- Accueil
- Volume 86 - Année 2017
- Articles
- Seasonal variation of community composition of zooplankton in the Palk strait, (9‐10 °N, Arabian sea, India).
Visualisation(s): 5599 (14 ULiège)
Téléchargement(s): 140 (0 ULiège)
Seasonal variation of community composition of zooplankton in the Palk strait, (9‐10 °N, Arabian sea, India).

Document(s) associé(s)
Version PDF originaleAbstract
The present investigation has been made to study the distribution, qualitative composition, of zooplankton from the eastern Arabian Sea of the habitat from Sundarapandian Pattinamand Manamelkudi (9° 40’ N; 69° 20’ E) along the Palk Strait of east coast of Tamil Naduduring. From July 2005 to June 2006, the zooplankton was sampled every three months for each monsoon seasons. The studied sites are located in one of the most productive zones of the world. Zooplankton species associations were studied using a null model species co‐occurrence. The results revealed the absence of regulators only for Zooplankton in station II in fixed-fixed model. In spite of these results would not agree with the literature descriptions, the cause would be the presence of many repeated species for studied sites. Ecological fisheries and resources management were discussed.
Table des matières
Introduction
1The eastern part of the Arabian Sea, along the southwestern coast of India, is one amongst the most productive regions in the world ocean, and is characterized by one of the largest bodies of oxygen deficient waters on the Earth (Pillai et al., 2000; Boll et al., 2015; Narale et al., 2015, Gupta et al., 2016). In this region, high fisheries activities are regulated by seasonal variations in secondary productivity (Pitchaikani & Lipton, 2015; Nair et al. 2015)
2At the eastern side of the Arabian Sea, the Gulf of Mannar and Palk Bay are both located between South‐East India and North Sri Lanka (Jyothibabu et al. 2014). The Gulf of Mannar is relatively deep (average depth 100 m) and extends ~190 km along the SE Indian coastline. It is connected to the Arabian Sea in the West, and to Palk Bay in the NE through a narrow strait, so called Palk Strait. The Palk Bay is an enclosed shallow basin (average depth 9m), which extends~260 km along the Indian coastline. It is connected to the Bay of Bengal in the NorthEast (Jyothibabu et al. 2013). Hydrographic parameters of Gulf of Mannar and Palk Bay were studied by Bindu Sulochanan and Muniyandi (2005).
3The secondary production of this region has been investigated several times until very recently (Santhakumari 1970; Thankaraj et al. 1979; Subramanian, 1987; Sanpathkumar et al. 2015), and good knowledge is available on zooplankton composition in the Palk Strait area (Kalaiarasi, 2011). Due to the presence of vulnerable biological resources, the Gulf of Mannar is now legally protected as Marine Biosphere Reserve (Jyothibabu et al. 2013). The aim of the present study is analyze the zooplankton occurrence at two stations along the Palk Strait, in the Tamil Nadu region of SE India (Fig. 1).
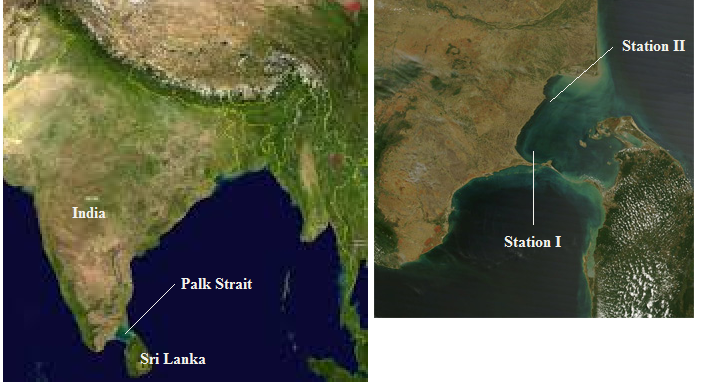
Fig. 1: Map of India (left) and the sampling sites on the east coast of India (right), state of Tamil Nadu, included in the present study; Palk Strait is the channel between India and Sri Lanka. Source: Google Earth, link accesed the 25th Jule 2016:
Material and Methods
4There were two study sites: the first, Station I, is at Sundarapandian Pattinam, a small fishing village located along Palk Strait on the east coast of the southern part of the Indian state of Tamil Nadu. This village is situated in the Ramanathapuram District and is known for its fishery resources (Kalaiarasi, 2011). The second site, Station II, at Manamelkudi, is at a village in the region Avudaiyarkoil Taluk of the Pudukkottai District, with a population of 10 072 (as per the 1991 census) (fig. 1). It extends over an area of 11.35 km2 and is situated close the Sethusamudram canal of Palk Strait (Rajamanickam, 2004; De los Ríos-Escalante et al., 2016).
5The rainfall in these regions is mainly due to North East and South West monsoon. These coastal areas have a minimal wave action. Turbidity of the seawater is moderately low and also they are rich in nutrients hence (year variation: NO3- = 4.66-5.71 M, PO4-3 = 3.64-4.83 M; chlorophyll “a” = 3.11-898 mg/L; Cf: Anantharaj et al., 2013), they serve as treasure houses for valuable marine resources like seagrass, seaweeds and invertebrates like coelenterates, echinoderms and shell fishes. The major occupation of the people is fishing activities (Kalaiarasi, 2011).
6Zooplankton sampling was carried out once in every three month at the middle of each season in function of monsoon due the precipitations associated to these seasons (Kalaiarasi, 2011; De los Ríos-Escalante et al., 2016). Zooplankton samples were collected by No. 10 plankton net (Mesh aperture size 150µm) bolting silk plankton nets for the period of one year from July 2005- June 2006 (Kalaiarasi, 2011; De los Ríos-Escalante et al., 2016). All collections were made on the early morning hours of full moon days (Kalaiarasi, 2011; De los Ríos-Escalante et al., 2016).
7An artisanal boat was used for plankton collection purposes; it could maintain a set speed and keep the net dragging distance the same on all sampling occasions. The construction and design of the net was similar to the net used by Wickstead (1968). The plankton collections were made by horizontal towing after reaching the location of the station, which was located at 50 m from the shore. The haul was made in first instance at approx. 20 m depth, and the towing distance was 100 m, whence approximately 27.5 m3 water was sampled. The sampling was done while maintaining a constant speed of the boat. All the zooplankton samples were preserved in 5% formaldehyde solution, and analyzed qualitatively using Sedgwick Rafter plankton counting cell, and identified in according to literature and counted by individuals under microscope (Davis 1955; Edmondson 1959; Omori & Ikeda 1984; Kasturirangan 1963; Newell & Newell, 1973; Kalaiarasi, 2011).
8A species absence/ presence matrix was constructed, with the species in rows and the sites in columns, and it calculated a Checkerboard score (“C‐score”). This is a qualitative index of occurrence that measures the extent to which species co‐occur less frequently than expected by chance. A community is structured by competition when the C‐score is significantly larger than expected by chance (Tiho & Johens, 2007, Gotelli & Entsminser 2009; De los Ríos-Escalante et al. 2016; Muñoz-Pedreros et al. 2015).It compared co-occurrence patterns with null expectations via simulation. In this contextGotelli & Entsminger (2009) proposed the following robust statistical null models:
9(1) Fixed‐Fixed: in this model, the row and column sums of the matrix are preserved. Thus, each random community contains the same number of species as the original community (fixed column), and each species occurs with the same frequency as in the original community (fixed row).
10(2) Fixed‐Equiprobable: in this algorithm, only the row sums are fixed, and the columns are treated as equiprobable. This null model considers all the samples (column) as equally available for all species.
11(3) Fixed‐Proportional: in this algorithm, the species occurrence totals are maintained as in the original community, and the probability that a species occurs at a site (column) is proportional to the column total for that sample. The null model analyses were applied using the software Ecosim version 7.0 (Gotelli & Entsminger, 2009).
Results and Discussion
12The results revealed the presence of high species richness in zooplankton, and in this context the copepods contributed with many species in comparison to other groups for all studied sites (Table 1).
Table 1: Zooplankton groups presence-absence species observed at two sampled sites in the present study.
|
Protozoa |
Station I |
Station II |
||||||
|
Premonsoon |
Monsoon |
Postmonsoon |
Summer |
Premonsoon |
Monsoon |
Postmonsoon |
Summer |
|
|
Favella philippinensis Roxas, 1941 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
Mollusca |
||||||||
|
Bivalve veligers |
0 |
1 |
1 |
0 |
1 |
1 |
1 |
0 |
|
Gastropods veligers |
0 |
1 |
1 |
0 |
1 |
1 |
1 |
0 |
|
Crustacea |
||||||||
|
Copepoda |
||||||||
|
Acartiasp. Dana, 1846. |
0 |
0 |
0 |
1 |
0 |
9 |
0 |
0 |
|
A. erythrea Giesbrecht, 1889 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
|
Calanus finmarchicus (Gunnerus, 1770) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Calocalanus pavo (Dana, 1852) |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Candacia armata Boeck, 1872. |
1 |
0 |
1 |
1 |
1 |
0 |
0 |
0 |
|
C. furcatus (Dana, 1849) |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
|
C. longicornis Mori, 1932 |
1 |
1 |
1 |
1 |
1 |
0 |
0 |
0 |
|
C. orsini Giesbrecht, 1889 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
|
C. typicus Kroyer, 1849 |
1 |
0 |
1 |
0 |
0 |
1 |
0 |
1 |
|
Centropages furcatus (Dana, 1849) |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
|
C. typicus Kroyer, 1849 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
|
Cyclopina longicornis Boeck, 1872 |
1 |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
|
Eucalanus elongatus (Dana, 1848) |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
Euchaeta concinna Dana, 1849 |
1 |
0 |
1 |
1 |
1 |
0 |
0 |
1 |
|
Eurytemora hirundinoides (Nordquist, 1888) |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
Isias clavipesBoeck, 1865 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
|
I. tropica Sewell, 1924 |
0 |
1 |
1 |
1 |
0 |
0 |
1 |
1 |
|
Labidocera acuta (Dana, 1849) |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
L. pavo Giesbrecht, 1889 |
1 |
1 |
1 |
1 |
1 |
0 |
1 |
1 |
|
Metacalanus aurivilli Cleve, 1901. |
1 |
1 |
1 |
1 |
1 |
0 |
0 |
1 |
|
Microcalanus pusillus Sars, G.O., 1903. |
1 |
1 |
1 |
0 |
1 |
0 |
0 |
0 |
|
Oithona brevicornis Giesbrecht, 1891 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
O. helgolandica (Claus, 1863) |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
O. plumífera Baird, 1843 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
O. rigida Giesbrecht, 1896 |
1 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
|
Paracalanus parvus (Claus, 1863) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Parapontella brevicornis Lubbock, 1857. |
1 |
0 |
0 |
1 |
0 |
1 |
1 |
0 |
|
Pontelladanae Giesbrecht, 1889. |
0 |
1 |
0 |
1 |
1 |
1 |
1 |
1 |
|
Pontenilla plumata Dana, 1849 |
1 |
1 |
1 |
1 |
0 |
0 |
1 |
0 |
|
Pseudocalanus elongatus (Boeck, 1865) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Pseudodiaptomus serricaudatus (Scott, T., 1854) |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
Rhinocalanus nasutus Giesbrecht, 1888 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
|
Temora longicornis (Müller O.F., 1785). |
1 |
1 |
0 |
1 |
1 |
0 |
1 |
1 |
|
T. turbinita (Dana, 1849). |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
|
Copepod nauplii |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
Malacostraca |
||||||||
|
Decapoda |
||||||||
|
Carcinus maenas (Linnaeus, 1758) |
0 |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
|
Inachus dorsettensis (Pennant, 1777) |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
|
Leucifer sp H. Milne Edwards, 1837. |
0 |
0 |
0 |
1 |
0 |
1 |
1 |
0 |
|
Lithodes maja (Linnaeus, 1758) |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
|
Pagurus sp. Fabricius, 1775 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
0 |
|
Penaeus indicus H. Milne Edwards, 1837. |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
0 |
|
Decapod nauplii |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
1 |
|
Peneid nauplii |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
1 |
|
Echinodermata |
||||||||
|
Bipinnaria larvae |
1 |
1 |
1 |
0 |
1 |
0 |
0 |
0 |
|
Echinospira larvae |
0 |
0 |
0 |
1 |
1 |
0 |
0 |
0 |
|
Chordata |
||||||||
|
Egg and fish larvae |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
13The results of null models revealed the presence of regulator patterns for both sampled stations, whereas for station I only fixed-fixed model revealed presence of regulator patterns, and for station II, all simulations revealed the absence of regulator patterns (Table 2).
Table 2: Results of null model analysis for studied sites (“P” values lower than 0.05 denotes structured patterns).
|
Zooplankton total |
||||
|
Model |
Observed index |
Mean index |
Standard effect size |
P |
|
Fixed-fixed |
2.075 |
2.009 |
3.516 |
0.008* |
|
Fixed-proportional |
2.075 |
1.956 |
1.708 |
0.010* |
|
Fixed-equiprobable |
2.075 |
1.980 |
1.587 |
0.024 * |
|
Zooplankton station I |
||||
|
Model |
Observed index |
Mean index |
Standard effect size |
P |
|
Fixed-fixed |
0.587 |
0.555 |
3.315 |
0.012 * |
|
Fixed-proportional |
0.587 |
0.548 |
1.192 |
0.055 n.s |
|
Fixed-equiprobable |
0.587 |
0.555 |
1.134 |
0.074 n.s |
|
Zooplankton station II |
||||
|
Model |
Observed index |
Mean index |
Standard effect size |
P |
|
Fixed-fixed |
0.689 |
0.689 |
-0.045 |
0.466 n.s |
|
Fixed-proportional |
0.689 |
0.654 |
1.061 |
0.087 n.s |
|
Fixed-equiprobable |
0.689 |
0.655 |
1.052 |
0.091 n.s |
14The results about species assemblages of zooplankton agree with literature descriptions about Arabian Sea, due high productivity, although there are marked seasonal density fluctuations due monsoons (Vineetha et al. 2015; Vishnu Radhanan et al. 2015; Gupta et al. 2016). Within the zooplankton species, Copepods by their sheer and diversity constitute the most important group in any zooplankton community (Anbazhagan, 1988; Jayalakshmi et al. 2015; Vineetha et al. 2015), because they occupy an important position in the trophic structure and play a major role in the energy transfer of any aquatic environment. Because of their predominance, copepods form the chief index of utilization of biotope at secondary level (Madhupratap & Haridas, 1975; Madhupratap, 1979; Gupta et al., 2016). About the seasonality of decapod larvae (Vineetha et al., 2015), and eggs and fish larvae (Abesamis et al., 2015; Samoilys et al., 2014), this condition would not agree with null results of model analysis. In the present study the result showed that the seasonal variations were occurred in the community structure is mainly due to the occurrence of many repeated species were present in the studied area and this results supported by null models.
15The obtained information is important for understand biological process, specifically fishes and invertebrates recruitment, especially for species with marked interest for local fisheries and aquaculture, that is an important activity in Palk Bay and Palk Strait. In this context it would be necessary implement management measures that will allow regulating human activities thatmight disturb the normal development of the ecosystem in these. In conclusion, it is necessary more detailed studies for preserve this ecosystem.
Acknowledgements.
16The present paper was funding by project MECESUP UCT 0804, and the authors express their gratitude to M.I for her valuable help.
References.
17Abesamis, R.A., C.R. L Jadloc, & G.R. Russ, 2015. Varying annual patterns of reproduction in four species of coral reef fish in a monsoonal environment. Marine Biology, 162: 1993-2006,
18Anbazhagan, P. 1988. Hydrobiology and Benthic ecology of Kodiakkarai coastal sanctuary (Southeast coast of India).Ph.D. Thesis, Annamalai University, India, 1988.
19Anantharaj, K., C. Govindasamy, G. Natanamurugaraj & S. Jeyachandran, 2013. Characteristics of water quality in the Palk Strait, South East coast of India. American-Eurasian Journal of Agricultural and Environmental Sciences, 13: 926-929.
20Bindu Sulochanan & K. Muniyandi, 2005. Hydrographic parameters of Gulf of Mannar and Palk Bay during an year of abnormal rainfall. Journal of Marine Biology Association of India. 47: 198-200.
21Boll, A., H. Schulz, P. Munz, T. Rixen, B. Gaye, K.C.Emeis, 2015. Constrasting sea surface temperature of summer and winter monsoon variability in the northern Arabia Sea over the last 25 ka. Palaeogeography Palaeoclimatology and Palaeoecology, 426: 10-21,
22Davis, C.C. 1955. Plankton.Western Reserves University.India.
23De los Ríos-Escalante, P., M. Kalaiarasi, P. Paul, & C. Stella, 2016. Faunistic studies on macrozooplankton at Sundaparandian Pattinam and Manamelkudi both locations along Palk Strait, Tamil Nadu, India (9-10° N, Arabian Sea. Crustaceana, 89: 1149-1160.
24Edmondson, W.T. 1974. Secondary production.Mitteilungen. Internationale Vereinung fur Limnolology 20: 229-272.
25Gotelli, N.J., & G.L. Entsminger. 2009. EcoSim: Null models software for ecology. Version 7. Acquired Intelligence Inc. &Kesey-Bear.Jericho, VT 05465.
26http://garyentsminger.com/ecosim.htm.
27Gupta, G.V.M., V. Sudheesh, K.V. Sudharma, N. Saravanane, V. Dhanya, K.R. Dhanya, M. Sudhakar, & S.W.A. Naqvi. Evolution to decay of upwelling and associated biogeochemistry over the southeastern Arabian Sea shelf. Journal of Geophysical Research and Biogeosciences, 121, 159–175, 2016.
28Jayalakshmi, K.J., P. Sabu, C.R. Asha Devi, & V.N. Sanjeevan. 2015. Response of micro-and mesozooplankton in the southwestern Bay of Bengal to a cyclonic eddy during the winter monsson 2005. Environmental Monitoring Assessment, 187: 473, 2015.
29Jyothibabu, R., P.M.Arya, L. Jagadeesan, A. Anjusha, K.R. Muraleedharan, K.R. Lallu, Kiran Krishna, & N. Ullas. 201. Ecology and trophic preferences of picoplankton and nanoplankton in the Gulf of Mannar and the Palk Bay, southeast coast of India. Journal of Marine Systems 111-112: 29-44.
30Jyothibabu, R., N.V. Madu, L. Jagadeesan, A. Anjusha, P.M. Arya, N. Ullas, K. Sudheesh, & C. Karnan. 2014. Why do satellite imageries show exceptionally high chlorophyll in the Gulf of Mannar and the Palk Bay during the Northeast Monsoon?. Environmental Monitoring Assessment 186: 7781-7792.
31Kalaiarasi M. 2011. Studies of zooplankton in S.P. Pattinam and Manamelkudi in Palk Strait. PhD. Thesis Alagappa University, India.
32Kasthurirangan LR. 1963. A key for the identification of the more common plank tonic copepod of Indian coastal waters. CSIR, New Delhi, India, 1963.
33Madhupratap M. 1979. Distribution, community structure and species succession of copepods from Cochin back wares. Indian Journal of Marine Sciences, 8: 1-8.
34Madhupratap, M., & P. Haridas, 1975. Composition and variations in abundance of zooplankton of back water from Cochin to Alleppey. Indian Journal of Marine Sciences, 4: 77-85.
35Narale, D.D., P.D. Naidu, A.C. Anil, & S.P. Godad, 2015. Evolution of productivity and monssonal dynamics in the eastern Arabian sea during the past 68 ka using dinoflagellate cyst records. Palaeogeography Palaeoclimatology and Palaeoecology, 435: 193-202.
36Muñoz-Pedreros, A., P. De los Ríos-Escalante, & P. Möller, 2015.. Zooplankton of the Highland bogs of Putana, a desert wetland of the high Puna, northern Chile. Crustaceana, 88: 1235-1244.
37Nair, V.R., K.K. Kusum, R. Gireesh, & M. Nair, 2015. The distribution of the chaetognath population and its interaction with environmental characteristics in the Bay of Bengal and the Arabian Sea.Marine Biology Research 11: 269-282.
38Newell, G.E., & R.C. Newell, 1973. Marine Plankton, a practical Guide. Hutchinson education Co, Ltd., London, UK.
39Omori, M. & T. Ikeda, 1974. Methods in marine zooplankton ecology. “A Wiley-Inter Science Publication John Willey & Sons.Japan.
40Pillai, V.N., V.K. Pillai, C.P. Gopinathan, & A. Nandakumar, 2000. Seasonal variations in the physico-chemical and biological characteristics of the eastern Arabian Sea. Journal of Marine Biology Association of India, 42: 1-20.
41Pitchaikani, S., & A.P. Lipton, 2015. Seasonal variation of zooplankton and pelagic fish catch in the fishing grounds off Tirichendur coast, Gulf of Mannar, India. Ecohydrology and Hydrobiology 15: 89-100.
42Rajamanickam G. Sethusamudram canal: The lifeline of Tamil Nadu.Proceedings of the National Seminary Ecology Balance and Sethusamudram Canal.9-15, 2004.
43Samoilys, M.A., D. Macharia, J. Robinson, G.W. Maina, & J. Bijoux, 2014. Confirmed sighting of a spawning aggregation of the brown-marbled grouper, Epinephelus fuscoguttatus in Kenya. Western Indian Journal of Marine Sciences 13: 189-203.
44Sampathkumar, P., S. Balakrishnan, K. Kamalakannan, R. Sankar, L. Ramkumar, S. Ramesh, N. Kabilan, T. Sureshkumar, C. Thenmozhi , M. Gopinath , S. Jayasudha, A. Arokiyasundram, T. Lenin, T. Balasubramanian, 2015. Hydrographical parameters and phytoplankton assemblages along the Pondicherry-Nagapattinam coastal waters, southeast coast of India. Advanced Climate Change Research 6: 36-45.
45Santhakumari P., 1970. Seasonal variations in the N:P ratios-in the surface water of the mouth of the Vellar estuary. Journal of Annamalai University, 28: 13-18.
46Subramanian, P. 1987. Spawner recruitment distribution of Penaeus indicus in Parangipetai coastal ecosystem. Journal of Marine Biological Association of India, 29: 23-26.
47Thangaraj, G.S., V. Sivakumar, R. Chandre, & R. Santhanam, 1979. Srikrishnadhas B,Ramamoorthi K. An Environmental inventory of Parto Nova coastal Zone. Proceedings of Symposium of Environmental Biology:75-87.
48Tiho, S., & J. Johens, 2007. Co-occurrence of earthworms in urban surroundings: a null models of community structure. European Journal of Soil Biology, 43: 84-90.
49Vineetha, G., N.V. Madhu, K.K. Kusum, & P.M. Sooria, 2015. Seasonal dynamics of the copepod community in a tropical monsoonal estuary and the role of sex ration in their abundance pattern. Zoological Studies, 54: 54.
50VishnuRadhan, R., S. Jerome, S. Ebin, P. Vethamony, P. Shirodkar, Z. Zainudin, & S. Shirodkar, 2015.. Southwest monsoon influences the water quality and waste assimilative capacity in the Mandovi estuary (Goa State, India). Chemical Ecology 31: 217-234.
51Wickstead, J.H. 1968. Temperate and tropical plankton a quantitative comparison. Journal of Zoology London, 155: 253-269.