- Accueil
- Volume 39 (2021)
- Numéro 1
- Evaluation of an Acacia mangium Provenance Trial after Seven Years, on the Ibi - Batéké Plateau, Democratic Republic of the Congo
Visualisation(s): 2257 (5 ULiège)
Téléchargement(s): 390 (0 ULiège)
Evaluation of an Acacia mangium Provenance Trial after Seven Years, on the Ibi - Batéké Plateau, Democratic Republic of the Congo

Document(s) associé(s)
Annexes
Résumé
Évaluation d’un essai de provenances d’Acacia mangium âgé de sept ans et établi à Ibi sur le plateau des Batéké en République Démocratique du Congo
Cette étude rapporte l'évaluation d’un essai de 46 provenances et variétés d'Acacia mangium plantées en 2006 sur le plateau d'Ibi - Batéké, en République Démocratique du Congo. Après sept ans, la hauteur et le diamètre des arbres, la biomasse et les stocks de carbone des 46 provenances d'Acacia mangium, ainsi que les concentrations de carbone et d'azote dans le sol, ont été comparés aux sols de savane dans lesquels les Acacia mangium n'étaient pas présents. Les hauteurs et les diamètres de 20 à 25 arbres par provenance ont été mesurés. Le carbone dans la biomasse a été déterminé par la méthode directe. Au total, 25 arbres ont été récoltés et pesés pour chaque compartiment de carbone (feuilles, branches, litière, troncs et racines). Quatre-vingt-dix échantillons de sol ont été recueillis à trois profondeurs différentes dans les parcelles des provenances et dans la savane, puis analysés pour déterminer leurs concentrations en C et en N. Il y avait des différences pour la croissance en hauteur et en diamètre ainsi que dans le stockage de carbone parmi les arbres selon leur provenance. De plus, le carbone du sol et l’azote différaient selon les provenances et les profondeurs. Le carbone et l'azote avaient tendance à diminuer avec la profondeur. Les résultats de l'étude ont révélé de meilleures performances pour les provenances originaires de Papouasie-Nouvelle-Guinée, d'Australie, de Malaisie, du Vietnam, de Chine, des Fidji et des Philippines.
Abstract
This study reports on the evaluation of 46 Acacia mangium provenances and varieties, which were planted in 2006 on the Ibi-Batéké Plateau, Democratic Republic of the Congo. After seven years, tree height and diameter, biomass and carbon stocks of the 46 Acacia mangium provenances, together with soil carbon and nitrogen concentrations, were compared with savannah soils in which Acacia was no present. Heights and diameters of 20 to 25 trees per provenance were measured. Carbon in the biomass was determined by the direct method. In total, 25 trees were harvested and weighed for each carbon compartment (leaves, branches, litter, trunks and roots). Ninety soil samples were collected at three different depths in the provenance plots and on the savannah and analyzed for their C and N concentrations. There were differences in height and diameter growth and in accumulated carbon among trees of different origins (provenances). Finally, soil C and N differed under different provenances, and with depth. Carbon and nitrogen tended to decrease with depth. The results of the study revealed better performance for provenances originating from Papua New Guinea, Australia, Malaysia, Vietnam, China, Fiji and the Philippines.
Table des matières
Introduction
1Brown salwood, Acacia mangium Willd. (Section Juliflorae, Fabaceae, Mimosoideae), is a low-elevation species, which usually occurs at altitudes below 300 m. Its natural range extends between 1° and 18°57’S, and between 125°22' and 146°17' E. Also known as sabah salwood, mangium (Malay), hickory wattle, krathin-thepa (Thai) and mangge hutan (Indonesian), among others, this species is found in northeast Queensland in Australia, through to the Western Province of Papua New Guinea (PNG) into the adjacent Indonesian province of Papua (formerly Irian Jaya), and the adjacent Maluku Islands (23). It is a fast-growing nitrogen (N2)-fixing tree species with high biomass production, and is capable of adapting to a wide range of environmental conditions, including acidic and nutrient-deficient soils (11, 17, 23).
2Salwood is a promising species for forest plantation establishment in many countries where degraded land requires rehabilitation or diverse climatic regimes occur (11). Reforestation with this species would reduce pressure on native forest ecosystems, while restoring soil fertility and ecosystem productivity (18).
3In 1980, seed collection of A. mangium from across its natural range was undertaken by the CSIRO (Commonwealth Scientific and Industrial Research Organization) of Australia and the Forest service of Papua New Guinea (23). The species has been introduced into many countries far beyond its original range (e.g., Central America and the Caribbean; Cameroon; Hawaii; Southeast Asia, including the Indian sub-continent and the Philippines).
4It has assumed an important role in reforestation programs in tropical and subtropical zones over the last few decades (2, 11). Acacia mangium was introduced into the Democratic Republic of Congo (DRC) in the early 1980s, primarily to reforest savannas and other marginal areas, and provide erosion control (17). At the same time, salwood plantings have produced fuelwood and food through the creation of agroforestry systems (17, 18).
5Multiple provenances of A. mangium exist and could be used in DRC, but there are few data in the literature to evaluate growth differences among them. Moreover, their ability to store biomass carbon, and their influence on soil organic carbon (SOC) and N concentrations remains unknown. This paucity of information needs to be addressed so that scientifically sound recommendations can be made regarding the potential of different provenances to produce wood, store carbon in their biomass, and affect SOC and soil N concentrations.
6This study evaluated a trial of 46 A. mangium provenances and varieties that had been established on the Batéké Plateau in 2006, as part of the Ibi Batéké Carbon Sink Plantation Project. The objective of this work was to identify, 7 years after planting, the most productive provenances of height and diameter at breast height (DBH, 1.30 m) accrual, together with the quantities of C stored in above- and belowground biomass.
7Effects of the introduction of these provenances on SOC and N concentrations were also addressed and compared to savannah soils where A. mangium had not been planted. We hypothesized that 1) certain provenances exhibited greater biomass production and, consequently, biomass C storage would vary among them; and 2) SOC and N concentrations would differ between savannah soils that had been planted with A. mangium versus those without A. mangium, and among soils that had been planted with different A. mangium provenances.
Materials and methods
Description of the area
8The study was conducted in Ibi Village, which is a rural commune that is located on the Batéké Plateau, 140 km east of the capital, Kinshasa. The site is located between 4°19’ and 4°25’S, and 16°4’ and 16°9’E (24). Ibi Village is situated on a plateau that rises slightly from South to North, averaging 700 m in elevation. The area is covered by grasses and shrubs (24). The climate is humid tropical - Type AW4, according to Köppen’s classification (19).
9Annual recorded rainfall is 1500 mm. Monthly average temperature has a very narrow range, i.e., 24-25 °C. The soils are acidic (pH < 5) and classified as Arenosols (FAO; Psamments, USDA equivalent) according to the World Reference Base for soil resources (35). These relatively nutrient-poor sandy soils contain very low quantities of N (< 0.5 g kg-1), organic C (< 10 g/kg-1) and phosphorus (P). Further, they have limited cation exchange capacity (CEC, < 10 cmol+ kg-1 soil) and are fragile due to their low organic matter content and low water retention capacity. The particle size distribution is about: 3.3 ± 0.2 % clay, 5.6 ± 0.5 % slit and 91.6 ± 0.5 % sand (14, 30).
Plantation establishment
10The CSIRO supplied the seedlots, which had originated from natural habitats and from orchards that were dedicated to improvement programs. Each seedlot was separately pretreated with hot water to break seed-coat dormancy and speed up germination (17). The seeds were then sown in a nursery in October 2006. Field-planting took place in February 2007, when seedlings were about 4-months-old, on land that had been previously cleared and where stumps were removed.
Experimental design
11The experiment was a randomized complete block layout, which is the most commonly used design for provenance trials (15). Our study was part of a larger experiment that included 92 provenances from three Acacia species (21 of A. auriculiformis, 24 of A. crassicarpa, and 46 of A. mangium). For each provenance, five trees were planted in rows at 3 m x 3 m spacing within each of the 12 blocks that formed the experimental design.
12Therefore, there were 92 rows (one for each provenance), containing five trees of the same provenance, in each block. The global experimental design contained 5520 trees that were planted over a ~5 ha area; 2760 trees were A. mangium. The plantation was surrounded by two extra rows of Acacia to minimize edge effects. No fertilizer was used during planting and manual weed control was frequently conducted when the plants were young.
13The current study deals with the 46 provenances of A. mangium detailed in Table S1. Given the large number of replicates (i.e., 12), we reduced the size of the initial experimental design and randomly selected five blocks to estimate tree biomass, the amount of C that was stored in tree biomass, and SOC and soil N concentrations.
14Provenance performance was evaluated by measuring two traits linked to productivity: tree height (m) and tree DBH (1.3 m; where DBH [cm] = circumference/π). Height and DBH were measured in August 2013, i.e., 6.5 years after planting. For trees with many stems, each stem was measured and overall tree circumference was obtained by taking the square root of the sum of each circumference-squared, according to the following equation (21): C130 = √(∑n, i Ci2), where C130 is the circumference (cm) at 1.30 m height of the individual with multiple stems, n is the number of stems and Ci is the circumference (cm) for stem i. All stems of A. mangium were measured within each block.
Tree biomass and biomass C
15We destructively sampled the trees and weighed their different components to obtain true biomass. This method, although difficult to conduct on a large scale, is by far the most accurate one for measuring tree biomass (26). We subsequently measured tree biomass and biomass C stocks on five of the 46 A. mangium provenances that were included in the trial. These provenances were selected on the basis of their DBH values. Four (i.e., Nos. 1, 37, 42 and 43) were selected randomly among the five provenances having the highest DBH, and one (No. 7) was chosen randomly from among the following 25 (see Table S2).
16For the five provenances that were selected, an individual was randomly chosen in each of the five blocks. Twenty-five trees were felled at ground level. Branches were cut and separated from the trunk, and the entire root system was manually excavated. Five biomass C compartments were considered: trunk, branches, leaves, litter and roots (26, 34).
17Fresh biomass of these five components was determined on site. Tree litter was harvested at the base of each tree using four 0.5 m2 quadrats that were placed around each individual. Samples of woody biomass were oven-dried at 105 °C to constant mass, which took about a week, while samples of non-woody biomass were oven-dried at 70°C to constant mass, which took about three days. Biomass C concentrations in each reservoir were estimated considering that C mass represents about 50 % of the dry biomass (34).
Soil carbon and nitrogen concentrations
18Soils were sampled at 1 m from the tree stems. We also sampled the soil under savannah vegetation without Acacia at five different locations that were adjacent to the plantation to act as a reference or control for evaluating the effects of A. mangium provenances on SOC and soil N. Soils were manually sampled with an auger at three depths, i.e., 0-10, 10-40 and 40-100 cm. A total of 90 soil samples were taken and sent to the laboratory for the quantification of N and organic C concentrations.
19Soils were air-dried and sieved to pass a 2-mm sieve. Fifteen g of air-dried soil were ground to <10 µm in a mortar and pestle. About 0.3 g and 0.5 g of ground soil was used respectively for N and organic-C analysis. Total soil N concentration was determined by flow-injection following Kjeldahl digestion (31). Soil organic C concentration was determined by dichromate oxidation (37).
Statistical analyses
20Statistical analyses were performed using SAS 9.3 (29). We used the Mixed Procedure in SAS to perform analysis of variance (ANOVA). Data were checked for normality of the residuals and homoscedasticity. Logarithmic transformation (log base 10) was performed to meet ANOVA assumptions, when needed. Soil depth was included in the model as a repeated measure factor. Tukey tests compared and detected differences between provenance means for each of the dependent variables that were measured. Dependent variables included tree height, tree DBH, dry mass and C storage of the whole tree and the five compartments, and SOC and soil N concentrations at each soil depths. Figures were created using Sigmaplot 12.0 (32).
Results
Tree height and DBH
21Means for tree height and DBH of the 46 provenances are presented in Table S2. Significant differences were found both for tree height (F = 2.08, P = 0.0001) and DBH (F = 1.97, P = 0.0004). Among the 20 best provenances for height, seven provenances were from Papua New Guinea (Nos. 20, 19, 18, 32, 37, 34 and 36), seven others from Queensland (Australia) (Nos. 6, 12, 7, 5, 11, 2 and 1), two were from Malaysia (Nos. 42 and 41), one was from Vietnam (No. 46) and one was from the Philippines (No. 43).
22The provenances with the least important heights were from Papua New Guinea (Nos. 25, 26, 24 and 28) and from the Philippines (No. 45). Regarding the DBH, among the 20 best provenances, nine were from Papua New Guinea (Nos. 37, 32, 31, 18, 19, 36, 20, 34 and 33), five were from Queensland (Nos. 2, 1, 10, 12 and 6), two were from Malaysia (No. 42 and 41), one was from Fiji (No. 40), one was from Vietnam (No. 46), and one was from China (No. 39). Provenances with the lowest DBH were from Papua New Guinea (No. 25, 26 and 28) and the Philippines (No. 44 and 45).
Tree biomass and biomass C concentrations
23We found significant differences between provenances for dry mass of the trunk, branches and roots (Fig. 1). No significant difference among provenances was found for either the leaves and the litter dry mass (data not shown). Trunks formed the largest compartment, with 65-75 % of total dry mass (Fig.1). Despite up to 1.6-fold differences in biomass, the trunks of provenances 1, 37, 42 and 43 had statistically similar dry masses, given a high degree of variation (i.e., 440 kg for provenance 43 vs 277 kg for provenance 37). Trunk mass for provenance 7 (i.e., 112 kg) was significantly lower than that of the others.
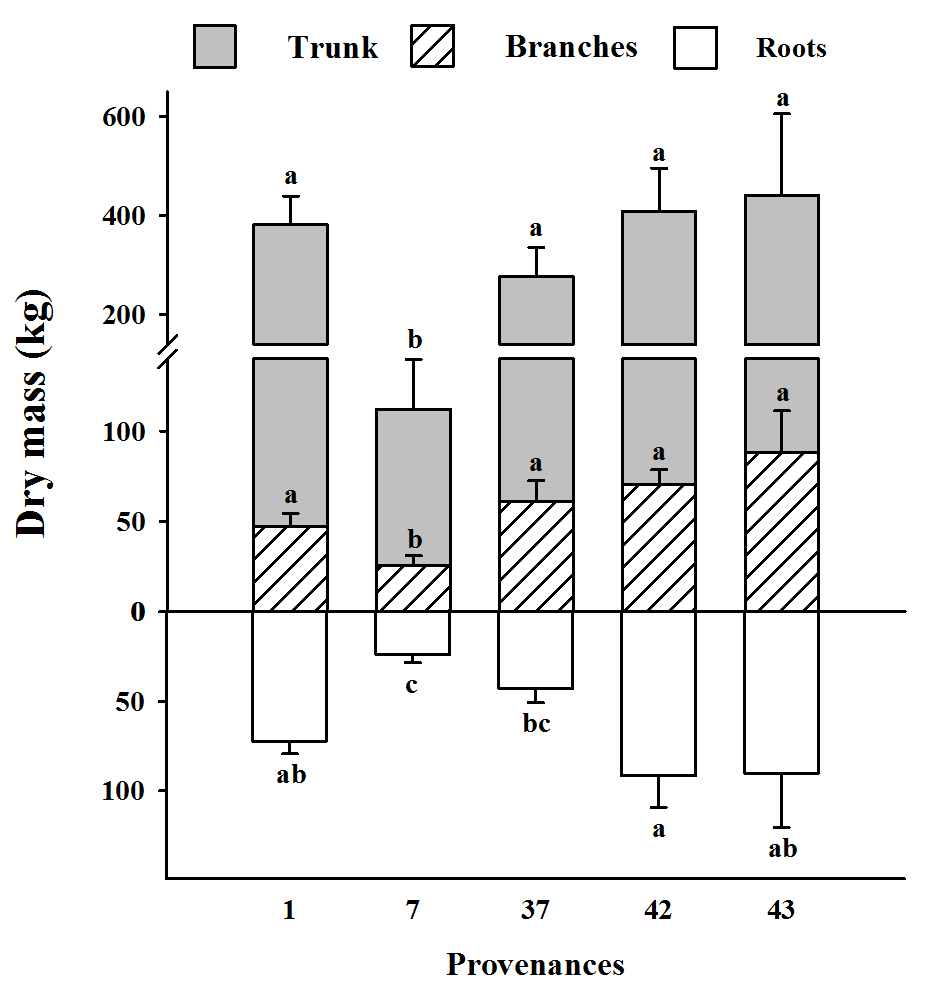
Figure 1: Dry mass of the roots, trunks and branches for each of the provenances
Means labelled with the same letter are not significantly different from one another following Tukey's multiple comparison tests at P < 0.05; measures of dispersion correspond to standard errors
24A similar pattern was observed for branch dry mass; there was no significant difference among provenances 1, 37, 42 and 43, despite 1.9-fold differences among treatment means (i.e., 88 kg for provenance 43 versus 47 kg for provenance 1), and a significantly lower value for provenance 7 with 26 kg. Provenances 42 and 43 had the highest root mass (~91 kg), although this did not differ from that of provenance 1 (72 kg). The lowest root masses were found for provenance 7 and 37, i.e., 24 kg and 43 kg, respectively.
25The differences found among tree compartments also reflected those of total tree biomass (Fig. 2).
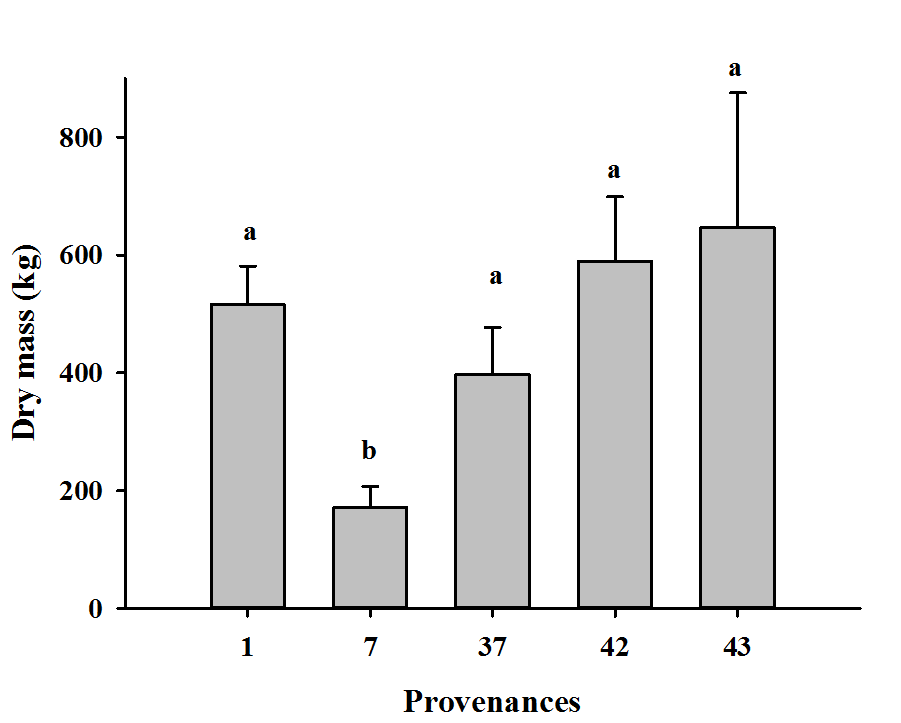
Figure 2: Dry mass of whole trees by provenance
Means labelled with the same letter are not significantly different from one another following Tukey's multiple comparison tests at P < 0.05; measures of dispersion correspond to standard errors
26Results showed that only provenance 7 had a total tree biomass (i.e., 171 kg) that was statistically lower than the others. Although differences among provenances were not statistically significant, provenance 43 produced the greatest biomass, with 649 kg compared to 590, 516 and 397 kg for provenances 42, 1 and 37, respectively. Similarly, quantities of C that was stored in tree biomass of provenances 43, 42, 1 and 37 did not differ significantly, but were greater than the quantity that was stored in provenance 7 (Table 3).
27Considering that planting are spaced at 3 x 3 m, one hectare of A. mangium would contain ~1000 trees. Consequently, plantations of provenances 1, 37, 42 and 43 could store 179, 232, 265 and 291 Mg C ha-1 in total tree biomass, respectively.
Table 3: Means carbon stocks of trees for each provenance
|
Provenance |
Moyenne (kg) |
|
Prov. 1 |
231,80 (29,56)a |
|
Prov. 7 |
77,00 (16,19)b |
|
Prov. 37 |
178,80 (35,57)a |
|
Prov. 42 |
265,40 (49,29)a |
|
Prov. 43 |
291,00 (102,85)a |
Means sharing the same letter are not statistically different (Tukey P 0.05). The values in parentheses correspond to the standard error
Soil organic C and total N concentrations
28Soil organic C concentrations significantly differed among provenances and the control (savannah) soil at 10–40 cm depth (F = 3.90, P = 0.012) and 40–100 cm depths (F = 6.12, P = 0.001) (Fig. 3). At 10–40 cm depth, provenance 37 had the highest SOC concentration, i.e., 14.8 g SOC kg-1 soil, while provenance 43 had the lowest, i.e., 7.9 g SOC kg-1 soil.
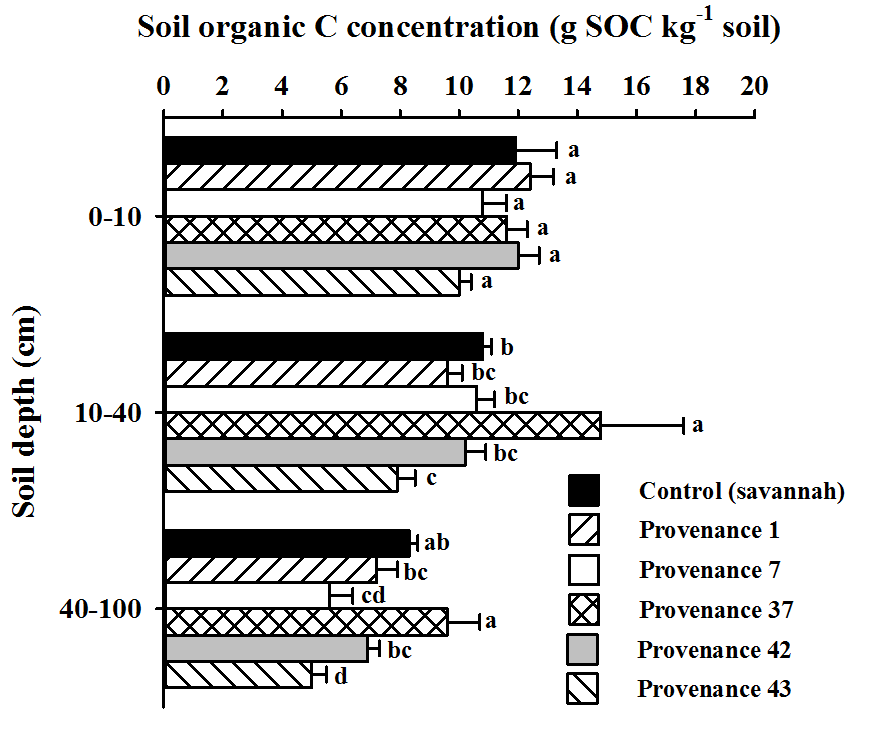
Figure 3: Soil carbon concentrations under the provenances, at 3 soil depths
Means labelled with the same letter are not significantly different from one another following Tukey's multiple comparison tests at P < 0.05; measures of dispersion correspond to standard errors
29Results for soils beneath other provenances, together with savannah soils without A. mangium, were not statistically different. Provenance 37 had the highest SOC concentration at 40-100 cm depth (9.6 g SOC kg-1 soil), although it was not statistically different from savannah soils without Acacia (8.3 g SOC kg-1 soil). The lowest SOC concentration was found under provenance 43 (5.0 g SOC kg-1 soil).
30Soil total N differed with depth (Fig. 4). At 0–10 cm depth (F = 3.88, P = 0.013), the highest soil N concentrations were found in the savannah soils without A. mangium and under provenance 42; these averaged about 0.60 g N kg-1 soil. At 10-40 cm depth (F = 5.69, P = 0.002), savannah soil without A. mangium and the soils under provenances 37 and 1 had the highest N concentrations, which were about 0.47 g N kg-1 soil.
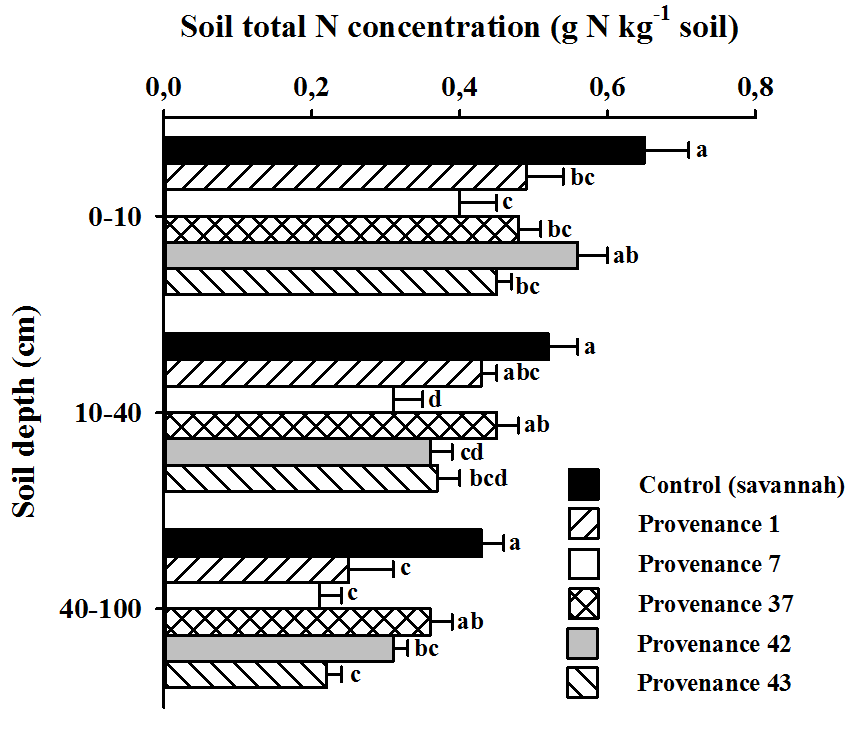
Figure 4: Soil nitrogen concentrations under the provenances, at 3 soil depths
Means labelled with the same letter are not significantly different from one another following Tukey's multiple comparison tests at P < 0.05; measures of dispersion correspond to standard errors
31Differences were also noted for soil N concentrations in the deepest soil layer, i.e., 40-100 cm depth (F = 6.62, P = 0.0009). Again, soils under savannah and provenance 37 had the highest N concentrations, i.e., 0.40 g N kg-1 soil. Provenance 7 had the lowest N concentrations at each soil depth that was investigated.
32Soil organic C and total N concentrations decreased with soil depth.
33The decrease was least pronounced for provenance 37, where SOC and soil total N concentrations at 40-100 cm depth were respectively 83 and 75 % of those that were determined at 0-10 cm depth, and 69 and 66 % of that measured for the savannah soil. For other provenances, SOC and total N concentrations in the deepest soil layer were 50 to 60 % of those determined in the surface soil layer.
Discussion
Tree height and DBH
34Our results indicate that 7 years after plantation establishment, provenances with the greatest tree height and DBH originated from Papua New Guinea, Queensland (Australia), Malaysia, Vietnam, Fiji, China and the Philippines, although there are differences in the performance of provenances originating from the same regions. Evaluation of provenance trials by Salazar and Mesen (28), and Leksono and Rosiawan (22) that were conducted in Costa Rica and Indonesia, respectively, showed better results for provenances from Queensland, particularly from Claudie River, together with those from Papua New Guinea, such as Oriomo River.
35Harwood and Williams (11) surveyed many international trials of A. mangium that had been established on 19 sites located in South Asia, Australia and Fiji. They noted that the most successful provenances were from Papua New Guinea and from Queensland, particularly Claudie River in the latter case.
36Other studies that were conducted on different experimental sites also recorded remarkable success for provenances from Papua New Guinea (12, 25, 33). Khasa et al. (18) evaluated many provenance trials of A. auriculiformis and A. mangium that had been established in the Democratic Republic of Congo, particularly on the Batéké Plateau.
37They noted that 21 months after planting, a general tendency towards greater success for A. mangium provenances that originated from Papua New Guinea. Superiority of these provenances could be explained by similarity of the climate of Ibi to the temperature regimes and rainfall of the source regions from which they originated. The geographical distribution of these provenances (see Table S1) was restricted to latitudes of 7°20’ and 16°45’S and longitudes of 141°15’ and 143°05’ E, in which the annual average rainfall is between 1400 and 2400 mm (6). Nevertheless, the least successful provenances were also from Papua New Guinea and the Philippines, suggesting substantial genetic variation both within- and among-populations (16). Their less successful performance could be explained by other factors, given that they were not well adapted to the soils of the Batéké Plateau.
38More information on edaphic conditions of the original sites of these provenances would be required, however, to explain their poor performance. Still, differences that were observed in the performance of provenances originating from the same region supports the need to proceed with trials when planning on using exotic species for plantations (7, 12). Moreover, the superiority of some provenances, such as 42, 43 and 37, could be explained by their plantation and seed orchard origins, which were dedicated to forest seed improvement programs and, therefore, which correspond to a certain level of genetic improvement.
394.2 Tree biomass and biomass C storage
40Tree trunks are the compartments that contributed most to total biomass, with an average contribution of 69.7 %. In contrast, branches and roots contributed respectively about 12.6 % and 13.8 % to total biomass. Bernhard-Reversat et al. (2) determined the root contribution of A. mangium was about 15.9 % of total tree biomass, which is very close to our findings. When averaged across provenances, our results indicate that 7-years old A. mangium could produce, on average, 464 kg of dry biomass for an average DBH of 23.8 cm. Bernhard-Reversat et al. (2) measured the biomass of A. mangium in Congo-Brazzaville for different circumference classes in ~7-year-old plantations that were growing under environmental conditions that were similar to those encountered in the present study. The three largest circumference classes in the aforementioned study were 72 cm, 83 cm and 94 cm; the last value corresponds to DBH = 29.9 cm.
41According to their research, trees belonging to this circumference classes could produce 380 kg of dry biomass. Our results are slightly higher, but sufficiently close to those obtained by Bernhard-Reversat et al. (2). However, we found significant differences between provenances with respect to total biomass production and biomass C storage (Figs. 2 and 3; Table 3), thereby confirming our first hypothesis. Provenance 7 produced the lowest total biomass and consequently stored the lowest quantity of C, whereas total biomass production and biomass C storage did not differ among the four other provenances.
42Most A. mangium biomass studies have used allometric equations to estimate tree biomass. The diversity of results reported by these various studies underscores not only the importance of choosing the appropriate biomass assessment method, but also the roles that site conditions, age and planting density play (7,13, 38).
Soil carbon and nitrogen concentrations
43Results showed significant differences in SOC and total soil N concentrations among provenances at different soil depths. We also noted a general trend towards decreasing SOC and soil N concentrations with depth, except for provenance 37. In this instance, SOC accumulated at 10-40 cm depth. Bernhard-Reversat (3) found results that were similar to our own in his study at Pointe Noire, which was located in a similar savannah.
44The values of carbon and nitrogen contents for Acacia mangium for 6- to 7-year-old and 7- to 9-year-old age classes were respectively 7 g SOC kg-1 and 0.46 g N kg-1 and 9.3 g SOC kg-1 and 0.58 g N kg-1 at 0-10 cm depth. In his study, nitrogen and carbon contents of natural vegetation and Acacia auriculiformis were not statistically different from those for Acacia mangium. Our results agreed with Sente (30), who investigated SOC and total soil N concentrations in the same soils and for trees that were the same age as ours, but for A. auriculiformis A. Cunn. ex Benth.
45Concentrations were 10.5 g SOC kg-1 and 0.67 g N kg-1, and 6.8 g SOC kg-1 and 0.41 g N kg-1 at 0-25 cm and 25-50 cm depths, respectively (30). In contrast, Kasongo et al. (14) sampled soils of the Batéké Plateau under 8-year-old A. auriculiformis and reported higher SOC and total soil N concentrations than those in the present study. Their average values were 18.7 g SOC kg-1 and 1.78 g N kg-1, and 9.7 g SOC kg-1 and 0.8 g N kg-1 at 0-25 cm and 25-50 cm depths, respectively.
46The two plantations of these studies succeeded a fallow rotation of Acacia auriculiformis, which was followed by one or two years of cultivation of maize (Zea mays L.) and cassava (Manihot esculenta Crantz). Apart from small differences in age, differences between the values that were obtained could be related to differences in planting density, which is correlated with litter decomposition (20).
47Planting density was higher in the study that was conducted by Sente (30) than that conducted by Kasongo et al. (14), i.e., about 1500 trees per hectare versus 833 trees per hectare. Other studies that were conducted elsewhere, and which were reporting on the effect of A. mangium on tropical soil properties, reported similar or higher SOC and total soil N concentrations; Bini et al. (5), in São Paulo, Brazil and Yamashita et al. (36), in Sumatra, Indonesia.
48As previously mentioned, such differences could be explained not only by age and plantation management, but also by differences in soil texture. Soils that were investigated by Bernhard-Reversat (3) were sandy soils, while those investigated by Bini et al. (5) and Yamashita et al. (36) had higher clay contents. A greater percentage of clay in the soil increases its capacity to retain organic matter; small differences in the quantity of clay could thus translate into greater differences in the quantities of SOC and soil N that are retained in the soil (3, 10).
49We found that SOC concentrations at 10–40 and 40–100 cm depths were the greatest under provenance 37. In contrast, SOC concentrations at the same depths were observed under provenance 43, even if the latter produced greater root biomass. The pattern similar for soil N, except that differences between 37 and the other provenances were less pronounced. Soils under savannah vegetation contained N concentrations that were similar to that under provenance 37. These results suggest that provenance 37 has significant carbon storage potential in soils, even at depth.
50These results also suggest differences in the functional traits of provenances, in particular the C/N ratio of the litter and its hemicellulose, cellulose and lignin contents, which could explain the differences in decay rates between provenances (1, 8). Several authors have suggested that the low litter decomposition rate under A. mangium was a factor limiting the capacity of this species to restore soil organic matter (4, 20).
51The savannah soils (without A. mangium) exhibited N and SOC concentrations that were similar to those planted with A. mangium. This is surprising considering that Acacia mangium is a leguminous tree, which is considered to be capable of restoring soil fertility. Contributions of biologically fixed-N2 to soil N concentrations after 7 years, therefore, would appear to be negligible. However, the soil was highly disturbed prior to plantation establishment; site preparation operations consisted of cutting down and removing trees and other woody vegetation, setting fires for stump removal, and light ploughing of the soil before planting the trees (30).
52These practices likely caused a reduction in soil organic matter concentrations and disturbance in the natural habitat for numerous plants and soil microorganisms, especially those in the surface soil layers (10, 27). Savannah soils from our study are colonized by perennial grasses that contribute to the restoration of their fertility (9). They did not undergo the same treatment that disturbed the soils under our provenance plantations. We hypothesize that disturbance of the soil could explain, in part, the non-significant effect of planting A. mangium on SOC and soil N concentrations compared to the savannah soils.
53Nevertheless, our results indicate that despite initial disturbance of the site at planting, A. mangium was capable of restoring soil fertility to a level comparable to that of the undisturbed savannah soil. Consequently, more than 7 years are required prior to tree harvesting to ensure efficient C storage in the soil and biomass, and to restore soil fertility.
Conclusion
54This objective of this study was to evaluate the performance of 46 provenances of Acacia mangium that had been established in Ibi. Although it was based upon a limited number of observations, this evaluation has demonstrated that differences existed among provenances in terms of height and DBH growth, and that there was a general trend towards better performance for provenances from Papua New Guinea, followed by those from Queensland (Australia), and those from Malaysia, Vietnam, Fiji and China.
55This study has also showed that differences among provenances were apparent for biomass and carbon stocks, as well as N and C concentrations in the soils. SOC concentrations were particularly high, even at depth, under provenance 37. The results of this study suggested that further research in A. mangium plantations should focus on quantifying rates SOC and soil N accumulation, while determining their levels of stability at different depths in the soil. The results suggest further research on the differences between functional traits of the different provenances.
Bibliographie
-
Bachega L.R., Bouillet J.P., de Cássia Piccolo M., Saint-André L., Bouvet J.M., Nouvellon Y., Gonçalves J.L.M., Robin D. & Laclau J.P., 2016, Decomposition of Eucalyptus grandis and Acacia mangium leaves and fine roots in tropical conditions did not meet the Home Field Advantage hypothesis. Forest Ecol. Manag. 359, 33-43.
-
Bernhard-Reversat F., Diangana D. & Tsatsa M., 1993, Biomasse, minéralomasse et productivité en plantation d’Acacia mangium et A. auriculiformis au Congo. Bois For. Trop. 238, 35-44.
-
Bernhard-Reversat F., 1996, Nitrogen cycling in tree plantations grown on a poor sandy savannah soil in Congo. Appl. Soil Ecol. 4(2), 161-172.
-
Bernhard-Reversat F., Harmand J.M. & Uguen K., 1998, Les litières et la dynamique de l’azote dans divers biotopes à Acacia d’Afrique occidentale pp. 205-219. In : L’acacia au Sénégal. Campa, C., Grignon, C., Gueye, M., Hamon, S. (Eds). ORSTOM, ISRA. Paris (Collection colloques et séminaires) ISBN 2-7099-1423-9.
-
Bini D., Dos Santos C.A., Bouillet J.P., de Morais Gonçalves J.L. & Cardoso E.J.B.N., 2013, Eucalyptus grandis and Acacia mangium in monoculture and intercropped plantations: Evolution of soil and litter microbial and chemical attributes during early stages of plant development. Appl. Soil Ecol. 63, 57-66.
-
Boland D.J., Brooker M.I.H., Chippendale G.M., Hall N., Hyland B.P.M., Johnston R.D., Kleinig D.A., McDonald M.W. & Turner J.D. (Eds.), 2006, Forest Trees of Australia, 5th edition, Forests and Forestry Australia, CSIRO Publishing, pp 164-165.
-
Coleman J.S., McConnaughay K.D. & Ackerly D.D., 1994, Interpreting phenotypic variation in plants. Trends Ecol. Evol. 9(5), 187-191.
-
De Deyn G.B., Cornelissen J.H.C. & Bardgett R.D., 2008, Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 11(5), 516-531.
-
Dugue P., 1998, Les graminées pérennes : Des plantes utiles pour des aménagements anti-érosifs en zone de savane. Le Flamboyant 48, 23-28.
-
Feller C., Fritsch E., Poss R. & Valentin C., 1991, Effet de la texture sur le stockage et la dynamique des matières organiques dans quelques sols ferrugineux et ferralitiques (Afrique de l’Ouest, en particulier). ORSTOM Série Pédologie 26(1), 25-36.
-
Harwood C.E. & Williams E.R., 1992, A review of provenance variation in growth of Acacia mangium. Aciar. Proc. 37, 22-30.
-
Harwood C.E., Aplegate G., Robson K. & Williams A.R., 1993, Early growth of provenances and progenies in Acacia mangium seed production areas in North Queensland, Australia. Acacias for Rural, Industrial and Environmental Development. FAO and Winrock International, Bangkok 113-122.
-
Heriansyah I., Miyakuni K., Kato T., Kiyono Y. & Kanazawa Y., 2007, Growth characteristics and biomass accumulations of Acacia mangium under different management practices in Indonesia. J. Trop. For. Sci. 19(4), 226-235.
-
Kasongo R.K., Van Ranst E., Verdoodt A., Kanyankagote P. & Baert G., 2009, Impact of Acacia auriculiformis on the chemical fertility of sandy soils on the Batéké plateau, DR Congo. Soil Use Manage. 25(1), 21-27.
-
Kemp R.H., 1977, Les essais de provenances [Provenance trials (in African Savannah)]. Unit of Tropical Silviculture, Commonwealth Forestry Institute, Oxford, UK. Boisement des savanes en Afrique, FAO (Food and Agriculture Organization) of the United Nations, Rome. pp 53-58.
-
Khasa P.D., Cheliak W.M. & Bousquet J., 1994a, Genetic variation in 26 populations of Racosperma auriculiforme and Racosperma mangium using allozymes. Can. J. Forest Res. 24(6), 1123-1132.
-
Khasa P.D., Vallée G. & Bousquet J., 1994b, Biological considerations in the utilization of Racosperma auriculiforme and Racosperma mangium in tropical countries with emphasis on Zaire. J. Trop. For. Sci. 6(4), 422-443.
-
Khasa P.D., Li P., Vallée G., Magnussen S. & Bousquet J., 1995, Early evaluation of Racosperma auriculiforme and R. mangium provenance trials on four sites in Zaire. Forest Ecol. Manag. 78(1-3), 99-113.
-
Köppen W., 1936, Das geographische System der Klimate. In: Köppen, W., Geiger, R. (eds) Handbuch der Klimatologie, 9-15.
-
Kunhamu T.K., Kumar B.M. & Viswanath S., 2009, Does thinning affect litterfall, litter decomposition, and associated nutrient release in Acacia mangium stands of Kerala in peninsular India ? Can. J. Forest Res. 39(4), 792-801.
-
Lejeune P. & Rondeux J., 1994, Modèle de cubage pour essences multi-tiges : application à des plantations d’acacia. Cah. Agric. 3, 189-194.
-
Leksono B. & Rosiawan H., 1997, Evaluasi uji provenansi Acacia mangium umur 30 bulan di Kampar km, Riau. [Evaluation of provenance test of 30-month-old Acacia mangium in Kampar km, Riau; In Bihasa Indonesia with English abstract]. Buletin Kehutanan [Forestry Bulletin] (32), 15-22.
-
National Research Council, 1983, Mangium and other Acacias of the Humid Tropics, Innovations in Tropical Reforestation. National Academy Press, Washington, DC, 64 p.
-
NOVACEL, 2006, Puits de carbone d’Ibi en République Démocratique du Congo, Plantation forestière dans la savane du plateau des Batéké. Note de présentation technique et financière, Nouvelles Sociétés d’Agriculture, Cultures et Élevage, 27 p.
-
Otsamo A., Ådjers G., Hadi T.S., Kuusipalo J. & Vuokko R., 1997, Evaluation of reforestation potential of 83 tree species planted on Imperata cylindrica dominated grassland–A case study from South Kalimantan, Indonesia. New Forests 14(2), 127-143.
-
Picard N., Saint-André L. & Henry M., 2012, Manuel de construction d’équations allométriques pour l'estimation du volume et la biomasse des arbres. CIRAD, FAO, Rome, Italie, 211p.
-
Poirier V., Angers D.A., Rochette P., Chantigny M.H., Ziadi N., Tremblay G. & Fortin J., 2009, Interactive effects of tillage and mineral fertilization on soil carbon profiles. Soil Sci. Soc. Am. J. 73(1), 255-261.
-
Salazar R. & Mesen F., 1991, Análisis de procedencias de Acacia mangium en Costa Rica. For. Syst. 1, 213-216.
-
SAS Institute, 2008, SAS System for PC, Version 9.2. SAS Institute Inc., Cary, NC, USA.
-
Sente A., 2011, Impact de l’Acacia auriculiformis sur les propriétés des sols sableux du plateau Batéké, République Démocratique du Congo. Mémoire de maîtrise, Faculté d’ingénierie biologique, agronomique et environnementale, Université catholique de Louvain, Louvain-la-Neuve, Belgium, 98 p.
-
Stewart J.W.B., Ruzicka J., Bergamin Filho H. & Zagatto E.A., 1976, Flow injection analysis: Part III. Comparison of continuous flow spectrophotometry and potentiometry for the rapid determination of the total nitrogen content in plant digests. Anal. Chim. Acta 81(2), 371-386.
-
Systat Software, 2013, Sigmaplot for PC, Version 12.0. Systat Software Inc., San Jose, CA, USA.
-
Tuomela K., Otsamo A., Kuusipalo J., Vuokko R. & Nikles G., 1996, Effect of provenance variation and singling and pruning on early growth of Acacia mangium Willd. plantation on Imperata cylindrica (L.) Beauv. dominated grassland. Forest Ecol. Manag. 84(1), 241-249.
-
Walker W., Baccini A., Nepstad M., Horning N., Knight D., Braun E. & Bausch A., 2011, Field Guide for Forest Biomass and Carbon Estimation. Version 1.0, Woods Hole Research Center, Falmouth, MA, USA, 43-49.
-
WRB (World Reference Base), 2006, World Reference Base for soil resources 2006, 2nd ed. World Soil Resources Report No. 103, FAO, Rome
-
Yamashita N., Ohta S. & Hardjono A., 2008, Soil changes induced by Acacia mangium plantation establishment: comparison with secondary forest and Imperata cylindrica grassland soils in South Sumatra, Indonesia. Forest Ecol. Manag. 254(2), 362-370.
-
Yeomans J.C. & Bremner J.M., 1988, A rapid and precise method for routine determination of organic carbon in soil. Comm. Soil Sci. Plan. 19(13), 1467-1476.
-
Zhang H., Guan D. & Song, M., 2012, Biomass and carbon storage of Eucalyptus and Acacia plantations in the Pearl River Delta, South China. Forest Ecol. Manag. 277, 90-97.
Pour citer cet article
A propos de : Etienne Yusufu Kachaka
Doctorant en sciences forestières à l’Université Laval et Chef de travaux à l’Université de Kinshas. Centre d'étude de la forêt, et Département des Sciences du bois et de la forêt, Université Laval, 2405 rue de la Terrasse, Québec, QC G1V 0A6, Canada. Département de gestion des ressources naturelles, Faculté des sciences agronomiques, Université de Kinshasa, Kinshasa XI, République démocratique du Congo. Corresponding Author: etienne-yusufu.kachaka.1@ulaval.ca,
A propos de : Vincent Poirier
Professeur AssociéUnité de recherche et de développement en agroalimentaire, Université du Québec en Abitibi-Témiscamingue, 79 rue côté, Notre-Dame-du-Nord, Québec, QC J0Z 3B0, Canada.
A propos de : Alison D. Munson
Professeure TitulaireCentre d'étude de la forêt, et Département des Sciences du bois et de la forêt, Université Laval, 2405 rue de la Terrasse, Québec, QC G1V 0A6, Canada
A propos de : Damase P. Khasa
Professeur Titulaire, Centre d'étude de la forêt, et Département des Sciences du bois et de la forêt, Université Laval, 2405 rue de la Terrasse, Québec, QC G1V 0A6, Canada.