- Accueil
- Volume 92 - Année 2023
- Numéro 1
- Diversity of hard tick (Acari: Ixodidae) infesting small ruminants in some breeding farms in Tizi-Ouzou area (Northern Algeria)
Visualisation(s): 4071 (11 ULiège)
Téléchargement(s): 101 (1 ULiège)
Diversity of hard tick (Acari: Ixodidae) infesting small ruminants in some breeding farms in Tizi-Ouzou area (Northern Algeria)
Diversité des tiques dures (Acari : Ixodidae) infestant les petits ruminants dans quelques fermes d’élevage dans la région de Tizi-Ouzou (Nord d’Algérie)

Document(s) associé(s)
Version PDF originaleRésumé
Les tiques dures sont des arthropodes hématophages et des ectoparasites obligatoires d’une répartition cosmopolite. Elles ont une grande importance d’intérêt médical et vétérinaire. L'étude de la composition de la faune des tiques est une étape cruciale pour établir des programmes de lutte antivectorielle. Dans ce contexte, un inventaire des tiques dures infestant les petits ruminants dans la région de Tizi-Ouzou a été réalisé sur une période d’une année, allant du mois de décembre 2020 au mois de novembre 2021. D’un total de 663 animaux examinés, 170 ont été infestés et un nombre de 1318 spécimens de tiques adultes a été collecté dans quatre sites échantillonnés, en utilisant la technique de la collecte directe sur hôte animal. Le résultat obtenu exprime une richesse de six espèces de tiques. L’espèce la plus abondante a été Rhipicephalus bursa (68,66%), suivie de Rhipicephalus sanguineus (29,74%) et Ixodes ricinus (1,36%). Les autres espèces ont été moins abondantes, elles présentent chacune une abondance de 0,08% de l’abondance totale. La richesse spécifique la plus élevée a été enregistrée dans le site d'Ain El Hammam, situé à haute altitude. Une corrélation positive a été observée entre l'altitude des fermes et la diversité des tiques. Le résultat de l'analyse AFC a montré que la distribution des tiques est liée à l'altitude et à l'habitat des espèces de tiques.
Abstract
Ticks are hematophagous arthropods and obligate ectoparasites with a cosmopolitan distribution. They are of great medical and veterinary importance. Studying the composition of the tick fauna is a crucial step in establishing vector control programs. In this context, an inventory of hard ticks infesting small ruminants in the Tizi-Ouzou area was carried out over a one-year period, from December 2020 to November 2021. Using the direct collection technique on the host animal, a total of 663 animals examined, 170 were infested and a total of 1318 adult tick’s specimens were collected in four sampling sites. The result shows a tick diversity comprising six different species. The most abundant species was Rhipicephalus bursa (68.66%), followed by Rhipicephalus sanguineus (29.74%) and Ixodes ricinus (1.36%). Other species were less abundant, each representing only 0,08% of the total abundance. The highest specific richness was recorded in the Ain El Hammam site located at high altitude. A positive correlation was observed between the farms’ altitude and the tick diversity. The result of FCA analysis showed that distribution of ticks is related to altitude and habitat of tick’s species.
Table des matières
1Manuscrit reçu le 6 septembre 2023 et accepté le 16 octobre 2023
2Article publié selon les termes et les conditions de la licence Creative Commons CC-BY 4.0
1. Introduction
3Ticks are arthropods that belong to the arachnid class, acarid group and metastigmata order (Tissot Dupont & Raoult, 1993). They are characterized by a wide diversity, containing around 900 different species (Boulanger et al., 2019) divided into three major families (Tissot Dupont & Raoult, 1993; Klompen et al., 1996; D.McCog & Boulanger, 2015, Boulanger et al., 2019), the Argasidae family with 193 species (Boulanger et al., 2019), the Ixodidae family with 700 species and only one species representing the Nuttalliellidae family (D.McCog & Boulanger, 2015; Boulanger et al., 2019). Ticks are obligate ectoparasites of terrestrial vertebrates (Klompen et al., 1996; Wall & Shearer, 2001). They are hematophagous at all stages of development (Klompen et al., 1996; Guiguen & Degeilh, 2001; Boulanger et al., 2019). The blood meal is essential for their life cycles (Peter & Brossard, 1998; Boulanger et al., 2019). Ticks play an important role in the transmission of bacteria, viruses, protozoa, and helminths that cause both human and animal disease (krčmar, 2019). These mites have an extremely varied global distribution, ranging from icy zones to the most inhospitable desert areas, and from lowland to high-altitude regions (Perez-Eid & Gilot, 1998; Boulanger et al., 2019). Currently, with global warming, environmental conditions are changing, which can have an impact on the distribution patterns and vector potential of ticks (Rehman et al., 2017).
4Numerous studies have been carried out on small ruminant ticks around the Mediterranean: in north-central Spain (Estrada-Pena et al., 2004), Turkey (Ozubek & Aktas, 2017), Lebanon (Dabaja et al., 2017), Iraq (Omer et al., 2007) and Tunisia (Elati et al., 2018). In Algeria, cattle ticks have been the primary subject of the majority of studies carried on farm animal ticks (Yousfi- Monod & Aeschlimann, 1986; Boulkaboul, 2003; Benchikh Elfegoun et al., 2007; Benchikh Elfegoun et al., 2013; Benchikh Elfegoun et al., 2019; Derradj & Kohil, 2020). Only a few research on small ruminant ticks have been done in the Adrar region (Bouhous et al., 2011), El Tarf (Leulmi et al., 2016), Sidi Bel Abbes and Saida (Abdelkadir et al., 2019), and Constantine (Foughali et al., 2021). In the Tizi-Ouzou area, the only studies conducted are those by Abdul Hussain et al. (2004) on cattle ticks and free-stage ticks on vegetation and Bedouhene et al. (2022) on cow ticks during their study in the western Djurdjura. This region lacks an inventory of the ixodid fauna of small ruminants, in this sense the current study was conducted.
5The goal of this preliminary work is to identify the different tick species infesting small ruminants in some farms in the Tizi-Ouzou area. This will serve as groundwork for further research in this field.
2. Materials and methods
2.1 Study area
6Tizi-Ouzou is a department in northern Algeria, it is limited by the Mediterranean Sea from the north, Bouira department from the south, Boumerdes department from the west and Bejaia department from the east. The area has a Mediterranean subhumid climate. Our work was carried out in four sites in the Tizi-Ouzou area, on various mixed goat and sheep farms. Their geographical characteristics are shown in table 1. The geographical location of the study sites is shown in figure 1.
Table 1: Geographical characteristics of study sites.
|
Sites |
Code of sites |
Geographical coordinates of sites |
Altitude (m) |
|
Ain El Hammam |
AEH |
36°28'55"N 4°17'44"E |
1019 |
|
Irdjen |
IR |
36°38'37"N 4°09'23"E |
507 |
|
Tizi Rached |
TR |
36°41'46"N 4°13'02"E |
117 |
|
Freha |
FR |
36°43'57"N 4°16'00"E |
132 |
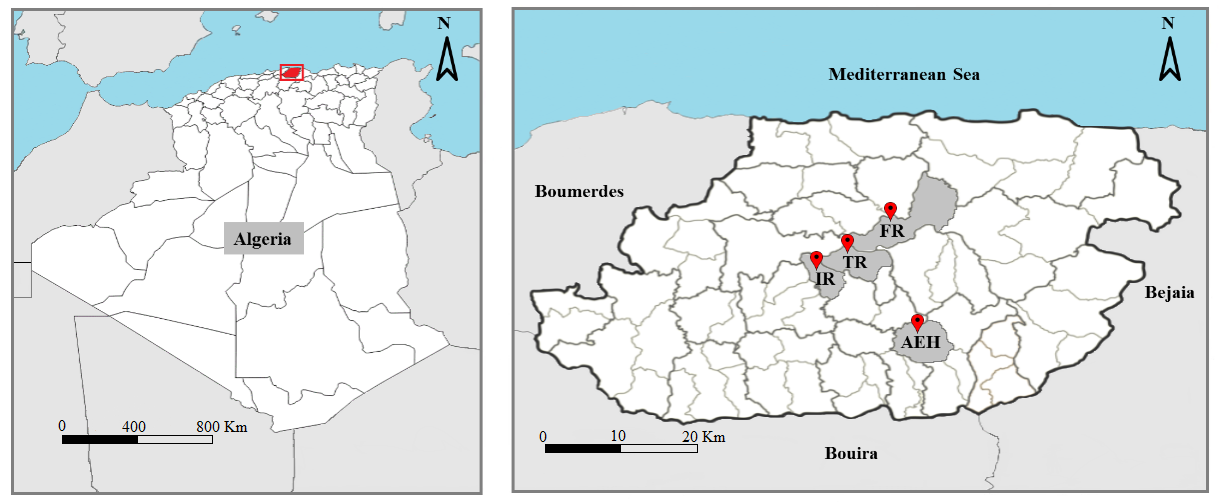
Figure 1: Geographical location of sites sampled in the Tizi-Ouzou department. (For abbreviation see table 1).
2.2 Tick collection and preservation
7Ticks were collected from small ruminants (goats and sheep) twice a month from December 2020 to November 2021. A random sampling method was used to choose animals on which the presence of ticks can be inspected. Then ticks were carefully removed with forceps and transferred to dry tubes containing a 70% ethanol solution to preserve their morphological characteristics. Each tube is labeled with the study site, collection date and host type.
2.3 Tick identification
8Collected specimens were transferred to the Laboratory of Ecology and Biology of Terrestrial Ecosystems LEBIOT of Mouloud Mammeri University, Tizi-Ouzou (Algeria), for morphological identification of genera and species. This procedure was completed using an OPTIKA stereomicroscope (WF 10X/21), with reference to the taxonomic key of Walker et al. (2003). Our identification was verified at the Parasitic Eco-epidemiology and population genetics laboratory of the Pasteur Institute of Algeria.
The identification process was carried out in the following steps:
-
First, the ticks were sorted out to determine their stage of development and gender. The adult stage of the ticks was determined on the basis of the presence of four pairs of legs, porose areas and a genital groove. Gender determination was carried out by observing the dorsal surface of the ticks. The male differs from the female by the presence of a chitinous plate, the scutum, on the entire dorsal surface, whereas in the female it covers only part of the back.
-
The genus was then identified on the basis of the morphological characteristics of certain parts of the tick's body:
● The position of the anal groove in front of the anus has made it possible to differentiate the genus Ixodes from other tick genera.
● The length of palp articles 2 is greater than the length of the palp articles 1 and 3 in the genus Hyalomma.
9● The hexagonal shape of the entire basis of the capitulum and the presence of the festoons on the posterior edge of the idiosoma have enabled the genus Rhipicephalus to be identified.
● The rectangular shape of the basis of the capitulum, the broad shape of the palp articles 2 and the morphology of the coxae of the legs 1 confirmed the genus Haemaphysalis.
-
Finally, species identification was based on certain morphological details. Here are the main morphological characteristics that helped to distinguish between the three predominant species in the study:
10● Genital groove between coxae 4 and the internal spur on coxa 1 is long in the female of Ixodes ricinus.
11● The shape of the eyes, scutum and spiraclular plates distinguish between females of both Rhipicephalus bursa and Rhipicephalus sanguineus.
12● Adanal plates shape is narrow and trapezoid in male of Rhipicephalus sanguineus, broad and curved in male of Rhipicephalus bursa.
2.4 Data analysis
2.4.1 Ecological indices
13The ixodidae population collected from the different sites of the study area, was examined using several indices:
14Specific richness (S) is one of the essential factors characterizing a population. The total richness of a biocenosis corresponds to all the species that make it up (Ramade, 1984).
Relative abundance (RA%) is the percentage of the number of individuals of a species (ni) in relation to the total number of individuals (N) (Faurie et al., 1980).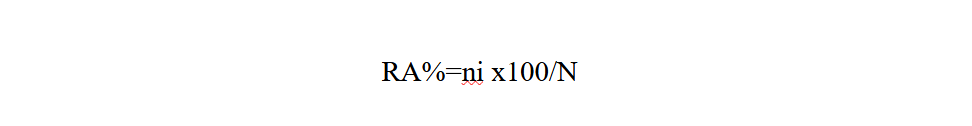
15ni: number of individuals of a species i taken into consideration
16N: total number of all individuals
17Frequency occurrence (FO) of each study site is calculated as the ratio of the number of records containing the study species (Pi) to the total number of records carried out (P), expressed as a percentage (Dajoz, 1975).
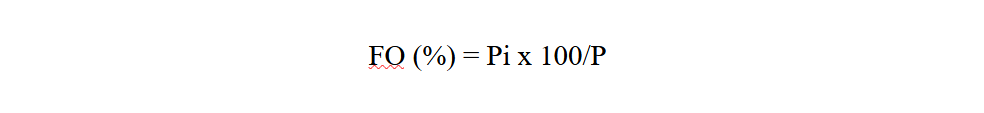 Pi: is the number of records containing the species
Pi: is the number of records containing the species
P: is the total number of records
18Based on FO values, we distinguish three classes of constancy. When FO>50% species are considered constant, as accessory when 25%<FO<50% and accidental when FO<25%.
Shannon diversity index (H') provides information on the effective diversity of a community and varies according to the number of species present (Ramade, 2003). It is calculated using the following formula: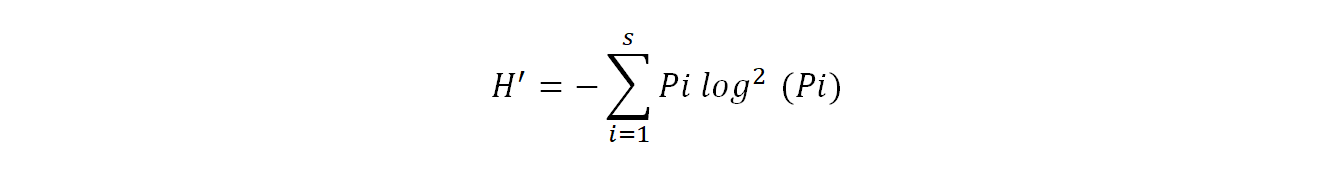
19Where pi= ni/N
20H': Shannon diversity index expressed in binary units
21ni: is the number of each species i
22N: is the total of the ni of all species combined
23Log2: logarithm - base 2.
Equitability index (E) gives the theoretical maximum diversity of a community, taking into account its specific richness (Ramade, 2003). It is calculated using the following formula: 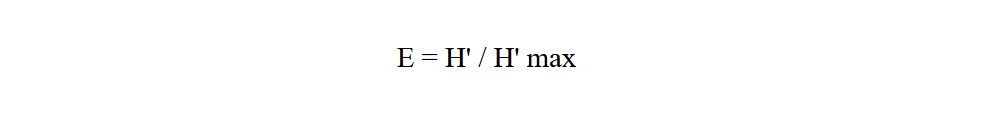
24With H' max = log 2 S
25H' max: is expressed in bits
26S: specific richness.
2.4.2 Statistical analysis
27Statistical analysis was carried out using R software, version 4.1.2. The Pearson correlation coefficient (Millot, 2018) was applied on the parameters to demonstrate the impact of altitude on species diversity. The factorial correspondence analysis (FCA) is used to summarize and visualize the information contained in the contingency table formed by two qualitative variables (Carricano et al., 2010). FCA is used to demonstrate the relationship between the altitudinal gradient and the distribution of species in the study area.
3. Results
28From a total of 663 animals examined, 170 were infested and a number of 1318 adult tick’s specimens were collected, of which 690 were males and 628 were females. All of the ticks were found on the ears.
3.1 Specific richness
29During this study, six tick species were recorded on sheep and goats. These species are represented in table 2 and illustrated in figure 2. The Ain El Hammam site (AEH: located at 1019 m) is the richest in terms of species, with six species recorded (Rhipicephalus bursa, Rhipicephalus sanguineus, Ixodes ricinus, Rhipicephalus turanicus, Hyalomma marginatum marginatum, Haemaphysalis punctata), followed by the Irdjen (IR: located at 507 m) and Tizi Rached (TR: 117 m) sites with two species each, namely Rhipicephalus bursa and Rhipicephalus sanguineus. On the other hand, the Freha site (FR: located at 132 m) is the least diversified, with just one species (Rhipicephalus sanguineus). Figure 3 displays the specific richness of hard ticks found at each location. As can be observed, altitude has an impact on the values of species richness. The Pearson test performed on the data revealed a positive correlation (r: 0,858) between altitude and tick diversity in the study area (Figure 4).
Table 2: Species richness of hard ticks recorded during this study.
|
Familly |
Genus |
Species |
|
Ixodidae |
Rhipicephalus |
Rhipicephalus sanguineus (Latreille, 1806) |
|
Rhipiciphalus bursa Canestrini & Fanzago, 1878 |
||
|
Rhipicephalus turanicus Pomerantsev, 1936 |
||
|
Hyalomma |
Hyalomma marginatum marginatum Koch, 1844 |
|
|
Ixodes |
Ixodes ricinus (Linnaeus, 1758) |
|
|
Haemaphysalis |
Haemaphysalis punctata Canestrini & Fanzago, 1878 |
30
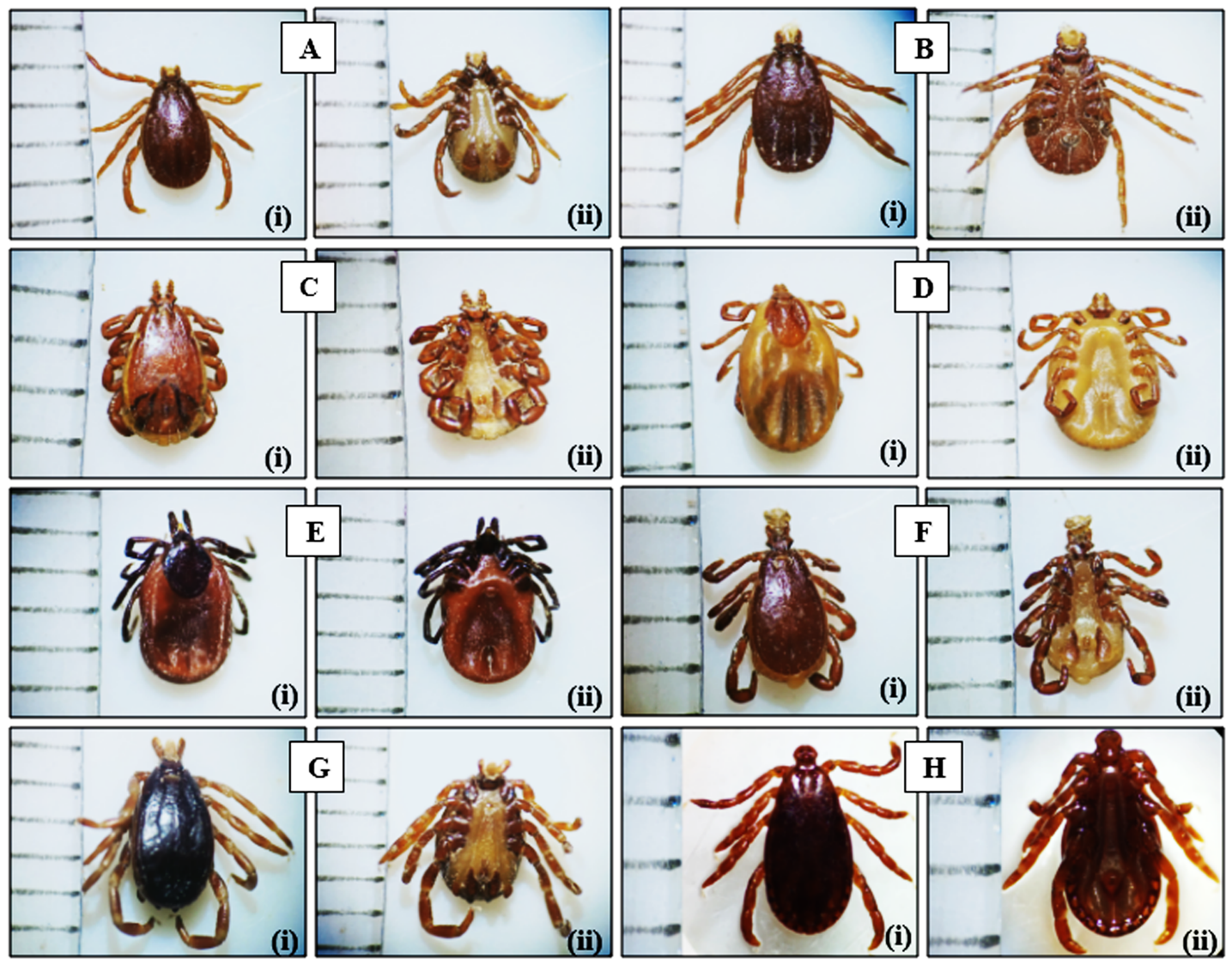
Figure 2: A. Rh. bursa male, (i) dorsal, (ii) ventral; B. Rh. bursa female, (i) dorsal, (ii) ventral; C. Rh. sanguineus male, (i) dorsal, (ii) ventral; D. Rh. sanguineus female, (i) dorsal, (ii) ventral; E. I. ricinus female, (i) dorsal, (ii) ventral; F. Rh. turanicus male, (i) dorsal, (ii) ventral; G. Hy. marginatum marginatum male, (i) dorsal, (ii) ventral. H. H. punctata male, (i) dorsal, (ii) ventral. Enlargement: A, B, C, D, E, F, G x20 and H x40. Scale bar: 1 mm.
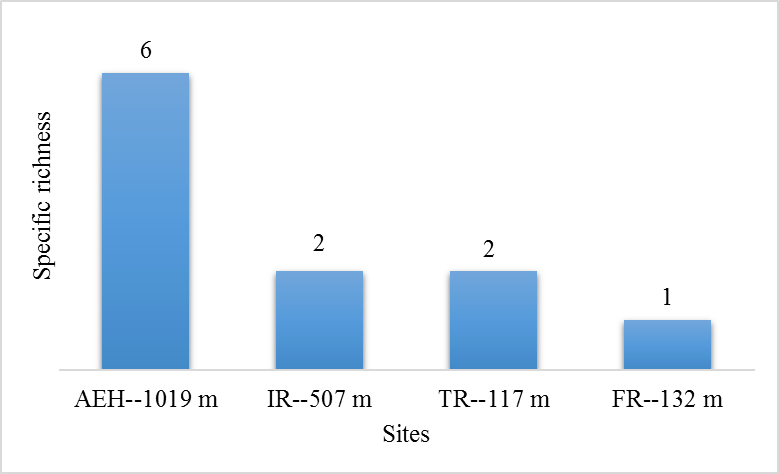
Figure 3: Specific richness of hard tick at the various study sites.
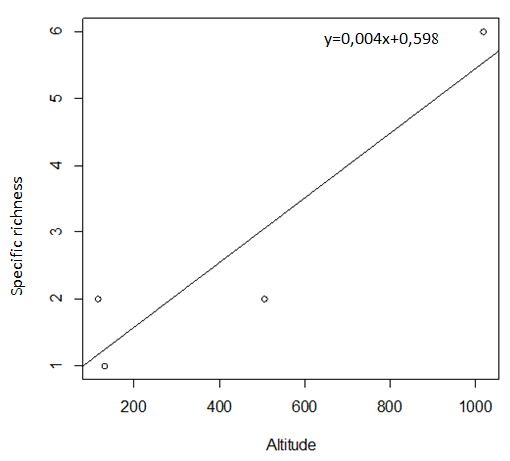
Figure 4: Pearson test showing the relationship between altitude and tick species richness recorded at each study site.
3.2 Relative abundance and frequency occurrence
31The most abundant species in this study was Rh. bursa at 68.66%, followed by Rh. sanguineus at 29.74% and I. ricinus at 1.36%. The other species were very low, with an abundance of 0.08% each (Figure 5).
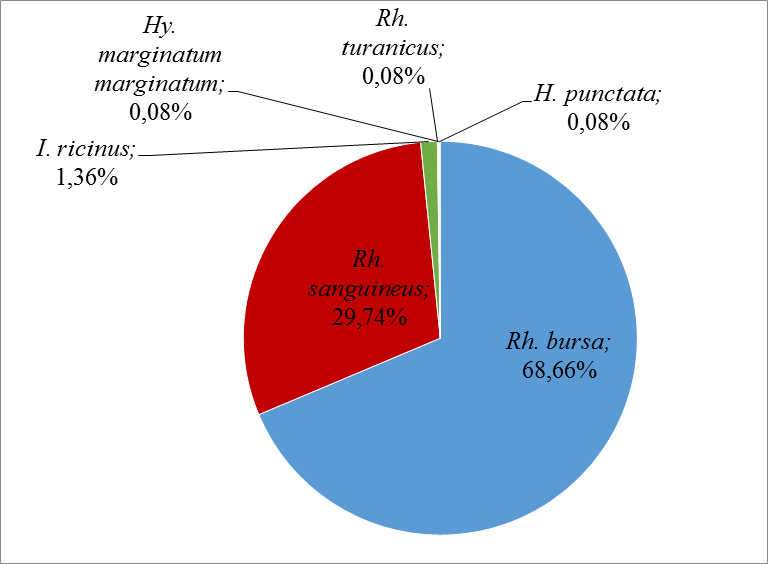
Figure 5: Relative abundances of the different tick’s species collected during this study.
32The relative abundances of each species collected per site are presented in table 3 and illustrated in figure 6. Rh. bursa is the most abundant species in all the study sites (AEH: 70.36%, IR: 69.89% and TR: 54.55%), except for the FR site which is represented by only one species (Rh. sanguineus). The coefficient of occurrence of each species was calculated in the different sites, revealing two classes of constancy in the study area (Table 3). The frequencies of occurrence calculated for the AEH site, show that the two species found (Rh. bursa and Rh. sanguineus) are qualified as accessory species, whereas the other species are accidental. The two species sampled at the IR site are classified as accessory species, while those found at the TR site are considered accidental. Only one accidental species was found at the FR site.
Table 3: Relative abundance (%) and Frequency occurrence of Ixodidae species collected at the various sampling sites.
|
Species |
AEH |
IR |
TR |
FR |
|
Rh. sanguineus |
27,29% (A) |
30,11% (A) |
45,45% (a) |
100 % (a) |
|
Rh. bursa |
70,36% (A) |
69,89% (A) |
54,55% (a) |
- |
|
Rh. turanicus |
0,11% (a) |
- |
- |
- |
|
Hy. marginatum marginatum |
0,11% (a) |
- |
- |
- |
|
I. ricinus |
2,01% (a) |
- |
- |
- |
|
H. punctata |
0,11% (a) |
- |
- |
- |
33(A): Accessory; (a): accidental.
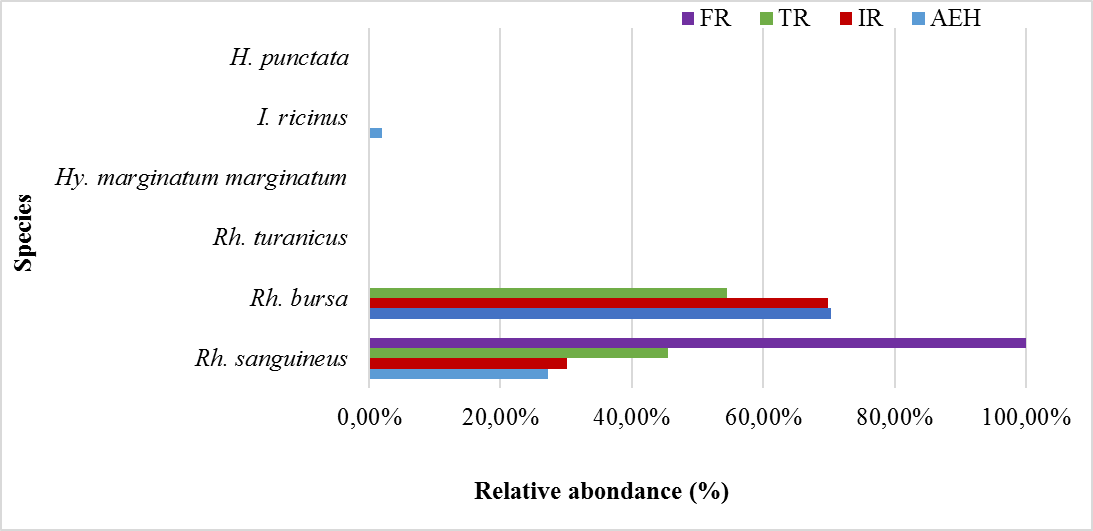
Figure 6: Relative abundance of each species collected per site.
3.3 Shannon diversity and equitability index
34The Shannon diversity and equitability index calculated for the four studied sites were shown in table 4. The values of these parameters fluctuated between 0 and 1,01 bits for the Shannon index, and between 0 and 0,39 for equitability in the different sites. The maximum values of the Shannon and equitability index were recorded in the site of AEH, which are located at high altitude. While, the low value of these two parameters were recorded in the site of FR, as only one species of tick was found.
Table 4: Shannon diversity and equitability index.
|
Site |
AEH |
IR |
TR |
FR |
|
H' (bits) |
1,01 |
0,88 |
0,99 |
0 |
|
H' max (bits) |
2,58 |
1 |
1 |
0 |
|
E |
0,39 |
0,88 |
0,99 |
0 |
3.3 Factorial correspondence analysis (FCA)
35The factorial correspondence analysis was performed on the various species found at the four study sites (Figure 7). It has revealed that the two factorial axes (Dim1xDim2 plane) are responsible for the greatest contributions, accounting for 80,70% and 19,30% of the cloud inertia, which corresponds to a cumulative total of 100%. Three groupings appear clearly in the Dim1xDim2 plane. These groupings mainly concern the different types of distribution of the tick species collected. The first one corresponds to the omnipresent species (Rh. bursa) in the three study sites AEH, IR and TR. The second one group corresponds to the one species (Rh. sanguineus) captured at FR at low altitude. As for the third one, it corresponds to the species recorded only at AEH at high altitude (Rh. turanicus, I. ricinus, Hy. marginatum marginatum and H. punctata).
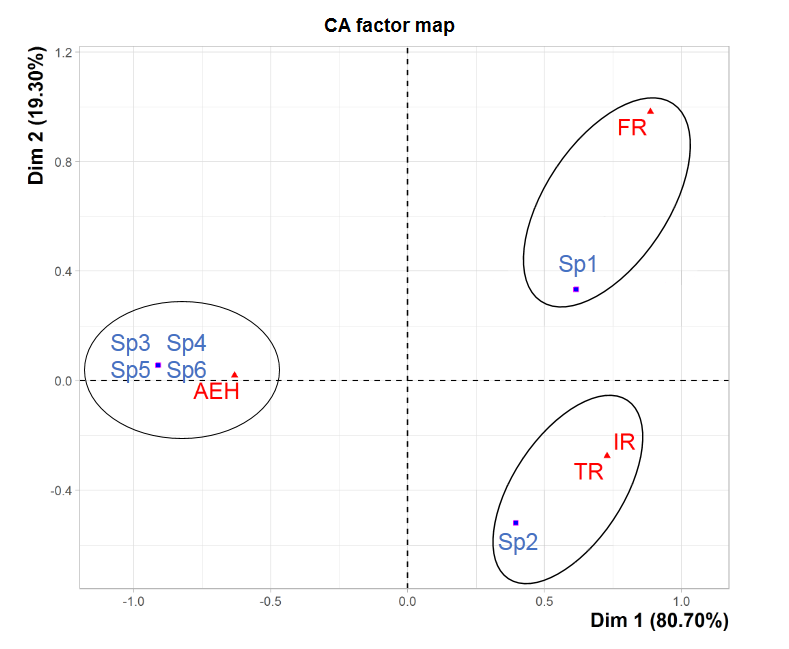
Figure 7: Factorial correspondence analysis of the ixodid distribution in the four study sites. Sp1: Rh. sanguineus, Sp2: Rh. bursa, Sp3: Rh. turanicus, Sp4: I. ricinus, Sp5: Hy. marginatum marginatum, Sp6: H. punctata.
4. Discussion
36In the present study, the species richness found is significant, especially in the forest site of AEH located at high altitude (1019 m) recorded with six species. However, the FR site has only one species as it is an agricultural site where acaricide treatments are often applied. Despite the application of these treatments, the presence of the Rh. sanguineus species is due to the presence of dogs in the farm. According to Walker et al. (2003), the preferred host for all stages of Rh. sanguineus species is dog, while other hosts are generally infested only when dogs are present to maintain a tick population.
37The Pearson test demonstrates that species richness rises with altitude. In fact, a significant change in altitude can cause significant climatic changes (Zeroual et al., 2014). This variability in climatic characteristics combined with adequate vegetation and host overpopulation provide suitable habitat and biotic conditions for several tick species (Estrada-Pena et al., 2004; Zeroual et al., 2014).
38In other studies, carried out on small ruminant ticks, Estrada-Pena et al. (2004) found nine species, while Bouhous et al. (2011); Arnaudov et al. (2014); Dabaja et al. (2017) and Chaligiannis et al. (2018) reported seven species. Five species were reported by Ozubek & Aktas in 2017, while Leulmi et al. (2016) and Foughali et al. (2021) mentioned four species. Only three species were reported by Abdelkadir et al. (2019) and two species by Elati et al. (2018).
39The Rh. bursa species was very abundant in our study with almost 70%. Indeed, according to Walker et al. (2003), Rh. bursa is one of the common Rhipicephalus species of livestock in North Africa. In Algeria, similar results have been found in several studies (Abdelkadir et al., 2019; Benchikh Elfegoun et al., 2019; Foughali et al., 2021; Bedouhene et al., 2022). While other studies have recorded low abundances of Rh. bursa, only 91/659 individuals were sampled by Leulmi et al. (2016) in the El Tarf region in north-eastern Algeria. In a previous study conducted in the Tizi-Ouzou region on cattle ticks and free-stage ticks on vegetation, Boophilus annulatus was the predominant species with 70.57% (Abdul Hussain et al., 2004). The latter, however, was not present during the investigation since it is a species that is often associated with cattle but can also occur with sheep and goats (Walker et al., 2003).
40Rh. bursa has also been very prevalent in numerous studies all over the world. In Iraq, 90% of ticks collected from sheep and goats were Rh. bursa (Omer et al., 2007). In Parvomai, southern Bulgaria, the same species was invasive and predominant in both animals (Arnaudov et al., 2014). This species was the most frequent on both hosts in Turkey with a rate of 63.4% (Ozubek & Aktas, 2017). However, Rh. bursa was less abundant in Lebanon and Greece (Dabaja et al., 2017; Chaligiannis et al., 2018). This species is implicated in the transmission of different pathogens, particularly Theileria (Aktas et al., 2006; Garcia-Sanmartin et al., 2008; M'ghirbi et al., 2010), Babesia (Altay et al., 2008; Garcia-Sanmartin et al., 2008; Aydin et al., 2015), Anaplasma (de la Fuente et al., 2004) and Rickettsia (Leulmi et al., 2016; Dib et al., 2019).
41Rh. sanguineus was the second most abundant species in the study which goes in tune with Leulmi et al. (2016). While other studies show that it is less abundant (Estradaa-Pena et al., 2004; Arnaudov et al., 2014). Rh. sanguineus is known to be the vector of Rickettsia conorii conorii, the causal agent of Mediterranean spotted fever (Sarih et al., 2008; Bitam et al., 2009) and Rickettsia massiliae (Bitam et al., 2009).
42I. ricinus was the third most abundant species in the study. Only one individual of it was collected from sheep in El Tarf in north-eastern Algeria by Leulmi et al. (2016). This species was among those inventoried on sheep in the region of Aragon (north-central Spain) (Estrada-Pena et al., 2004), and was also found on sheep and goats in Parvomai, southern Bulgaria (Arnaudov et al., 2014). I. ricinus transmits Borrelia garinii (Benredjem et al., 2014) and Rickettsia spp. (Dib et al., 2009; Benredjem et al., 2014; Dib et al., 2019).
43As for the other species, they were very sparse. They were limited to a single individual recorded for each species: Rh. turanicus, Hy. marginatum marginatum and H. punctata. According to Bitam et al. (2009), Hy. marginatum marginatum is implicated in the transmission of Rickettsia aeschlimannii and Rh. turanicus transmits Rickettsia massiliae. The species H. punctata is a vector of Rickettsia slovaca (Leulmi et al., 2016).
44The results obtained for the coefficient of occurrence of each species in the different sites reveal that four species (Rh. turanicus, I. ricinus, Hy. marginatum marginatum and H. punctata) were identified as accidental species in the region. This is probably due to the fact that these species are much more frequently sampled on cattle in the Tizi-Ouzou area (Abdul Hussain et al., 2004; Bedouhene et al., 2022).
45The preferred attachment site for ticks in this study was the ears. Several studies have found similar results. For instance, in Iraq, 85% of sheep and goat ticks (mainly Rh. bursa) were collected on the ears, 9.8% on the mammal and 5.1% on the hind legs and around the eyes (Omer et al., 2007). According to Elati et al. (2018), the common attachment site for Rhipicephalus ticks was near the ear tag, whereas Hyalomma ticks were mainly collected on the sternum.
46The results obtained for the Shannon index show that there is no significant diversity in the studied sites. The value of equitability at AEH site is 0.39, which shows that there is a dominant species (Rh. bursa) in relation to the other species. Whilst, at the TR and IR sites, the species tend to be in equilibrium, since equitability is close to 1.
47The factorial correspondence analysis shows that the spatial organization of the ticks sampled in the farms of the four sites in the study area, is directly related to altitude and the habitat of the species. In fact, vegetation indirectly impacts the tick's reproductive cycle due to the microclimate that plants generate, which controls both the humidity and temperature in which a tick grows. Some plant species can serve as indicators of the presence of ticks, but not directly through their ability to prevent them from desiccation; rather, they do so indirectly through the attraction they exert on specific hosts on which certain tick species feed (D.McCoy & Boulanger, 2015).
5. Conclusion
48In the light of our results, we conclude that small ruminants (goats and sheep) are infested by six hard tick species in the Tizi-Ouzou area. The current data provide an important baseline for future epidemiological studies. As vectors of numerous diseases and arthropods that cause serious damage to small ruminants, particular attention should be paid to tick control and prevention. In the future, further studies should be carried out on ticks to examine their possible role in the transmission of various pathogens in our study area.
Acknowledgments
49The corresponding author would like to thank all animal’s farmers of study sites of Tizi-Ouzou who accepted to carry out our sampling on their animals. We also thank Dr. Massinissa Faci for his valuable advice and comments on improving the manuscript.
ORCID identifiers of authors
50Thinhinane DJOUAHER: https://orcid.org/0000-0003-1203-0592
51Soumeya CHAHED: https://orcid.org/0000-0002-8505-6032
52Naouel EDDAIKRA: https://orcid.org/0000-0003-1328-3508
Author contributions
53T.D.: Conceptualization, Investigation, Methodology, Formal analysis, Validation, Writing - original draft, Writing - review & editing. S.C.: Formal analysis, Writing-review & editing. A.B.: Methodology. N.E.: Writing - review & editing, Validation. K.B.: Conceptualization, Supervision, Validation, Writing-review & editing. All authors read and approved the final manuscript.
Conflicts of interest
54The authors declare no conflict of interest
References
55Abdelkadir, K., Palomar, A.M., Portillo, A., Oteo, J.A., Ait-Oudhia, K. & Khelef, D. (2019). Presence of Rickettsia aeschlimannii, ‘Candidatus Rickettsia barbariae’ and Coxiella burnetii in ticks from livestock in Northwestern Algeria. Ticks and Tick-borne Diseases, 10, 924-928. https://doi.org/10.1016/j.ttbdis.2019.04.018
Abdul Hussain, A.S., Bitam, I., Abdul Hussain, M.S. & Cozma, V. (2004). Aperçu sur la dynamique des tiques Ixodidés dans la région de Tizi Ouzou, Algérie. Scientia Parasitologica, 1-2, 175-179.
56Aktas, M., Altay, K. & Dumanli, N. (2006). PCR-based detection of Theileria ovis in Rhipicephalus bursa adult ticks. Veterinary Parasitology, 140, 259-263.
57https://doi.org/10.1016/j.vetpar.2006.04.005
58Altay, K., Aktas, M. & Dumanli, N. (2008). Detection of Babesia ovis by PCR in Rhipicephalus bursa collected from naturally infested sheep and goats. Research in Veterinary Science, 85(1), 116-119. https://doi.org/2 10.1016/j.rvsc.2007.08.002
59Arnaudov, D.Y., Arnaudov, A.D., Kirin, D.A. & Gospodinova, S.G. (2014). Ixodidae ticks of small ruminants in the region of Parvomai, Southern Bulgaria. Bulgarian Journal of Agricultural Science, 20(3), 590-594.
60Aydin, M.F., Aktas, M. & Dumanli, N. (2015). Molecular identification of Theileria and Babesia in ticks collected from sheep and goats in the Black Sea region of Turkey. Parasitoogyl Research, 114, 65-69. https://doi.org/10.1007/s00436-014-4160-x
61Bedouhene, A., Kelanemer, R., Medrouh, B., Kernif, T., Saidi, F., Tail, G. & Ziam, H. (2022). Seasonal Dynamics and Predilection Sites of Ticks (Acari: Ixodidae) Feeding on Cows in the Western Parts of the Djurdjura, Algeria. Frontiers in Tropical Diseases, 3, 1-11.
62https://doi.org/10.3389/fitd.2022.856179
63Benchikh Elfeggoun, M.C., Benakhla, A., Bentounsi, B., Bouattour, A. & Piarroux, R. (2007). Identification et cinétique saisonnière des tiques parasites des bovins dans la région de Taher (Jijel) Algérie. Annales de Médecine Vétérinaire, 151, 209-214.
64Benchikh Elfegoun, M.C., Gharbi, M., Djebir, S. & Kohil, K. (2013). Dynamique d’activité saisonnière des tiques ixodidés parasites des bovins dans deux étages bioclimatiques du nord-est algérien. Revue d’élevage et de médecine vétérinaire des pays tropicaux, 66(4), 117-122.
65Benchikh Elfegoun, M.C., Kohil, K., Gharbi, M., Afoutni, L. & Benachour, M.L. (2019). Cinétique d’infestation par les tiques des bovins de la région subhumide de Constantine en Algérie. Revue d’élevage et de médecine vétérinaire des pays tropicaux, 72(1), 41-45.
66Benredjem, W., Leulmi, H., Bitam, I., Raoult, D., & Parola, P. (2014). Borrelia garinii and Rickettsia monacensis in Ixodes ricinus Ticks, Algeria. Emerging Infectious Diseases, 20(10), 1776- 1777. https://doi.org/10.3201/eid2010.140265
67Bitam, I, Kernif, T, Harrat, Z., Parola, P., & Raoult, D. (2009). First detection of Rickettsia aeschlimannii in Hyalomma aegyptium from Algeria. Clinical Microbiology and Infection, 15(2), 253-254. https://doi.org/10.1111/j.1469-0691.2008.02274.x
68Bouhous, A., Aissi, M. & Harhoura, K. (2011). Prevalence of Ixodidae in sheep brought for slaughter in Adrar municipal abattoir, Southwest Algeria. Scientia Parasitologica., 12(4), 197-201.
69Boulkaboul, A. (2003). Parasitisme des tiques (Ixodidae) des bovins à Tiaret, Algérie. Revue d’élevage et de médecine vétérinaire des pays tropicaux, 56(3-4), 157-162.
Boulanger, N., Boyer, P., Talagrand-Reboul, E. & Hansmann, Y. (2019). Ticks and tick-borne diseases. Tiques et maladies vectorielles à tiques. Médecine et maladies infectieuses, 1-11. https://doi.org/10.1016/j.medmal.2019.01.007
70Carricano, M., Poujol, F. & Bertrandias, L. (2010). Analyse de données avec SPSS. 2ème édition. Ed. Pearson, Paris, 235 p.
71Chaligiannis, Ι., Fernández de Mera, I.G., Papa, A., Sotiraki, S. & De la Fuente, J. (2018). Molecular identification of tick-borne pathogens in ticks collected from dogs and small ruminants from Greece. Experimental and Applied Acarology, 74, 443-453. https://doi.org/10.1007/s10493-018-0237-z
72Dabaja, M.F., Tempesta, M., Bayan, A., Vesco, G., Greco, G., Torina, A., Blanda, V., La Russa, F., Scimeca, S., Lelli, R., Ezzedine, M., Mortada, H., Raoult, D., Fournier, P.E. & Mortada, M. (2017). Diversity and distribution of ticks from domestic ruminants in Lebanon. Veterinaria Italiana, 53, 147-155.
73Dajoz R. (1975). Précis d’écologie. 3ème édition. Ed. Gauthier-villars, Paris, 549p.
74de la Fuente, J., Naranjo, V., Ruiz-Fons, F., Vicente, J., Estrada-Pena, A., Almazan, C., Kocan, K.M., Martin, M.P., Gortazar, C. (2004). Prevalence of tick-borne pathogens in ixodid ticks (Acari: Ixodidae) collected from European wild boar (Sus scrofa) and Iberian red deer (Cervus elaphus hispanicus) in central Spain. European Journal of Wildlife Research, 50, 187-196. https://doi.org/10.1007/s10344-004-0060-1
75Derradj, L. & Kohil, K. (2020). Identification and incidence of hard tick species during summer season 2019 in Jijel Province (northeastern Algeria). Journal of Parasitic Diseases.
76https://doi.org/10.1007/s12639-020-01296-4
77Dib, L., Bitam, I., Bensouilah, M., Parola, P. & Raoult, D. (2009). First description of Rickettsia monacensis in Ixodes ricinus in Algeria. Clinical Microbiology and Infection, 15(2), 261-262. https://doi.org/10.1111/j.1469-0691.2008.02277.x
78Dib, L., Lafri, I., Boucheikhchoukh, M., Dendani, Z., Bitam, I. & Benakhla, A. (2019). Seasonal distribution of Rickettsia spp. in ticks in northeast Algeria. New Microbes and New Infections, 27(C), 48-52. https://doi.org/10.1016/j.nmni.2018.10.008
79D.McCoy, K. & Boulanger, N. (2015). Tiques et maladies à tiques : Biologie, écologie évolutive, épidémiologie. Ed. IRD, Marseille, 336p.
80Elati, K., Ayad, A.A., Khamassi Khbou, M., Jdid, M., Rekik, M. & Gharbi, M. (2018). Population dynamics of ticks infesting sheep in the arid steppes of Tunisia. Revue d’élevage et de médecine vétérinaire des pays tropicaux, 71(3), 131-135.
81Estrada-Pena, A., Quilez, J. & Sanchez Acedo, C. (2004). Species composition, distribution, and ecological preferences of the ticks of grazing sheep in north-central Spain. Medical and Veterinary Entomology, 18, 123-133.
82Faurie, C., Ferra, C., Medori, P. & Devaux, J. (1980). Écologie approche scientifique et pratique. Ed. Lavoisier, Paris, 488p.
83Foughali, A.A., Jedidi, M., Dhibi, M., Mhadhbi, M., Sassi, L., Berber, A., Bitam, I., & Gharbi, M. (2021). Infection by haemopathogens and tick infestation of sheep during summer season in Constantine region, Northeast Algeria. Veterinary Medicine and Science, 1-9.
84https://doi.org/10.1002/vms3.551
85Garcia-Sanmartin, J., Barandika J.F., Juste, R.A., Garcia-Perez, A.L., & Hurtado, A. (2008). Distribution and molecular detection of Theileria and Babesia in questing ticks from northern Spain. Medical and Veterinary Entomology, 22, 318-325. https://doi.org/10.1111/j.1365-2915.2008.00748.x
Guiguen, C. & Degeilh, B. (2001). Les tiques d’intérêt médical : rôle vecteur et diagnose de laboratoire. Revue Française des Laboratoires, (338), 49-57.
86Klompen, J.S.H, Black, W.C.I.V., Keirans, J.E. & Oliver, J.H. (1996). Evolution of ticks. Annual Review of Entomology, 41, 141-161. https://doi.org/10.1146/annurev.en.41.010196.001041
87Krčmar, S. (2019). Diversity, ecology, and seasonality of hard ticks (Acari: Ixodidae) in eastern Croatia. Journal of Vector Ecology, 44(1), 18-29. https://doi.org/10.1111/jvec.12325
88Leulmi, H., Aouadi, A., Bitam, I., Bessas, A., Benakhla, A., Raoult, D., & Parola, P. (2016). Detection of Bartonella tamiae, Coxiella burnetii and rickettsiae in arthropods and tissues from wild and domestic animals in northeastern Algeria. Parasites & Vectors, 9, 1-8. https://doi.org/ 10.1186/s13071-016-1316
89M’ghirbi, Y., Hurtado, A. & Bouattour, A. (2010). Theileria and Babesia Parasites in Ticks in Tunisia. Transboundary and Emerging Diseases, 57, 49-51.
90https://doi.org/10.1111/j.1865-1682.2010.01110.x
Millot, G. (2018). Comprendre et réaliser les tests statistiques à l’aide de R. Manuel de biostatistique. 4ème édition. Ed. DeBoeck Supérieur, Paris, 960p.
91Omer, L.T., Kadir, M.A.A., Seitzer, U. & Ahmed, J.S. (2007). A survey of ticks (Acari: Ixodidae) on cattle, sheep and goats in the Dohuk governorate, Iraq. Parasitology Research, 101(2), 179-181. https://doi.org/10.1007/s00436-007-0690-9
92Ozubek, S., & Aktas, M. (2017). Molecular and parasitological survey of ovine piroplasmosis, including the first report of Theileria annulata (Apicomplexa: Theileridae) in sheep and goats from Turkey. Journal of Medical Entomology, 54(1), 212-220. https://doi.org/10.1093/jme/tjw134
93Perez-Eid, C & Gilot B. (1998). Les tiques : cycles, habitats, hôtes, rôle pathogènes, lutte. Médecine et Maladies Infectieuses, 28, 335-343.
Péter, O. & Brossard, M. (1998). Lutte contre les tiques. Médecine et Maladies Infectieuses, 28, 383-386.
94Ramade, F. (1984). Éléments d’écologie-écologie fondamentale. Ed. Mc Graw-Hill, Paris, 397p.
95Ramade, F. (2003). Éléments d’écologie- écologie fondamentale. 3ème édition. Ed. Dunod, Paris, 690p.
96Rehman, A., Nijhof, A.M., Sauter-Louis, C., Schauer, B., Staubach, C. & Conraths, F.J. (2017). Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasites & Vectors, 10(190), 1-15. https://doi.org/10.1186/s13071-017-2138-0
97Sarih, M., Socolovschi, C., Boudebouch, N., Hassar, M., Raoult, D., & Parola P. (2008). Spotted Fever Group Rickettsiae in Ticks, Morocco. Emerging Infectious Diseases, 14(7), 1067- 1073. https://doi.org/10.3201/eid1407.070096
Tissot Dupont, H. & Raoult, D. (1993). Maladies transmises par les tiques. La Revue de Medecine Interne, 14, 300-306.
98Walker, A.R., Bouattour, A., Camicas, J.L., Estrada-Pena, A., Horak, G., Latif, A.A., Pergram, R.G. & Preston, P.M., (2003). Tiks of Dometic Animals in Africa: a guide to identification of Spicies. 221p.
99Wall, R. & Shearer, D. (2001). Veterinary Ectoparasites: Biology, Pathology and Control.
2nd edition. Ed. BLackwell Science, 262p.
100Yousfi-Monod R. & Aeschlimann A. (1986). Recherche sur les tiques (Acarina, Ixodidae), parasites de bovidés dans l’Ouest Algérien. Inventaire systématique et dynamique saisonnière. Annales de Parasitologie Humaine et Comparée, 61(3), 341-358.
101Zeroual, F., Bitam, I., Ouchene, N., Leulmi, H., Aouadi, A. & Benakhla, A. (2014). Identification and seasonal dynamics of ticks on wild boar (Sus scrofa) in the extreme north east of Algeria. Bulletin de la Société Zoologique de France, 139(1-4), 245-253.