- Portada
- Volume 17 (2013)
- numéro 3
- Soil model systems used to assess fouling, soil adherence and surface cleanability in the laboratory: a review
Vista(s): 4974 (39 ULiège)
Descargar(s): 141 (1 ULiège)
Soil model systems used to assess fouling, soil adherence and surface cleanability in the laboratory: a review

Notes de la rédaction
Received on September 4, 2012; accepted on March 19, 2013
Résumé
Synthèse bibliographique des souillures modèles utilisées en laboratoire pour l’évaluation de l’adhérence des souillures, l’encrassement et la nettoyabilité des surfaces. L’encrassement des surfaces est un problème chronique dans les industries de transformation. L’état de propreté de surface est donc un paramètre critique à l’égard des performances du processus de production et de la qualité finale d’un produit. De ce fait, le nettoyage et la désinfection sont indispensables. La stratégie la plus importante de réduction de l’encrassement, d’optimisation du nettoyage et de la désinfection est de comprendre leurs mécanismes. Ceci permet de trouver des moyens de réduction, voire d’élimination de l’encrassement ou d’amélioration des procédés de nettoyage et de désinfection. Ce document présente une synthèse de différents modèles de salissures répertoriés dans la littérature spécialisée pour l’étude des phénomènes d’encrassement et de nettoyage. Les modèles de salissures organiques, minérales, microbiologiques, particulaires et de salissures composées sont présentés. Ces modèles sont particulièrement pertinents pour l’étude en laboratoire de l’encrassement ou de l’adhérence des salissures et le nettoyage des surfaces. Les principales caractéristiques de ces modèles de salissures ainsi que leurs avantages et leurs inconvénients, l’application des résultats de laboratoires en industrie sont examinés et discutés.
Abstract
Surface fouling is a chronic problem in processing industries. The hygienic state of surfaces is thus a critical parameter with respect to the performance of the production process and to the final quality of the product. For this reason, cleaning and disinfection are essential. The most important first step in implementing a fouling mitigation strategy through cleaning and disinfection is to understand the mechanisms of fouling. This allows ways to be found to reduce, even to eliminate fouling, or to improve the effectiveness of cleaning and disinfection. This paper reviews the relevant literature and summarizes a selection of soil model systems used to aid such improvements. Organic, mineral, microbial, particulate, and composite soil model systems are presented. These soil model systems are of particular relevance in the study of fouling, cleaning or soil adhesion onto solid surfaces in the laboratory environment. The key features of the models, as well as their practical advantages and disadvantages, are described and discussed.
Tabla de contenidos
1. Introduction
1In processing industries, surface hygienics are undertaken primary to remove all undesirable material from surfaces. Such undesirable materials, which may include food residues, microorganisms, foreign bodies, and cleaning chemicals, are generally referred to as “soil”, and may derive from normal production processes, spillages, line-jams, equipment maintenance, packaging or general environmental contamination such as dust and dirt (Holah, 2000). In order to achieve successful surface hygienics, it is essential to understand the nature of the soil to be removed. Soils have been characterized by their chemical composition e.g. carbohydrate, fat, protein, starch or mineral. Microorganisms may either be incorporated into the soil or be attached to surfaces, ultimately forming layers known as biofilms. In order to control the fouling process better, studies have paid particular attention to the parameters affecting both the formation of a particular type of soil and how its formation might be retarded or prohibited. These parameters include the role of soluble macromolecules, calcium sequestrants, pH, preheating, moisture level, temperature and flow rate.
2No one cleaning agent is able to perform all the necessary functions to achieve the successful removal of all existing soil types. Several studies have been undertaken to determine fouling-related costs in industrialized countries. For the United States and New Zealand, the corresponding total annual costs are approximately 0.25% and 0.15% of the country’s Gross National Product (GNP), respectively. The severe problem of fouling in heat exchanger equipment is known to have blocked normal levels of industrial production; the corresponding cost of fouling in China’s total industry is more than 10 billion RMB per year (Bansal et al., 2008; Zhao et al., 2011). In France, the total cost of fouling in the dairy industry in 1991 was estimated at more than 152 million Euros (Cliaudagne, 1991). In membrane filtration applications, fouling reduces membrane flux with a consequent increase in energy costs and the need for early membrane replacement (Al-Amoudi, 2010).
3Lalande et al. (1989) are among many researchers to have undertaken a large number of successful investigations providing a better understanding of the fouling process. However, a real breakthrough, i.e. complete control over fouling, has not yet been achieved. This is mainly due to the types of soil systems investigated to date and to the lack of a full understanding of fouling mechanisms. The objective of this paper is to review the existing knowledge on soil models in the area of fouling, to establish the fundamental factors involved and to present an up-to-date view on the processes taking place.
2. Soiling
2.1. Definition
4Soil has been defined as any matter that is out of place on a defined solid surface. This surface is referred to as the substrate (Jennings, 1965). In the food industry, a soil can be a product residue or raw material, often in combination with a mineral deposit. This residue may have been altered to a greater or lesser extent by the processing conditions, by interaction with a cleaning solution, by subsequent contamination or by microbial contamination (Kulkarni et al., 1975). For this reason, the nature of the soil and the process that generates it must be understood before determining the treatment to be applied (cleaning-in-place, impinging jets, etc.) (Rowan, 2005). In membrane filtration applications, fouling is the process resulting in a deterioration in membrane performance (reduced membrane flux) due to the deposit of suspended or dissolved substances on its surface and pores (Al-Amoudi, 2010).
2.2. Types of soil
5Soils can be classified according to different criteria. They have been divided into organic, biological, mineral and composite soils, with cleaning processes being matched accordingly. Soils have also been categorized as being free, adherent or embedded. However, although this classification facilitates the identification of appropriate cleaning techniques in general, it is far from being exhaustive. Fryer et al. (2009) classified soils into five types according to the mechanism of deposit formation. The HRS group (2011) classified soils into four common and readily identifiable types, thus making possible the grouping and comparison of data from different industries:
6– chemical soils or reaction fouling occur when chemical changes within the fluid cause a fouling layer to be deposited onto the substrate. This occurs in areas as diverse as milk (protein denaturation) and crude oil fouling;
7– biological soils or soiling is caused by the growth of organisms within the fluid that is deposited onto the substrate, across several length scales, from the adhesion of single organisms to the development of biofilms and seaweed;
8– deposit soils or particulate fouling occur when particles contained within the fluid settle onto the substrate. Examples of this can be seen in the deposition of suspended magnetite particles from cooling water, dust from the air or protein aggregates onto surfaces. In membrane fouling (reverse osmosis, nanofiltration, ultrafiltration and microfiltration membranes), particulate matter in water or mixed liquids can be classified as settleable solids (> 100 µm), supra-colloidal solids (1 µm to 100 µm), colloidal solids (10-3 µm to 1 µm) and dissolved solids (< 10-3 µm). A full characterization of feed waters and mixed liquids may be used as a tool to predict membrane performance and to characterize the main mechanisms to which the soils are subjected (Potts et al., 1981; Gao et al., 2013);
9– crystallization or precipitation fouling occur when a component of the fluid is deposited when its solubility limit is reached, such as calcium carbonate from boiling water or calcium phosphate (whose solubility decreases with temperature). A subset of this type of fouling is “solidification fouling”, which occurs when the fluid or its components become solidified on the surface. Examples of this can be seen in the solidification of ice from water, starch from a food fluid or, as in the processing of personal care products, the deposition of toothpaste or waxes from cosmetics (Fryer et al., 2009);
10– corrosion soils occur when a layer of corrosion products builds up on the substrate, forming an extra layer of usually high thermal resistance material. Often corrosion products are conveyed by fluid to form fouling deposits elsewhere via a particulate fouling mechanism.
11In order to aid development of the understanding of cleaning mechanisms and to improve industrial practice, Fryer et al. (2009) presented soil types and cleaning methods in a two-dimensional representation with the axes as follows: soil material properties (the severity of fouling differs and will depend on the deposit properties) and cleaning fluid (deposit properties will determine which fluid should be used for cleaning) (Figure 1). The shaded area in figure 1 shows the three types of soil that are the most difficult to clean.
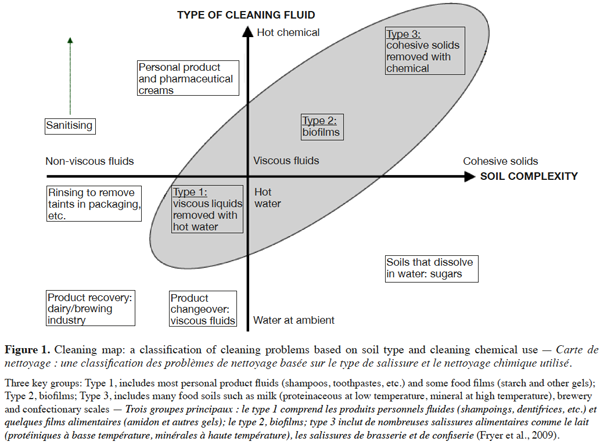
12Rowan (2005) classified various types of soil encountered in the food industry according to their solubility in water (sugars, certain starches and salts), in acid solution (limestone, most mineral deposits) and in alkaline solution (with proteins, fats and emulsions). The classification of Quittet et al. (1999) is based on the nature of the soil: mineral (tartar, etc.), organic (lipid, protein, carbohydrate), microbiological (spores, viruses, molds, yeasts, bacteria, etc.), composite (consisting of a mixture of at least two of the previously listed soil classes) and other soils deriving from the environment (dust, heavy metals, etc.). Jennings (1965) suggested a simplified classification of different types of soil into two groups: homogeneous and composite soils. Paria (2003), meanwhile, proposed the most appropriate classification for textile research: lipid, particulate and composite soils.
13None of the classifications proposed above is fully exhaustive or totally wrong. Indeed, the different types of soil can be defined by all these different classifications. The nature of soils and their respective solubility have been shown to be very important but the form of the soil itself must also be taken into account when cleaning treatment is planned: solid particulates (more or less hydrated powder), hydrated matrix (gel), biofilms, etc. In the following part of the review, we present different models of soiling systems used in the literature to assess substrate fouling, soil adherence or removal techniques in the laboratory.
3. Soil model systems
3.1. Organic soils
14Organic soils can be defined as any undesirable matter of a large class of gaseous, liquid or solid chemical compounds whose molecules contain carbon (combined with hydrogen, oxygen or nitrogen) such as carbohydrates, lipids and proteins, which is associated with living things (of biological origin: flora, fauna, food, human), and is out of place on a defined solid surface.
15Many studies have been undertaken looking at the retention and cleanability of soil material using a basic soil consisting of starch as it is a source of carbohydrate (Boyd et al., 2001; Detry et al., 2011), using milk soil as it contains a mixture of carbohydrates, fatty acid esters, and proteins (Kon et al., 1961; Boyd et al., 2001) and using edible oil (Detry et al., 2007). In studying some surface-related aspects of stainless steel cleaning or heat transfer surfaces fouled by proteins, the proteinaceous soil models generally used have been whey protein, β-lactoglobulin (β-LG), egg albumin and heat induced whey gels used as a simple system model of a fouling agent (Fickak et al., 2011). Initial studies have been carried out on a pilot scale plate heat exchanger (PHE), described in detail by Christian et al. (2002), and using whey protein concentrate solution as a processing fluid to simulate milk fouling. Gordon et al. (2012) used a gelatin and egg yolk solution as the model material to investigate enzyme-based cleaning of protein soils. Organic soils are often studied in paste form. Thus, baked tomato paste with a known composition (protein, carbohydrate, sugar, fat, fiber and water) and bread dough (60.6% plain flour, 1.8% wet yeast, 1.2% salt and 36.4% water) have been used in a model to investigate the effect of surface treatment on the removal of a food soil (Saikhwan et al., 2006). Liu et al. (2002; 2006) used the same soil models to study the development and use of a micromanipulation technique for measuring the force required to disrupt deposits and to remove, for example, a fouling deposit of whey protein concentrate.
16Oily soil models used to evaluate the cleanability of solid surfaces have involved the use of food grade sunflower oil sprayed or applied as a film deposition (Boulangé-Petermann et al., 2006; Detry et al., 2007), food grade mineral oil (Dillan et al., 1979) or hexadecane. Motor oil has also been used to study its removal in laundry detergent at low salinity. The relationship between micro-emulsion phase behavior and detergency has been studied for oily soils (Tongcumpou et al., 2005; Tanthakit et al., 2009), and for concentrated food emulsion (oil-in-water) (mayonnaise composition) including virgin olive oil, sunflower oil or soybean oil (Michalski et al., 1999). Basu et al. (1996) used bitumen to investigate oil displacement on solid surfaces in an aqueous environment. Both Liu et al. (2006) and Jurado-Alameda et al. (2012) used a fatty food (pork lard) as a fatty soil model to assess the capacity of ozone to facilitate the removal of fatty soils from hard surfaces and to develop effective cleaning procedures involving ozone.
17Organic fouling remains a significant challenge in the membrane filtration application to desalination, drinking water, wastewater, food and other industrial applications. Developing strategies for fouling control has always been a major challenge in membrane research. Alginate and humic have been identified as major organic components in natural water, seawater and wastewater effluents. And these substances have been extensively used as model organic foulants to study membrane fouling in forward osmosis, reverse osmosis, pressure-driven membrane, ultrafiltration, nanofiltration and filtration processes (Ang et al., 2006; Resosudarmo et al., 2013; She et al., 2013). Alginate is used in membrane fouling research to represent polysaccharides and hydrophilic mater, while humic represents hydrophobic and natural organic matter. The influence of other types of organic matter on membrane fouling such as proteins and fatty acids has been studied in order to further develop understanding of overall fouling mechanisms and cleaning procedures, and the role of various physical and chemical interactions, such as intermolecular adhesion forces, initial permeate flux, and membrane orientation. In these studies, bovine serum albumin and octanoic acid, representing, respectively, proteins and fatty acids, were used as the organic foulant model (Mi et al., 2008; Ang et al., 2011).
18All the soil models mentioned above can be used to generate very useful data for the study of fouling and cleaning in controlled conditions. Such data may explain:
19– the mechanisms of action of a chemical agent in the breaking down of a soil;
20– the surface parameters influencing the cleaning process (roughness, hydrophobicity);
21– the adhesive strength of a soil to various substrates;
22– the effect of soil aging on its adherence or the determination of the appropriate surface modification that will mitigate fouling;
23– how to reduce soil adhesion or facilitate soil removal.
24However, the adhesion between deposit and substrate varies with the surface characteristics, inducing lack of reproducibility from one surface to another. The surfaces investigated in the studies described exhibited varying degrees of robustness towards repeated fouling and cleaning in manufacturing, indicating that some of the results of fouling and cleaning cycles in the laboratory obtained in limited numbers may be extended to real-life situations. A strong influence of organic compounds in the media can modify their adhesion behavior and thus, their cleanability. The use of oils, proteins and carbohydrates separately or as a mixture for soiling makes the method even more realistic: the attachment of microorganisms to equipment surfaces depends on environmental conditions, amongst other criteria. Fouling may also result from more than one mechanism. For example, proteins and calcium phosphate interact with surfaces during milk processing (Rosmaninho et al., 2008) and the deposition of protein aggregates involves both reaction and particulate fouling. Membrane fouling in the presence of organic matter can be influenced by:
25– membrane characteristics, including surface structure as well as surface chemical properties;
26– the chemistry of the feed solution, including ionic strength, pH and the concentration of monovalent ions and divalent ions;
27– the properties of organic matter, including molecular weight and polarity;
28– hydrodynamic and operating conditions including permeate flux, pressure, concentration polarization;
29– the mass transfer properties of the fluid boundary layer (Al-Amoudi, 2010).
3.2. Mineral fouling
30Mineral scale formation or fouling is a process in which unwanted mineral materials, originally dissolved in processed fluids, are deposited onto heat transfer surfaces (Helalizadeh et al., 2000). The term “mineral scale” is used to differentiate fouling due to inorganic salt deposits from organic fouling and biofouling. Mineral scale is a recurring problem in processing plants, water heat exchangers, membrane filtration applications, household equipment and steam generation units (Kazi et al., 2010; Zhao et al., 2011). Inorganic fouling on heat exchanger surfaces and on filtration membranes is one of the most frequent problems in processing industries due to the inverse solubility of salts. Crystallization fouling is usually caused by the precipitation of salts, when their solubility limit is reached. The mechanism of crystallization fouling lies at the heart of many natural and technological processes, from the production of pharmaceuticals, food and nano-materials to oil refinery or crude oil treatment, and gas, petrochemical and scale deposition (Fiona et al., 2009), etc. Although crystallization is the underlying mechanism for scale formation, the process of deposition in exchangers is different from that occurring in industrial crystallizers (Sheikholeslami, 2003). Many researchers and industrialists have been challenged by scaling problems and have witnessed their complexity (Zhao et al., 2011). Mineral fouling has been studied for many years. On reviewing the large body of information available in this area, it appears that calcium salts (calcium carbonate, calcium sulfate, etc.) are the main types of mineral scale in heat exchangers and in filtration membrane applications. Investigations have indicated that the solubility of calcium sulfate is strongly affected by the presence and concentration of other ions in the system; water quality greatly affects induction times and the precipitation of calcium sulfate. In an attempt to optimize the thermal treatment processes of milk, several studies have been undertaken to improve the understanding and reduction of the mineral fouling process and to improve cleaning processes (Christian et al., 2002; Kazi et al., 2009). The main mineral soil models used for all the studies cited above were solutions of differing mineral content: whey protein concentrated solution with calcium and phosphorus; oxalate monohydrate (COM) and amorphous silica (SiO2) in aqueous solution; calcium chloride solution for calcium carbonate deposition and sodium dihydrogen phosphate solution for calcium phosphate deposits in addition to calcium chloride, iron oxides and other calcium and mineral salts, oils or light greases. In filtration, automotive and heavy equipment testing, Arizona road dust (ARD) has been widely used as a standard test dust (aerosol). This is also an excellent choice for studying sand ingestion in jet engines as it has very similar properties to sands found throughout the world and is readily available (Reagle et al., 2012).
31Mineral fouling on surfaces in processing industries is still a complicated phenomenon and the mechanism is not yet fully understood. Most of the soil models used are generally too basic to reflect the reality of the industrial environment, even though they provide a partial understanding of mineral fouling and cleaning mechanisms.
3.3. Microbial foulants
32Microbial adhesion to surfaces and the consequent formation of biofilms has been documented in many different environments. Biofilms remain a concern in a broad range of areas and specifically in the food, environmental and biomedical fields (Verran, 2002; Maukonen et al., 2003). There is a natural tendency of microorganisms to attach to wet surfaces, to multiply and to embed themselves in a slimy matrix composed of self-produced extracellular polymeric substances (EPS), forming a biofilm. Studies investigating microbial adhesion and cleaning or disinfection are generally carried out using microbial soil models such as microbial cell and spore suspension, biofilm or other material inoculated with spores.
33Microbial cell suspension. Adhesion of cells to each other and to foreign surfaces is the key to multicellular development, colonization and pathogenesis. Specific cell surface proteins, called adhesins, predominantly confer adhesive properties (Brückner et al., 2012). In the hygienic assessment of polymeric coating, cleanability assessments of surfaces in industries using a microbiological approach involving different bacteria cell suspensions have included: Saccharomyces cerevisiae (Callewaert et al., 2005; Guillemot et al., 2006; Brückner et al., 2012), Enterococcus faecalis (Boulangé-Petermann et al., 2004), Escherichia coli (Foschino et al., 2003), Campylobacter and Salmonella species (De Cesare et al., 2003), Pseudomonas aeruginosa and Staphylococcus aureus (Verran et al., 2001), Streptrococcus bovis, Streptrococcus waus and Streptrococcus thermophilus (Flint et al., 2000), Bacillus stearothermophilus (Flint et al., 2001). Some of the findings of these studies were as follows:
34– S. cerevisiae is a model system suitable for studying not only the mechanisms and regulation of cell adhesion, but also the role of this process in microbial development, ecology and evolution (Brückner et al., 2012);
35– bacterial survival is governed by environmental factors such as relative humidity and the presence of nutrients in coatings, with the surface properties of the coatings playing a secondary role (Boulangé-Petermann et al., 2004);
36– the type of surface finish on AISI 304 stainless steel (shot treated or not) had no significant effect on the cleanability of stainless steel. In the tested conditions, AISI 304 stainless steel was confirmed as a suitable material for the food industry, since it showed a remarkable ease of removal of bacteria before they could develop into a biofilm (Foschino et al., 2003);
37– both the contact surface and the level of organic matter can influence the survival and persistence of C. jejuni and Salmonella species on food contact surfaces (De Cesare et al., 2003);
38– unavoidable “sticky” compounds present on the S. cerevisiae cell wall could not be completely removed during successive washings of a rehydrated cell suspension before use. This led to a dramatic alteration of the surface properties of the bacterium and to a modification of its adhesive strength on 316L stainless steel, thus clearly demonstrating the necessity to work with yeast from fresh cultures (Guillemot et al., 2006).
39Bacterial spore suspension. Bacterial spores are among the most resistant forms of living organisms. Their resistance favors their long-term persistence and prolonged survival during food processing. This contamination of equipment with bacterial spores resistant to cleaning operations is favored by the strong adhesive properties of many species of endospore forming bacteria, such as Bacillus cereus, and by their ability to form biofilms (Auger et al., 2009). Packaging material may also harbor bacterial spores and contaminate the processed product (Pirttijärvi et al., 1996). The hygienic risk associated with microbial soil on surfaces is often evaluated by spore suspension, for example: Aspergillus niger (Foschino et al., 2003), and Bacillus (Faille et al., 2007; Blanpain-Avet et al., 2011). Pseudomonas fragi, Photobacterium leiognathi, Bacillus thuringiensis and Bacillus stearothermophilus suspended in phosphate buffer or in saline have also been chosen for trial work, as part of a program focusing on developing test conditions for materials, soil application methods and cleaning protocols that could be standardized. These spores are known to attach well to surfaces. They have been detected after cleaning trials and have been found to be relatively safe for controlled aerosol dispersal during spray cleaning (Holah, 2000). Mercier-Bonin et al. (2011) used B. cereus spores as a simplified spore model to determine their adhesive force on AISI 316L stainless steel. The authors reported that the ability of the spores to attach to AISI 316L stainless steel was mainly affected by the presence (and number) of appendages such as the existence of discrete bonds or of local clusters of anchoring sites and heterogeneity within the spores.
40Biofilms as microbial soil models. Biofilms constitute a protected mode of growth that allows microorganisms to survive in hostile environments, their physiology and behavior being significantly different from their planktonic counterparts. In industry, biofilms may be a source of recalcitrant contamination, causing food spoilage. They also represent a possible source of public health problems such as outbreaks of food borne pathogens. Biofilms are difficult to eradicate due to their resistant phenotype. Moreover, conventional cleaning and disinfection regimens may also contribute to ineffective biofilm control and to the dissemination of resistance. Consequently, new control strategies are constantly emerging, focusing mainly on the use of biosolutions (enzymes, phages, interspecies interactions and antimicrobial molecules of microbial origin) (Simoes et al., 2010). Biofilms are problematic in particular food industry sectors such as brewing, dairy processing, fresh products, poultry and red meat processing (Somers et al., 2004; Chen et al., 2007). Different soil models have been used as part of a biofilm approach to evaluate the cleanability of certain substrates. Beresford et al. (2001) used a Listeria monocytogenes cell suspension to investigate the adhesion of Listeria cells in biofilm to 17 different food-use approved materials representing metals, rubbers and polymers. It has been shown that adhesion to a wide range of materials is time-dependent and characterized by reversible and irreversible stages. A mixed biofilm of L. monocytogenes and Flavobacterium spp. was used by Bremer et al. (2001) to study the survival of L. monocytogenes attached to stainless steel surfaces in the presence or absence of Flavobacterium spp. That study showed that Flavobacterium spp. increased significantly the number of L. monocytogenes cells attached to stainless steel. Adoue et al. (2007) investigated the role of surface protein in bacterial adhesion to stainless steel. The authors used protein-coated amine latex microbeads as a simplified bacterial model within the context of a long-term study of biofilm formation in a marine environment. Results showed that the BSA-coated latex beads promoted an increased adhesion to stainless steel in comparison with bare beads. Knight et al. (2010) developed and trialed a soil model system for evaluating the efficacy of disinfectants to inactivate bacteria present in biofilm on surfaces within a dairy factory environment. The soil model system used was generated using a single or mixed isolates (pseudomonas, coliforms and staphylococci) added to 10% (v/v) UHT whole milk. Flint et al. (2001) reported an investigation into the use of conductance methods to monitor the development of biofilms of thermophilic streptococci on a stainless steel surface. They observed biofilm formation on the internal surfaces of plate heat exchangers used in milk processing and examined some of the factors involved in the formation of a biofilm of B. stearothermophilus. Gibson et al. (1999) and Frank et al. (2001) studied the effectiveness of cleaning techniques used in the food industry through the removal of bacterial biofilms using a soil model biofilm of P. aeruginosa or S. aureus. Parkar et al. (2004) used a biofilm of B. thermophilus spp. to investigate the mechanism of removal and inactivation of biofilms that optimally grow on surfaces (stainless steel). Results indicated that the biofilm was more difficult to remove than milk-based soil.
41Microorganisms in biofilms are more resilient to biocides, and these biofilms can therefore be a persistent source of contamination in biomedical food application. Preventing biofilm formation by minimizing the adhesion of microorganisms to surfaces represents a key strategy in reducing the risk of contamination. The development of anti-adhesive and/or anti-microbial materials is highly valuable and is therefore of major interest, constituting an important field of investigation. Numerous strategies have been used to minimize the adhesion of microorganisms, such as minimizing surface roughness, and coating surfaces with anti-microbial agents and anti-adhesive compounds. The use of natural biological macromolecules as antifouling coatings has recently been demonstrated. Meyer et al. (2013) showed that conditioning stainless steel surfaces with an aqueous extract of fish proteins could prevent adsorption and reduce bacterial adhesion to stainless steel. Increasing attention is being paid to silver-based products, due to the broad-spectrum biocidal activity of silver toward many bacteria, fungi, and viruses. In studies by Mercier-Bonin et al. (2012) and by Saulou et al. (2012), plasma-mediated coatings, containing silver nanoparticles embedded in an organosilicon or silica-like matrix, were deposited onto stainless steel in order to evaluate their anti-adhesive potentialities towards E. coli and S. cerevisiae. These studies revealed the promising capacity of deposited nanosilver-containing films in curbing the formation of biofilms on stainless steel. This technology may find its place as anti-adhesive and/or anti-microbial coatings for a wide variety of food industry, biomedical and general use applications involving spoilage or pathogenic microorganisms (Saulou et al., 2012).
42Attention should also be paid to surface nanomechanical properties, which could also influence spore removal. Such a focus would help facilitate further identification of morphological, nanomechanical and physico-chemical factors involved in spore adhesion, leading to better cleaning strategies for processing equipment in the future.
3.4. Particulate soil models
43Particulate fouling is defined as the deposition of unwanted material (particles) on a surface. In the literature, the term “solid particle” is used to designate organic or heavy precipitated minerals in dry state. In fabric detergency, for example, the soils present may be classified as particulates (solids, usually inorganic), oils (usually organic and liquid, sometimes also waxy solids) or stains (unwanted dyestuffs) (Carrol, 1996). Particulate adhesion and removal have been widely studied. Price et al. (2002) and Saint-Lorant et al. (2007) investigated the adhesion of powder to substrate surfaces using mannitol, maltitol, lactose monohydrate, pregelatinised starch, polyethyleneglycol and heavy precipitated calcium carbonate powder. Rennie et al. (1998) used an agglomerated whole milk powder to study the adhesion of dairy powders to equipment surfaces during the drying process. In a study of the use of surfactants for particulate soil removal in dry-cleaning with carbon dioxide, Van Roosmalen et al. (2004) used clay particles as the particulate soil model. Polystyrene latex particles have been used as a model suspension in many studies including deposition, adsorption and/or kinetics of particle deposition (Adamczyk et al., 2007; Szyk-Warszynska et al., 2007). Xue et al. (2012) used gum arabic (GA) powder to investigate the fouling of a copper surface. Elzo et al. (1996) studied mineral particle deposition on a membrane surface using glass particles as mineral particles. Polystyrene latex, melamine, glass beads and polymethylmethacrylate (Pasquino et al., 2013) are commonly used in the laboratory as particulate models. These particles are also utilized for their physico-chemical properties in a variety of further applications, including electrostatic forces generated by a net charge, an electric potential, Lifshitz–van der Waals forces, acid/basic properties, density, refractive indices, elastic properties, topography and hydrophobicity, all of which directly influence the adhesion process. Parameters such as particle shape, size, roughness, amorphous content and crystalline form may also affect adhesion and the subsequent cleaning process.
44The physics of transport, deposition, detachment and re-entrainment of particles suspended in a fluid are of great interest in many areas of fluid engineering: fouling of heat exchangers, contamination of nuclear reactors, plugging of filtration membranes, occlusion of human veins, and deposits in the micro-electronics and the paper industries. Although many models have been developed to predict the occurrence of these particular phenomena in industrial applications, we are still far from having a comprehensive description of the interplay between all the physico-chemical mechanisms involved in particulate fouling. In order to gain a better understanding of the adhesion and cleaning mechanisms of particulate soils, it seems appropriate to look at all the elements of the environment in which or from which the soils are derived, or to examine the nature of the particulate soils. The studies conducted to date have often focused on the particle itself, disregarding the involvement of other elements such as minerals or organic elements from the external environment.
3.5. Composite soils
45Composite soils can be considered as artificial complex soils reflecting the industrial or domestic reality. However, according to the literature, it is uncommon or even impossible to find an exact reproduction of industrial or domestic soils when studying soil adhesion or removal. Thus, in their aim of achieving a better understanding of adhesion and cleaning mechanisms, researchers are forced to approximate real-life situations by developing artificial soil models. Studies to assess the fouling, cleanability and disinfection of dairy processing equipment have used the following: stirred yoghurt inoculated with spores (Leclercq-Perlat et al., 1994), buttermilk inoculated with spores of B. stearothermophilus (Holah, 2000; Frank et al., 2001), milk inoculated with spores (Wong, 1998), pudding inoculated with spores of B. cereus (Bénezéck et al., 2002), suspensions of whole milk powder (Boyd et al., 2001; Verran et al., 2001), reconstituted milk (Wong, 1998; Chen et al., 2004), milk (Yang et al., 1991; Flint et al., 2001), yellow film encountered in a dairy (Maxcy, 1972), and whole milk mixed with isolates of pseudomonas, coliforms and staphylococci (Knight et al., 2010).
46Composite soils are generally used to provide a more complicated soil, and they are cleaned from selected samples using different methods. In order to assess the cleanability of stainless steel, Boyd et al. (2001) and Liu et al. (2006) used a concentrate starch soil suspension combined with the S. aureus bacterium, baked tomato paste with a known composition (proteins, carbohydrates, sugars, fat, fiber and minerals). The authors found that cleanability was affected by temperature, the concentration of the cleaning agent and the type of the cleaning process, exposure time, surface roughness, and the size of the starch molecules and of the cells of the bacterium. Gram et al. (2007) used L. monocytogenes in cold-smoked salmon juice or in an emulsion of a meat sausage to evaluate the effectiveness of cleaning and disinfecting products against L. monocytogenes attached to food-soiled inert surfaces in a laboratory model. Results showed that the efficacy of cleaning and disinfection products against L. monocytogenes was strongly influenced by the food matrix. Dourou et al. (2011) used beef fat, lean tissue and ground beef inoculated with E. coli to examine the attachment and biofilms formation on food-contact surfaces encountered in beef processing environments. The results of that study indicated that E. coli attachment to beef-contact surfaces was influenced by the type of soiled substrate and by temperature. In a study of the use of surfactants for particulate soil removal in dry-cleaning with carbon dioxide, Van Roosmalen et al. (2004) used as their soil models sebum (skin fat) colored with carbon black (oily and greasy soils combined with particulate soils), egg yolk (proteins and starchy soils, oily and greasy soils), butterfat with colorant (oily and greasy soils) and vegetable oil colored with chlorophyll (oily and greasy soils combined with oxidizable or bleachable soils). The authors concluded that the charged surfactant particles that formed were responsible for the removal of soil particles from the textiles. In 2000, initial work within the study concentrated on developing test conditions for materials. Three organic soils were chosen for comparative studies, as these were shown to be easily created, stable, easy to apply and relatively detectable after cleaning. These were: “Campden” soil, a mixture of milk, starch and oil; a margarine based soil for use in the assessment of moderately sized closed equipment and a soured milk based soil for use in the EHEDG Cleanability Test Method for small sized closed equipment (Holah, 2000).
47When dealing with problems relating to fouling or its removal, it is beneficial to use a complex approach, because fouling may result from more than one mechanism. For example, proteins and calcium phosphate interact in milk fouling (Rosmaninho et al., 2008), and the deposition of protein aggregates involves both reaction and particulate fouling. Fouling also depends on various parameters such as solution or environmental composition (source, presence of suspended particles, effect of supersaturation, pH, etc.), operating parameters (flow velocity, thermal flux, surface and bulk temperature, etc.) and surface characteristics (energy, roughness, etc.). Although often approximating the industrial reality, the use of composite soils and methods of cleaning and disinfection that give excellent results in the laboratory rarely provide the expected results in real-life application in industry. Transferring deposition or removal results from laboratory scale facilities to large-scale processing units raises different challenges from those normally addressed when dealing with heat exchanger surfaces. In the laboratory, using a simple tube or surface unit test rig, we can readily approach the Reynolds numbers with known wall shear stress for a sample or tube heat exchanger. However, it is much more difficult to match the high Reynolds numbers or wall shear stress in other processing units, particularly with vapor phase flow. Although elements of the fouling mechanisms may be the same (e.g. transport, adhesion, aging), scale-up issues can be very different from the case of projecting single-tube laboratory results. Challenges in translating laboratory adhesion or removal data to plant units include not only the usual scale-up difficulties, but also problems in understanding the behavior of soils consisting of many components. The process by which deposits are formed in the systems represents a further challenge. For example, Watkinson et al. (2011) showed that for heavy hydrocarbon fouling from the vapor-phase, laboratory results taken in the laminar or low turbulent flow (compared to those in industry) indicated physical condensation on the wall as the main cause of deposition. At plant scale, bulk condensation appeared to be the primary cause of fouling. Although velocities in the laboratory were close to those in the plant, the high Reynolds numbers and wall shear stress of the plant could not be matched. Simple transport models show the importance of droplet size in plant conditions, but do not account for the adhesion process.
4. Real-life conditions of application
48All the aforementioned laboratory soil model systems can be used to generate very useful information in the study of cleaning and fouling in controlled conditions. This information may include:
49– the mechanisms of action of a chemical in breaking down soil;
50– the surface parameters influencing the cleaning process;
51– the adhesive strength of a soil to various substrates;
52– the effect of soil aging on its adherence or the determination of the appropriate surface modification that will mitigate fouling;
53– reducing soil adhesion or facilitating soil removal.
54However, the experimental data are generally obtained for selected soil models. Furthermore, except perhaps in the case of soil panels, these experimental data cannot be easily applied “as generated” raw data to soils with a complex composition in order to predict how the equipment will become fouled or to identify the appropriate cleaning method. This was well illustrated by Watkinson et al. (2011), who attempted to extend concepts of fouling from heat exchangers to processing equipment. The study revealed that for heavy hydrocarbon fouling from the vapor-phase, for example, laboratory results taken in the laminar or low turbulent flow (compared to those in industry) indicated that physical condensation on the wall was the root cause of deposition. For the plant, bulk condensation appeared to be the primary cause of fouling. Although velocities in the laboratory were close to those in the plant, the high Reynolds numbers and wall shear stress of the plant could not be matched. This was due more to the geometry of the actual channels in the plant, leading to a complex flow, than it was due to the mode of flow. However, the level of shear stress in real time can be effectively reproduced in the laboratory, and even amplified. For example, a turbulent flow has a level of average shear stress somewhat lower than the shear stress of a laminar flow in a low height channel (Lorthois et al., 2001). Simple transport models have shown the importance of droplet size for plant conditions, but have not accounted for the adhesion process. Indeed, other phenomena associated with soil, such as solution or environmental composition (source, presence of suspended particles, effect of supersaturation, pH, etc.) (Kazi et al., 2010), operating parameters (flow velocity, thermal flux, surface and bulk temperature, etc.) and surface characteristics (energy, roughness, etc.) (Albert et al., 2011) have been suggested to play a non-negligible role in soiling and cleaning. Pilot and full-scale studies are expensive but valuable; smaller scale experiments are often problematic for a range of reasons, as outlined below:
55– Deposits on which experiments are conducted are often not truly representative of industrial systems. It is difficult to create reproducible fouling deposits. Furthermore, soil aging is not well understood, so the condition of real-life deposits can often not be reproduced. Model deposits, such as whey proteins used to simulate pasteurizer behavior, can give different results to real-life soils (Christian et al., 2002);
56– results from experiments carried out are in a form that is difficult to scale-up, and the scale-up rules themselves are not known. The relationship between the cleaning rate and extent at a different scale (enabling the prediction of industrial cleaning times from pilot or lab scale data) is unclear. However, quantitative indications, for example, of the shear stress threshold necessary for the detachment of the soils are provided. The shear stress threshold value depends on the environmental conditions. The usual techniques developed in the laboratory to obtain quantitative information on soil adhesion and detachment are represented by hydrodynamic systems (flow cell, impinging jets, rotating disks, fluid dynamic gauging), optical methods (atomic force microscopy, confocal laser scanning microscopy, optical tweezers), and ultrasonic methods;
57– the difficulty of comparing the cleaning of different soils in a way that generates an understanding of the mechanisms involved, for example, allowing the cleaning time of one soil to be reliably predicted from the cleaning time of another;
58– the lack of effective online measurement methods available for process validation and for identifying the cleaning endpoint. It is, of course, possible to dismantle the equipment and find residues of deposit or any organisms present by swabbing and assaying. This is often the only way of measuring cleaning efficacy in the pharmaceutical industry. It is not feasible to undertake this process online, as generally, standards are set at plant start-up and some swabbing done as part of QA (quality assurance) procedures. To date, industry has evaluated the state of fouling with reference to general measurements such as pressure drop or the thermal performance drift of heat exchangers. For the first time, the National Institute of Agronomic Research (INRA, 2013) has developed a fouling sensor based on the principle of the hot wire. It is simple to install and use. This sensor is used to assess levels of contamination at critical points during the use of equipment and makes it possible to follow online the fouling of any installation treating a body of fluid: a fluidic communication pipe or any type of engine (heat exchanger, recessing engine, etc.). Further research is being conducted in order to develop new solutions;
59– scaling up lab or pilot plant data is difficult (if the mechanism of cleaning is not understood) and so scaling up is going to be an empirical process (Fryer et al., 2009). The practical consequences of this are that the best way of cleaning each type of soil has been developed largely independently, so information on how to clean one particular material is not applied so easily to the cleaning of others.
5. Conclusion
60The existing different soil model systems used for testing cleaning methods, and for assessing adhesion and cleanability have been reviewed. This may be useful in developing a basic understanding of the principles of cleaning soils. The soil models described in this review provide recommended test soils that can be used as a basis for comparing or verifying the cleanability of new and existing food-processing equipment. Furthermore, the use of such soil models should facilitate the assessment of the comparative cleanability of different items of equipment assessed at different times or in different laboratories. While the method has been shown to be reproducible in the laboratory, new researchers may require a degree of familiarization with the specific techniques. These soil model systems can also be readily adapted to investigate the influence of cleaning parameters, such as application time, concentration of cleaning products, temperature, product type and interactions between cleaning products and disinfectants. The soil models generally used are sometimes versatile, and there are many avenues of investigation still to be explored, such as the effect of soluble macromolecules on soil adherence.
61Acknowledgements
62The authors are grateful to Professor Paul G. Rouxhet for his time and for the rich and useful discussions throughout this study. They are also grateful to Mrs Lynn Doran for her English-language assistance.
Bibliographie
Adamczyk Z. et al., 2007. Characterization of poly(ethylene imine) layers on mica by the streaming potential and particle deposition methods. J. Colloid Interface Sci., 313, 86-96.
Adoue M. et al., 2007. Experimental methodology for analysing macromolecular interactions in the context of marine bacterial adhesion to stainless steel. Chem. Eng. Res. Des., 85(A6), 792-799.
Al-Amoudi A.S., 2010. Factors affecting natural organic matter (NOM) and scaling fouling in NF membranes: a review. Desalination, 259, 1-10.
Albert F., Augustin W. & Scholl S., 2011. Roughness and constriction effects on heat transfer in crystallization fouling. Chem. Eng. Sci., 66, 499-509.
Ang W.S., Lee S. & Elimelech M., 2006. Chemical and physical aspects of cleaning of organic-fouled reverse osmosis membranes. J. Membr. Sci., 272, 198-210.
Ang W.S., Tiraferri A., Chen K.L. & Elimelech M., 2011. Fouling and cleaning of RO membranes fouled by mixtures of organic foulants simulating wastewater effluent. J. Membr. Sci., 376, 196-206.
Auger S. et al., 2009. Biofilm formation and cell surface properties among pathogenic and nonpathogenic strains of the Bacillus cereus group. Appl. Environ. Microbiol., 75, 6616-6618.
Bansal B., Chen X.D. & Müller-Steinhagen H., 2008. Analysis of “classical” deposition rate law for crystallisation fouling. Chem. Eng. Process. Process Intensif., 47, 1201-1210.
Basu S., Nandakumar K. & Masliyah J.H., 1996. A study of oil displacement on model surfaces. J. Colloid Interface Sci., 182, 82-94.
Bénézech T. et al., 2002. A new test method for in-place cleanability of food processing equipment. J. Food Eng., 54, 7-15.
Beresford M.R., Andrew P.W. & Shama G., 2001. Listeria monocytogenes adheres to many materials found in food-processing environments. J. Appl. Microbiol., 90, 1000-1005.
Blanpain-Avet P., Faille C., Delaplace G. & Bénézech T., 2011. Cell adhesion and related fouling mechanism on a tubular ceramic microfiltration membrane using Bacillus cereus spores. J. Membr. Sci., 385-386, 200-216.
Boulangé-Petermann L., Robine E., Ritoux S. & Cromières B., 2004. Hygienic assessment of polymeric coatings by physico-chemical and microbiological approaches. J. Adhes. Sci. Technol., 18(2), 213-225.
Boulangé-Petermann L., Gabet C. & Baroux B., 2006. On the respective effect of the surface energy and micro-geometry in the cleaning ability of bare and coated steels. Colloids Surf., A, 272, 56-62.
Boyd R.D. et al., 2001. The cleanability of stainless steel as determined by X-ray photo-electron spectroscopy. Appl. Surf. Sci., 172, 135-143.
Bremer J.P., Ian M. & Osborne M.C., 2001. Survival of Listeria monocytogenes attached to stainless steel surfaces in the presence or absence of Flavobacterium spp. J. Food Prot., 64(9), 1369-1376.
Brückner S. & Mösch H.U., 2012. Choosing the right lifestyle: adhesion and development in Saccharomyces cerevisiae. FEMS Microbiol. Rev., 36, 25-58.
Callewaert M., Rouxhet P.G. & Boulangé-Petermann L., 2005. Modifying stainless steel surfaces with responsive polymers: effect of PS-PAA and PNIPAAM on cell adhesion and oil removal. J. Adhes. Sci. Technol., 19(9), 765-781.
Carrol B., 1996. The direct study of oily soil removal from solid substrates in detergency. Colloid Surf., A, 114, 161-164.
Chen X.D., Li D.X.Y., Lin S.X.Q. & Necati Ö., 2004. On-line fouling/cleaning detection by measuring electric resistance - equipment development and application to milk fouling detection and chemical cleaning monitoring. J. Food Eng., 61, 181-189.
Chen J., Rossman M.L. & Pawar D.M., 2007. Attachment of enterohemorragic Escherichia coli to the surface of beef and a culture medium. LWT – Food Sci. Technol., 40, 249-254.
Christian G.K., Changani S.D. & Fryer P.J., 2002. The effect of adding minerals on fouling from whey protein concentrate development of a model fouling fluid for a plate heat exchanger. Food Bioproducts Process., 80, 231-239.
Cliaudagne D., 1991. Fouling costs in the field of heat exchange equipment in the French market. In: Bohnet M. et al., eds. Fouling mechanisms: theoretical and practical aspects. Paris: Éditions Européennes Thermique et Industrie, 21-25.
De Cesare A., Sheldon B.W., Smith K.S. & Jaykus L.A., 2003. Survival and persistence of Campylobacter and Salmonella species under various organic loads on food contact surfaces. J. Food Prot., 66(9), 1587-1594.
Detry J.G. et al., 2007. Cleanability assessment of model solid surfaces with a radial-flow cell. Colloids Surf., A, 302, 540-548.
Detry J.G. et al., 2011. Physico-chemical mechanisms governing the adherence of starch granules on materials with different hydrophobicities. J. Colloid Interface Sci., 355, 210-221.
Dillan K.W., Goddard E.D. & McKenzie D.A., 1979. Oily soil removal from a polyester substrate by aqueous nonionic surfactant systems. J. Am. Oil Chem. Soc., 56, 59-70.
Dourou D. et al., 2011. Attachment and biofilm formation by Escherichia coli O157:H7 at different temperatures, on various food-contact surfaces encountered in beef processing. Int. J. Food Microbiol., 149, 262-268.
Elzo D., Schmitz P., Houi D. & Joscelyne S., 1996. Measurement of particle/membrane interactions by a hydrodynamic method. J. Membr. Sci., 109, 43-53.
Faille C., Tauveron G., Le Gentil-Lelièvre C. & Slomianny C., 2007. Occurrence of Bacillus cereus spores with a damaged exosporium: consequences on the spore adhesion on surfaces of food processing lines. J. Food Prot., 70(10), 2346-2353.
Fickak A., Al-Raisi A. & Chen D.X., 2011. Effect of whey protein concentration on the fouling and cleaning of a heat transfer surface. J. Food Eng., 104, 323-331.
Fiona C. & Richard P.S., 2009. Now you see them. Science, 322, 1802-1803.
Flint S.H., Brooks J.D. & Bremer P.J., 2000. Properties of the stainless steel substrate, influencing the adhesion of thermo-resistant streptococci. J. Food Eng., 43, 235-242.
Flint S. et al., 2001. The growth of Bacillus stearothermophilus on stainless steel. J. Appl. Microbiol., 90, 151-157.
Foschino R. et al., 2003. Comparison of surface sampling methods and cleanability assessment of stainless steel surfaces subjected or not to shot peening. J. Food Eng., 60, 375-381.
Frank J.F. & Chmielewski R., 2001. Influence of surface finish on the cleanability of stainless steel. J. Food Prot., 64(8), 1178-1182.
Fryer P.J. & Asteriadou K., 2009. A prototype cleaning map: a classification of industrial cleaning processes. Trends Food Sci. Technol., 20, 255-262.
Gao W.J. et al., 2013. Characteristics of wastewater and mixed liquor and their role in membrane fouling. Bioresour. Technol., 128, 207-214
Gibson H., Taylor J.H., Hall K.E. & Holah J.T., 1999. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J. Appl. Microbiol., 87, 41-48.
Gordon P.W. et al., 2012. Elucidating enzyme-based cleaning of protein soils (gelatin and egg yolk) using a scanning fluid dynamic gauge. Chem. Eng. Res. Des., 90, 162-171.
Gram L. et al., 2007. Influence of food soiling matrix on cleaning and disinfection efficiency on surface attached Listeria monocytogenes. Food Control, 18, 1165-1171.
Guillemot G. et al., 2006. Shear-flow induced detachment of Saccharomyces cerevisiae from stainless steel: influence of yeast and solid surface properties. Colloids Surf. B, 49, 126-135.
Helalizadeh A., Müller-Steinhagen H. & Jamialahmadi M., 2000. Mixed salt cristallization fouling. Chem. Eng. Process., 39, 20-43.
Holah J., 2000. Food processing equipment design and cleanability. Dublin: The National Food Center.
HRS Group, 2011. Fouling factors in heat exchangers, http://www.hrs-heatexchangers.com/en/resources/fouling-factors-in-heat-exchangers.aspx, (07/01/12).
INRA, 2013. Capteur pour l'étude et le suivi de l'encrassement dans les procédés continus, http://www.inra-transfert.fr/offres-technologiques.php?optim=capteur-pour-_xe9-tude-suivi-encrassement-5yc1t, (21/01/13).
Jennings W.G., 1965. Theory and practice of hard-surface cleaning. Adv. Food Res., 14, 325-458.
Jurado-Alameda E., García-Román M., Altmajer-Vaz D. & Jiménez-Pérez J.L., 2012. Assessment of the use of ozone for cleaning fatty soils in the food industry. J. Food Eng., 110, 44-52.
Kazi S.N., Duffy G.G. & Chen X.D., 2009. Fouling and fouling mitigation on different heat exchanging surfaces. In: Müller-Steinhagen H., Malayeri M.R. & Watkinson A.P., eds. Proceedings of International Conference on Heat Exchanger Fouling and Cleaning VIII, 14-19 June 2009, Schladming, Austria, 367-377.
Kazi S.N., Duffy G.G. & Chen X.D., 2010. Mineral scale formation and mitigation on metals and a polymeric heat exchanger surface. Appl. Therm. Eng., 30, 2236-2242.
Knight G.C. & Craven H.M., 2010. A model system for evaluating surface disinfection in dairy factory environments. Int. J. Food Microbiol., 137, 161-167.
Kon S.K. & Cowie A.T., 1961. Milk: the mammary gland and its secretion. New York, USA: Ed. Academic Press.
Kulkarni S.M., Maxcy R.B. & Arnold R.G., 1975. Evaluation of soil deposition and removal processes: an interpretive review. J. Dairy Sci., 58(12), 1922-1936.
Lalande M., Rene F. & Tissier J.P., 1989. Fouling and its control in heat exchangers in the dairy industry. Biofouling, 1, 233-250.
Leclercq-Perlat M.N. & Lalande M., 1994. Cleanability in relation to surface chemical composition and surface finishing of some materials commonly used in food industries. J. Food Eng., 23, 501-517.
Liu W., Christian G.K., Zhang Z. & Fryer P.J., 2002. Development and use of a micromanipulation technique for measuring the force required to disrupt and remove fouling deposits. Food Bioprod. Process., 80(C4), 286.
Liu W., Christian G.K., Zhang Z. & Fryer P.J., 2006. Direct measurement of the force required to disrupt and remove fouling deposits of whey protein concentrate. Int. Dairy J., 16, 164-172.
Lorthois S., Schmitz P. & Anglés-Canoy E., 2001. Experimental study of fibrin/fibrin-specific molecular interactions using a sphere/plane adhesion model. J. Colloid Interface Sci., 241, 52-62.
Maukonen J. et al., 2003. Methodologies for the characterization of microbes in industrial environments: a review. J. Ind. Microbiol. Biotechnol., 30, 327-356.
Maxcy R.B., 1972. Nature and cause of yellow film occuring on dairy equipment. J. Dairy Sci., 56, 164-167.
Mercier-Bonin M., Dehouche A., Morchain J. & Schmitz P., 2011. Orientation and detachment dynamics of bacillus spores from stainless steel under controlled shear flow: modelling of the adhesion force. Int. J. Food Microbiol., 146, 182-191.
Mercier-Bonin M. et al., 2012. Dynamics of detachment of Escherichia coli from plasma-mediated coatings under shear flow. Biofouling, 28, 881-894.
Meyer R.L. et al., 2013. Physicochemical characterization of fish protein adlayers with bacteria repelling properties. Colloids Surf. B, 102, 504-510.
Mi B. & Elimelech M., 2008. Chemical and physical aspects of organic fouling of forward osmosis membranes. J. Membr. Sci., 320, 292-302.
Michalski M.C., Desobry S., Babak V. & Hard J., 1999. Adhesion of food emulsions to packaging and equipment surfaces. Colloids Surf., A, 149, 107-121.
Paria S., 2003. Studies on surfactant adsorption at the cellulose - water interface. PhD. Thesis: Indian Institute of Technology, Bombay (India).
Parkar S.G., Flint S.H. & Brooks J.D., 2004. Evaluation of the effect of cleaning regimes on biofilms of thermophilic Bacilli on stainless steel. J. Appl. Microbiol., 96, 110-116.
Pasquino R., Panariello D. & Grizzuti N., 2013. Migration and alignment of spherical particles in sheared viscoelastic suspensions. A quantitative determination of the flow-induced self-assembly kinetics. J. Colloid Interface Sci., 394, 49-54.
Pirttijärvi T.S.M., Graeffe T.H. & Salkinoja-Salonen M.S., 1996. Bacterial contaminants in liquid packaging boards: assessment of potential for food spoilage. J. Appl. Bacteriol., 81, 445-458.
Potts D.E., Ahlert R.C. & Wang S.S., 1981. A critical review of fouling of reverse osmosis membranes. Desalination, 36, 235-264.
Price R., Young P.M., Edge S. & Staniforth J.N., 2002. The influence of relative humidity on particulate interactions in carrier-based dry powder inhaler formulations. Int. J. Pharm., 246, 47-59.
Quittet C. & Nelis H., 1999. HACCP pour PME et artisans : secteur produits laitiers. Gembloux, Belgique : Les Presses agronomiques de Gembloux.
Reagle C. et al., 2012. A novel optical technique for measuring the coefficient of restitution of microparticle impacts in a forced flowfield. In: ASME Turbo Expo 2012: Turbine Technical Conference and Exposition, Volume 7: Structures and Dynamics, Parts A and B, June 11-15, 2012, Copenhagen, Denmark, paper n°GT2012-68252, 1-9.
Rennie P.R., Chen X.D. & Mackereth A.R., 1998. Adhesion characteristics of whole milk powder to a stainless steel surface. Powder Technol., 97(31), 191-199.
Resosudarmo A., Ye Y., Le-Clech P. & Chen V., 2013. Analysis of UF membrane fouling mechanisms caused by organic interactions in seawater. Water Res., 47, 911-921.
Rosmaninho R. & Melo L.F., 2008. Protein calcium phosphate interactions in fouling of modified stainless-steel surfaces by simulated milk. Int. Dairy J., 18, 72-80.
Rowan C., 2005. Cleaning and sanitizing. Food Beverage Int., 46-49.
Saikhwan P. et al., 2006. Effect of surface treatment on cleaning of a model food soil. Surf. Coat. Technol., 201(3-4), 943-951.
Saint-Lorant G., Leterme P., Gayot A. & Flament M.P., 2007. Influence of carrier on the performance of dry powder inhalers. Int. J. Pharm., 334, 85-91.
Saulou C. et al., 2012. Plasma-mediated nanosilver-organosilicon composite films deposited on stainless steel: synthesis, surface characterization, and evaluation of anti-adhesive and anti-microbial properties on the model yeast Saccharomyces cerevisiae. Plasma Process. Polym., 9, 324-338.
She Q., Wong Y.K.W., Zhao S. & Tang C.Y. , 2013. Organic fouling in pressure retarded osmosis: experiments, mechanisms and implications. J. Membr. Sci., 428, 181-189.
Sheikholeslami R., 2003. Nucleation and kinetics of mixed salts in scaling. AIChE J., 49(1), 194-202.
Simoes M., Simoes L.C. & Vieira M.J., 2010. A review of current and emergent biofilm control strategies. LWT - Food Sci. Technol., 43, 573-583.
Somers E.B. & Wong A.C., 2004. Efficacy of two cleaning and sanitizing combinations on Listeria monocytogenes biofilms formed at low temperature on a variety of materials in the presence of ready-to-eat-meat residue. J. Food Prot., 67, 2218-2229.
Szyk-Warszynska L. & Trybala A., 2007. Deposition of core latex particles encapsulated in polyelectrolyte shells at modified mica surfaces. J. Colloid Interface Sci., 314, 398-404.
Tanthakit P. et al., 2009. Micro-emulsion formation and detergency with oily soil: V. Effects of water hardness and builder. J. Surfactants Deterg., 12, 173-183.
Tongcumpou C. et al., 2005. Micro-emulsion formation and detergency with oily soils: III. Performance and mechanisms. J. Surfactants Deterg., 8(2), 147-156.
Van Roosmalen M.J.E., Woerlee G.F. & Witkamp G.J., 2004. Surfactants for particulate soil removal in dry-cleaning with high-pressure carbon dioxide. J. Supercrit. Fluids, 30, 97-109.
Verran J., 2002. Biofouling in food processing: biofilm or biotransfer potential? Food Bioprod. Process., 80, 292-298.
Verran J., Rowe D.L. & Boyd R.D., 2001. The effect of nanometer dimension topographical features on the hygienic status of stainless steel. J. Food Prot., 64(8), 1183-1187.
Watkinson A.P., Fan Z. & Petkovic B., 2011. Extending fouling concepts from heat exchangers to process equipment. In: Malayeri M.R., Müller-Steinghagen H. & Watkinson A.P., eds. Proceedings of International Conference on Heat Exchanger Fouling and Cleaning, June 5-10, 2011, Crete Island, Greece, 15-22.
Wong A.C.L., 1998. Biofilms in food processing environments. J. Dairy Sci., 81, 2765-2770.
Xue H.S., Fan J.R. & Hu Y.C., 2012. Particulate fouling during the pool boiling heat transfer of MWCNT nanofluid. Heat Mass Transfer, 48, 875-879.
Yang J., McGuire J. & Kolbe E., 1991. Use of the equilibrium contact angle as an index of contact surface cleanliness. J. Food Prot., 54, 879-884.
Zhao X. & Chen X.D., 2011. A critical review of basic crystallography to salt crystallization fouling in heat exchangers. In: Malayeri M.R., Müller-Steinhagen H. & Watkinson A.P., eds. Proceedings of International Conference on Heat Exchanger Fouling and Cleaning, June 5-10, 2011, Crete Island, Greece.
Para citar este artículo
Acerca de: Yetioman Toure
Univ. Liege - Gembloux Agro Bio-Tech. Analysis Quality and Risk Unit. Laboratory of Agro-food Quality and Safety. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: yetioman.toure@doct.ulg.ac.be
Acerca de: Nicolas Mabon
Univ. Liege - Gembloux Agro Bio-Tech. Analysis Quality and Risk Unit. Laboratory of Agro-food Quality and Safety. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Marianne Sindic
Univ. Liege - Gembloux Agro Bio-Tech. Analysis Quality and Risk Unit. Laboratory of Agro-food Quality and Safety. Passage des Déportés, 2. B-5030 Gembloux (Belgium).





