos- éthyl sur les termites ravageurs de l’hévéa (Hevea brasiliensis Müll. Arg.). Science et technique, Sciences naturelles et agronomie, 30 (2), 13-25.
Tano Y., Yapi A & Kouassi K. P. 2005. Diversité biologique et importance des termites (Isoptères) dans les écosystèmes de savane et de foret de Côte d’Ivoire. Bioterre, 5 (1), 44-64.
Tra Bi C. S. 2013. Diversité spécifique et dégâts des termites dans les cacaoyères (Theobroma cacao L., 1759) de la région d’Oumé en Côte D’ivoire. Thèse de Doctorat d’Université, Université Félix Houphouët-Boigny, Côte d’Ivoire, 243 p + annexes.
Tra-Bi C. S., Boga J.-P., Akpesse A. A. M., Konaté S., Kouassi P. & Tano Y. 2012. Diversité et effet de la litière sur l’assemblage des termites (Insecta: Isoptera) épigés le long d’un gradient d’âge de la cacaoculture (Theobroma Cacao L.) en Moyenne Côte d’Ivoire, Oumé. European Journal of Scientific Research, 79 (4), 519- 530.
Tra-Bi C. S., Akpesse A. A. M., Boga J.-P., Yapi A., Konaté S., Kouassi P. & Tano Y. 2014. Diversity and abundance of Hypogenous termites (Insecta: Isoptera) in cocoa plantation (Theobroma cacao L.) in semi deciduous forest zone (Oumé, Côte d’Ivoire). Pensee Journal, 76 (1), 138-146.
Tsukamoto J. & Sabang J. 2005. Soil macro-fauna in an Acacia mangium plantation in comparison to that in a primary mixed dipterocarp forest in the lowlands of Sarawak, Malaysia. Pedobiologia, 49, 69-80.
Voko Bi D., Ahonzo-Niamke S. & Zeze A. 2013. Impact des propriétés physicochimiques des sols de culture du manioc sur l’abondance et la diversité des communautés de champignons Mycorhiziens à arbuscules dans la zone agroécologique d’Azaguié, sud-est de la Côte d’Ivoire. Agronomie Africaine 25 (3), 251-264.
Yeo K. 2006. Dynamique spatiale et diversité des fourmis de la litière et du sol dans une mosaïque de savane en Côte d’Ivoire (Lamto, Côte d’Ivoire). Thèse de doctorat en cotutelle des Universités de Paris VI (France) et d’Abobo-Adjamé (Côte d’Ivoire), 212 p.
Yoboué K., Kouadio K., Kabore D. & Yao-Kouamé A. 2019. Nature minéralogique et niveau d’évolution d’un Cambisol développé sur schiste dans la région d’Azaguié, Sud-Est de la Côte d’Ivoire. International Journal of Innovation and Applied Studies 26 : 1170-1179.