- Portada
- Volume 78 (2025)
- Description of epigeous insects on the slopes of Mount Tonkoui (western Cote d'Ivoire)
Vista(s): 199 (0 ULiège)
Descargar(s): 38 (0 ULiège)
Description of epigeous insects on the slopes of Mount Tonkoui (western Cote d'Ivoire)

Documento adjunto(s)
Version PDF originaleRésumé
Cette étude examine l’entomofaune épigée des pentes du mont Tonkoui, situé dans l’ouest de la Côte d’Ivoire. Des inventaires d’insectes ont été réalisés à l’aide de pièges-fosses et de pièges jaunes le long d’un gradient altitudinal couvrant les principaux types de végétation. La diversité et l’abondance de l’entomofaune ont été évaluées à trois niveaux : forêt dégradée au niveau 1 (600–700 m), cacaoyère au niveau 2 (800–900 m) et forêt de montagne au niveau 3 (1000–1100 m) d’altitude, entre le 19 juin et le 24 juillet 2023.Tous les insectes capturés ont été identifiés au niveau taxonomique de la famille. Au total, 1 133 insectes ont été collectés dans la forêt dégradée (niveau 1), 2 628 dans les cacaoyères (niveau 2) et 1 354 dans la forêt de montagne (niveau 3). Ces insectes se répartissent en sept ordres et 86 familles au niveau 1, huit ordres et 113 familles au niveau 2 et huit ordres et 83 familles au niveau 3. Les Hyménoptères sont les plus abondants en nombre d’individus tandis que les Diptères présentent la plus grande diversité au niveau familial. La forêt de montagne s’avère être le milieu le plus diversifié avec un indice de Shannon de 2,794. Ces résultats soulignent l’importance écologique du mont Tonkoui en tant que réservoir de diversité entomologique et mettent en évidence la nécessité de conserver à la fois les habitats naturels et agroforestiers qui abritent des communautés variées d’insectes.
Abstract
This study investigates the epigeous entomofauna of the slopes of Mount Tonkoui in western Côte d'Ivoire. Insect inventories were carried out using pitfall and yellow traps along an altitudinal gradient in the main vegetation facies. Entomofaunal diversity and abundance in the three levels surveyed, i.e., degraded forest at level 1 (600-700 m), cocoa farm at level 2 (800-900 m) and highland forest at level 3 (1000-1100 m) above sea level were assessed between 19 June and 24 July 2023. All captured insects were identified to family taxonomic level. A total of 1,133 insects were collected in degraded forest (level 1), 2,628 insects in cocoa farms (level 2) and 1,354 insects in highland forest (level 3). These insects are divided into seven orders and 86 families at level 1, eight orders and 113 families at level 2, and eight orders and 83 families at level 3. Hymenoptera are most abundant in individuals, and Diptera are greatest family-level diversity. Highland Forest is the most diverse environment, with a Shannon index of 2.794. These findings highlight the ecological importance of Mont Tonkoui as a reservoir of insect diversity and underline the need for the conservation of both natural and agroforestry habitats that sustain diverse insect communities.
Reçu le 20 mars 2025 , accepté le 27 octobre 2025, mis en ligne le 12 novembre 2025
Cet article est distribué suivant les termes et conditions de la licence CC-BY (http://creativecomons.org/licenses/by/4.0/deed.fr)
INTRODUCTION
1Tropical mountain forests are among the world’s most threatened and least documented ecosystems (Bruijnzeel & Veneklaas, 1998). They represent natural laboratories for studying biodiversity distribution, as altitudinal gradients encompass steep variations in abiotic factors such as temperature, radiation, and precipitation (Mille & Louppe, 2015). These gradients provide unique opportunities to investigate how organisms respond to environmental changes on small spatial scales (Hájek et al., 2011), making them crucial for understanding biodiversity patterns and conservation (Al Karkouri, 2017; Kanga et al., 2021).
2In West Africa, mountain ecosystems such as the Nimba Mountains, Mount Cameroon, and Mount Tonkoui in Côte d’Ivoire are recognized as biodiversity hotspots, characterized by high species turnover across elevation and unique insect assemblages (Philips et al., 2023; Luiselli et al., 2025). Their vegetation ranges from lowland rainforest to mountain forest and grasslands, but they are increasingly threatened by deforestation, cocoa farming, and other human disturbances (Koné et al., 2021).
3Insects, which play essential ecological roles as pollinators, decomposers, and regulators of trophic interactions, are widely used as bioindicators of habitat change (Noriega et al., 2018; Didham et al., 2020). Yet, despite their importance, insect diversity in tropical mountain forests remains poorly studied, particularly in Côte d’Ivoire, where most research has focused on lowland forests and agroecosystems (Macera, 2024). Recent studies emphasize that mountain systems in Africa harbor diverse but fragile insect communities highly sensitive to environmental change (Janzen & Hallwachs, 2019; Ouin et al., 2022). However, baseline data from Côte d’Ivoire are scarce, limiting our understanding of the ecological roles and conservation value of mountain insects.
4To address this knowledge gap, our study provides the first systematic inventory of epigeous insects on Mount Tonkoui, a mountain ecosystem under increasing anthropogenic pressure in western Côte d’Ivoire. Specifically, we aimed to (i) assess insect diversity and abundance across three altitudinal ranges (600–700 m, 800–900 m, and 1000–1100 m above sea level) and (ii) compare taxonomic composition among major habitat types, namely degraded forest, cocoa farm, and highland forest.
MATERIAL AND METHODS
Location of study area
5The study was conducted on Mount Tonkoui (7°31′N, 8°31′W), the second highest peak in Côte d’Ivoire, located in the Tonkoui region about 15 km from the city of Man and close to the borders with Guinea and Liberia (Arnaud & Vennetier, 1978). Rising to 1190 m above sea level, Mount Tonkoui forms part of the Upper Guinean Forest zone of West Africa. The area has a humid tropical climate, with a mean annual temperature of around 25 °C (Moretto et al., 2021) and bimodal rainfall ranging from 1,600 to 2,400 mm annually, peaking between May–July and September–October. Vegetation is dominated by semi-deciduous moist forest, with remnants of mountain forest at higher elevations, while the lower slopes are increasingly affected by anthropogenic disturbances, particularly cocoa cultivation and forest degradation.
Sampling methods
6To evaluate insect diversity on Mount Tonkoui, three altitudinal habitats were surveyed: degraded forest (600–700 m), cocoa farms (800–900 m), and highland forest (1000–1100 m). In each habitat, five 100 m transects were established, spaced 30 m apart, with 10 sampling points placed every 10 m (Underwood and Fisher, 2006). Insects were captured using 30 pitfall traps (plastic cups, 7 × 9 cm, partly filled with 70% alcohol) and 20 yellow pan traps (20 × 5 cm plates filled with soapy water), installed at regular intervals on the same transect procedure-stratified random described by several authors (Bonneau, 2008; Lozano et al., 2013). Traps were exposed simultaneously for 48 h during June–July 2023 to minimize temporal bias. All specimens were preserved in 70% alcohol and later identified in the laboratory.
Specimen identification
7Identification and enumeration of captured specimens were carried out at the Laboratoire d'Écologie et du Développement Durable (LEDD) of Nangui ABROGOUA University Abidjan and identified down to family taxonomic level. All the insects caught were classified by order, using illustrations by Delahaye et al. (2006), Delvare & Aberlenc (1989) and (Leraut, 2003). Using a binocular magnifying glass, they were then separately identified down to the family level with taxon-specific determination keys (Delvare & Aberlenc, 1989).
Data analysis
8The data obtained were processed using Microsoft Excel. The abundance of each insect order according to the surveyed biotopes was calculated and represented graphically. The number of individuals per family was counted and abundances determined (Magurran, 2004). Diversity and evenness indices were calculated using the software Ecological Methodology following Shannon and Pielou formulas (Shannon & Weaver, 1949; Pielou, 1966). Differences in insect assemblages between habitats were assessed using one-way ANOVA, followed by Tukey’s HSD post-hoc tests. All statistical analyses were performed with R software version 4.4.0.
RESULTS
Overall abundance and taxonomic group of insects captured
9A total of 5,115 insects were collected, belonging to eight Orders. These orders were: Dermaptera, Homoptera, Lepidoptera Heteroptera, Orthoptera, Coleoptera, Diptera, Hymenoptera. Of these 8 orders, 146 families have been identified. The orders Coleoptera, Hymenoptera and Diptera are the most abundant. The most abundant families are Formicidae (2306 individuals), Dolichopodidae (326 individuals), Phoridae (273 individuals), Staphylinidae (179 individuals), Drosophilidae (150 individuals), Scolytidae (143 individuals) and Gryllidae (122 individuals) (table 1).
10Relative abundances in the three levels are dominated by the order Hymenoptera, followed by Diptera, Coleoptera and Orthoptera. The Formicidae family is the most represented, accounting for 52.5%, 46.5% and 36% respectively of the insects collected in level 1 (degraded forest), level 2 (cocoa) and level 3 (high forest).
Table 1: Diversity and relative abundance of insects collected in all habitats
|
DIVERSITY |
ABUNDANCE |
||||
|
Orders |
Families |
Level 1 (DF) |
Level 2 (CF) |
Level 3 (HF) |
|
|
Hymenoptera |
Aphelinidae |
4 |
3 |
2 |
|
|
Apidae |
1 |
1 |
1 |
||
|
Apidae Meliponinae |
0 |
3 |
0 |
||
|
Bethylidae |
1 |
4 |
11 |
||
|
Braconidae |
4 |
6 |
1 |
||
|
Ceraphronidae |
1 |
1 |
0 |
||
|
Chalcididae |
1 |
5 |
5 |
||
|
Cynipidae |
3 |
4 |
5 |
||
|
Diapriidae |
4 |
4 |
17 |
||
|
Diprionidae |
0 |
0 |
1 |
||
|
Dryinidae |
0 |
2 |
0 |
||
|
Encyrtidae |
0 |
1 |
0 |
||
|
Eucharidae |
0 |
1 |
1 |
||
|
Eulophidae |
1 |
0 |
0 |
||
|
Eupelmidae |
1 |
2 |
0 |
||
|
Eurypelmidae |
0 |
1 |
0 |
||
|
Eurytomidae |
0 |
1 |
0 |
||
|
Formicidae |
595 |
1223 |
488 |
||
|
Ichneumonidae |
7 |
9 |
32 |
||
|
Megaspilidae |
2 |
5 |
0 |
||
|
Mutillidae |
1 |
1 |
0 |
||
|
Mymaridae |
8 |
15 |
2 |
||
|
Platygastridae |
0 |
0 |
1 |
||
|
Pompilidae |
0 |
0 |
2 |
||
|
Ptéromalidae |
2 |
1 |
0 |
||
|
Scelionidae |
5 |
0 |
3 |
||
|
Serphidae |
0 |
1 |
0 |
||
|
Siricidae |
1 |
1 |
0 |
||
|
Sphecidae |
2 |
0 |
3 |
||
|
Tenthredinidae |
0 |
1 |
0 |
||
|
Torymidae |
1 |
0 |
3 |
||
|
Trichogrammatidae |
2 |
0 |
2 |
||
|
Vespidae |
1 |
0 |
0 |
||
|
Total |
648 |
1296 |
580 |
||
|
Heteroptera |
Alydidae |
2 |
1 |
0 |
|
|
Anthocoridae |
0 |
0 |
1 |
||
|
Aphididae |
2 |
0 |
0 |
||
|
Aradidae |
0 |
2 |
0 |
||
|
Cimicide |
1 |
1 |
1 |
||
|
Coreidae |
0 |
2 |
0 |
||
|
Cydnidae |
3 |
1 |
0 |
||
|
Lygaeidae |
6 |
7 |
3 |
||
|
Nepidae |
0 |
0 |
1 |
||
|
Pentatomidae |
0 |
4 |
0 |
||
|
Reduviidae |
0 |
19 |
0 |
||
|
Saldidae |
1 |
0 |
0 |
||
|
Total |
15 |
37 |
6 |
||
|
Diptera |
Agromysidae |
4 |
36 |
7 |
|
|
Anthomyiidae |
8 |
5 |
23 |
||
|
Asilidae |
2 |
0 |
0 |
||
|
Athericidae |
0 |
2 |
1 |
||
|
Bibionidae |
1 |
1 |
7 |
||
|
Bombyliidae |
0 |
0 |
1 |
||
|
Calliphoridae |
0 |
0 |
1 |
||
|
Cecidomyiidae |
25 |
8 |
1 |
||
|
Ceratopogonidae |
1 |
12 |
1 |
||
|
Chironomidae |
0 |
4 |
0 |
||
|
Chloropidae |
2 |
0 |
8 |
||
|
Conopidae |
1 |
4 |
0 |
||
|
Culicidae |
2 |
1 |
17 |
||
|
Curtonotidae |
11 |
1 |
26 |
||
|
Diopsidae |
9 |
16 |
10 |
||
|
Dolichopodidae |
53 |
63 |
210 |
||
|
Drosophilidae |
34 |
87 |
29 |
||
|
Ephydridae |
0 |
4 |
0 |
||
|
Gasterophilidae |
1 |
0 |
0 |
||
|
Keroplatidae |
0 |
0 |
1 |
||
|
Lauxaniidae |
23 |
69 |
0 |
||
|
Lonchaeidae |
0 |
11 |
0 |
||
|
Milichiidae |
0 |
1 |
0 |
||
|
Muscidae |
22 |
20 |
15 |
||
|
Mycetophilidae |
16 |
19 |
44 |
||
|
Otitidae |
6 |
0 |
0 |
||
|
Phoridae |
78 |
162 |
33 |
||
|
Psychodidae |
5 |
40 |
0 |
||
|
Sarcophagidae |
3 |
0 |
1 |
||
|
Sciaridae |
3 |
7 |
6 |
||
|
Sciomyzidae |
3 |
42 |
1 |
||
|
Simuliidae |
4 |
44 |
4 |
||
|
Stratiomyidae |
0 |
3 |
1 |
||
|
Syrphidae |
4 |
0 |
6 |
||
|
Tachinidae |
0 |
5 |
0 |
||
|
Thephritidae |
2 |
3 |
13 |
||
|
Therevidae |
0 |
1 |
1 |
||
|
Tipulidae |
1 |
6 |
0 |
||
|
Total |
324 |
677 |
468 |
||
|
Coleoptera |
Aderidae |
0 |
0 |
3 |
|
|
Anthicidae |
0 |
5 |
0 |
||
|
Attelabidae |
0 |
0 |
1 |
||
|
Bostrichidae |
0 |
4 |
5 |
||
|
Bruchidae |
0 |
1 |
0 |
||
|
Buprestidae |
0 |
2 |
1 |
||
|
Byrrhidae |
1 |
5 |
2 |
||
|
Cantharidae |
5 |
7 |
4 |
||
|
Carabidae |
3 |
13 |
5 |
||
|
Cerambycidae |
0 |
2 |
1 |
||
|
Chrysomelidae |
4 |
3 |
8 |
||
|
Cicindelidae |
0 |
1 |
0 |
||
|
Cleridae |
1 |
0 |
0 |
||
|
Curculionidae |
3 |
8 |
6 |
||
|
Cybocephalidae |
0 |
1 |
0 |
||
|
Dytiscidae |
1 |
8 |
0 |
||
|
Geotrupidae |
4 |
6 |
7 |
||
|
Gyrinidae |
1 |
0 |
19 |
||
|
Histeridae |
0 |
38 |
0 |
||
|
Hydrophilidae |
1 |
8 |
6 |
||
|
Lycidae |
0 |
1 |
0 |
||
|
Melandryidae |
1 |
0 |
0 |
||
|
Meloidae |
0 |
2 |
1 |
||
|
Mordellidae |
3 |
7 |
9 |
||
|
Nitidulidae |
0 |
17 |
3 |
||
|
Pselaphidae |
0 |
1 |
0 |
||
|
Psephenidae |
0 |
0 |
2 |
||
|
Ptinidae |
1 |
5 |
0 |
||
|
Scarabaeidae |
3 |
16 |
1 |
||
|
Scolytidae |
0 |
141 |
1 |
||
|
Silphidae |
1 |
2 |
2 |
||
|
Silvanidae |
2 |
0 |
0 |
||
|
Staphylinidae |
16 |
109 |
54 |
||
|
Tenebrionidae |
4 |
1 |
2 |
||
|
Trogidae |
1 |
24 |
0 |
||
|
Total |
56 |
438 |
143 |
||
|
Orthoptera |
Acrididae |
9 |
11 |
21 |
|
|
Eumastacidae |
1 |
4 |
8 |
||
|
Euschmidtiidae |
4 |
0 |
0 |
||
|
Gryllidae |
19 |
55 |
48 |
||
|
Pamphagidae |
2 |
0 |
0 |
||
|
Pyrgomorphidae |
5 |
2 |
2 |
||
|
Tetrigidae |
4 |
10 |
0 |
||
|
Tridactylidae |
0 |
1 |
0 |
||
|
Tettigoniidae |
16 |
7 |
38 |
||
|
Total |
60 |
90 |
117 |
||
|
Homoptera |
Aleyrodidae |
0 |
1 |
0 |
|
|
Aphididae |
0 |
7 |
0 |
||
|
Cercopidae |
11 |
14 |
15 |
||
|
Cicadellidae |
17 |
20 |
11 |
||
|
Cixiidae |
1 |
0 |
0 |
||
|
Lachnidae |
0 |
0 |
1 |
||
|
Psyllidae |
0 |
6 |
2 |
||
|
Total |
29 |
48 |
29 |
||
|
Dermaptera |
Apachyidae |
0 |
5 |
4 |
|
|
Forficulidae |
0 |
4 |
0 |
||
|
Lariduridae |
0 |
4 |
4 |
||
|
Total |
0 |
13 |
8 |
||
|
Lepidoptera |
Lycaenidae |
0 |
1 |
2 |
|
|
Nepticulidae |
0 |
2 |
0 |
||
|
Noctuidae |
0 |
2 |
0 |
||
|
Papilionidae |
0 |
0 |
1 |
||
|
Oecophoridae |
0 |
1 |
0 |
||
|
Pyralidae |
0 |
5 |
0 |
||
|
Saturniidae |
1 |
8 |
0 |
||
|
Sphingidae |
0 |
1 |
0 |
||
|
Tineidae |
0 |
6 |
0 |
||
|
Tortricidae |
0 |
3 |
0 |
||
|
Total |
1 |
29 |
3 |
||
Diversity index of habitats explored
11Shannon diversity index varies with altitude. They increase from bottom to top. All levels have high taxonomic diversity at family level; The highest diversity index was observed in the highland forest (level 3), while the lowest occurred in the degraded forest (level 1). Intermediate values were recorded in cocoa farms (level 2). (Figure 1A). Evenness followed the same trend, with the highland forest showing the highest value and the degraded forest the lowest (Figure 1B).
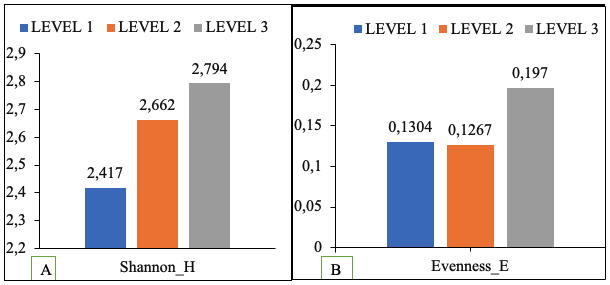
Figure 1: Shannon index and Shannon evenness by altitude level
A : Shannon index ; B : Evenness
Individuals and taxonomic diversity in degraded forest (level 1)
12In the degraded forest, a total of 1131 insects belonging to seven orders and 86 families were recorded. Hymenoptera dominated in abundance, whereas Lepidoptera showed the lowest representation. Regarding taxonomic diversity, Diptera comprised the highest number of families, while Lepidoptera and Homoptera were the least diverse (Figure 2).
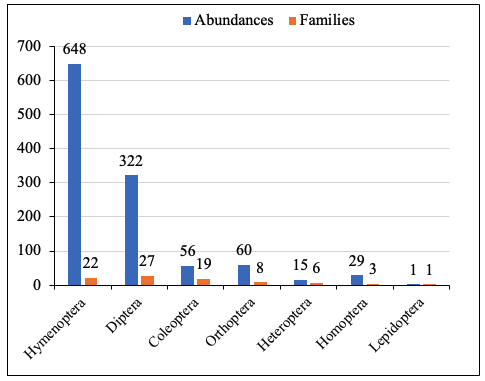
Figure 2: Abundance and families of taxonomic groups by orders in degraded forest
Individuals and taxonomic diversity in cocoa farms (level 2)
13In cocoa farms, a total of 2628 insects were collected, belonging to eight orders and 113 families. Hymenoptera were the most abundant, while Dermaptera had the lowest abundance. In terms of diversity, Diptera and Coleoptera comprised the highest number of families, whereas Dermaptera showed the lowest representation (Figure 3).
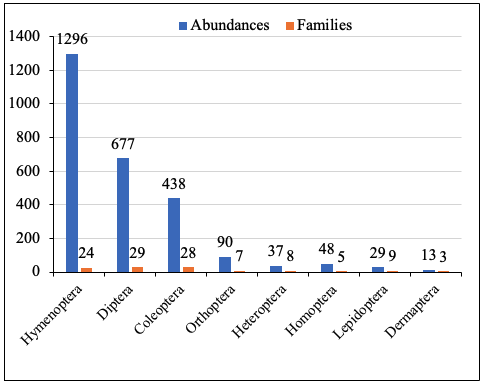
Figure 3: Abundance and families of taxonomic groups by orders in cocoa farm
Individuals and taxonomic diversity in highland forest (level 3)
14In the highland forest, 1352 insects were recorded, belonging to eight orders and 83 families, similar to those found in cocoa farms. Hymenoptera were the most abundant, whereas Lepidoptera and Heteroptera were least represented. Regarding diversity, Diptera and Coleoptera included the highest number of families, while Lepidoptera and Dermaptera were the least diverse (Figure 4).
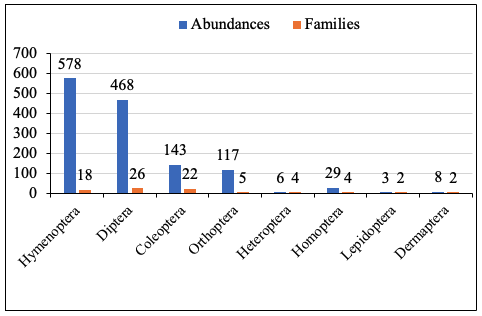
Figure 4: Abundance and families of taxonomic groups in the highland forest
Comparison of entomofaunal diversity and abundance as a function of habitats
15The habitats explored depending on altitude were degraded forest, cocoa farms and highland forest. One-way analyses of variance (ANOVA) revealed that there was a significant difference in taxon richness according to levels (F = 4.83 ; Df = 2 ; p = 0.03). Post-hoc Tukey’s HSD revealed significant differences between Highland Forest and Cocoa Farm (p = 0.03), while Degraded Forest was intermediate and did not differ significantly from either Highland Forest (p = 0.22) or Cocoa Farm (p = 0.14). (Figure 5A). Also for abundances, the analyses reveal that there is a highly significant difference between the different altitude levels (F = 8.45 ; Df = 2 ; p = 0.005). This difference is found between cocoa farm and highland forest and disturbed forest; while there is no significant difference between disturbed forest and highland forest (Figure 5B).
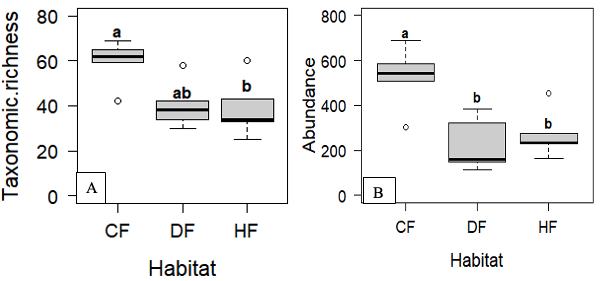
Figure 5: Taxonomic richness and abundance of insects according to habitat
A: taxonomic richness; B: abundance; CF: cocoa farm, DF: disturbed forest; HF: highland forest
The-box plots having the different letter “a; b” indicate significant difference. The-box plots having the same letter “b” indicate no significant difference
DISCUSSION
16The results obtained in this study constitute the first data on insects captured in Mount Tonkoui and aim to report on the diversity of epigeous insects in the threatened forest relict of Mount Tonkoui. The list of insects obtained can therefore be used as a reference for monitoring entomological biodiversity in the Mount Tonkoui classified forest, in order to determine the real impact of degradation on entomofauna and mountain ecosystems.
17Entomological surveys have identified several insect taxa in the Mount Tonkoui classified forest. These insects cohabit in different proportions at different altitudes. The results showed that the three levels surveyed, i.e., degraded forest at level 1 (600-700 m), cocoa farm at level 2 (800-900 m) and highland forest at level 3 (1000-1100 m) above sea level, differ in terms of insect groups. Most individuals were obtained in cocoa farms, followed by highland forest. These variations are linked to changes in abiotic and biotic factors and are probably related to the availability of resources favorable to insect development (egg-laying sites, light, temperature, etc.) as well as the insect groups characteristic of each biotope (Mavoungou et al., 2016; Lukoki et al., 2022). In degraded forests, the loss of structural complexity and plant diversity reduces ecological niches, leading to lower insect abundance and diversity (Adeoye et al., 2021). Cocoa agroforests maintain intermediate diversity, as shade trees and heterogeneous vegetation provide nesting sites, floral resources, and a more stable microclimate that sustains part of the forest insect community (Asare et al., 2021; Egbe et al., 2022). In contrast, mountain forests harbor the highest diversity due to their complex vertical structure, stable microclimates, and abundance of specialized niches (Houehanou et al., 2023; Sodhi et al., 2020). Similar studies in West Africa confirm that habitat heterogeneity and resource availability are key drivers of insect assemblages in forest–agroforestry landscapes (Boafo et al., 2022; Kouakou et al., 2023).
18Most number of families were obtained in cocoa farms, followed by degraded forest, while highland forest recorded the lowest number of families. According to Adjaloo and colleagues (2012) the presence of decomposing matter, banana stems, cocoa leaf litter and cocoa shells (at pod-breaking points) on farms may have provided a variety of microhabitats that hosted different insect species such as cocoa pollinators. The Formicidae are the most represented in the biotopes explored. This overestimation is explained by the use of an index system for counting ants. To overcome this issue in future studies, more standardized sampling protocols such as direct individual counts, pitfall trapping, or transect-based collection could be used. Combining multiple complementary methods would help reduce bias and provide more accurate estimates of ant abundance (Lozano et al., 2013; Yeo et al., 2019).
19Shannon’s diversity index varies with altitude, showing high diversity and low evenness. This variation could be explained by the differentiation of habitats according to altitude. Similar results were observed for termites, where the diversity index varied according to habitat (Anantharaju et al., 2014).
20Analyses of variance between altitudes reveal a significant difference in taxonomic group between habitats as a function of altitude. This difference is found between the high-altitude forest and the cocoa farm. The abundance of insects in cocoa farms may also be linked to the availability of diverse organic substrates such as banana stems, cocoa leaf litter, cocoa leaves, and cocoa shells, which provide shelter and breeding sites. Beyond the simple presence of plant residues, habitat complexity and microclimatic conditions created by shade trees contribute significantly to insect diversity and abundance (Bisseleua et al., 2009; Asare et al., 2021).
21The taxonomic richness of disturbed forest is intermediate between highland forest and cocoa farm. In fact, abiotic factors such as temperature, humidity, and light availability, together with biotic factors including vegetation structure and plant diversity, strongly influence insect assemblages by shaping resource availability and microhabitats. In addition, human activities such as land-use change, logging, and agricultural intensification alter habitat quality and connectivity, thereby impacting insect diversity and community composition (Sodhi et al., 2020; Adeoye et al., 2021 ; Kouakou et al., 2023).
22Analysis of variance for the three levels reveals highly significant differences in abundance depending on altitude. This difference is between the cocoa farm and the degraded forest and the highland forest, but there is no significant difference between the degraded forest and the highland forest. This distribution may be linked to the differentiation of landscapes, as the structure of habitats can generate specific microhabitats that are more or less favorable to the development of certain insect species (Acapovi-Yao et al., 2017; Moretto et al., 2021).
23Indeed, the different insect communities residing in cocoa farms potentially respond to the heterogeneity of their habitat. In cocoa farms, we recorded certain insect groups that are often associated with pest activity, such as Aphididae and some Formicidae, which were absent or much less represented in the degraded and mountain forests. While our sampling was not designed to specifically identify pest species, the presence of these groups suggests that cocoa agroecosystems may be more prone to pest-related pressures compared to natural habitats (Dosso et al., 2018).
24This pattern may be related to the fact that degradation reduces habitat availability, while at the same time, highland forests impose environmental constraints such as lower temperatures and variable humidity. These opposing effects could balance each other, resulting in no significant differences in insect abundance between the two forest types. Although altitude is expected to influence insect assemblages, the relatively narrow altitudinal range studied (600–1100 m) may not have generated strong enough climatic contrasts to drive marked differences. The temperature (20–25 °C) and humidity (41–98.5%) recorded fall within the tolerance limits of most insect groups, which could buffer against strong compositional turnover. Moreover, some taxa such as Diptera and Hymenoptera are known for their broad ecological plasticity, enabling them to persist across habitats despite microclimatic variation (Hodkinson, 2005; Brehm et al., 2019). In contrast, insect groups with narrower ecological requirements (e.g., Lepidoptera, Orthoptera) are more sensitive to microclimatic changes (Bonebrake et al., 2010), but their low representation in our samples may explain why no significant differences were detected (Chen et al., 2009; Avalos, 2018). Our survey reveals the faunistic significance of Mont Tonkoui, with key pollinator and indicator groups such as Apidae and Nymphalidae present (Coulibaly et al., 2023). The occurrence of Scarabaeidae of conservation concern (Ajong et al., 2025) confirms the area’s value as a biodiversity hotspot. Although no invasive taxa were detected, the presence of agricultural pests in cocoa farms and forests suggests ecological permeability between natural and cultivated habitats (Engels & Wood, 2023).
CONCLUSION
25This study provides the first baseline inventory of epigeous insects on Mount Tonkoui and highlights significant differences in diversity and composition across habitats. Although preliminary, these results represent a valuable reference for developing ecological monitoring strategies aimed at sustainable management and conservation of montane ecosystems.
26The observed differences among habitats reflect the influence of landscape heterogeneity, which creates more or less favorable conditions for insect development. Regular monitoring of ecological indices will therefore be essential for managers to assess ecosystem dynamics over time and to adjust conservation actions accordingly. The present species list can serve as a benchmark for tracking entomofaunal changes and evaluating the impact of habitat degradation in the Mount Tonkoui classified forest.
27Future research should extend this work to other mountains of the Man region, incorporating complementary sampling techniques and accounting for seasonal variations, in order to build a more comprehensive understanding of insect biodiversity in West African mountain ecosystems.
Acknowledgements
We would like to thank the management of the Société de Développement des Forêts (SODEFOR), for the trust they have shown in us by granting the necessary permits for our research. We are indebted to Mr. GBA Gouê Emile, president of the young people of the village of Gouimpleu, who hosted us during our stay and is as keen as we are to protect the Tonkoui. Thanks to DOUA Serge and GUEU Firmin who helped us with our research: the success of our missions owes them a great deal. We are grateful to the International Foundation for Science (IFS) for funding through a research grant awarded to Mr. Mamadou KONE (ref: I-1-D-6494-1).
Bibliographie
Adeoye A. O., Kehinde T., & Samways M. J., 2022. Effects of forest degradation on insect diversity and ecosystem functioning in tropical landscapes. Ecological Indicators, 127, 107753. https://doi.org/10.1016/j.ecolind.2021.107753
Adjaloo M.K., Oduro W. & Mochiah M.B., 2012. Spatial distribution of insect assemblage in cocoa farms in relation to natural forest Journal of Applied Biosciences, 54, 3870– 3879.
Ajong S.N., Kouadio K. & Kouamé N., 2025. Conservation importance of Scarabaeidae in West African montane forests. African Journal of Ecology, 63(1), 34–45.
Al Karkouri J., 2017. Les milieux montagneux marocains à l’épreuve du changement climatique (cas de la montagne rifaine). Hespéris-Tamuda, 52(1), 237-267.
Anantharaju T., Kaur G., Gajalakshmi S. & Abbasi S., 2014. Sampling and identification of termites in Northeastern Puducherry. Journal of Entomology Zoology Studies, 2(3), 225-230.
Arnaud J. & Vennetier P., 1978. Atlas de la Côte d'Ivoire. Éditions Jeune Afrique.
Asare R., Avornyo, V. K., Anim-Kwapong, G. J., & Nkrumah, E. E. (2021). Tree diversity and insect assemblages in shaded cocoa farms in Ghana. Agroforestry Systems, 95(5), 841–853. https://doi.org/10.1007/s10457-020-00575-2
Avalos G., 2018. Still searching the rich coast: Biodiversity of Costa Rica, numbers, processes, patterns, and challenges. In Global biodiversity pp. 101-135
Boafo A., Oppong, S. K., & Oduro, W. (2022). Influence of habitat heterogeneity on insect diversity in cocoa landscapes of Ghana. Biodiversity and Conservation, 31(6), 1599–1617. https://doi.org/10.1007/s10531-022-02383-3
Bonebrake T.C., Ponisio L.C., Boggs C.L. & Ehrlich P.R., 2010. More than just indicators: A review of tropical butterfly ecology and conservation. Biological Conservation, 143(8), 1831–1841.
Bonneau P., 2008. Mes pièges à Insectes. Ou comment se débrouiller avec les moyens du bord.
Brehm G., Zeuss D. & Colwell R.K., 2019. Moth diversity gradients along tropical elevational gradients. Ecography, 42(4), 1163–1175.
Bruijnzeel L. & Veneklaas E.J., 1998. Climatic conditions and tropical montane forest productivity: the fog has not lifted yet. Ecology, 79(1), 3-9.
Chen I-C., Shiu H-J., Benedick S., Holloway JD., Chey VK., Barlow HS. & Thomas C.D. 2009. Elevation increases in moth assemblages over 42 years on a tropical mountain. 106(5), 1479-1483.
Coulibaly A., Kouadio K. & Dosso K., 2023. Pollinator insects of highland forests in Côte d’Ivoire: Diversity and conservation perspectives. African Invertebrates, 64(1), 1–12.
Delahaye N., Drumont A. & Sudre J., 2006. Catalogue des Prioninae du Gabon ( Coleoptera, Cerambycidae). Revue Internationale d'Entomologie, 1-33.
Delvare G. & Aberlenc H.P., 1989. Les insectes d'Afrique et d'Amérique tropicale: clés pour la reconnaissance des familles. Editions Quae.
Dosso K., Koffi K. K. S., & Tiho S., 2018. Etude préliminaire du peuplement de coléoptères bousiers inféodés aux excréments de buffles dans la réserve scientifique de Lamto. Revue de l’Environnement et de la Biodiversité-PASRES, 3, 44-53.
Egbe A. E., Tchuenguem, F. N., & Bisseleua, H. B. D. (2022). Insect assemblages and ecological functions in cocoa-based agroforestry in Cameroon. Environmental Entomology,51(3), 456–468. https://doi.org/10.1093/ee/nvac015
Engels B. & Wood A., 2023. Invasive insects and agroecosystem permeability in African mountain landscapes. Journal of Applied Ecology, 60(1), 54–65.
Hájek M., Roleček J., Cottenie K., Kintrová K., Horsák M., Poulíčková A., Dítě D., 2011. Environmental and spatial controls of biotic assemblages in a discrete semi‐terrestrial habitat: comparison of organisms with different dispersal abilities sampled in the same plots. Journal of Biogeography, 38(9), 1683-1693.
Hodkinson I.D., 2005. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biological Reviews, 80(3), 489–513.
Houehanou T. D., Sinsin, B., & Fischer, M. (2023). Biodiversity patterns along elevational gradients in montane forests of West Africa. Journal of Insect Conservation, 27(2), 181–196. https://doi.org/10.1007/s10841-022-00393-7
Janzen D. H., & Hallwachs W., 2019. Perspective: Where might be many tropical insects? Biological Conservation, 233, 102-108.
Kanga K.P., Kouamé N., Zogbassé P., Gongomin B., Agoh K., Kouamé A., Rödel M., 2021. Amphibian diversity of a West African biodiversity hotspot: An assessment and commented checklist of the batrachofauna of the Ivorian part of the Nimba Mountains. Amphibian Reptile Conservation, 15(1), 71-107.
Kouakou N. K., Koné, I., & N’Guessan E. K., 2023. Landscape complexity and insect pollinator diversity in West African cocoa agroforests. Global Ecology and Conservation, 44, e02458. https://doi.org/10.1016/j.gecco.2023.e02458
Leraut P., 2003. Nouvelle contribution à l'étude des pyrales paléarctiques [Lepidoptera, Pyraloidea]. 25(2), 67-79.
Lozano D.P., Bosquée E., Lopes T., Chen J., Fa C.D., Yong L., Francis F., 2013. Evaluation de la diversité de l’entomofaune en cultures maraîchères dans l’est de la Chine. Entomologie faunistique-Faunistic Entomology. 66, 27-37.
Luiselli L., Fa, J. E., Le Duc, O. & Eniang E. A. (2025). Red Listing African Goliath Beetles: Assessing Threats and Conservation Needs. African Journal of Ecology. https://doi.org/10.1111/aje.70018
Lukoki H.N., Ilunga J., Nsabatien V., Wetshindjadi H., Muyeka P. & Lukoki F., 2022. Diversité de l’entomofaune terricole de la galerie forestière du Monastère Notre Dame de l’Assomption (RD Congo/Kinshasa). Revue Marocaine des Sciences Agronomiques et Vétérinaires 10: 437-47
Macera L., 2024. Restauration des écosystèmes de mangroves: évaluation et amélioration des pratiques à travers une étude comparative de projets à l'échelle mondiale Université Côte d'Azur].
Magurran A.E., 2004 Measuring Biological Diversity. Blackwell Science, Oxford.
Mavoungou J.F., Acapovi-Yao G.L., Koumba Z.C., Mbang N.O. & M’batchi, B., 2016. Diversité de l’entomofaune de la zone pétrolifère de Ntchengué à Port-Gentil au Gabon. Afrique SCIENCE, 12(2), 151-163.
Mille G. & Louppe D., 2015. Mémento du forestier tropical. Éditions Quae.
Moretto P., Cosson B., Takano H., Basquin P., Bordat P., Boucher S., Eitschberger U., 2021. Un refuge forestier menacé: La forêt d’altitude à Parinari du Mont Tonkoui en Côte d’Ivoire. Évaluation de la biodiversité entomologique, botanique et ornithologique. Valeurs de conservation du site. Catharsius La Revue, 1-123.
Noriega J. A., Hortal, J., Azcárate, F. M., Berg, M. P., Bonada, N., Briones, M. J. I., Santos, A. M. C. (2018). Research trends in ecosystem services provided by insects. Basic and Applied Ecology, 26, 8–23. https://doi.org/10.1016/j.baae.2017.09.006
Ouin A., Soro, D., & Kouadio, K. (2022). Insect diversity and habitat disturbance in West African forest landscapes. African Journal of Ecology, 60(3), 511–523. https://doi.org/10.1111/aje.12997
Philips T. K., Bowen, J. G., & Soumah, A. G. (2023). Dung beetle (Coleoptera: Scarabaeidae, Scarabaeinae) diversity of the highest elevation in West Africa: The Nimba Mountain Range. Journal of Tropical Insect Science. https://doi.org/10.1007/s42690-023-01017-3
Sodhi N. S., Brook, B. W., & Bradshaw, C. J. A. (2020). Tropical biodiversity loss and its implications for insect communities. Biological Conservation, 241, 108258. https://doi.org/10.1016/j.biocon.2019.108258
Underwood E.C. & Fisher B.L., 2006. The role of ants in conservation monitoring: if, when, and how. Biol. Conser., 132(2), 166-182.
Yeo K., Kouakou L.M.M., Touao M.K., Gauze E., Ouattara K. & Soro A.N., 2019. Ants response to mining prospection disturbances across vegetation zones in tropical mountain chains of Mount Nimba, Guinea, West Africa. International Journal of Biological Chemical Sciences, 13(2), 899-913.
(41 Ref.)