- Home
- Volume 43 (2025)
- Valorization of water hyacinth (Eichhornia crassipes) leaves in the diet of Clarias gariepinus fry: Digestibility, zootechnical performances and incorporation rate
View(s): 182 (3 ULiège)
Download(s): 0 (0 ULiège)
Valorization of water hyacinth (Eichhornia crassipes) leaves in the diet of Clarias gariepinus fry: Digestibility, zootechnical performances and incorporation rate

Abstract
The valorization in terms of digestibility and incorporation rate into the diet of Clarias gariepinus was the subject of this study. The Apparent Digestibility Coefficients for dry matter, proteins and lipids (ADCDM, ADCCP and ADCCL) were determined for the experiment diet and with water hyacinth leaves, they are respectively as follows: ADCDM= 80.33%; ADCCP= 80.02%; ADCCL= 86.54% and ADCDM= 75.65%, ADCcP= 77.4%; ADCcL= 78.14%. Concerning the determination of the optimal rate of incorporation of water hyacinth leaves in the diet of C. gariepinus, 6 iso-protein and iso-lipidic treatments (T0%, T2%, T4%, T6%, T8%, WH), including a reference consisting of an imported brand feed "Gouessant" were formulated. The zootechnical parameters varied significantly (p < 0.05). The Daily Weight Gain (DWG) varied from 5.68±0.21 g (T5) to 8.40±0.19 g (T0). the Feed Conversion Rate (FCR) and the Protein Efficiency Rate (PER) varied respectively from 2.34±0.11 (T5) to 1.14±0.09 (T0) and from 1.26±0.14 (T5) to 2.38±0.11 (T0). For the Survival Rate (SR), no significant difference was observed (p > 0.05). The evaluation of the Specific Growth Rate and Feed Efficiency according to the treatments show that after 4% incorporation (6% and 8%) there is a considerable drop of zootechnical parameters.
Table of content
Introduction
1In 2022, global aquaculture production reached a record high of 130.9 million tonnes, comprising 94.4 million tonnes of aquatic animals and 36.5 million tonnes of algae, an increase of 6.6% compared to 2020 (FAO, 2024). For the first time in history, according to the same source, the production of farmed animal species (51%) surpassed that of fisheries. This growth is primarily attributed to fish farming, as fisheries production has remained relatively constant since the late 1980s (FAO, 2024). This rise in production has allowed the average consumption per person per year to increase from 9.0 kg in 1961 to 20.7 kg in 2022 (FAO, 2024). Despite the fact that aquaculture is booming nowadays, aquacultural production remains insufficient compare to its demand. This situation hinders the profitability of aquaculture production. To remedy this, aquaculture farms and aquaculture research institutions are trying to find alternatives to reduce the cost of feed, which can go up to 75% of aquaculture expenses. Among these alternatives are the use of several local animal or vegetable by-products as sources of protein, lipids, energy and others (Abou et al., 2007a; Akadiri et al., 2022; El-Saidy et al., 2003; Richter et al., 2003; Vodounnou et al., 2025a). The use of macrophytes such as algae, ferns and aquatic plants is also an alternative due to their nutritional values. For example, we have the use of azolla (Abou et al., 2010; Djissou et al., 2017) in are fish feed, of Lemna minor (Nor et al., 2019). Among these macrophytes, there is the Water hyacinth (WH) (Eichhornia crassipes) which is a very invasive aquatic plant originally from South America and which has spread all over the world especially in Africa (Dagno et al., 2007; Tchiaze & Priso 2016). Many studies have been conducted on this plant for its eradication because of the damage of its proliferation on an aquatic ecosystem (Tchiaze & Priso 2016, Vodounnou et al., 2024). Although water hyacinth is considered as weed in many countries and responsible for many problems, some studies have managed to turn this threat of water hyacinth proliferation into an opportunity and find useful application of this plant. Water hyacinth is an aquatic plant rich in mineral elements and has some significant properties. For this reason, it is used for various purposes. It plays a purifying role in wastewater treatment station. In agriculture, it is used as a soil amendment for agricultural production. In addition, it is used as a protein supplement in the feed of ruminants and rabbits. This plant can also be used in the production of biofuel (Shanab et al., 2018). In aquaculture, few studies have addressed its use as aquaculture ingredients (Hontiveros & Serrano, 2015; Saha & Ray, 2011). But the incorporation of an ingredient in feed requires information on its bromatological composition, its optimal rate of incorporation and its digestibility (Vodounnou et al., 2025b). This is crucial in order to assess the ability of the ingredient to be valued by an animal organism in terms of availability and use of nutrients. The provided digestibility gives information on the utilization of the nutrients in an ingredient or a feed by an animal and constitutes the first step in the evaluation of the nutritional quality of this feed (Luo et al., 2009; Rahman et al., 2016). It therefore permits during the process of digestion to reduce macronutrients (carbohydrates, proteins, lipids) into micronutrients (Carbohydrates, amino acids, fatty acids and glycerols) and their passage through the intestinal villi to the blood medium. Digestibility depends not only on the animal species but also on the animal's diet (Fayeofori & Bob, 2013). Clarias gariepinus is a widespread omnivorous species in aquaculture farms in Africa. It is a species very resistant to environmental fluctuations with an opportunistic diet. This is what justifies the valorization of water hyacinth in its diet, thence this research on the valorization of the leaves of Water Hyacinth (Eichhornia crassipes) in the diet of Clarias gariepinus fry. This study aims on the one hand to fight against the proliferation of water hyacinth by giving it a nutritional utility which will be the object of its frequent harvesting in the natural environment and on the other hand to study the use of feeds containing Water Hyacinth by Clarias gariepinus through its digestibility and the expression of the zootechnical performance of fish fed with these feeds.
Material and methods
2This study was conducted in the Aquaculture and Fisheries Management Research Unit (URAGeP) of the School of Aquaculture (EAq) at the National University of Agriculture (UNA) located in the center of the department of Oueme in Benin Republic. The leaves of Water Hyacinth were chosen following a nutritional analysis of the different parts of this plant (roots, stems and leaves). Parameters such as proteins, ashes, lipids and fibers (Table 1) were searched in each part of WH in order to choose the part with the best nutritional value. The WH was collected on the banks of the Ouémé River and stripped of stems and roots. The leaves obtained were dried in the sun for 5 days before grinding.
Table 1: Nutritional composition of water hyacinth from the Ouémé River, Benin
|
Parameters |
Plant part |
||
|
Leaves |
Root |
Stem |
|
|
Lipids (%) |
0.37 |
0.14 |
0.29 |
|
Ashes (%) |
17.66 |
39.32 |
20.55 |
|
Proteins (%) |
14.30 |
6.40 |
6.47 |
|
Fibers (%) |
15.04 |
10.71 |
22.76 |
Digestibility test
Preparation of diets
3WH leaves were incorporated into the reference diet. The reference diet (Table 2) was formulated base on the nutritional need of Clarias gariepinus (Uys, 1989; Wilson & Moreau Y,1996). These diets contain 1% Chromic oxide (Cr2O3) as an inert marker. The experiment diet was composed of 70% of the reference diet and 30% of the ingredient tested (water hyacinth). The various ingredients used were ground and mixed dry. To this mixture are added red palm oil and water before extrusion. The food produced was 2 mm in diameter. The food thus produced was packaged in boxes and stored in the refrigerator at a temperature of 5°C.
Table 2: Diet composition (%) for digestibility test
|
Ingredients (%) |
R0 (%) |
R1 (%) |
|
Fish meal |
30 |
_ |
|
Chicken viscera meal |
20 |
_ |
|
Soyabean meal |
15 |
_ |
|
Cottonseed meal |
13 |
_ |
|
Wheat bran |
9 |
_ |
|
Corn flour |
4 |
_ |
|
Moringa leaves |
1 |
_ |
|
Starch |
2 |
_ |
|
Concentrated Methionine |
0.5 |
_ |
|
Concentrated Lysine |
0.5 |
_ |
|
Iron Sulphate |
0.5 |
_ |
|
Dicalcium Phosphate |
0.5 |
_ |
|
Premix (Vit+ Min) * |
1.5 |
_ |
|
Palm oil |
1.5 |
_ |
|
Cr2O3 |
1 |
_ |
|
R0 |
_ |
70 |
|
WH leaves |
_ |
30 |
|
Total (%) |
100 |
100 |
R0: reference diet with Chromic oxide (Cr2O3); R1: test diet
* premix (vitamin – mineral) contains (‰):Vitamin A 4,000,000 U.I; Vitamin D 800,000 IU; Vitamin E 40,000IU; Vitamin K3 1600mg; Vitamin B1 4000mg; Vitamin B2 3000mg; Vitamin B6 3800mg; Vitamin B12 3mg; Vitamin C 60,000mg; Biotin 100mg; Inositol 10,000mg Pantothenic acid 8,000mg; Nicotinic acid 18,000mg; Folic acid 800mg; Choline chloride 120,000mg; Colbat carbonate 150mg; Ferrous sulphate 8000mg; Potassium iodide 400mg; Manganese oxide 6000mg; Copper 800mg; Sodium selenite 40 mcg; Lysine 10,000mg; Methionine 10,000mg; Zinc Sulphate 8000mg
Feces collection
4The collection of feces began 3 days after the start of the experiment in order to allow the fish to completely evacuate the digestive content of the feed previously consumed (Koprucu & Ozdemir, 2005; Vodounnou et al., 2025b). The collection of feces was carried out by manual stripping twice a day. The collected feces were immediately stored in the freezer at a temperature of -20°C.
Chemical analyzes of diets and feces
5Diets and feces were analyzed. Proteins, lipids and dry matter were measured in all diets as well as feces. The standard method AOAC, 1990 (Association of Official Analytical Chemists) was used. The determination of chromic oxide in diets and feces was done according to the method described by Furukawa & Tsukahara, 1966.
Experimental conditions and conduct of the experiment
6For the digestibility trial, a total of six rectangular-shaped aquariums with a water volume of 60 liters each were used. The experimental device is composed of a random Fisher block of two treatments with three repetitions each. C. gariepinus fry with an average individual weight of 6.38±0.09 g was used. The stocking density was twenty fry per tank, one fry for 2.5 L The fries were fed three times a day to apparent satiety for 35 days. The amount of feed consumed per day is calculated at the end of the day. The physicochemical parameters (temperature, dissolved oxygen and pH) were recorded every day mornings and evenings to assess the quality of the water.
Digestibility parameters
7The Apparent Digestibility Coefficients (ADC) in dry matter, protein and lipid of feed nutrients and Water Hyacinth leaves for C. gariepinus fry were determined according to the equation of Cho et al.,1985.
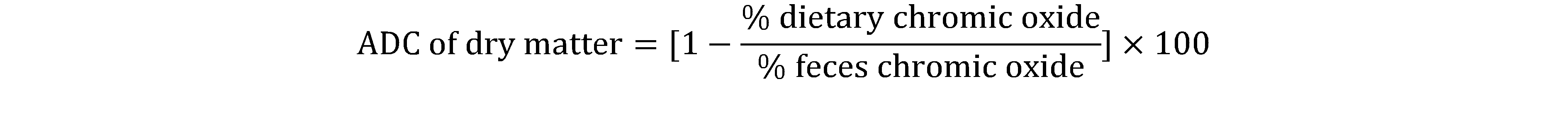
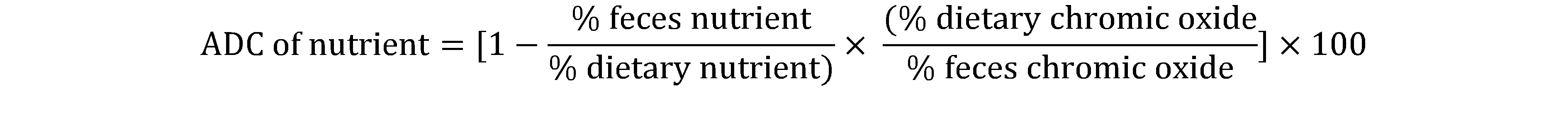
8The ADC of the test ingredients was calculated based on the digestibility of the Reference Diet and Test Diet using the equation (Forster, 1999):
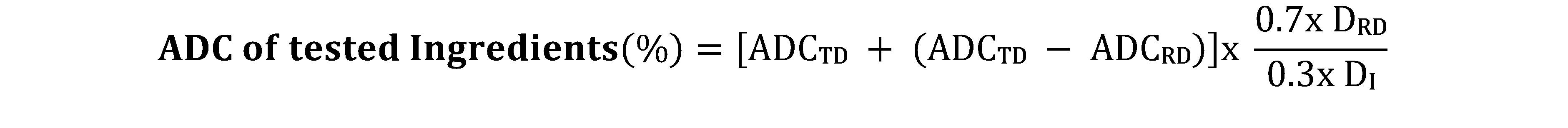
Where:
ADCTD = ADC of Test Diet
ADCRD = ADC of Reference Diet
DRD = % nutrient of the Reference Diet
DI = % nutrients of the test Ingredients.
Optimal rate of incorporation and zootechnical performance
Formulation and diet preparation
9The diets (Table 3) were formulated considering the nutritional need of Clarias gariepinus (Uys, 1989; Wilson & Moreau, 1996). A total of 6 iso-protein, iso-lipid and iso-energy experimental diets were formulated. The experimental diets consist of 4 diets containing the Water Hyacinth leaves, a control diet without the Water Hyacinth leaves and a reference diet consisting of an imported feed brand "Gouessant ®". The WH leaves were incorporated following an arithmetic progression of 2% from 0 to 8%. The various ingredients used were ground and mixed dry. To this mixture were added red palm oil and water before extrusion. The food produced was 2mm in diameter. It was then packaged in boxes and stored in the refrigerator at a temperature of 5°C.
Table 3: Dietary formula for the determination of the optimal rate of incorporation of WH leaves
|
Ingredients (%) |
T0* |
T1 (0%) |
T2(2%) |
T3(4%) |
T4 (6%) |
T5 (8%) |
|
Fish meal |
_ |
30 |
29 |
28 |
28 |
27 |
|
Chicken viscera meal |
_ |
20 |
20.5 |
21 |
21 |
21.5 |
|
Soyabean meal |
_ |
15.5 |
13.5 |
12.5 |
12 |
11 |
|
Cottonseed meal |
_ |
13 |
14 |
13.5 |
13 |
13 |
|
Wheat bran |
_ |
9 |
8.5 |
8.5 |
7,5 |
7 |
|
Corn flour |
_ |
4 |
4 |
4 |
4 |
4 |
|
Moringa leaves |
_ |
1 |
1 |
1 |
1 |
1 |
|
WH leaves |
_ |
0 |
2 |
4 |
6 |
8 |
|
Starch |
_ |
2 |
2 |
2 |
2 |
2 |
|
Concentrated Methionine |
_ |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Concentrated Lysine |
_ |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Iron Sulphate |
_ |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Dicalcium Phosphate |
_ |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Premix (Vit+ Min)* |
_ |
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
|
Palm oil |
_ |
2 |
2 |
2 |
2 |
2 |
|
Total (%) |
100 |
100 |
100 |
100 |
100 |
|
|
Protein (%) |
40,14 |
40,01 |
39,8 |
39,4 |
39,22 |
|
|
Lipid (%) |
13,9 |
13,6 |
13,4 |
13,2 |
13,03 |
|
T1: Diet without WH leaf, T2: Diet with 2% WH leaf, T3: Diet with 4% WH leaf, T4: Diet with 6% WH leaf, T5: Diet with 8% WH leaf
* "Le Gouessant ®" commercial food for C. gariepinus
**premix (vitamin – mineral) contains (‰):Vitamin A 4,000,000 U.I; Vitamin D 800,000 IU; Vitamin E 40,000IU; Vitamin K3 1600mg; Vitamin B1 4000mg; Vitamin B2 3000mg; Vitamin B6 3800mg; Vitamin B12 3mg; Vitamin C 60,000mg; Biotin 100mg; Inositol 10,000mg Pantothenicacid 8,000mg; Nicotinic acid 18,000mg; Folicacid 800mg; Cholinchloride 120,000mg; Colbat carbonate 150mg; Ferroussulphate 8000mg; Potassium iodide 400mg; Manganeseoxide 6000mg; Copper 800mg; Sodium selenite 40 mcg; Lysine 10,000mg; Methionine 10,000mg; Zinc Sulphate 8000mg
Experimental conditions and conduct of experiment
10To determine the optimal rate of incorporation of WH leaves in the diet of C. gariepinus, 18 rectangular-shaped aquariums with a water volume of 60 liters each were used. The experimental apparatus was composed of a random Fisher block of six treatments with three repetitions each. C. gariepinus fries with an average individual weight of 6.38±0.09 g were used. The stocking density was twenty fries per tank, one fry for 2.5 l. They were fed three times a day to apparent satiety for 35 days. Growth Control was done every 7 days. The water is renewed at a rate of 2 l / min during the day. The amount of feed consumed per day was calculated at the end of the day. The physicochemical parameters (temperature, dissolved oxygen and pH) were recorded every day mornings and evenings to assess the quality of the water. At the end of the experiment, the zootechnical and feed utilization parameters such as: Survival Rate (SR), Daily Weight Gain (DWG), Specific Growth Rate (SGR), Feed Conversion Rate (FCR) and the Protein Efficiency Rate (PER) were calculated to assess the feeds performance.
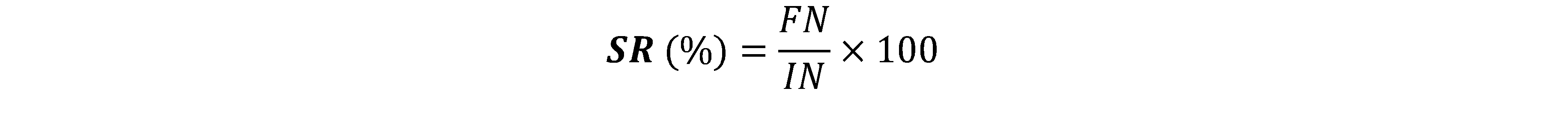
IN: Initial number of fish and FN: Final number of fish.
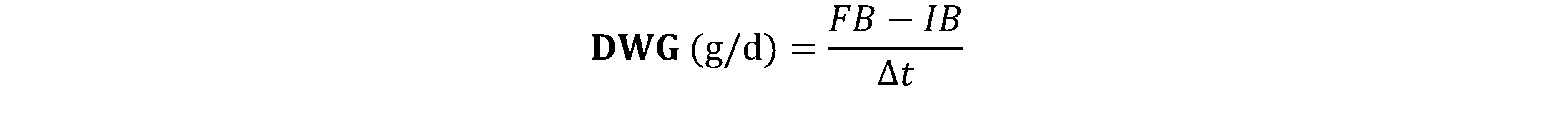
IB: Initial Biomass (g), FB: Final Biomass (g) and Δt: the duration of the experiment in number of days
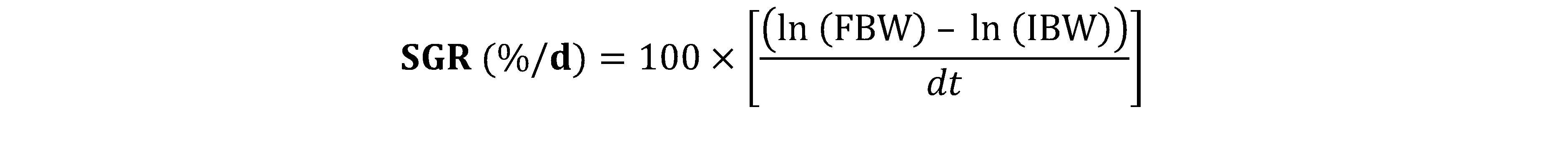
ln: Natural logarithm, dt: total duration of the experiment.
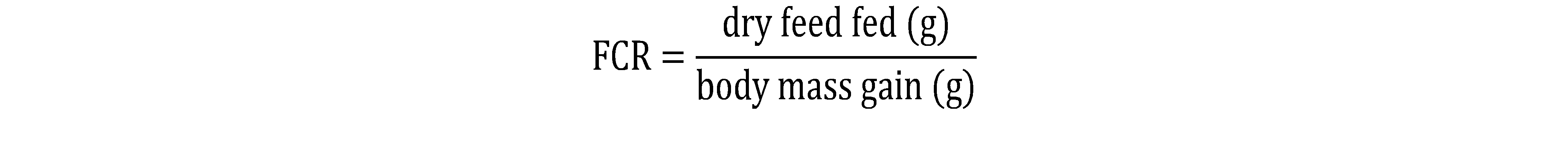
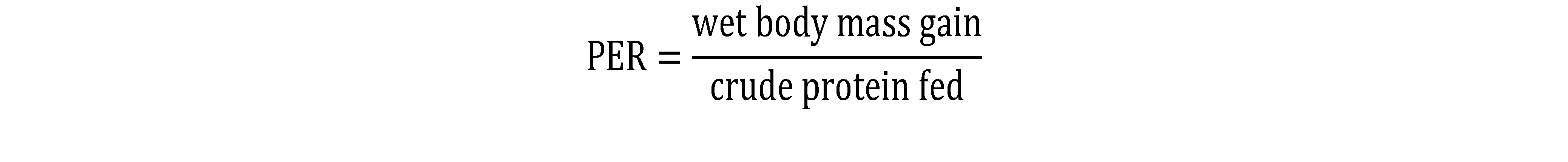
11The optimal rate of incorporation of Water Hyacinth leaves in the diet of C. gariepinus was determined based on the analysis of the variation of the Specific Growth Rate and the Feed Efficiency according to the treatment. The effect of the dose and the response obtained through the expression of the Specific Growth Rate and the Feeding Efficiency according to the treatments were used to determine the optimal rate of incorporation of the Water Hyacinth leaves in the diet of C. gariepinus.
Statistical analysis
12For each parameter, the mean along with the standard deviation was calculated. Statistical analyzes were performed using the STATVIEW software version 5.01 by the method of analysis of variance with one classification criterion (ANOVA 1). The zootechnical data obtained were subjected to Fisher's LSD tests to compare the different means calculated at the 5% threshold.
Results
Physicochemical parameters of water
13The physicochemical parameters of the water in the tanks for the study of the optimal rate of incorporation of Water Hyacinth leaves and digestibility were recorded throughout the experiment in order to the ensure optimal breeding conditions of fishes (Table 4). These parameters are pH, temperature and dissolved oxygen. The average temperature is 26.97 ± 0.55 °C. The average pH is 6.75 ± 0.08 and the average dissolved oxygen level is 6.28 ± 0.19 mg/l.
Table 4: Values of physico-chemical water parameters
|
Parameters |
T0 |
T 1 |
T2 |
T3 |
T4 |
T5 |
R0 |
R1 |
|
|
pH |
6.79 |
6.68 |
6.73 |
6.74 |
6.74 |
6.81 |
6.75 |
6.73 |
|
|
T(°C) |
27.53 |
27.70 |
26.33 |
26.63 |
26.68 |
26.97 |
27.80 |
27.75 |
|
|
O2 (mg/l) |
6.25 |
6.30 |
6.21 |
6.23 |
6.32 |
6.29 |
6.23 |
6.18 |
|
Digestibility parameters
14The Apparent Digestibility Coefficients in dry matter, proteins and lipids were determined in the reference diet (R0) and the test diet (R1) and in the Water Hyacinth leaves (Table 5). The results showed that the coefficients of apparent digestibility with regard to dry matter, proteins and lipids in the reference diet (86.75%, 84.45%, 90.14%) are respectively higher than the results observed in the test diet for the same parameters respectively (80.33%, 80.02%, 86.54%). We also note that the Apparent Digestibility Coefficients of the Water Hyacinth leaves for the different parameters considered are lower than those observed in the test diet and are as follows: ADCDM: 75.65%, ADCCP: 77.4%, ADCCL: 78.14%.
Table 5: Apparent Digestibility Coefficient (ADC) in Dry Matter (ADCDM), Protein (ADCCP) and Lipids (ADCLP) for test diets and for the Water Hyacinth leaves (WH)
|
ADC |
R0 |
R1 |
|
Reference Test |
||
|
ADCDM (%) |
86.75±0.41 |
80.33±0.33 |
|
ADCCP (%) |
84.45±0.12 |
80.02±0.18 |
|
ADCCL (%) |
90.14±0.21 |
86.54±0.15 |
|
|
Ingredient (WH) |
|
|
ADCDM (%) |
- |
75.65±0.87 |
|
ADCCP (%) |
- |
77.4±0.75 |
|
ADCCL (%) |
- |
78.14±0.94 |
Zootechnical and feed utilization parameters
15Concerning the study of the optimal rate of incorporation of WH leaves, the zootechnical and feed utilization parameters were calculated (Table 6). The final biomass varied from 325.58±2.45 g (T5) to 420.58±1.49 g (T0). We note a significant difference (p < 0.05) between the different treatments after an incorporation of 4% of the WH with other treatments. The Daily Weight Gain (DWG) varied from 5.68±0.21 g (T5) to 8.40±0.19 g. A significant difference (p < 0.05) is observed between the different treatments. After a substitution of 4%, we notice a considerable drop in the DWG. The same observation is made with regard to the Feed Conversion Rate (FCR) and the Protein Efficiency Rate (PER) which varied respectively from 2.34±0.11 (T5) to 1.14±0.09 (T0) and from 1.26±0.14 (T5) to 2.38±0.11 (T0). As for the Survival Rate (SR), no significant difference was observed between the treatments. The SR varied from 85.33% (T0) to 95.33% (T4).
Table 6: Growth performances and feed utilization of Clarias gariepinus fingerlings fed with experimental diets
|
Paramètres |
T0 |
T1(0%) |
T2(2%) |
T3(4%) |
T4(6%) |
T5(8%) |
|
IB (g) |
126.50±0.59 a |
126.55±0.48 a |
126.68±0.39 a |
127.10±0.66 a |
126.75±0.75 a |
126.65±0.74 a |
|
FB (g) |
420.58±1.49 a |
410.48±1.88 a |
408.52±1.67 a |
406.66±1.55 a |
375.15±2.09 b |
325.58±2.45b |
|
DWG (g/J) |
8.40±0.19 a |
8.11±0.14 a |
8.05±0.22 a |
7.99±0.11 a |
7.10±0.17 b |
5.68±0.21 b |
|
FCR |
1.14±0.09 a |
1.22±0.06 a |
1.24±0.08 a |
1.30±0.07 a |
1.81±0.10 b |
2.34±0.11 b |
|
PER |
2.38±0.11 a |
2.29±0.14 a |
2.30±0.09 a |
2.25±0.12 a |
1.61±0.08 b |
1.26±0.14 b |
|
SR (%) |
85.33±3.44a |
90.66±2.50 a |
90.33±0,2.50 a |
90.33±3.33 a |
95.33±2.66 a |
90.66±2.33 a |
Values are expressed as means ± standard deviation. Values with the same alphabetical letters on the same row are not significantly different at the threshold of p = 0.05.
Determination of the optimal rate of incorporation of WH leaves in the diet of C. gariepinus
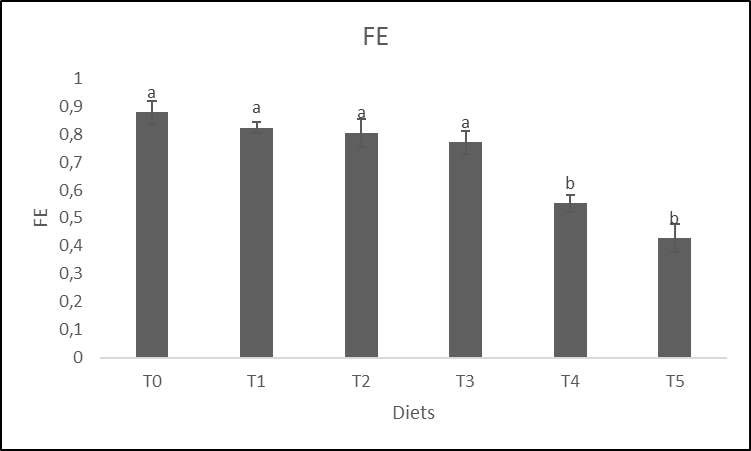
16The effect of the dose and the response obtained through the result of the Specific Growth Rate (SGR) (Figure 1) and the Feed Efficiency (FE) (Figure 2) according to the treatments shows that there is no difference significant (p > 0.05) for WH incorporation rates below 4% (T1, T2 and T3). For incorporation rates greater than 4% (T4 and T5), no significant difference is observed either (p > 0.05). But the significant difference is noted rather between the groups of incorporations of WH lower than 4% and the groups of incorporations of WH higher than 4%.
Figure. 1 : Variation of the Specific Growth Rate of Clarias gariepinus fingerlings fed with experimental diets
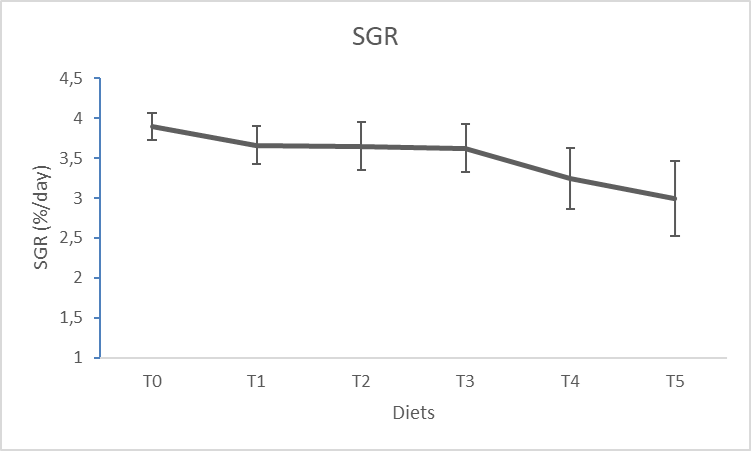
Figure. 2 : Variation of the Feed Efficiency of Clarias gariepinus fingerlings fed with experimental diets
Discussion
17The water quality during the experiment (WH digestibility and optimal incorporation rate test) with respect to the average of parameters like temperature, pH and dissolved oxygen are respectively: 26.97 ± 0.55°C, 6.75 ± 0.08 and 6.28 ± 0.19 mg/l-1. These parameters remained within the range accepted by the species according to Oké et al., 2016.
18WH leaves have a nutritional profile that can be used in fish feeding. Its protein rate (14.30%) is close to that of wheat bran (15.10%) and is higher than that of rice bran (14.10%), corn flour (8.7%) but slightly lower than that of sweet potato leaves (16.6%) and cassava leaves (22.30%) (9). Some studies have been able to show the use of Water Hyacinth in fish feed without studying its digestibility and the optimal rate of its incorporation into the feed (Hasan et al., 1990; Mahmood et al., 2016). The study of the digestibility of WH leaves in the diet of Clarias gariepinus showed that the coefficients of apparent digestibility with regard to dry matter, proteins and lipids respectively in the control diet (86.75%, 84.45%, 90.14 %) are superior to the results observed in the reference diet without WH for the same parameters respectively (80.33%, 80.02%, 86.54%). The coefficients of apparent digestibility directly related to the leaves of the WH (75.65%, 77.4% and 78.14%) are lower than those observed in the test diet for the same parameters considered. According to the results of Da et al., 2013, the digestibility coefficient obtained by using sweet potato leaves (79.3% and 71.8%) and cassava leaves (79.4% and 63.6%) respectively for dry matter and protein content to feed the fry of Pangasianodon hypoththalamus is close to those obtained in the present study with WH leaves (75.65%, 77.4%) for the same parameters. WH leaves can therefore be used in the same way as cassava leaves and sweet potato leaves based on their coefficient digestibility of dry matter and protein in the diet of the African catfish. On the other hand, duckweed (Lemna sp.) has a high digestibility (81.7%) with regard to proteins (Da et al., 2013) compared to WH leaves. This difference can be explained by the fact that duckweed (Lemna sp.) has a more balanced amino acid composition compared to WH leaves (Mbagwu & Adeniji 1988). The same observation is made with respect to the digestibility of ingredients of animal origin which show better digestibility compared to ingredients of plant origin (Nor et al., 2019; Panini et al., 2017; Sklan et al., 2004). On the other hand, the apparent digestibility coefficients of protein-enriched WH leaves showed better digestibility when feeding the Oreochromis niloticus according to studies by Hontiveros & Serrano, 2015. These results show that digestibility is not only a function of the ingredient tested but also of the species studied.
19The incorporation of WH leaves influenced the expression of zootechnical parameters (DWG, FCR, PER). In general, the more the incorporation rate of WH leaves increases, the more a slight drop is noticed on the zootechnical performance up to 4%. After 4% incorporation (6% and 8%) there is a significant drop in the same parameters considered. This finding can be explained by the imbalance in the amino acid profile of WH leaves (A-Rahman et al., 2012; Sayed-Lafi et al., 2018) The presence of antinutritional factors as in most proteins of plant origin also limit high incorporation of leaf ingredients in fish feed (Francis et al., 2001; Siddhuraju et al., 2001; Akande et al., 2010). On the other hand, according to studies by Chavez et al., 2014, the incorporation of water hyacinth to feed shrimp (Penaeus monodon) as a substitute for soy flour at 75% and in the feed at 48% does not significantly affect the growth parameters of these shrimps. According to this author, the water hyacinth leaves do not present any disadvantage in the feeding of shrimps up to 48% incorporation. This may be due to the specificity of the shrimp diet and their farming environment. The present study has shown that the incorporation of more than 4% of WH leaves in the diet of Clarias gariepinus can lead to a drop in zootechnical performance considering the Specific Growth Rate and the Feed Efficiency which drop sharply, in our study, after an incorporation of 4%. Furthermore, the incorporation of WH leaves in the diet of C. gariepinus up to 8% incorporation has no negative effects on the survival rate, which was 90% on average.
Conclusion
20The incorporation of WH leaves in the diet of Clarias gariepinus showed that it can be used as an ingredient in the composition of aquaculture feeds of C. gariepinus based on its digestibility. But for an optimal valorization of this feed by Clarias gariepinus, its incorporation rate should not exceed 4%.
Acknowledgments
21We thank Green Keeper Africa which financed part of this research for its ecological control project against the proliferation of water hyacinth and the Research Unit in Aquaculture and Fisheries Management (URAGEP).
-
Abou Y., Fiogbe E.D., Aïna P. N., Buldgen A. & Micha J-C., 2010, Evaluation of nitrogen and phosphorus wastes produced by Nile tilapia (Oreochromis niloticus L.) fed Azolla-diets in concrete tanks. Int. J. Biol. Chem. Sci 4(1), 42-50. http://ajol.info/index.php/ijbcs
-
Abou Y., Fiogbe E.D., Micha J-C., 2007 a, Effects of stocking density on growth, yield and profitability of farming Nile tilapia,Oreochromis niloticus L., fed Azolla diet, in earthen ponds. Aquaculture Research 38, 595-604. DOI:10.1111/j.1365-2109.2007.01700.x
-
Abou Y., Fiogbé E.D., Micha J-C., 2007 b, A preliminary assessment of growth and production of Nile tilapia, Oreochromis niloticus L., fed Azolla-based-diets in earthen ponds. Journal of Applied Aquaculture 19(4), 55-69. DOI:10.1300/J028v19n04_03
-
Akadiri F. J., Aboh A.B., Olounlade P.A., Assogba F. & Houndonougbo F., 2022, Valeurs nutritives, propriétés antioxydantes et cytotoxicité des cossettes de Dioscorea dumetorum pour la valorisation en alimentation animale et humaine au Bénin. Tropicultura 40 (1), 1-16. DOI: 10.25518/2295-8010.1963
-
Akande K.E., Doma U.D., Agu H.O., Adamu H.M., 2010, Major antinutrients found in plant protein sources, their effect on nutrition. Pakistan Journal of Nutrition 9(8):827– 832. https://doi.org/10.3923/pjn.2010.827.832
-
AOAC, 1990, Official Methods of Analysis. 15th edn., Washington, DC, USA. approaches to research and development. Ottawa, Ontario, IDCR-233e, IDRC, Canada, 154 pp. https://idl-bnc-idrc.dspacedirect.org/handle/10625/7242
-
A-Rahman M.E., Abol-Munafi A.B., Amiza A.M., Khoda Bakhsh H. & Adam H.M., 2012, Apparent digestibilty coefficient of pelleted fish feed incorporated with water hyacinth (Eichhornia crassipes). Online Journal of Animal and Feed Research 2, 30-33.
-
Chavez N.N., Traifalgar R. F., Gonzaga J. V. & Corre V. L. J., 2014, Growth performance of Penaeus monodon fed diets containing water hyacinth leaf protein concentrate. ABAH Bioflux 6, 195-201.
-
Cho C.Y., Cowey C.B., Watanabe T., 1985, Finfish nutrition in Asia: methodological sturgeon (Acipenser baerii Brandt), compared by two chromic oxide analyses methods. Aquac Nutr 15:650–6.
-
Da C.T., Lundh T., Lindberg J. E., 2013, Digestibility of dietary components and amino acids in plant protein feed ingredients in feed in striped catfish (Pangasianodon hypothalamus) fingerlings. Aquaculture Nutrition 19, 619–628. DOI:10.1111/anu.12011
-
Dagno K., Lahlali R., Friel D., Bajji M. & Haïssam Jijakli M., 2007, Bibliographic summary: problem of water hyacinth Eichchornia crassipes, in tropical and subtropical regions of the world, in particular its eradication by biological control in means of phytopathogens. Biotechnol. Agron. Soc. Environ 11(4), 299–311. https://popups.uliege.be/1780-4507/index.php?id=1706
-
Djissou, A.S.M., Ochiai, A., Koshio, S., Fiogbe, E.D. (2017). Effect of total replacement of fishmeal by earthworm and Azolla filiculoides meals in the diets of Nile tilapia Oreochromis niloticus (Linnaeus, 1758) reared in concrete tanks. Indian Journal of Fisheries, 64(1), 31-36. https://doi.org/10.21077/ijf.2017.64.1.55317-05
-
El-Saidy D.M.S.D., Gaber, M.M.A., 2003, Replacement of fish meal with a mixture of different plant protein sources in juvenile Nile tilapia Oreochromis niloticus (L.) diets. Aquaculture Research 34, 1119– 1127. DOI:10.1046/j.1365-2109.2003.00914.x
-
FAO, 2024. The State of World Fisheries and Aquaculture. Rome, Italy: Food and Agriculture Organization of the United Nations. www.fao.org
-
Fayeofori G., Bob M., 2013, Methods using in digestibility evaluation on fish diet: A reviews and challenges. Continental J. Fisheries and Aquatic Science 7(2), 25 – 37. https://doi.org/10.5281/zenodo.3526678
-
Forster I., 1999, A note on the method of calculating digestibility coefficients of nutrients provided by single ingredients to feeds of aquatic animals. Aquacult. Nutr 5,143-145. https://doi.org/10.1046/j.1365-2095.1999.00082.x
-
Francis G., Makkar H.P.S., Becker K., 2001, Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199,197–227. DOI:10.1016/S0044-8486(01)00526-9
-
Furukawa A., Tsukahara H., 1966, On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility in fish feed. Bull Jpn Soc Sci Fish 32, 502–506.
-
Hasan M.R., Moniruzzaman M., Omar Farooque A.M., 1990, Evaluation of leucaena and water hyacinth leaf meal as dietary protein sources for the fry of Indian major carp, Labeo rohita (Hamilton). In: R. Hirano & I. Hanyu, eds. The Second Asian Fisheries Forum. Manila, Asian Fisheries Society, pp. 275-278 https://doi.org/10.21608/ejnf.2015.105109
-
Hontiveros G.J.S., Serrano J.A.E., 2015, Nutritional value of water hyacinth (Eichhornia crassipes) leaf protein concentrate for aquafeeds. AACL Bioflux 8(1), 26-33. http://www.bioflux.com.ro/aacl
-
Koprucu K., Ozdemir Y., 2005, Apparent digestibility of selected feed ingredients for Nile tilapia (Oreochromis niloticus). Aquaculture 250, 308–316. https://doi.org/10.1016/j.aquaculture.2004.12.003
-
Luo Z., Li X.D., Gong S.Y., Xi W.Q., 2009, Apparent digestibility coefficients of four feed ingredients for Synechogobius hasta. Aquac Res 40, 558–65.
-
Mbagwu I. G., Adeniji H. A., 1988, The nutritional content of duckweed (Lemna paucicostata Hegelm) in the Kainji Lake area, Nigeria. Aquatic Botany 29, 357–366. https://doi.org/10.1016/0304-3770(88)90079-4
-
Mahmood S., Khan N., Iqbal K.J., Ashraf M., & Khalique A., 2016. Evaluation of water hyacinth (Eichhornia crassipes) supplemented diets on the growth, digestibility and histology of grass carp (Ctenopharyngodon idella) fingerlings. Journal of Applied Animal Research, 46(1),24–28. https://doi.org/10.1080/09712119.2016.1256291
-
Nor F.N., Zulkifli M., Seng Lim L., Shapawi R., Yong S. A., Senoo S., Devic E., Kawamura G. & Mustafa S., 2019, Apparent digestibility coefficient of black soldier fly (Hermetia illucens) larvae in formulated diets for hybrid grouper (Epinephelus fuscoguttatus ♀ x Epinephelus lanceolatus ♂). AACL Bioflux 12 (2), 513-522
-
Oké V., Abou Y., Adité A., Kabré A.T. & 2016, Growth Performance, Feed Utilization and Body Composition of Clarias gariepinus (Burchell 1822) Fed Marine Fish Viscera-based-diet in Earthen Ponds. Fisheries and Aquaculture Journal 7 (4), 1-7. DOI:10.4172/2150-3508.1000183
-
Panini R.L., Freitas L.E.L., Guimarães A.M., Rios C., da-Silva M. F.O., Viera F.N., Fracalossi D. M., Samuels R.I., Predêncio E.S., Silva C.P. & Amboni R.D. M.C., 2017, Potential use of mealworms as an alternative protein source for Pacific white shrimp: Digestibility and performance. Aquaculture 473,115-120.
-
Rahman M., Hyon-Sob H., Kang-Woong K., Kyoung-Duck K., Bong-Joo L. & Sang-Min L., 2016, Apparent digestibility coefficients of the extruded pellet diets containing various fish meals for olive flounder, Paralichthys olivaceus. Fisheries and Aquatic Sciences 19(27), 1-8. DOI:10.1186/s41240-016-0027-7
-
Richter N., Siddhuraju P., Becker K., 2003, Evaluation of nutritional quality of Moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.). Aquaculture. 217, 599-611. DOI:10.1016/S0044-8486(02)00497-0
-
Saha S., Ray A. K., 2011, Evaluation of nutritive value of water hyacinth (Eichhornia crassipes) leaf meal in compound diets for rohu, Labeo rohita (Hamilton, 1822) fingerlings after fermentation with two bacterial strains isolated from fish gut. Turkish Journal of Fisheries and Aquatic Sciences 11, 199-207. DOI:10.4194/trjfas.2011.0204
-
Sayed-Lafi R.M., Al-Tameemi R.A. Gowdet A.I., 2018, Evaluation of raw and fermented water hyacinth (Eichhornia crassipes) incorporated diets on growth and feed efficiency of young grass carp (Ctenopharyngodon Idella). Basrah J. Agric. Sci 31(1), 31-39. DOi:10.21276/basjas
-
Shanab S.M.M., Hanafy E.A., Shalaby E.A., 2018, Water Hyacinth as Non-edible Source for Biofuel Production. Waste Biomass Valor 9, 255-264 . https://doi.org/10.1007/s12649-016-9816-6
-
Siddhuraju, P., & K. Becker, 2001, Preliminary nutritional evaluation of Mucuna seed meal (Mucuna pruriens var. utilis) in common carp (Cyprinus carpio L.): An assessment by growth performance and feed utilisation. Aquaculture 196:105–123. http://dx.doi.org/10.1016/S0044-8486(00)00577-9
-
Sklan D., Prag T., Lupatsch I., 2004, Apparent digestibility coefficients of feed ingredients and their prediction in diets for tilapia, Oreochromis niloticus x Oreochromis aureus (Teleostei, Cichlidae). Aquaculture Research 35, 358- 364. DOI:10.1111/j.1365-2109.2004.01021.x
-
Tchiaze I.A.V., Priso R.J., 2016, Distribution and valuation of invasisive macrophytes in the coastal region (Cameroon): case of Eichhornia crassipes (Mart.) Solms-Laubach. Journal of Applied Biosciences 100, 9522 – 9534. http://dx.doi.org/10.4314/jab.v100i1.4
-
Uys W., 1989, Nutritional Physiology and Dietary Requirements of Juvenile and Adult Clarias. PhD thesis, Rhodes Univ., Grahamstown, South Africa. https://core.ac.uk/download/pdf/145047341.pdf
-
Vodounnou, J.V., Dossa V., Djissou, C., Kpogue, D., Agadjihouede, H., Fiogbe, E.D. & Micha, J-C., 2024, Feeding Optimization of Water Hyacinth (Eichhornia crassipes) Leaves as Rearing Substrate for the Production of Black Soldier Fly (Hermetia illucens) Larvae. Waste and Biomass Valorization 15, 3569–3578 https://doi.org/10.1007/s12649-023-02408-w
-
Vodounnou, J.V., Iko, R., Okou, G., Kpogue, D., Ahouansou Montcho, S., & Micha, J-C., 2025a, Complete Substitution of fish meal with black soldier flies Hermetia illucens (L. 1758) larvae meal at varying incorporation rates for feeding Oreochromis niloticus raised in captivity. Aquaculture science and management, 2:1, https://doi.org/10.1186/s44365-024-00004-0
-
Vodounnou, J.V., Tokeme, N., Iko, R., Coulibaly, S., Ahouansou Montcho, S., & Micha, J.-C., 2025b, Utilising water hyacinth (Eichhornia crassipes) in Nile tilapia (Oreochromis niloticus) diets: Effect on growth, digestibility, and optimal inclusion rate. Aquatic Research, 8(3), 207-217. https://doi.org/10.3153/AR25020
-
Wilson R.P., Moreau Y., 1996, Nutritional requirements of catfishes (Siluroidei). Aquat. Liv. Resources 9,103-111. https://www.alr-journal.org/articles/alr/pdf/1996/05/alr96hs07.pdf
To cite this article
About: Juste Vital Vodounnou
Hydrobiology and Aquaculture, Research Unit in Aquaculture and Fisheries Management (URAGEP), Ecole d'Aquaculture, Université Nationale d'Agriculture (UNA), Benin
Corresponding author: justekingjv@gmail.com
About: Romaric Iko
Research Unit in Aquaculture and Fisheries Management (URAGEP), Ecole d'Aquaculture, Université Nationale d'Agriculture (UNA), Benin
About: Diane Kpogue
Hydrobiology and Aquaculture, Research Unit in Aquaculture and Fisheries Management (URAGEP), Ecole d'Aquaculture, Université Nationale d'Agriculture (UNA), Benin
About: Elie Montchowui
Hydrobiology and Aquaculture, Research Unit in Aquaculture and Fisheries Management (URAGEP), Ecole d'Aquaculture, Université Nationale d'Agriculture (UNA), Benin
About: Emile Didier Fiogbe
Hydrobiology and Aquaculture, Laboratory of Research on Wetlands, (LRZH) of Abomey-Calavi University (UAC), Benin
About: Jean-Claude Micha
Research Unit in Environmental and Evolutionary Biology (URBE), Institute of Life, Earth and Environment (ILEE), University of Namur, Belgium