- Home
- Volume 93 - Année 2024
- No 1
- Effect of copper on ornamental plants Agrostis stolonifera L., Centaurea cyanus L., Godetia grandiflora Lindl.
View(s): 1923 (10 ULiège)
Download(s): 68 (0 ULiège)
Effect of copper on ornamental plants Agrostis stolonifera L., Centaurea cyanus L., Godetia grandiflora Lindl.

Attached document(s)
original pdf fileAbstract
Ornamental plants are widely used in horticulture and urban greening. However, when plants are used in urban greening, it is necessary to select varieties with increased tolerance to urban conditions. Copper is one of the most toxic metals to plants, so the assessment of plant resistance to copper is important for urban environments. High sensitivity of the studied plants to copper was shown. Godetia grandiflora is the most resistant to copper among the species studied. Agrostis stolonifera and Centaurea cyanus had comparable resistance to copper.
The effect of copper on calli of Agrostis stolonifera was also studied.
Table of content
1† Authors (Gladkov E.A., Gladkova O.V.) contributed equally to this work and share first authorship
2Manuscrit reçu le 16 octobre 2023 et accepté le 30 janvier 2024
3Article publié selon les termes et les conditions de la licence Creative Commons CC-BY 4.0
1. Introduction
4Plants have long been valued for their decorative qualities. The color palette of the plants has many shades. The color of flowers is related to the tissue structure of a petal and the type and amount of pigments in the petal cells (Zhao and Tao 2015).
5However, generating the blue color of the flower is difficult, requiring a complex chemical pathway, where six anthocyanins together with six co-occurring molecules form a ring around two central metal ions (Dyer et al. 2021). Ornamental plants are widely used in horticulture and urban greening. When plants are used in urban greening, it is necessary to select varieties with increased tolerance to urban conditions. When ornamental plants are used in urban greening in conditions of increased soil pollution, it is difficult to maximize the aesthetic impact due to the reduction of ornamental qualities of plants.
6Copper is one of the main pollutants of urban ecosystems (Gladkov et al, 2021; 2022). The soils of many cities are indeed contaminated with copper. Soil contamination by copper is one of the main problems of urban landscaping, as copper has significantly higher phytotoxicity compared to other priority heavy metals (zinc and lead).
7Soil contamination with copper is characteristic of some large European cities. Copper is one of the soil pollutants in the old part of the Copenhagen metropolitan area ( Li et al, 2013).
8Copper is one of the priority pollutants of Moscow soils among heavy metals. In 2010, the maximum copper content in the study areas was 400 mg/kg, with an average value of 19 mg/kg (Report on the state of the environment in the city of Moscow in 2010).
9Copper is one of the most toxic metals to plants. Even a slight excess of Cu can be harmful to plants (Ye et al. 2014). Copper has an inhibitory effect on mineral nutrition, chlorophyll biosynthesis and antioxidant enzyme activity (Mir et al. 2021). High levels of copper in the plant contribute to oxidative stress by generating reactive oxygen species (Kumar et al. 2021).
10Copper pollution of soil results in changes in plant growth and development. Growth inhibition of various plant species by high concentrations of copper has been shown (Gladkov et al. 2019; Huo et al. 2020). The effect of copper was investigated for some ornamental plants. Plants Dendranthema xgrandiflorum L., Rosa x hybrida L., Pelargonium x hortorum L. showed sensitivity to copper (Zheng et al 2004).
11The response of various crops to elevated Cu concentrations varies depending upon the nature of the cultivars used (Adrees et al. 2015). Therefore, the assessment of plant resistance to copper is important. By determining the copper resistance of different plant species and cultivated varieties it is possible to assess their potential for use in different urban ecosystems. We used urban flowering plants and lawn grass as study objects. The main object of the study was the lawn grass Agrostis stolonifera.
12Agrostis stolonifera L. is a perennial grass species from the Poaceae family.
13Centaurea cyanus L. (cornflower pink) is a representative of the Asteraceae (Aster family). It is widely cultivated as an ornamental plant. It is used for alpine slides and rockeries, for group plantings, and has pink inflorescences.
14Godetia grandiflora Lindl. belongs to the Onagraceae family. The flowers of Godetia grandiflora are up to 5-8 cm in diameter. Godetia grandiflora looks spectacular against the background of the lawn. Godetia is grown in flowerbeds and mixborders. Godetia grandiflora is grown as bedding plant for landscaping purposes and also as a cut flower (Sharma et al. 2016).
15These plant species can also be used to create a flowering lawn. Flowering lawns are a mix of lawn grass and low-growing flowers (Ramer et al. 2019); creating rural landscape elements in the city. The effect of copper on calli may be of interest for biotechnological studies. However, the effects of copper on callus and plants may differ. In a preliminary approach, the calli data presented herein can be used to determine selective copper concentration for obtaining copper-tolerant plants.
2. Materials and Methods
2.1Assessment of copper phytotoxicity
16Seeds were placed in Petri dishes on filter paper moistened with copper solution.
17The phytotoxicity of copper was evaluated by measuring shoot growth.
18CuSO4∙5H2O was used in the experiments (in all experiments copper concentrations are given). Under control conditions, seeds were placed in Petri dishes on filter paper moistened with water. Each variant of the experiment was carried out 4-5 times depending on the plant species.
2.2 Callus induction
19To obtain the calli of Agrostis stolonifera, seeds were used. They were previously soaked in ethyl alcohol for10- 60 seconds . Then they were sterilized with commercial bleach solution (Sodium hypochlorite ≤ 5%, sodium hydroxide ≤ 2% named Belizna®), for 10-20 minutes and finally rinsed three times 10 min in distilled water. The sterilized seeds were spread in Petri dishes on the surface of agar medium and cultured at 26°C and 70% humidity with a daylight length of 16 hours.
20Calli of Agrostis stolonifera were obtained on Murashige and Skoog modified medium with 3 mg/l 2,4- dichlorophenoxyacetic acid. The sucrose content was 30 g/L, casein hydrolysate was 500 mg/L, agar - agar was 7 g/L.
Figure 1: Callus of Agrostis stolonifera
2.3 Assessment of copper toxicity for calli
21Murashige and Skoog modified medium supplemented with 1 mg/L 2,4- dichlorophenoxyacetic acid was used for callus growth. Controls were calli cultured on medium without copper. Calli were cultured on Murashige and Skoog modified medium supplemented with CuSO4∙5H2O. Determination of the sensitivity of callus cultures to copper was carried out based on calli growth. Calli were evaluated by the increase in crude weight after 30 days of cultivation.
3. Results and discussion
22High sensitivity of the studied plant species was shown in aqueous copper solutions. The inhibitory concentration, slowing shoot growth by more than 25- 30% was 30-50 mg/L copper depending on the plant species (Figure 2).Copper tolerance of the studied species differed. This is consistent with the literature data (Gladkov et al. 2019). For example, cabbage plants presented higher tolerance to increased Cu levels in the growing environment than beet plants (Schmitt et al, 2022). However, we did not observe very significant differences among the studied plant species. Godetia grandiflora is the most resistant to copper among the species studied. Agrostis stolonifera and cornflower pink had comparable resistance to copper.
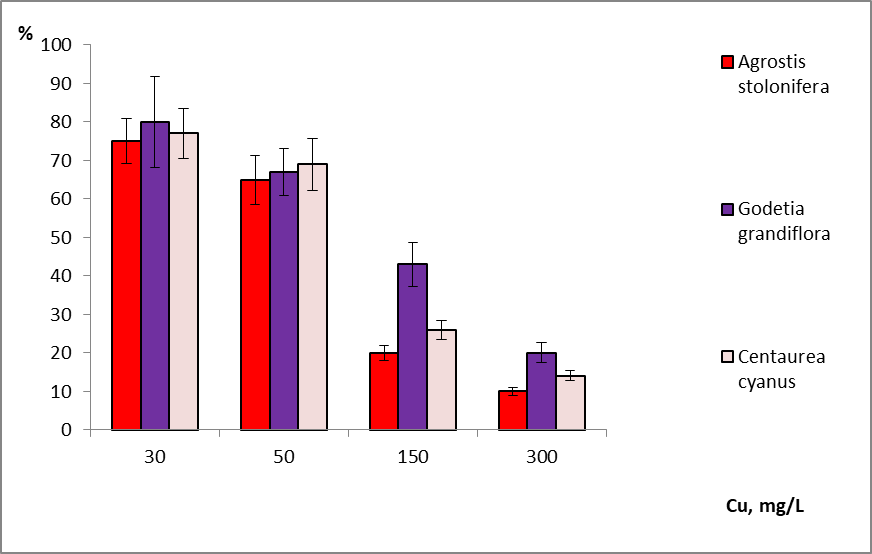
Figure 2: Effect of copper on shoot growth in aqueous solutions (% of control).
23Calli of Agrostis stolonifera were cultured on Murashige-Skoog modified medium with 150 mg/L copper.
24The study showed that Agrostis stolonifera plants and calli had different resistance (Table 1). Copper had a great effect on plant growth in aqueous solution (Table 1).
Table 1: Comparison of the effects of copper toxicity on Agrostis stolonifera plant and callus growth at 150 mg /L copper (% of control)
|
Plants |
202 % |
|
Calli |
371,5 % |
25The differential resistance to copper may be attributed to different mechanisms of resistance to copper at the cellular, tissue, organismal levels (interaction with parts and organs of the whole plant).
26High sensitivity of the studied plants to copper was demonstrated. However, the studied plant species showed more resistance to copper than Chrysanthemum carinatum (Gladkov et al, 2019). Copper pollution is one of the limiting factors for herbaceous urban plants. Therefore, it is necessary to select varieties of urban plants with greater resistance to copper pollution and the use of technologies that increase the tolerance of plants to copper (Gladkov et al. 2021; 2022). With proper choice of species and varieties, plants will be less sensitive to the effects of copper in urban parks, gardens and near residential buildings. For example, in most parts of Moscow, copper will not have a very significant effect on plants with the right choice of plants. However, in areas with very high soil contamination, copper will have a negative effect on plants.
4. Ethics approval and consent to participate
27Not applicable.
28
5. Consent for publication
29Not applicable
6. Funding
30Gladkov E. was supported within the state assignment of the Ministry of Science and Higher Education of the Russian Federation (theme 122042600086-7).
31
7. Competing Interest
The authors declare that they have no competing interests.
8. Availability of data and materials
All data generated or analysed during this study are included in this published article
9 References
32Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Zia-Ur-Rehman M, Irshad MK, Bharwana SA.(2015) The effect of excess copper on growth and physiology of important food crops: a review. Environmental Science and Pollution Research . 22(11):8148-62. DOI: 10.1007/s11356-015-4496-5.
33Dyer AG, Jentsch A, Burd M, Garcia JE, Giejsztowt J, Camargo MGG, Tjørve E, Tjørve KMC, White P and Shrestha M (2021) Fragmentary Blue: Resolving the Rarity Paradox in Flower Colors. Front. Plant Sci. 11:618203.
34DOI: 10.3389/fpls.2020.618203
35Gladkov EA, Tashlieva II, Gladkova OV (2022) Cell selection for increasing resistance of ornamental plants to copper. Environmental Science and Pollution Research 29: 25965–25969. DOI: org/10.1007/s11356-022-19067-4
36Gladkov Evgeny A., Tashlieva Ilina I., Gladkova Olga Victorovna (2019) Copper resistance of lawn grass and Chrysanthemum Carinatum plants. Archives for Technical Sciences. DOI: 10.7251/afts.2019.1121.063G
37Gladkov EA, Tashlieva II, Dolgikh YI, Gladkova OV (2021) Increasing the tolerance to copper of ornamental plants and lawn grasses in urban ecosystems. Bull Soc Royale Sci Liège 90:181–188.
38Huo K, Shangguan X, Xia Y, Shen Z, Chen C. Excess copper inhibits the growth of rice seedlings by decreasing uptake of nitrate. Ecotoxicology and Environmental Safety. 2020 190:110105. DOI: 10.1016/j.ecoenv.2019.110105.
39
40Kumar V, Pandita S, Singh Sidhu GP, Sharma A, Khanna K, Kaur P, Bali AS, Setia R (2021). Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere. 262:127810. DOI:10.1016/j.chemosphere.2020.127810
41Li Lijun, Holm Peter E., Marcussen Helle, Hansen Hans Christian Bruun (2014),
42Release of cadmium, copper and lead from urban soils of Copenhagen,
43Environmental Pollution, 187 : 90-97.DOI : org/10.1016/j.envpol.2013.12.016.
44Mir AR, Pichtel J, Hayat S. (2021) Copper: uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 34(4):737-759.
45DOI: 10.1007/s10534-021-00306-z.
46Ramer, H., Nelson, K. C., Spivak, M., Watkins, E., Wolfin, J., & Pulscher, M. (2019). Exploring park visitor perceptions of ‘flowering bee lawns’ in neighborhood parks in Minneapolis, MN, US. Landscape and Urban planning 189: 117-128.
47Report on the state of the environment in the city of Moscow in 2010 (2011). Department of Natural Resources and Environmental Protection of the City of Moscow, Moscow
48Sharma, P., Gupta, Y. C., Dhiman, S. R., Sharma, P., & Bhargava, B. (2016). Variation in growth, flowering and seed yield of satin flower (Godetia grandiflora) planted on different dates. The Indian Journal of Agricultural Sciences, 86(2)
49Schmitt OJ, Andriolo JL, Silva ICB, Tiecher TL, Chassot T, Tarouco CP, Lourenzi CR, Nicoloso FT, Marchezan C, Casagrande CR, Drescher GL, Kreutz MA, Brunetto G (2022) Physiological responses of beet and cabbage plants exposed to copper and their potential insertion in human food chain. Environ. Sci. Pollution Res. Int. 29(29):44186-44198. DOI: 10.1007/s11356-022-18892-x.
50Ye N, Li H, Zhu G, Liu Y, Liu R, Xu W, Jing Y, Peng X, Zhang J.(2014) Copper suppresses abscisic acid catabolism and catalase activity, and inhibits seed germination of rice. Plant Cell Physiol. 55(11):2008-16. DOI: 10.1093/pcp/pcu136
51Zhao D and Tao J (2015) Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 6:261.
52DOI: 10.3389/fpls.2015.00261
53Zheng, Y., Wang, L., & Dixon, M. A. (2004). Response to Copper Toxicity for Three Ornamental Crops in Solution Culture. HortScience 39(5): 1116-1120. DOI : org/10.21273/HORTSCI.39.5.1116
54±±