First in situ biocontrol attempt against Cephalcia tannourinensis on Cedrus libani
Abstract
Alarming outbreaks of the insect defoliator Cephalcia tannourinensis are threatening the survival of the cedars of Lebanon, Cedrus libani. As a first in situ biocontrol attempt against this cedar web-spinning sawfly, inundative treatment using entomopathogenic nematodes (EPNs) were applied from 2014 to 2016 in Tannourine cedars forest. The treatments consisted of applying three times per year a commercial strain of Heterorhabditis bacteriophora in treated sites and tap water in control sites. The success of this treatment was assessed by monitoring the C. tannourinensis populations by measuring the density of underground larvae, emerging adults and the associated foliar damage. High fluctuations of the pest’s population occurred during the years of treatment in all sites. No statistical difference resulted between the EPNs treated sites and the control sites for the density of underground larvae or emerging adults. Although foliar damage in EPNs treated sites decreased significantly, the EPNs inundative treatments against C. tannourinensis in the Tannourine forest is still at experimental phase. Nevertheless, this study provides better guidance for future strategic biocontrol plans against insect forest pests.
Introduction
1Climate change observed worldwide has effects on insects such as species distribution, increases in population abundance, earlier outbreaks and enhanced winter survival (Battisti, 2008; Kurz et al., 2008; Lindner et al., 2010; Larson, Tinghitella and Taylor, 2019; Peterson and Cipollini 2020). In Lebanon, the life cycle of an insect defoliator, Cephalcia tannourinensis (Chevin 2002) (Hymenoptera: Pamphilidae), is mainly affected by the fluctuations in temperatures over the years. Starting 2013, alarming new outbreaks have been observed following short periods of snow cover in winters and dry summers with high temperatures (Nemer et al., 2014). These outbreaks are threatening Cedrus libani (Richard 1823) (Pinales: Pinaceae), a vulnerable plant species according to the 2000 Red List (Gardner, 2013).
2This web-spinning sawfly causes intense defoliation that weakens cedars, declines their growth and threatens their survival if attacked for three consecutive years. The adults of C. tannourinensis emerge from the ground in early spring, once the snow melts. After mating, females lay eggs on the new cedars’ needle clusters called buds. After hatching, the larvae feed on these buds and move to lower branches progressively forming a silky thread, from April till mid-June. The mature larvae drop to the ground, enter the soil at 5 to 20 cm deep (Battisti, 1994) under the cedar’s crown and prepare for diapause in an earth – walled chamber. Next spring, individuals with annual diapause cycle (called pronymphs) develop pupal eye and become pupae, ready for adult emergence. The other individuals (called eonymphs) will remain in the soil at deeper levels for their multiple year diapause cycles (Battisti, 1993).
3The first outbreaks of C. tannourinensis have been observed in the early 90s, in one of the north cedars’ forests in Lebanon known as Tannourine forest nature reserve (Nemer et al., 2005). This forest is one of the twelve main cedar forests in Lebanon (Hajar et al., 2010), protected under nature reserve areas legislation (Lebanese Ministry of Environment, Law 558, 24/7/96). Since its discovery, several paths were explored to take back the control of C. tannourinensis populations: chemical control using aerial spraying of the diflubenzuron growth inhibitor was applied in the forest between 1994 and 2004 (Nemer et al., 2005) causing a mortality rate of 80%. Moreover, biological control studies were conducted in vitro using entomopathogenic agents as Beauveria bassiana (Vuillemin 1912) (Hypocreales: Cordycipitaceae) (ARSEF 152, clade C), naturally occurring entomopathogenic fungi in the Tannourine cedars forest. It is the first identified natural enemy of C. tannourinensis causing 93 to 100% in vitro mortality rates on aerial populations (eggs and the first three instar larvae) (Abdo et al., 2008) and 70 to 76% in vitro cumulative mortality rate on pronymphs and eonymphs (Al Khoury et al., 2019). Entomopathogenic nematodes (EPNs) were also tested in vitro against this sawfly: a commercial strain of Heterorhabditis bacteriophora (Poinar 1976) (Rhabditida: Heterorhabditidae) (HbCOM supplied by Koppert) and a local one found nearby the Tannourine cedars forest (LB04) caused respectively 80% in vitro mortality rate (Noujeim, Rehayem and Nemer, 2015); a local strain of Steinernema feltiae (Filipjev 1934) (Rhabditida: Steinernematidae) found in the Tannourine forest (LB132) caused 60% in vitro mortality rate (Rehayem et al., 2018). Finally, small-scale experiments conducted by Rehayem et al. (2018) in jars under Tannourine forest’s environment showed 85% mortality rate for C. tannourinensis prepupae using 625 000 Infective Juveniles (IJs) / m2 of a commercial strain of H. bacteriophora.
4In light of these results, biological control seems a promising attempt to control C. tannourinensis avoiding the use of chemicals in a nature reserve. To our knowledge, no biological control treatment was conducted in situ on a large scale against C. tannourinensis. Therefore, this study aims to control biologically the populations of C. tannourinensis in different chosen sites of Tannourine cedars forest nature reserve, by applying a commercial strain of H. bacteriophora. The efficacy of these inundative EPNs treatments in controlling this devastating pest will be evaluated by monitoring (i) the underground population of C. tannourinensis; (ii) its aerial population and (iii) the cedar damage it causes.
Materials and methods
Area of study
5This study was carried out entirely in the Tannourine cedars forest nature reserve. An area with high C. tannourinensis outbreak was located by the forest managers and had been previously surveyed with no autochthone EPNs found, but only indigenous entomopathogenic fungi B. bassiana (Rehayem et al., 2018). The habitat in this section is mostly dense cedar stands mixed with broadleaved trees, large rocks and a slope going from medium to high (15 to 30%). Eight reachable sites were then selected by the forest’s managers within this section (Fig. 1): each site is 100 m2 and includes approximately 7 cedar trees. All 8 sites have the same climatic and environmental conditions and are distant from each other by at least 4m. Due to the forest’s topography and the habitat composition, the sites were not overlooking each other’s. These sites were then differentiated into two types: the first includes 4 sites treated using EPNs (thereafter described as EPNs sites); the second includes 4 other sites treated using only tap water (described as control sites).
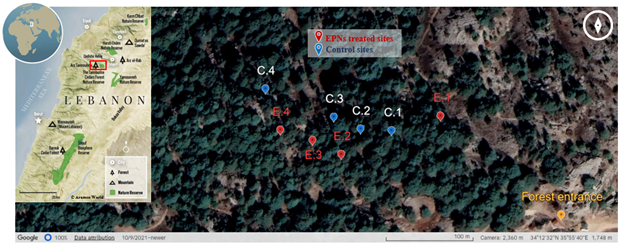
Fig. 1 Area of study and chosen sites in the Tannourine cedar forest
Sites in red are EPNs treated sites; sites in blue are control sites treated with tap water. The red rectangle on map shows the location of the Tannourine cedar forest. E. 1: EPNs treated site 1; E. 2: EPNs treated site 2; E. 3: EPNs treated site 3; E. 4: EPNs treated site 4; C. 1: Control site 1; C.2: Control site 2; C. 3: Control site 3; C. 4: Control site 4. Lebanese map © Aramco World (Chad, 2019)
EPN treatments
6Following the studies conducted for the control of C. tannourinensis via the EPNs in vitro and in a small-scale trial (Noujeim, Rehayem and Nemer, 2015; Rehayem et al., 2018), a commercial strain of H. bacteriophora was chosen for the current study (H. bacteriophora LARVANEM; purchased from Koppert B.V., Berkel en Rodenrijs, the Netherlands). This strain was applied at a rate of 625 000 IJs / m2 using a watering can, from autumn 2014 till autumn 2016. Each treatment consists of two applications separated by one week and was applied after rain or following the irrigation of the site, to increase soil humidity and provide a suitable environment for the EPNs. Three treatments were conducted per year, each targeting a specific stage of the web spinning sawfly. The autumn treatment targets the prepupae overwintering; the spring treatment targets the pupae in preparation for their metamorphosis into adults. Summer treatment targets the larvae who just dropped from the cedar trees in preparation for their diapause in the soil. Prior to each treatment, the viability and the concentration of the EPNs suspension were checked. Establishment of EPNs and their ability to find and infest the larvae once in the soil was also checked: Ten perforated Eppendorf tubes containing two Galleria mellonella (Linnaeus 1758) (Lepidoptera: Pyralidae) larvae, commonly used biological filter for EPNs (Sturhan and Mráček, 2000), were introduced into the soil of each site before each EPNs application at a depth between 5 to 20 cm. They were then collected after 3 days. The cadavers of G. mellonella larvae were then placed individually on the modified white traps (Kaya and Stock, 1997) for emergence of the IJs. Emerging nematodes were used to infest fresh G. mellonella larvae to confirm Koch’s postulates of pathogenicity. A minimum 70% mortality rate of the G. mellonella larvae was expected to consider the EPNs application successful (which means IJs were able to penetrate the soil and infest the larvae). If not, another treatment was rescheduled.
7The success of the EPNs treatments during the 3 years of study was evaluated by monitoring the evolution of C. tannourinensis populations (measuring the density of prepupae and the emerging adults) and the percentage of the cedar damage it causes.
Monitoring the underground population of C. tannourinensis
8The underground population of C. tannourinensis is directly targeted by the EPNs treatments. Measuring the underground density of prepupae is a commonly used monitoring method for sawflies (Battisti, 1994; Nemer et al., 2005; Holuša, 2011; Meterc, Borkovič and Jurc, 2014;). In this study, 1 m2 was defined under a cedar branch bearing traces of larval feeding. The litter layer (up to 5 cm depth), when existent, was removed and the presence of C. tannourinensis larvae in that layer was checked. 16 soil samples (V = 25 x 25 x 15 cm3) were extracted from this 1m2. The number of C. tannourinensis prepupae was counted for each sample. This experiment was conducted once during the autumn period of each year (during the month of November as cited by Nemer (2008)). The measured parameter is the density of prepupae found in 1 m2.
Monitoring the aerial population of C. tannourinensis
9The adult population of C. tannourinensis was collected using yellow sticky traps (20 x 10 cm): This accessible and easy technique is commonly used by foresters for the monitoring of large populations of sawflies (Battisti and Rodeghiero, 1998; Nemer et al., 2005; Holusa and Drapela, 2006). These traps were hung in the middle of each site from April till June. They were replaced every two weeks. This experiment was conducted once every year. The parameter measured is the number of emerging adults found on the yellow trap of each site.
Monitoring the cedar damage caused by C. tannourinensis
10Monitoring the cedar damage caused by C. tannourinensis helps in evaluating the efficacy of the treatment: 10 cedar branches were collected randomly from each site during the beginning of July every year. The following parameters were counted: for each branch, the total number of buds and the damaged buds showing bites.
Statistical analysis
11Statistical analysis was conducted using the Minitab program (version 18). Before conducting analysis, data were checked for homogeneity of variance. T test was applied to compare the difference between the years in prepupae density and between the two types of sites (p < 0.05). Kruskal-Wallis test was applied to compare the difference in adult outbreaks between years and types of sites (p < 0.05). Analysis of variance (ANOVA) was applied to compare the difference between the two types of sites and between the cedar damage (p < 0.05).
Results
Preparation for the EPNs treatment
12Prior to each EPNs application for each treatment, the viability and the concentration of the EPNs suspension as well as the 70% minimum mortality rate for the G. mellonella larvae traps were checked. These three criteria were met and no treatment was rescheduled.
Monitoring the underground population of C. tannourinensis
13The monitoring of prepupae was affected by snowstorms which lead to missing data, especially for the control sites for the year 2014. Same goes for 2016 (the underground density could only be measured for two sites (Table 1).
Table 1 Underground density of C. tannourinensis larvae /m²
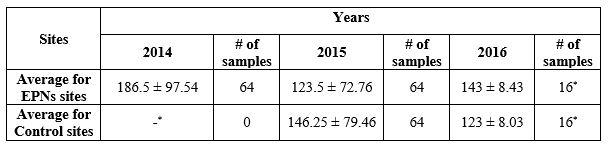
-* Data missing due to snowstorms
14During 2014, the lowest underground larvae density among the EPNs sites was 68 larvae/m2 while the highest was 303 larvae/m2. As for 2015, 58 larvae/m2 was the lowest larvae density while the highest was 220 larvae/m2. Among the control sites, the lowest and highest larvae densities were 33 and 203 larvae/m2 respectively during 2015. No statistically significant difference was detected between 2014 and 2015 for the prepupae at the EPNs sites (p>0.05). As for the year 2015, no statistically significant difference was observed between the EPNs sites and the control sites (123.5 ± 72.8 and 146.3 ± 79.5 respectively, p = 0.690). This experiment cannot confirm the efficacy of the EPNs treatment.
Monitoring adult population of C. tannourinensis
15The year 2015 witnessed alarming outbreaks of C. tannourinensis with the highest numbers of adults emerging from both types of sites (respectively 52.98% and 76.62% higher for EPNs sites and control sites compared to 2014). In 2016, a decrease of 57.81% was observed at EPNs sites and 54.52% at control sites compared to 2015. Comparing the two types of sites, the number of adults emerging from the control sites was higher than those emerging from EPN treated sites for the years 2015 and 2016 (respectively 19.84% and 29.21%) (Fig. 2). Nevertheless, this number is not significantly different between the two types of sites (p>0.05). Moreover, no significant difference was noted between the years for each type of sites. No conclusion can be made yet concerning the success of the EPN treatments in controlling the adult populations of C. tannourinensis following this experiment.
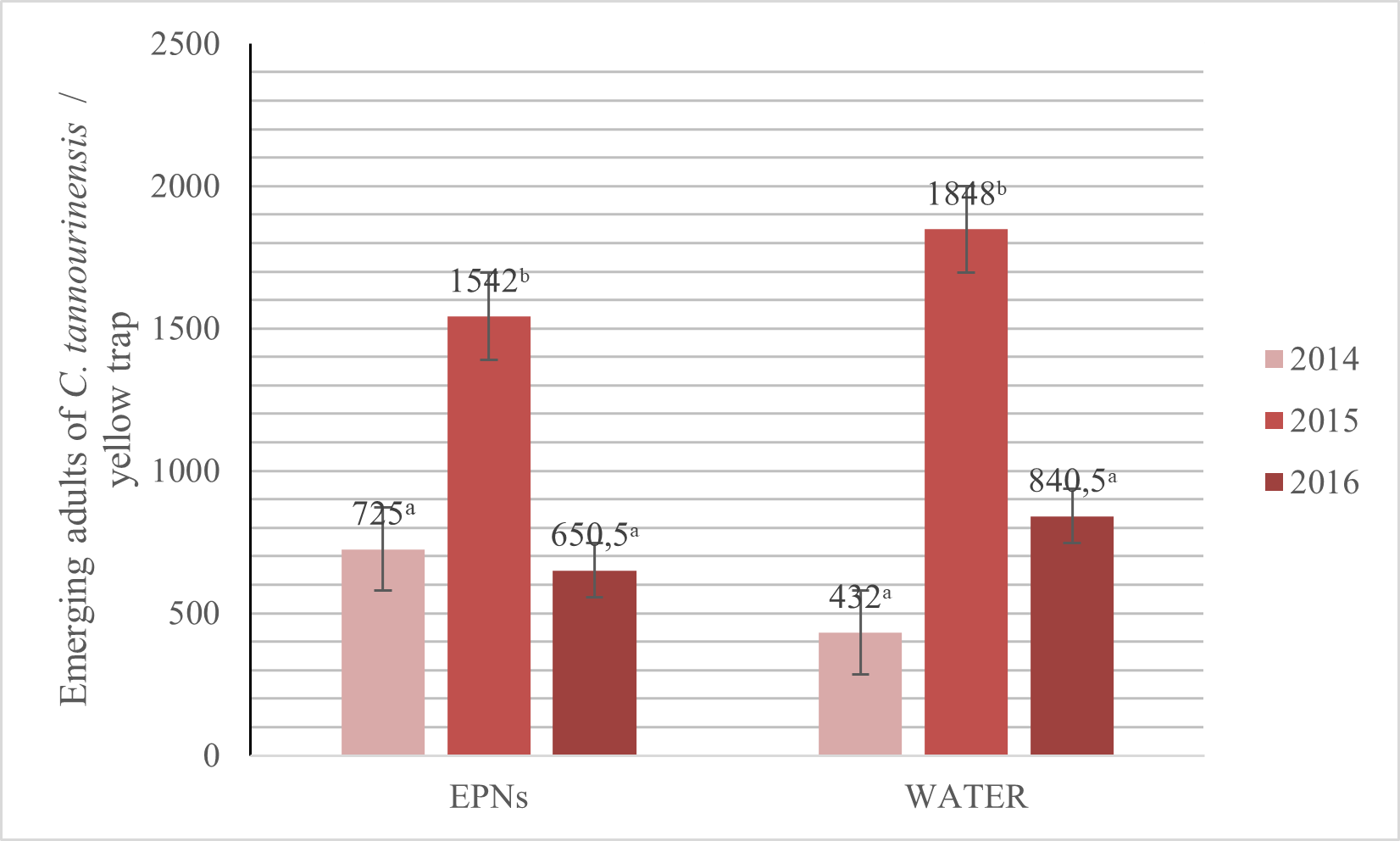
Fig. 2 Evolution of aerial population of C. tannourinensis in EPNs and control sites.
For each year, data shown is the number of adults emerging calculated for 100 m2 of each type of sites
Monitoring the damage on cedars caused by C. tannourinensis
16Percentage of foliar damage in the EPNs treated sites decreased significantly by about 40% from 2014 till 2016 (F=11.92, df=2, p<0.001). As for the control sites, the foliar damage significantly increased by about 13% in 2015 and then significantly decreased by about 34% in 2016 (Fig. 3) (F=11.92, df=2, p<0.001).
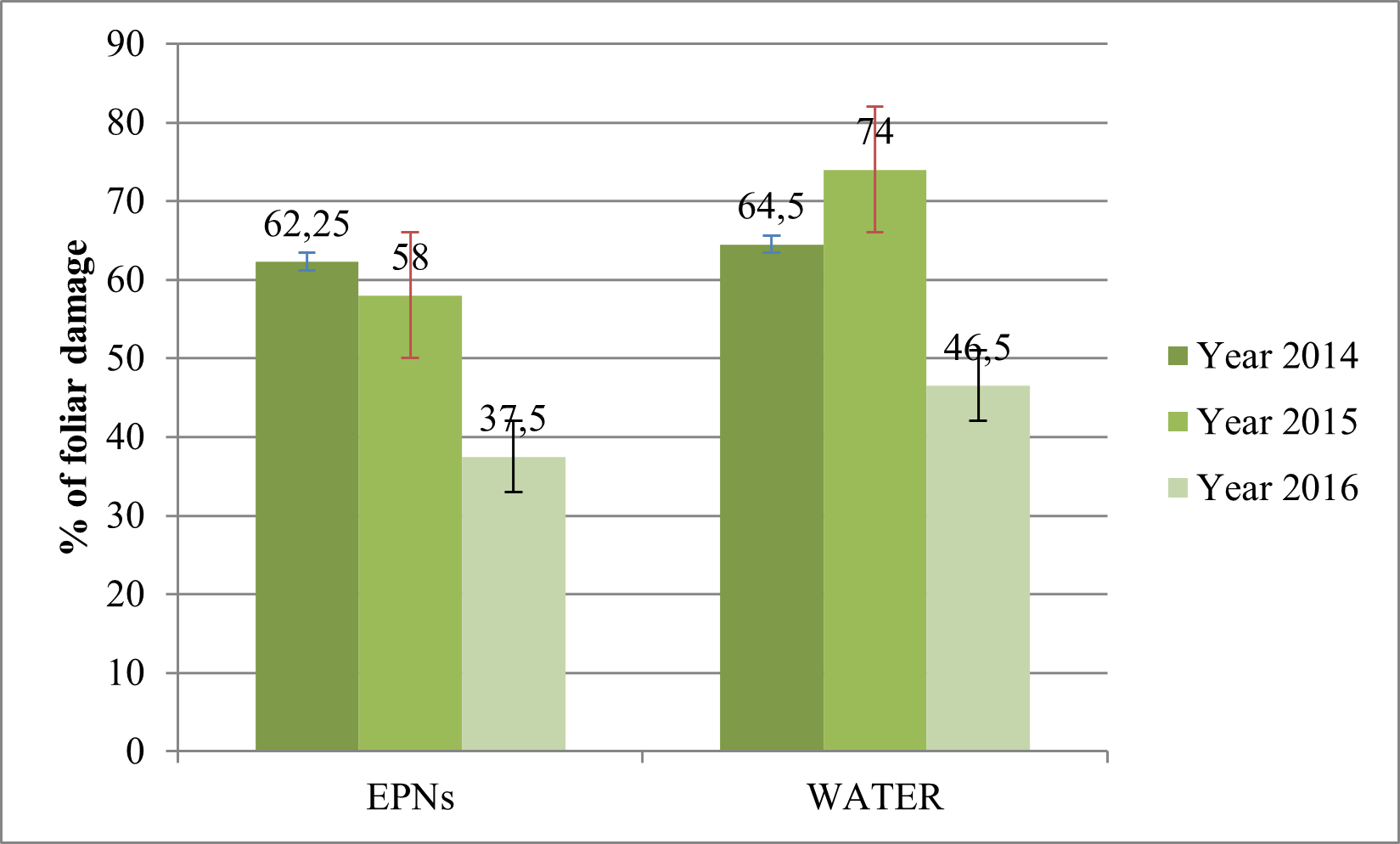
Fig. 3 Evolution of the foliar damage caused by C. tannourinensis on cedars in EPNs and control sites.
For each year, data shown is the percentage of foliar damage calculated for 100m2 for each type of sites
Discussion
17C. tannourinensis is the main cedar defoliator threatening the growth and the viability of the Tannourine cedars forest since 1997. This study represents the first biological control attempt against C. tannourinensis on a large scale (100m2 sites). Underground and aerial populations of the pest were monitored as well as the foliar damage on cedars following inundative EPNs’ treatments.
18Considering the underground population, results show that most of the samples had larvae density higher than the defoliation threshold of C. tannourinensis which is 70 prenymph / m2 (Nemer, 2008). This proves how urgent it is to find a way to control this devastating pest. In the EPNs’ treated sites, the number of prepupae decreased without any significant difference from 2014 to 2015, following one year of treatment. At first sight, it seems that one year of treatment is not enough to control the underground population of this pest. But with limited data collected in this experiment due to snowstorms, no strict conclusion can be made. This decrease can also be attributed to the usual winter mortalities due to climatic and pedological parameters (Gruppe, 1996; Gill, Goyar and Chahil, 2017).
19As for the aerial population of C. tannourinensis, the number of emerging adults increased in 2015 and then significantly decreased in 2016 in both EPNs and control sites. This change might reflect the pest dynamic enhanced by the high temperature observed in 2014. The lack of difference between EPNs and control sites can be attributed to the timing of the treatment and the pest dynamic. Other authors found similar results for example with pheromone traps to control the outbreaks of the bark beetle, Ips typographus (Linnaeus 1758) (Coleoptera: Curculionidae) (Grodzki et al., 2006); with massive releases of rodenticides to control the outbreaks of the common vole Microtus arvalis (Pallas 1778) (Rodentia: Cricetidae) (Olea et al., 2009) or with aerial spraying of insecticides to control outbreaks of the pine processionary moth Thaumetopoea pityocampa (Denis & Schiffermüller 1775) (Lepidoptera: Notodontidae) (Cayuela, Hodar and Zamora, 2011).
20The peak of C. tannourinensis dynamic was also shown in the evolution of the foliar damage caused on the cedar buds. The percentage of foliar damage in the control sites increased significantly in 2015 then decreased significantly in 2016. As for the EPNs treated sites, it decreased from 2014 to 2015 and significantly from 2015 to 2016. This might show the effect of the EPNs inundative treatments after 3 years of treatment but still does not confirm their effectiveness as a curative strategy during alarming outbreaks of C. tannourinensis. The reason why significant differences were detected in the foliar damage experiment and not with the aerial population of C. tannourinensis experiment remains unclear. It can be attributed to the pest dynamic hiding the effects of the EPNs’ inundative treatments. Battisti and Rodeghiero (1998) reported male predominance of Cephalcia arvensis (Panzer 1805) (Hymenoptera: Pamphilidae) in their catches which might be the case for the experiment of this current study. This can be explained by the fact that males of C. tannourinensis are more active and emerge earlier than females (Nemer et al., 2005).
21Regardless of the efficacy of the EPNs’ inundative treatments on C. tannourinensis, urgent strategies are needed to control any future outbreaks of this pest. Following the actual global warming, climate conditions in forests in high altitudes are becoming warmer thus more suitable for the insects’ development in areas where they used to be absent (Battisti, 2008; Cayuela, Hodar and Zamora, 2011; Pureswaran, Roques and Battisti, 2018; Choi et al., 2019). This is the case of the north cedars’ forests of Lebanon, such as the Ehden nature reserve and the cedar protected area of Bcharreh. In these two forests above 2000 m of altitude, long snow coverage duration and low temperatures prevented the outbreak of C. tannourinensis population until recently (personal communication by forest managers): high temperatures enhanced alarming outbreaks of C. tannourinensis in these forests, where chemical treatments were applied in 2015 in Bcharreh as a last resort to limit the damages on the “cedars of God” (Nemer et al., 2014). As an alternative to chemicals, pheromone traps controlling the emerging adults might be a more suitable strategy (Faccoli and Stergulc, 2008; Galko et al., 2016). Future studies on the C. tannourinensis' pheromone, previously identified and extracted from the female adults (Nemer et al., 2007), are highly encouraged.
22With its trials and errors, this study offers insights that can guide the implementation of future biocontrol strategies against forest pests like C. tannourinensis. It highlights the impact of climate change on pest dynamics and the urgency to take it into account for future control strategies, particularly in high – altitude forests where rising temperatures have led to increased outbreaks in previously unaffected areas. The findings of this study suggest that managing pests during the high fluctuation of its population might hide the efficacy of the treatments. Therefore, it is crucial to establish a defined efficient monitoring plan for the pest’s population before attempting any treatment. The monitoring methods should be labor – efficient and less influenced by climatic conditions. The effectiveness of this monitoring plan helps with early interventions and therefore prevents outbreaks from reaching damaging levels. Finally, biocontrol treatments still face challenges under natural forest conditions. This study advocates for continuous research to understand the behavior of entomopathogenic agents under these conditions.
Conclusion
23As a conclusion, the efficacy of the EPNs inundative treatments in Lebanese cedars forests especially in the Tannourine forest is still at experimental phase. Therefore, it is still not recommended when facing alarming outbreaks of C. tannourinensis. With its trials and errors, this study can guide future strategic biocontrol plans against forest pests.
Statements and Declarations
Author contributions
24MR conceived and designed the project. MR collected the data, performed analysis. The first draft of the manuscript was written by MR and then edited by RCM and FF. All authors read and approved the final manuscript.
Ethics approval and consent to participate
25Not applicable.
Data Sharing and Data Accessibility
26The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
27The authors have no conflict of interest.
Funding
28This research was supported by grants from the National Council for Scientific Research (CNRS Conseil National de la Recherche Scientifique) in Lebanon and by the Programme Hubert Curien CEDRE franco-libanais [grant number 32953UL]. The Tannourine Cedars Forest Nature Reserve partially supported the field work. Field experiments were supervised by Bernad Duvic, Olivier Thaler, Nabil Nemer and Elise Noujeim.
Acknowledgments
29Field experiments were supervised by Bernad Duvic, Olivier Thaler, Nabil Nemer and Elise Noujeim. Special thanks to Wael Yammine and Benjamin Delattre.
Bibliographie
Abdo, C., Nemer, N., Nemer, G., Abou Jawdah, Y., Atamian, H., and Kawar, N. (2008) Isolation of Beauveria species from Lebanon and evaluation of its efficacy against the cedar web-spinning sawfly, Cephalcia tannourinensis. BioControl, 53, 341-352 https://doi.org/10.1007/s10526-006-9062-0
Al Khoury, C.A., Rehayem, M., Abou Jaoude, C., Noujeim, E. and Nemer, N. (2019) Efficacy of local strains of entomopathogenic Beauveria bassiana (Bals.) Vuill. and Steinernema feltiae (Filipjev) on the pronymphs and eonymphs of Cephalcia tannourinensis (Chevin) under laboratory conditions. Academia Journal of Agricultural Research, 7(3), 054-060 10.15413/ajar.2019.0103
Battisti, A. (1994) Effects of entomopathogenic nematodes on the spruce web – spinning sawfly Cephalcia arvensis Panzer and its parasitoids in the field. Biocontrol Science and Technology, 4(1), 95-102 https://doi:10.1080/09583159409355317
Battisti, A. (1993) Bionomics of the spruce web-spinning sawfly Cephalcia arvensis Panzer in Northeastern Italy (Hym.: Pamphiliidae). Journal of Applied Entomology, 115(1-5), 52-61 https://doi.org/10.1111/j.1439-0418.1993.tb00363.x
Battisti, A. (2008) Forest and climate change – lessons from insects. iForest – Biogeosciences and Forestry, 1(1), 1-5 https://doi.org/10.3832/ifor0210-0010001
Battisti, A. and Rodeghiero, M. (1998) Monitoring spruce web – spinning sawflies Cephalcia spp.: the correlation between trap catches and soil sampling. Entomologia Experimentalis et Applicata, 88(3), 211-217 https://doi.org/10.1046/j.1570-7458.1998.00365.x
Cayuela, L., Hodar, J.A. and Zamora, R. (2011) Is insecticide spraying a viable and cost-efficient management practice to control pine processionary moth in Mediterranean woodlands?. Forest Ecology and Management, 261(11), 1732-1737 https://doi.org/10.1016/j.foreco.2011.01.022
Chad, S. (2019). Cedrus libani forever? AramcoWorld retrieved October 6, 2023, https://www.aramcoworld.com/Articles/May-2019/Cedrus-libani-Forever
Choi, W.I., Nam, Y., Lee, C.Y., Choi, B.K., Shin, Y.J., Lim, J-H., Koh, S-H., and Park, Y-S. (2019) Changes in Major Insect Pests of Pine Forests in Korea Over the Last 50 Years. Forests, 10(8), 692 https://doi:10.3390/f10080692
Faccoli, M. and Stergulc, F. (2008) Damage reduction and performance of mass trapping devices for forest protection against the spruce bark beetle, Ips typographus (Coleoptera Curculionidae Scolytinae). Annals of Forest Science, 65, 309 https://doi.org/10.1051/forest:2008010
Galko, J., Nikolov, C., Kunca, A., Vakula, J., Gubka, A., Zubrik, M., Rell, S. and Konôpka, B. (2016) Effectiveness of pheromone traps for the European spruce bark beetle: a comparative study of four commercial products and two new models. Lesnícky Časopis Forestry Journal, 62, 207-215 https://doi:10.1515/forj-2016-0027
Gardner, M. (2013) Cedrus libani. The IUCN Red List of Threatened Species 2013: e.T46191675A46192926. Available at: http://dx.doi.org/10.2305/IUCN.UK.2013-1.RLTS.T46191675A46192926.en (Accessed: 01 October 2023).
Gill, H.K., Goyal, G. and Chahil, G. (2017) Insect Diapause: A Review. Journal of agricultural science and technology, A 7 https://doi:10.17265/2161-6256/2017.07.002
Grodzki, W., Jakus, R., Lajzova, E., Sitkova, Z., Mackza, T. and Skvarenina, J. (2006) Effects of intensive versus no management strategies during outbreak of the bark beetle Ips typographus (L.) (Col.: Curculionidae, Scolytinae) in the Tatra Mts. in Poland and Slovakia. Annals of Forest Science, 63(1), 55-61 https://doi.org/10.1051/forest:2005097
Gruppe, A. (1996) Model of the importance of exogenic factors influencing the variable dormancy of prepupae of Cephalcia abietis (Hymenoptera: Symphyta: Pamphiliidae). Entomologia Generalis, 21(1-2), 95-105
Hajar, L., François, L., Khater, C., Jomaa, I., Déqué, M., and Chaddadi, R. (2010) Cedrus libani (A. Rich) distribution in Lebanon: Past, present and future. Comptes Rendus Biologies, 333(8), 622-630 https://doi.org/10.1016/j.crvi.2010.05.003
Holuša, J. (2011) Preimaginal development of Cephalcia lariciphila during an outbreak in the Czech Republic. Bulletin of Insectology, 64, 55-61
Holuša, J. and Drápela, K. (2006) Yellow sticky boards: a possible way of monitoring little spruce sawfly (Pristiphora abietina) (Hymenoptera: Tenthredinidae). Journal of Forest Science, 52(1), 13-21 http://dx.doi.org/10.17221/4482-JFS
Kaya, H.K. and Stock. S.P. (1997) ‘Techniques in insect nematology’ in Lacey, L. (ed.) Manual of Techniques in Insect Pathology. London: Academic Press, 281-324.
Kurz, W.A., Dymond, C., Stinson, G., Rampley, G., Neilson, E., Carroll, A., Ebata, T. and Safranyik, L. (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature, 452, 987-990 https://doi.org/10.1038/nature06777
Larson, E.L., Tinghitella, R.M. and Taylor, S.A. (2019) Insect Hybridization and Climate Change. Frontiers in Ecology and Evolution, 7, 348 https://doi.org/10.3389/fevo.2019.00348
Lindner, M., Maroschek, M., Netherer, S., Kremer, A., Barbati, A., Garcia – Gonzalo, J., Seidl, R., Delzon, S., Corona, P., Kolstroma, M., Lexer, M.J. and Marchetti, M. (2010) Climate change impacts, adaptive capacity and vulnerability of European forest ecosystems. Forest Ecology and Management, 259, 698-709 https://doi.org/10.1016/j.foreco.2009.09.023
Meterc, G., Borkovič D. and Jurc, M. (2014) Outbreak of the spruce web-spinning sawfly Cephalcia arvensis (Hymenoptera: Pamphilidae) in Slovenia. Sumarski List, 138, 293-299.
Nemer, N. (2008) Biologie et écologie chimique de deux nouveaux ravageurs en cédraies libanaises: Cephalcia tannourinensis Chevin (Hymenoptera: Pamphiliidae) et Ernobius libanensis n. sp. (Coleoptera: Anobiidae). Doctoral thesis, Université de Paris XI, France, p. 218.
Nemer, N., Demolin, G., Kawar, N., Kfoury, L. and Zakhour, E. (2005) ‘Monitoring of the new cedar web-spinning sawfly, Cephalcia tannourinensis n. sp. in cedar forests of Lebanon’ in Lieutier, F. and Ghaioule, D. (eds.) Entomological Research in Mediterranean Forest Ecosystems. France: INRA, 247-255.
Nemer, N., El Beyrouthy, M., Lahoud, C., Mnif, W., Bashour, I. and Kawar, N. (2014) The influence of soil properties on the development of Cephalcia tannourinensis Chevin (Hym. Pamphiliidae) infesting the Cedar Forests in Lebanon. African Journal of Biotechnology, 13(47), pp. 4369-4381 https://doi.org/10.5897/ajb2013.12088
Nemer, N., Kawar, N., Kfoury, L. and Frerot, B. (2007) Evidence of sexual attraction by pheromone in the cedar web-spinning sawfly. Canadian Entomology, 139, 713-721 https://doi.org/10.4039/N06-042
Noujeim, E., Rehayem, M. and Nemer, N. (2015) Comparison of indigenous and exotic entomopathogenic nematode strain for control of the cedar web-spinning sawfly, Cephalcia tannourinensis in vitro. Journal of Biocontrol Science and Technology, 25, 843-851 https://doi.org/10.1080/09583157.2015.1019832
Olea, P., Sánchez-Barbudo, I., Viñuela, J., Barja, I., Mateo-Tomás, P., Piñeiro, A., Mateo, R. and Purroy, F. (2009) Lack of scientific evidence and precautionary principle in massive release of rodenticides threatens biodiversity: old lessons need new reflections. Environmental Conservation, 36(1), 1-4 https://doi.org/10.1017/S0376892909005323
Peterson, D.L. and Cipollini, D. (2020) Larval Performance of a Major Forest Pest on Novel Hosts and the Effect of Stressors. Environmental Entomology, 49(2), 482-88 https://doi.org/10.1093/ee/nvz160
Pureswaran, D.S., Roques, A. and Battisti, A. (2018) Forest Insects and Climate Change. Current Forestry Reports, 4(2), 35-50 https://doi.org/10.1007/s40725-018-0075-6
Rehayem, M., Noujeim, E., Nemer, N., Pages, S., Ogier, J-C., Thaler, O. and Duvic, B. (2018) New insights in biocontrol strategy against Cephalcia tannourinensis, the principal insect defoliator of Lebanese cedars. Forest Science, 64(4), 383-391 https://doi.org/10.1093/forsci/fxx018
Sturhan, D. and Mráček, Z. (2000) Comparison of the Galleria baiting technique and a direct extraction method for recovering Steinernema (Nematoda: Rhabditida) infective-stage juveniles from soil. Folia Parasitologica, 47(4), 315-318 https://doi.org/10.14411/fp.2000.055

