- Home
- Volume 37 (2019)
- Numéro 3
- Assessment of water quality and bacteriological levels in nile tilapia (oreochromis niloticus) of aiba reservoir, nigeria, west africa
View(s): 18013 (27 ULiège)
Download(s): 0 (0 ULiège)
Assessment of water quality and bacteriological levels in nile tilapia (oreochromis niloticus) of aiba reservoir, nigeria, west africa

Résumé
Évaluation de la qualite de l'eau et des niveaux bacteriologiques dans le tilapia du nil (oreochromis niloticus) du reservoir d'aiba, nigeria, afrique de l'ouest.
Les activités anthropiques constituent une menace majeure pour la qualité des masses d'eau. Cette étude a évalué la qualité de l'eau et les niveaux bactériologiques dans le foie, les branchies et les tissus d'Oreochromis niloticus dans le réservoir d'Aiba. Le réservoir a été divisé à dessein en fonction des activités anthropiques; site d'atterrissage des poissons et accessibilité dans trois zones d'échantillonnage marquées Zone 1 (entrée), Zone 2 (centre) et Zone 3 (sortie) avec chaque zone ayant un site d'atterrissage, Gaa Fulani, site d'atterrissage et Oju odo respectivement. Des échantillons de poisson d'un poids moyen de 200 g et des échantillons d'eau ont été recueillis au hasard tous les quinze jours en trois exemplaires pendant six mois. Les valeurs moyennes de l'analyse de l'eau mesurées se situaient dans la plage tolérable pour les poissons, bien qu'il y ait eu de légères variations dans les zones qui n'étaient pas significatives (P <0.05) et significatives entre les mois. Escherichia coli et Staphylococcus aureus ont été observés sur le foie, les tissus et les branchies des échantillons de poisson et peuvent causer de la dysenterie, de la fièvre, de la diarrhée et une insuffisance rénale chez les humains. Les tissus des échantillons de poisson présentaient le nombre total moyen de microbes et de coliformes le plus élevé. Il est donc opportun que des stratégies de surveillance soient utilisées pour assurer le strict respect du niveau de polluants autour du réservoir afin d'assurer une consommation sûre du poisson et l'utilisation de l'eau.
Abstract
Anthropogenic activities pose a major threat to the quality of water bodies. This study assessed the water quality and bacteriological levels in the liver, gills and tissues of Oreochromis niloticus (O. niloticus) in Aiba reservoir. The reservoir was purposively partitioned based on anthropogenic activities; fish landing site and accessibility into three sampling zones tagged Zone 1(inlet), Zone 2 (middle) and Zone 3 (outlet) with each zone having a fish landing site namely Gaa Fulani, landing site and Oju odo respectively. Fish samples with average weight of 200g and water samples were randomly collected fortnightly in triplicates for six months. Mean values of water analysis measured were within the tolerable range for fish although there were slight variations within the zones which were not significant (P<0.05) and significant among the months. Escherichia coli and Staphylococcus aureus were observed on the liver, tissues and gills of fish samples and can cause dysentery, fever, diarrhea and renal failure in humans. The tissues of fish samples had the highest mean total microbial and coliform count. It is therefore expedient that monitoring strategies be employed for strict adherence to level of pollutants around the reservoir to ensure safe consumption of fish and usage of water.
Table of content
Introduction
1Biota is principally affected by the prevailing water quality of the ecosystem. In general terms, water quality is defined as the suitability of water for the survival and growth of fish and is governed by only a few variables (3). Traditionally, water quality referred to only its chemical characteristics and toxicological properties related to drinking water or aquatic life uses, but presently have included both its physical, chemical, and biological characteristics (8). Fishes are key units in many natural food webs serving as important sources of food. They are sources of low fat protein, rich in omega-3 fatty acids, vitamins, calcium, phosphorus and various minerals (8; 22). They have an impact on the physical and chemical properties of the system in which they dwell, they can affect other aquatic organisms (8) and can serve as environmental indicator of the state of water bodies (5).
2Tilapia species have become increasingly important components of tropical inland fisheries production and are spread either by deliberate introduction or by accident. The farming of these species including Nile tilapia is the most widespread of aquaculture in the world especially in Asia and Africa (17). Tilapia culture has many economic benefits including food security, employment creation and poverty reduction. In Nigeria and Ghana, farmed tilapia is being used to reduce hunger and create economic opportunities (7). Tilapia remains a popular product in the retail sector in many parts of the globe (18); they are very tolerant to a wide variety of conditions (4; 22); can withstand crowding and utilize a variety of feeds from zooplanktons to specially mixed pellet feeds (20).
3The physico-chemical and bacteriological parameters influencing fish and water entails the analysis of chemicals and microbes present in the reservoir and on the body of the fish (26). The pH of most natural waters ranges from 6.5 - 8.5 and the fluctuation in the optimum pH ranges may lead to an increase or decrease in the toxicity of chemicals that may be present in the water bodies (12; 24). Bacteria are important components of the aquatic systems and their exposure to variation in habitats can affect their survival and significance (23; 25).
4Aiba Reservoir is the second oldest impoundment of Osun river basin and was created to provide portable water to indigenes and its’ environs with fisheries production as an additional benefit which is a cheap quality source of protein. The rapid increase in population and other social development such as educational institutions, banks, market places and media houses within the locality has posed an utmost concern about the state of the environment (10). It was reported that developments such as these has posed a serious problem on the quality of water (9) and can in turn affect the fish biodiversity and microbial load in the water and on the fish. Water borne diseases due to infectious agents associated with pathogenic bacteria, viruses or other parasites in water polluted by human, animal faeces or urine have been reported (10). With paucity of documented information regarding the microbial load of the reservoir; this study assessed the water quality of the reservoir and bacterial load on the tissues, gills and liver of O. niloticus which is most abundant fish species in the reservoir so as to ascertain the effects of these anthropogenic and developmental activities on the state of the water and other resources. These results will be useful in drawing inferences about the health status of the reservoir in terms of water quality and microbial load and fish and proffer necessary management solutions where needed as the indigenes are dependent on the river for water supply and fish.
Materials and methods
Study Area
5The reservoir is located between coordinates 07o38’579’N and 004o12’570’ E in Iwo, Nigeria with Onikan and Osun streams as tributaries. It is encompassed with abundance of vegetative cover and various anthropogenic activities with Gaa Fulani, landing site and Oju odo as the major fish landing sites. The reservoir was purposively partitioned based on anthropogenic activities, accessibility and fish landing sites into three sampling zones tagged Zone 1(inlet), Zone 2 (middle) and Zone 3(outlet). Water samples and O. niloticus species were fortnightly collected in triplicates for a period of six months from each sampling zone (Fig. 1).
6Description of zones:
-
Zone 1 (inlet) was at the North-Eastern area where the stream enters the reservoir. The flow of water was fast and the area was densely surrounded with vegetation; with little or no human activites except fishing with Gaa Fulani as fish landing site in the zone.
-
Zone 2 (middle) was at the Northern area which is the middle of the reservoir. The area was peculiar for effluent discharge and litters, dense vegetation, fecal materials and wastes and anthropogenic activities such as washing of clothes and automobiles. Fishing activities were also observed with landing site as the fish landing site.
-
Zone 3 (outlet) was located at the southwestern part of the reservoir towards the spill way. Fishing and sale of water were evident with little or no washing and refuse dumping activities although crops were grown around this area with Oju odo as the landing site in this zone.
Water quality Parameters
7The water samples collected from the sampling zones were measured for total hardness, Dissolved oxygen, Temperature and pH.
Dissolved oxygen
8It was measured using Winklers titrimetric method and was expressed in mg L-1 (6). Sterile sampling bottles were filled to the brim with water samples from each zone and were fixed in situ by adding 2 ml Winklers A reagent (Manganous sulphate solution) and B reagent (Potassium Iodide and Sodium Hydroxide solution). The resultant observation on the samples was formation of a white precipitate and was transported to the laboratory for further analysis (6; 10).
Total Hardness (Calcium and Magnesium)
9Water samples from each zone were measured for Total Hardness using
10Ethylenediaminetetra acetic acid (EDTA) method (2; 29). 50 ml of the sample was pipette into a conical flask of 250 ml and diluted with deionized water to 100 ml. 4 ml of buffer solution and 6 drops of Modrant black 11 solutions were later added. The samples were titrated with EDTA solution to an end point with distinct blue colour (29).
Temperature
11It was measured insitu using a mercury-in-glass thermometer calibrated in degrees centigrade (oC) (10). This was achieved by immersing the thermometer to a depth of 10 mm into the river and allowed to stay for about two minutes (21).
pH
12It was measured using a buffered electronic pH meter (model number Metrohm Herisau E520). The meter was first calibrated based on the manufacturer’s direction and probe inserted into the water sample. Readings were taken when the meter reading indicator was steady (10).
Collection of Fish samples
13The abundance of O. niloticus amongst Chrysichthys nigrodigitatus; Hepsetus odoe; Morymyrus; Channa obscura, Labeo cubie and Clarias gariepinus from the three landing sites influenced the choice of species for bacteriological analysis. They were randomly selected in triplicates with average weight of 200 g from each landing site within the sampling zones.
Processing and Enrichment of samples for Bacteriological analysis
14The fish samples were dissected and tissue, gill and liver were collected and stored in saline solution. The mashed gills and liver in separate mortar and pestle were diluted with sterile water in relation to the weight of the samples to be analyzed (15). On each sub-sample, a serial dilution experiment was done and kept in a cool place for further analysis (14).
Determination of Total Viable Count
150.5 ml of each diluted sample was inoculated onto the nutrient agar using Spread-plate method. Incubation of the plates was at 37 oC for 24 - 48 hours. The total viable count was determined by multiplying the number of colonies with the dilution factor (14).
Determination of Total Coliform Count
16Eosin Methylene Blue Agar was a selective medium used to determine the presence of gram negative intestinal pathogenic bacteria (14). 37.5 g of the agar was dispersed in 1 litre of distilled water. After soaking for 10 minutes, the solution was swirled to mix and sterile by autoclaving the solution at 121 oC for 15 minutes. It was later cooled to 47 oC and gently agitated to ensure even distribution of the precipitate, before pouring the distribution into sterile petri-dishes. The colonies that develop on the medium were counted by visual observation after incubation to assess the presence of fecal bacteria (14, 15).
Statistical analysis
17Data obtained from the sampling zones were analyzed using two-way Analysis of variance to determine the level of significance (95%) between spatial and temporal relationships.
Results and Discussion
Water quality parameters
18The mean values of water quality parameters and standard error are shown in Table 1 with values from World Health Organization (30) as standards. There were slight variations in parameters measured from the sampling zones of the reservoir. The mean water temperature from the study (27.65 °C) was within the acceptable range of 21 - 30 °C (8; 30). Statistically, there was no significance (P > 0.05) within the sampling zones but significance between the sampling months (21; 27). Despite the anthropogenic activities, the mean dissolved oxygen concentration recorded the sampling zones was within the recommended range of 5-8 mg L-1 (11; 30). This concentration of dissolved oxygen was attributed to the fast rate of flow of water and aeration of water by aquatic plants which constantly replenish the oxygen content in the water. There were slight variations in pH levels recorded from the sampling zones and were attributed to human activities. The values were within the recommended range of 6.6-8.9 (30). Generally, human activities can affect the pH concentration of surrounding water (9; 28). The mean total hardness (43.83 mg L-1 CaCO3) and the slight differences recorded across the study sites was a result of the human activities peculiar to the sites (9). Significant differences (P > 0.05) occurred within the sampling months and no significance within the across the sampling zones for Dissolved oxygen, pH and Total hardness, respectively.
Table 1: Mean values of water quality parameters from the sampling zones
|
Parameters |
Zone 1 (Inlet) |
Zone 2 (Middle) |
Zone 3 (Outlet) |
Mean |
WHO (30) |
|
Temperature ( oC) |
27.50 ± 0.05 ab |
27.80± 0.07 ac |
27.66 ± 0.07 ab |
27.65 ± 0.16 |
21-30 |
|
Dissolved oxygen (mg O2 L-1) |
6.45 ± 0.06 ab |
6.21 ± 0.04 ac |
6.44 ± 0.12 ad |
6.36 ± 0.11 |
5-8 |
|
Total Hardness (mg L-1 CaCO3) |
45.15 ± 1.45 ab |
43.18 ±1.02 ac |
43.16 ± 1.79 ad |
43.83 ± 0.19 |
75 - 200 |
|
pH |
7.19 ± 0.23 ab |
7.21 ± 0.15 ac |
7.11 ± 0.18ad |
7.17 ± 0.10 |
6.6-8.5 |
Superscripts with different letters are significantly different (P < 0.05)
Fish samples for Bacteriological analysis
19The species of bacteria isolated from the samples of gill, tissue and liver were Escherichia coli and Staphylococcus aureus. The Metallic green sheen pigmentation observed on the bacteria isolates from tissues, liver and gills when observed on the Eosin Methylene Blue agar plate indicated the presence of Escherichia coli which was significant (Table 3) (13). The yellowish colour on the fish which resulted from the fermentation of the Mannitol salt agar medium by the bacteria colonies implied the presence of Staphylococcus aureus and the population was insignificant. The mean highest total microbial and coliform count was found on the tissues of O. niloticus with 6.3 x 102 CFU ml-1 and 3.9 x 101 CFU ml-1 respectively (Table 2 - 3) around zone 2 (figure 1). These resulted from the presence of fecal materials from animals, humans and wastes from the environment peculiar to this zone. The elevated levels of microbial load in the tissues when compared to other parts analysed was as a result of the constant exposure of the fish to the pollutants in the environment (1; 14). The elevated levels can be traced to the anthropogenic activities around the catchment area that increased the bacteria population around it (16; 8, 10). The presence of faecal coliform bacteria in the gills, liver and tissues are indicators of pollution in a water body due to animal and human faecal decomposition (19; 16; 28). These bacteria can cause various diseases in human when consumed; S. aureus can cause septicemia and osteomyelitis (14; 28) and E. coli causing diarrhea, dysentery, fever and renal failure (1; 14).
Table 2: Mean Total Bacteria count (CFU ml-1) from the Liver, gills and tissues of O. niloticus across the sampling zones
|
Zone |
Liver (CFU ml-1) |
Gills (CFU ml-1) |
Tissues (CFU ml-1) |
|
1 |
5.5 x 102 |
1.5 x 106 |
6.1 x 102 |
|
2 |
5.3 x 102 |
1.3 x 106 |
6.3 x 102 |
|
3 |
5.0 x 102 |
1.1 x 106 |
6.1 x 102 |
20
Table 3: Mean Total Bacteria count (CFU ml-1) from the liver, gills and tissues of O. niloticus across the sampling zones
|
Zone |
Liver (CFU ml-1) |
Gills (CFU ml-1) |
Tissues (CFU ml-1) |
|
1 |
3.2 x 101 |
1.0. x 103 |
3.2 x 101 |
|
2 |
3.6 x 101 |
0.9 x 103 |
3.9 x 101 |
|
3 |
3.1 x 101 |
1.1 x 103 |
3.5 x 101 |
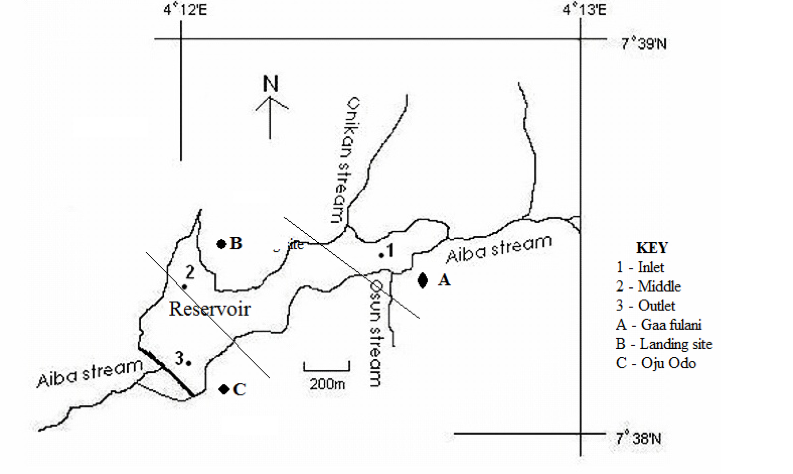
Figure 1: Map of Aiba reservoir with sampling zones (1 – 3) with landing sites (A – C).
Conclusion
21It was evident the reservoir received effluents which has altered the quality of water over time and residual effects on the microbial load of fish. These may pose health challenges to people who depend on the reservoir for protein intake. Public enlightenment on the dangers of unhealthy environment and the use of adequate waste disposal systems (such as landfills, incinerators, and refuse collection sites e.t.c) is essential for sustenance of healthy environment. Monitoring and Inspection is also a vital tool to ensure compliance with environmental laws.
References
-
Adebayo-Tayo, B.C., Adegoke, A.A. and Akinjogunla, O.J. (2009). Microbial and physico-chemical quality of powdered soy milk samples in Akwa Ibom , South-Southern Nigeria. Afr. J. Biotech., 8(13),3066-3071.
-
Ademoroti, C.M.A. (2006). Standard Methods for Water and Waste Effluents Analysis, Foludex Press, Ibadan, Nigeria.
-
Ahmed, Z.F., Wahab, M.A., Miah, M.A.H. and Azim, M.E. (2000). Investigation of water quality parameters in carp nursery under two different pond condition. Pak .J. Biological Science, 3:1349-1351.
-
Alsalim, M.S.D.H. (2007). The impact of sewage pollution on the water resources of Abha metropolitan area. In MA thesis, King Khalid University, Geography Department,1-30.
-
APHA, (1995). Standard Methods for the Examination of Water and Wastewater. 19th ed.Washington, DC: American Public Health Association.
-
APHA, AWWA, WEF. (2012). Standard Methods for examination of water and wastewater. 22nd ed.Washington: American Public Health Association, 1360 pp. ISBN 978-087553-013-0 http://www.standardmethods.org
-
Asiedu B., Failler P. and Yolaine, B. (2015). Enhancing aquaculture development: mapping the Tilapia aquaculture Value Chain in Ghana. Reviews in Aquaculture. 7: 1-9.
-
Atobatele, O.E. (2008). Physico-chemical parameters, plankton, macrozoobenthos and some aspects of the biology of two Chrysichthys (BAGRIBAE) of Aiba reservoir, Iwo, Osun State.2008
-
Atobatele, O.E., Morenikeji, O.A. and Ugwumba, O.A. (2005). Spatial variation in physical, chemical parameters and benthic macroinvertebrate fauna of river Ogunpa, Ibadan. The Zoologizt; 3:58-67.
-
Atobatele, O.E. and Owoseni, A.A. (2012). Distribution and Diversity of Bacteria in a Small Tropical Freshwater Body (Aiba Reservoir) in Iwo, Osun State, Nigeria. Nature and Science 2012; 10:(12) 92 - 97. http://www.sciencepub.net/nature.
-
Ayoade, A.A., Fagade, S.O. and Adebisi, A.A. (2006). Dynamics of limnological features of two man-made lakes in relation to fish population. African Journal of Biotechnology 10:1013-1021.
-
Banwo, K. (2006). Nutrient Load and Pollution Study of Some Selected Stations along Ogunpa River in Ibadan, Nigeria. M.Sc Dissertation, University of Ibadan, Ibadan, Nigeria, 2006 p. 107 (unpublished).
-
Buxton, A. and Fraser, G. (1997). Ascomycetes in Animal Bacteriology. Immunology, Bacteriology, Mycology and Diseases of Fish and Laboratory Methods. Edited by Buxton .A., Fraser .G., Oxford, UK: Blackwell scientific Publications; 301-302.
-
Egbebi, A.O., Muhammad, A.A., Ugbodaga, M. and Oyama, M.O. (2016). Bacteriological analysis of catfish (C. gariepinus) in Owo area, Ondo state, Nigeria. Journal of Biological Sciences 2(10)71-80.
-
Egbere, O.J., Kadir, A., Oyero, T., Steve, K., Odewumi, O. and Zakari, H. (2010). Bacteriological Quality of Catfish in Jos Metropolis, Nigeria. Intl J. Biosc., 5 (2), 2010, 95-103
-
Exner, C.O. (2002). Restoring and Protecting the World’s Lakes and Reservoirs. World Bank Technical Paper No. 289. The World Bank, Washington DC; 85p.
-
FAO. (2014). The State of World Fisheries and Aquaculture 2014. Rome. 223 pp.
-
FAO. (2016). The State of World Fisheries and Aquaculture 2014. Rome. 190 pp.
-
Hurst, C.J. et al. (1997). Manual of Environmental Microbiology. American Society for Microbiology. Washington DC. ; 849p
-
Ipinmoroti, M.O. and Iyiola, A.O. (2011). Organic production of zooplankton from locally sourced cassava peels using no supplemental nutrients. A. Leu, H. Lee, H, Z. Zhou, P. Villegas and L. Zuck (Eds). Procedings (precomference) of the 17th IFOAM Organic World Congress held at Gyeonggi Paldang, Republic of South Korea, 26th September – 5th October, 2011. Aquaculture, p376-380(d3015).
-
Iyiola, A.O. (2015). Human Impact on the Water Quality and Benthic Macro-Invertebrate Compositions in Ogunpa River, Nigeria. Journal of Agriculture and Ecology Research International 2(2): 120-128, Article no.JAERI.2015.013 DOI: 10.9734/JAERI/2015/14323
-
Kistemann, T., Classen, T., Koch, C., Dangendorf, F., Fischeder, R., Gebel, J., Vacata, V., and Exner, M. (2002). Microbial Load of Drinking Water Reservoir Tributaries During Extreme Rainfall and Run-off. Applied Environ Microbial 65(5):251-264.
-
Leff, L.G, McArthur, J.V. Shimkets, L.J. (1998). Persistence and dissemination of introduced bacteria freshwater microcosms. Microbial Ecology 36:02-211.
-
Lewis, Jr W.M. (2005). Basis for the Protection and Management of Tropical Lakes. Lakes and Reservoirs: Research and Management 5:35-48.
-
Ortiz, R.M. (2007). Assessment of Microbial and Chemical Water Quality of Individual and Small System Groundwater Supplies in Arizona. PhD Thesis, University of Arizona, Department of Soil and Environment (unpublished).
-
Stevens, M., Ashbolt, N. and Cunliffe, D. (2003). Review of coliforms and microbial indicators of drinking water quality- Recommendations to change the use of coliforms as microbial indicators of drinking water quality. NHRMS, Biotext Pty Ltd, Canberra, Australia 2003; 1-42.
-
Udeze, A.O., Talatu, M., Ezediokpu, M.N., Nwanze, J.C., Onoh, C. and Okonko, I.O.(2012). The effect of Klebsiella pneumoniae on catfish (Clarias gariepinus). Res. 4(4),51-59.
-
Ugwumba, A.A.A., Ogidiaka, E., and Esenowo, I.K. (2011). Physico-chemical parameters and benthic macro-invertebrates of Ogunpa River at Bodija, Ibadan, Oyo state. European Journal of Scientific Research 85(1):89-97
-
World Health Organization (WHO) (1999). Determination of Hardness of water. Method WHO/M/26.R1. http://www.who.int/whopes/quality/en/MethodM26.pdf. Retrieved on 12th April, 2018.
-
World Health Organization (WHO) (1994). Guidelines for drinking quality water, drinking water quality control in small community supplies, Geneva. 3:221
To cite this article
About: A. O. Iyiola
Nigerian, PhD - Fisheries Ecology and Climate Change (on-going), Assistant Lecturer, Department of Fisheries and Wildlife Management, College of Agriculture, Osun State University, P.M.B. 4494, Osogbo, Osun state, Nigeria. Email: adams.ovie.iyiola@gmail.com; adams.iyiola@uniosun.edu.ng
About: B. Asiedu
Ghanaian, PhD, Senior Lecturer, Department of Fisheries and Water Resources, University of Energy and Natural Resources, P.O. Box 214, Sunyani-Ghana. Email: berchieasiedu@yahoo.com; berchie.asiedu@uenr.edu.gh
About: A. S. Kolawole
Nigerian, PhD Fisheries Ecology (on-going), Research Assistant, Department of Aquaculture and Fisheries Management, Faculty of Renewable Natural Resources, University of Ibadan, Oyo state, Nigeria. kolawoleayotunde01@gmail.com
About: P. Failler
French, PhD, Professor of Economics and Director of the Centre for Blue Governance, Portsmouth Business School, University of Portsmouth, Richmond Building, Portland Street, Portsmouth, PO1 3DE, United Kingdom. Email: pierre.failler@port.ac.uk