- Startpagina tijdschrift
- Volume 26 (2023)
- number 1-2
- The Cretaceous–Paleogene transition in spiny-rayed fishes: surveying “Patterson’s Gap” in the acanthomorph skeletal record
Weergave(s): 7236 (68 ULiège)
Download(s): 2115 (31 ULiège)
The Cretaceous–Paleogene transition in spiny-rayed fishes: surveying “Patterson’s Gap” in the acanthomorph skeletal record
André Dumont medalist lecture 2018

Abstract
In contrast to the rich collections of articulated spiny-rayed fishes from early Late Cretaceous and Eocene Lagerstätten, similar skeletal remains are sparse in Maastrichtian–Paleocene strata. Here we coin this poorly understood span “Patterson’s Gap” and review known articulated skeletons from it, summarizing available information on their phylogenetic affinities, age, and environmental context. Roughly fifty percent of taxa in both the Maastrichtian and Paleocene come from Europe and North America, with percomorphs representing around 60% of the skeletal acanthomorph taxa in each interval. This is higher than the only pre-Maastrichtian assemblage with a reasonable sample of percomorphs, but lower than most Eocene and younger sites. Fossils from Patterson’s Gap show a steady accumulation of the principal lineages of spiny-rayed fishes. Material from Paleocene or older strata provides evidence for most of the roughly 20 major acanthomorph divisions recovered by molecular studies. Many fossils from Patterson’s Gap remain undescribed and unnamed, and almost none have been included within formal phylogenetic analyses. Revision of existing material, combined with additional fieldwork, should be a priority for future efforts seeking to clarify this murky but significant interval in the evolutionary history of a major vertebrate radiation.
Inhoudstafel
1One genus . . . from the uppermost Cretaceous . . . marks the dawn of the higher groups, and must have been contemporaneous with many unknown Acanthopterygii which rapidly became differentiated into the various families in seas of which the sediments still remain undiscovered or unexplored.
2Arthur Smith Woodward (1901, p. xi–xii)
3Major events in acanthomorph phylogeny had obviously taken place between the Campanian and the late Paleocene, but the fossil record has little to say about them beyond placing some minimum ages on various lineages.
4Colin Patterson (1993, p. 52)
1. Introduction
5The spiny-rayed fishes, or Acanthomorpha, number roughly 20,000 living species (Dornburg & Near, 2021) that represent nearly 60 percent of extant fish diversity (Fricke et al., 2022). Among acanthomorphs, over 95 percent of species belong to the perch-like fishes, or percomorphs. The taxonomic diversity of acanthomorphs is complemented by their equally remarkable anatomical and ecological variety (Wainwright & Longo, 2017). Establishing the roots of acanthomorph diversity—in terms of both kind and form—is central to understanding the origin of the modern vertebrate fauna. As with many groups of backboned animals (Alroy, 1999; Prum et al., 2015; Upham et al., 2019; Foley et al., 2023), the late Mesozoic and early Cenozoic represent a critical episode in the evolution of extant acanthomorph diversity (Friedman, 2010; Alfaro et al., 2018; Ghezelayagh et al., 2022). This interval is marked by substantial taxonomic turnover in marine and terrestrial vertebrate faunas, with the Cretaceous–Paleogene (K–Pg) mass extinction and subsequent evolutionary recovery in the early Paleogene often implicated in mediating these changes. Acanthomorphs went from being minor components of marine fish faunas during the early Late Cretaceous to being the primary actinopterygian group in these settings by the early parts of the Paleogene (Patterson, 1993). Drawing on data from fossils (Friedman, 2010; Carnevale & Johnson, 2015) and living species (Alfaro et al., 2018; Ghezelayagh et al., 2022), a growing body of studies present a range of conclusions about the timing and pattern of spiny-rayed fish diversification over this interval. Paleontology plays a critical role in these debates, with the first appearance of lineages—and by extension minimum ages of evolutionary origin and divergence from immediate relatives—representing its most basic contribution. However, a major and enduring deficiency of the fossil record obscures our understanding of this significant episode of acanthomorph history. Several well-studied sites dating to the latest Early Cretaceous (latest Albian; López-Palomino et al., 2021) and first three-quarters of the Late Cretaceous (Cenomanian–Campanian; 100–72 Ma) yield articulated fishes recording the initial phase of acanthomorph evolution, while rich assemblages dating to the earliest Eocene (56 Ma; Danil’chenko, 1960; Bannikov et al., 2017; El-Sayed et al., 2021) and just after (54–48 Ma; Bonde et al., 2008; Friedman et al., 2015; Friedman & Carnevale, 2018) record the establishment of acanthomorph faunas closely resembling those of the modern day, with a preponderance of percomorphs. Many have touched on the paleontological deficiencies of this span, but none so directly as Colin Patterson. Writing three decades ago, he recognized the sparsely fossiliferous deposits of the Maastrichtian–Paleocene as a “gap . . . in our knowledge of acanthomorph history” (1993, p. 51). Patterson illustrated this hiatus with a stratigraphic column depicting the most noteworthy marine and freshwater assemblages from the Early Cretaceous to the mid-Eocene yielding complete teleost skeletons (1993, fig. 2, reproduced here with minor modifications as Fig. 1), and communicated its impact on our understanding of acanthomorph paleontology. Although Patterson’s framing of the problem was the most explicit, it was not the first; a similar hiatus in the record was readily apparent to earlier workers (Woodward, 1901).
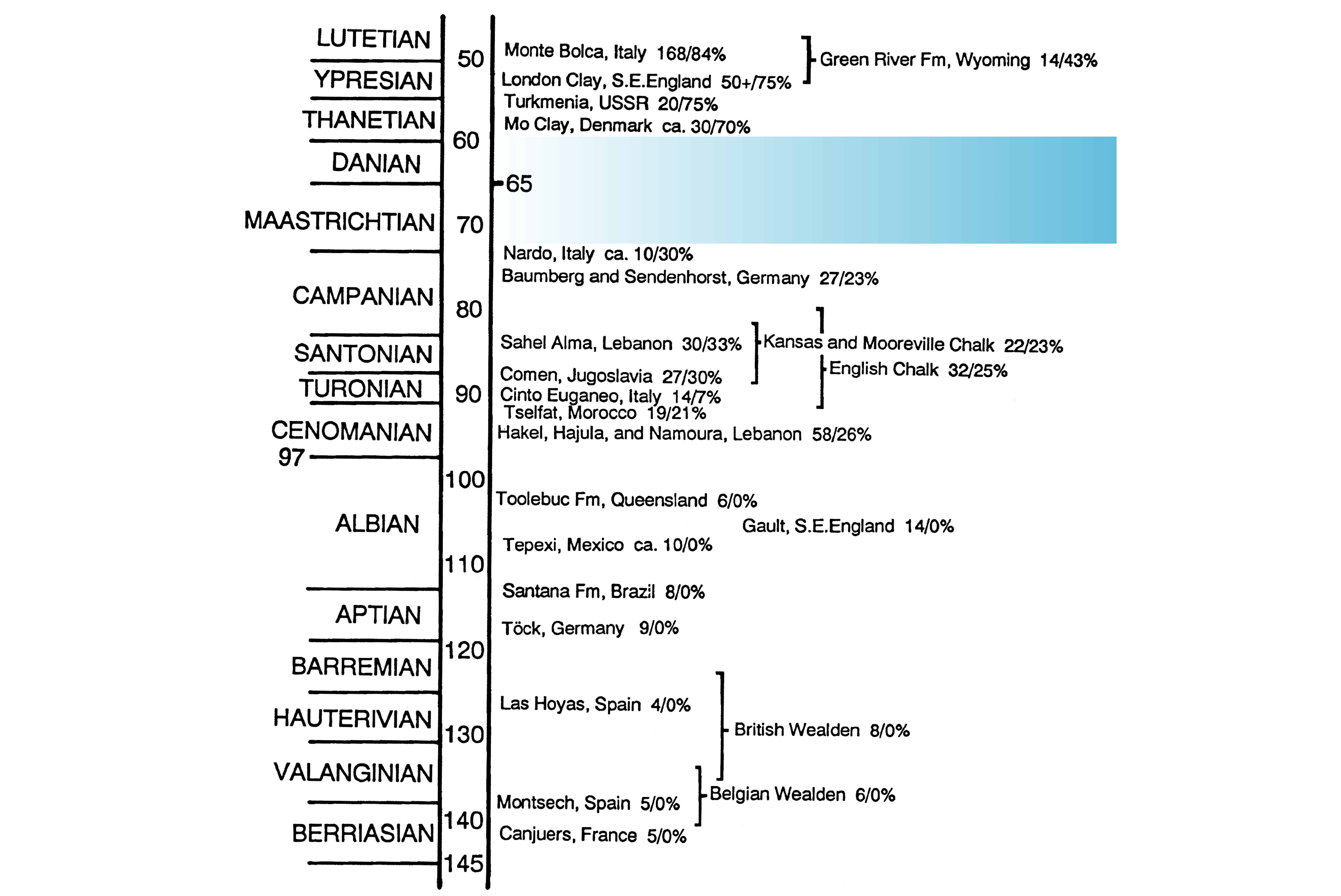
Figure 1. Diagram from Patterson (1993, fig. 1; reproduced from Bulletin of Marine Science with permission of the publisher) illustrating important marine and freshwater sites yielding articulated teleost skeletons. Numerals immediately to the right of locality names indicate the number of genera known to Patterson, followed by the percentage of these genera that he interpreted as acanthomorphs. The “Mo Clay” (= Fur Formation, Denmark) and “Turkmenia” (Danata Formation, Turkmenistan) localities, thought by Patterson to date to the late Paleocene, are now known to be early Eocene in age, while the Nardò sites likely lie well within the Campanian. The major hiatus in the record spanning the Maastrichtian–Paleocene interval indicated by shading is termed Patterson’s Gap here.
6There is something of a tradition in vertebrate paleontology of naming gaps in the record—either real or perceived—after the workers whose research led to their recognition. Talimaa’s Gap in the vertebrate fossil record around the Ordovician–Silurian boundary (Turner et al., 2004), Romer’s Gap for tetrapods in the Tournaisian–Visean stages of the Carboniferous (Coates & Clack, 1995), and Olson’s Gap in the Permian record of terrestrial vertebrates (Lucas & Heckert, 2001) represent some notable examples. By identifying and explicitly delimiting perceived gaps, these naming exercises encourage further investigation that might either refute their existence (Benton, 2012) or catalyze efforts to populate them through the discovery of new fossils or the reexamination of old ones (Smithson et al., 2012).
7In the interest of sharpening research focus on what we regard as the significant—and currently deficient—skeletal fossil record of acanthomorphs in the latest Cretaceous and Paleocene, we coin this sparse interval “Patterson’s Gap.” Our goal in doing so is to direct renewed attention on the material already known from this interval, as well as encourage additional fieldwork specifically targeting major stratigraphic, geographic, and paleoenvironmental lacunae in the record. We present the necessary foundation for these future efforts: a summary of the principal sites yielding articulated fish skeletons dating to Patterson’s Gap. While the otolith record (and, to a lesser degree, the ichthyolith record; Sibert & Norris, 2015; Sibert et al., 2018) provides important data bearing on the evolutionary history of fishes (Nolf, 1985; Nolf, 2013), we focus on the record of articulated fishes because of the high information content of intact skeletons. They have a critical role in recording patterns of morphological diversity and represent the principal source of fossil-based minima for divergence-time analyses. We begin with a brief historical overview of the skeletal record of acanthomorphs highlighting the persistence of Patterson’s gap, followed by more detailed accounts of finds from this significant interval in acanthomorph history.
2. Contextualizing Patterson’s Gap: a brief history of the Late Cretaceous–early Paleogene fossil record of acanthomorphs
8Numerous threads in the study of fossil fishes trace back to Louis Agassiz and his Recherches sur les Poissons fossiles (Agassiz, 1833–1843). Agassiz’s “Généalogie de la Classe de Poissons” indicates the geological ranges of major divisions of fishes as he understood them within the accepted stratigraphy of the time (Fig. 2). This diagram reveals a striking pattern: the simultaneous appearance of many modern divisions of fishes in the Chalk period, corresponding to—with some important differences noted below—the Cretaceous of modern usage. To Agassiz, this simply reflected to a new interval of creation. In an evolutionary framework, however, this pattern is suggestive of prolific origination of new groups, increased preservation potential, or some combination of the two. This was not lost on Darwin, who agonized over this apparently sudden appearance of diverse teleost lineages and its consequences for his gradual model of change (Darwin, 1859).
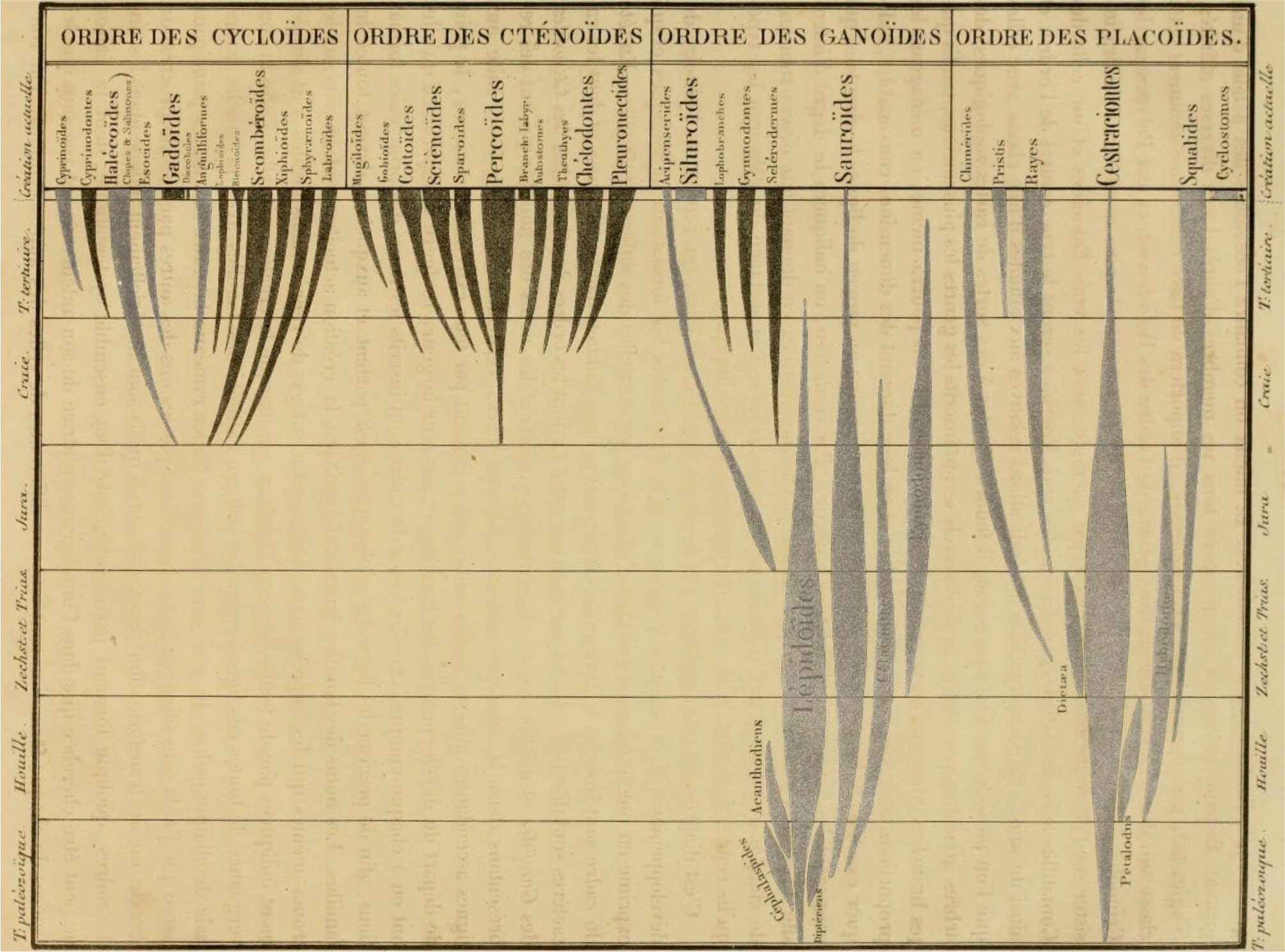
Figure 2. Agassiz’s depiction of major groups of fishes, plotted with respect to the geological record (Agassiz, 1833–1843). This image has been modified by muting non-acanthomorph groups, highlighting the apparently rapid appearance of many acanthomorph groups in the fossil record.
9Unpacking the true significance of this pattern requires consideration of the lineages responsible for it. Agassiz’s higher-level divisions, based on scale morphology, bear little resemblance to the classifications applied by some of his contemporaries to living fishes or to the frameworks used by today’s systematic ichthyologists. His families, however, show a closer correspondence to modern groupings. Figure 2 is modified relative to the original so that fishes now considered acanthomorphs are indicated by the darkest spindles. This simple exercise indicates that, with only a few minor exceptions, the pattern that vexed Darwin was not of the sudden appearance of teleosts per se, but rather of the apparently rapid proliferation of acanthomorphs. Agassiz included in his Chalk period several prominent faunas now known to be Paleogene in age (most notably Bolca), but this modification only shifts the problem stratigraphically: the appearance of new groups—mostly percomorphs—is concentrated in the early Cenozoic rather than the Cretaceous.
10The key faunas of the Late Cretaceous and early Paleogene known to Agassiz include one-third of the Late Cretaceous–Eocene localities referenced over 150 years later in Patterson’s (1993) depiction of the gap on either side of the Cretaceous–Paleogene boundary (Fig. 1). Major new faunas would be added to this list over the coming years, but by and large Agassiz’s inaugural sample established the strengths and comparative deficiencies of the early acanthomorph record. The most ancient acanthomorph skeletons reported by Agassiz derived from the Cenomanian Sannine Formation of Lebanon; these remained the oldest examples of spiny-rayed fishes found for more than 175 years, until the recent discovery of slightly earlier fossils in the latest Albian of México (González-Rodríguez & Feilitz, 2008; López-Palomino et al., 2021). Agassiz reported a healthy sample of acanthomorphs from the Cenomanian–Campanian record of Europe, followed by a hiatus that ended with the rich faunas of the early Eocene London Clay Formation and Bolca that record a shift to marine assemblages dominated by acanthomorphs in general and percomorphs in particular, a paradigm that has continued into the present (Wainwright & Longo, 2017). Then as now, fossils in intervening strata now recognized as Maastrichtian–Paleocene in age were sparse. Patterson’s Gap has thus been apparent from the dawn of systematic study of the fossil fish record.
11The following 150 years of work enriched the acanthomorph record in a highly uneven fashion. Discoveries of diverse new faunas overwhelmingly centered in the intervals already well-represented by available fossil material. By contrast, the critical span around the Cretaceous–Paleogene boundary remained thinly populated, growing only through a slow and intermittent stream of finds. The discovery of articulated marine fishes from the Danian strata of Limhamn Quarry in southern Sweden represented the first substantial addition to this sparse interval in the decades following Agassiz. From these chalks, which were then considered latest Cretaceous in age, Davis (1890) described a modest fauna containing a handful of acanthomorph taxa, some of which he regarded as congeneric with fossils best-known from earlier Cretaceous deposits elsewhere in Europe. Subsequent reports of articulated acanthomorph specimens in other rocks of latest Cretaceous and earliest Paleogene age tended to be of single taxa, with no substantial faunas of the sort otherwise well-represented on either side of this gap.
12Thus the understanding—or, perhaps more aptly, lack of understanding—of Maastrichtian–Paleocene acanthomorph diversity changed little from Woodward’s summary in his synoptic Catalogue of Fossil Fishes in the British Museum (Natural History) (1901), to Patterson’s (1964) monographic treatment of Cretaceous spiny-rayed fishes, and then finally to his later review of the early paleontological history of acanthomorphs (Patterson, 1993). Arguably the most important discovery made during the 20th century bearing on Patterson’s Gap does not fall within this interval but rather helps define its base: the Nardò assemblage of southern Italy (Sorbini, 1978), which comprises multiple fossil sites in the Apulia region (Cava, Canale, and Porto Selvaggio) (Fig. 3). The Campanian-age Nardò localities, along with the Sendenhorst and Coesfeld members of Sendenhorst, Westphalia, Germany known to Agassiz (Siegfried, 1954), yield the youngest substantial marine teleost faunas of the Late Cretaceous represented by complete, articulated skeletons. The composition of the Westphalian acanthomorph assemblage is like that of faunas from older Cretaceous strata, comprising a modest assortment of polymixiiforms, †sphenocephaliforms, and trachichthyiforms. The more diverse fauna of Nardò (Belmonte et al., 2016; Fig. 3) likewise includes deep branches within acanthomorph phylogeny: lampriforms (†Aspesaipichthys, †Nardovelifer: Sorbini & Sorbini, 1999; Taverne, 2004), zeiforms (†Cretazeus: Tyler et al., 2000), and putative trachichthyiforms (†Lissoberyx, †Bannikovperca: Taverne, 2003; Taverne, 2014). But the Nardò assemblage contains a healthy assortment of definitive percomorphs that set it apart from Sendenhorst and earlier Cretaceous faunas. These include the syngnathiform †Gasterorhamphosus (Sorbini, 1981), the putative tetraodontiform †Cretatriacanthus (Tyler & Sorbini, 1996), and the incertae sedis forms †Nardoichthys, †Johnsonperca, and †Zorzinperca (Sorbini & Bannikov, 1991; Taverne, 2010). The age of the Nardò assemblages is not well constrained at present. Initial reports of microfossils were used to argue for a latest Campanian–earliest Maastricthian (ca. 72 Ma) age (Sorbini, 1981) that has been repeated in subsequent literature (Patterson, 1993; Benton et al., 2015). Overlap of currently understood ranges of the calcareous nannofossils enumerated by Sorbini (1981) for the Cava and Canale sites restrict Nardò to UC15–UC16 (www.mikrotax.org), spanning much of the Campanian and consistent with a broad age constraint of roughly 81–72 Ma (Gale et al., 2020); it therefore seems likely that the base of Patterson’s Gap lies deeper in time than his diagram indicates. Additional work also shifts the top of the interval, with evidence currently indicating early Ypresian—rather than late Thanetian—ages for the fish-bearing portions of the Danata and Fur formations (Gavrilov et al., 2003; Westerhold et al., 2009). This revised chronology indicates a lengthier hiatus in the acanthomorph record than even Patterson recognized.
13Fossil finds have begun to slowly populate the Maastrichtian–Paleocene gap in the record of spiny-rayed fishes in the years after Patterson (1993), but like most earlier discoveries these generally consist of single taxa (López‐Arbarello et al., 2003; Andrews et al., 2023) or small assemblages containing only a few acanthomorphs (Carnevale & Johnson, 2015). The most substantial addition during this period is, in fact, an old discovery: the Tenejapa-Lacandón Unit of Chiapas, México (Alvarado-Ortega et al., 2015). Contemporary study of fish remains from this marine deposit is less than a decade old, but these Danian fossils appear to have been known to the Mayan inhabitants of Palenque several centuries ago (Alvarado-Ortega et al., 2018). At present, the four genera of acanthomorphs from the Tenejapa-Lacandón Unit, along with the similarly modest assemblage from Limhamn Quarry, represent the most extensive faunas of early Paleocene acanthomorphs preserved as intact skeletons (Cantalice et al., 2022). Each is substantially smaller than the richest acanthomorph faunas of either the Late Cretaceous or Eocene.

Figure 3. Select marine acanthomorphs from the Nardò localities (Campanian), Apulia region, Italy. A, the lampriform †Aspesaipichthys cavensis (Museo Civico di Storia Naturale, Verona, Italy, MCSNV Na 507). B, the lampriform †Nardovelifer altipinnis (MCSNV Na 519). C, the putative trachichthyiform †Lissoberyx nardoensis (MCSNV Na 240). D, the incertae sedis percomorph †Nardoichthys francisci (MCSNV Na 514). E, the syngnathiform percomorph †Gasterorhamphosus zuppichinii (MCSNV Na T 877). F, the putative tetraodontiform percomorph †Cretatriacanthus guidottii (MCSNV 1377). Scale bars equal 10 mm.
3. Maastrichtian sites yielding articulated acanthomorphs
3.1. Europe
14The European record provides the greatest variety of articulated Maastrichtian acanthomorphs, whether gauged in terms of overall richness or phylogenetic diversity (Fig. 4). At least seven Maastrichtian spiny-rayed fish taxa are represented by articulated skeletons from Europe, with more than half of these attributable to percomorphs. Deposits near the village of Trebiciano in the region of Friuli-Venezia Giulia in northeastern Italy yield the most significant fauna of articulated Maastrichtian acanthomorphs in Europe. These strata capture a shallow marine setting adjacent to a source of freshwater (Sorbini & Bannikov, 1996), and have recently been attributed to the informal ‘Liburnia formation’ (Jurkovšek et al., 1996; Carnevale & Johnson, 2015). Acanthomorphs from Trebiciano include the incertae sedis paracanthopterygian †Trebiciania roseni (Sorbini & Bannikov, 1996; Borden et al., 2013), the ophidiiform †Pastorius methenyi (Carnevale & Johnson, 2015; Fig. 4A), and the batrachoidiform-like †Bacchiaichthys zucchiae (Bannikov & Sorbini, 2000; Carnevale & Collette, 2014). A Maastrichtian age is currently proposed for the fishes of Trebiciano (Carnevale & Johnson, 2015), but this is poorly constrained. The presence of the benthic foraminiferan †Murciella suggests a Late Cretaceous age, with Dalla Vecchia (2008) proposing a late Campanian–early Maastrichtian age on the basis of regional correlations. More broadly, the ‘Liburnia formation’ ranges in age from Maastrichtian to Paleocene, with the uppermost parts of the sequence possibly of Thanetian age (Jurkovšek et al., 2016).

Figure 4. Select marine acanthomorphs from the Maastrichtian of Europe represented by articulated remains. A, the ophidiiform †Pastorius methenyi, ‘Liburnia formation,’ Trebiciano, Italy (Museo di Geologia e Paleontologia, Università degli Studi di Torino, Italy, MGPT-PU 130334; image provided by Giorgio Carnevale). B, the trachichthyiform †Hoplopteryx sp., early Maastrichtian Ciply-Malogne Phosphatic Chalk Formation, Mons Basin, Wallonia, Belgium (Royal Belgian Institute of Natural Sciences, Brussels, Belgium, RBINS P 8783). C, undescribed polymixiid, Vijlen Member, Gulpen Formation, Limburg, the Netherlands (Natuurhistorisch Museum Maastricht, Maastricht, the Netherlands, NHMM 2006 025). D, the incertae sedis percomorph †Prolates heberti, Mont-Aimé Vertus Formation, Grand Est, France (The Natural History Museum, London, United Kingdom, NHMUK PV P62781). E, unnamed percomorph, presumably from the Mont-Aimé Vertus Formation, Grand Est, France (Geologisch-Paläontologisches Institut Tübingen, Tübingen, Germany, GPIT-PV-51145; image provided by Anna Krahl). Scale bars equal 10 mm.
15A series of additional finds complement the modest fauna of articulated acanthomorphs from Trebiciano. Multiple horizons within the marine chalks of the Netherlands and Belgium yield rare acanthomorph skeletons. The oldest of these is material identified as belonging to the trachichthyiform †Hoplopteryx or a closely related taxon from the early Maastrichtian Ciply-Malogne Phosphatic Chalk Formation (Friedman, 2012; Fig. 4B). This fossil remains undescribed, and future study of both it and the named species of †Hoplopteryx should test whether these geographically and stratigraphically widely distributed taxa form a clade. The Ciply-Malogne Phosphatic Chalk Formation lies within the †Belemnella obtusa Zone of the early Maastrichtian, roughly correlative with the lower parts of the Vijlen Member of the Gulpen Formation in the Netherlands (Robaszynski, 2006); eccentricity cycles established by studies of bioclast concentration suggest a refined age estimate of approximately 70.4 Ma (Keutgen, 2018). The Vijlen Member itself yields an undescribed †Omosoma-like polymixiid (Fig. 4C), while a †Hoplopteryx-like trachichthyiform is reported from the terminal Maastrichtian Maastricht Formation of the Netherlands (Friedman, 2012). Highly fragmentary material in the Natuurhistorisch Museum Maastricht suggests additional, small-bodied acanthomorphs from the Maastricht Formation, but these remains are not diagnostic in an unprepared state (pers. obs. M. Friedman). Friedman (2012) incorrectly attributed bony plates from the Maastricht Formation to tetraodontiforms based on comparison with ostraciids (boxfishes); these in fact represent dermal scutes of batoids. Material from the Paris Basin joins these examples from Belgium and the Netherlands. Deposits in the Grand Est region of northern France belonging to the shallow marine Mont-Aimé Vertus Formation yield a modest fauna of articulated fishes including the percomorph †Prolates heberti (Fig. 4D). Represented by numerous, well-preserved fossils (Priem, 1898; Priem, 1908), this taxon has not been re-examined in detail since its description over a century ago. A unique specimen in the collection of the Paläontologisches Museum, Tübingen (GPIT-PV-51145; Fig. 4E) is clearly distinct from †Prolates and represents a second acanthomorph from the Mont-Aimé Vertus Formation; like †Prolates, it is clearly a percomorph. Historical literature describes the age of this deposit as Montian, a disused regional stage corresponding to the Danian. However, foraminiferans, ostracodes, and chondrichthyans reported from the Mont-Aimé Vertus Formation are inconsistent with persistent interpretations of an early Paleocene age (Bignot, 1987), and the deposit is more likely Maastrichtian (Guernet & Villier, 2017).
3.2. North America
16Articulated Maastrichtian acanthomorphs are rare in North America (Stewart, 1996). The only marine examples are highly incomplete material identified as †‘Beryx’ insculptus from New Jersey, United States, the type of which is a patch of flank scales from the Maastrichtian Navesink Formation. Rapp (1946) assigned this species to the trachichthyiform genus †Hoplopteryx but provided no rationale for this identification. Biostratigraphy and strontium isotope stratigraphy provide an estimated age of 69.3 Ma for the base of the Navesink Formation (Sugarman et al., 1995).
17†Lindoeichthys albertensis (Fig. 5A), from the Scollard Formation of Alberta, Canada, represents the only articulated freshwater acanthomorph of Maastrichtian age described from North America and the earliest intact percopsiform (Murray et al., 2020). †Lindoeichthys co-occurs with other complete fishes at the Pisces Point locality in the lower part of the Scollard Formation, which is radioistopically dated to 66.88–66.04 Ma (latest Maastrichtian) (Eberth & Kamo, 2019).

Figure 5. Select marine and freshwater acanthomorphs from the Maastrichtian of North America, South America, and Asia represented by articulated remains. A, percopsiform †Lindoeichthys albertensis, Scollard Formation, Pisces Point Locality, Dry Buffalo Jump Provincial Park, Alberta, Canada (Royal Tyrrell Museum of Palaeontology, Drumheller, Canada, TMP 2013.046.1421; photograph provided by Don Brinkman). B, the incertae sedis percomorph †Indiaichthys bamanborensis, Deccan intertrappean beds, Bamanbor, Mawal, Gujarat, India (Laboratory of Vertebrate Paleontology, University of Jammu, Jammu, India, VPL/JU/IF/1a; reproduced with permission of the publisher from Arratia et al. 2004). C, incertae sedis percomorph †Eoserranus hislopi, Lameta Formation, Dongargaon, Maharashtra, India (NHMUK PV P10726). D, incertae sedis ?percomorph †Saldenioichthys remotus, Saldeño Formation, Mendoza, Argentina (Cátedra de Paleontología de la Facultad de Ciencias Exactas y Naturales de la Universidad de Buenos Aires, Buenos Aires, Argentina, CPBA-V-14099; image provided by Beatriz Aguirre-Urreta). Scale bars equal 10 mm.
3.3. Asia
18The fossil record of India yields several Maastricthian freshwater acanthomorphs represented by complete or partially articulated remains (Fig. 5B, C). Significantly, all the Indian fossils appear to be percomorphs. The sedimentary units yielding these remains are variously intermingled with the flood basalts of the Deccan Traps; stratigraphic information in older literature typically describes their positions as “infratrappean” (i.e., located below the basalts, historically taken as secure evidence of Cretaceous age) and “intertrappean” (i.e., bounded above and below by basalts, and potentially of Late Cretaceous or Paleocene age). The first examples reported are large, three-dimensionally preserved crania and some postcranial remains of †Eoserranus hislopi (Fig. 5C) from the Maastrichtian infratrappean Lameta Formation of central India at Dongargaon, Mawal in the state of Maharashtra. Questionably described as serranids (Woodward, 1908), limited anatomical evidence available at present suggests they are best regarded as incertae sedis percomorphs (Patterson, 1993). Available material of †Eoserranus has the potential to be informative through additional preparation or computed tomography scanning. The section yielding these fossils is interpreted as near—or possibly traversing—the boundary between magnetic polarity chrons C29R and C30N (Kapur & Khosla, 2019), placing them roughly 300–200 kyr prior to the Cretaceous–Paleogene boundary (Sprain et al., 2015). Younger intertrappean localities near Bamanbor and Ninama in the Surendranagar region of Gujarat state, western India, yield additional percomorph specimens. Palynological evidence supports a late Maastrichtian age for these fish-bearing horizons (Samant et al., 2014), although the sequence might extend into the earliest Paleocene (Kapur & Khosla, 2019). The first acanthomorphs reported from the intertrappean sediments include two species of the questionable pristolepid †Palaeopristolepis and a skull very doubtfully referred to Perca (Borkar, 1973a; Borkar, 1973b). Accounts of this material are brief and these specimens—like those of †Eoserranus—require restudy. More recent collecting from the beds near Bamanbor yielded additional acanthomorph fossils: multiple specimens assigned to †Indiaichthys bamanborensis (Fig. 5B) plus a single individual of an indeterminate percomorph (Arratia et al., 2004). These later discoveries were compared to literature accounts, but not the original material, of †Palaeopristolepis; the taxonomic and phylogenetic status of these intertrappean taxa from Bamanbor relative to one another is unclear.
19There are no described marine acanthomorphs represented by articulated skeletons from the Maastrichtian of Asia. However, specimens reported from the Muwaqqar Chalk Marl Formation of east-central Jordan suggest that these deposits might yield a rich acanthomorph fauna important from both a stratigraphic and environmental perspective. Published photographs of privately held fossils show at least two deep-bodied acanthomorphs (Kaddumi, 2009). One of these is certainly a percomorph, while the other is identified as a ‘beryciform’ sensu lato but it shows some clear similarities with veliferids. The Jordanian acanthomorphs co-occur with a rich fauna of non-acanthomorph teleosts, chondrichthyans, and marine reptiles, all represented by remarkable articulated specimens. The age of the Muwaqqar Chalk Marl Formation is well constrained based on ammonite biostratigraphy. Restricted to a narrow window between 66.5–66.1 Ma (Jagt et al., 2017), this deposit seems particularly significant for understanding patterns of turnover in marine settings associated with the Cretaceous–Paleogene extinction.
3.4. South America
20The single incomplete postcranium of †Saldenioichthys remotus (Fig. 5D) represents the only articulated acanthomorph specimen from the Maastrichtian of South America. This unique fossil is interpreted as a percomorph and derives from calcareous sandstones of the Saldeño Formation in the high cordillera of Mendoza, Argentina. These strata represent a shallow marine setting under brackish influence (López-Arbarello et al., 2003), and palynomorphs and nannofossils restrict the deposit to the Maastrichtian but provide no narrower constraints on age (Tunik et al., 2004).
3.5. Africa
21The record of articulated teleosts in the Maastrichtian record of Africa is sparse, apparently limited to a single specimen from a crater lake deposit in South Africa interpreted as a galaxiid (Anderson, 1998). A variety of teleosts, including putative acanthomorphs, are reported on the basis of fragmentary remains from marine deposits in the northern half of Africa corresponding to the southern Tethys and the Trans-Saharan Seaway (Khalloufi et al., 2017; O’Leary et al., 2019). Some of these deposits seem unlikely to yield articulated fishes, although rare examples are known from lithologically comparable units elsewhere (e.g., Ciply-Malogne Phosphatic Chalk).
3.6. Remaining landmasses
22The trachichthyiform †Antarctiberyx seymouri represents the only intact Maastrichtian acanthomorph reported from Antarctica and comprises an incomplete but three-dimensionally preserved skull from the López de Bertodano Formation of Seymour (Marambio) Island (Grande & Chatterjee, 1987). †Antarctiberyx derives from a stratigraphic interval called Unit 9, representing the penultimate Maastricthian division within the López de Bertodano Formation and situated entirely within C30N of the geomagnetic timescale (Cione et al., 2018; Reguero, 2019). This provides a narrow age constraint of roughly 66.4–68.2 Ma (Gale et al., 2020). It seems likely that additional collecting in the Maastrichtian of Seymour Island might yield additional acanthomorph material based on past reports (Zinsmeister, 1998) and abundant fish material collected on more recent paleontological expeditions (K. Claeson and M. Lamanna, pers. comm.).
4. Paleocene sites yielding articulated acanthomorphs
4.1. Europe
23Rocks in northern Denmark and southern Sweden yield the best record of articulated Paleocene acanthomorphs from Europe (Fig. 6). Indeed, for some time fossils from chalks of the København Limestone Formation historically exposed in Limhamn Quarry, southern Sweden, represented the only appreciable fauna of articulated marine teleost fishes of Danian age. The København Limestone Formation dates to the late Danian, spanning nannofossil zones P4 to P5 (Jakobsen et al., 2017). Davis (1890) provided the first account of the fauna, and subsequent work remains restricted to the superficial re-examination of select taxa (Patterson, 1964; Patterson, 1968; Taverne, 2005) or broad overviews of the assemblage (Adolfssen et al., 2017; Bonde & Leal, 2017). The lampriform †Bathysoma (Fig. 6A) and the percomorph †Proserranus (Fig. 6B) are the best-known acanthomorphs from Limhamn. These are joined by a polymixiiform and trachichthyiform, referred by Davis (1890) to the genera well-represented in older deposits of the English Chalk: †Berycopsis and †Hoplopteryx. It is probable that the Danian fossils are generically distinct from the Cretaceous examples (Bonde & Leal, 2017). An additional undescribed percomorph from Limhamn, differing from †Proserranus in having a very small, anteriorly placed mouth, appears in Bonde & Leal (2017). The entire Limhamn fish fauna is in dire need of revision, but unfortunately the quarry is now filled and therefore not accessible for further collecting. The København Limestone Formation yields additional articulated remains outside of Limhamn Quarry, but these are rare. Examples include a specimen of the lampriform †Palaeocentrotus preserved in a flint nodule (Bonde et al., 2008; Fig. 6C) plus other, less complete fishes (Bonde & Leal, 2017).
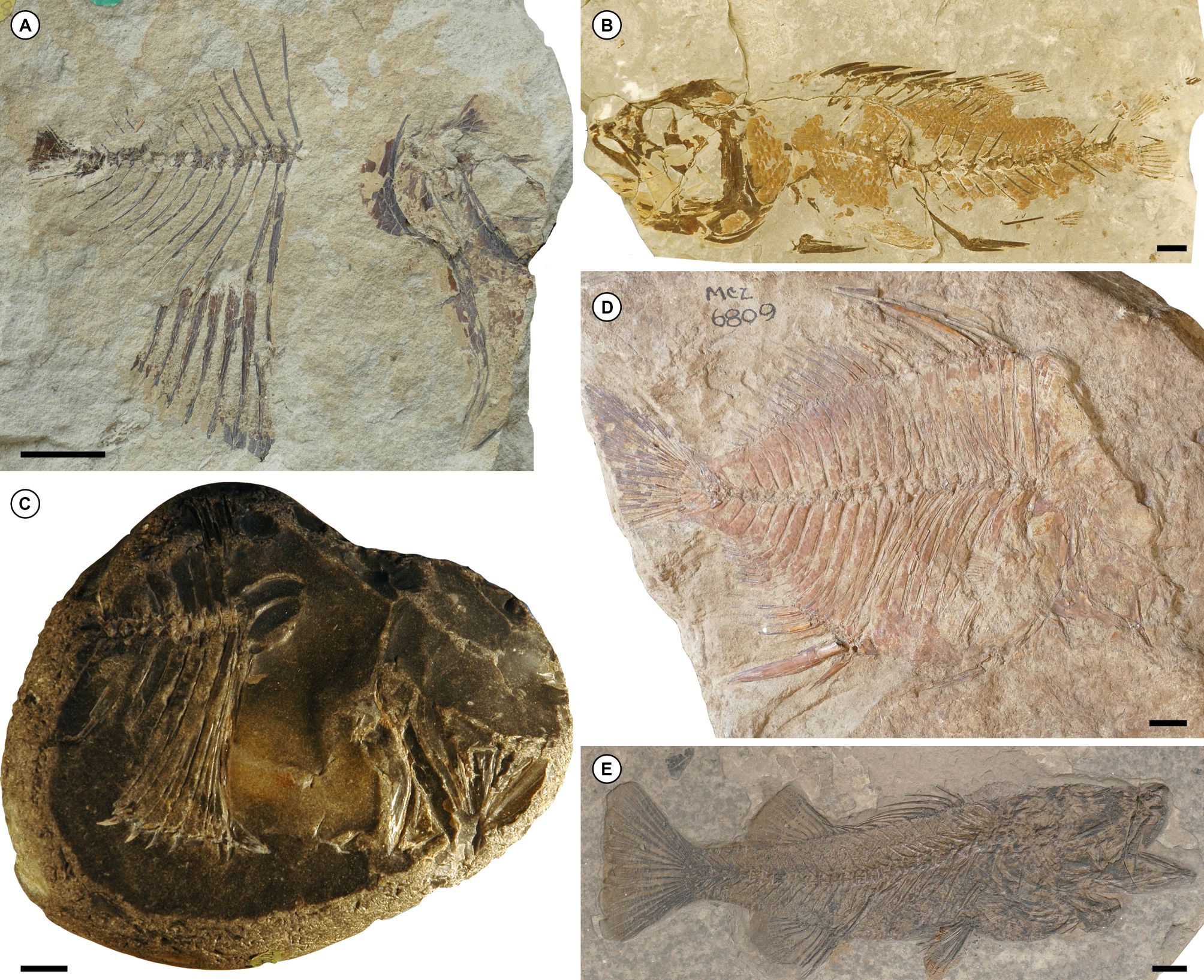
Figure 6. Select marine and freshwater acanthomorphs from the Paleocene of Europe represented by articulated remains. A, Danian lampriform †Bathysoma lutkeni, København Limestone Formation, Limhamn Quarry, Scania, Sweden (NHMUK PV P9948). B, Danian incertae sedis percomorph †Proserranus lundensis, København Limestone Formation, Limhamn Quarry, Scania, Sweden (Lund University, Lund, Sweden, LO 900 T). C, Danian lampriform †Palaeocentrotus sp. in flint likely derived from København Limestone Formation (Natural History Museum of Denmark, Copenhagen, Denmark, NHMD DK4); D, Selandian unnamed percomorph, likely Lellinge Greensand Formation, Scania, Sweden (Museum of Comparative Zoology, Cambridge, United States, MCZ PF-6809; image credit: Museum of Comparative Zoology, Harvard University; ©President and Fellows of Harvard College; shared under CC BY-NC-SA 3.0 license: https://creativecommons.org/licenses/by-nc-sa/3.0/). E, Selandian–Thanetian incertae sedis percomorph †Properca angusta, Menat, Auvergne-Rhône-Alpes region, France (NHMUK PV 27736). Scale bars equal 10 mm.
24A smattering of isolated finds from younger and older strata in Scandinavia complement the fauna of the København Limestone Formation. The oldest of these is a partial skeleton from the Fiskeler Member (the so-called “Fish Clay”) of the Rødvig Formation at Stevns Klint, immediately above the Cretaceous–Paleogene boundary. Missing both its skull and caudal fin, this earliest Danian (P0, ca. 66 Ma) fossil is compared by Schwarzhans & Milàn (2017) with beryciforms generally and Centroberyx more specifically. The early Selandian Lellinge Greensand Formation (upper NP4–lower NP5; Sheldon et al., 2012) of southern Sweden also appears to bear articulated acanthomorph material, but the high-energy depositional setting of this unit suggests that such examples will be rare. A single deep-bodied percomorph specimen (Museum of Comparative Zoology VPF-6809; Fig. 6D) preserved in a glauconitic sandstone is recorded as being from the greensand of Scania, southern Sweden; this fossil almost certainly derives from the Lellinge Greensand Formation. Incorrectly labeled as Scatophagus, this specimen resembles the putative leiognathid †Leiognathoides, represented by several species from Oligocene–Miocene deposits in Germany, Switzerland, Poland, Russia, Turkey, and Azerbaijan (Micklich et al., 2017; Kovalchuk et al., 2021). †Bathysoma, best known from Limhamn Quarry, is also represented by a single specimen hosted on an erratic boulder derived from the Lellinge Greensand Formation (Bonde & Leal, 2017).
25Articulated Paleocene acanthomorphs are nearly unknown in Europe outside of Scandinavia. The most prominent example is †Properca angusta (Gaudant, 1979; Fig. 6E), known from many specimens from the lacustrine Lagerstätte of Menat, in the Auvergne-Rhône-Alpes region of France. Biostratigraphic information from pollen and vertebrates, as well as more indirect data from radiometric dating, suggest a Selandian–Thanetian age for Menat (Wedmann et al., 2018). The ostensibly Paleocene–Miocene †Properca requires revision, and attribution of the genus to Percichthyidae is similarly problematic. Pre-cladistic concepts of the family encompassed a polyphyetic assemblage, and extant members of the group are now restricted to freshwater in Australia and South America (Johnson, 1984).
4.2. North America
26The record of articulated Paleocene spiny-rayed fishes from North America—inclusive of Greenland and Central America—is the most diverse in terms of both numbers of taxa and their spread across acanthomorph phylogeny, as well as the variety of environments they represent. The shallow marine limestones of the Tenejapa-Lacandón Unit of Chiapas, México provide what is arguably the most significant collection of Paleocene acanthomorphs from North America (Alvarado-Ortega et al., 2015; Fig. 7). This assemblage contains four genera of spiny-rayed fishes, all of which are percomorphs: the syngnathiform †Eekaulostomus (Cantalice & Alvarado-Ortega, 2016; Fig. 7A), the incertae sedis †Kelemejtubus (Cantalice & Alvarado-Ortega, 2017; Fig. 7B), the possible serranid †Paleoserranus (Cantalice et al., 2018; Fig. 7C), and the pomacentrid †Chaychanus (Cantalice et al., 2020; Fig. 7D). There are currently no biostratigraphic constraints on the age of the fish-bearing horizon within the Tenejapa-Lacandón Unit. Instead, an estimated age of 63 ± 1.5 Ma (mid-Danian) derives from strontium isotope analysis of fish teeth (Alvarado-Ortega et al., 2015).
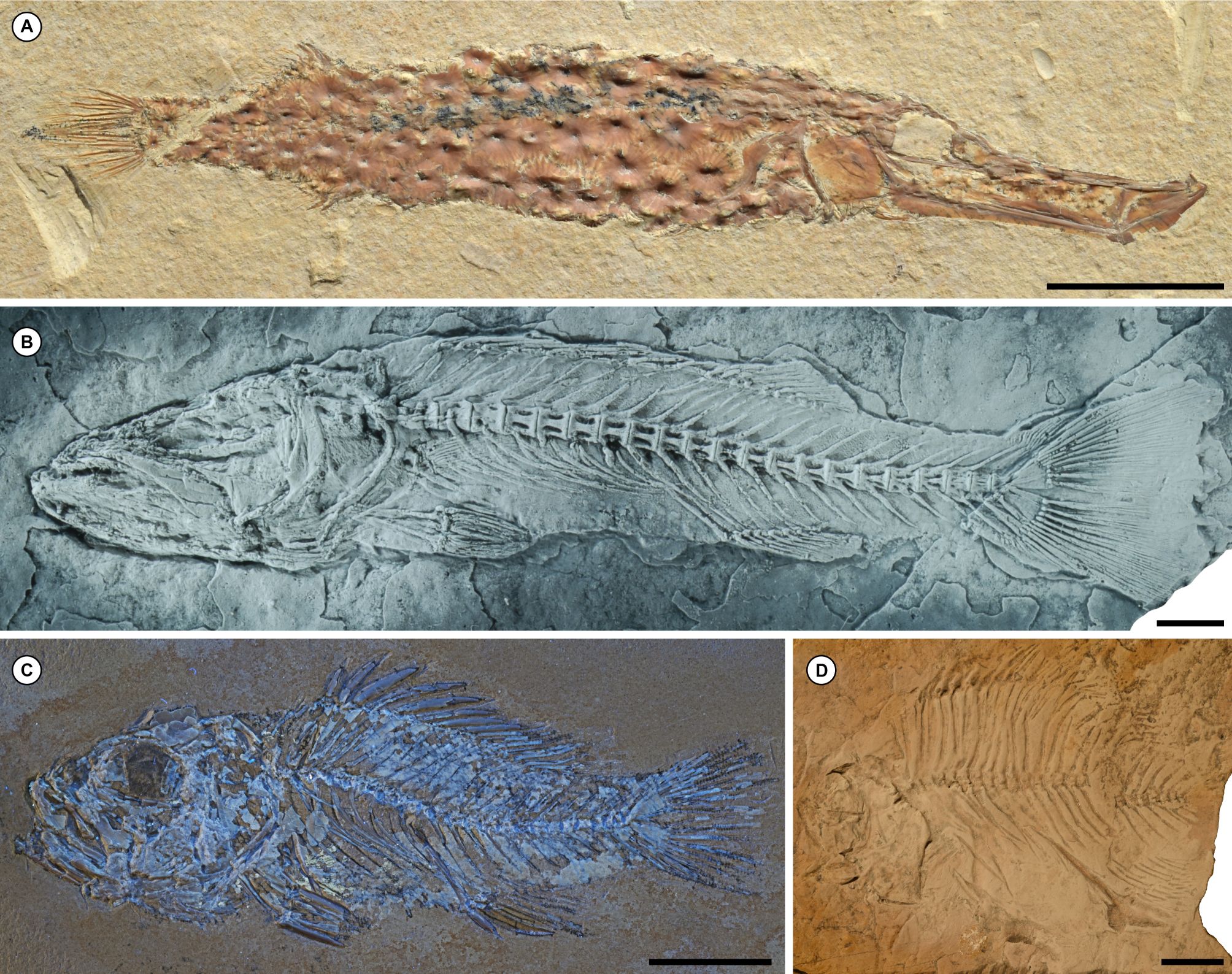
Figure 7. Articulated marine acanthomorphs from the Paleocene (Danian) Tenejapa-Lacandón Unit of Chiapas, México. A, the syngnathiform †Eekaulostomus cuevasae (Instituto de Geología, Universidad Nacional Autónoma de México, México City, México, IGM 4716). B, the incertae sedis percomorph †Kelemejtubus castroi (IGM 4864). C, the putative serranid percomorph †Paleoserranus lakamhae (IGM 4550). D, the pomacentrid percomorph †Chaychanus gonzalezorum (IGM 1183). All images provided by Kleyton Cantalice. Scale bars equal 10 mm.
27In contrast to the paleotropical fauna recovered from the Tenejapa-Lacandón Unit, other marine horizons yielding articulated acanthomorphs derive from higher paleolatitudes. The Eqalulik Formation of the Nuussuaq Peninsula of western Greenland yields a sparse, offshore marine fish fauna (Bendix-Almgreen, 1969; Rosenkrantz, 1970; Capobianco et al., 2021). The assemblage includes a single acanthomorph, represented by an articulated skeleton of a gadiform fish (Rosen & Patterson, 1969), informally dubbed “Protocodus” (Cohen, 1984) and reported to resemble a morid (Fedotov & Bannikov, 1989). Support for a late Danian (lower NP3–upper NP4; ca. 63–62 Ma) age for these fossils comes from palynological and nannoplankton biostratigraphy (Nøhr-Hansen & Sheldon, 2000). “Protocodus” represents the oldest known gadiform and requires formal study and description. The only other reasonably complete marine Paleocene acanthomorph fossil from North America outside of Chiapas is from the basal Hornerstown Formation of New Jersey, United States and represents the stem holocentrid †Iridopristis (Fig. 8A). The age of the deposit yielding this specimen is controversial due to reworking of Maastrichtian fossils from underlying strata, but current evidence favors an earliest Danian age (Andrews et al., 2023).
28A handful of freshwater acanthomorph taxa represented by articulated skeletons from the northern United States and southern Canada complement these marine examples. Like all articulated freshwater acanthomorphs from North America, these Paleocene fossils are percopsiforms (Borden et al., 2013; Grande et al., 2013) and derive from fluvial deposits. The Tullock Member of the Fort Union Formation of Montana, United States yields a single, three-dimensionally preserved specimen of †Mcconichthys longipinnis (Fig. 8B), which is regarded as branching from the common stem of Aphredoderus and Ambyplosidae (Borden et al., 2013). The Tullock Member extends from the Cretaceous–Paleogene boundary to a lignite horizon known as the ‘U coal’ that is dated radioistopically to 64.866 ± 0.023 Ma (Sprain et al., 2015), indicating an early Danian age for †Mcconichthys. The Paskapoo Formation of Alberta, Canada bears two genera of percopsids found at separate localities. A mass-mortality layer of fishes at Joffre Bridge yields over 1,750 specimens of †Massamorichthys wilsoni (Murray, 1996; Fig. 8C), while †Lateopisciculus turrifumosus derives from the Smoky Tower site (Murray & Wilson, 1996). Biostratigraphic evidence from plants (Christophel, 1976) and mammals (Fox, 1990), plus radiometric dates ranging from 62.5–61.5 Ma (Dawson et al., 1994), indicate a late Paleocene age for the Paskapoo Formation, corresponding to the Tiffanian North American Land Mammal age and the late Danian–early Selandian of the global timescale.

Figure 8. Select marine and freshwater acanthomorphs from the Paleocene of the United States and Canada represented by articulated remains. A, earliest Danian holocentroid †Iridopristis parrisi, Hornerstown Formation, Sewell, New Jersey, United States (New Jersey State Museum, Trenton, United States, NJSM GP12145). B, Danian percopsiform †Mcconichthys longipinnis, Tullock Member, Fort Union Formation, Montana, United States (Field Museum, Chicago, United States, FMNH PF12916). C, Selandian–Thanetian percopsiform †Massamorichthys wilsoni, Paskapoo Formation, Smoky Tower, Alberta, Canada (University of Alberta Laboratory of Vertebrate Paleontology, Edmonton, Canada, UALVP30842b; photograph by John Bruner). Scale bars equal 10 mm.
4.3. Asia
29The record of articulated Paleocene acanthomorphs from Asia is restricted to a single three-dimensionally preserved cranium from Jabal Umm Himar, southern Hijaz Province, Kingdom of Saudi Arabia (Greenwood, 1983; Greenwood, 1995). Greenwood’s descriptions of †Palaeopercichthys arabis compare it with “serranid-like fishes,” while acknowledging these comparisons are essentially phenetic in nature. Computed tomography scans of other fish material from this locality yield useful results (M. Friedman, pers. obs. of the osteoglossid †Magnigena arabica NHMUK PV OE PAL 2007-1), suggesting that this approach might help to better constrain the affinities of †Palaeopercichthys. Vertebrate biostratigraphy suggests a late Paleocene age for the Umm Himar Formation, which appears to be estuarine in origin (Madden et al., 1995).
4.4. Africa
30The African record of articulated Paleocene acanthomorphs is poor. Nevertheless, the sedimentary sequences of Cacongo (formerly appearing as Lȃndana), Cabinda Province, Angola once represented the most significant source of taxonomically diagnostic marine acanthomorph skeletons from the Paleocene outside of Europe (Dartevelle & Casier, 1943; Dartevelle & Casier, 1949; Dartevelle & Casier, 1959; Fig. 9A, B). Among more fragmentary remains of other acanthomorphs, these strata yield skulls and a partial postcranium of †Landanichthys, a probable crown scombrid with similarities to taxa from the early Eocene of the United Kingdom and Turkmenistan grouped as †Eocoelopomini (Monsch & Bannikov, 2012; Beckett & Friedman, 2016). There are two nominal species of †Landanichthys, although distinctions between them are questionable. Unfortunately, the type specimens of both species cannot be found in the Museum for Central Africa, Tervuren, Belgium, and are assumed lost (M. Friedman, pers. obs.). Surviving material is restricted to the material described and figured by Dartevelle & Casier (1959): a partial skull identified only as †Landanichthys sp. (Fig. 9A) but likely referrable to the type species, plus an additional skull (Fig. 9B) identified as †L. lusitanicus that, while a percomorph, is clearly not a scombrid based on the presence of a broad field of alveoli along the dentary indicating a pavement of small teeth rather than the single marginal tooth row characteristic of scombrids (Johnson, 1986). Although past work refers to †Landanichthys as Danian (Patterson, 1993), recent biostratigraphic revision of the Cacongo sequence indicates the oldest occurrences of the genus are Selandian in age (planktonic foraminiferal zone P3c; Solé et al., 2019), dating to roughly 62–61 Ma (Speijer et al., 2020).
31Priem (1907) and Astre (1927) reported material of the moonfish †Mene phosphatica from phosphates in Tunisia historically regarded as Montian (= Danian) in age. However, Friedman & Johnson (2005) concluded a late Paleocene or early Eocene age as more probable, based on the stratigraphic distribution of phosphates in the Gafsa Basin of Tunisia (Kocsis et al., 2013). While Moroccan phosphates of Paleocene age yield abundant fragmentary fish material (Arambourg, 1952), articulated acanthomorphs are not yet known from these deposits.
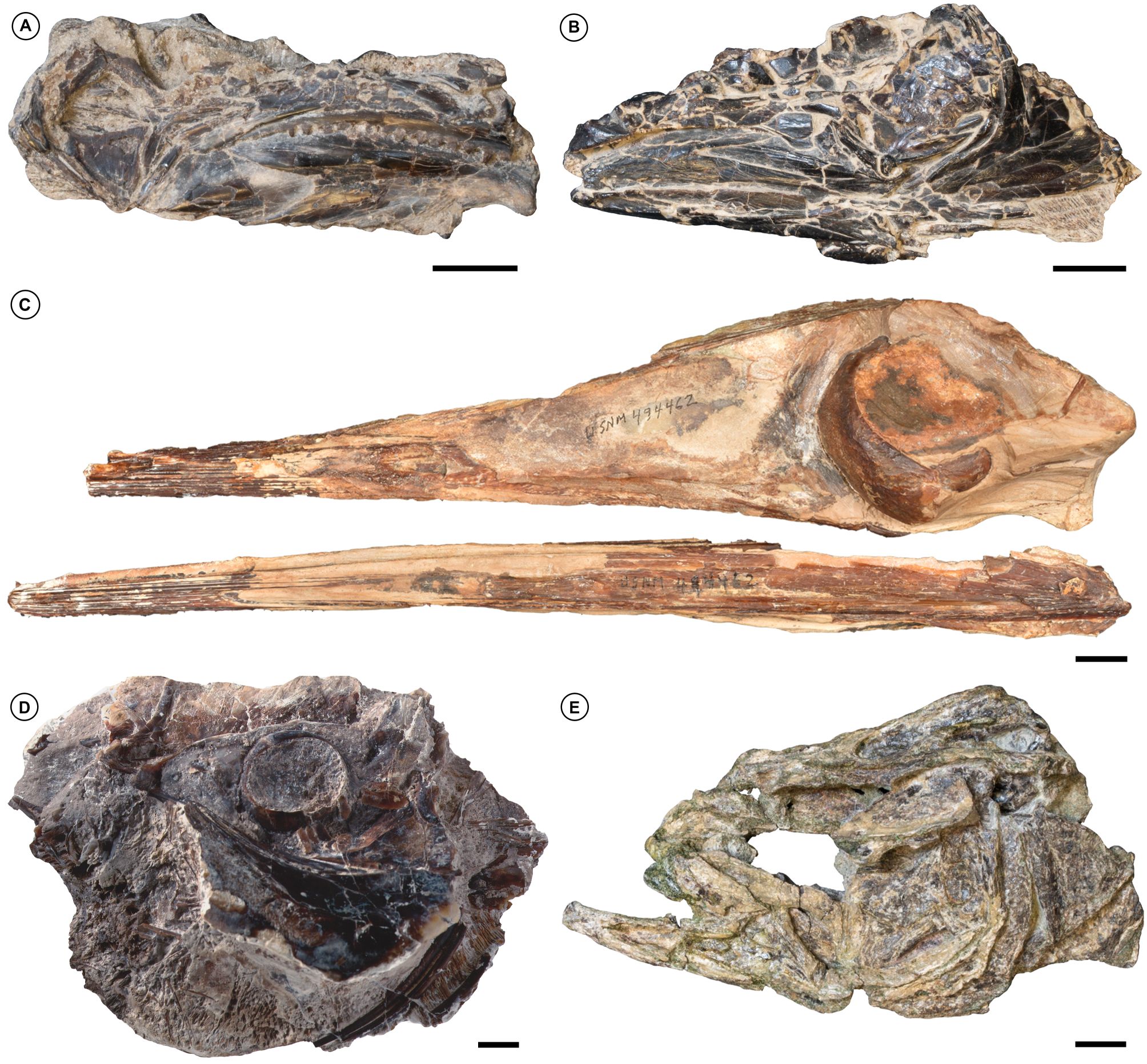
Figure 9. Select marine acanthomorphs from the Paleocene of Africa and South America. A, the Selandian scombrid †Landanichthys sp., Cacongo (formerly Lȃndana), Cabinda Province, Angola (Royal Museum for Central Africa MRAC 9166). B, undetermined percomorph referred incorrectly to †Landanichthys lusitanicus, Cacongo (formerly Lȃndana), Cabinda Province, Angola (Royal Museum for Central Africa, Tervuren, Belgium, MRAC 9165). C, ?Thanetian †Blochius-like xiphioid percomorph, Talara Province, northwestern Perú (National Museum of National History, Washington, United States, USNM PAL 494462). D, ?Thanetian menid percomorph †Mene purydi, Talara Province, northwestern Perú (USNM PAL 494403). E, late Danian–early Selandian percomorph, Waipara Greensand, central South Island, New Zealand (Canterbury Museum, Canterbury, New Zealand, CMC 1997.233.1). Scale bars equal 10 mm.
4.5. South America
32A pair of three-dimensionally preserved crania constitute the only plausible Paleocene marine records of articulated acanthomorphs from South America (Fig. 9C, D). Friedman & Johnson (2005) described †Mene purdyi (Fig. 9D) from Talara Province in the Piura Region of northwestern Perú and indicated that a similarly preserved but undescribed partial skull of a †Blochius-like xiphioid (Fig. 9C) was collected from the same locality. Planktonic foraminiferans from the specimen of Mene restrict these fossils to zones P4c–P5; Friedman & Johnson (2005) concluded this indicated a late Thanetian age, but subsequent revision of the timescale places the youngest portions of this interval in the Ypresian and so an early Eocene age cannot be ruled out. These two fossils were collected incidentally by an invertebrate paleontologist, indicating that this region of northwestern Perú is a promising candidate for future field efforts. Deposits of the Pernambuco-Paraíba Basin of northeastern Brazil also merit renewed attention. What appear to be early Paleocene coastal marine deposits of the Maria Farinha Formation have yielded one intact skull belonging to the albulid elopomorph †Farinichthys (Gallo & De Figueiredo, 2003). Although articulated remains of acanthomorphs are unknown from this deposit, less complete material of percomorphs—tentatively identified as serranids—suggests that further excavation might prove productive (Gallo et al., 2001).
33In terms of freshwater acanthomorphs, Gayet & Meunier (1998) reported and figured an articulated specimen from the Danian La Palca locality of the El Molino Formation of southwestern Bolivia. They noted similarities to the †Percichthys hondoensis from the Eocene of Argentina (Schaeffer & Simpson, 1947), and tentatively identified the Bolivian fossil as belonging to that taxon. The El Molino Formation spans the Danian and much of the Maastrichtian (Sempere et al., 1997); the putative percichthyid remains derive from both Late Cretaceous and Paleocene portions of the sequence (Gayet & Meunier, 1998). The articulated specimen figured by Gayet & Meunier (1998, fig. 25) is Danian in age, and the condition of material from Maastrichtian parts of the sequence is unclear. Skeletons of another putative percichthyid, †Percichthys lonquimayiensis, from freshwater deposits of Lonquimay, Araucanía Region, southern Chile were historically regarded as late Paleocene in age (Chang et al., 1978; Arratia, 1982). These strata are now recognized as the Río Pedregoso Formation, and dated to the Miocene based on detrital zircons and fossil mammals (Pedroza et al., 2017).
4.6. Remaining landmasses
34There are few promising localities for articulated acanthomorphs elsewhere in the world. The Waipara Greensand of New Zealand, best known for its fossils of Paleocene penguins (Mayr et al., 2017), yields partial cranial remains of percomorph fishes. The best of these is an undescribed three-dimensionally preserved skull of uncertain affinities (Fig. 9E) from a locality in the central portion of the South Island (Te Waipounamu) (Canterbury Museum CMC 1997.233.1). This specimen derives from the Mt Ellen Member, placed in the middle part of the New Zealand Teurian stage that corresponds to the late Danian–early Selandian of the global timescale.
35In Antarctica, no acanthomorph skeletons are yet known from fish-bearing Danian portions of the López de Bertodano Formation or the overlying Paleocene strata of the Sobral Formation and the lower Seymour Island Group (Reguero, 2019). However, associated teleost remains from these early Cenozoic deposits (e.g., British Antarctic Survey D5.1295.2, representing a partial aulopiform skull) suggest that investigation of unstudied material or collection of new specimens might produce informative acanthomorph material.
5. Discussion
5.1. Maastrichtian–Paleocene acanthomorph skeleton assemblages: compositional, geographic, and environmental patterns
36There are no acanthomorph faunas from the Maastrichtian or Paleocene with comparable taxonomic diversity approaching the richest examples from the Eocene or the preceding portions of the Late Cretaceous (Forey et al., 2003; Carnevale et al., 2014; Friedman et al., 2015); in this sense, Patterson’s Gap persists. However, a few points emerge from this smattering of Maastrichtian–Paleocene fossils, accompanied by the obvious taphonomic and sampling caveats relating to such a limited set of remains.
37First, the cumulative Maastrichtian and Paleocene records of acanthomorph skeletons are broadly similar in terms of magnitude and taxonomic breakdown at the coarsest level. Each provides roughly the same number of distinct taxa, with percomorphs representing a narrow majority of acanthomorphs for both the Maastrichtian and Paleocene. This is only a modest increase in the taxonomic share of percomorphs relative to the Campanian Nardò localities, and differs from their clear dominance in early Eocene acanthomorph assemblages (Friedman, 2010; Friedman et al., 2015). Maastrichtian and Paleocene percomorphs include examples identified as belonging to extant orders, families, or even genera, although many of the percomorph fossils during this interval are “generalized percoids” of historical use and thus of highly uncertain placement.
38Second, the Maastrichtian and Paleocene records feature similarly uneven sampling of geographic regions that reflect well-known paleontological biases (Raja et al., 2022; Ye & Peters, 2023): a majority of taxa from Patterson’s Gap derive from marine rather than continental sediments, with finds heavily skewed toward rocks in North America and Europe (Argyriou & Davesne, 2021; Friedman, 2022; Fig. 10). Thus, our understanding of acanthomorph diversity in particular regions and environments is severely limited. This is not a unique feature of the Maastrichtian–Paleocene record but is exacerbated relative to the same biases in older and younger strata by the relatively sparse sampling of this interval.
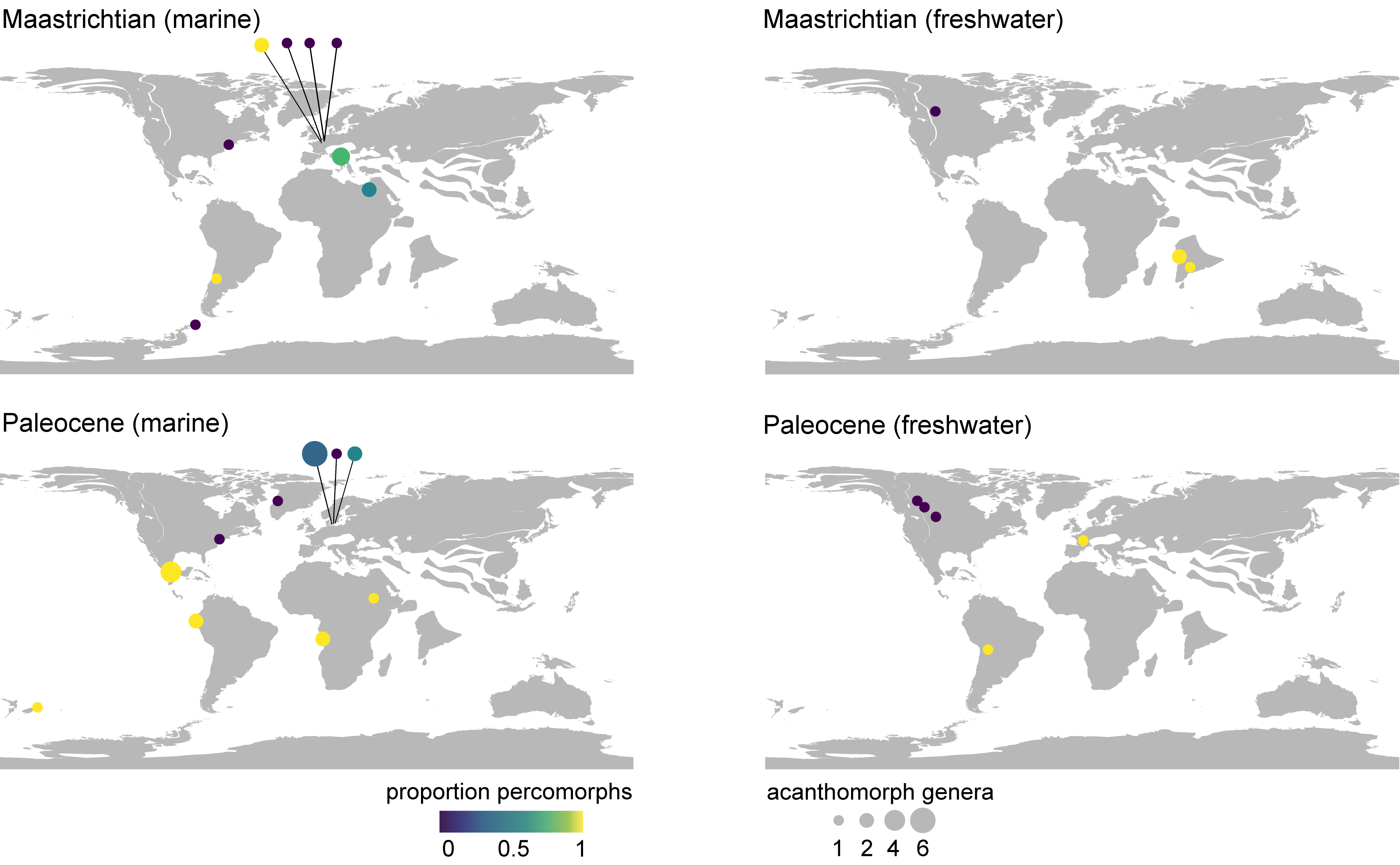
Figure 10. Compositional, paleogeographic, and paleoenvironmental structure of Maastrichtian–Paleocene assemblages of skeletal acanthomorph fossils. Taxonomic data are considered at the generic level. Disc size reflects relative generic richness of acanthomorphs and disc color indicates the proportion of acanthomorph genera that are percomorphs. An early Eocene age cannot be excluded for the marine fauna from the west coast of South America (Talara Province, northwestern Perú) shown here as Paleocene. Paleogeographic reconstructions for the Maastrichtian (69 Ma) and Paleocene (61 Ma), generated using R packages mapast (Varela & Rothkugel, 2018) and chronosphere (Kocsis & Raja, 2021).
39Third, there are significant differences in faunal composition related to paleogeography, paleoenvironment, or both (Fig. 10). In marine settings, this is best illustrated by the contrast between the ‘chalk’ faunas of northern Europe and those from other depositional settings at lower latitudes both within Europe and other continents. The small acanthomorph fauna of the Maastrichtian chalks of the Netherlands and Belgium is comparable to that of much older, but lithologically similar deposits of the United Kingdom (Friedman et al., 2015). The Danian assemblage from the København Limestone Formation agrees in many respects with these more ancient faunas of spiny-rayed fishes from northern European chalks. The Limhamn assemblage, which for over a century provided the only meaningful skeletal data for marine fishes from the early Paleocene, includes only a single described percomorph, with nearly all remaining acanthomorphs assigned to deep phylogenetic branches well-represented in older deposits: lampriforms, polymixiiforms, and trachichthyiforms. A late-surviving †dercetid aulopiform further amplifies the Cretaceous aspect of the fauna (Taverne, 2005). These assemblages stand in contrast to those of similar age at lower latitudes in the Caribbean or the European portion of the Tethys, both of which were in communication via the circum-global equatorial current (Iturralde-Vinent, 2003). While the shallow-water faunas of Trebiciano and Chiapas do not share any acanthomorph taxa in common, they nevertheless both differ from their higher-latitude contemporaries in terms of composition: the acanthomorph component of each consists principally of percomorphs, and include fishes unquestionably aligned with well-circumscribed, anatomically specialized extant lineages rather than ‘generalized’ taxa of unclear placement. These patterns within Patterson’s Gap match similar contrasts in Campanian strata; the more northerly, relatively deep deposits (Hübner & Müller, 2010) of Sendenhorst yield a variety of non-percomorph groups but no percomorphs (Siegfried, 1954), while the coeval but shallower, West Tethyan Nardò assemblage includes a healthy sample of percomorphs, including representatives of highly specialized lineages (Belmonte et al., 2016). Quantitative comparisons among latest Cretaceous–early Paleogene marine localities reinforce these qualitative impressions, showing that sites like Trebiciano and Chiapas are more comparable to Eocene and younger faunas than they are northern European chalk assemblages dating to the same Maastrichtian–Danian interval (El-Sayed et al., 2021).
40The meaning of compositional differences in continental settings is less clear due to the sparse record of freshwater deposits yielding articulated material. With that caveat in mind, the dominance of percopsiforms in North America presents a striking contrast with the prominence of percomorphs in South American, Asian, and European freshwater strata from around the Cretaceous–Paleogene boundary. Fragmentary remains from North American continental deposits extend the record of percopsiforms to the late Campanian (Neuman & Brinkman, 2005; Brinkman et al., 2013). Paracanthopterygians more generally represent the earliest acanthomorphs to inhabit freshwater settings, beginning with the separate continental invasion by the lineage including the Cenomanian †Spinocaudichthys from the Kem Kem beds of Morocco (Filleul & Dutheil, 2001; Davesne et al., 2018). In this sense, the continued dominance of percopsiforms in North American assemblages in the Maastrichtian and Paleocene might be an archaic aspect of the freshwater fauna of the continent. Fragments assigned to †“Priscacara” from the Maastrichtian Hell Creek and Scollard formations (Brinkman et al., 2014; Brinkman et al., 2021) do show that percomorphs were present in North American freshwater ecosystems by the close of the Cretaceous, with the exceptional Green River Lagerstätte demonstrating that percomorphs had become the dominant acanthomorph group in at least some North American freshwater settings by the early Eocene (Grande, 2013). The presence of diverse acanthomorphs in continental deposits from landmasses separated by marine barriers during much or all of the Cretaceous—North America, South America, Africa, and India—suggests several independent colonizations of freshwater prior to the end of the Mesozoic, with most examples representing percomorphs. This is broadly consistent with teleost-wide analyses that infer substantial invasion of freshwater settings in the Late Cretaceous and Paleogene based on ancestral range estimation used in conjunction with time-calibrated molecular phylogenies (Miller & Román-Palacios, 2021).
5.2. Implications for the emergence of major acanthomorph lineages
41The strong contrasts between the best-known faunas of Late Cretaceous age and the exceptional assemblages of the early Eocene implicate the intervening interval as a critical episode in acanthomorph evolutionary history (Patterson, 1993). Substantial debate focuses on fish diversification in the Late Cretaceous and early Paleogene, especially what role—if any—the end-Cretaceous mass extinction played in modulating macroevolutionary patterns at this time. These questions have been examined using fossils alone (Cavin & Martin, 1995; Friedman, 2009; Friedman, 2010; Carnevale & Johnson, 2015; Sibert & Norris, 2015; Sibert et al., 2018), as well as with data from living species where paleontology provides important temporal constraints (Miya et al., 2013; Near et al., 2013; Price et al., 2014; Alfaro et al., 2018; Ribeiro et al., 2018; Friedman et al., 2019; Ghezelayagh et al., 2022). The record of Patterson’s Gap is especially relevant, since the Cretaceous–Paleogene boundary is centered within it.
42The skeletal fossil record outside of Patterson’s Gap provides some basic constraints on our understanding of acanthomorph diversification around this time. First, the taxonomic profile of marine fish faunas shifts substantially between the end of the Campanian and the earliest Eocene. Over this span, acanthomorphs go from being a minority component of faunas to representing the dominant group, gauged from relative taxonomic richness. Over the same span, percomorphs increase their share of the acanthomorph fauna to the point that they are the principal spiny-rayed lineage in Eocene and younger faunas (Friedman et al., 2015). Second, the overall morphological diversity among acanthomorphs increases between the Cretaceous and the Paleogene, with much of this elevated variety attributable to percomorphs (Friedman, 2010). However, fossil evidence unambiguously indicates that numerous highly specialized acanthomorph—and more specifically, percomorph—body plans originated well before the end of the Cretaceous (Sorbini, 1981; Carnevale & Johnson, 2015).
43Considering the 21 major divisions of acanthomorph fishes recognized by Dornburg & Near (2021) and Ghezelayagh et al. (2022), the total groups of at least four of these are represented from uncontroversial articulated skeletal remains predating the Maastrichtian: Lampriformes, Zeiformes, Trachichthyiformes, and Syngnathiformes (Fig. 11). The exact status of Cretaceous polymixiids and beryciforms are unsettled (Schrøder et al., 2022; Andrews et al., 2023), although there are plausible candidate members of each group in Campanian or older strata (Patterson, 1993) and so we regard these groups as having a pre-Maastrichtian record. Collectively, these groups represent some of the most deeply diverging lineages of acanthomorphs. If the assignment of †plectocretacicoids to tetraodontiforms is correct, this would indicate the pre-Maastrichtian appearance of one of the most nested of the major acanthomorph lineages, the percomorph group Acanthuriformes sensu Dornburg & Near (2021). The radically different implications for spiny-rayed fish evolution stemming from alternative phylogenetic placements of these taxa (Tyler & Sorbini, 1996; Santini & Tyler, 2003; Benton et al., 2015; Arcila & Tyler, 2017; Troyer et al., 2022) indicate that resolution of the †plectocretacicoid problem should be a priority for future systematic investigation of Cretaceous acanthomorphs.
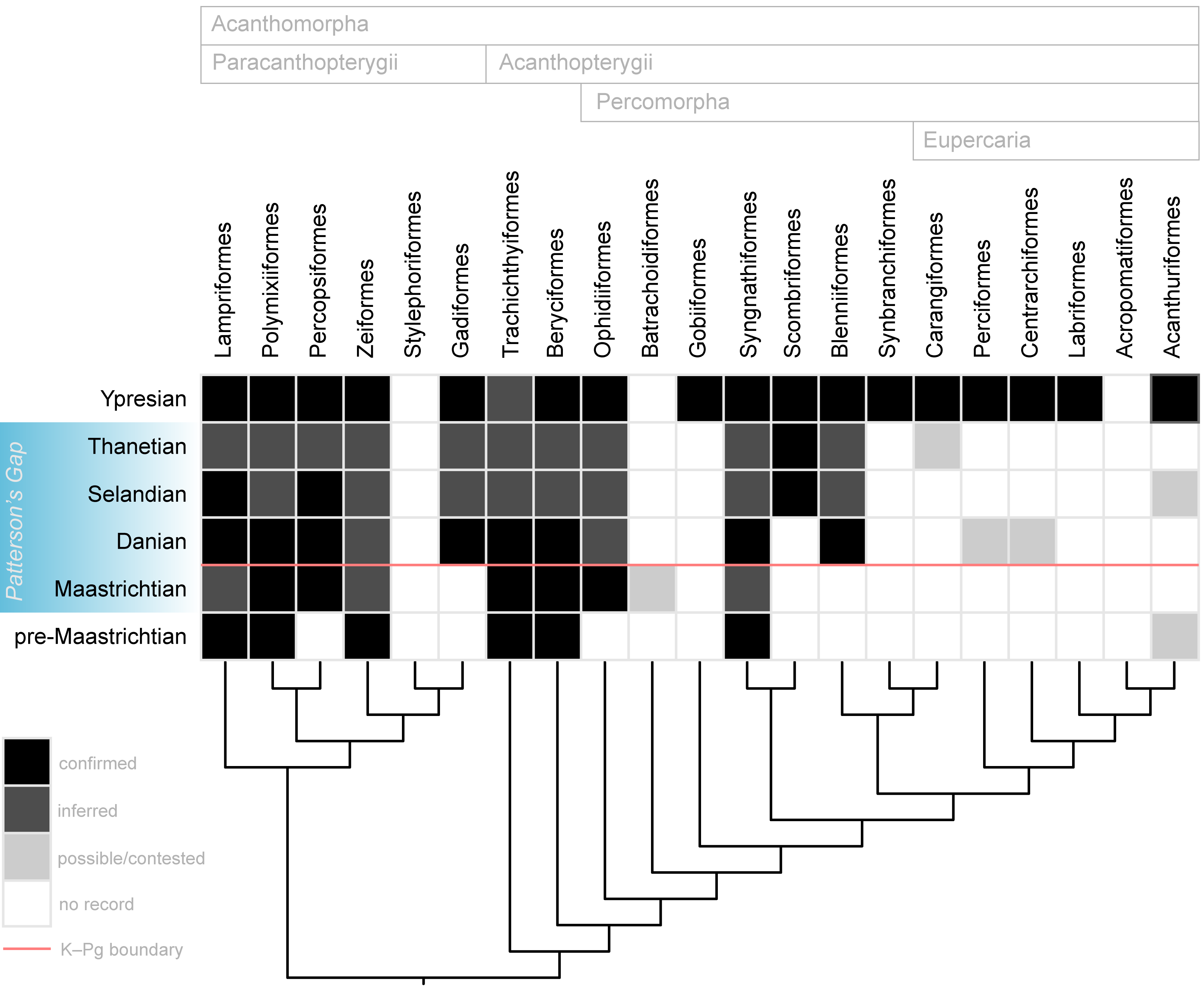
Figure 11. Summary of the articulated skeletal record of major acanthomorph lineages in the Late Cretaceous and early Paleogene. Topology and classification largely adapted from Dornburg & Near (2021) and Ghezelayagh et al. (2022). Skeletal occurrences outside of Patterson’s Gap based on multiple sources. Boxes marked as “inferred” are intervals for which lineages are inferred to be present based on earlier, definitive occurrences but are not yet represented by articulated remains in deposits of the age represented by the box.
44Within Patterson’s Gap, Maastrichtian rocks yield the oldest fossil skeletons belonging to Percopsiformes and the most deeply diverging percomorph lineage Ophidiiformes, as well as a potential example of Batrachoidiformes. It is possible that well-preserved but anatomically generalized “percoids” from latest Maastrichtian strata like †Eoserranus, †Indiaichthys, and †Prolates represent the first skeletal records of other major lineages, but their phylogenetic positions remain unsettled. Gadiformes, Blenniiformes, and possibly Perciformes make their skeletal record debut during the Danian, represented by “Protocodus,” †Chaychanus, and †Paleoserranus, respectively. Centrarchiformes, if attribution of Bolivian skeletons to Percichthys is correct, first appear in the Danian. The Selandian record yields the oldest articulated Scombriformes. If the undescribed percomorph from the Lellinge Greensand proves close to †Leiognathoides, and that genus is indeed related to leiognathids, this would represent an articulated occurrence for Acanthuriformes in the Selandian. Stratigraphic uncertainties lead to an ambiguous Paleocene record for Carangiformes, with clear material of billfishes and Mene that might be Thanetian in age. Thus, a majority of the principal acanthomorph groups appear in the skeletal record before the end of the Paleocene. Exceptions that are likely due to taphonomic biases include the lineages leading to Stylephorus and Synbranchiformes; these have either sparse or non-existent fossil records over their inferred evolutionary histories stemming from distributions in environmental settings (the deep sea in the case of Stylephorus) or geographic regions (freshwaters of Southeast Asia, Africa, and South America in the case of Synbranchiformes) with limited availability or sampling in the sedimentary record. Most remaining lineages not known with certainty from Patterson’s Gap—Labriformes, Acropomatiformes, and Acanthuriformes—form a clade within a group called Eupercaria, which occupies an apical position within percomorph phylogeny. Early Eocene rocks yield unambiguous examples of each of these groups (Danil’chenko, 1968; Bonde et al., 2008; Carnevale et al., 2014; Friedman et al., 2015; Friedman & Carnevale, 2018).
45Thus, despite the sparse record within Patterson’s Gap, this record is still sufficient to place some basic constraints on the patterns of acanthomorph—and especially percomorph—diversification around the Cretaceous–Paleogene boundary. The paleontological record clearly supports the origin of most higher-level percomorph lineages within the Late Cretaceous, consistent with molecular phylogenies calibrated principally with Eocene and younger percomorph fossils (Near et al., 2013; Alfaro et al., 2018; Hughes et al., 2018; Ghezelayagh et al., 2022). Likewise, at least some divergent morphologies had arisen among percomorphs in the Mesozoic (Carnevale & Johnson, 2015), although the more complete repertoire of body plans within the group is not apparent until the early Eocene (Friedman, 2010; Ghezelayagh et al., 2022). This combination of deep evolutionary divergences, coupled with delayed anatomical diversification across multiple lineages, matches patterns inferred for other vertebrate groups that apparently radiated in association with major mass extinctions, including placental mammals (dos Reis et al., 2013; Goswami et al., 2022), and early ray-finned fishes (Sallan & Friedman, 2012; Giles et al., 2023), and, to a lesser extent, birds (Field et al., 2020).
5.3. Implications for the geographic and environmental axes of acanthomorph evolution
46The fossil record offers some insights on acanthomorph evolution beyond the timing of divergences that are not so easily discerned from molecular phylogenies. First, paleontology provides evidence for contrasting patterns of extinction between groups. There are no obvious examples of major acanthomorph lineages present in the final stages of the Late Cretaceous that do not persist until the Paleogene. This stands in contrast to elevated extinction reported for several groups of non-acanthomorphs, particularly large-bodied pelagic predators (Friedman, 2009). It is therefore possible that much of the Cenozoic success of acanthomorphs stems from this apparent resilience in comparison to other lineages, leaving them with ample opportunities for subsequent diversification into new ecological roles (Harrington et al., 2016; Ribeiro et al., 2018; Friedman et al., 2019; Cantalice et al., 2022). Second, fossils from Patterson’s Gap can place important constraints on the composition of faunas, in terms of the relative dominance of lineages as well as the contrasts between assemblages as a function of habitat, geography, or both. The fossil occurrences reviewed here, although limited, imply potential spatial heterogeneity in percomorph diversification not apparent when faunal records are considered as a global aggregate. Studies of the fossil invertebrate record routinely identify shallow-water settings (Jablonski & Bottjer, 1990) and the tropics (Jablonski et al., 2006) as the locus for the origin of new lineages, with subsequent expansions to more offshore environments or higher latitudes. Percomorphs are reasonably well represented, if still uncommon, in shallow marine settings in the latest Cretaceous, but they are entirely absent from the few pelagic assemblages of this age that are known (Argyriou & Davesne, 2021; Argyriou et al., 2022). It is only in the Cenozoic that percomorphs represent a significant enough component of offshore faunas that they are regularly found in these deposits (Danil’chenko, 1968), with lineages like scombrids and carangids sometimes thought to have diversified in response to vacated ecological roles after the end-Cretaceous extinction (Friedman, 2009; Ribeiro et al., 2018; Friedman et al., 2019). These contrasts find a parallel in comparisons between low- and high-latitude marine assemblages during the Paleocene, with the tropical assemblage of Chiapas having a more modern aspect than the northerly fauna of Scandinavia and its clear links to older Cretaceous assemblages. Documenting the first occurrences of lineages still represents a significant exercise, but future work should focus on the other kinds of information that fossils uniquely provide about past geographic distributions and faunal compositions.
5.4. Priorities for future research
47Despite their apparent significance, many of the fossils known from Patterson’s Gap have limited capacity to impact hypotheses of acanthomorph diversification because they are poorly known, with unclear affinities to modern taxa. This will require dedicated revision of existing material, with many of the fossils reviewed in this contribution representing promising candidates for chemical, mechanical, or virtual preparation that could yield high-quality morphological data bearing on their relationships. However, even detailed anatomical information for fossils cannot overcome another challenge: lack of clear osteological characters for many recognized acanthomorph lineages (Johnson, 1984; Johnson & Patterson, 1993; Patterson, 1993; Betancur-R. et al., 2017). This problem is most acute for clades recognized based on genetic data, but for which no anatomical characters have been proposed (Miya et al., 2013; Harrington et al., 2016). The situation for fossils from Patterson’s Gap is especially challenging, since it is plausible that many of the fossils from this interval belong to the common stems of phenotypically heterogeneous clades that seem to share few, if any, obvious derived features. Renewed investigation of morphology in the light of molecular phylogenies (Borden et al., 2013; Davesne et al., 2016; Girard et al., 2020; Pastana et al., 2021) provides hope that sufficient evidence might be available to paleontologists to eventually identify fossil members of these groups with confidence.
48The study of known fossils alone is probably insufficient to substantially refine our perception of acanthomorph evolution around the Cretaceous–Paleogene boundary. The spatially and environmentally heterogeneous patterns shown by sporadic existing records suggest that further sampling of the Maastrichtian–Paleocene record outside of established localities will yield new faunas compositionally distinct from known ones. A better understanding of the distribution of acanthomorph groups across sites and habitats represents a critical piece in documenting the transition from the Cretaceous fauna to the early Eocene one that so clearly foreshadows the modern assemblage. Additional field-based efforts are required to address these questions. Work in the marine record should prioritize the search for new low-latitude faunas from nearshore settings (Cantalice et al., 2022). Based on current understanding of the record, it is sites fitting this description that stand the best chance of capturing the first appearances of lineages critical for calibrating the acanthomorph evolutionary timescale, in terms of the divergence of lineages and establishment of new ecological and functional roles. The fossil history of freshwater acanthomorphs shows even more glaring gaps in coverage than that of marine species (Davesne et al., 2018), mirroring a general pattern of bias in the record (Friedman, 2022). In the case of freshwater taxa, any new fossils from outside of the handful of known localities seem likely to provide significant insights, and could have a vital bearing on longstanding biogeographic debates (Capobianco & Friedman, 2019). While Patterson’s Gap is perhaps not as sparse as earlier accounts suggest, it remains a relatively understudied interval. We are optimistic that renewed efforts in the field, museum collections, and research laboratories will yield important new paleontological data contributing to our understanding the evolution of the remarkable diversity of spiny-rayed fishes.
Acknowledgments
49We thank the editors of Geologica Belgica for their invitation to provide this paper, and to G. Carnevale (University of Turin, Italy) and A. Murray (University of Alberta, Edmonton, Canada) for their helpful feedback on an earlier version of this contribution. Work on acanthomorphs from the Maastrichtian and Paleocene has benefitted from conversations with numerous colleagues and access to several collections, but we would particularly like to recognize: A. Schulp and J. Jagt (Natuurhistorisch Museum, Maastricht, the Netherlands); A. Folie and T. Smith (Royal Belgian Institute of Natural Sciences, Brussels, Belgium); N. Bonde, B. Lindow, and S.-L. Jakobsen (Natural History Museum of Denmark, Copenhagen, Denmark); E. Bernard, Z. Johanson, and M. Richter (Natural History Museum, London, United Kingdom); G. Carnevale; L. Grande and W. Simpson (Field Museum, Chicago, United States); F. Mets (Museum for Central Africa, Tervuren, Belgium); Paul Schofield (Canterbury Museum, Canterbury, New Zealand); C. Byrd, J. Cundiff, and S. Pierce (Museum of Comparative Zoology, Harvard University, Cambridge, United States); A. Gishlick and J. Maisey (American Museum of Natural History, New York, United States); D. Ehret and R. Pellegrini (New Jersey State Museum, Trenton, United States). We thank C. Abraczinskas (University of Michigan Museum of Paleontology) for providing a high-quality image for Fig. 1. This study was supported by the National Science Foundation (grant DEB 2017822 to M. Friedman), the Rackham Graduate School of the University of Michigan (Rackham Merit Fellowships to J. Andrews and H. Saad), and the Egyptian Ministry of Higher Education (Fellowship to S. El-Sayed).
Author contributions
50M. Friedman conceived the topic of the article and composed the first draft, with major contributions on stratigraphy by J. V. Andrews and S. El-Sayed. H. Saad produced paleogeographic reconstructions. All authors provided feedback on the manuscript and contributed to the composition of figures.
Data availability
51Fossil specimens are available in the collections and institutions cited in the text.
References
52Adolfssen, J.S., Milan, J. & Friedman, M., 2017. Review of the Danian vertebrate fauna of southern Scandinavia. Bulletin of the Geological Survey of Denmark, 65, 1–23. https://doi.org/10.37570/bgsd-2017-65-01
53Agassiz, L., 1833–1843. Recherches sur les Poissons fossiles. Imprimerie de Petitpierre, Neuchatel, 188 p. https://doi.org/10.5962/bhl.title.4275
54Alfaro, M.E., Faircloth, B.C., Harrington, R.C., Sorenson, L., Friedman, M., Thacker, C.E., Oliveros, C.H., Černý, D. & Near, T.J., 2018. Explosive diversification of marine fishes at the Cretaceous–Palaeogene boundary. Nature Ecology & Evolution, 2, 688–696. https://doi.org/10.1038/s41559-018-0494-6
55Alroy, J., 1999. The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation. Systematic Biology, 48, 107–118. https://doi.org/10.1080/106351599260472
56Alvarado-Ortega, J., Cuevas-García, M., Melgarejo-Damián, M., Cantalice, K.M., Alaniz-Galvan, A., Solano-Templos, G. & Than-Marchese, B.A., 2015. Paleocene fishes from Palenque, Chiapas, southeastern Mexico. Palaeontologia Electronica, 18.2.39A, 1–22. https://doi.org/10.26879/536
57Alvarado-Ortega, J., Cuevas-García, M. & Cantalice, K.M., 2018. The fossil fishes of the archaeological site of Palenque, Chiapas, southeastern Mexico. Journal of Archaeological Science: Reports, 17, 462–476. https://doi.org/10.1016/j.jasrep.2017.11.029
58Anderson, M.E., 1998. A Late Cretaceous (Maastrichtian) galaxiid fish from South Africa. Special Publication, J. L. B. Smith Institute of Ichthyology, 60, 1–12.
59Andrews, J.V., Schein, J.D. & Friedman, M., 2023. An earliest Paleocene squirrelfish (Teleostei: Beryciformes: Holocentroidea) and its bearing on the timescale of holocentroid evolution. Journal of Systematic Palaeontology, 21, 2168571. https://doi.org/10.1080/14772019.2023.2168571
60Arambourg, C., 1952. Les vertébrés fossiles des gisements de phosphates (Maroc-Algérie-Tunisie). Mémoires du Service géologique du Maroc, 92, 1–372.
61Arcila, D. & Tyler, J.C., 2017. Mass extinction in tetraodontiform fishes linked to Palaeocene-Eocene thermal maximum. Proceedings of the Royal Society B, 284, 20171771. https://doi.org/10.1098/rspb.2017.1771
62Argyriou, T. & Davesne, D., 2021. Offshore marine actinopterygian assemblages from the Maastrichtian–Paleogene of the Pindos Unit in Eurytania, Greece. PeerJ, 9, e10676. https://doi.org/10.7717/peerj.10676
63Argyriou, T., Alexopoulos, A., Carrillo-Briceño, J.D. & Cavin, L., 2022. A fossil assemblage from the mid–late Maastrichtian of Gavdos Island, Greece, provides insights into the pre-extinction pelagic ichthyofaunas of the Tethys. PLoS ONE, 17, e0265780. https://doi.org/10.1371/journal.pone.0265780
64Arratia, G., 1982. A review of freshwater percoids from South America (Pisces, Osteichthyes, Perciformes, Percichthyidae, and Perciliidae). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 540, 1–52.
65Arratia, G., López-Arbarello, A., Prasad, G.V.R., Parmar, V. & Kriwet, J., 2004. Late Cretaceous-Paleocene percomoprhs (Teleostei) from India - Early radiation of Perciformes. In Arratia, G., Wilson, M.V.H. & Cloutier, R. (eds), Recent Advances in the Origin and Early Radiation of Vertebrates. Verlag Dr. Friedrich Pfeil, Munich, 635–663.
66Astre, G., 1927. Le carangidé de phosphates tunisiens. Bulletin de la Société d’Histoire naturelle de Toulouse, 56, 501–504.
67Bannikov, A.F. & Sorbini, C., 2000. Preliminary note on a lower Paleocene fish fauna from Trebiciano (Trieste - north-eastern Italy). Atti del Museo Civico di Storia Naturale di Trieste, 48, 15–30.
68Bannikov, A.F., Tyler, J.C., Arcila, D. & Carnevale, G., 2017. A new family of gymnodont fish (Tetraodontiformes) from the earliest Eocene of the Peri-Tethys (Kabardino-Balkaria, northern Caucasus, Russia). Journal of Systematic Palaeontology, 15, 129–146. https://doi.org/10.1080/14772019.2016.1149115
69Beckett, H.T. & Friedman, M., 2016. The one that got away from Smith Woodward: cranial anatomy of Micrornatus (Acanthomorpha: Scombridae) revealed using computed microtomography. In Johanson, Z., Barrett, P.M., Richter, M. & Smith, M. (eds), Arthur Smith Woodward: His Life and Influence on Modern Vertebrate Palaeontology. Geological Society, London, Special Publications, 430, 337–353. https://doi.org/10.1144/SP430.16
70Belmonte, G., Capasso, L. & Taverne, L.P., 2016. Salento Cretaceous fish in Italian museums and other scientific institutions. Museologia Scientifica, nuova serie, 10, 94–98.
71Bendix-Almgreen, S.E., 1969. Notes on the Upper Cretaceous and Lower Tertiary fish faunas of northern West Greenland. Meddelelser fra Dansk Geologisk Forening, 19, 204–217.
72Benton, M.J., 2012. No gap in the Middle Permian record of terrestrial vertebrates. Geology, 40, 339–342. https://doi.org/10.1130/G32669.1
73Benton, M.J.D., Donoghue, P.C.J., Asher, R.J., Friedman, M., Near, T.J. & Vinther, J., 2015. Constraints on the timescale of animal evolutionary history. Palaeontologia Electronica, 18.1.1FC, 1–107. https://doi.org/10.26879/424
74Betancur-R., R., Wiley, E.O., Arratia, G., Acero, A., Bailly, N., Miya, M., Lecointre, G. & Ortí, G., 2017. Phylogenetic classification of bony fishes. BMC Evolutionary Biology, 17, 162. https://doi.org/10.1186/s12862-017-0958-3
75Bignot, G., 1987. Les paléoenvironnements et les paléogéographies du bassin de Paris au Danien, d’après les Foraminifères du Mont Aimé (Marne, France). Revue de Micropaléontologie, 30,150–176.
76Bonde, N. & Leal, M.E.C., 2017. Danian teleosteans of the North Atlantic Region - compared with the Maastrichtian. Research & Knowledge, 3, 50–54.
77Bonde, N., Andersen, S., Hald, N. & Jakobsen, S.L., 2008. Danekræ - Danmarks Bedste Fossiler. Gyldendal, Copenhagen, 225 p.
78Borden, W.C., Grande, T. & Smith, W.L., 2013. Comparative osteology and myology of the caudal fin the Paracanthopterygii (Teleostei: Acanthomorpha). In Arratia, G., Schultze, H.-P. & Wilson, M. V. H. (eds), Mesozoic Fishes 5 - Global Diversity and Evolution. Verlag Friedrich Pfeil, Munich, 419–455.
79Borkar, V.D., 1973a. Fossil fishes from the inter-trappean beds of Surendranagar District, Saurashtra. Proceedings, Indian Academy of Sciences, B, 78, 181–193. https://doi.org/10.1007/BF03045500
80Borkar, V.D., 1973b. New fossil fishes from the intertrappean beds in Surendrangar district, Gujarat state. Current Science, 42, 422–423.
81Brinkman, D.B., Newbrey, M.G., Neuman, A.G. & Eaton, J.G., 2013. Freshwater Osteichthyes from the Cenomanian to late Campanian of Grand Staircase-Escalante National Monument, Utah. In Titus, A.L. & Lowen, M.A. (eds), At the Top of the Grand Staircase: The Late Cretaceous of Southern Utah. Indiana University Press, Bloomington, Indiana, 195–236.
82Brinkman, D.B., Newbrey, M.G. & Neuman, A.G., 2014. Diversity and paleoecology of actinopterygian fish from vertebrate microfossil localities of the Maastrichtian Hell Creek Formation of Montana. In Wilson, G.P., Clemens, W.A., Horner, J.R. & Harman, J.H. (eds), Through the End of the Cretaceous in the Type Locality of the Hell Creek Formation in Montana and Adjacent Areas. Geological Society of America Special Papers, 503, 247–270. https://doi.org/10.1130/2014.2503(09)
83Brinkman, D.B., Divay, J.D., Demar, D.G., Jr. & Wilson Mantilla, G.P., 2021. A systematic reappraisal and quantitative study of the nonmarine teleost fishes from the late Maastrichtian of the Western Interior of North America: evidence from vertebrate microfossil localities. Canadian Journal of Earth Sciences, 58, 936–967. https://doi.org/10.1139/cjes-2020-0168
84Cantalice, K.M. & Alvarado-Ortega, J., 2016. Eekaulosomus cuevasae gen. and sp. nov., an ancient armored trumpetfish (Aulostomoidea) from Danian (Paleocene) marine deposits of Belisario Domínguez, Chiapas, southeastern Mexico. Palaeontologia Electronica, 19.3.53A, 1–24. https://doi.org/10.26879/682
85Cantalice, K.M. & Alvarado-Ortega, J., 2017. Kelemejtubus castroi, gen. et sp. nov., an ancient percomorph (Teleostei, Actinopterygii) from the Paleocene marine deposits near Palenque, Chiapas, southeastern Mexico. Journal of Vertebrate Paleontology, 37, e1383265. https://doi.org/10.1080/02724634.2017.1383265
86Cantalice, K.M., Alvarado-Ortega, J. & Alaniz-Galvan, A., 2018. Paleoserranus lakamhae gen. et sp. nov., a Paleocene seabass (Perciformes: Serranidae) from Palenque, Chiapas, southeastern Mexico. Journal of South American Earth Sciences, 83, 137–146. https://doi.org/10.1016/j.jsames.2018.01.010
87Cantalice, K.M., Alvarado-Ortega, J. & Bellwood, D.R., 2020. †Chaychanus gonzalezorum gen. et sp. nov.: A damselfish fossil (Percomorphaceae; Pomacentridae), from the Early Paleocene outcrop of Chiapas, Southeastern Mexico. Journal of South American Earth Sciences, 98, 102322. https://doi.org/10.1016/j.jsames.2019.102322
88Cantalice, K.M., Alvarado-Ortega, J., Bellwood, D.R. & Siqueira, A.C., 2022. Rising from the ashes: the biogeographic origins of modern coral reef fishes. BioScience, 72, 769–777. https://doi.org/10.1093/biosci/biac045
89Capobianco, A. & Friedman, M., 2019. Vicariance and dispersal in southern hemisphere freshwater fish clades: a palaeontological perspective. Biological Reviews, 94, 662–699. https://doi.org/10.1111/brv.12473
90Capobianco, A., Foreman, E. & Friedman, M., 2021. A Paleocene (Danian) marine osteoglossid (Teleostei: Osteoglossomorpha) from the Nuussuaq Basin of Greenland, with a brief review of Palaeogene marine bonytongue fishes. Papers in Palaeontology, 7, 625–640. https://doi.org/10.1002/spp2.1291
91Carnevale, G. & Collette, B.B., 2014. †Zappaichthys harzhauseri, gen. et sp. nov., a new Miocene toadfish (Teleostei, Batrachoidiformes) from the Paratethys (St. Margarethen in Burgenland, Austria), with comments on the fossil record of batrachoidiform fishes. Journal of Vertebrate Paleontology, 34, 1005–1017. https://doi.org/10.1080/02724634.2014.854801
92Carnevale, G. & Johnson, G.D., 2015. A Cretaceous cusk-eel (Teleostei, Ophidiiformes) from Italy and the Mesozoic diversification of percomorph fishes. Copeia, 103, 771–791. https://doi.org/10.1643/CI-15-236
93Carnevale, G., Bannikov, A.F., Marramà, G., Tyler, J.C. & Zorzin, R., 2014. The Pesciara-Monte Postale Fossil-Lagerstätte: 2. Fishes and other vertebrates. Rendiconti della Società Paleontologica Italiana, 4, 37–63.
94Cavin, L. & Martin, C., 1995. Les Actinoptérygiens et la limite Crétacé-Tertiaire. Geobios MS, 28, 183–188. https://doi.org/10.1016/S0016-6995(95)80110-3
95Chang, A., Arratia, G. & Alfaro, G., 1978. Percichthys lonquimayiensis n. sp. from the upper Paleocene of Chile (Pisces, Perciformes, Serranidae). Journal of Paleontology, 52, 727–736.
96Christophel, D.C., 1976. Fossil floras of the Smoky Tower locality, Alberta. Palaeontographica Abteilung B, 157, 1–43.
97Cione, A.L., Santillana, S., Gouiric-Cavalli, S., Acosta Hospitaleche, C., Gelfo, J.N., López, G.M. & Reguero, M.A., 2018. Before and after the K/Pg extinction in West Antarctica: New marine fish records from Marambio (Seymour) Island. Cretaceous Research, 85, 250–265. https://doi.org/10.1016/j.cretres.2018.01.004
98Coates, M.I. & Clack, J.A., 1995. Romer’s gap: tetrapod origins and terrestriality. Bulletin du Muséum national d’Histoire naturelle, 4ème série – section C – Sciences de la Terre, Paléontologie, Géologie, Minéralogie, 17, 373–388.
99Cohen, D.M., 1984. Gadiformes: overview. In Moser, H.G., Richards, W.J., Cohen, D.M., Fahay, M.P., Kendall, A.W., Jr. & Richardson, S.L. (eds), Ontogeny and Systematics of Fishes. Allen Press, Lawrence, Kansas, 259–265.
100Dalla Vecchia, F.M., 2008. I dinosauri del Villaggio del Pescatore (Trieste): Qualche aggiornamento. Atti del Museo Civico di Storia Naturale di Trieste, 53, 111–130.
101Danil’chenko, P.G., 1960. Kostistye ryby Maikopskikh otlozhenii Kavaza. Trudy Paleontologicheskogo Instituta, 78, 1–208.
102Danil’chenko, P.G., 1968. Fishes from the upper Paleocene of Turkmenia. In Ocherki po filogenii i sistematike iskopaemykh ryb i beschelyustnykh. Nauka, Moscow, 113–156.
103Dartevelle, E. & Casier, E., 1943. Les Poissons fossiles du Bas-Congo et des régions voisines. 1ère partie. Annales du Musée du Congo belge, A Ser. 3, 2, 1–200.
104Dartevelle, E. & Casier, E., 1949. Les Poissons fossiles du Bas-Congo et des régions voisines. 2e partie. Annales du Musée du Congo belge, Tervuren (Belgique), A Ser. 3, 2, 201–255.
105Dartevelle, E. & Casier, E., 1959. Les Poissons fossiles du Bas-Congo et des régions voisines. 3e partie. Annales du Musée du Congo belge, Tervuren (Belgique), A Ser. 3, 3, 257–568.
106Darwin, C., 1859. On the Origin of Species by Means of Natural Selection. John Murray, London, 502 p.
107Davesne, D., Gallut, C., Barriel, V., Janvier, P., Lecointre, G. & Otero, O., 2016. The phylogenetic intrarelationships of spiny-rayed fishes (Acanthomorpha, Teleostei, Actinopterygii): fossil taxa increase the congruence of morphology with molecular data. Frontiers in Ecology and Evolution, 4, 129. https://doi.org/10.3389/fevo.2016.00129
108Davesne, D., Geuriau, P., Dutheil, D. & Bertrand, L., 2018. Exceptional preservation of a Cretaceous intestine provides a glimpse of the early ecological diversity of spiny-rayed fishes (Acanthomorpha, Teleostei). Scientific Reports, 8, 8509. https://doi.org/10.1038/s41598-018-26744-3
109Davis, J.W., 1890. On the fossil fishes of the Cretaceous formations of Scandinavia. Scientific Transactions of the Royal Dublin Society, (2) 4, 363–434.
110Dawson, F.M., Kalkreuth, W.D. & Sweet, A.R., 1994. Stratigraphy and coal resource potential of the upper Cretaceous to Tertiary strata of northwestern Alberta. Geological Survey of Canada, Bulletin, 466, 1–68. https://doi.org/10.4095/194029
111Dornburg, A. & Near, T.J., 2021. The emerging phylogenetic perspective on the evolution of actinopterygian fishes. Annual Review of Ecology, Evolution, and Systematics, 52, 427–452. https://doi.org/10.1146/annurev-ecolsys-122120-122554
112dos Reis, M., Inoue, J., Hasegawa, M., Asher, R.J., Donoghue, P.C.J. & Yang, Z., 2013. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proceedings of the Royal Society B, 279, 3491–3500. https://doi.org/10.1098/rspb.2012.0683
113Eberth, D.A. & Kamo, S. L., 2019. First high-precision U-Pb CA-ID-TIMS age for the Battle Formation (Upper Cretaceous), Red Deer River valley, Alberta, Canada: implications for ages, correlations, and dinosaur biostratigraphy of the Scollard, Frenchman, and Hell Creek formations. Canadian Journal of Earth Sciences, 56, 1041–1051. https://doi.org/10.1139/cjes-2018-0098
114El-Sayed, S., Friedman, M., Anan, T., Faris, M. & Sallam, H., 2021. Diverse marine fish assemblages inhabited the paleotropics during the Paleocene-Eocene Thermal Maximum. Geology, 49, 993–998. https://doi.org/10.1139/cjes-2018-0098
115Fedotov, V.F. & Bannikov, A.F., 1989. On phylogenetic relationships of fossil Gadidae. In Cohen, D.M. (ed.), Papers on the Systematics of Gadiform Fishes. Natural History Museum of Los Angeles County, Los Angeles, 187–195.
116Field, D.J., Benito, J., Chen, A., Jagt, J.W. & Ksepka, D.T., 2020. Late Cretaceous neornithine from Europe illuminates the origins of crown birds. Nature, 579, 397–401. https://doi.org/10.1038/s41586-020-2096-0
117Filleul, A. & Dutheil, D., 2001. Spinocaudichthys oumtkoutensis, a freshwater acanthomorph from the Cenomanian of Morocco. Journal of Vertebrate Paleontology, 21, 774–780. https://doi.org/10.1671/0272-4634(2001)021[0774:SOAFAF]2.0.CO;2
118Foley, N.M., Mason, V.C., Harris, A.J., Bredemeyer, K.R., Damas, J., Lewin, H.A., Eizirik, E., Gatesy, J., Karlsson, E.K., Lindblad-Toh, K., Zoonomia Consortium, Springer, M.S. & Murphy, W.J. 2023. A genomic timescale for placental mammal evolution. Science, 380, eabl8189. https://doi.org/10.1126/science.abl8189
119Forey, P.L., Yi, L., Patterson, C. & Davies, C.E., 2003. Fossil fishes from the Cenomanian (Upper Cretaceous) of Namoura, Lebanon. Journal of Systematic Palaeontology, 1, 227–330. https://doi.org/10.1017/S147720190300107X
120Fox, R.C., 1990. The succession of Paleocene mammals in western Canada. Geological Society of America Special Papers, 243, 51–70. https://doi.org/10.1130/SPE243-p51
121Fricke, R., Eschmeyer, W.N. & Fong, J.D., 2022. Eschmeyer’s Catalog of Fishes: Genera/Species by Family/Subfamily [Online]. http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp, accessed December 2022.
122Friedman, M., 2009. Ecomorphological selectivity among marine teleost fishes during the end-Cretaceous extinction. Proceedings of the National Academy of Sciences of the USA, 106, 5218–5223. https://doi.org/10.1073/pnas.0808468106
123Friedman, M., 2010. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proceedings of the Royal Society B, 277, 1675–1683. https://doi.org/10.1098/rspb.2009.2177
124Friedman, M., 2012. Ray-finned fishes (Osteichthyes, Actinopterygii) from the type Maastrichtian, the Netherlands and Belgium. In Jagt, J.W.M., Donovan, S.K. & Jagt-Yazykova, E.A. (eds), Fossils of the type Maastrichtian (Part 1). Scripta Geologica, Leiden, Special Issue, 8, 112–142.
125Friedman, M., 2022. The macroevolutionary history of bony fishes: a paleontological view. Annual Review of Ecology, Evolution, and Systematics, 53, 353–377. https://doi.org/10.1146/annurev-ecolsys-111720-010447
126Friedman, M. & Carnevale, G., 2018. The Bolca Lagerstätten: shallow marine life in the Eocene. Journal of the Geological Society, 175, 569–579. https://doi.org/10.1144/jgs2017-164
127Friedman, M. & Johnson, G.D., 2005. A new species of Mene (Perciformes: Menidae) from the Paleocene of South America, with notes on paleoenvironment and a brief review of menid fishes. Journal of Vertebrate Paleontology, 25, 770–783. https://doi.org/10.1671/0272-4634(2005)025[0770:ANSOMP]2.0.CO;2
128Friedman, M., Beckett, H.T., Close, R.A. & Johanson, Z., 2015. The English Chalk and London Clay: two remarkable British bony fish Lagerstätten. In Johanson, Z., Barrett, P. M., Richter, M. & Smith, M. (eds), Arthur Smith Woodward: His Life and Influence on Modern Vertebrate Palaeontology. Geological Society, London, Special Publications, 430, 165–200. https://doi.org/10.1144/SP430.18
129Friedman, M., Feilich, K.L., Beckett, H.T., Alfaro, M.E., Faircloth, B.C., Cerny, D., Miya, M., Near, T.J. & Harrington, R.C., 2019. A phylogenomic framework for pelagiarian fishes (Acanthomorpha: Percomorpha) highlights mosaic radiation in the open ocean. Proceedings of the Royal Society B, 286, 20191502. https://doi.org/10.1098/rspb.2019.1502
130Gale, A.S., Mutterlose, J. & Batenburg, S., 2020. The Cretaceous Period. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D. & Ogg, G.M. (eds), Geologic Time Scale 2020. Elsevier, Amsterdam, 1023–1086. https://doi.org/10.1016/B978-0-12-824360-2.00027-9
131Gallo, V. & De Figueiredo, F.J., 2003. †Farinichthys gigas, a new albulid fish (Teleostei: Elopomorpha) from the Paleocene of the Pernambuco-Paraiba Basin, northeastern Brazil. Journal of Vertebrate Paleontology, 22, 747–758. https://doi.org/10.1671/0272-4634(2002)022[0747:FGANAF]2.0.CO;2
132Gallo, V., De Figueiredo, F.J., De Carvalho, L.B. & De Azevedo, S.A.K., 2001. Vertebrate assemblage from the Maria Farinha Formation after the K-T boundary. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 219, 261–284. https://doi.org/10.1127/njgpa/219/2001/261
133Gaudant, J., 1979. Mise au point sur l’ichthyofaune paléocène de Menat (Puy-de-Dôme). Comptes rendus des Séances de l’Académie des Sciences, Série D, Sciences naturelles, 288, 1461–1464.
134Gavrilov, Y.O., Shcherbinina, E.A. & Oberhänsli, H., 2003. Paleocene-Eocene boundary events in the northeastern Peri-Tethys. Geological Society of America Special Papers, 369, 147–168. https://doi.org/10.1130/0-8137-2369-8.147
135Gayet, M. & Meunier, F.J., 1998. Maastrichtian to early late Paleocene freshwater Osteichthyes of Bolivia: additions and comments. In Malabarba, L.R., Reis, R.E., Vari, R.P., Lucena, Z.M.S. & Lucena, C.a.S. (eds), Phylogeny and Classification of Neotropical Fishes. EDIPUCRS, Porto Alegre, Brazil, 85–110.
136Ghezelayagh, A., Harrington, R.C., Burress, E.D., Campbell, M.A., Buckner, J.C., Chakrabarty, P., Glass, J.R., Mccraney, W.T., Unmack, P.J., Thacker, C.E., Alfaro, M.E., Friedman, S.T., Ludt, W.B., Cowman, P.F., Friedman, M., Price, S.A., Dornburg, A., Faircloth, B.C., Wainwright, P.C. & Near, T.J., 2022. Prolonged morphological expansion of spiny-rayed fishes following the end-Cretaceous. Nature Ecology & Evolution, 6, 1211–1220. https://doi.org/10.1038/s41559-022-01801-3
137Giles, S., Feilich, K.L., Warnock, R.C.M., Pierce, S.E. & Friedman, M., 2023. A Late Devonian actinopterygian suggests high lineage survivorship across the end-Devonian mass extinction. Nature Ecology & Evolution, 7, 10–19. https://doi.org/10.1038/s41559-022-01919-4
138Girard, M.G., Davis, M.P. & Smith, W.L., 2020. The phylogeny of carangiform fishes: morphological and genomic investigations of a new fish clade. Copeia, 108, 265–298. https://doi.org/10.1643/CI-19-320
139González-Rodríguez, K.A. & Feilitz, C., 2008. A new species of acanthomorph fish from the Upper Cretaceous Muhi Quarry, Hidalgo, central Mexico. In Arratia, G., Schultze, H.-P. & Wilson, M.V.H. (eds), Mesozoic Fishes 4 - Homology and Phylogeny. Verlag Dr. Friedrich Pfeil, Munich, 399–411.
140Goswami, A., Noirault, E., Coombs, E.J., Clavel, J., Fabre, A.-C., Halliday, T.J.D., Churchill, M., Curtis, A., Watanabe, A., Simmons, N.B., Beatty, B.L., Geisler, J., Fox, D.L. & Felice, R.N., 2022. Attenuated evolution of mammals through the Cenozoic. Science, 378, 377–383. https://doi.org/10.1126/science.abm7525
141Grande, L., 2013. The Lost World of Fossil Lake: Snapshots from Deep Time. University of Chicago Press, Chicago, 425 p. https://doi.org/10.7208/chicago/9780226922980.001.0001
142Grande, L. & Chatterjee, S., 1987. New Cretaceous fish fossils from Seymour Island, Antarctic Peninsula. Palaeontology, 30, 829–837.
143Grande, T., Borden, W.C. & Smith, W.L., 2013. Limits and relationships of Paracanthopterygii: a molecular framework for evaluating past morphological hypotheses. In Arratia, G., Schultze, H.-P. & Wilson, M.V.H. (eds), Mesozoic Fishes 5 - Global Diversity and Evolution. Verlag Dr. Friedrich Pfeil, Munich, 385–418.
144Greenwood, P.H., 1983. A new Palaeocene fish from Saudi Arabia. Journal of Natural History, 17, 405–418. https://doi.org/10.1080/00222938300770271
145Greenwood, P.H., 1995. A Paleocene percoid fish, tentatively referred to the family Serranidae, from Jabal Umm Himar, Kingdom of Saudi Arabia. U. S. Geological Survey Bulletin, 2093-B, B1–B3.
146Guernet, C. & Villier, L., 2017. Les ostracodes de la collection de P. Margerie et l’âge des couches à Laffittéines du Mont Aimé (bassin de Paris, France). Geodiversitas, 39, 225–249. https://doi.org/10.5252/g2017n2a4
147Harrington, R.C., Faircloth, B.C., Eytan, R.I., Smith, W.L., Near, T.J., Alfaro, M.E. & Friedman, M., 2016. Phylogenomic analysis of carangimorph fishes reveals flatfish asymmetry arose in a blink of the evolutionary eye. BMC Evolutionary Biology, 16, 224. https://doi.org/10.1186/s12862-016-0786-x
148Hübner, T. & Müller, A., 2010. Selachian teeth from Campanian sediments (Upper Cretaceous) of the Münsterland Cretaceous Basin (NW-Germany). Paläontologische Zeitschrift, 84, 437–455. https://doi.org/10.1007/s12542-010-0056-y
149Hughes, L.C., Ortí, G., Huang, Y., Sun, Y., Baldwin, C.C., Thompson, A.W., Arcila, D., Betancur-R., R., Li, C., Becker, L., Bellora, N., Zhao, X., Li, X., Wang, M., Fang, C., Xie, B., Zhou, Z., Huang, H., Chen, S., Venkatesh, B. & Shi, Q., 2018. Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Proceedings of the National Academy of Sciences of the USA, 115, 6249–6254. https://doi.org/10.1073/pnas.1719358115
150Iturralde-Vinent, M.A., 2003. A brief account of the evolution of the Caribbean seaway: Jurassic to present. In Prothero, D.R., Ivany, L.C. & Nesbitt, E.A. (eds), From Greenhouse to Icehouse: the Marine Eocene-Oligocene Transition. Columbia University Press, New York, 386–396.
151Jablonski, D. & Bottjer, D.J., 1990. Onshore-offshore trends in marine invertebrate evolution. In Ross, R.M. & Allmon, W.D. (eds), Causes of Evolution: A Paleontological Perspective. University of Chicago Press, Chicago, 21–75.
152Jablonski, D., Roy, K. & Valentine, J.W., 2006. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science, 314, 102–106. https://doi.org/10.1126/science.1130880
153Jagt, J.W.M., Jagt-Yazykova, E.A., Kaddumi, H.F. & Lindgren, J., 2017. Ammonite dating of latest Cretaceous mosasaurid reptiles (Squamata, Mosasauroidea) from Jordan—preliminary observations. Alcheringa, 42, 587–596. https://doi.org/10.1080/03115518.2017.1308011
154Jakobsen, P.R., Rhode, M.M. & Sheldon, E., 2017. Structures and stratigraphy of Danian limestone, eastern Sjælland, Denmark. Geological Survey of Denmark and Greenland Bulletin, 38, 21–24. https://doi.org/10.34194/geusb.v38.4391
155Johnson, G.D., 1984. Percoidei: development and relationships. In Moser, H.G., Richards, W.J., Cohen, D.M., Fahay, M.P., Kendall, A.W. & Richardson, S.L. (eds), Ontogeny and Systematics of Fishes. American Society of Ichthyologists and Herpetologists, Lawrence, Kansas, 464–498.
156Johnson, G.D., 1986. Scombroid phylogeny: an alternative hypothesis. Bulletin of Marine Science, 39, 1–43.
157Johnson, G.D. & Patterson, C., 1993. Percomorph phylogeny: a survey of acanthomorphs and a new proposal. Bulletin of Marine Science, 52, 554–626.
158Jurkovšek, B., Toman, M., Ogorelec, B., Sribar, L., Drobne, K., Poltjak, M. & Sribar, L., 1996. Geological map of the southern part of the Trieste-Komen Plateau. Cretaceous and Paleogene carbonate rocks. 1:50 000. Institut za Geologijo, geotekniko in geofiziko, Ljubljana, Slovenia.
159Jurkovšek, B., Biolchi, S., Furlani, S., Kolar-Jurkovšek, T., Zini, L., Jež, J., Tunis, G., Baveca, M. & Cucchi, F., 2016. Geology of the classical Karst region (SW Slovenia–NE Italy). Journal of Maps, 12, 352–362. https://doi.org/10.1080/17445647.2016.1215941
160Kaddumi, H.F., 2009. Fossils of the Harrana Fauna and the Adjacent Areas. Eternal River Museum of Natural History, Amman, Jordan, 324 p.
161Kapur, V.V. & Khosla, A., 2019. Faunal elements from the Deccan volcano‐sedimentary sequences of India: A reappraisal of biostratigraphic, palaeoecologic, and palaeobiogeographic aspects. Geological Journal, 54, 2797–2828. https://doi.org/10.1002/gj.3379
162Keutgen, N., 2018. A bioclast-based astronomical timescale for the Maastrichtian in the type area (southeast Netherlands, northeast Belgium) and stratigraphic implications: the legacy of P.J. Felder. Netherlands Journal of Geosciences, 97, 229–260. https://doi.org/10.1017/njg.2018.15
163Khalloufi, B., Brito, P.M., Cavin, L. & Dutheil, D., 2017. Revue des ichthyofaunes mésozoïques et cénozoïques marocaines. Mémoires de la Société géologique de France, 180, 167–248.
164Kocsis, Á.T. & Raja, N.B., 2021. chronosphere: Earth System History Variables. R package version 0.4.1 ed.
165Kocsis, L., Ounis, A., Chaabani, F. & Salah, N.M., 2013. Paleoenvironmental conditions and strontium isotope stratigraphy in the Paleogene Gafsa Basin (Tunisia) deduced from geochemical analyses of phosphatic fossils. International Journal of Earth Sciences, 102, 1111–1129. https://doi.org/10.1007/s00531-012-0845-5
166Kovalchuk, O.M., Świdnicka, E. & Stefaniak, K., 2021. Early Miocene ponyfishes (Acanthuriformes, Leiognathidae) of the Carpathian Basin. Paleontological Journal, 55, 421–428. https://doi.org/10.1134/S0031030121040092
167López-Arbarello, A., Arratia, G. & Tunik, M.A., 2003. Saldenoichthys remotus gen. et sp. nov. (Teleostei, Perciformes) and other acanthomorph remains from the Maastrichtian Saldeño Formation (Mendoza, Argentina). Fossil Record, 6, 161–168. https://doi.org/10.5194/fr-6-161-2003
168López-Palomino, I., González-Rodríguez, K.A., Schultze, H.-P., Palma-Ramírez, A. & Contreras-Cruz, D., 2021. Ammonites from the La Negra Facies (El Doctor Formation, late Albian) of the Muhi Quarry, Hidalgo, central Mexico. Journal of South American Earth Sciences, 111, 103400. https://doi.org/10.1016/j.jsames.2021.103400
169Lucas, S.G. & Heckert, A.B., 2001. Olson’s gap: A global hiatus in the record of Middle Permian tetrapods. Journal of Vertebrate Paleontology, 21(S3), 75A.
170Madden, C.T., Whitmore, F.C., Jr., Schmidt, D.L. & Naqvi, I.M., 1995. The Umm Himar Formation (Paleocene) of Saudi Arabia and associated strata: stratigraphy, vertebrate fauna, and paleoenvironment. U. S. Geological Survey Bulletin, 2093-A, A1–A19.
171Mayr, G., Scofield, R.P., De Pietri, V.L. & Tennyson, A.J.D., 2017. A Paleocene penguin from New Zealand substantiates multiple origins of gigantism in Sphenisciformes. Nature Communications, 8, 1927. https://doi.org/10.1038/s41467-017-01959-6
172Micklich, N., Bannikov, A. & Yabumoto, Y., 2017. First record of ponyfishes (Perciformes: Leiognathidae) from the Oligocene of the Grube Unterfeld ("Frauenweiler") clay pit. Paläontologische Zeitschrift, 91, 375–398. https://doi.org/10.1007/s12542-017-0340-1
173Miller, E.C. & Román-Palacios, C., 2021. Evolutionary time best explains the latitudinal diversity gradient of living freshwater fish diversity. Global Ecology and Biogeography, 30, 749–763. https://doi.org/10.1111/geb.13253
174Miya, M., Friedman, M., Satoh, T.O., Takeshima, H., Sado, T., Iwasaki, W., Yamanoue, Y., Nakatani, M., Mabuchi, K., Inoue, J.G., Poulsen, J.Y., Fukunaga, T., Sato, Y. & Nishida, M., 2013. Evolutionary origin of the Scombridae (tunas and mackerels): members of a Paleogene adaptive radiation with 14 other pelagic fish families. PLoS ONE, 8, e73535. https://doi.org/10.1371/journal.pone.0073535
175Monsch, K.A. & Bannikov, A.F., 2012. New taxonomic synopses and revision of the scombroid fishes (Scombroidei, Perciformes), including billfishes, from the Cenozoic of the territories of the former USSR. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 102, 253–300. https://doi.org/10.1017/S1755691011010085
176Murray, A.M., 1996. A new Paleocene genus and species of percopsid, †Massamorichthys wilsoni (Paracanthopterygii) from Joffre Bridge, Alberta, Canada. Journal of Vertebrate Paleontology, 16, 642–652. https://doi.org/10.1080/02724634.1996.10011354
177Murray, A.M. & Wilson, M.V.H., 1996. A new Palaeocene genus and species of percopsiform (Teleostei: Paracanthopterygii) from the Paskapoo Formation, Smoky Tower, Alberta. Canadian Journal of Earth Sciences, 33, 429–438. https://doi.org/10.1139/e96-032
178Murray, A.M., Brinkman, D.B., Newbrey, M.G. & Neuman, A.G., 2020. Earliest North American articulated freshwater acanthomorph fish (Teleostei: Percopsiformes) from Upper Cretaceous deposits of Alberta, Canada. Geological Magazine, 157, 1087–1096. https://doi.org/10.1017/S0016756819001328
179Near, T.J., Dornburg, A., Eytan, R.I., Keck, B.P., Smith, W.L., Kuhn, K.L., Moore, J.A., Price, S.A., Burbrink, F.T., Friedman, M. & Wainwright, P.C., 2013. Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. Proceedings of the National Academy of Sciences of the USA, 110, 12738–12743. https://doi.org/10.1073/pnas.1304661110
180Neuman, A.G. & Brinkman, D.B., 2005. Fishes of the fluvial beds. In Currie, P.J. & Kopplelhus, E.B. (eds), Dinosaur Provincial Park, A Spectacular Ancient Ecosystem Revealed. Indiana University Press, Bloomington, Indiana, 167–185.
181Nøhr-Hansen, H. & Sheldon, E., 2000. Palyno- and nannostratigraphic dating of the marine Paleocene succession in the Nuussuaq Basin, West Greenland. GFF, 122, 115–116. https://doi.org/10.1080/11035890001221115
182Nolf, D., 1985. Otolithi Piscium. Gustav Fischer Verlag, Stuttgart, Handbook of Paleoichthyology, 10, 145 p.
183Nolf, D., 2013. The Diversity of Fish Otoliths, Past and Present. Royal Belgian Institute of Natural Sciences, Brussels, 222 p.
184O’Leary, M.A., Bouaré, M.L., Claeson, K.M., Heilbronn, K., Hill, R.V., Mccartney, J., Sessa, J.A., Sissoko, F., Tapinala, L., Wheeler, E. & Roberts, E.M., 2019. Stratigraphy and paleobiology of the Upper Cretaceous-lower Paleogene sediments from the trans-Saharan seaway in Mali. Bulletin of the American Museum of Natural History, 436, 1–177. https://doi.org/10.1206/0003-0090.436.1.1
185Pastana, M.N.L., Johnson, G.D. & Datovo, A., 2021. Comprehensive phenotypic phylogenetic analysis supports the monophyly of stromateiform fishes (Teleostei: Percomorphacea). Zoological Journal of the Linnean Society, 195, 841–963. https://doi.org/10.1093/zoolinnean/zlab058
186Patterson, C., 1964. A review of Mesozoic acanthopterygian fishes, with special reference to those of the English Chalk. Philosophical Transactions of the Royal Society of London B, 247, 213–482. https://doi.org/10.1098/rstb.1964.0003
187Patterson, C., 1968. The caudal skeleton in Mesozoic acanthopterygian fishes. Bulletin of the British Museum (Natural History) Geology, 17, 47–102.
188Patterson, C., 1993. An overview of the early fossil record of acanthomorphs. Bulletin of Marine Science, 52, 29–59.
189Pedroza, V., Le Roux, J.P., Gutiérrez, N.M. & Vicencio, V.E., 2017. Stratigraphy, sedimentology, and geothermal reservoir potential of the volcaniclastic Cura-Mallín succession at Lonquimay, Chile. Journal of South American Earth Sciences, 77, 1–20. https://doi.org/10.1016/j.jsames.2017.04.011
190Price, S.A., Schmitz, L., Oufiero, C.E., Eytan, R.I., Dornburg, A., Smith, W.L., Friedman, M., Near, T.J. & Wainwright, P.C., 2014. Two waves of colonization straddling the K–Pg boundary formed the modern reef fish fauna. Proceedings of the Royal Society B, 281, 20140321. https://doi.org/10.1098/rspb.2014.0321
191Priem, F., 1898. Sur la faune ichthyologique des Assises Montiennes du Bassin de Paris et en particulier sur Pseudolates heberti Gervais sp. Bulletin de la Société géologique de France, 3e série, 26, 399–412.
192Priem, F., 1907. Sur des vertébrés de l’Eocène d’Egypte et Tunisie. Bulletin de la Société géologique de France, 4e série, 7, 412–419.
193Priem, F., 1908. Étude des Poissons fossiles du bassin parisien. Masson et Cie, Paris, Publications des Annales de Paléontologie, 144 p.
194Prum, R.O., Berv, J.S., Dornburg, A., Field, D.J., Townsend, J.P., Lemmon, E.M. & Lemmon, A.R., 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature, 526, 569–573. https://doi.org/10.1038/nature15697
195Raja, N.B., Dunne, E.M., Matiwane, A., Ming Kahn, T., Nätscher, P.S., Ghilardi, A.M. & Chattopadhyay, D., 2022. Colonial history and global economics distort our understanding of deep-time biodiversity. Nature Ecology & Evolution, 6, 145–154. https://doi.org/10.1038/s41559-021-01608-8
196Rapp, W.F., Jr., 1946. Check list of the fossil fishes of New Jersey. Journal of Paleontology, 20, 510–513.
197Reguero, M.A., 2019. Antarctic Paleontological Heritage: Late Cretaceous Paleogene vertebrates from Seymour (Marambio) Island, Antarctic Peninsula. Advances in Polar Science, 30, 328–355.
198Ribeiro, E., Davis, A. M., Rivero-Vega, R. A., Ortí, G. & Betancur-R, R., 2018. Post-Cretaceous bursts of evolution along the benthic-pelagic axis in marine fishes. Proceedings of the Royal Society B, 285, 20182010. https://doi.org/10.1098/rspb.2018.2010
199Robaszynski, F., 2006. Maastrichtian. Geologica Belgica, 9, 63–72.
200Rosen, D.E. & Patterson, C., 1969. The structure and relationships of the paracanthopterygian fishes. Bulletin of the American Museum of Natural History, 141, 357–474.
201Rosenkrantz, A., 1970. Marine Upper Cretaceous and lowermost Tertiary deposits in West Greenland. Bulletin of the Geological Society of Denmark, 19, 406–453.
202Sallan, L.C. & Friedman, M., 2012. Heads or tails: staged diversification in vertebrate evolutionary radiations. Proceedings of the Royal Society B, 279, 2025–2032. https://doi.org/10.1098/rspb.2011.2454
203Samant, B., Mohabey, D.M., Srivastava, P. & Thakre, D., 2014. Palynology and clay mineralogy of the Deccan volcanic associated sediments of Saurashtra, Gujarat: Age and paleoenvironments. Journal of Earth System Science, 123, 219–232. https://doi.org/10.1007/s12040-013-0390-z
204Santini, F. & Tyler, J.C., 2003. A phylogeny of the families of fossil and extant tetraodontiform fishes (Acanthomorpha, Tetraodontiformes), Upper Cretaceous to Recent. Zoological Journal of the Linnean Society, 139, 565–617. https://doi.org/10.1111/j.1096-3642.2003.00088.x
205Schaeffer, B. & Simpson, G.G., 1947. An Eocene serranid from Patagonia. American Museum Novitates, 1331, 1–9.
206Schrøder, A.E., Rasmussen, J.A., Møller, P.R. & Carnevale, G., 2022. A new beardfish (Teleostei, Polymixiiformes) from the Eocene Fur Formation, Denmark. Journal of Vertebrate Paleontology, e2142914. https://doi.org/10.1080/02724634.2022.2142914
207Schwarzhans, W. & Milàn, J. 2017. After the disaster: Bony fish remains (mostly otoliths) from the K/Pg boundary section at Stevns Klint, Denmark, reveal consistency with teleost faunas from later Danian and Selandian strata. Bulletin of the Geological Society of Denmark, 65, 59–74. https://doi.org/10.37570/bgsd-2017-65-05
208Sempere, T., Butler, R.F., Richards, D.R., Marshall, L.G., Sharp, W. & Swisher, C.C., III, 1997. Stratigraphy and chronology of Upper Cretaceous–lower Paleogene strata in Bolivia and northwest Argentina. GSA Bulletin, 109, 709–727. https://doi.org/10.1130/0016-7606(1997)109<0709:SACOUC>2.3.CO;2
209Sheldon, E., Gravesen, P. & Nøhr-Hansen, H., 2012. Geology of the Femern Bælt area between Denmark and Germany. GEUS Bulletin, 26, 13–16. https://doi.org/10.34194/geusb.v26.4740
210Sibert, E.C. & Norris, R.D., 2015. New Age of Fishes initiated by the Cretaceous-Paleogene mass extinction. Proceedings of the National Academy of Sciences of the USA, 112, 8537–8542. https://doi.org/10.1073/pnas.1504985112
211Sibert, E.C., Friedman, M., Hull, P., Hunt, G. & Norris, R.D., 2018. Two pulses of morphological diversification in Pacific pelagic fishes following the Cretaceous-Palaeogene mass extinction. Proceedings of the Royal Society B, 285, 20181194. https://doi.org/10.1098/rspb.2018.1194
212Siegfried, P., 1954. Die Fisch-Fauna des Westfälischen Ober-Senons. Palaeontographica Abteilung A, 106, 1–36.
213Smithson, T.R., Wood, S.P., Marshall, J.E.A. & Clack, J.A., 2012. Earliest Carboniferous tetrapod and arthropod faunas from Scotland populate Romer’s Gap. Proceedings of the National Academy of Sciences of the USA, 109, 4532–4537. https://doi.org/10.1073/pnas.1117332109
214Solé, F., Noiret, C., Desmares, D., Adnet, S., Taverne, L., De Putter, T., Mees, F., Yans, J., Steeman, T., Louwye, S., Folie, A., Stevens, N.J., Gunnell, G.F., Baudet, D., Yaya, N.K. & Smith T., 2019. Reassessment of historical sections from the Paleogene marine margin of the Congo Basin reveals an almost complete absence of Danian deposits. Geoscience Frontiers, 10, 1039–1063. https://doi.org/10.1016/j.gsf.2018.06.002
215Sorbini, L., 1978. New fish bed localities of latest Campanian age (Lecce - South Italy - A preliminary paper). Bollettino del Museo Civico di Storia Naturale di Verona, 5, 607–608.
216Sorbini, L., 1981. The Cretaceous fishes of Nardò. 1°. Order Gasterosteiformes (Pisces). Bollettino del Museo Civico di Storia Naturale di Verona, 8, 1–27.
217Sorbini, L. & Bannikov, A.F., 1991. The Cretaceous fishes of Nardò. 2°. An enigmatic spiny-rayed fish. Bollettino della Società Paleontologica Italiana, 30, 239–249.
218Sorbini, L. & Bannikov, A.F., 1996. A new percopsiform-like paracanthopterygian fish from the early Paleocene of Trieste Province, north-eastern Italy. Atti del Museo Civico di Storia Naturale di Trieste, 47, 309–317.
219Sorbini, C. & Sorbini, L., 1999. The Cretaceous fishes of Nardò. 10°. Nardovelifer altipinnis gen. nov. et sp. nov. (Teleostei, Lampridiformes, Veliferidae). Studi e Ricerche sui Giacimenti Terziari di Bolca, 8, 11–27.
220Speijer, R.P., Pälike, H., Hollis, C.J., Hooker, J.J. & Ogg, J.G., 2020. The Paleogene period. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D. & Ogg, G.M. (eds), Geologic Time Scale 2020. Elsevier, Amsterdam, 1087–1140. https://doi.org/10.1016/B978-0-12-824360-2.00028-0
221Sprain, C.J., Renne, P.R., Wilson, G.P. & Clemens, W.A., 2015. High-resolution chronostratigraphy of the terrestrial Cretaceous-Paleogene transition and recovery interval in the Hell Creek region, Montana. GSA Bulletin, 127, 393–409. https://doi.org/10.1130/B31076.1
222Stewart, J.D., 1996. Cretaceous acanthomorphs of North America. In Arratia, G. & Viohl, G. (eds), Mesozoic Fishes - Systematics and Paleoecology. Dr. Friedrich Pfeil, Munich, 383–394.
223Sugarman, P.J., Miller, K.G., Bukry, K.G. & Feigenson, M.D., 1995. Uppermost Campanian–Maestrichtian strontium isotopic, biostratigraphic, and sequence stratigraphic framework of the New Jersey Coastal Plain. GSA Bulletin, 107, 19–37. https://doi.org/10.1130/0016-7606(1995)107<0019:UCMSIB>2.3.CO;2
224Taverne, L., 2003. Les poissons crétacés de Nardò. 14°. Lissoberyx pugliensis sp. nov. (Teleostei, Beryciformes, Trachichthyidae). Bollettino del Museo Civico di Storia Naturale di Verona, Geologia Paleontologia Preistoria, 27, 3–13.
225Taverne, L., 2004. Les poissons crétacés de Nardò. 17°. Aspesaipichthys cavaensis gen. et sp. nov. (Teleostei, Acanthomorpha, Aipichthyoidea). Bollettino del Museo Civico di Storia Naturale di Verona, Geologia Paleontologia Preistoria, 28, 3–15.
226Taverne, L., 2005. Les poissons crétacés de Nardò. 21. Ophidercetis italiensis gen. et sp. nov. (Teleostei, Aulopiformes, Dercetidae). Une solution ostéologique au problème des genres Dercetis et Benthesikyme (=Leptotrachelus). Bollettino Museo Civico di Storia Naturale di Verona, Geologia Paleontologia Preistoria, 29, 55–79.
227Taverne, L., 2010. Les poissons crétacés de Nardò. 32°. Trois nouveaux Perciformes : Zorzinperca weverberghi gen. et sp. nov., Johnsonperca annavaccarii gen. et sp. nov. et Bannikovperca apula gen. et sp. nov. (Teleostei, Percomorpha). Bollettino del Museo Civico di Storia Naturale di Verona, Geologia Paleontologia Preistoria, 34, 51–80.
228Taverne, L., 2014. Les poissons crétacés de Nardò. 37. Compléments à l’étude de Bannikovperca apula Taverne, 2010. Bollettino del Museo Civico di Storia Naturale di Verona, Geologia Paleontologia Preistoria, 38, 17–26.
229Troyer, E.M., Betancur-R, R., Hughes, L.C., Westneat, M.W., Carnevale, G., White, W. T., Pogonoski, J.J., Tyler, J.C., Baldwin, C.C., Ortí, G., Brinkworth, A., Clavel, J. & Arcila, D., 2022. The impact of paleoclimatic changes on body size evolution in marine fishes. Proceedings of the National Academy of Sciences of the USA, 119, e2122486119. https://doi.org/10.1073/pnas.2122486119
230Tunik, M.A., Concheyro, A., Ottone, E.G. & Aguirre-Urreta, B., 2004. Paleontología de la Formación Saldeño (Maastrichtiano), Alta Cordillera de Mendoza, Argentina. Ameghiniana, 41, 143–160.
231Turner, S., Blieck, A. & Nowlan, G.S., 2004. Vertebrates (agnathans and gnathostomes). In Webby, B.D., Paris, F., Droser, M.L. & Percival, I.G. (eds), The Great Ordovician Biodiversification Event. Columbia University Press, New York, 327–335.
232Tyler, J.C. & Sorbini, C., 1996. New superfamily and three new families of tetraodontiform fishes from the Upper Cretaceous: the earliest and most morphologically primitive plectognaths. Smithsonian Contributions to Paleobiology, 82, 1–59. https://doi.org/10.5479/si.00810266.82.1
233Tyler, J.C., Bronzi, P. & Ghiandoni, A., 2000. The Cretaceous fishes of Nardò 11°. A new genus and species of Zeiformes, Cretazeus rinaldii, the earliest record for the order. Bollettino del Museo Civico di Storia Naturale di Verona, Geologia Paleontologia Preistoria, 24, 11–28.
234Upham, N.S., Esselstyn, J.A. & Jetz, W., 2019. Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biology, 17, e3000494. https://doi.org/10.1371/journal.pbio.3000494
235Varela, S. & Rothkugel, K.S., 2018. mapast: combine paleogeography and paleobiodiversity.
236Wainwright, P.C. & Longo, S.J., 2017. Functional innovations and the conquest of the oceans by acanthomorph fishes. Current Biology, 27, R550–R557. https://doi.org/10.1016/j.cub.2017.03.044
237Wedmann, S., Uhl, D., Lehmann, T., Garrouste, R., Nel, A., Gomez, B., Smith, K. & Schaal, S.F.K., 2018. The Konservat-Lagerstätte Menat (Paleocene; France) – an overview and new insights. Geologica Acta, 16, 189–213. https://doi.org/10.1344/GeologicaActa2018.16.2.5
238Westerhold, T., Röhl, U., Mccarren, H.K. & Zachos, J.C., 2009. Latest on the absolute age of the Paleocene–Eocene Thermal Maximum (PETM): New insights from exact stratigraphic position of key ash layers +19 and −17. Earth and Planetary Science Letters, 287, 412–419. https://doi.org/10.1016/j.epsl.2009.08.027
239Woodward, A.S., 1901. Catalogue of the Fossil Fishes in the British Museum (Natural History). Part IV. Containing the Actinopterygian Teleostomi of the Suborders Isospondyli (in part), Ostariophysi, Apodes, Percesoces, Hemibranchii, Acanthopterygii, and Acanthini. Trustees of the British Museum (Natural History), London, 636 p. https://doi.org/10.5962/bhl.title.61854
240Woodward, A.S., 1908. On some fish-remains from the Lameta Beds at Dongargaon, Central Provinces. Palaeontologia Indica, 3.
241Ye, S. & Peters, S.E., 2023. Bedrock geological map predictions for Phanerozoic fossil occurrences. Paleobiology. https://doi.org/10.1017/pab.2022.46
242Zinsmeister, W.J., 1998. Discovery of fish mortality horizon at the K-T Boundary on Seymour Island: Re-evaluation of events at the end of the Cretaceous. Journal of Paleontology, 72, 556–571. https://doi.org/10.1017/S0022336000024331
243Manuscript received 09.02.2023, accepted in revised form 24.03.2023, available online 16.06.2023.






