Bioprotection of Cucumis melo from Alternaria leaf spot by Glomus mosseae and Trichoderma harzianum
Résumé
Bioprotection de Cucumis melo contre maladie des taches foliaires par Glomus mosseae et Trichoderma harzianum.
Alternaria alternata, responsable de l’alternariose des taches foliaires, est une propagation mondiale notamment en Irak. Cette maladie se caractérise par la formation de lésions sur les feuilles, entraînant des pertes substantielles de rendement et de qualité chez plusieurs espèces végétales telles que Cucumis melo (melon). Glomus mosseae et Trichoderma harzianum sont considérés comme une alternative écologique et respectueuse de l'environnement par rapport à l'utilisation des fongicides en raison de leur capacité intrinsèque à induire des voies antistress natives chez les plantes. Dans ce contexte, ces travaux de recherche ont été menées pour évaluer l'efficacité des bio-agents testés, G. mosseae et T. harzianum (en combinaison ou séparément), sur les melons infectés par A. alternata dans des conditions sous serre. G. mosseae et T. harzianum ont fourni de puissants effets inhibiteurs contre la maladie des taches foliaires, en diminuant le pourcentage de gravité (DSI = 31,35 et 40,39 %, respectivement) et l'indice de la maladie (DI = 1,30 et 1,49, respectivement). Les résultats obtenus ont révélé que le traitement du melon séparé contre cet agent pathogène aéroporté en utilisant G. mosseae et T. harzianum a généré la plus forte activité de la catalase (CAT = 5,678 et 3,389 unités/mg protéine/min, respectivement) et de la peroxydase (POX = 9,948 et 7,542 unités/g/ml/min, respectivement). Cependant, les résultats en cours ont révélé que la combinaison de G. mosseae et de T. harzianum réduisait de manière négligeable le DSI (47,04 %) et le DI (2,08) et augmentait la CAT (1,962 unités/mg de protéine/min) et la POX (3,08 unités/g/ ml/min). Pour lutter contre A. alternata dans le cadre de stratégies de lutte intégrée contre les ravageurs, des agents de lutte biologique (tels que G. mosseae et T. harzianum) doivent être pris en considération.
Abstract
Alternaria alternata, responsible for Alternaria leaf spot, is spread worldwide, notably in Iraq. This disease is characterized by lesion formation on the leaves, leading to substantial yield and quality losses in several plant species such as Cucumis melo (melon). Glomus mosseae and Trichoderma harzianum are considered eco-friendly and bio-safe alternatives to traditional chemical fungicides due to their intrinsic ability to induce native anti-stress pathways in plants. This research study was conducted to evaluate the efficacy of the tested bio-agents, G. mosseae and T. harzianum (in combination or separately), on cucumber infected with A. alternata under greenhouse conditions. G. mosseae and T. harzianum provided strong inhibitory effects against leaf spot disease, decreasing disease severity index (DSI = 31.35 and 40.39%, respectively) and disease index (DI = 1.30 and 1.49, respectively). Obtained results revealed that the separate treatment of melon against this airborne pathogen using G. mosseae and T. harzianum generated the strongest activity of catalase (CAT = 5.678 and 3.389 units/mg protein/min, respectively) and peroxidase (POX = 9.948 and 7.542 units/g/ml/min, respectively). However, the ongoing results revealed that the combination of G. mosseae and T. harzianum negligibly reduced DSI (47.04%) and DI (2.08) and increased CAT (1.962 units/mg protein/min) and POX (3.08 units/g/ml/min) activities. To control A. alternata within integrated pest management strategies, biological control agents (such as G. mosseae and T. harzianum) should be considered.
Introduction
1In Iraq, melon (Cucumis melo L.) contributes fairly to the agricultural economy. However, significant yield fluctuations were recorded from year to year, mainly due to several factors such as biotic and abiotic stresses. Amongst biotic stress, Alternaria alternata (Fr.) Keissl. responsible for leaf spot disease affected melon crop by reducing yield and fruit quality (Rhouma et al., 2020; Matrood & Rhouma, 2021).
2To achieve the nutritional needs of an increasing population from the available cultivable land , intensive agricultural practices, including the use of fungicides, have multiplied. Besides, use of fungicides also helps in disease reduction. Still, their ill-advised applications negatively affect the natural balance of soil ecosystems, contaminate soils and develop resistance in phytopathogens. In this context, disease management using biological control agents (BCAs) offers a great promise. These agents represent a vital component of sustainable agriculture, which does not contain any toxic residues, unlike fungicides (Egberongbe et al., 2010; Martínez-Medina et al., 2011; Dehariya et al., 2015; Rhouma et al., 2018).
3Arbuscular mycorrhizal fungi and Trichoderma spp. signify one of the most promising BCAs. Trichoderma spp. represent useful avirulent saprophytes that act as BCAs against several phytopathogenic fungi through various mechanisms such as antibiotic and enzyme production, mycoparasitism, induced resistance and rhizosphere competition. In addition to disease control, Trichoderma spp. are acknowledged to have beneficial effects in promoting growth and physiological activities of plants (Altomare et al., 1999; Rhouma et al., 2018).
4Bioprotection mediated by arbuscular mycorrhizal fungi has been exploited as a key practice for disease control. Induced systemic resistance (ISR) is the primary phenomenon involving plant protection by arbuscular mycorrhizal fungi. The mechanism of ISR by arbuscular mycorrhizal fungi focuses on nutritional effects, morphological changes in roots and root tissues, competition for infection sites, microbial changes in mycorrhizosphere, and changes in chemical constituents in plant tissues (Ames et al., 1983; Egberongbe et al., 2010).
5The interaction between arbuscular mycorrhizal fungi and Trichoderma spp. documented stimulation or inhibition of the population/colonization of certain species. Information on mutually interactive species of arbuscular mycorrhizal fungi and Trichoderma spp. could be beneficial for developing a successful biological control strategy that will overcome the harmful effects associated with the use of chemical fungicides and fertilizers (Egberongbe et al., 2010; Martínez-Medina et al., 2011; Dehariya et al., 2015).
6The aims of the present study were to evaluate the potential biological control of T. harzianum and G. mosseae against A. alternata on enhancing enzyme activities and disease intensity under greenhouse conditions.
Materials and Methods
7The pathogen (Alternaria alternata) and the antagonistic fungi (Trichoderma harzianum) used in the present research were obtained from the Laboratory of Plant Protection, College of Agriculture, Basra, Iraq. Fungal materials were isolated from symptomatic leaves (pathogen), and rhizosphere (antagonistic fungi) collected from experimental field cultivated with cucumber plants in Basra, Iraq. The arbuscular mycorrhizal fungi (Glomus mosseae) were obtained from the Ministry of Science and Technology, Agricultural Research Department, Iraq.
Inoculum preparation
8A. alternata and T. harzianum were grown on potato dextrose agar (PDA) medium at 25°C for 4 days until sporulation, and then, an Erlenmeyer flask containing 50 ml of potato dextrose broth was inoculated with four pieces individually. Spore production was induced in an orbital shaker, and the spores were recovered from culture by filtration. A hemocytometer was used to determine the concentration of the spores (106 spores/ml) (Rhouma et al., 2018).
9G. mosseae was grown on sterilized medium composed of wheat (Triticum aestivum L.) seeds. For inoculum preparation, wheat seeds were soaked for 12 h in distilled water and then air-dried. Seeds were transferred to one liter capacity flasks, which were subsequently autoclaved three successive times at 120ºC for one hour. Flasks were incubated at 25ºC for 16 weeks until complete colonization of wheat seeds by the arbuscular mycorrhizal. Flasks were shaken manually once a week to avoid clustering of inoculum. The colonized wheat seeds were used as an inoculum (Egberongbe et al., 2010; Dehariya et al., 2015).
10To know the number of G. mosseae spores, we first carried out the spores extraction from the inoculated wheat seeds according to the method described by Jmal et al. (2015). 100 g of seeds were mixed with 200 ml water, agitated on a magnetic stirrer for 5 min, and washed through nested 1.6 mm, 0.25 mm et 0.038 mm sieves. The supernatant retained on the 0.038 mm sieve were collected and placed into Petri dishes (90 mm). The materials were distributed in other Petri dishes (60 mm) containing distillated water and enumerated under a stereomicroscope at a magnification of 40.
Greenhouse assessment of Trichoderma harzianum and Glomus mosseae efficacy
11The experiment was carried out in the greenhouse as a randomized complete block design (three blocks). Melon seedlings cv. Amal (15 days old) were placed in a pot containing peat and vermiculite (1:1); at the rate of one seedling in each pot. When the melon plants turned a month old, different treatments were applied to them.
12Treatments consisted of:
13T+: melon plants were inoculated only with pathogen (positive control);
14T-: melon plants were treated only with sterilized and distilled water (negative control);
15T1: melon plants were sprayed with 10 ml of T. harzianum. After seven days, the same melon plants were sprayed with 10 ml of A. alternata. The spores used were recovered from the culture filtrate;
16T2: 30 g of wheat seeds containing approximately 130 spores were placed in the pot at 5-10 cm depth. After seven days, the same melon plants were sprayed with 10 ml of A. alternata;
17T3: melon leaves were sprayed with 10 ml of T. harzianum and the melon roots were inoculated with 30 g of wheat seeds. After seven days, the same melon plants were sprayed with 10 ml of A. alternata.
18Immediately after inoculation of the melon plants with the pathogen, the pots were covered with plastic bags for 24 h to optimize the conditions suitable for infection to occur (Rhouma et al., 2018; Matrood et al., 2021).
Disease severity assessment
19Assessments were performed three weeks after inoculation of the pathogen. Observed areas symptoms were scored for disease index (DI) using a scale from 0 to 4; 0 = no lesions; 1 = lesions covering about 1-10% of the leaf surface; 2 = lesions covering about 11-25% of the leaf surface; 3 = lesions covering about 26-50% of the leaf surface; 4 = lesions covering about 51-100% of the leaf surface. The disease severity index (DSI) was calculated for each block by the formula of McKinney (1923): DSI (%) = (Σvn)/(20 × V) × 100. v is the numeric value of DI scale, n is the number of plants assigned to the disease index scale, and V is the numeric value of the highest disease index scale (Matrood et al., 2021).
Enzymes Activity
20For the enzyme activities, plant tissue extract was prepared by freezing 0.1 g of sample leaves in liquid nitrogen to stop the proteolytic activity, then homogenized with extraction buffer (1:5) (0.1 M phosphate buffer + 0.5 mM EDTA (pH = 7.5)) and centrifuged at 15,000 × g for 20 min at 4°C. The supernatant was collected, transferred to new Eppendorf tubes and assayed for enzyme activity.
21Catalase (CAT) activity was estimated according to Bhuvaneshwari et al. (2015). A decline in absorbance was reported at 240 nm.
22Peroxidase (POX) activity was evaluated according to the method of Velazhahan and Vidhyasekaran (1994). Three ml of reaction mixture composed of 0.5 ml guaiacol, 1 ml phosphate buffer, 0.5 ml H2O2, 0.1 ml enzyme extract, and 0.9 ml water and the absorbance was measured at 470 nm.
Statistical analysis
23Statistical analysis was performed using the mean values of the replicates. The data were analyzed by ANOVA using SPSS version 20.0 statistical software (SPSS, SAS Institute, USA). Homogeneity of variances and normality was checked by applying Duncan’s Multiple Range Test. Duncan’s Multiple Range Test determined differences between treatments. All statistical tests were performed with a significance level of 1% (P ≤ 0.01).
Results
24Significant reduction in disease index (DI) and disease severity index (DSI) and improvement in catalase and peroxidase activities was recorded when G. mosseae and T. harzianum were applied against A. alternata (Figures 1, 2, 3 and 4).
25Results revealed that melon plants treated separately by G. mosseae and T. harzianum against A. alternata showed less disease symptoms on the vegetative parts decreasing the disease index (1.30 and 1.49, respectively) and the disease severity index (31.35% and 40.39%, respectively). However, the combination between G. mosseae and T. harzianum was less efficient, recording DI of 2.08 and DSI of 47.04% (Figures 1 and 2).
26Glomus mosseae and T. harzianum exhibited higher catalase and peroxidase activities in the presence of A. alternata; recording 5.68 units/mg protein/min (CAT) and 9.95 units/g/ml/min (POX) for G. mosseae and 3.39 units/mg protein/min (CAT) and 7.54 units/g/ml/min (POX) for T. harzianum, as presented in Figures (3) and (4). On the other hand, the lowest activities of catalase (1.96 units/mg protein/min) and peroxidase (3.08 units/g/ml/min) were recorded in melon plants treated with both G. mosseae and T. harzianum (Figures 3 and 4).
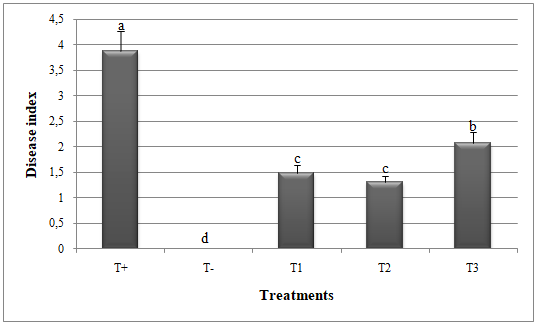
Figure 1: Disease index in melon plants treated by Glomus mosseae and Trichoderma harzianum (separately or in combination ) against Alternaria alternata. Small letters are used to compare different treatments.
Different letters above bars indicate statistically significant differences within the experiments (P≤ 0.5) according to the Duncan’s Multiple Range Test. Bars without letter are not significantly different. T+: Positive control; T-: Negative control; T1: T. harzianum + A. alternata; T2: G. mosseae + A. alternata; T3: G. mosseae + T. harzianum + A. alternata
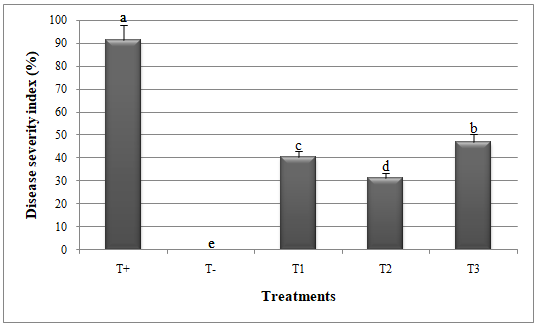
Figure 2: Disease severity index in melon plants treated by Glomus mosseae and Trichoderma harzianum (separately or in combination ) against Alternaria alternata.
Small letters are used to compare different treatments. Different letters above bars indicate statistically significant differences within the experiments (P≤ 0.5) according to the Duncan’s Multiple Range Test. Bars without letter are not significantly different. T+: Positive control; T-: Negative control; T1: T. harzianum + A. alternata; T2: G. mosseae + A. alternata; T3: G. mosseae + T. harzianum + A. alternata
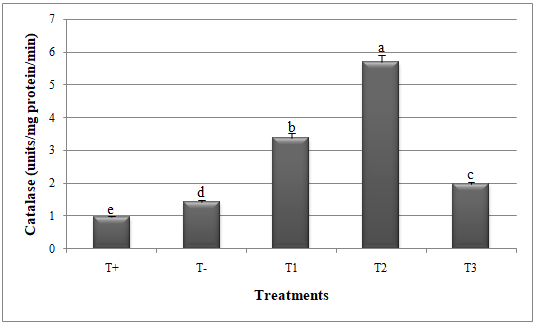
Figure 3: Catalase activity in melon plants treated by Glomus mosseae and Trichoderma harzianum (separately or in combination ) against Alternaria alternata.
Small letters are used to compare different treatments. Different letters above bars indicate statistically significant differences within the experiments (P≤ 0.5) according to the Duncan’s Multiple Range Test. Bars without letter are not significantly different. T+: Positive control; T-: Negative control; T1: T. harzianum + A. alternata; T2: G. mosseae + A. alternata; T3: G. mosseae + T. harzianum + A. alternata
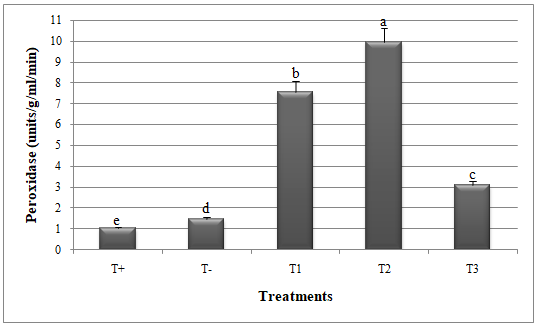
Figure 4 : Peroxidase activity in melon plants treated by Glomus mosseae and Trichoderma harzianum (separately or in combination ) against Alternaria alternata.
Small letters are used to compare different treatments. Different letters above bars indicate statistically significant differences within the experiments (P≤ 0.5) according to the Duncan’s Multiple Range Test. Bars without letter are not significantly different. T+: Positive control; T-: Negative control; T1: T. harzianum + A. alternata; T2: G. mosseae + A. alternata; T3: G. mosseae + T. harzianum + A. alternata
Discussion
27This study presents the assessment of G. mosseae and T. harzianum (applied separately) to enhance resistance and reduce disease severity of melon against A. alternata, the causal agent of leaf spot disease in Iraq.
28The accumulation of peroxidase and catalase results in a defensive reaction (Kloepper et al., 2004). Peroxidase is known to play a role in deposition of lignin and suberin in plant cell walls (Vieira et al., 2003). Catalase represents one of the few enzymes that exhibit dual enzyme activity. They play a role of specific peroxidase, and their function is to protect cells from toxic effects of hydrogen peroxide (Luhova et al., 2003).
29The effect of Trichoderma spp. could be explained by the production of antibiotic substances to influence the growth of phytopathogens, as reported by Sreedevi et al. (2011) and Rhouma et al. (2018). The beneficial effect of Trichoderma spp. is due to direct mycoparasitism on pathogenic fungi and indirect effects on plants, such as promoting plant development and stimulating plant resistance (Harman, 2000; Harman et al., 2004). Hajieghrari et al. (2008) documented that Trichoderma spp. produced a number of antibiotics such as trichodernin (Kucuk & Kivanc, 2004) and some cell walls degrading enzymes such as chitinase and glucanase that break down polysaccharides and chitins, thereby destroying cell wall integrity (Elad, 2000). Boughalleb-M’Hamdi et al. (2018) and Rhouma et al. (2018) demonstrated the promising results for Trichoderma spp. in the biological control of plant diseases applying the mechanisms of antibiosis, competition of nutrients availability, production of inhibitory compounds, and mycoparasitism mediated by hydrolytic enzymes. Trichoderma spp. could stimulate plant growth independently of any plant diseases (Shivanna et al., 1996).
30Chitinolytic enzyme activity expressed by arbuscular mycorrhizal fungi and Trichoderma spp. has long been recognized among factors contributing to plant disease control. The bio-agents directly attack the plant pathogens by secreting lytic enzymes, including chitinases. These enzymes hydrolysis the pathogen cell wall as chitin in principal and other components as glycan causing dramatic effect on pathogenic fungi (Harman, 2000; Harman et al., 2004; Hajieghrari et al., 2008).
31These findings are in agreement with Morandi (1990) and Siqueira et al. (2002). They pointed out that the role of arbuscular mycorrhizal fungi in controlling plant diseases doesn't increase nutrient absorption only, but one of the factors that contribute to enhancing their role in controlling plant diseases is production of phenolic or secondary inhibitory compounds during their interaction with plants.
32In retrospect, it could be concluded that the bioagents G. mosseae and T. harzianum, regardless of the application method, may be considered aspotent antagonists against A. alternata used in this study, due to their role as a bioagent through nutrient absorption augmentation, mycoparasitism, competition for space and nutrients or as plant growth promoter, along with other potentialities which include, secretion of antibiotics, production of phenolic compoundsand secretion of enzymes to degrade fungal cell wall (Harman et al., 2004). It also produces plant growth regulators (Ahmad et al., 2008). These mechanisms protect plants and make them healthy with a good translocation rate of macronutrients from roots to shoots (Ahmad et al., 2008; Harman et al., 2004).
33However, the combination between G. mosseae and T. harzianum was revealed to be less efficient. This could be due to inhibition of G. mosseae parasitism by T. harzianum. As a biocontrol agent, the action mechanism of T. harzianum used in this study is known to be based on mycoparasitism. It could, therefore, be possible for Trichoderma spp. to have had a deleterious effect on the arbuscular mycorrhizal fungi as reported by Rousseau et al. (1996). Similar negative interference between arbuscular mycorrhizal fungi and Trichoderma spp. on the growth of plants and their resistance to several phytopathogens has been demonstrated in many studies (Green et al., 1999; Mar Vázquez et al., 2000; Martinez et al., 2001; Tchameni et al., 2011).
Conclusion
34In view of the discussion above, lines of evidence have been provided to show that a single application of G. mosseae and T. harzianum revealed a series of morphological and biochemical changes that are considered part of the melon plant defence response against A. alternata. However, the ability of dual application with G. mosseae and T. harzianum to enhance disease severity reduction and induce systemic resistance of plants was less functional, showing their possible incompatibility to occupy the same rhizosphere.
Bibliographie
Ahmad F., Ahmad I. & Khan M.S., 2008. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 163, 173-181.
Altomare C., Norvell W.A., Björkman T. & Harman G.E., 1999. Solubilization of phosphates and micronutrients by the plant-growth promoting biocontrol fungus Trichoderma harzianum. Appl. Environ. Microbiol. 65, 2926-2933.
Ames R.N., Reid C.P., Porterf P.L.K. & Cambardella C., 1983. Hyphal uptake and transport of nitrogen from two 15N-labelled sources by Glomus mosseae, a vesicular-arbuscular mycorrhizal fungus. New Phytol. 95, 381-396.
Bhuvaneshwari V., Amsaveni R., Kalaiselvi M., Rajeshwari R. & Paul P.K., 2015. Induced resistance by neem extracts in plants. Int. J. Biosci. Nanosci. 2, 221-224.
Boughalleb-M’Hamdi N., Salem I.B. & M’Hamdi M.,2018. Evaluation of the efficiency of Trichoderma, Penicillium, and Aspergillus species as biological control agents against four soil-borne fungi of melon and watermelon. Egypt J. Biol. Pest Contr. 28, 1-12.
Dehariya K., Shukla A., Sheikh I.A. & Vyas D., 2015. Trichoderma and arbuscular mycorrhizal fungi based biocontrol of Fusarium udum Butler and their growth promotion effects on Pigeon Pea. J. Agric. Sci. Technol. 17, 505-517.
Egberongbe H.O., Akintokun A.K., Babalola O.O. & Bankole M.O., 2010. The effect of Glomus mosseae and Trichoderma harzianum on proximate analysis of soybean (Glycine max (L.) Merrill.) seed grown in sterilized and unsterilized soil. J. Agric. Ext. Rural Dev. 2, 54-58.
Elad Y., 2000. Biological control of foliar pathogen by means of Trichoderma harzianum and potential mode of action. J. Crop Prot. 19, 709-714.
Green H., Larsen J., Olsson P.A., Jensen D.F. & Jakobsen I., 1999. Supression of the biocontrol agent Trichoderma harzianum by mycelium of the arbuscular mycorrhizal fungus Glomus intraradices in root-free soil. Appl. Environ. Microbiol. J. 65, 1428-1434.
Hajieghrari B, Torabi-Giglou M., Mohammadi M.R. & Davari M., 2008. Biological potential of some Iranian Trichoderma isolates in control of soil borne plant pathogenic fungi. Afr. J. Biotechnol. 7, 967-972.
Harman G.E., 2000. Myths and dogmas of biocontrol. Plant Dis. 84, 377-391.
Harman G.E., Howell C.R., Viterbo A., Chet I. & Lorito M., 2004. Trichoderma species opportunistic, a virulent plant symbionts. Nat. Rev. Microbiol. 2, 43-56.
Jmal Z., Labidi S., Dalpe Y., Slim S., Lounes-Hadj Sahraoui A. & Ben Jeddi F., 2015. Diversité de champignons mycorhiziens arbusculaires d’un bas fond halomorphe en Tunisie. J. New Sci. 18, 648-657.
Kloepper J.W., Ryu C.M. & Zhang S., 2004. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 94, 1259-1266.
Luhova L., Lebeda A., Hedererova D. & Pec P., 2003. Activities of amine oxidase, peroxidase and catalase in seedlings of Pisum sativum L. under different light conditions. Plant Soil Environ. 49, 151-157.
Mar Vázquez M., Sonia C., Azcón R. & Barea J.M., 2000. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 15, 261-272.
Martinez C., Blanc F., Le Claire E., Besnard O., Nicole M. & Baccou J.C., 2001. Salicylic acid and ethylene pathways are differentially activated in melon cotyledons by active or heat-denatured cellulase from Trichoderma longibrachiatum. Plant Physiol. 127, 334-344.
Martínez-Medina A., Roldán A. & Pascual J.A., 2011. Interaction between arbuscular mycorrhizal fungi and Trichoderma harzianum under conventional and low input fertilization field condition in melon crops: Growth response and Fusarium wilt biocontrol. Appl. Soil Ecol. 47, 98-105.
Matrood A.A.A. & Rhouma A., 2021. Efficacy of foliar fungicides on controlling early blight disease of Eggplant, under laboratory and greenhouse conditions. Nov. Res. Microbiol. J. 5, 1283-1293.
Matrood A.A.A., Ramírez Valdespino C.A., Al-Waeli M.A., Khrieba M.I. & Rhouma A., 2021. Pathogenicity and chemical control of Alternaria sp. on date palm (Phoenix dactylifera L.). Plant Sci. Today. 8, 386-391.
Mckinney H.H., 1923. Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J. Agric. Res. 26, 195-218.
Morandi D., 1990. Effect of endomycorrhizal infection and biocides on phytoalexin accumulation in soybean roots. Agric. Ecosyst. Environ. 29, 303-305.
Rhouma A., Ben Salem I., M’Hamdi M. & Boughalleb-M’Hamdi N., 2018. Antagonistic potential of certain soilborne fungal bioagents against Monosporascus root rot and vine decline of watermelon and promotion of its growth. Nov. Res. Microbiol. J. 2, 85-100.
Rhouma A., Mougou I. & Rhouma H., 2020. Determining the pressures on and risks to the natural and human resources in the Chott Sidi Abdel Salam oasis, southeastern Tunisia. Euro-Mediterr. J. Environ. Integr. 5, 1-11.
Rousseau A., Benhamou N., Chet I. & Piché Y., 1996. Mycoparasitism of the extramatrical phase of Glomus intraradices by Trichoderma harzianum. Phytopathology. 86, 434-443.
Shivanna M.B., Meera M.S. & Hyakumachi M., 1996. Role of root colonization ability of plant growth promoting fungi in the suppression of talke-all and common root rot of wheat. J. Crop Prot. 15, 497-504.
Siqueira J.O., Lambais M.R. & Sturrmer S.L., 2002. Fungos micorrízicos arbusculares: características, associação simbiótica e aplicação na agricultura. Biotecnol. Ciênc. Desenvolv. 25, 12-21.
Sreedevi B., Charitha-Devi M. & Saigopal D.V.R., 2011. Isolation and screening of effective Trichoderma spp. against the root rot pathogen Macrophomina phaseolina. J. Agric. Technol. 7, 623-635.
Tchameni S.N., Ngonkeu M.E.L., Begoude B.A.D., Wakam Nana L., Fokom R., Owona A.D., Mbarga J.B., Tchana T., Tondje P.R., Etoa F.X. & Kuaté J., 2011. Effect of Trichoderma asperellum and arbuscular mycorrhizal fungi on cacao growth and resistance against black pod disease. J. Crop Prot. 30, 1321-1327.
Velazhahan R. & Vidhyasekaran P., 1994. Role of phenolic compounds, peroxidase and polyphenol-oxidase in resistance of groundnut to rust. Acta Phytopathol. Entomol. Hung. 29, 23-29.
Vieira D.S.C., Letousey P., Delavault P. & Thalouarn P., 2003. Defense gene expression analysis of Arabidopsis thaliana parasitized by Orobanche ramosa. Phytopathology. 93, 451-457.

