- Accueil
- Volume 75 (2022)
- Bioecology of Orthopterans (Orthoptera, Insecta) in the biological reserve of Djebel Ouahch, Beni Hamidene and Grarem Gouga (Eastern Algeria)
Visualisation(s): 2362 (11 ULiège)
Téléchargement(s): 210 (0 ULiège)
Bioecology of Orthopterans (Orthoptera, Insecta) in the biological reserve of Djebel Ouahch, Beni Hamidene and Grarem Gouga (Eastern Algeria)

Document(s) associé(s)
Version PDF originaleRésumé
Une étude bioécologique sur le peuplement des orthoptères a été effectuée au niveau de trois stations dans l’est Algérien (Djebel Ouahch, Beni Hamidene et Grarem Gouga) entre Février et Juillet 2019. Les investigations ont révélé la présence de 25 espèces appartenant à cinq familles (Pamphagidae, Acrididae, Tetrigidae, Tettigoniidae et Gryllidae) dont la famille Acrididae s’est montrée quantitativement la plus abondante avec 18 espèces. L’étude de la diversité et de la structure du peuplement d’orthoptères montre que la région d’étude est diversifiée et la majorité des espèces sont accidentelles. L’espèce Pamphagus milevitanus (Benkenana & Massa, 2017), récemment découverte en Algérie, a été recensée au niveau de la station de Grarem Gouga. L’étude des oothèques des femelles de cette espèce géante indique qu’elle a une forte fécondité. L’influence des variables météorologiques sur l’abondance des orthoptères révèle le rôle important de la température sur le développement de la plupart des espèces acridiennes, notamment l’espèce Pamphagus milevitanus.
Abstract
This article presents a bioecological study on the orthoptera population at three stations in eastern Algeria (Djebel Ouahch, Beni Hamidene and Grarem Gouga) from February to July 2019. The investigation results indicate 25 species belonging to five families (Pamphagidae, Acrididae, Tetrigidae, Tettigoniidae and Gryllidae), of which the family Acrididae was quantitatively the most abundant with 18 species. The study of the diversity and structure of the orthopteran population shows that the study area is diverse, and the majority of species are accidental. The species Pamphagus milevitanus (Benkenana & Massa, 2017), recently discovered from Algeria, was recorded at the station Grarem Gouga. The study of oothecae of females of this giant species indicates that it has a high fecundity. The influence of meteorological variables on orthopteran abundance reveals the important role of temperature on the development of most locust species, including Pamphagus milevitanus.
Table des matières
Reçu le 20 juillet 2021, accepté le 28 mars 2022
Cet article est distribué suivant les termes et conditions de la licence CC-BY (http://creativecommons.org/licenses/by/4.0/deed.fr)
INTRODUCTION
1The Djebel Ouahch reserve is a natural heritage, the lung of Constantine. This massif is part of a series of small chains known as Numidian or Constantine (Boudy, 1955). It is a forest that abounds with 43 ornamental and noble forest species of Algerian, European and American origin (Hachiche, 2014), including Quercus ilex L., 1753 and Pinus halepensis Mill., 1768. It has a high diversity of forest vegetation and habitats, suggesting an equal variety of orthopterans. However, there is no in-depth faunistic study in this region, hence our interest in this topic which aims to shed some light on the orthopteran fauna in the Djebel Ouahch region. The orthopteran fauna occupies an important place in the ecosystems. They are economically significant and constitute a food resource for many vertebrates and invertebrates (Joern et al., 2006; Gandar, 1982). They are recyclers of plant material, using the remains of dead plants at one stage or another of their life cycle (Belovsky & Slade, 2002). They are also of ecological interest, representing good indicators of imbalances and climate change (Samways & Sergeev, 1997).
2Orthopterans are important crop pests in different parts of the world, causing economic losses and threatening the food security of the population (Wright, 1986; Brader et al., 2006; Millist & Abdalla, 2011; Latchininsky, 2013). Climatic conditions favour their proliferation (Benkenana, 2006). This work presents the list of orthopteran species recorded and some environmental factors influencing their development in Djebel Ouahch and in two others localities (Beni Hamidene in Constantine and Grarem Gouga in Mila).
MATERIALS AND METHODS
The geographical setting of the study area
3The Djebel Ouahch region is located in the east of Constantine city (36°27’59’N, 6°44’13’’E and altitude 994 m). It covers an area of 66.535 hand concerns seven communes, namely: Zighoud Youcef, Didouche Mourad, Constantine, El Khroub, Ibn Badis, Aïn Abid and Ouled Rahmoune. These communes are part of the Wilaya of Constantine. The ecological analyses were carried out on three localities, including Beni Hamidene located in the north-west of Constantine (36°30’20’N, 6°32’59’’E, altitude 540m) and Grarem Gouga situated in the north of the Wilaya of Mila (36°30’26’’N, 6°21’32’’E, altitude 770 m) (Figure 1).
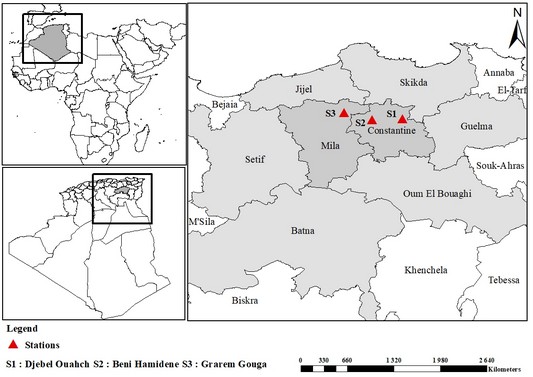
Figure 1: Presentation of the study locations
4The climatic data used for the study period were collected from the national meteorological office completed by the database of the website (https://www.infoclimat.fr/climatologie/) (Table 1).
Table 1: Climatic data in the geographical zone of the study area
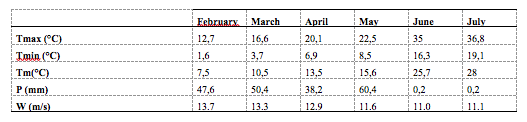
Tmax: maximum temperature, Tmin: minimum temperature, Tm: mean temperature, P: precipitations, W: wind
Sampling and identification
5The study was conducted from February to July 2019, with trips completed four times a month. Grasshoppers were captured by two sampling methods: by hand or using a sweep net for flying species. sampling took place during the day (10:00 to 17:00) under favourable weather conditions.
6Specimens were placed in labelled bottles. They were pinned and pricked on polystyrene blocks. Systematic analysis of species was carried out using several identification keys; Chopard (1943), Jago (1963), Launois (1979), Massa et al. (2012). We used two sites (http://orthoptera.speciesfile.org) and (https://acrinwafrica.mnhn.fr) for updating the classification and nomenclature.
Methods of analysis and data processing
7The structure and diversity of orthopteran populations in the study area were assessed using ecological and statistical descriptors: frequency of occurrence, Shannon index, habitat amplitude and Pearson correlation coefficient. The analyses were performed using PAST v.1.81 on a matrix based on locust abundance (Hammer et al., 2001).
Frequency of occurrence and ecological status of inventoried species
8According to Dajoz (1985), the frequency of occurrence of a species is the ratio, expressed as a percentage, between the number of samples in which this species is noted and the total number of samples collected. The formula proposed by Faurie et al. (2003) is defined as follows:
9C (%) = (Pi x 100) / P
10C : Constance ;
11Pi: Number of samples containing the species collected;
12P: Total number of samples collected;
13Ubiquitous species: C% = 100%;
14Constant species: 75% ≤ C% < 100;
15Regular species: 50% ≤ C < 75%;
16Accessory species: 25% ≤ C< 50%;
17Accidental species: C < 25.
Habitat amplitude (HA) and ecological barycentre (G)
18The specific distribution was examined using the habitat amplitude parameter (HA) for each species to reflect the amplitude of the spatial niche. According to Blondel (1979), this parameter has the following formula:
19HA = eᴴ'
20e: base of the Naperian logarithm;
21H': -Σ Pi. Log2 (Pi) (Shannon and Weaver, 1949);
22Pi: the proportion of individuals of the species in the environment i.
23According to Blondel (1979), the barycentre measures the centre of gravity of the species’ distribution along with a descriptor. It locates the average position of each species along a gradient (Ramade, 1984). It makes it possible to identify the ecological optimum of species statistically and with precision (Felzines, 1982). It depends closely on the a priori definition of the number of resource classes. The formula was defined by Lheritier et al. (1979) as follows:
24g = (x1+ 2x2+ 3x3. .... nxn)/ Ʃx
25X1: centesimal frequency of species E in class 1 of the factor considered;
26X2: centesimal frequency of species E in class 2 of the factor considered, etc.
27Lheritier et al. (1979) reported that the habitat amplitude and the barycentre better understand how each species is distributed along the ecological succession.
Study of morphometric traits and oviposition characteristics of Pamphagus milevitanus females
28Measurements of the different body parts of the female Pamphagus milevitanus species were taken with a precision measuring magnifier and graph paper. The following parameters were measured: overall height, head size, thorax and abdomen, length and width of the hind femur. We investigated the oothecae of three females (number and size of eggs) to study fecundity and egg-laying capacity.
RESULTS
Composition of the orthopterological fauna
29The orthopteran fauna recorded in the study area shows 25 species belonging to nine subfamilies and five families. The Acrididae was quantitatively the most abundant, being represented by 18 species (Table 2).
Table 2: Orthopteran species recorded in the three study sites
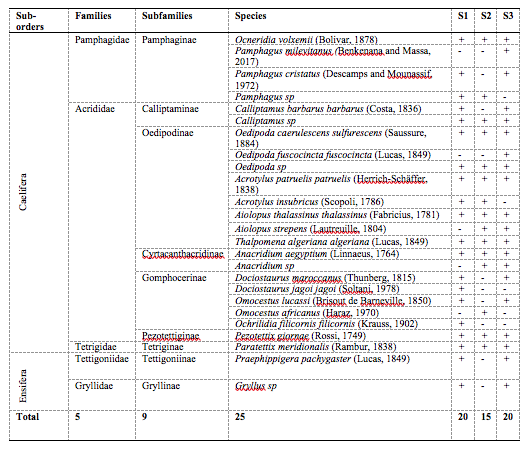
S1: Djebel Ouahch, S2: Beni Hamidene, S3: Grarem Gouga
Frequency of occurrence and ecological status of inventoried species
30Table 3 lists the frequencies of occurrence of orthopteran fauna. The environment of the Djebel Ouahch site has, out of a total of 20 species, 12 accidental species, five accessory species, two regular species and one constant species. At the Beni Hamidene site, out of 15 species, there are seven accidental species, four accessory species, three regular species and one constant species. At the Grarem Gouga site, out of 20 species, accidental species are predominant (14); there are also three accessory species, two regular species and one constant species.
Table 3: Assessment of the ecological status of orthopteran species in the study areas
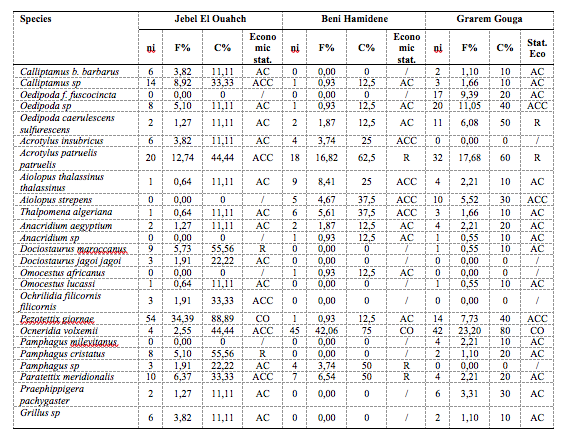
AC: Accidental, ACC: Accessory, R: Regular, Co: Constant, OMN: Omnipresent
Habitat amplitude (HA) and ecological barycentre (G)
31The results summarised in Table 4 indicate that each environment has preferential species. Nevertheless, of the 25 species inventoried, almost half are found in the locality of Djebel Ouahch (g>2), followed by the locality of Beni Hamidene (g>1). These two environments attract almost all the species (23 species). Taking into account all the species (25) confirms these observations. Furthermore, Table 4 shows that the species whose HA=1 are species restricted to the locality of Grarem Gouga. On the other hand, species with 1<HA2 are species whose spatial niche (ecological plasticity) encompasses two localities, Grarem Gouga and Beni Hamidene. On the other hand, species with 2<AH3 are species whose spatial niche (ecological plasticity) encompasses two localities: Beni Hamidene and Djebel Ouahch. It can be noted that the most significant habitat amplitude belongs to the species Acrotylus patruelis patruelis, with a value of 2.90.
Table 4: Distribution of Orthoptera species along the altitudinal gradient of the study areas
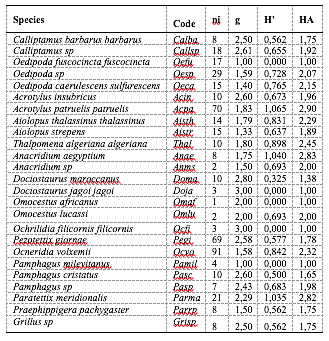
ni: number of individuals, g: ecological barycentre, H’: Shannon’s index, HA: Habitat amplitude
32Figure 2 indicates an almost complete turnover of the population between the second and third biotope. The lowest HA values are for Pamphagus milevitanus (Pamil) and Oedipoda f. fuscocinta (Oefu). While the highest values are for the species Dociostaurus jagoi jagoi (Doja) and Ochrilidia filicornis filicornis (Ocfi), which have the highest values, they characterise the locality of Djebel Ouahch.
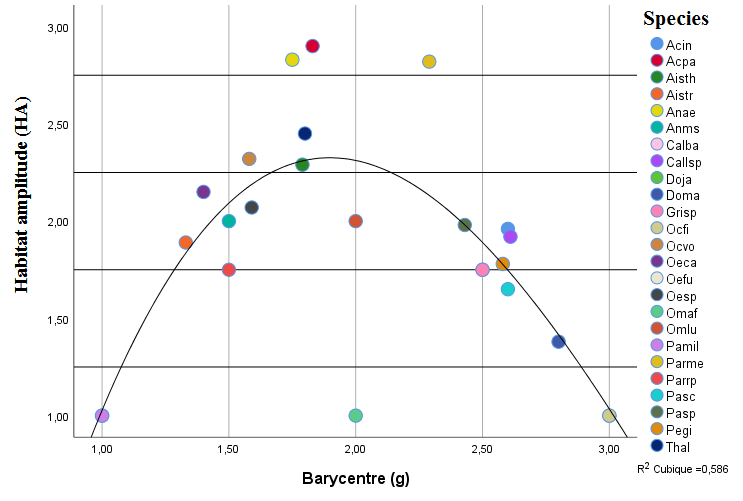
Figure 2: Relationship between maximum abundance (g) and species dispersal (HA)
Study of morphometric traits and oviposition characteristics of Pamphagus milevitanus females
33The coefficient of variation (CV) was calculated to express better the morphometric measurements of the female and her litter (oothecae) (Figure 3). A CV below 15% indicates the true values of the measurements. For each measurement, the CVs were checked at 15%. Measurements that exceeded the norms were processed by reducing the outliers (*). Table 5 shows the final measurements of the different morphometric traits and oviposition characteristics of Pamphagus milevitanus females.
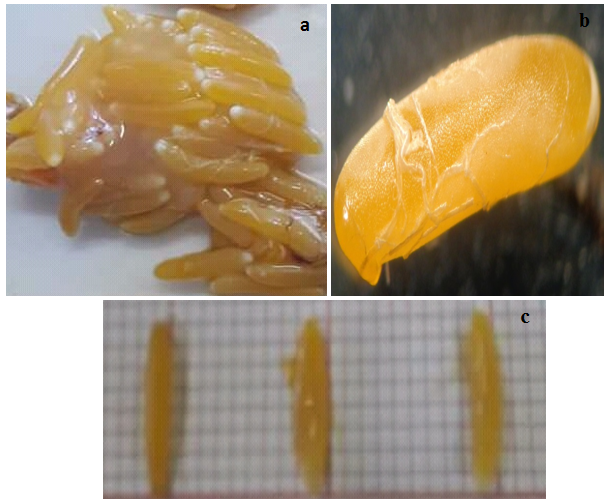
Figure 3: Ootheca of the species Pamphagus milevitanus (x40)
a: Oothecae, b: Egg, c: Size of eggs
Table 5: Morphometric analyses of female individuals of the species Pamphagus milevitanus
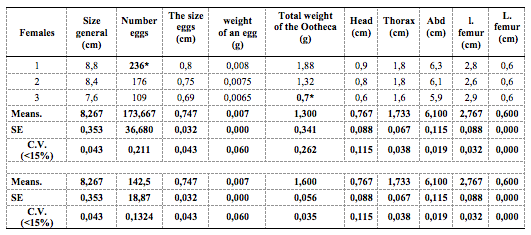
I: width, L: Length, Abd: Abdomen
Influence of environmental parameters on orthopteran settlement
34The impact of the variation of certain climatic parameters on the availability of orthopterans was studied, including temperature, precipitation and wind. Furthermore, the relationship between orthopteran availability and climatic factors was verified by drawing a regression graph. The R2 value indicates whether the climate variable explains the orthopteran abundance variable. The Pearson correlation coefficient (r) calculation showed the influence of temperature, precipitation, and wind on orthopteran abundance (Figure 4a-b-c). Figure 4a shows that higher temperature favours a higher abundance of the whole orthopteran community, while Figure 4b shows that the precipitation factor negatively impacts the abundance of orthopterans. Figure 4c shows that wind does not influence the abundance of the orthopteran fauna.
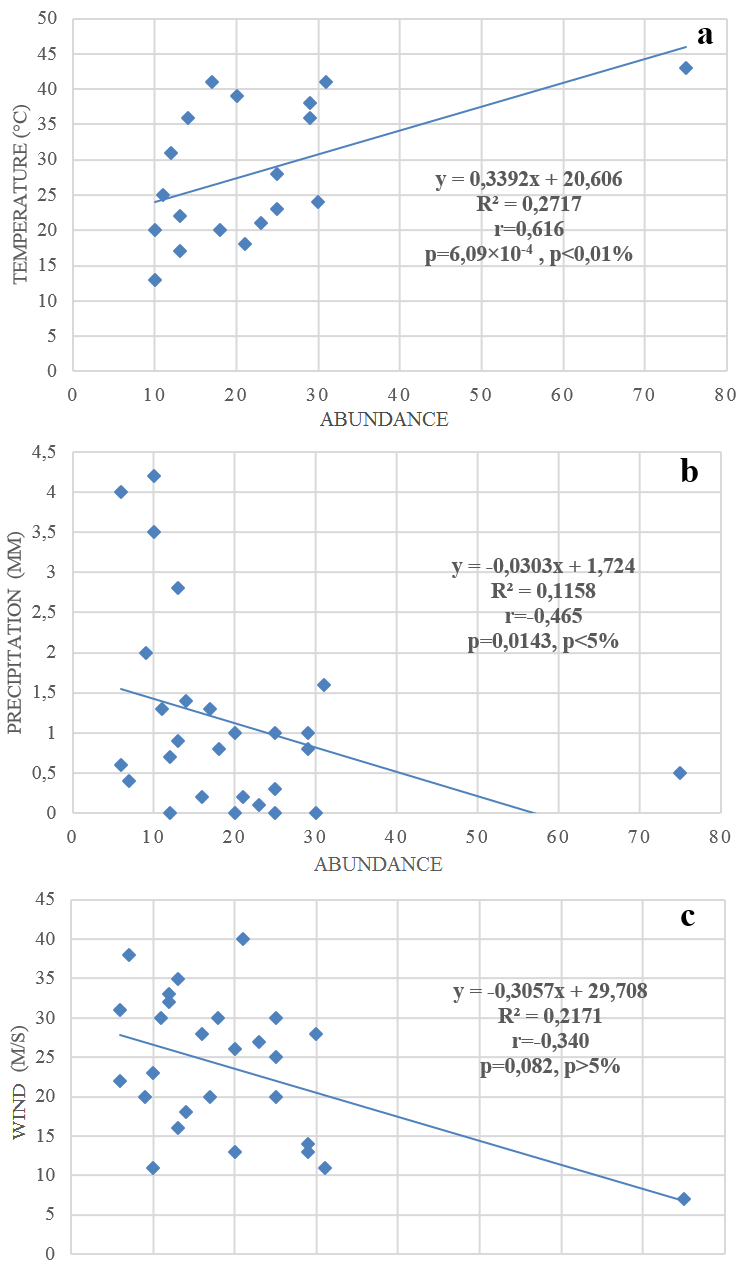
Figure 4: Impact of some environmental parameters on orthopteran abundance
DISCUSSION
35This study shows that the study area is very diverse, with 25 species of orthopterans recorded belonging to five families and nine subfamilies. In general, the Acrididae shows clear dominance in terms of taxonomic richness. It is represented by 18 species divided into five subfamilies, which its adaptation can explain the environmental conditions of the area and the diversified vegetation. The subfamily Oedipodinae is the most widespread with eight species. These results are similar to those made by many researchers in Eastern Algeria, namely Benkenana (2006), Benkenana & Harrat (2009) and Benkenana et al. (2019), where the dominance of the family Acrididae is reported. Betina et al. (2017) reported 21 species in Batna, while Mahloul et al. (2016) inventoried 17 species in Oum El Bouaghi. The distribution of species between the three study sites indicates a high diversity in the two sites; Djebel Ouahch Park and Beni Hamidene. In contrast, the Grarem Gouga site has low diversity. This difference is linked to the environment and climatic conditions.
36The study of the frequency of occurrence aims to define the structuring species of the procession. At the Djebel Ouahch station level, the study of the frequency of occurrence shows that the species: Pezotettix giornae is the most abundant, with a value of 34.39. This means that this species is less disadvantaged than the others by the ecological conditions of the station. It is a species that occurs during most of the year in dry places, grasses, and low bushes (Chopard, 1943). Overall, the species belonging to the family Acrididae dominate the park’s population. This family is the most represented in all the work carried out in Algeria (Chopard, 1943; Ould El Hadj, 2004; Moussi et al., 2011). The regularity of the surveys allowed us to distinguish four groups of species: only one constant species; Pezotettix giornae, two regular species; Dociostaurus maroccanus and Pamphagus cristatus, five accessory species; Calliptamus sp, Acrotylus patruelis patruelis, Ochrilidia filicornis filicornis, Ocneridia volxemii and Paratettix meridionalis, and ten accidental species.
37For the Beni Hamidene station, the study of the frequency of each species made it possible to point out that certain species have high frequencies compared to others. It follows that this station contains one constant species; Ocneridia volxemii, which is the most frequent species. According to Benkenana (2013), Ocneridia volxemii has been recorded in 24 stations in eastern Algeria, and has been found in the semi-arid, subhumid and humid bioclimatic stages, when the temperature is lower than 6°C. It also holds three regular species; Acrotylus patruelis patruelis, Pamphagus sp and Paratettix meridionalis, four accessory species and seven accidental species.
38At the Grarem Gouga station, it appears that it contains only one constant species Ocneridia volxemii, two regular species; Oedipoda caerulescens sulfurescens and Acrotylus patruelis patruelis, three accessory species and 14 accidental species.
39The habitat amplitude (HA) was used to estimate each species’ spatial niche and thus see their distribution. The species Acrotylus patruelis patruelis is the most observed orthopteran in the study area. It is an essentially Mediterranean species (Bellmann & Luquet 1995). According to Defaut (1999), it is present throughout Africa. However, the species: Pamphagus milevitanus, Ochrilidia filicornis filicornis, Omocestus africanus, Dociostaurus jagoi jagoi and Oedipoda fuscocincta fuscocincta have a reduced spatial niche (HA=1), they are the most vulnerable, and this may be since they are influenced by the less favourable ecological conditions prevailing in the geographical area studied.
40Pamphagus milevitanus, an endemic species of the family Pamphagidae, described by Benkenana & Massa in 2017. It was reported for the first time in north-eastern Algeria in Mila. The study of morphometric and oothecae analyses of females shows that this species is giant with high fecundity. The number of eggs per ootheca exceeds 173. According to Bounechada (2007), the species Ocneridia volxemii of the same family as P. milevitanus, the number per ootheca varies between 30 and 35. This difference may be related to size, as Ocneridia volxemii is small in size; the diet is also different. Bounechada (2007) found that environmental conditions, including the quantity and quality of food consumed, influence the number of eggs in the oothecae. According to Benkenana (2013), Ocneridia volxemii is graminivorous, feeding mainly on Poaceae. On the other hand, species of the genus Pamphagus have a preference for Fabaceae.
41Climatic factors have a significant influence on the availability and distribution of orthopterofauna. According to Dusoulier (2002), orthopterans show a high sensitivity to environmental conditions. The Pearson correlation coefficient (r) calculation allowed us to deduce that the temperature variable positively affects orthopteran abundances (r = 6.09×10-4). This correlation indicates that the variable precipitation negatively influences Orthopteran abundance (r = -0.465). Wind speed’s climatic variable does not seem to affect orthopteran availability (r-value is very low (r = -0.340). Temperature is a key factor for many orthopteran species (Bellmann & Luquet, 1995; Boitier, 2003).
CONCLUSION
42This study highlighted an important richness of orthopterans in the study area. The number of species recorded in the three stations surveyed indicates a considerable diversity (25 species). The results reveal a clear difference in biodiversity between the three study stations. The family Acrididae is the richest in terms of number of species and individuals in the three surveyed stations. Pezotettix giornae is a constant and very abundant species in the park of Djebel Ouahch. The species Pamphagus milevitanus shows a high fecundity. This giant species, prey to various predators (birds, reptiles, etc.), has established itself in several sites. The sampling procedure and the ecological and statistical analyses proposed in this article allow an estimate of the spatial distribution of species, the structure and diversity of orthopteran populations in the study area. It is recommended to extend and deepen the research in the entire Eastern Algeria region to complete the results.
Bibliographie
Bellmann H. & Luquet G., 1995. Guide des Sauterelles, Grillons et Criquets d'Europe occidentale. Paris: Delachaux & Niestlé.
Belovsky G.E. & Slade J.B., 2002. An ecosystem perspective on grasshopper control: possible advantages to no treatment. Journal of Orthoptera Research, 11, 29–35.
Benkenana N., 2006. Analyse bio systématique, écologique et quelques aspects de la biologie des espèces acridiennes d’importance économique dans la région de Constantine. Magister thesis: University Frères Mentouri Constantine1 (Algeria).
Benkenana N. & Harrat A., 2009. Contribution to the systematic study of grasshopper fauna (Orthoptera, Caelifera) and some bio-ecological aspects of economic importance of species in the Constantine region (Eastern Algeria). Emirates Journal of Food and Agriculture, 21(1), 40-47.
Benkenana, N., 2013. Inventaire et analyse bio systématique de la famille des Pamphagidae (Orthoptera, Caelifera) de l’Est algérien. PhD Thesis: University Frères Mentouri Constantine 1 (Algeria).
Benkenana N., Harrat A. & Petit D., 2013. Analysis of the number of sensilla on the labrum and the diet of grasshoppers belonging to the family Pamphagidae (Orthoptera). European Journal of Entomology, 110(2), 355–364. DOI: 10.14411/eje.2013.097
Benkenana N. & Massa B., 2017. A new species of Pamphagus (Orthoptera: Pamphagidae) from Algeria with a key to all the species of the genus. Zootaxa, 3168, 22–38. https://doi.org/10.11646/zootaxa.4254.1.6
Benkenana N., Benchiheub S. & Zaabat N., 2019. Contribution à la connaissance de la faune acridienne (Orthoptera, Caelifera) dans la région de Mila (Est algérien). Revue Agrobiologia, 9(1), 1302-1310.
Betina S.I., Harrat A. & Petit D., 2017. Analysis grasshopper diversity and associated factors involved in grasshopper diversity in arid Aurès mountains (Batna, Algeria). Journal of Entomology and Zoology Studies, 5(5), 339-348.
Blondel J., 1979. Biogéographie et écologie. Paris : Masson.
Boitier E., 2003. Caractérisation écologique et faunistique des peuplements d’Orthoptères en montagne auvergnate. Diplôme d’étude et de recherche. Science de la vie et de la terre. Limoges University (France).
Boudy P., 1955. Economie forestière Nord-africaine. Tom IV. Description forestière de l’Algérie et de la Tunisie. Paris : Larose.
Bounechada M., 2007. Recherches sur les Orthoptères. Etude bioécologique et essais de lutte biologique sur Ocneridia volxemi Bol. (Orthoptera, Pamphagidae) dans la région de Sétif. PhD thesis : University Ferhat Abbas Setif (Algeria).
Brader L. et al., 2006. Towards a more effective response to desert locusts and their impacts on food security, livelihoods and poverty. In Proceedings of Independent Multilateral Evaluation of the 2003–2005 Desert Locust Campaign, FAO, Rome.
Chopard L., 1943. Orthoptéroïdes de l'Afrique du Nord. Faune de l'empire français 1. Paris : La rose.
Dajoz R., 1985. Précis d’écologie. Paris : Dunod.
Defaut B., 1999. Synopsis des Orthoptères de France. Matériaux entomocénotiques, Bédeilhac, 2,1-88.
Dusoulier F., 2002. Les insectes peuvent-ils servir de bioindicateurs climatiques, L’exemple des orthoptères en Bretagne. Publications de l’Association Internationale de Climatologien, 14, 245-252.
Eades D.C. et al., 2011.Orthoptera Species File Online. Version 2.0/4.0. http://orthoptera.speciesfile.org/ (20/05/2021).
Faurie C., Ferra C. Medori P. Devaux J. & Hemptinne J.L., 2003. Ecologie approche scientifique et pratique. Paris : Lavoisier.
Felzines J.C., 1982. Etude dynamique, sociologique et écologique de la végétation des étangs du Centre-Est de la France. Importance de la compétition interspécifique dans l'organisation de la végétation et la distribution des espèces et des associations. PhD thesis: Lille University of Science & Technology (France).
Gandar M.V., 1982. The dynamics and trophic ecology of grasshoppers (Acridoidea) in a South African savanna. Oecologia, 54, 370–378.
Hachiche L., 2014. Restauration et réhabilitation de la réserve biologique de Djebel Ouahch, Constantine. Master thesis, University Frères Mentouri Constantine 1 (Algeria).
Hammer D.A.T. et al., 2001. Paleontological statistics software package for education and data analysis. Palaeontologica Electronica (9) http://palaeoelectronica.org/ (08/06/2021).
Jago N.D., 1963. A revision of the genus Calliptamus (Orthoptera, Acrididae). Bulletin of the British Museum (Natural History) Entomology, 13(9), 289-350.
Joern A., Danner B.J., Logan J.D. & Wolesensky W., 2006. Natural History of Mass-Action in Predator-Prey Models: A Case Study from Wolf Spiders and Grasshoppers. The American Midland Naturalist, 156(1), 52–64.
Kirch W. Ed., 2008. Pearson’s Correlation Coefficient BT-Encyclopedia of Public Health. P. 1090-1091. Dordrecht: Springer.
Latchininsky A.V., 2013. Locusts and remote sensing: a review. Journal of Applied Remote Sensing, 7, 075099. https://doi.org/10.1117/1.JRS.7.075099
Launois M., 1979. Manuel pratique d’identification des principaux acridiens du Sahel. Paris : Ministère de la coopération.
Lheritier J.N., Debussche M. & Lepart J., 1979. L'avifaune nicheuse des reboisements de Pin noir du Causse Méjean. La revue française d’ornithologie, 49, 185-211
Louveaux A. et al., 2021. Orthoptères Acridomorpha de l'Afrique du Nord-ouest. http://acrinwafrica.mnhn.fr/ (06/06/2021).
Mahloul S., Harrat A. & Petit D., 2016. Diversity of grasshoppers (Caelifera) recorded on the banks of a Ramsar listed temporary Salt Lake in Algeria. European Journal of Entomology, 113, 158-172. https://doi.org/10.14411/eje.2106.020
Massa B., Fontana P. Buzzetti F.M. Kleukers R. & Ode B., 2012. Fauna d’Italia. XLVIII. Orthoptera. Bologna: Calderini.
Météo en temps réel., 2021. Données climatologiques. https://www.infoclimat.fr/climatologie/ (25/12/2020).
Millist N. & Abdalla A., 2011. Benefit-cost analysis of Australian plague locust control operations for 2010–11. ABARES report prepared for the Australian Plague Locust Commission.
Moussi A., Abba A., Harrat A. & Petit D., 2011. Desert acridian fauna (Orthoptera, Acridomorpha): comparison between steppic and oasian habitats in Algeria. Comptes Rendus Biologies, 334, 158-167. https://doi.org/10.1016/j.crvi.2010.12.001
National meteorological office., 2021. Données climatologiques. https://www.meteo.dz/ (25/12/2020).
Ould El Hadj M.D., 2004. Le problème acridien au Sahara algérien. PhD thesis: Higher National Agronomic School El-Harrach (Algeria).
Ramade F., 1984. Eléments d’écologie : écologie fondamentale. Paris : Mc. Graw & Hill.
Samways M. & Sergeev M.G., 1997. Orthoptera and landscape change. In: Gangwere SK, Muralirangan MC, Muralirangan M, editors. The bionomics of grasshoppers, katydids and their kin. Wallingford (UK) : CAB International ; p. 147–162.
Shannon, C. E. and Weaver, W., 1949. The mathematical theory of communication. Illinois: University of Illinois Press.
Wright D.E., 1986. Economic assessment of actual and potential damage to crops caused by the 1984 locust plague in south-eastern Australia. Journal of Environmental Management, 23, 293-308.
(42 Réf.)