A survey on the presence of undesirable botanical substances in feed in the European Union
RIKILT. Institute of Food Safety. P.O. Box 230. NL-6700 AE Wageningen (The Netherlands). E-mail: Leo.vanraamsdonk@wur.nl
Federal Agency for the Safety of the Food Chain (FASFC). Leuvensesteenweg, 17. B-3080 Tervuren (Belgium).
Danish Plant Directorate (DPD). Department for Feedingstuffs and Fertilizers. Laboratory for Feed and Fertilizers. Skovbrynet, 20. DK-2800 Lyngby (Denmark).
Abstract
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed lists a range of substances from botanical origin (weed seeds) and additionally some chemical compounds directly originating from specific weeds. In order to examine the actual status of enforcement and of the present occurrence of these botanical substances, a survey was carried out. A questionnaire was sent to 103 laboratories, including official control labs from all member states of the European Union. The results, indicating the frequency of occurrence as far as reported, are compared to the publications of the EU Rapid Alert System for Food and Feed (RASFF). A total of 44 questionnaires was returned (42.7%) from 22 member states. Ten member states predominantly from north-western Europe appeared to have an active monitoring of botanical undesirable substances. The questionnaire results did not indicate that the other member states enforce this part of Directive 2002/32/EC. Reports on the frequency of occurrence include: a few to 25-50% of the samples contain traces of ergot (8 member states), a few to 24% contain at least some traces of thorn apple (6 member states), zero to 17% contain some castor oil plant seeds (3 member states), zero to a few samples contain Crotalaria seeds (3 member states), and zero to 6% contain traces of Sareptian mustard (4 member states). One member state conducted extra surveillance since several cases of animal intoxications have been reported. In some cases a coincidence with undesirable botanical substances was found.
1. Introduction
1Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed lists a range of substances from botanical origin (weed seeds) and additionally some chemical compounds directly originating from specific weeds. A difference between these two categories is made from the analytical point of view. The substances from botanical origin are described in terms as seeds, fruits, seed hulls or processed products from seeds, and limits are described as traces or at the level of mg.kg-1. This paper will focus on these substances, which are detected by non-chemical methods, e.g. visual inspection.
2The Scientific Committee on Animal Nutrition has published an opinion (SCAN, 2003) on the entire list of substances mentioned in this directive, including those of botanical origin (indicated as “Botanical impurities”). SCAN concluded that the current practice of microscopic screening for whole seeds or parts thereof should be replaced by a quantitative chemical analysis of the harmful substances contained in these seeds. The majority of the listed species is considered to have only historical interest or poses no real hazard. It was therefore advised to rephrase the current specific listing to a more general statement for the prevention of these unwanted seeds, in order to accommodate for changes in agricultural practice. Besides the desire for dedicated chemical analysis, SCAN concludes that microscopy should be the primary method for the detection of botanical contamination for its flexibility and possibilities to handle emerging problems. Directive 2002/32/EC was not updated until now (January 2007) concerning the botanical substances1.
3A survey of the occurrence of undesirable substances of botanical origin in feeds can only be based on data from visual inspection, including microscopy. The current amount of data is scarce, at first glance. From networks of microscopists it can be concluded that not every member state of the European Union enforces actively the monitoring of these undesirable substances, although this is requested by Directive 882/2004/EC (formerly 95/53/EC). Before drawing conclusions on the occurrence or on being (almost) extinct of certain weed species, more information are desired on the status of monitoring programs and of their results.
4In this report a survey is presented on the current status of monitoring for undesirable substances of botanical origin in feeds, and on the results of these programs. The results, indicating the frequency of occurrence as far as available, are compared to the publications of the EU Rapid Alert System for Food and Feed, in which alert and information notifications are published from the competent authorities on a weekly basis. The full results have been published in a separate report (van Raamsdonk, 2007) with seven tables.
2. Methodology and organization
5During Spring 2006 a questionnaire was developed for collecting information on the status, scope and results of the monitoring program, if available on the laboratory of the addressees. This questionnaire consisted of three sections:
6– questions on the existence and scope of an active monitoring program for visual examination for undesirable substances of botanical origin. If no monitoring program was carried out, the respondent was asked to skip section 2,
7– tables for registering results and additional remarks for every individual weed seed,
8– questions concerning proposed deletions from or additions to Directive 2002/32/EC, and concerning required activities for maintaining or increasing expertise.
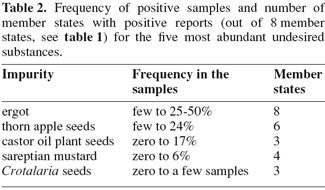
9This questionnaire was presented at the annual meeting of the IAG working group "Microscopy in Rostock", Germany, June 2006. After updating the text, it was sent to a total of 103 laboratories known to have microscopic expertise. At the closing date of 1 December 2006 a total of 44 returned questionnaires was received at RIKILT.
10The lists of the EU Rapid Alert System for Food and Feed (RASFF) are based on Regulation EC/178/2002, and are weekly published at: http://ec.europa.eu/food/food/rapidalert/index_en.htm. The lists of 2005 and 2006 were examined for any notification concerning undesirable substances of botanical origin.
3. Results and discussion
3.1. Response
11Forty-four laboratories returned their questionnaires, i.e. a response rate of 42.7%. Only three member states out of 25 did not return a questionnaire. There is good dispersion among member states.
12Almost 80% of the received questionnaires were returned by official control laboratories. In order to get an overview of the occurrence of the specific species undesirable substances of botanical origin, the results from these two categories have been pooled. In most cases the results from the year 2005 and/or 2006 were sent in. Some respondents, e.g. from member state B, sent in detailed lists of positive samples from the last ten years, or commented on average occurrences during a larger period of time.
3.2. Active maintenance of monitoring
13Twenty-seven out of 44 laboratories stated to have an active monitoring for undesirable substances of botanical origin. Of these laboratories, 9 indicated to have zero sample examined, which means that no effective monitoring is carried out. A total of 18 laboratories out of 44 respondents have actually examined samples of feeds and/or feed raw materials and eventually found undesirable botanical substances. One laboratory provided the results of a survey separately.
14The mentioned 18 labs reporting effective monitoring originate from 10 member states (see table 1). The results from the evaluations carried out by IRL and ES were not available for this survey. This means that actual results can be discussed in this report from 8 member states. Some of these 8 member states have organized the maintenance of the monitoring at regional levels. It appears in these cases that a part of these regions performs monitoring, whereas other regions in the same member states did not evaluate samples for the presence of undesirable substances.

15Directive 2002/32/EC requires macroscopic (by eye or at low magnifications) or microscopic (at high magnifications) evaluation of the samples, depending on the type of prohibition: either as whole seeds only or after processing as well. All reporting 18 laboratories stated that macroscopic examinations are carried out. Three of these laboratories did not perform microscopic examinations. In this way several botanical undesirable substances can almost not be encountered, especially the mustard species.
3.3. Occurrence of undesirable substances
16In the following paragraphs some of the botanical undesirable substances as listed in Directive 2002/32/EC (see table 2) will be discussed in more detail.
17Rye ergot - Claviceps purpurea (Fr.) Tul. Ergot is the most frequently mentioned undesirable substance: 12 laboratories reported either numbers of evaluated samples, or frequencies of occurrence, or both. Ergot appears to be present in low or relevant frequencies, up to 25-50%. The remark was made that ergot occurrence seems to have increased in recent years. As far as reported in the survey, a considerable amount of rye samples appeared to be contaminated. Some other cereal grains as well as compound feeds can contain ergot as well. Only one laboratory proposed to delete ergot from the list of undesirable substances provided that a solid chemical test for the detection of the alkaloids involved can take its place. More precise information on occurrence of Claviceps in feed materials, of ergot alkaloid distribution and on toxic effects are desired. This research need is also indicated in the opinion of the Scientific Panel on Contaminants in Food Chain on ergots (EFSA, 2005).
18In the questionnaire one laboratory reported 3 samples for 2005 and 2006 with amounts exceeding the legal limit according to Directive 2002/32/EC (1,000 ppm). These samples were not (yet) reported in the lists of the RASFF system.
19Thorn apple - Datura stramonium L. Eight laboratories reported data on the occurrence of thorn apple. In all cases it was found at least occasionally, with varying frequencies over the years. A variety of feed ingredients that can pose threats for the presence of thorn apple were mentioned: wheat, maize, soybean, linseed, sunflower, rapeseed and compound feeds. Some respondents provided detailed lists of results. One respondent reported the occurrence of thorn apple in maize grits at a level of 0.1% in 2006, which is at the legal limit of 1,000 ppm.
20The lists of RASFF include notifications for the presence of thorn apple during autumn 2006: it was found six times in millet samples (Urochloa ramosa (L.) T.Q.Nguyen, 5 samples) and canned green beans (1 sample) originating from HU and A, all for human consumption. Thorn apple at a high concentration level was reported once in red millet seeds for feeding purposes by D originating from HU. The absence of such reports in the returned questionnaires can be due to the fact that some German laboratories did not respond. In addition, atropine and scopolamine, the main alkaloids, were found twice in buckwheat flour during summer 2006. In the same periods of 2005 no reports were made in the RASFF listings. This absence in the notifications can be due to differing natural occurrences over the years, or to the absence of active monitoring in 2005. The occurrence of thorn apple in maize grit as mentioned in one questionnaire was not reported in the RASFF system. The presence in materials for human consumption can obviously not be reported in monitoring programs for feeds, but the combined data for all materials (food and feed) indicate that thorn apple is certainly not eradicated. Its occurrence and toxicity imply the need for a legal limit.
21Castor oil plant - Ricinus communis L. and Crotalaria spp. Only 4 laboratories indicated to perform monitoring for Ricinus or castor oil plant (see figure 1), and Crotalaria species. Material of these species is reported to occur occasionally by some countries. Although castor oil beans are usually very easy to recognize, seeds of Crotalaria species are much more difficult to detect. Respondents propose to keep these species in the list of undesirable substances because of their high toxicity. A member state found (in the years 1996 and 1998) two occurrences of R. communis that coincided with animal health incidences.

22Mustard species - Brassica spp. L. Between 2 and 4 laboratories reported to search for seeds of mustard species, depending on the species. Reported occurrences were (much) below 5%, except for one report from B (17%) for Sareptian mustard (Brassica juncea (L.) Czern. & Coss. ssp. juncea). Frequencies below 5% in an amount of 2-3 samples per year mean actually that no mustard seeds were found. It can be questioned whether some of these mustard seeds would need to be included on the list of undesirable substances, since they are used as spices for human consumption and for their moderate toxicity. A member state found that in 1999 two occurrences of B. juncea ssp. juncea coincided with chicken death incidences.
23Purghera - Jatropha curcas L. and Croton - Croton tiglium L. Only 2 laboratories reported active control for these species. From these two species, only croton was found very occasionally. Notwithstanding these very low frequencies of occurrence, it was proposed to keep them in the list of undesirable substances because of their high toxicity.
24Other species not listed in Directive 2002/32/EC. The responding laboratory from HU reported a series of additional toxic or noxious weeds in 25 samples examined in 2006. Among the latter category, Galium aparine L. (7 samples, 28%), Polygonum spp. L. (three different species present in 10 samples, 40%, in 6 samples in combination with G. aparine), Ambrosia elatior L. (4 samples, 16%) and Cannabis sativa L. (1 sample, 4%) were the most predominant. The Galium and Ambrosia species were also suggested as possible additions to the list of undesirable substances by other respondents.
3.4. Requirements for modifying Directive 2002/32/EC
25Proposals for deletion. Some respondents propose to delete the following species from the list in Directive 2002/32/EC: mowrah (Madhuca longifolia (L.) J.F.Macbr. and Madhuca indica J.F.Gmel.), Lolium temulentum L. and Lolium remotum Schrank, apricots (Prunus armeniaca L.), bitter almond (Prunus dulcis (Mill.) D.A.Webb var. 'amara' (DC.) Focke), beech mast (Fagus silvatica L.) and camelina (Camelina sativa (L.) Crantz). One laboratory mentioned that species can be deleted only when not found over the last 25 years, and after an indication of no risk according to EFSA. On the other hand, 6 laboratories proposed to keep all current species on the list of 2002/32/EC. Mustard species can be deleted as well in the view of one respondent, whereas another laboratory proposed to keep them as one item: mustard seeds.
26Proposals for addition. There is a range of proposals for additions to the list of undesirable substances. Highlights are Ambrosia spp. (5 laboratories), a list of alkaloid containing seeds (2 laboratories) and Galium aparine (2 laboratories). Member state HU maintains a large list of weed seeds which are prohibited according to national legislation. Ambrosia and G. aparine are part of this list. Another species listed is Datura ferox L., which can hardly be distinguished from Datura stramonium L. D. ferox is proposed to be added to the list by the Hungarian respondent.
27The survey was focusing on weed seeds as category of undesirable substances according to the Directive. Other weed contaminants such as common ragwort (Senecio jacobaea L.) can pose threats in certain feed sources (e.g. fodder, grass meal). Control measures for the increasing problem of ragwort can be set as an addition to item 14 (“Weed seeds and unground and uncrushed fruits containing alkaloids, glucosides or other toxic substances separately or in combination including”: followed by a list of three species) of Directive 2002/32/EC. The absence of any discussion in this survey does not imply that these weeds are not relevant in this context.
3.5. Expertise maintenance and improvement
28The respondents maintain their level of expertise in several ways. Textbooks and internet as sources for information are most frequently mentioned (25 and 23 indications, respectively). Ten respondents report the implementation of knowledge or expert systems, without any further comments. It could be possible that these indications point to the use of this type of systems in general. It is known that only three of these ten respondents use the expert system ARIES (Animal Remains Identification and Evaluation System, van Raamsdonk et al., 2004. Available from RIKILT, Institute of Food Safety, Wageningen. http://workplace.wur.nl/aries) for identification of animal by-products. A survey for collecting further information on the type of systems used would be favorable.
29Training on the identification of botanical undesirable substances is indicated as the most important source of expertise development (28 respondents). A network of colleagues (24 respondents) is indicated as almost evenly important. Knowledge or expert systems for increasing knowledge are desired by 15 laboratories. Dedicated software for the identification of weed seeds is in development at RIKILT. Combination of different activities is proven to be profitable, e.g. training by using software programs.
4. Conclusion and recommendations
30The enforcement of the control according to Directive 882/2004/EC is not consistent among the EU member states. In the current survey there are hardly any results reported by respondents from southern and eastern European member states. In order to have a proper evaluation of the list of botanical undesirable substances in Directive 2002/32/EC, an effective monitoring in all member states for at least one or two years is recommended. The results of this monitoring together with risk assessments by EFSA would allow the composition of a new updated list with undesirable botanical substances in feed.
31The current list of undesirable botanical substances can be divided in four parts, based on the results of the returned questionnaires in the current study. Parameters for this division are the frequency of occurrence as far as active control is enforced, and the general level of toxicity:
32– moderate or frequent occurrence, moderate to high toxicity: rye ergot (Claviceps purpurea), thorn apple (Datura stramonium), castor oil plant (Ricinus communis), and Crotalaria spp.
33– low occurrence, high toxicity: purghera (Jatropha curcas), and croton (Croton tiglium).
34– low occurrence, low to moderate toxicity: mowrah (Madhuca longifolia and Madhuca indica), Lolium temulentum and Lolium remotum, apricots (Prunus armeniaca), bitter almond (Prunus dulcis var. 'amara'), beech mast (Fagus silvatica) and camelina (Camelina sativa).
35– variable occurrence, insufficiently known toxicity: mustard species (Brassica spp.).
36The taxonomy of the Brassica species is revised since the publication of the Directive. Although the taxa are still recognized at the species level, the subspecific division of Brassica juncea (Sareptian, Indian and Chinese mustard) is not yet supported (SCAN, 2003). Based on the results presented in the current study, the first two categories would be worth considering for a future new list. The third category can be considered as candidates for deletion, but only after a proper risk assessment. The in- or exclusion of the mustard species and the newly proposed species such as G. aparine, Polygonum spp. and Ambrosia spp. are recommended for an extensive risk assessment. Common ragwort (Senecio jacobaea) can be added to this recommendation. When reconsidering the list of undesirable botanical impurities, one must always have in mind that even if a toxic impurity has not been identified in several years it may suddenly appear because of changes in the supply of raw materials to countries where specific toxic plants are more common.
37Microscopy is an effective technique for detection at macroscopic as well as microscopic level. The development and application of validated chemical detection methods of the toxic compounds should be encouraged. However, microscopic examination is still considered valuable for monitoring of emerging risks of new weed seeds and for all those listed seeds for which no chemical detection is available, as is also concluded in the SCAN report (SCAN, 2003).
38Four occurrences of rye ergot and thorn apple with amounts at or over the legal limit in 2005 and 2006 were reported in the survey. These findings were not included in the RASFF notifications. On the other hand, one report of thorn apple in red millet seed was not mentioned in the returned questionnaires. The laboratory that reported this occurrence to the RASFF might not have sent in its questionnaire for the current survey.
39There is a need for improving knowledge levels. This can be achieved by organizing training sessions, raising colleague networks and the development of dedicated expert systems, or a combination of these three activities.
Bibliographie
EFSA, 2005. Opinion of the scientific panel on contaminants in food chain on a request from the Commission related to ergot as undesirable substance in animal feed. EFSA J., 225, 1-27, http://www.efsa.eu.int.
European Commission, 2002. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed as regards dioxins and dioxin-like PCBs. Off. J. Eur. Communities, L 140, 30.05.2002, 10.
European Commission, 2004. Regulation (EC) n°882/2004 of the European Parliament and of the Council on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare. Off. J. Eur. Communities, L 191, 28.05.2004.
European Commission, 2008. Directive 2008/76/EC of 25 July 2008 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council on undesirable substances in animal feed as regards dioxins and dioxin-like PCBs. Off. J. Eur. Communities, L 198, 26.07.2008, 37-40.
SCAN, 2003. Opinion of the scientific committee on animal nutrition on undesirable substances in feed. Brussels: European Commission, Health & Consumer Protection Directorate-General.
van Raamsdonk L.W.D. et al., 2004. The microscopic detection of animal proteins in feeds. Biotechnol. Agron. Soc. Environ., 8(4), 241-247.
van Raamsdonk L.W.D., 2007. A survey on the presence of undesirable botanic substances in feed in the European Union. Report 2007.004. Wageningen, The Netherlands: RIKILT.
