- Accueil
- Volume 76 (2023)
- Study on the foraging and pollinating activities of Apis mellifera L., 1758 (Hymenoptera: Apidae) on Solanum aethiopicum L., 1763 (Solanaceae) in Dang (Ngaoundéré, Adamawa, Cameroon)
Visualisation(s): 2257 (1 ULiège)
Téléchargement(s): 132 (0 ULiège)
Study on the foraging and pollinating activities of Apis mellifera L., 1758 (Hymenoptera: Apidae) on Solanum aethiopicum L., 1763 (Solanaceae) in Dang (Ngaoundéré, Adamawa, Cameroon)

Document(s) associé(s)
Version PDF originaleRésumé
Des observations ont été faites à Dang (Adamaoua, Cameroun) sur les fleurs de Solanum aethiopicum au cours des saisons 2020 et 2021 pour évaluer l'influence des activités de butinage et de pollinisation de Apis mellifera sur la production. Quatre traitements de 120 fleurs chacun ont été établis selon la protection (T2 et T6) ou non (T1 et T5) des fleurs contre les visites d'insectes, et l'ouverture puis la refermeture des fleurs après avoir reçu une visite d’A. mellifera (T3 et T7) ou sans visite d’insectes et de tout autre organisme (T4 et T8). Les visites florales d’A. mellifera ont été étudiées et les paramètres agronomiques évalués et comparés entre les traitements. Apis mellifera est apparu comme le principal insecte visiteur et pollinisateur de S. aethiopicum au cours des deux années d’investigation, avec une abondance relative moyenne de 41,07%. Cette espèce d’abeille récoltait exclusivement les grains de pollen sur les fleurs de S. aethiopicum. Son activité journalière débutait à 6 h avec l’épanouissement des fleurs et se terminait à 15 h avec leur fanaison ; un pic de visites florales a été noté entre 8 h et 9 h. La densité moyenne des abeilles était d'environ 340 individus/1000 fleurs et la vitesse de butinage variait de 4,1 à 6,77 fleurs/minute. Grâce à son efficacité pollinisatrice, A. mellifera a augmenté le taux de fructification, le taux moyen de graines par fruit et le taux de graines normales de S. aethiopicum de 15,46 %, 6,84 % et 7,60 % respectivement. La protection d’A. mellifera est essentielle pour l'amélioration des rendements au champ de S. aethiopicum.
Abstract
Observations were made in Dang (Adamawa, Cameroon) on Solanum aethiopicum flowers during the 2020 and 2021 seasons for assessing the influence of Apis mellifera foraging and pollination activities on yields. Four treatments of 120 flowers each were established according to the protection (T2 and T6) or not (T1 and T5) of the flowers from insect visits and isolating, opening, and reclosure of flowers after receiving a visit from A. mellifera (T3 and T7) or no visitor whatsoever (T4 and T8). Floral visits by A. mellifera were studied and the agronomic parameters were evaluated and compared between treatments. With a relative abundance of 41.07%, this bee species harvested exclusively pollen grains on S. aethiopicum flowers. The daily activity of workers occurred between 6 a.m. and 3 p.m., with a peak of floral visits at 8 - 9 a.m. time interval. The mean density of bees was about 340 individuals/1000 flowers and the foraging speed varied from 4.1 to 6.77 flowers/minute. Through its pollination efficiency, A. mellifera increased the fruiting rate, the average rate of seeds per fruit, and the rate of normal seeds of S. aethiopicum by 15.46%, 6.84%, and 7.60% respectively. Protection of A. mellifera is essential for the improvement of S. aethiopicum field yields.
Table des matières
INTRODUCTION
1Insects are key factors in food security in the world and essential links in the food chains (Nicolas et al., 2013). Among these invertebrates, bees in general and singularly honeybees Apis mellifera Linnaeus, 1758 are the most important visitors and efficient vectors of pollen grains on plant species from flower to flower (Rosa et al., 2010). Apis mellifera is therefore the major pollinator of several crops and wild plant species (Ollerton et al., 2011). By visiting the flowers in searching for proteins and carbohydrates, workers facilitate the host plant breeding through its pollination activities (Klein et al., 2007). Several previous findings are illustrative of the prominence of honeybees as the pollinator of wild plant species and crops: Malus domestica Stark Brothers, 1916 (Jacob-Remacle, 1989); Cucumis sativus Linnaeus, 1753 (Stanghellini et al., 1998a,b, Gingrass et al., 1999); Anacardium occidentale Linnaeus, 1753 Freitas et al., 2002; Citrullus lanatus (Thunberg) Mansf, 1916 (Kremen et al., 2004; Azo’o et al., 2017); Helianthus annuus Linnaeus, 1753 (Greenleaf and Kremen, 2006; Tchuenguem et al., 2009a,b); Ximenia americana Linnaeus, 1927 and Syzygium guineense var. guineense Willd, 1828 (Djonwangwé et al., 2011a,b); Phaseolus coccineus Linnaeus, 1753 (Pando et al., 2011a,b); Cucumeropsis mannii Naudin, 1815 (Azo’o and Messi, 2012); Callistemon rigidus Robert Brown, 1857 (Fameni et al., 2012); Vigna unguiculata (Linnaeus) Walpers, 1843 (Kengni et al., 2015a,b); Gossypium hirsutum Linnaeus, 1763 (Basga et al., 2019); Bidens steppia Serff, 1923 (Mbere et al., 2022). The pollination service of honeybees is an unvaluable contribution for maintaining biological balance in nature and enables animals, plants, and humans to thrive (Gallai et al., 2009). Alongside the pollination activity on flowers, honeybees produce several products such as honey, propolis, wax, venom, and royal jelly (Tyburce, 1996). These products have multifunctional uses and are considered beneficial to health purposes (Molan, 2001).
2One-third of human food and three-quarters of fruit, legume, oilseed, and protein crops depend on entomophilous pollination for their production (Terzo, 2007). African vegetables have been neglected for a long time and are now gradually being taken into account nationally and internationally (Seck, 2007). Cultivation of vegetables plays an important role in the income diversification strategies of urban and peri-urban populations (Kahane et al., 2005). Growing them in home gardens is an effective way to improve the livelihoods of peasants in alleviating poverty (Betti et al., 2016). Of the 275 most important vegetable species in tropical Africa, 207 are consumed for their leaves, approximately 37 for their fruits and over 31 species are used for their roots or tubers, especially their fruits such as the African eggplant Solanum aethiopicum (Kahane et al., 2005).
3Also called scarlet eggplant or Ethiopian eggplant, S. aethiopicum is a plant of the Solanaceae family (Silva et al., 2004). It is mainly a fruit vegetable, but its hairless leaves are also eaten in southern Senegal and other African countries (Seck, 2007). Its edible fruits can be eaten at an advanced stage of ripening (Chen et al., 2000). The fruits of S. aethiopicum are richer than those of tomato and European eggplant in calories, proteins, carbohydrates, and ash (Toury et al., 1965). The roots and fruits are used as carminative and sedative hypertensives, antiemetic and antidiarrheal (Agbankpe et al., 2014). Leaf sap is also used as a sedative to treat uterine ailments (Jouzier, 2005; Diatta et al., 2020).
4The enhancement of crop yields using insects as a production factor still failed to appreciate in the rural sector by peasants in Cameroon (Tchuenguem et al., 2001). Yet, bees are nowadays recognized as indisputable agricultural inputs which allow a substantial increase in yields while preserving the integrity of the environment (Haubruge et al., 2007). Before our research, published data on the mutualism between S. aethiopicum and flower-visiting insects are from Nigeria (Oyelana and Ogunwenmo, 2012), Ivory Coast (Konan, 2013; Obodji et al., 2016; Felicia et al., 2019), and India (Latif et al., 2009). Apart from current studies, only works of Kengni et al. (2022) have been carried out on the foraging and pollination activity of an unidentified carpenter bee Xylocopa sp. on the Solanaceae in the Far-North region of Cameroon. The present study was defined to show the importance of honeybee activities in pollination and the production of African eggplant in the field study. Specific objectives were to i) determine the rank of honeybees A. mellifera in the floral entomofauna of the host plant; (ii) study the floral activity of bee workers on the flowers of the crop and, (iii) appreciate the consequence of the floral activity of A. mellifera on the pollination and yields of S. aethiopicum.
MATERIALS AND METHODS
Materials
5Investigations were carried out from May to September 2020 and from May to August 2021 in Dang (Adamawa Region of Cameroon). This region belongs to the ecological zone with the high Guinean savannas (Djoufack-Manetsa, 2011). The climate in the Adamawa is of the Sudano-Guinean type, which has two seasons: a rainy season (April to October) and a dry season (November to March). The average annual temperature (°C) is 22±1 and the average annual humidity (%) is 70±16 (Ngaoundéré-airport meteorological station, 2022). The study site was an experimental field with 437 m2 corresponding to the following geographical coordinates: latitude: 7°24'22.6''N, longitude: 13°32'52.3''E, altitude: 1085 m.
6In addition to the insects naturally present in the environment of the study site, the animal material was enriched with an apiary of 36 and 42 colonies of A. mellifera in 2020 and 2021 respectively. The plant material consisted of S. aethiopicum seeds purchased in a store for the sale of agricultural equipment in Ngaoundéré. Technical equipment was used for setting up the experimental plot. Entomological equipment such as an entomological hand net, gauze bags, 70% diluted ethanol, and entomological boxes were used for capturing and preserving African eggplant flower-visiting insect specimens.
7In Laboratory of Applied Zoology, University of Ngaoundéré, bee identifications were done by Prof. Tchuenguem Fohouo Fernand-Nestor, using reference collection and identification books (Delvare & Aberlenc, 1989; Borror & White, 1991; Eardley et al., 2010).
Determination of the reproduction mode of African eggplant
8At the appearance of the flower buds (August 29, 2020, and July 14, 2021), 240 of them were randomly selected on 15 stands per subplot for two treatments of 120 flowers each; a treatment X [T1 (2020) or T5 (2021)] with open-pollinated flowers and a treatment Y [T2 (2020) or T6 (2021)] where flowers were covered using 1 mm2 mesh gauze bags (Figure 1). At maturity, the number of ripe fruits was counted, and the fruiting index (Ifr) was calculated in both treatments using the following formula: Ifr = Fb / Fa, where Fb is the number of fruits formed and Fa is the number of flowers initially labeled (Tchuenguem et al., 2001). The difference in fruiting indexes between treatments enables estimating the rate of allogamy (TC) and autogamy (TA) using both equations of Demarly (1977): TC = {[(IfrX - IfrY) / IfrX] * 100}, where IfrX and IfrY are the fruiting indexes in treatments X and Y respectively and the deduced TA = (100 – TC).
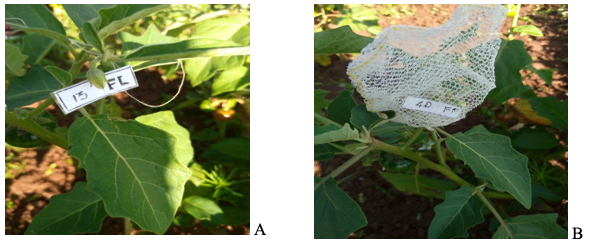
Figure 1: Solanum aethiopicum plant showing unprotected flower (A) and protected with gauze bag (B)
Ranking of honeybees in the floral entomofauna of eggplant
9During the flowering period of African eggplant, from August 29 to September 15, 2020 and from July 14 to August 29, 2021, observations were made every day on the flowers of treatments T1 and T3, following six daily time intervals: 6 - 7 a.m., 8 - 9 a.m., 10 - 11 a.m., 12 - 1 p.m., 2 - 3 p.m., and 4 - 5 p.m. The different flower-visitor species found were counted using the direct method and the cumulative results of counts were expressed as the number of visits (Tchuenguem et al., 1997). Data on the frequency of forager visits enabled us to determine the rank of each one of them in the floral entomofauna of S. aethiopicum. The frequency of visits of a given insect i to the flowers of S. aethiopicum (Fi) was evaluated using the following equation: Fi = [(Vi / Vt) * 100], where Vi is the number of visits of the insect i on the flowers from treatments X and Vt the number of visits of all the insects recorded (Tchuenguem et al., 1997).
Activity of Honeybees on the flowers of African eggplant
10The proportion of collecting floral products (pollen or nectar) by A. mellifera on S. aethiopicum was noted when recording the length of visit per flower (Tchuenguem et al., 2010). The nectar-sicker plunged its proboscis towards the nectaries while pollen collectors scraped the anthers using its mandibles and legs. The rhythm of honeybee activity according to the rhythm of eggplant blossoming daily was highlighted; moreover, the variation of the daily activity of foragers as a function of the time intervals was assessed. The duration of the floral products harvested was timed at the same dates as the frequency of visits, during the daily time slots mentioned above. The stopwatch, reduced to zero, was started as soon as a forager landed on a flower and stopped as soon as its lefts it. The duration of an individual visit corresponds to the value read on the stopwatch. The greatest number of individuals simultaneously active on a flower was recorded following direct counts. The abundance of foragers per 1000 flowers (A1000) was inferred using the following equation: A1000 = [(Ax / Fx) * 1000], where Fx and Ax are respectively the numbers of blooming flowers and the number of foragers counted on opened flowers at time x (Tchuenguem et al., 2004). The foraging speed (Vb) or the number of flowers visited per minute was also recorded (Jacob-Remacle, 1989); the chronometer reduced to zero was triggered as soon as a forager landed on a flower and was stopped as soon as it was lost sight of; the number of flowers visited was counted during the foraging trip. Vb = [(Fi / di) * 60], where di is the duration given by the stopwatch (in seconds) and Fi is the number of flowers corresponding to di (Jacob-Remacle, 1989).
11The foraging ecology which was considered consisted of the study of the abiotic factors influences such as temperature and relative humidity on the daily foraging rhythm of A. mellifera. During each day of observation, the temperature and relative humidity of the study station were recorded twice per observation time slot using a thermo-hygrometer. In addition, disruption of the activity of foragers by competitors or predators and the attractiveness exerted by other plant species on A. mellifera was assessed by direct observations (Tchuenguem, 2005). For the second parameter, the number of times that the bee left the Solanaceae flowers to another plant species and vice versa was noted through the investigation period (Tchuenguem, 2005).
Repercussion of the foragers on production of African eggplant
12In parallel with the implementation of treatments X and Y, 240 flower buds were labeled to constitute two other treatments consisting of 120 flowers each: a treatment A [T3 (2020) or T7 (2021)] whose flowers were intended for the pollinating efficiency of A. mellifera workers. As soon as each flower bloomed, the gauze bag placed the day before was removed to favor a single visit from a worker after which the flower was covered again; a Z treatment [T4 (2020) or T8 (2021)] whose initially protected flowers were opened and then reclosed without any visiting insect (Tchuenguem et al., 2018).
13At maturity, the fruits from treatments A and Z were harvested and compared. The assessment of the impact of flower-visiting insects on the fruit and seed production of S. aethiopicum was based on the estimates and comparison of the fruiting rate, the average number of seeds, and the percentage of normal seeds per fruit between treatments X, Y, and Z. for a given treatment, the fruiting rate results from the ratio between the number of fruits formed and the number of flowers considered. The contribution of insect floral activity on the fruiting rate (Fri) was deduced using the equation: Fri = {[(FX - FZ) / (FX + FY - FZ)] * 100}, where FX, FY, and FZ are the fruiting rates in treatments X, Y and Z (Diguir et al., 2020). Using the same method as for fruiting rate, the percentage of normal seeds due to flowering insects was deduced.
14The contributions of A. mellifera to the fruiting rate, the mean number of seeds per fruit, and the percentage of normal seeds were also evaluated. The fruiting rate due to A. mellifera (Fra) was calculated using the following formula: Fra = {[(FA - FZ) / FA] * 100} (Djakbé et al., 2017). The percentage of normal seeds due to the pollinating action of honeybees was estimated using the same reasoning.
Data analysis
15Data were compiled in Excel 2013 and analyzed through descriptive statistics. The following statistic tests were hereby used: (i) the t-test of Student for comparing means between two samples, (ii) the Chi-square test (χ2) for the comparison of proportions between two samples, and (iii) the Pearson correlation coefficient (r) for establishing linear relationships between two data series from two equal size samples. The actual analyzes were facilitated using the software R 2.13.0.
RESULTS
Reproduction mode of Solanum aethiopicum
16Fruiting indexes were 0.97, 0.86, 0.98 and 0.88 in treatments T1, T2, T5 and T6 respectively. The deduced TC and TA using these indexes were TC = 11.34% and TA = 88.66% in 2020 and TC = 10.20% and TA = 89.80%. The cumulative results from both years were TC = 10.77% and TA = 88.93%. Overall, S. aethiopicum shows a mixed reproduction mode with the prominence of autogamy on allogamy.
Rank of Apis mellifera in the floral entomofauna of Solanum aethiopicum
17In 2020 and 2021, 565 and 522 visits of 5 and 7 insect species were registered on 120 flowers of S. aethiopicum. Results from table 1 are illustrative of insect species recorded and their relative abundance yearly. In both years, A. mellifera was the first flower visitor of S. aethiopicum with 41.07% of the frequency of visits. There is no significant difference between both proportions year to year (χ2 = 0.67; df = 1; p ˃ 0,001).
Table 1: Flower-visitors of Solanum aethiopicum and their frequencies in 2020 and 2021
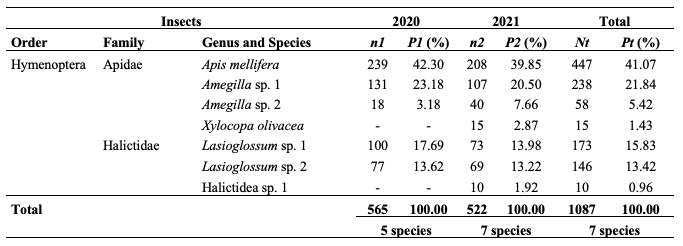
n1: number of visits in 2020; n2: number of visits in 2021; Nt: total sum of visits in 2020 and 2021; P1: percentages of visits in 2020; P2: percentages of visits in 2021; Pt: total sum of percentages of visits in 2020 and 2021; sp.: unidentified species; P1 = (n1/565) * 100; P2 = (n2/(522) * 100.
Floral products harvested
18During their floral visits, workers of A. mellifera highly and exclusively collected pollen of S. aethiopicum. Indeed, the results recorded showed that among 200 visits studied for floral preference, the workers were only observed in the posture of collecting pollen (Figure 2).

Figure 2 : Posture for pollen harvesting by Apis mellifera on an eggplant flower.
Rythm of honeybee visits according to the daily rhythm of eggplant blossoming
19Figure 3 shows the variation of the number of blooming flowers and the number of A. mellifera workers visits depending on the observation days. From this figure, the number of daily visits by A. mellifera was proportional to the number of daily opened flowers of S. aethiopicum. The correlation between both parameters was positive and significant in 2020 (r = 0.72; df = 4; p < 0.01) and in 2021 (r = 0.82; df = 4; p < 0.01). This result highlights the good attractiveness of the pollen of S. aethiopicum towards A. Mellifera.
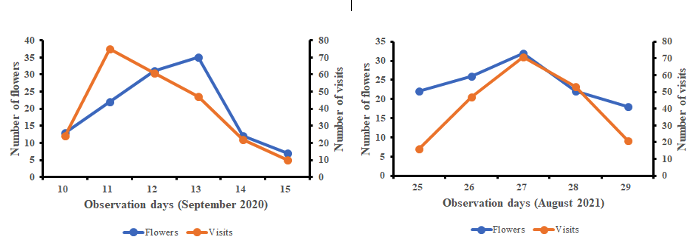
Figure 3: Seasonal variations of Solanum aethiopicum opened flowers and the honeybee worker visits.
Daily rythm of worker visits as a function of time interval
20Figure 4 shows the distribution of A. mellifera visits on the flowers of S. aethiopicum following the daily time slots defined. The floral activity of honeybees started at down 6 - 7 a.m. (flower anthesis) and stopped at 2 - 3 p.m. (flower wilting). An important peak of this daily activity occurred at the 8 - 9 a.m. time interval. There is a similarity in the daily rhythm of worker visits during both years.
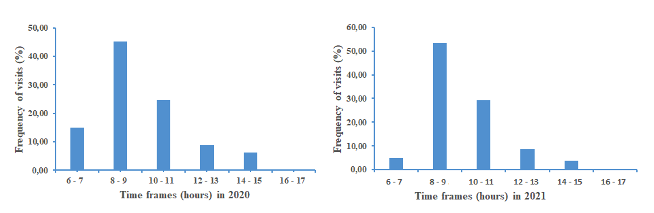
Figure 4: Rythm of floral activity of honeybees on eggplant following the daily time slots.
Density of workers
21The average density of workers per flower of S. aethiopicum was 1 (n = 54; s = 0) in 2020 and 2021 as well (n = 63; s = 0). The mean abundance per 1000 flowers were 337.03 (n = 54; s = 153.32) in 2020 and 317.46 flowers (n = 63; s = 155.06) in 2021. The difference of the two latter means between both years was not significant (t = 0.68; df = 115; p ˃ 0.05) which is expressive of the stability of the field experiment yearly.
Mean duration of a honeybee visits per flower
22The mean length of a floral visit of A. mellifera varied from 35.16 sec (n = 100; s = 26.37) in 2020 to 35.03 sec (n = 113; s = 24.41) in 2021. The difference between both values was not significant (t = 0.04; df = 211; p ˃ 0.05).
Mean foraging speed
23On S. aethiopicum, a bee worker visited 1 to 51 flowers/min in 2020 then, 1 to 53 flowers/min in 2021 during a foraging trip. The mean foraging speed was 6.71 flowers/min (n = 111; s = 3.36) in 2020 and 4.10 flowers/min (n = 132; s = 2.59) in 2021. The difference was highly significant between both mean values (t = 6.77; df = 241; p < 0.001) and shows the variation of the foraging speed of workers from year to year.
Influence of abiotic and biotic factors on the daily rhythm of activity of workers
24The correlations were not significant between the A. mellifera visits and the temperature in 2020 (r = 0.04; df = 4; p > 0.05) and in 2021 (r = 0,02; df = 4; p > 0.05), as well as between these visits and the relative humidity in 2020 (r = 0.35; df = 4; p > 0,05) and in 2021 (r = 0.61; df = 4; p > 0.05).
25Workers of A. mellifera were disturbed in their foraging activity by other foragers of the same species or those from other species that were competitors for S. aethiopicum pollen. In 2020, for 100 visits of A. mellifera, 2 and 1 were interrupted by A. mellifera and Amegilla sp. 1 Friese, 1897 respectively. In 2021, for 113 visits, 3.53%, 1.76%, 0.88% and 0.88% were interrupted by A. mellifera, Amegilla sp. 1, Lasioglossum sp. 1 Curtis, 1833 and Xylocopa olivacea Fabricius, 1787 respectively.
26During the flowering season of S. aethiopicum, flowers of many other plant species surrounding the field of this Solanaceae were visited by A. mellifera, for nectar (ne) or pollen (po). Among these plants were Bidens pilosa L., 1753 (Asteraceae, ne and po), Hibiscus sabdarifa L., 1753 (Malvaceae, ne) and Zea mays L., 1753 (Poaceae, po). During the two years of study, we observed no passage of A. mellifera from S. aethiopicum flowers to flowers of another plant species and vice versa.
Impact of flowering insect including Apis mellifera on the pollination and production of eggplant
27During pollen harvesting, the worker constantly meets the reproductive organs of S. aethiopicum and moves from flower to flower with pollen grains adhered to its hair tegument. Therefore, honeybees were identified in this study as one of the active pollen transferors on S. aethiopicum flowers.
28Table 2 shows the fruit set rates, the mean number of seeds per fruit, and the percentage of normal seeds from flowers of S. aethiopicum according to treatments T1 and T2 in 2020 then T5 and T6 in 2021. Overall, the fruit set rate was higher in treatments with open-pollinated flowers (T1 and T5) than those whose flowers were covered for excluding insect foraging activity (T2 and T6). The difference of the fruit set rate was highly significant between T1 and T2 (χ2 = 10.03; df = 1; p < 0.01) and between T5 and T6 (χ2 = 9.64; df = 1; p < 0.01). The values of the fruit set rates due to the foraging activity of insects were 11.76% in 2020 and 17.32% in 2021, with a cumulative mean value of 14.54%.
Table 2: Production of eggplant according to treatments T1, T2, T5 and T6.
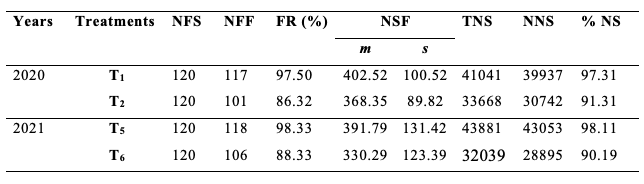
NFS: number of flowers studied; NFF: number of fruits formed; FR: Fruiting rate; NSF: number of seeds/fruit; TNS: total number of seeds; NNS: number of normal seeds; %NS: percentage of normal seeds; T1 and T5: unprotected flowers; T2 and T6: protected flowers; m: mean; s: standard deviation.
29From the same table 2, the mean number of seeds per fruit was higher in treatments T1 and T5 than in treatments T2 and T6. The difference between the mean value of seeds per fruit from both treatments was significant in 2020 (t = 2.54; df = 216; p < 0.05) and high significant in 2021 (t = 3.60; df = 222; p < 0.001). The deduced mean number of seeds per fruit of eggplant which resulted from the floral activity of its visitors was 7.06% in 2020 and 6.83% in 2021, with a cumulative mean value of 6.94%.
30The percentage of normal seeds was less important in bagged clusters than on exposed-pollinated flowers of corresponding treatments. The insects' floral activity was responsible for the percentage improvement of normal seeds by 9.57% in 2020 and 5.35% in 2021, with a cumulative result of 7.46% for both experiments.
Pollination efficiency of Apis mellifera on Solanum aethiopicum
31Table 3 shows the fruit set rate, the mean number of seeds per fruit, and the percentage of normal seeds of S. aethiopicum according to treatments T3 and T4 in 2020 and T7 and T8 in 2021. Overall, the fruit set rate was higher in treatments with flowers visited by A. mellifera (T3 and T7) than those whose flowers were protected, uncovered and rebagged without visit of insect or any other organism (T4 and T8). The difference of the fruit set rate was highly significant between T3 and T4 (χ2= 20.07; df = 1; P < 0.001) and between T7 and T8 (χ2 = 24.52; df = 1; P < 0.001). The value of the fruit set rate due to the foraging activity of A. mellifera was 13.04% in 2020 and 17.89% in 2021, with a cumulative mean value of 15.46%.
Table 3: Production of eggplant according to treatments T3, T4, T7 and T8.
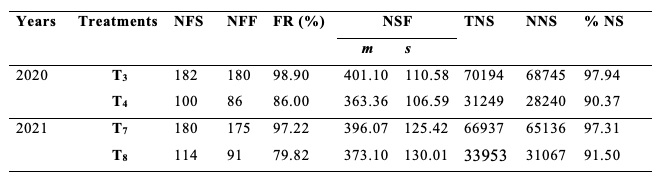
NFS: number of flowers studied; NFF: number of fruits formed; FR: Fruiting rate; NSF: number of seeds/fruit; TNS: total number of seeds; NNS: number of normal seeds; %NS: percentage of normal seeds; T3 and T7: flowers protected then uncovered, visited once by Apis mellifera and reprotected; T4 and T8: flowers bagged then uncovered and rebagged without visit by insect or any other organism; m: mean; s: standard deviation.
32From the same table 3, the mean number of seeds per fruit was higher in treatments T3 and T7 than in treatments T4 and T8. The difference between the mean number of seeds per fruit from both treatments was highly significant in 2020 (t = 2.81; df = 264; p ˂ 0.01) and not significant in 2021 (t = 1.33; df = 264; p ˃ 0.05). The deduced mean number of seeds per fruit for eggplant which resulted from the floral activity of A. mellifera was 7.72% in 2020 and 5.97% in 2021, with a cumulative mean value of 6.84%.
33The percentage of normal seeds was less important in bagged clusters visited by A. mellifera than in flowers protected, uncovered and rebagged without visit of insect or any other organism of corresponding treatments. The A. mellifera' floral activity was responsible for the percentage improvement of normal seeds by 9.41% in 2020 and 5.80% in 2021, with a cumulative result of 7.60% for both experiments.
DISCUSSION
34Our findings on the honeybee pollination of eggplant in Dang are once more the confirmation of the mixt reproduction mode autogamy-allogamy characterizing the crop and which is globally consistent with previous information on plant species of the family Solanaceae (Salunkke et al., 1987; Charrier et al., 1997; Smith and Knapp, 2002). Moreover, previous results on the Solanaceae pollinator interactions corroborate the importance of bee species as prominent visitors and efficient pollinators of Solanaceae species. According to Shanika et al. (2017), the Solanaceae family is among plant families which are pollinated by bees. In Western Belize (Centre America), seven Solanaceae species belonging to both genus Solanum L., 1753 (S. erythrotrichum, S. lanceifolium, S. rudepannum, S. cordovense, S. nudum) and Lycianthes (L. hypoleuca and L. gorgonea) were mentioned to be pollinated exclusively by 17 different bee species belonging to Colletidae, Halictidae and Apidae families (Smith and Knapp, 2002). In Oklahoma (Norman), several species in the genus Bombus Latreille, 1802 were the primary pollen vector of S. rostratum (Solís-Montero, 2013). In Kenya, Gemmill-Harren and Ochieng (2008) identified two native bee species namely Macronomia rufipes Smith, 1875 and Xylocopa caffra L., 1767 as effective pollinators of S. melongena. Oyelana and Ogunwenmo (2012) highlighted the implication of wild bee species Megachile latimanus Thomas Say, 1823 in the pollination of S. aethiopicum in Nigeria. In Britain, Solanaceae were predominantly pollinated by bees and flies (Edmonds and Chweya, 1997). Mamoudou et al. (2021) have already shown the outbreak of A. mellifera on S. nigrum flowers as the main pollinator of this crop in Maroua (Cameroon); moreover, the findings of Kengni et al. (2022) are illustrative of the prominence of a Carpenter bee Xylocopa sp. as the main visitor and pollinator of S. aethiopicum which are not consistent with the present result in the same plant species with honeybees as the principal anthophilous insect. Of the above, there is a difference in terms of the number of insects and their diversity, plants, periods and geographical areas. In all, the floral entomofauna can vary from plant species to plant species inside the same botany family, for a given plant species from year to year, and for the same year from season to season or site to site (Klein et al., 2007; Ollerton et al., 2011).
35The result in exclusively pollen-feeding behavior of A. mellifera in the flowers of S. aethiopicum is related to the absence of nectar production by this plant species as already ascertained by Mein (2021). Our results highlighted a contrast with those of Mamoudou et al. (2021) which are indicative of the dominance of pollen harvesting on the nectar foraging by A. mellifera in S. nigrum flowers at Meskine (Maroua, Cameroon). The pollen collection by honeybee workers was higher between 8 and 9 a.m which is within the daily period of the highest availability of pollen in the flowers of S. aethiopicum.
36During their floral activity on S. aethiopicum, workers commonly scrabbled and trigged anthers and thus could increase self-pollination of the flowers visited. Visiting a given flower, honeybee individuals were dusted with pollen grains of S. aethiopicum. During a floral trip, the high values of the foraging speed which was relatively up to 5 flowers/minute are illustrative of the implication of foragers in the cross-pollination of the flowers visited. Moreover, as a social bee, the pollination ethology of A. mellifera workers, which is related to the important number of congeners inside a colony and the ability of explorers to communicate with and recruit several individuals toward an interesting source of booty (Louveaux, 1984) is justificative of the high mean value of the honeybee density on S. aethiopicum flowers. This influx of A. mellifera foragers visiting eggplant flowers for pollen harvesting implies that this bee species is one of the best pollinator of S. aethiopicum in the Adamawa Region of Cameroon. According to Vaissière et al. (1996), pollen grains retained by the honeybee hair are efficient for crop pollination.
37Overall, the pollination activity of A. mellifera impacted positively the reproduction ability and yield improvement of the plant studied. Indeed, the increase of pollen load on the stigmatic surface of each flower visited due to honeybee workers enables the establishment of an important fruiting rate and seed set in open-pollinated flowers comparatively with flowers of S. aethiopicum which were bagged to avoid insect visits. Several other studies on plant-pollination networks highlighted the positive influence of anthophilous insect species in increasing fruit and seed production worldwide. A quantitative assessment of the increase in production due to pollinating insects on cotton is known to be 53% in India (Mahadevan and Chandy, 1957). The honey bee increases the yields of Physalis peruviana L., 1753 by 7% in Colombia (Chautá-Mellizo et al., 2012). In Cameroon, insects have increased the fruit production of Physalis minima L., 1753 by 23% (Djakbé et al., 2017) and that of Solanum nigrum by 16% (Mamoudou et al., 2021).
38During the collection of pollen on each flower, A. mellifera workers were regularly in contact with the stigma and anthers. They could thus enhance self-pollination, which has been demonstrated in the past by Edmonds and Chweya (1997), by applying pollen of one flower on its stigma. Apis mellifera could provide allogamous pollination through carrying of pollen within their hairs, silk, legs, mouthparts, thorax and abdomen, which is then deposited on flowers belonging to a different plant of the same species (geitonogamy) (Edmonds and Chweya, 1997). The positive and significant contribution of A. mellifera in the production of S. aethiopicum is justified by the action of this bee on the pollination of visited flowers.
CONCLUSION
39In Dang, S. aethiopicum has a mixt reproduction mode including the autogamy which predominated the allogamy. Moreover, A. mellifera was the main pollen harvester and pollinator on this plant species studied. Honeybee workers enabled the self and cross-pollination to occur, hence helped in boosting the fruiting rate, the mean number of seeds per fruit and the rate of normal seeds per fruit of S. aethiopicum by 15.46%, 6.84% and 7.60%. The installation of A. mellifera colonies at the vicinity of S. aethiopicum fields is recommended to increase fruit quantity and seed production, as well as to improve pollen production as a hive product.
Acknowledgments
The authors thank all those who facilitated this work, especially the University of Ngaoundéré which provided us the plot for experiments.
Bibliographie
Agbankpé A.J., Dougnon, T.V., Bankole H.S., Yèhouénou B., Yedomonhan H., Legonou M. & Dougnon T.J., 2014. Etude ethnobotanique des légumes feuilles thérapeutiques utilisés dans le traitement des diarrhées au sud-Bénin (Afrique de l’Ouest). International Journal of Chemical Sciences, 8(4), 1784-1795.
Amougou J.A., Abossolo S.A. & Tchindjang M., 2015. Variability of precipitations at Koundja and Ngaoundere based on temperature changes of Atlantic Ocean and El NINO. Ivoiry Coast Review of Science and Technology, 25, 110-124.
Azo’o E.M. & Messi J., 2012. Yield responses of Cucumeropsis mannii (Cucurbitaceae) to the presence or the absence of the insect foraging activity at Nkolbisson in Cameroon. Journal of Animal and Plant Sciences, 13(3), 1791-1799.
Azo’o E.M., Tchuenguem F.F.-N. & Messi J., 2017. Biological diversity of the entomofauna associated with Citrullus lanatus (Cucurbitaceae) flowers and assessment of its impact on yields. Journal of Entomology and Zoology Studies, 5(5), 810-815.
Basga E., Fameni T.S., Otiobo E.N. & Tchuenguem F.F.-N., 2019. Efficacité pollinisatrice de Apis mellifera Linné (Hymenoptera: Apidae) sur les fleurs de Gossypium hirsutum (Malvaceae) à Djamboutou (Garoua, Cameroun). Journal of Applied Biosciences, 138, 14123-14136.
Betti J.L., Ngankoué C.M., Dibong S.D. & Singa A.E., 2017. Etude ethnobotanique des plantes alimentaires spontanées vendues dans les marchés de Yaoundé, Cameroun. International Journal of Biological and Chemical Sciences, 10(4), 1678-1693.
Borror D.J. & White R.E. (1991). Les insectes de l’Amérique du Nord (au nord du Mexique).Broquet, Laprairie, 408 p.
Charrier A., Jacquot M., Hamon S. & Dominique N.D., 1997. L'amélioration des plantes tropicales. CIRRAD (Centre de coopération internationale en recherche agronomique pour le développement) et ORSTOM (Institut français de recherche scientifiques pour le développement en coopération). Repères, Montpellier.
Chautá-Mellizoa A., Campbell S.A., Bonillaa M.A., Thaler J.S. & Poveda K., 2012. Effects of natural and artificial pollination on fruit and offspring quality. Basic and Applied Ecology, 13, 524-532, http://dx.doi.org/10.1016/j.baae.2012.08.013.
Chen W., Wu Z., Su M. & Zhu J., 2000. Post-harvest technology, transport and marketing of bananas in China. Agrociencia, 36(2), 169-180.
Delvare G. & Arbelenc H.P. 1989. Les insectes d’Afrique et d’Amérique tropicale.Clés pour la reconnaissance des familles. Prifas,CIRAD-GERDAT, Montpellier, 302 p.
Demarly Y. 1977. Génétique et amélioration des plantes. Masson, Paris, p. 577.
Diatta K., Diatta W., Fall A.D., Dieng S.I.M., Mbaye A.I., Sarr A. & Adegbindin M.A., 2020. evaluation of the antioxidant activity of stalk and fruit of Solanum aethiopicum L. (Solanaceae). Asian Journal of Research in Biochemistry, 6(1), 6-12.
Diguir B.B., Pando, J.B., Fameni, T.S. & Tchuenguem, F.F-N. (2020). Pollination efficiency of Dactylurina staudingeri (Hymenoptera: Apidae) on Vernonia amygdalina (Asteraceae) florets at Dang (Ngaoundéré, Cameroon). International Journal of Research Studies in Agricultural Sciences, 6(2), 22-33.
Djakbé D.D., Ngakou A., Wékéré C., Faibawa E. & Tchuenguem F.F-N., 2017. Pollination and yield components of Physalis minima (Solanaceae) as affected by the foraging activity of Apis mellifera (Hymenoptera: Apidae) and compost at Dang (Ngaoundéré, Cameroon). International Journal of Agronomy and Agricultural Research, 11(3), 43- 60.
Djonwangwé D., Tchuenguem F.F.-N., Messi J. & Brückner D. 2011a. Foraging and pollination activities of Apis mellifera adansonii Latreille (Apidae) on Syzygium guineense var. guineense (Myrtaceae) flowers at Ngaoundéré (Cameroon). Journal of Animal and Plant Sciences, 10, 1325-1333.
Djonwangwé D., Tchuenguem F.F.-N. & Messi J., 2011b. Foraging and pollination activities of Apis mellifera adansonii Latreille (Hymenoptera: Apidae) on Ximenia americana (Olacaceae) flowers at Ngaoundéré (Cameroon). International Research Journal of Plant Science, 2(6), 170-178.
Djoufack-Manetsa V., 2011. Étude multi-échelle des précipitations et du couvert végétal au Cameroun: Analyses spaciales, tendances temporelles, facteurs climatiques et anthropiques de variabilité du Normalized Difference Vegetation Index. Thèse de Doctorat d’État, Université de Yaoundé I - Université de Bourgogne.
Djoufack V., Fontaine B., Martiny N. & Tsalefac M., 2012. Climatic and demographic determinants of vegetation cover in northern Cameroon. International Journal of Remote Sensing, 21, 6904-6926.
Eardley C.D., Kuhlmann M. & Pauly A. (2010). Les genres et sous-genres d’abeilles de l’Afrique subsaharienne (The bee genera and subgenera of sub-Saharan Africa). Abc, Taxa 9, 144 p.
Edmonds J.M. & Chweya J.A., 1997. Black Nightshade. Solanum nigrum L. and related species. Promoting the conservation and use of underutilized and neglected crops. XV. Institute of Plant Genetic and Crop Plant Research, Gatersleben/Internationl Plant Genetic Resource Institute, Rome, p. 112.
Fameni T.S., Tchuenguem F.F.-N. & Brückner D., 2012. Pollination efficiency of Apis mellifera adansonii (Hymenoptera: Apidae) on Callistemon rigidus (Myrtaceae) flowers at Dang (Ngaoundéré, Cameroon). International Journal of Tropical Insect Science, 32(1), 2-11.
Félicia J., Adolphe G.G., Boga J.P. & N’Goran A., 2019. Incidence des insectes et des nématodes sur la production de l’aubergine Solanum aethiopicum Linné, 1756 variété Djamba F1 dans la zone périurbaine d’Abidjan, Côte d’Ivoire. International Journal of Multidisciplinary Research and Development, 6(1), 06-11.
Freitas B.M., Robert J.P. & Holanda-Neto J.P., 2002. Identfying pollinators among an array of flower visitors, and the case of inadequate cashew pollination in Ne Brazil. In: Kevan P & Imperatriz Fonseca VL (eds). Pollinating Bees, the conservation link between agriculture and nature. Ministry of Environment/Brasília. p. 229-244.
Gallai N., Salles J.M., Settele J. & Vaissière B.E., 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecological Economics, 68, 810-821.
Gemmill-Harren B. & Ochieng A.O., 2008. Role of native bees and natural habitats in eggplant (Solanum melongena) pollination in Kenya. Agriculture Ecosystem and Environment, 127(1-2), 31-36.
Gingrass D., Gingrass J. & Olivera D., 1999. Visits of honeybees (Hymenoptera: Apidae) and their effects on cucumber yields in the field. Journal of Economic Entomology, 92, 435-438.
Greenleaf S.S. & Kremen C., 2006. Wild bees enhance honey bees' pollination of hybrid sunflower. Proceedings of the National Academy of Sciences, USA, 103, 13890- 13895.
Haubruge E., Nguyen B.K., Widart J., Thome J.P., Fickers P. & Depauw E., 2006. Le dépérissement de l’abeille domestique, Apis mellifera L., 1758 (Hymenoptera: Apidae): faits et causes probables. Notes Fauniques de Gembloux, 59(1), 3-21.
Jacob-Remacle A., 1989. Comportement de butinage de l'abeille domestique et des Abeilles sauvages dans des vergers de pommiers en Belgique. Apidologie, 20(4), 271-285.
Jouzier É., 2005. Solanacées médicinales et philatélie. Bulletin de la Société de Pharmacie, 144, 311-332.
Kahane R., Temple L., Brat P. & De Bon H., 2005. Les légumes feuilles des pays tropicaux : diversité, richesse économique et valeur santé dans un contexte très fragile. In «Les légumes: un patrimoine à transmettre et à valoriser» (Colloque Angers 7-9 septembre 2005), pp. 1-9.
Kengni B.S., Tchuenguem F.F.-N. & Ngakou A., 2015a. Pollination and yield attributes of (cowpea) Vigna unguiculata L. Walp. (Fabaceae) as influenced by the foraging activity of Xylocopa olivacea Fabricius (Hymenoptera: Apidae) and inoculation with Rhizobium in Ngaoundere, Cameroon. International Journal of Agronomy and Agricultural Research, 6(2): 62-76.
Kengni B.S., Tchuenguem F.F.-N. & Ngakou A., 2015b. Impact of the foraging activity of Apis mellifera adansonii Latreille (Hymenoptera: Apidae) and Bradyrhizobium fertilizer on pollination and yield components of Glycine max L. (Fabaceae) in the field. International Journal of Biological Research, 3(2), 64-71.
Kengni B.S., Kodji I.I., Azo’o E.M. & Tchuenguem F.F.-N., 2022. Influence of Xylocopa sp. (Hymenoptera: Apidae) on Solanum aethiopicum L., 1763 production in Far-North, Cameroon. Trends in Entomology, 18, 104-114.
Klein A.M., Vaissière B.E., Cane J.H., Steffan-Dewenter I., Cunningham S.A., Kremen C. & Tscharntke, T., 2007. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society, London (B), 274, 303-313.
Klinkhamer P.G.L. & De Jong T.J., 1993. Attractiveness to pollinators: a plant’s dilemma. Oikos, 66, 180-184.
Kremen C., Williams M.N., Bugg R.L., John P.F. & Thorp R.W., 2004. The area requirements of an ecosystem service: crop pollination by native bee communities in California. Ecology Letters, 7, 1109-1119.
Konan Y.D., 2013. Inventaire des principaux insectes ravageurs des aubergines et leurs impacts sur le rendement de trois accessions d’aubergine en voie de sélection à Anguédédou (Sud, Côte d’Ivoire). Mémoire de DEA, Université Félix Houphouët- Boigny, Abidjan.
Latif M.A., Rahman M.M. & Alam M.Z., 2010. Efficacy of nine insecticides against shoot and fruit borer, Leucinodes orbonalis Guenee (Lepidoptera: Pyralidae) in eggplant. Journal of Pest Science, 83(4), 391-397.
Lobreau-Calllen & Coutin 1987. Ressources florales exploitées par quelques Apoïdes des zones cultivées en savane arborée sénégalaise durant la saison des pluies. Agronomie, 7 (4), 231-246.
Louveaux J., 1984. L’abeille domestique dans ses relations avec les plantes cultivées. In: «Pollinisation et productions végétales». Pesson P. & Louveaux J. (éds), INRA, p. 527-555.
Mahadevan V. & Chandy K.C., 1957. Preliminary studies on the increase in cotton yield due to honeybee pollination. Madras Agriculture Journal, 47, 23-26
Mamoudou J., Fameni S.T., Basga E. & Tchuenguem F.F.-N., 2021. Pollination efficiency of Apis mellifera (Hymenoptera: Apidae) on Solanum nigrum (Solanaceae) at Miskine (Maroua, Cameroon). International Journal of Biological and Chemical Sciences, 15(3), 1073-1089.
Mbere N., Azo’o E.M., Tchobsala & Tchuenguem F.F.-N., 2020. Floral activity of Apis mellifera (Hymenoptera: Apidae) on Bidens steppia (Asteraceae), Cordia africana (Boraginaceae), Pittosporum viridiflorum (Pittosporaceae) and Psychotria mahonia (Rubiaceae) in Nyambaka (Adamaoua, Cameroon). African Journal of Agricultural Research, 16(9), 1278-1288.
Mein H., Opoku K.N., Bissah N.A.B. & Su T., 2021. Solanum aethiopicum: the nutrient-rich vegetable crop with great economic, genetic piodiversity and pharmaceutical potential. Horticulturae, 126(7), 1-7.
Molan P.C., 2001. Why honey is effective as a medicine. 2 - The scientific explanation of its effects. Bee World, 82(1): 22-40.
Nicolas S., Paul-André C., Denis T. & Frédéric M.-P., 2013. Interactions insectes-plantes. Éditions Quae, Versailles Cedex, France, 785 p.
Noubissié T.J.-B., Bell J.M. & Yadji H.T., 2012. Studies on variability and gene effects of seed protein contents in Cameroonian bean (Phaseolus vulgaris L.) cultivars. Journal of Agriculture and Social Sciences, 8, 17-23.
Obodji A., Aboua L.R.N., Tano D.K.C. & Seri-Kouassi B.P., 2016. Inventory of entomofauna associated with african eggplant (Solanum aethiopicum) according to the phenological stages and assessment of damages caused by insect pests. Journal of Advanced Studies in Agricultural and Environmental Sciences, 3(2), 12-21.
Ollerton J., Winfree R. & Tarrant S., 2011. How many flowering plants are pollinated by animals? Oikos, 120, 321-326.
Oyelana O.A. & Ogunwenmo K.O., 2012. Floral biology and the effects of plant-pollinator interaction on pollination intensity, fruit and seed set in Solanum. African Journal of Biotechnology, 11(84), 14967-14981.
Pando J.B., Tchuenguem F.F.-N. & Tamesse J.L., 2011a. Foraging and pollination behavior of Xylocopa calens Lepeletier (Hymenoptera: Apidae) on Phaseolus coccineus L. (Fabaceae) flowers at Yaoundé (Cameroon). Entomological Research, 41, 185-193.
Pando J.B., Tchuenguem F.F.-N. & Tamesse J.L., 2011b. Pollination and yield responses of pigeon pea (Cajanus cajan L. Millsp.) to the foraging activity of Chalicodoma cincta cincta (Hymenoptera: Megachilidae) in Yaoundé (Cameroon). Journal of Animal and Plant Sciences, 11, 1346-1357.
Pouvreau A., 2004. Les Insectes pollinisateurs. Delachaux et Niestle (ed.), Paris, 192 p.
Rosa A.S., Blochtein B., Ferreira NR. & Witter S., 2010. Apis mellifera (Hymenoptera: Apidae) as a potential Brassica napus pollinator (cv. Hyola 432) (Brassicaceae), in Southern Brazil. Brazilian Journal of Biolgy, 70(4), 1075-1081.
Seck A., 2007. Recherche et étude génétique de la résistance de l’aubergine africaine (Solanum aethiopicum) aux acariens phytophages. Réseau Africain pour le Développement de l’Horticulture, 12 p.
Shanika U.J.M., Jayasinghe R., Saumya T.H., Silva E., Inoka W.A. & Karunaratne P., 2017. Buzzing wild bee visits enhance seed set in Eggplant, Solanum melongena. Journal of Entomology, 1-7.
Salunkhe O.K., Oesai B.B. & Bhat N.R., 1987. Vegetable and flower, seeds production. New Dehli Agricole Publishing Academy, 486 p.
Smith S.D., & Knapp S., 2002. The natural history of reproduction in Solanum and Lycianthes (Solanaceae) in a subtropical moist forest. Bulletin of Natural History Museum, 32(2): 125-136.
Solís-Montero L., 2013. Pollination ecology and mating system of Solanum rostratum (Solanaceae) in North America. PhD thesis, University of Stirling.
Stanghellini M.S., Ambrose J.T. & Schultheis J.R., 1998a. Seed production in watermelon: a comparison between two commercially available pollinators. Horticultural Science, 33, 28-30.
Stanghellini M.S., Ambrose J.T. & Schultheis J.R., 1998b. Using commercial bumble bee colonies as backup pollinators for honey bees to produce cucumbers and watermelon. Hort Technology, 8, 590-594.
Terzo M. & Rasmont P., 2007. Suivi, étude et vulgarisation sur l'interaction entre les MAE et les abeilles sauvages. Malvas, Direction Générale de L'Agriculture, Région Wallonne, 77 p.
Toury J., Giorgi R., Favier J.C., & Savina J.F., 1965. Aliments de l’Ouest Africain : tables de composition. Annales de la Nutrition et de l’Alimentation, 21, 73-127.
Tchuenguem F.F.-N., Mapongmetsem P.M., Hentchoya H.J. & Messi J., 1997. Activité de Apis mellifica L. (Hymenoptera: Apidae) sur les fleurs de quelques plantes ligneuses à Dang (Adamaoua-Cameroun). Cameroon Journal of Biological and Biochemical Sciences, 7(1), 86-91.
Tchuenguem F.F.-N., Mapongmetsem P.M., Hentchoya H.J. & Messi J., 2004. Exploitation des fleurs de quatre plantes oléagineuses par Apis mellifera adansonii à Ngaoundéré (Cameroun): Bombax pentandrum, Vitellaria paradoxa, Lophira lanceolata et Dacryodes edulis. Procédés Biologiques et Alimentaires, 2, 27-36.
Tchuenguem F.F.-N., 2005. Activité de butinage et de pollinisation d’Apis mellifera adansonii Latreille (Hymenoptera: Apidae, Apinae) sur les fleurs de trois plantes à Ngaoundéré (Cameroun): Callistemon rigidus (Myrtaceae), Syzygium guineense var. macrocarpum (Myrtaceae) et Voacanga africana (Apocynaceae). Thèse de Doctorat d’Etat, Université de Yaoundé I.
Tchuenguem F.F.-N., Djonwangwé D., Messi J. & Brückner D., 2009a. Activité de butinage et de pollinisation de Apis mellifera adansonii Latreille (Hymenoptera: Apidae, Apinae) sur les fleurs de Helianthus annuus (Asteraceae) à Ngaoundéré (Cameroun). Cameroon Journal of Experimental Biology, 5(1), 1-9.
Tchuenguem F.F.-N., Ngakou A. & Kengni B.S., 2009b. Pollination and yield responses of cowpea (Vingna unguiculata L. Walpers) to the foraging activity of Apis mellifera adansonii (Hymenoptera: Apidae) at Ngaoundéré (Cameroon). African Journal of Biotechnology, 8(9), 1988-1996.
Tchuenguem F.F.-N., Fameni T.S., Pharaon M.A., Messi J. & Brückner D., 2010. Foraging behaviour of Apis mellifera adansonii (Hymenoptera: Apidae) on Combretum nigricans, Erythrina sigmoidea, Lannea kerstingii and Vernonia amygdalina flowers at Dang (Ngaoundéré, Cameroon). International Journal of Tropical Insect Science, 30(1), 40-47.
Tchuenguem F.F.-N., Djakbé D.J., Ngakou A., Wékéré C. & Faïbawa E., 2018. Impact de l’activité de butinage de Apis mellifera Linné (Hymenoptera: Apidae) sur la pollinisation et les rendements fruitier et grainier de Ceratotheca sesamoides Endl. (Pedaliaceae) à Dang (Ngaoundéré, Cameroun). Cameroon Journal of Experimental Biology, 12(1), 22-31.
Tyburce B., 1996. Transformation des sucres par l’abeille, du nectar au miel. L’abeille de France, 81, 211-215.
Vaissière B.E., Rodet G., Cousin M., Botella L. & Torré G.J.P., 1996. Pollination effectiveness of honey bees (Hymenoptera: Apidae) in a Kiwifruit orchard. Journal of Economic Entomology, 89(2), 453-461.
Wékéré C., Kingha T.B.M., Dongock N.D., Djakbé J.D., Faïbawa E. & Tchuenguem F.F.-N., 2018. Exploitation of Jatropha curcas, Senegalia polyacantha and Terminalia schimperiana flowers by Apis mellifera (Hymenoptera: Apidae) at Dang (Ngaoundéré, Cameroon). Journal of Entomology and Zoology Studies, 6(2), 2072-2078.
75 Réf.