- Home
- Volume 26 (2023)
- number 1-2
- An exceptional concentration of marine fossils associated with wood-fall in the Terhagen Member (Boom Formation; Schelle, Belgium), Rupelian of the southern North Sea Basin
View(s): 5042 (37 ULiège)
Download(s): 1905 (10 ULiège)
An exceptional concentration of marine fossils associated with wood-fall in the Terhagen Member (Boom Formation; Schelle, Belgium), Rupelian of the southern North Sea Basin

Abstract
A large fragment of driftwood was discovered in the marine Terhagen Member (Boom Formation, NP23) at Schelle (Belgium), representing the first well-documented case of wood-fall in the Rupelian of the North Sea Basin. This trunk with a side-branch, identified as Cupressinoxylon sp. (Cupressaceae), caused a large irregularity on the sea bottom, creating a unique microenvironment which allowed colonization by some taxa virtually absent elsewhere in the Boom Formation. The fossils were further concentrated in a silty lens against the trunk by the effects of prolonged wave-driven turbulence. This lens comprised a large set of compartmental plates of the turtle barnacle Protochelonibia hermani Gale sp. nov., possibly part of a single colony originally attached to a turtle. The material includes the best preserved plates of Protochelonibia known to date, yielding new information on the construction of its shell. Additionally, a disarticulated tooth set of 154 teeth of Carcharias contortidens (Agassiz, 1843) was found, the first such discovery in more than 100 years. An articulated dentition of this taxon, initially studied by Leriche (1910), is refigured herein. Some very rare valves of the bivalve Palliolum permistum (Beyrich, 1848) are identified and the gastropod Amblyacrum cf. roemeri (von Koenen, 1867) is reported here for the first time from the Belgian Rupelian. The teleost otolith assemblage comprises ca 30,000 specimens belonging to 11 species only, of which Trachurus reineckei Hoedemakers sp. nov. is new to science and Myoxocephalus primas (Koken, 1891) and Capros siccus Schwarzhans, 2008 are new for the Belgian Rupelian. The new species represents the earliest record of the thermophilic genus Trachurus in the Oligocene of the North Sea Basin. Liparis minusculus Nolf, 1977 is synonymized with Myoxocephalus primas, whereas Erythrocles ohei Schwarzhans, 1994 is transferred to the genus Trachurus.
Table of content
1https://zoobank.org/urn:lsid:zoobank.org:pub:A35B27E5-A745-4BBA-AA8F-E7F01C684B0C
2This paper is dedicated to the memory of Dr Jacques Herman (1948–2022), for his longstanding contributions to the knowledge of extinct and extant elasmobranch fishes.
1. Introduction
3The essentially clayey Boom Formation was deposited in an open marine environment at the southern border of the North Sea Basin during the Rupelian (early Oligocene). It is one of the thickest and most intensively studied stratigraphic units of Flanders (northern Belgium) and is of considerable scientific and industrial interest. The Boom Clay is studied in the subsurface as a potential host rock for nuclear waste. For more than two hundred years, the clay has been excavated for the brick and roof tile industry in both the Rupel (province of Antwerp) and Waasland regions (province of Oost-Vlaanderen); numerous abandoned clay pits shape the landscape today. The thick clay deposits around the Rupel River are the historical stratotype for the international Rupelian stage (Van Simaeys & Vandenberghe, 2006; Speijer et al., 2020). However, the Rupelian Global Boundary Stratotype Section (GSSP) in the Italian Massignano section is about 1.5 to 2 million years older than the base of the historical stratotype (Speijer et al., 2020). The Boom Formation is known for its diverse macrofauna, including elasmobranchs (e.g. Van Beneden, 1860; Leriche, 1910; Steurbaut & Herman, 1978; Hovestadt & Hovestadt-Euler, 1995), teleost fishes (e.g. Nolf, 1977, Steurbaut & Herman, 1978, Taverne et al., 2006) and molluscs (e.g. Nyst, 1835, 1845; De Koninck, 1838; Vincent, 1930; Glibert, 1955, 1957; Marquet, 2010, 2016). In the 19th and early 20th centuries, the clay was generally extracted manually, resulting in the discovery of numerous spectacular fossils, including associated elasmobranch and teleostean remains. From the Boom Formation, Leriche (1910) mentioned an articulated dentition of Carcharias contortidens (Agassiz, 1843), commonly listed in the palaeontological literature with the specific name of Carcharias acutissima (Agassiz, 1843) and refigured herein. Although macrofossils in the Boom Formation are generally sparsely distributed, some levels proved to contain slightly higher concentrations of isolated fossils (Steurbaut & Herman, 1978).
4In 2008, an exceptional discovery was made in the Ceulemans clay pit, located in the municipality of Schelle (Fig. 1). A large fragment of driftwood, about three meters long, was found on top of the pink ‘R horizon’ in the Terhagen Member (Boom Formation). It was surrounded by a small silty lens, containing a rich assemblage of elasmobranch and teleost remains, molluscs and barnacles. Many of these finds are very rare; the irregularity caused by the driftwood on the seabed provided a unique opportunity to preserve a palaeontological biocoenosis of species that lived together in the vicinity of the sunken trunk during a constrained period of time. Some species are new to science. In the present paper, a general overview of the recorded fossil assemblage is presented and ecological considerations are made based on the recorded invertebrates and teleost otoliths.
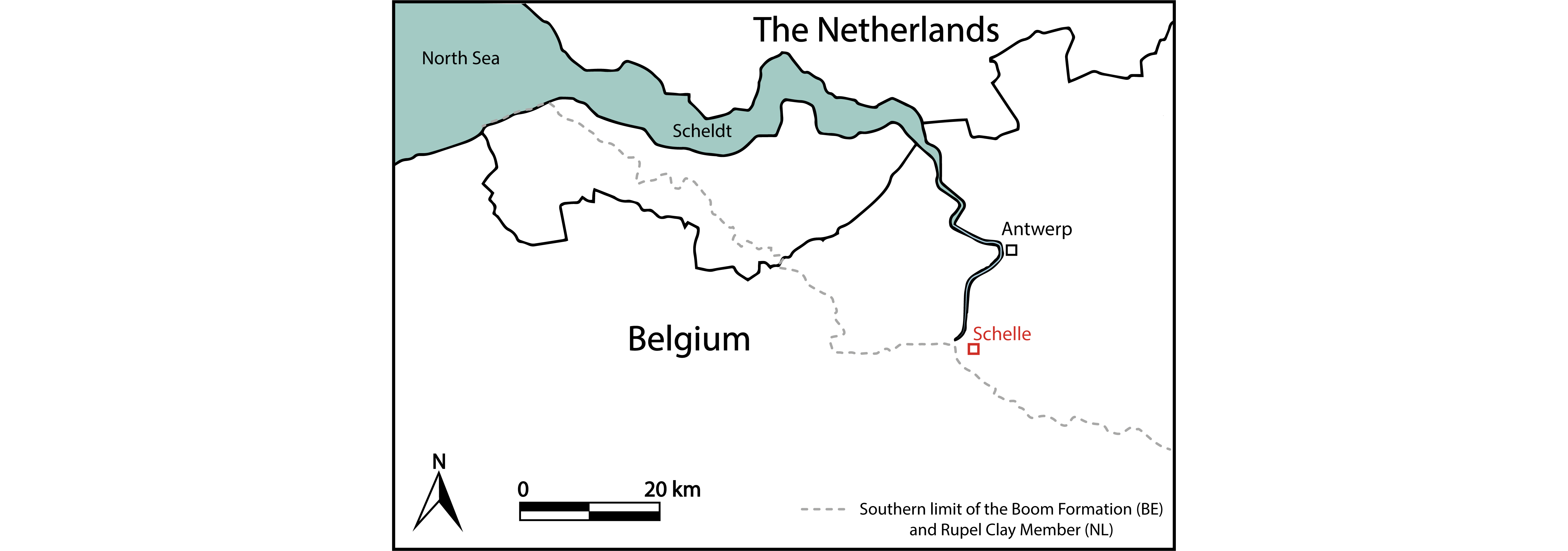
Figure 1. Location of the Ceulemans clay pit at Schelle (51°07'01.22'' N, 4°21'21.30'' E). The southern limit of the Boom Formation is based on Vis et al. (2016) and Vandenberghe (2017). The former also mapped the southern limit of the Rupel Clay Member, the Dutch equivalent of the Boom Formation.
2. Geological background
5A strong global cooling occurred around the NP21/NP22 biochron boundary (Prothero et al., 2003), about 200,000 years after the start of the Oligocene (Bohaty et al., 2012; Hutchinson et al., 2021). As the Antarctic icecap was rapidly expanding, the global sea level dropped several tens of meters (Miller et al., 1991, 2005). In the Antwerp–Rupel area (northern Belgium), this global sea-level drop is reflected in a regression marked by an erosive surface (Vandenberghe et al., 2003; Vandenberghe, 2017). Afterwards clayey to silty lagoonal sand (Wintham Silt?) was deposited in the study area, covered by the shallow marine sand of the Ruisbroek Member (lowstand systems tract) during biochron NP22 (Steurbaut, 1992; Vandenberghe et al., 2003). At the time of the NP22/NP23 biochron boundary, sedimentation ceased and the sea bottom became cemented, probably due to the upwelling of phosphate. When transgression resumed, this apatite-hardground was broken up, slightly reworked and covered by the silty clays of the Boom Formation (Vandenberghe et al., 2002; Herman & Marquet, 2012; Herman et al., 2013).
6The Boom Formation is the historical unit stratotype of the Rupelian. It consists of laterally continuous, banded layers with rhythmic variations in silt/clay content, carbonate and organic material (Vandenberghe, 1978), representing glacio-eustatic sea-level oscillations (Vandenberghe et al., 1997; 1998). Abels et al. (2007) showed that these glacio-eustatic sea-level oscillations were largely driven by the 41 ka obliquity cycle, with a secondary influence of the 100 and 405 ka eccentricity. Lower frequency grain-size cycles could also be influenced by vertical tectonics in and around the basin (Vandenberghe & Mertens, 2013). Lithostratigraphically, the Boom Formation is subdivided into the very silty Belsele-Waas, the grey Terhagen, the black Putte and the silty Boeretang members, with the latter being only present in the Campine subsurface, where the Boom Formation can reach a thickness of up to 140 m (Vandenberghe et al., 2001, 2014). The Terhagen Member, of further interest in the present paper, was deposited within calcareous nannoplankton zone NP23 (Steurbaut, 1992, fig. 8).
7In the past decades, the sequence stratigraphy of the Boom Formation has been widely established, and multiple third-order eustatic sequences have been recognized within its grain-size distributions (Vandenberghe et al., 1998, 2004). Starting from the base of the Boom Formation (the phosphate bed), a grain-size fining upward trend is observed throughout the Belsele-Waas and lower Terhagen members. Maximum fining is reached within the pink R-horizon (Terhagen Member) (Fig. 2), which is consequently interpreted as the first maximum flooding surface, with the deepest bathymetry of the Terhagen Member (Vandenberghe et al., 1998, 2014). Above, the sediment coarsens slightly upwards until the very silty, fine sandy Double Band (DB), which is a key level in the Putte Member. Studying benthic foraminifera in these successions, De Man & Van Simaeys (2004) estimated water depths of around 100 m and noted that deposition occurred in a normal marine shelf environment with open marine connections to the oceanic realm. Moreover, they mainly recovered cold- to cold-temperate taxa, estimating that the bottom water palaeotemperature always remained between 5 and 10 °C (De Man & Van Simaeys, 2004, fig. 4). Vandenberghe et al. (2014) suggested that water depths varied between 50 m in the silty clay layers and 150 m in the pure clay layers. The sea bottom was periodically within and beyond the wave turbulence base and silty beds formed when the wave turbulence reached the sea bottom.

Figure 2. Stratigraphy of the Ceulemans clay pit (Schelle) in June 2008. The Putte and Terhagen members are indicated, together with some stratigraphic reference levels (S = Septaria levels; DB = Double band; R = pink R-level; after Vandenberghe et al., 2014). The sunken trunk lay on top of the R-horizon in the Terhagen Member (indicated by the arrow).
3. Location and stratigraphy of the clay pit
8The Ceulemans clay pit at Schelle (51°07'01.22'' N, 4°21'21.30'' E), formerly known as the Steenbakkerij Damman, is located in the province of Antwerp, northwestern Belgium (Fig. 1). In this quarry, both the Terhagen Member and the lower part of the Putte Member are exposed. The fossil assemblage was encountered on top of the pink R-horizon (base of bed 22 sensu Vandenberghe et al., 2014, fig. 12) in the Terhagen Member (Fig. 2), which can be attributed to the early Rupelian NSO3 dinoflagellate biozone (between 31.6–30.9 Ma) of Van Simaeys et al. (2005) and the NP23 calcareous nannoplankton zone (Steurbaut, 1992; Vandenberghe et al., 2014), and is dated at approximately 31.5 Ma (based on Lagrou et al., 2004 and Speijer, et al., 2020).
4. Material and methods
9In June 2008, a large fragment of driftwood was observed on the surface of the clay pit; unfortunately it had already been partially shattered by the dredger. The large trunk, measuring around 3 m in length and oriented in a north-south direction, was carefully excavated (Plate 1). A side-branch of ca 80 cm in length was oriented in a south-east direction. Enclosed between the main trunk and the side-branch, a locally restricted fossiliferous lens of more coarse, silty sediment was encountered. The entire lens, almost 300 kg in mass, was sampled in plastic bags. The silty sediment was then broken into smaller pieces, dried and subsequently wet-sieved through mesh sizes of 2, 1 and 0.3 mm. This whole process took around three months. The residues were then handpicked for fossils (Fig. 3). A sediment sample is stored at the Geological Survey of Belgium to allow micropalaeontological analyses in the future.
10The fossil material figured in this paper is housed in the collections of the Royal Belgian Institute of Natural Sciences (RBINS; Brussels) under registration numbers IRSNB P 10299 to P 10349, 7705 to 7736 and b9669–9670 (see Table 1). In addition, some representative specimens of Cocculina reineckei and Palliolum permistum have been donated to the palaeontological collection of Senckenberg Forschungsinstitut (Frankfurt, Germany).

Figure 3. Fossil accumulation after sieving, yielding wood fragments, shark teeth, mollusc debris and otoliths, ready to be handpicked.
5. Palaeontology
5.1. Plantae (JS & VK)
11Systematics follows Christenhusz et al. (2011).
12Subclass Pinidae Cronquist, Takhtajan & Zimmermann, 1966 (= conifers)
13Order Cupressales Link, 1829
14Family Cupressaceae Gray, 1822
15Genus Cupressinoxylon Göppert, 1850
16Cupressinoxylon sp.
17Plate 2.A–F (Sample: IRSNB b9669) and Fig. 4 (IRSNB b9670)
18By the absence of (1) normal resin canals and spirals on tracheid walls, and the presence of (2) rather abundant axial parenchyma, (3) uniseriate abietoid pitting on radial tracheid walls, and (4) cupressoid cross-field pitting (Plate 2), we can confidently assign the wood to the family Cupressaceae (see Teodoridis & Sakala, 2008, p. 300), more precisely to the Cupressaceae sensu stricto (see Sakala, 2003). According to the key presented by Vaudois & Privé (1971), (1) the absence of both juniperoid and callitroid thickenings, (2) the regular presence of axial parenchyma with smooth transverse end walls, (3) cupressoid cross-field pits, (4) smooth horizontal ray walls, and (5) the roundish shape of the tracheids in cross-section strongly point in the direction of the Cupressinoxylon A/Tetraclinoxylon complex. Hence, regarding its preservation, where some key features are missing or only hardly visible, we propose to leave our wood in open nomenclature and designate it as Cupressinoxylon sp. This fossil genus is regularly recorded in the Tertiary floras of Europe (e.g. Dolezych & Schneider, 2006; Kłusek, 2014).
19We used the following combination of features sensu Esteban et al. (2004): P5 (axial parenchyma with resin) and R34 (ray parenchyma with resin), which are both very conspicuous in our fossil (Plate 2A–E). Compared to similar types of modern wood, such a combination is present in Hesperocyparis (= Cupressus) bakeri Jeps., Cupressus goveniana Gordon var. goveniana, C. guadalupensis S. Watson, C. lusitanica Mill var. benthamii (Endl.) Carrière, and C. sempervirens L. (Esteban et al., 2004). Since this fragment of Rupelian driftwood does not allow to define a more specific relationship with present-day species, we refrain from a detailed comparison or illustration. Interestingly, all aforementioned species, except for C. sempervirens, are typical of today’s North American region (Román-Jordán et al., 2016).
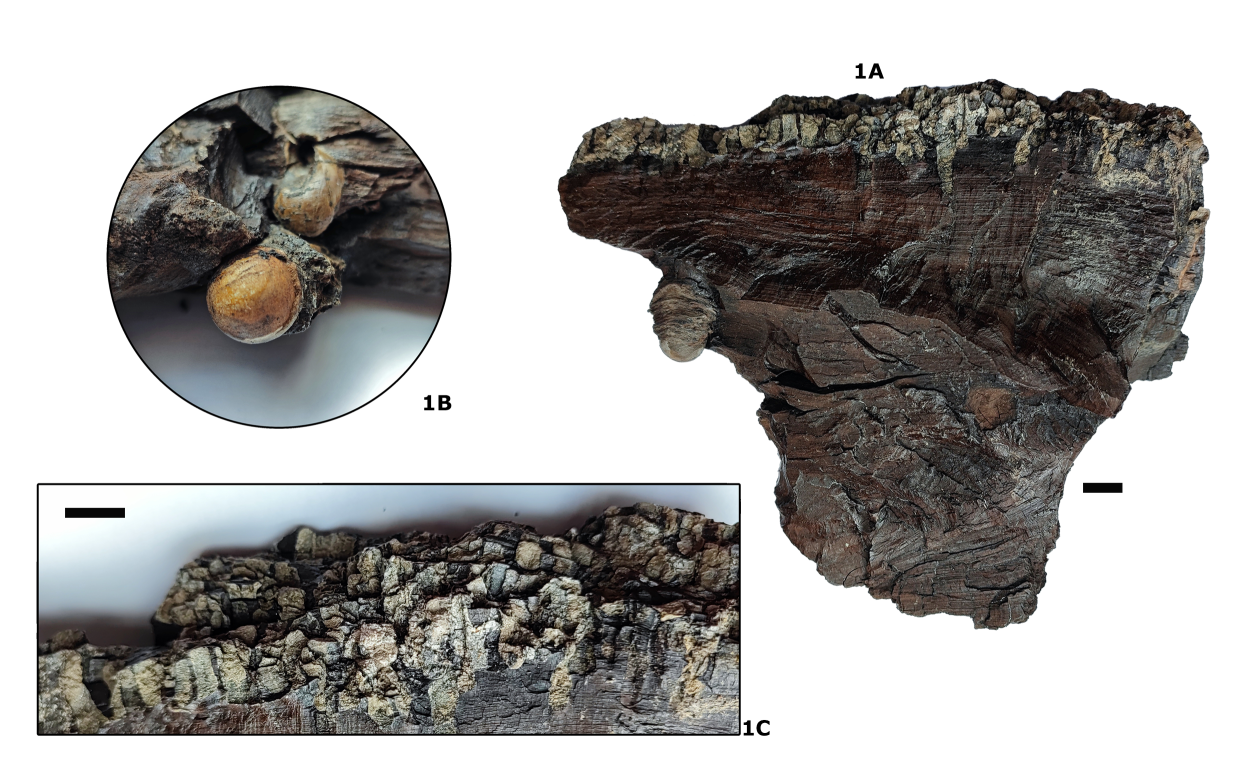
Figure 4. Cupressinoxylon sp. wood fragment (IRSNB b9670). 1A. General view. 1B. Detail of globular casts of teredinid shells (maximum diameter 12 mm). 1C. Detail of dense network of teredinid borings in the bark. Some borings penetrate the wood to depths of 6 cm. Scale bars = 10 mm.
5.2. Cirripedia (AG)
20Class Thecostraca Gruvel, 1905
21Infraclass Thoracica Darwin, 1854
22Superorder Thoracicalcarea Gale, 2015
23Order Balanomorpha Pilsbry, 1916
24Superfamily Coronuloidea Leach, 1817
25Family Chelonibiidae Pilsbry, 1916
26Remarks. Harzhauser et al. (2011) created the subfamily Protochelonibiinae to accommodate their genus Protochelonibia. However, as discussed below, the genus is remarkably close morphologically to Chelonibia itself. Furthermore, molecular studies (Pérez-Losada et al., 2014) place only two extant genera in the Chelonibiidae, Chelonibia itself and Stephanolepas Fischer, 1886, a small barrel-shaped taxon which embeds in turtles and was previously referred to the Platylepadidae (e.g. Hayashi, 2013). This genus is morphologically very different from both Chelonibia and Protochelonibia, and Zonneveld et al. (2022) do not consider it to be a chelonibiid. Therefore, I provisionally leave all three taxa as Chelonibiidae without any subfamilial designation.
27Genus Protochelonibia Harzhauser & Newman, 2011
28Diagnosis. Shell wall made up of 8 plates, unfused rostrum and rostromarginals; profile low, sides straight to weakly concave; articular sutures between compartments simple; septa thin, do not reach basal margin. Inner lamina absent.
29Type species. P. submersa Harzhauser & Newman, in Harzhauser et al., 2011.
30Included species. In addition to the type species, Chelonibia melleni Zullo, 1982, P. starnesi Perreault et al., 2022; P. hermani sp. nov. and possibly Chelonibia capellinii De Alessandri, 1895.
31Discussion. There is some uncertainty as to the number of species of Protochelonibia and precisely how these are diagnosed. Harzhauser et al. (2011) used the “depressed profile” as a diagnostic character of P. submersa as distinguishing it from the higher profile of P. capellinii (De Alessandri, 1895); however, as the type material of P. submersa is flattened by compaction (see Harzhauser et al., 2011, fig. 2)—the valves are broken and some separated—this is not really a valid argument. Harzhauser et al. (2011) also stated that the internal ribbing in P. submersa showed irregular development, as compared to the regular primary, secondary and tertiary ribbing in P. capellinii; however, a further specimen of P. capellinii figured by De Alessandri (1906, pl. 18, fig. 4) also has irregular ribbing. Both species are of early Miocene age, and were found approximately 600 km apart, no great distance for cirripedes attached to swimming sea turtles. For these reasons, P. submersa is probably a subjective junior synonym of Chelonibia capellinii De Alessandri, 1895. A decision must await redescription and better illustration of C. capellinii.
32Zullo (1982) described Chelonibia melleni from the Lower Oligocene of Mississippi, USA, on the basis of five isolated compartments, including a carina, a rostrum, two rostromarginals and a marginal or carinomarginal plate. This species was subsequently referred to Protochelonibia (Collareta & Newman, 2020), and P. melleni was later identified from the Oligocene (Rupelian) of Germany, on the basis of a crushed colony (Collareta et al., 2021) and specimens attached to a turtle (Collareta et al., 2022b) from the Rauenberg lagerstätte in southern Germany. Collareta & Newman (2020) argued that Protochelonibia differed from Chelonibia in the unfused rostrum-rostromarginals, and in the acutely triangular apices of these compartments. They also considered that P. melleni and P. submersa differed primarily in the presence of a corrugated basal compartmental margin on a single plate (Zullo, 1982, figs 1–2). Recently, Perreault et al. (2022) described a new species of Protochelonibia, P. starnesi, from the Early Oligocene of Mississippi, USA, from which they also recorded and figured a well-preserved carinomarginal of P. melleni (Perreault et al., 2022, fig. 3). This specimen provides details of the interior structure of the plate, refigured here (Fig. 5) for comparison with P. hermani sp. nov. (Plate 3.1–3, 5–7; Plate 4.1–7; Plate 5.1, 3, 5–7; see below).
33New investigations of extant Chelonibia show that the rostrum and rostrolaterals are in fact unfused in C. testudinaria forma patula (Plate 3.4, 8), although the sutures are not visible on the external surface (Plate 3.4B). Also, the apices of the rostral and rostromarginal parietes are equally acutely triangular in both C. patula and Protochelonibia (compare Plate 3.2, 5–7 with Plate 3.4, 8). Chelonibia and Protochelonibia differ most significantly in the following characters:
-
In lateral profile, Protochelonibia has a low, domed form with slightly concave sides ventrally (Plate 3.3; Plate 5.6) and straight sides dorsally (Plate 5.1C, 3C). Chelonibia, in contrast, has the form of a rounded dome with convex sides (Plate 5.2, 4), less convex ventrally.
-
The sutures between compartments are different in the two genera. In Protochelonibia the contact between the rostrum and rostromarginals (Plate 5.6) is smooth, showing only growth lines, whereas in C. testudinaria forma patula the plates articulate by fine ridges and intervening grooves (Plate 5.4). Articulations between the rostromarginals, marginals and carinomarginals are complex in C. testudinaria forma testudinaria (e.g. Plate 5.2C) which has an oval, elongated facet on the ala bearing divergent grooves and ridges for articulation with the ridged radial margin of the adjacent plate (a in Plate 5.2C). The homologous surface is poorly developed in Protochelonibia (Plate 5.5). A further surface is present in C. testudinaria forma testudinaria (Plate 5.2C) which comprises interlocking denticles, entirely absent in Protochelonibia (Plate 5.5).
-
The external surface of the parietes of Protochelonibia species (e.g. Plate 3.2B, 5B; Plate 4.1B, 3A; see also Zullo, 1982, figs 14, 5, 8, 11; Harzhauser et al., 2011, fig. 2a; Collareta et al., 2021, fig. 2) bears an apicobasal sculpture comprising either very fine ribs when worn (e.g. Plate 3.1–2) or more widely separated narrow grooves between low, weakly convex ribs (e.g. Plate 5.1B). In contrast, the external surfaces of Chelonibia testudinaria are either smooth with weak commarginal growth lines (Plate 3.4B, 8B, 9B) or weakly reticulate (Plate 5.2B).
-
The septa have very different structures in the two genera (Perreault et al., 2022). In Protochelonibia, these are thin (notably crushed in some specimens—e. g. Plate 3.5A), smooth, and do not all descend to the basal surface. In Chelonibia, the septa are robust, striated vertically, and flush with the basal margin (Plate 5.2A). The ribbing is expressed internally as interpenetrant V-shaped bundles of calcite crystals (Collareta et al., 2022a, fig. 5c).
-
In Chelonibia an inner lamina descends from the sheath to the basal margin—C. testudinaria forma testudinaria (Plate 5.2A), or as a series of flat prongs separated by U-shaped spaces in C. t. forma patula (Plate 3.4A, 9B). In Protochelonibia, the rostrum and rostromarginals lack an inner lamina, but in P. hermani sp. nov. a short, vertically striated flange descends from the base of the sheath in the marginals and carinomarginals (Plate 4.1A; Plate 5.1A). In the carinae, a series of short, striated flanges descend from the sheath (Plate 4.7), but are broken away in some specimens (e.g. Plate 5.3B).
34In conclusion, Chelonibia and Protochelonibia are closely related, and the former evolved from the latter (Harzhauser et al., 2011; Collareta et al., 2021) by developing complex articulation structures between compartments, an inner lamina and strengthened septa which all descend to the basal margin and are supported by vertical ribs. New material probably representing C. submersa from the Miocene of the Netherlands is morphologically intermediate between Oligocene Protochelonibia and Miocene to present day Chelonibia.
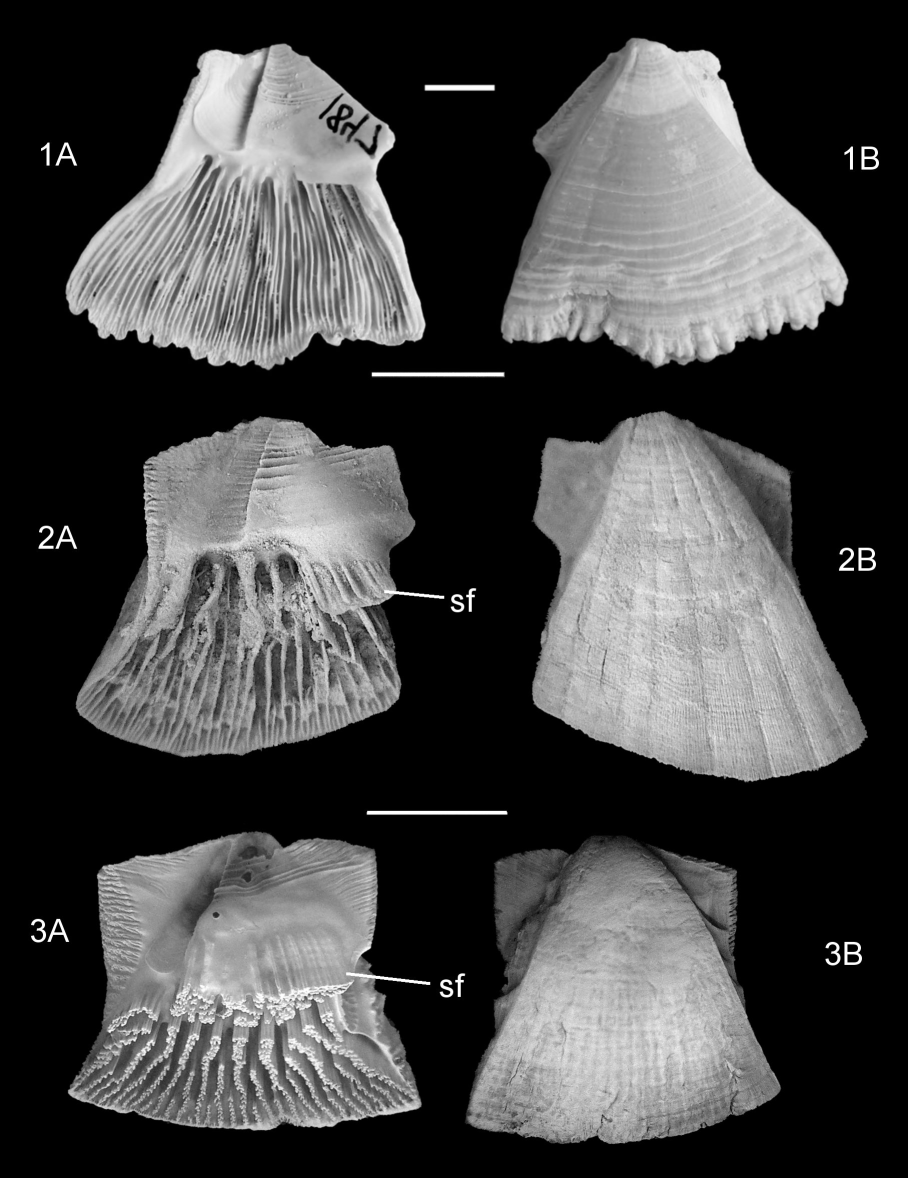
Figure 5. Comparison of the parietal structure of Protochelonibia melleni (Zullo, 1982), P. hermani sp. nov. and Chelonibia testudinaria forma testudinaria (Linnaeus, 1758). 1A. Internal, 1B, external views of carinomarginal of P. melleni, figured after Perreault et al., 2022, fig. 3. Marianna Formation, Rupelian, Smith County, Mississippi, USA (MMNS-IP 1847). 2A. Internal, 2B, external views of holotype marginal of P. hermani sp. nov. (IRSNB 7718). 3A. Internal, 3B, external views of marginal of Chelonibia testudinaria forma testudinaria. Present day, North Carolina, USA. Scale bars = 5 mm.
Note presence of a striated flange (sf) descending from the base of the sheath in P. hermani sp. nov. (2A), incorporated into the sheath in C. testudinaria (3A). Also note crenulated basal margin in P. melleni (1A, 1B), smooth in P. hermani sp. nov. (2A, 2B) and the denser internal ribbing in P. melleni (1A).
35Protochelonibia hermani Gale sp. nov.
36https://zoobank.org/urn:lsid:zoobank.org:act:E43C6AFC-5011-4A9C-9F12-C1BAF6F8A59F
37Plate 3.1–3, 5–7; Plate 4.1–7; Plate 5.1, 3, 5–7; Fig. 5.2
38Diagnosis. Protochelonibia in which the marginals, carinomarginals and carinae bear one or several striated processes descending from the base of the sheath.
39Types. The marginal plate figured here (Plate 5.1) is the holotype (IRSNB 7718), all other figured specimens are paratypes (IRSNB 7705–7717, 7719–7722).
40Material. 68 isolated compartmental plates and partially articulated individuals.
41Locus typicus. Ceulemans clay pit at Schelle, 51°07'01.22'' N, 4°21'21.30'' E.
42Stratum typicum. Silty lens associated with a tree trunk on top of pink R-horizon, Terhagen Member, Boom Formation, Rupelian (NP23), Oligocene.
43Derivatio nominis. In honour of Dr Jacques Herman (1948–2022), for his friendship and longstanding contributions to the knowledge of extinct and extant elasmobranchs.
44Description. Shell with low profile, sides flat to weakly concave (Plate 3.3), rostrum and rostromarginals unfused, united by flat articulation surface (Plate 5.6). External surfaces of compartments bear shallow, broad apicobasal ribs, separated by narrow grooves (Plate 3.2B, 5B; Plate 4.1B, 3A; Plate 5.1B, 3A, 7A); slightly eroded surfaces display fine ribbing (Plate 3.1A, 2B). Internal septa numerous, thin, smooth, not organised into well-defined sets, do not extend to basal surface (Plate 3.5A, 7; Plate 5.3B, 7). Sheaths proportionately tall (40–70% total compartment height), variable in height (compare Plate 3.6; Plate 4.4–7). Short, striated flange extends from base of sheath on marginals and carinomarginals (Plate 4.1A; Plate 5.1B); variably developed as series of short flanges on carinae (Plate 4.7). Superior margins of radii variably angled to lateral margins (Plate 4.4–7). Sutural margins of radii strongly denticulate, articulate with notched groove on alar surfaces (Plate 5.5). Height of rostra 1.5–2× greater than breadth; rostromarginals bear broad alae (Plate 3.5; Plate 4.4–6). Marginals (Plate 5.1A–C) and carinomarginals (Plate 4.1A–C) similar, distinguished by angle between upper and lateral alar margins (100o in marginals, 160o in carinomarginals). Both asymmetrically triangular and have convex basal margins. Marginals in specimens which were crowded by adjacent individuals strongly asymmetrical (Plate 4.2A, B) with triangular extension and very high sheath. Carinae (Plate 4.7; Plate 5.3A–C) with flat external surface, short alae, sheath angled to external surface.
45Discussion. P. hermani sp. nov. differs from P. submersa in the presence of broad apicobasal ribbing on the parietes and details of compartmental articulation. P. hermani sp. nov. differs from P. melleni most importantly in the development of striated flanges descending from the base of the sheath in the carinae, carinomarginals and marginals (compare Fig. 5.1A, 2A). These are absent on the rostromarginals and rostrum in both species. Additionally, the basal margin of P. melleni is crenulate (see also Collareta & Newman, 2020), but smooth in P. hermani sp. nov. (compare Fig. 5.1B, 2B). The density of the internal ribbing on the marginals and carinomarginals is much greater in P. melleni (50+ per plate) than in P. hermani sp. nov. (25+ per plate). In the extant species Chelonibia testudinaria, the striated flange is incorporated into the lower part of the sheath (Fig. 5.3A). P. hermani sp. nov. is thus displays internal characters which are intermediate in morphology between Protochelonibia and Chelonibia.
46P. melleni was recorded from the Oligocene (Rupelian) of Germany (Collareta et al., 2021) on the basis of crushed colony of specimens. In my view, these are too poorly preserved for specific identification.
47Palaeoecology. Chelonibia lives epizoically on turtles and also occurs on manatees; Chelonibia testudinaria forma patula attaches to crustaceans and the chelicerate arthropod Limulus (Ross & Frick, 2007). It has also been found attached to mammalian bones (Collareta & Bianucci, 2021). Molecular study has shown that many of the “species” of Chelonibia are actually ecomorphs of a single species, C. testudinaria (Zardus et al., 2014). Protochelonibia attached to turtles, as demonstrated by specimens attached to an indeterminate cheloniid from the Oligocene of Germany (Collareta et al., 2022b). Diverse turtles are known from the Boom Clay (Smets, 1886a, 1886b, 1887a, 1887b, 1888) and would offer suitable substrates for Protochelonibia. The material described here may form part of a single association of individuals (“colony”) originally attached to one turtle.
5.3. Mollusca (SE)
48The molluscan assemblage contains several species that are poorly known from the Boom Formation, one has never been cited from the Belgian Rupelian. Many shells are broken or fragmented, which is especially the case for thin fragile pectinids. The gastropods are represented by some very small species and juvenile specimens of larger species. It can be noted that no Pteropoda were encountered, despite the fact that Clio blinkae Janssen, 1989 can be abundant in the underlying pink R-horizon (Gürs & Janssen, 2004).
49Class Bivalvia Linnaeus, 1758
50Subclass Protobranchia Pelseneer, 1889
51Order Nuculida Dall, 1889
52Family Nuculidae Gray, 1824
53Genus Nucula Lamarck, 1799
54Type species. Arca nucleus Linnaeus, 1758
55Nucula duchasteli Nyst, 1835
56Plate 6.7 (IRSNB 7730)
571835 Nucula Duchastelii Nyst, p. 16, pl. 3, fig. 64.
581845 Nucula Chastelii Nyst, p. 235, pl. 9, fig. 1.
591957 Nucula duchasteli Nyst, 1835; Glibert, p. 11, pl. 1, fig. 4.
602010 Nucula (Nucula) duchasteli Nyst, 1835; Marquet, p. 256, pl. 3, fig. 2.
61Material. 4 disarticulated valves, 4 bivalved specimens, 12 fragments.
62Description. Typical asymmetric nuculid with opistogyrate umbo and a taxodont hinge, with fine hinge teeth. The dimensions of the largest shell are 10 mm in height (H) × 15 mm in length (L). The umbo lies at ca 1/3 of the dorsal margin. The valves have a triangular shape, as the ventral margin makes a gentle angle with the anterior and posterior margins. A fine crenulation is present on the ventral margin. Nucula duchasteli is characterized by its particular ornamentation, consisting of strong, irregular concentric ribs, making it easily distinguishable from the other Nucula species in the Belgian Oligocene (Glibert, 1957; Marquet et al., 2012).
63Remarks. Besides Nucula duchasteli, also Nucula orbignyi Glibert, 1955 is present in the Boom Formation. The former is more common than the latter (Marquet, 2010). The extant European Nucula species have a broad bathymetric distribution, occurring from a few meters to as much as 400 meters water depth (Poppe & Goto, 1993). The presence of articulated specimens points to a calm environment in which the shells could be buried in (quasi) life position.
64Subclass Autobranchia Grobben, 1894
65Order Myida Stoliczka, 1870
66Family Teredinidae Rafinesque, 1815
67Teredinidae indet.
68Plate 6.6 (IRSNB 7729); Plate 7.3 (IRSNB 7725)
692010 Teredinidae indet.; Marquet, p. 269.
70Material. 2 valve fragments and more than 30 tube fragments from the sediment surrounding the tree trunk and numerous burrows in pieces of wood.
71Description. Calcareous, hollow tubular fragments with diameters ranging between 1 and 5 mm. Locally, the wood surface is very densely covered with burrows (up to 6 cm deep). In a broken wood fragment, globular casts of the reduced shells are observed, with a maximum diameter of 12 mm (Fig. 4). The small, reduced inequilateral shells have a characteristic shape, with a distinct small apophysis. Probably due to damage, no distinct auricle (= posterior lobe) is observed. The anterior lobe is covered by fine ridges, running parallel to the hinge. These ridges make an angle of about 90° with the longitudinal ridges on the anterior disc. This type of ornamentation is absent on the median disc. Unfortunately, the valve is too fragmented to allow further identification.
72Remarks. Teredinidae, often called ‘shipworms’, are a group of highly specialized, obligate xylophagous bivalves colonizing driftwood, existing since the Cretaceous at least (Robin et al., 2018). They are common in (sub)tropical regions in a wide range of environments, from the intertidal zone up to fully marine conditions (Robin et al., 2018). Teredinidae were already mentioned from driftwood in the S30 level of the Terhagen Member at Niel/Schelle (Marquet, 2010), only a few meters below the horizon of this study. Marquet (2010) and Marquet & Herman (2012) also reported Teredinidae from “transitional layers” above the Putte Member in the Mol borehole, nowadays attributed to the Boeretang Member.
73Family Corbulidae Lamarck, 1818
74Genus Varicorbula Grant & Gale, 1931
75Type species. Tellina gibba Olivi, 1792
76Varicorbula gibba (Olivi, 1792)
77Plate 7.1 (IRSNB 7723)
781792 Tellina gibba Olivi, p. 101.
791845 Corbula gibba Oliv.; Nyst, p. 65.
801957 Corbula (Varicorbula) gibba Olivi, sp. 1792; Glibert, p. 46.
812010 Corbula (Varicorbula) gibba gibba (Olivi, 1792); Marquet, p. 269.
822010 Varicorbula gibba (Olivi, 1792); Moerdijk et al., p. 243, fig. 462.
832012 Corbula (Varicorbula) gibba gibba (Olivi, 1792); Marquet et al., p. 85, pl. 32, fig. 3.
84Material. 55 disarticulated valves, 55 bivalved specimens.
85Description. Juvenile, small (figured specimen H = 3.4 mm, L = 3.8 mm), tumid asymmetric shells with an oval to subtriangular shape. The left valve is smaller than the right valve. Some concentric ribs are observed on the right valves. The articulated specimens are pyritized. See also the description by Marquet (2005, p. 89).
86Remarks. Varicorbula gibba is a true living fossil, already occurring during the Paleogene in a multitude of palaeoenvironments (e.g. Moerdijk et al., 2010; Marquet, 2010). The species has a broad bathymetry, occurring from the intertidal zone to depths of 200 m (e.g. Marquet, 2005; Hrs-Brenko, 2006). It is characteristic of stressed environments with a low biodiversity, due to its good tolerance for eutrophic conditions (nowadays a pollution indicator), low oxygen levels and turbidity. After catastrophic anoxic events, Varicorbula can ‘boom’ and dominate during recovery periods of the benthic community, due to its capacity of producing large amounts of eggs (Hrs-Brenko, 2006). This might be a partial explanation for the local occurrence of dense, almost monospecific, small clusters of Varicorbula gibba in the Boom Formation between S30 and S40 (Marquet, 2010). Indeed, periods with low oxygen levels did occur during the deposition of the Boom Formation, which is reflected in the mollusc fauna of the Putte Member (Marquet & Herman, 2012). However, it should be noted that the bottom waters of the Rupelian sea were not anoxic but suboxic (Vandenberghe et al., 2014).
87Order Pectinida Gray, 1854
88Family Pectinidae Rafinesque, 1815
89Genus Palliolum Monterosato, 1884
90Type species. Pecten incomparabilis Risso, 1826
91Palliolum permistum (Beyrich, 1848)
92Plate 6.8–10 (IRSNB 7731–7733)
931848 Pecten permistus Beyrich, p. 60.
941868 Pecten permistus Beyr.; von Koenen, 231, pl. 7, fig. 20a–c.
951930 Chlamys (Aequipecten) permista Beyrich; Vincent, p. 4, fig. 4a–b.
961943 Pecten cf. permistus Beyrich; Albrecht & Valk, p. 120, pl. 11, fig. 363–366.
971957 Chlamys permista Beyrich, sp. 1848; Glibert, p. 20.
981973 Chlamys (Chlamys) permista (Beyrich, 1848); Neuffer, p. 40, pl. 5, figs 16–17.
992000 Palliolum (s.lat.) permistum (Beyrich, 1848); Moths, p. 46, pl. 16, fig. 6.
1002011 Chlamys permista (Beyrich, 1848); Müller, p. 31, pl. 16, figs 8–9.
1012010 Palliolum permistum (Beyrich, 1848); Marquet, p. 263.
102Material. 7 valves, 19 fragments.
103Description. Fragile and thin, nearly equilateral shells. The auricles are mostly damaged or missing. The largest valve measures 21 mm (H) × 20 mm (L), the smallest 6 × 6 mm. The disc is almost circular and rather flat. The shells show a distinct ornamentation, with many fine and closely interspaced radial ribs, often covered by abundant fine scales. The presence and abundance of these scales are highly variable. Also, the number of ribs can strongly vary: ca 35 ribs were counted on the smallest specimen, ca 70 on the largest specimen.
104Remarks. Vincent (1930) mentioned ten specimens of Chlamys permista from the Boom Formation, exhibiting a maximum height of 24 mm; one is refigured here (IRSNB IST 1804 or 1805, Fig. 6.3). Our specimens closely resemble those figured by Vincent (1930, fig. 3). Since Vincent (1930), no additional occurrences have been reported for the Boom Formation (see Marquet, 2010). Our specimens match well with the original description of Beyrich (1848), with the exception of the generally higher number of radial ribs. However, Beyrich studied only two shells, including a bivalved specimen with 25 radial ribs on the left valve, and more than 40 on the right valve. In contrast, von Koenen (1868) mentioned a right valve with more than 70 ribs. Also, Neuffer (1973) figured valves with similar high rib numbers, while the specimen figured by Moths (2000) has only ca 40 ribs. Albrecht & Valk (1943) mention the presence of ca 25 ribs on the left valve and ca 50 on the right valve. Vincent (1930) also noted a strong variability in the presence or absence of scales on the ribs, which is also the case in the specimens figured by Müller (2011). Given all these similarities and the inferred intraspecific variability, an identification of our material as Palliolum permistum seems appropriate. However, it should be taken into account that the current status of Beyrich’s type material remains unknown, as it is probably lost (see also the remark of Neuffer, 1973). Given the large number of specimens we recovered of this otherwise very rare species, it can be supposed that a small community was present in the vicinity of the sunken trunk.

Figure 6. Some important Pectinidae from the Boom Formation in the RBINS collection, refigured after Vincent (1930). 1. Palliolum deshayesi (Nyst, 1836), fragment with posterior auricle, IRSNB IST 1801 (as “Chlamys picta Goldfuss” in Vincent, 1930, fig. 1). 2A–B. Idem, fragment with anterior auricle, IRSNB IST 1802 (Vincent, 1930, fig. 2). 3. Palliolum permistum (Beyrich, 1848), IRSNB IST 1804-1805 (other specimen unrecognisable due to pyrite decay; as “Chlamys permista Beyrich” in Vincent, 1930, fig. 4A). 4. Palliolum delheidi (Vincent, 1930), IRSNB IST 1806-1807 (other syntype refigured by Marquet, 2010, pl. 3, fig. 6; as “Chlamys delheidi” in Vincent, 1930, fig. 5B). Scale bars = 5 mm.
105Palliolum delheidi (Vincent, 1930)
106Plate 6.11 (IRSNB 7734)
1071930 Chlamys (Hilberia) Delheidi Vincent, p. 6, fig. 5.
1081957 Chlamys delheidi Vincent, E., 1930; Glibert, p. 19.
1092010 Palliolum delheidi Vincent, 1930; Marquet, p. 263.
110Material. 1 fragment.
111Description. Thin, flat fragment (25 mm) of a much larger shell. Faint, wide radial ribs are present, together with concentric growth lines (see also Vincent, 1930).
112Remarks. In the caption of his text-figure 5, Vincent (1930) stated that both syntypes (IRSNB IST 1806–1807, former Delheid collection) originate from the Steendorp clay pit. However, the original labels in the RBINS collection mention the Niel pit, which was also adopted by Glibert (1957) and Marquet (2010). The latter refigured one of both syntypes, the other is shown here (Fig. 6.4). In total, 6 specimens are known from Niel, Noeveren and Steendorp (Vincent, 1930; Glibert, 1957). Marquet (2010) did not encounter this species in his recent surveys. Therefore, our fragment is the first published record from the Boom Formation in a century.
113Palliolum indet.
114Material. 1 nearly complete specimen, 10 fragments.
115Description. Fragments with variable ornamentation, all without scales on the ribs. One more complete valve (L = 22 mm) with broken ventral margin, displaying very fine, faint radial ribs, disappearing towards the centre and outer edges of the disc.
116Remarks. These specimens cannot be identified to the species level, as we do not have enough material to properly assess the range in intraspecific ornamental variability of Palliolum permistum. It is likely that many fragments actually belong to this species. However, Palliolum deshayesi (Nyst, 1836) cannot be excluded either, as it is more common in the Belgian Oligocene. The latter species was mentioned by Vincent (1930) and Glibert (1957) as Chlamys picta (Goldfuss, 1834) (forma diomedes d’Orbigny, 1852), but synonymized with P. deshayesi by Marquet (2010). The large valves (maximum H = 65 mm, L = 70 mm) described from the Boom Formation by Vincent (1930) show little ornamentation, with the exception of concentric growth lines (Fig. 6.1–2) and some (very) faint radial ribs on the oldest parts of the shells. In contrast, Neuffer (1973, pl. 6) showed extreme variation within the sculpture of this species, which is also the case for the Recent Palliolum tigerinum (Müller, 1776) (Janssen & Dijkstra, 1996). Given the wide variation and existing ambiguities, our fragments are kept in open nomenclature.
117Class Gastropoda Cuvier, 1797
118Subclass Heterobranchia Burmeister, 1837
119Familia Pyramidellidae Gray, 1840
120Genus Odostomia Fleming, 1813
121Type species. Turbo plicatus Montagu, 1803.
122Odostomia cf. acutiuscula (Braun in Walchner, 1851)
123Plate 7.7 (IRSNB 7728)
1241851 Actaeon acutiusculum Braun in Walchner, p. 1123.
1251863 Odontostoma acutiusculum A. Braun sp.; Sandberger, p. 170, pl. 15, fig. 1.
1261954 Odostomia (Megastomia) acutiusculum Sandberger, sp., 1863; Glibert & De Heinzelin, p. 360.
1272000 Odostomia (Megastomia) acutiuscula (Braun, 1850); Moths, p. 36, pl. 12, fig. 6.
1282012 Odostomia acutiuscula (Braun, 1850); Lozouet & Maestrati, p. 294, fig. 191.
1292012 Odostomia acutiuscula (Braun, 1850); Marquet & Herman, 2012, p. 111.
1302016 ? Odostomia cf. acutiuscula (Braun in Walchner, 1851); Marquet et al., p. 77, pl. 21, fig. 4.
131Material. 4 specimens.
132Description. Small (figured specimen H = 2.0 mm, width (W) = 0.9 mm), elongated shells with 4 to 5, rather flat, only slightly convex whorls. The last whorl displays a carina and is a little less than half the height of the shell. A small columellar tooth is present. No visible sculpture/ornamentation. Small heterostrophe protoconch.
133Remarks. Marquet & Herman (2012) mentioned Odiostoma acutiuscula from the Boom Formation, but only from the septaria level S50 in the Putte Member. Unfortunately, no images were provided. This genus had not been previously described from the Boom Formation, probably because collecting was mainly done visually during the 19th and early 20th centuries. As a result, very small shells were often overlooked in the field. Lozouet & Maestrati (2012) figured a specimen of Odiostoma acutiuscula from the sandy French Rupelian (“Stampien”), which is very similar to our specimens. However, several other Odostomia species occur in the lowermost Oligocene of Belgium (Glibert & de Heinzelin, 1954; Marquet et al., 2016). Due to the large diversity within this genus and the many species described in older works, an identification of Odostomia species is often difficult. For now, we cautiously maintain the name Odostomia cf. acutiuscula for the specimens of the Boom Formation, following Marquet & Herman (2012) and Marquet et al. (2016). However, it cannot be excluded that a future revision may reveal that this material actually belongs to other species. For example, the whorls of the specimens figured by Sandberger (1863, plate XV, fig. 1) and Moths (2000) are more convex than observed in our material. The original description by Braun in Walchner (1851) brings no clarity. Extant Odostomia spp. live in a broad range of habitats (from 0 to 700 m water depth, van Aartsen et al., 1998) and are known as ectoparasites on both molluscs and polychaetes (Cole & Hancock, 1955)
134Subclass Caenogastropoda Cox, 1960
135Order Littorinimorpha Golikov & Starobogatov, 1975
136Family Naticidae Guilding, 1834
137Genus Euspira Agassiz, 1837
138Type species. Natica glaucinoides Sowerby, 1812
139Euspira cf. achatensis (De Koninck, 1838)
140Plate 7.6 (IRSNB 7727)
1411838 Natica achatensis De Koninck, p. 9.
1421943 Polynices (Lunatia) achatensis (Récluz); Albrecht & Valk, p. 53, pl. 4, figs 91–96.
1431957 Natica (Lunatia) achatensis (Recluz) De Koninck, sp., 1837; Glibert, p. 57, pl. 6, fig. 12.
1442000 Polinices (Lunatia) achatensis (Koninck, 1838); Moths, p. 20, pl. 3, fig. 2.
1452012 Euspira achatensis (de Koninck, 1837); Lozouet & Maestrati, p. 286, pl. 185, figs 7–9.
1462016 Euspira helicina achatensis (De Koninck, 1837); Marquet, p. 17.
1472016 Euspira achatensis (De Koninck, 1837); Marquet et al., p. 17, pl. 3, fig. 4.
148Material. 5 specimens.
149Description. Juvenile, very small (figured specimen H = 2.1 mm, W = 2.1 mm) globular shells. Apex barely protruding. Distinct suture. Tumid, rather contiguous whorls, giving the transition between the whorls a rather smooth appearance. No visible sculpture/ornamentation is present on the whorls. The specimens display little callus and have a small umbilicus with some very fine lines/folds in the curvature.
150Remarks. Euspira achatensis was described by De Koninck (1838) from the Boom Formation. It is the most common Naticidae in this formation, and was encountered in almost all levels (Glibert, 1957; Marquet, 2016). Albrecht & Valk (1943) described this species from the Oligocene of the Netherlands (southern Limburg). In Germany, the species is very common in the Rupelian clay of Malliß (Mecklenburg-Vorpommern) (Moths, 2000). In addition, Lozouet & Maestrati (2012) figured it from the sandy “Stampien” of the Paris Basin. Given the (very) juvenile life stage of the shells, we maintain them as Euspira cf. achatensis.
151Order Neogastropoda Wenz, 1938
152Family Mangeliidae Fischer, 1883
153Genus Amblyacrum Cossmann, 1889
154Type species. Pleurotoma rugosa Deshayes, 1834
155Amblyacrum cf. roemeri (von Koenen, 1867)
156Plate 6.12 (IRSNB 7736)
1571867 Mangelia roemeri von Koenen, p. 95, pl. 6, fig. 9a–d.
1581979 Amblyacrum roemeri (Koenen, 1867); Janssen, p. 325, pl. 18, fig. 71–72.
1591987 Amblyacrum roemeri (von Koenen, 1867); Schnetler & Beyer; p. 205.
1601998 Sorgenfreispira roemeri (Koenen, 1867); Welle, p. 96; pl. 18, fig. 4.
1612000 Sorgenfreispira roemeri (Koenen, 1867); Moths, p. 32, pl. 10, fig. 2.
162Material. 5 specimens.
163Description. Juvenile, very small fusiform shells (figured specimen H = 2.3 mm, W = 1.2 mm). The shells are narrow and elongated, with a rather short siphonal canal and a relatively deep suture. The protoconch is dome-shaped, with ca 3 smooth convex whorls. The nucleus is very small. Although its surface is not well preserved, no obvious sculpture/ornamentation is present. The transition to the teleoconch is vaguely demarcated, some very short, vague lines (possible spirae?) were observed on the last whorl of the protoconch. On the teleoconch, ca 10 widely interspaced, pronounced ophistocline axial ribs are present per whorl, covered by 5–6 coarse spiral ribs on the first teleoconch whorl. The sinus of the growth lines is slightly curved (elongated, inverted S-shape).
164Remarks. Von Koenen (1867) mentioned this species from the German Rupelian (Freienwalde) and Chattian (Sternberger Gestein, Krefeld, Hohenkirchen, etc.). The protoconch consists of 2½ to 3 smooth whorls. The Rupelian specimens differ from the Chattian shells by their more compact shape, and by the absence of finer spiral lines between the broader spiral ribs on the last whorls (von Koenen, 1867). Janssen (1979) described and figured Amblyacrum roemeri from the German Chattian: the protoconch of his material consisted of 3 smooth whorls, followed by 7 spiral ribs (more than on our specimens). Moths (2000) identified this species in the Rupelian of Malliß, but mentioned that his material resembled the Chattian form with a fine sculpture on the protoconch. The latter characteristic was not observed in our material. Amblyacrum roemeri also occurs in the Chattian Brejning Member (Vejle Fjord Formation) of Denmark (Schnetler & Beyer, 1987), but no images were provided. Welle (1998) described 15 specimens (as Sorgenfreispira roemeri) from the Chattian of Schacht 8 (Sophia Jacoba mine) near Erkelenz (Germany). His figured shell is similar, but not identical to our material: more abundant, fine spiral ribs are present. This genus and species are new for the Belgian Oligocene; neither was ever mentioned by Glibert & de Heinzelin (1954), Glibert (1957) and Marquet & Herman (2012). Given the juvenile nature of our material and the subtle differences between the forms mentioned in the literature, we attribute our specimens to Amblyacrum cf. roemeri.
165Subclass Neomphaliones (see also Bouchet et al., 2017)
166Order Cocculinida Haszprunar, 1987
167Family Cocculinidae Dall, 1882
168Genus Cocculina Dall, 1882
169Type species. Cocculina rathbuni Dall, 1882.
170Cocculina reineckei Marquet, 2016
171Plate 7.4 (IRSNB 7726) and Plate 7.5 (specimen lost during scanning)
1722016 Cocculina (Cocculina) reineckei Marquet, p. 15, pl. 1, figs 3–4.
173Material. 35 specimens.
174Description. Very small limpet shells with the umbo close to the posterior margin. The figured specimen has a total shell length of 3.0 mm. For a more detailed description, see Marquet (2016). In our material, some specimens with damaged protoconchs are present (Plate 7.5). Unfortunately, the outer shell layers are damaged and partly missing, displaying no ornamentation/sculpture on the protoconch.
175Remarks. The discovered assemblage represents the largest known association of this species. Marquet (2016) described 15 shells associated with driftwood in the S30 level of the Terhagen Member. Our material was found in a similar setting, only a little higher in the same succession (Fig. 2). Extant Cocculinidae and Pseudococculinidae are opportunistic deep-water limpets typical of bathyal and abyssal depths, colonizing decaying driftwood sinking from shallow water into the aphotic zone (e.g. Marshall, 1985; McLean & Harasewych, 1995; Ardila & Harasewych, 2005). These limpets exploit decomposing wood as a substrate, and most probably feed on the microbes involved in the decaying process (Marshall, 1985). At a presumed water depth of ca 100 m, the cocculinid population of the Boom Formation lived in a relatively shallow environment compared to many of its extant relatives. Similar limpets are well known from the Oligocene of the North Sea Basin; Marquet (2016) mentioned Acmaea schreiberi Welle, 2009 and Cocculina papyracea (Sandberger, 1861) from the German Rupelian, and Lepetella helgae Schnetler & Beyer, 1990, Lepetella jyttae Schnetler & Beyer, 1990 and Cocculina megapolitana (Wiechmann, 1868) from the Danish and German Chattian. Also in the Neogene, multiple (pseudo)cocculinids occur, e.g. very rare Cocculina dittmeri (Anderson, 1964) and Cocculina miocaenica Boettger, 1901 from the Langhian of Miste (the Netherlands) (Janssen, 1984). However, recent literature assigns these Langhian species to the genera Pseudococculina and Notocrater respectively, both Lepetelloidea (Stein et al., 2016). Although historically classified together with the Cocculinoidea (including the Cocculinidae and Bathysciadiidae) in the Cocculiniformia, molecular phylogeny showed that the latter group was paraphyletic (McArthur & Harasewych, 2003) and that the Lepetelloidea and Collucinoidea belong to the subclasses of the Vetigastropoda and the Neomphaliones respectively (Bouchet et al., 2017). Nevertheless, the shells of Cocculinidae and Pseudococculinidae are similar; the morphology of the protoconch and radula is therefore often used to separate these families (McLean & Harasewych, 1995). Unfortunately, the protoconch is often damaged or even missing (e.g. the holotype of C. reineckei) and the radula does not fossilize. The protoconch of Cocculinidae (e.g. Cocculina, Coccopigya) generally has a rather short and broad apical fold with a free protoconch tip, while the Pseudococculinidae (e.g. Pseudococculina, Notocrater) display a long and narrow protoconch apical fold, and a fused protoconch tip (Marshall, 1985). The general shape of the protoconch of our specimen resembles that of the Cocculinidae. Unfortunately, the surface layers of the protoconch are missing, making it unknown whether the typical reticulate sculpture of the Cocculinidae was present.
176Class Scaphopoda Bronn, 1862
177Order Dentaliida Starobogatov, 1974
178Family Rhabdidae Chistikov, 1975
179Genus Rhabdus Pilsbry & Sharp, 1897
180Type species. Dentalium rectius Carpenter, 1864
181Rhabdus parallelus (Zinndorf, 1928)
182Plate 7.2 (IRSNB 7724)
1831928 Dentalium parallelum Zinndorf, p. 38, pl. 1, fig. 8.
1841978 Rhabdus aff. parallelum Zinndorf, 1928; Janssen, p. 140.
1851996 Rhabdus aff. parallelum Zinndorf, 1928; Moths et al., p. 14, pl. 1, fig. 4.
186Material. 1 specimen.
187Description. Thin calcareous tube with a length of 2.85 mm and a diameter of 0.69 mm. No ornamentation. The core of the tube is filled with pyrite.
188Remarks. These very small Scaphopoda are often overlooked and confused with worm tubes. Zinndorf (1928) described this species based on four specimens found in the “Rupelton” at Offenbach am Main. His longest specimen attained a length of 11 mm and a diameter of 0.5 mm. Rhabdus aff. parallelus is also known from the Chattian of Germany (Janssen, 1978; Moths et al., 1996).
5.4. Elasmobranchii (PDS)
189Systematics follows Nelson et al. (2016), whereas anatomical tooth terminology follows Cappetta (2012). Despite the high number of recovered elasmobranch teeth, only five species are represented (Table 1). For additional illustrations of these taxa, the reader is referred to Hovestadt & Steurbaut (2023).
190Class Chondrichthyes Huxley, 1880
191Order Lamniformes Berg, 1958
192Family Carchariidae Müller & Henle, 1838
193Genus Carcharias Rafinesque, 1810
194Type species. Carcharias taurus Rafinesque, 1810, by original monotypy.
195Carcharias contortidens (Agassiz, 1843)
196Plate 8.1–12 (IRSNB P 10299–10310)
1971843 Lamna (Odontaspis) contortidens Agassiz, p. 294, vol. 3, tab. 37a, figs 17–23.
1981910 Odontaspis acutissima L. Agassiz, 1844; Leriche, p. 245, 261, figs 73–76, pl. 14, figs 1–27.
1991988 Synodontaspis acutissima (Agassiz, 1844); Nolf, p. 140, pl. 44, figs 1–9.
2001999 Synodontaspis acutissima (Agassiz, 1844); Baut & Génault, p. 16, pl. 3, figs 1–2.
2012001 Carcharias acutissimus (Agassiz, 1844); Reinecke et al., p. 11, pls 10–15.
2022010 Carcharias acutissima (Agassiz, 1843); Hovestadt et al., figs 3–4.
2032020 Carcharias contortidens (Agassiz, 1843); Höltke et al., p. 11, pl. 2, figs 8–10, pl. 3, figs 1–3.
204Material. 154 teeth representing all tooth positions.
205Description. The tooth set comprises 154 disarticulated teeth, which range in size from 2 mm (posterior) to 25 mm (lower anterior). A representative sample of all tooth positions is shown in Plate 8. As observed in Carcharias taurus, the dentition of C. contortidens consists of three upper (UA1-UA3) and four lower anterior teeth (LA1-LA4) (see also Reinecke et al., 2011, fig. 10). Multiple intermediate files may have been present, but this feature is very variable within the genus Carcharias, as is the number of lateral and posterior files (e.g. Applegate, 1965; Sadowsky, 1970).
206Comparisons with dental characters of the extant sand tiger shark C. taurus (see Cunningham, 2000) revealed that all tooth positions are represented in our fossil sample. The marked degrees of dignathic and monognathic heterodonty simplified the determination of each tooth position. Elongated, more slender teeth were separated from those with a shorter crown and were assigned to anterior positions. The upper and lower teeth were grouped based on the amount of lingual curvature of the crown, recurvature of the crown-tip, and angle between the root-lobes. Teeth with a strong lingual curvature of the crown and strong lingual protuberance of the root were assigned to lower positions. The remaining teeth, having strongly recurved crown tips, were assigned to the upper jaw. Their position was then determined by considering the increasing angle of root-lobe divergence in distal direction. Some teeth are preserved only as thin enamel “shells” lacking the root. These incomplete teeth represent replacement teeth.
207The UA1 (IRSNB P 10299 - Plate 8.1A–C) is smaller in size than the remaining upper anterior teeth. In distal view (Plate 8.1A), the strongly labially recurved crown tip, characteristic of upper tooth positions, is clearly noticeable. The UA2 (IRSNB P 10300 - Plate 8.2A–C) has relatively short root lobes compared to the remaining anterior ones. The UA3 (IRSNB P 10301 - Plate 8.3A–C) has a distinctive morphology with a long and elongated mesial root lobe and a compressed distal one. The crown is distally directed but exhibits a slight mesial slant. The concave mesial edge of the crown is to conform to the distal margin of the anterior hollow in the palatoquadrate (see Siverson, 1999, fig. 3a). Three intermediate teeth are present, two of which are well preserved (IRSNB P 10303 - Plate 8.5A–B & IRSNB P 10304 - Plate 8.6A–C). Interestingly, both specimens display a secondary distal cusplet. Upper lateral teeth (IRSNB P 10302 - Plate 8.4A–C) have a distally directed crown.
208The LA1 (IRSNB P 10305 - Plate 8.7A–C) is the smallest anterior tooth position. It is strongly mesiodistally compressed with a much longer distal root lobe. The LA2 (IRSNB P 10306 - Plate 8.8A–C) and LA3 (IRSNB P 10307 - Plate 8.9A–C) are the largest teeth in the jaw and very similar to each other in morphology, the former being more symmetrical than the latter, which is slightly distally directed. The LA4 (IRSNB P 10308 - Plate 8.10A–C) is strongly distally directed. Lower lateral teeth (IRSNB P 10309 - Plate 8.11A–C) have a short, straight crown. Posterior teeth are significantly smaller in size compared to the lateral teeth and possess very short and low crowns (IRSNB P 10310 - Plate 8.12A–B). Some teeth bear marginal folds at the labial crown base (Plate 8.12B).
209Remarks. The family Odontaspididae Müller & Henle, 1839 traditionally consists of two extant (Carcharias and Odontaspis Agassiz, 1838) and numerous extinct genera (see Cappetta & Nolf, 2005). However, based on both molecular (e.g. Naylor et al, 1997; Vélez-Zuazo & Agnarsson, 2011) and morphological data (e.g. Stone & Shimada, 2019), the family Carchariidae Müller & Henle, 1838 was resurrected to separate the genus Carcharias from the family Odontaspididae sensu stricto for Odontaspis (e.g. Adolfssen & Ward, 2015).
210There is still disagreement about the use of the genus name Carcharias for the many fossil species currently attributed to it (e.g. Adolfssen & Ward, 2015). A reclassification of the fossil ‘odontaspidids’ is needed, but beyond the scope of this study. However, C. contortidens probably belongs to the same lineage as the present-day C. taurus, which is fairly common since the early–middle Miocene (e.g. Bor et al., 2012; Everaert et al., 2019), as teeth of both species are morphologically very similar (e.g. Cappetta & Nolf, 1991; Ward & Bonavia, 2001).
211For a long time, these teeth were attributed to Carcharias acutissima (Agassiz, 1843) (e.g. Cappetta, 2012) or, following the International Code of Zoological Nomenclature (ICZN), C. acutissimus (e.g. Reinecke et al., 2001). However, both syntypes of C. acutissimus are morphologically very close to Carcharoides catticus (Philippi, 1846), with the exception of the folds on the lingual crown surface, which are well visible on Agassiz’s illustrations (1843, pl. 37a, figs 33–34) (see Höltke et al., 2020). Since Agassiz’s work, teeth of this type have not been reported in literature (Höltke et al., 2020). In the same volume, Agassiz (1843, p. 294–295, pl. 37a, figs 17–23) also described and figured a series of teeth that he assigned to Carcharias contortidens. The latter is much more commonly found and morphologically very similar to the mass occurrences of teeth labelled as C. acutissimus in the available literature. The type material of both species is probably lost, but based on the different morphology of the teeth, C. acutissimus and C. contortidens are regarded as separate species (Höltke et al., 2020). Consequently, also the C. acutissimus teeth from Oligocene deposits of Belgium (e.g. Leriche, 1910) should be reassigned to C. contortidens.
212The largest part of our material probably belongs to a single individual based on the same size range, morphology and preservation state. All teeth occurred on the same bedding plane between the trunk and side-branch, within a limited area of less than 1 m2. In contrast to this concentration, larger shark teeth are generally very rarely found nowadays and scattered in the Boom Clay. Unfortunately, no vertebrae or jaw remains were discovered. Nevertheless, a number of teeth seem to belong to smaller individuals which can be expected in such an accidental association. In addition, the Boom Formation has yielded several associated tooth sets in the past (see Leriche, 1910).
213Leriche (1910, p. 264, text-figs 73–76, plate XIV) described and figured an important articulated tooth set of C. contortidens (IRSNB P 678, as Odontaspis acutissima), found in Niel, situated only 2 km south of the clay pit at Schelle. That specimen, featuring fragments of the palatoquadrate (upper jaw) and Meckel’s cartilage (lower jaw), is refigured in Figure 7. While the lateral teeth detached during excavation (Leriche, 1910, p. 264), the anterior tooth files display their original arrangement. Figure 7A–B (IRSNB P 678f, Leriche 1910, pl. XIV, figs 6, 6a, 6b) represent the lower symphysis, consisting of the left jaw half, including four anterior tooth files (LA1 to LA4) and the right jaw half showing the first three anterior tooth files (LA1 to LA3) (LA1 = ‘symphyseal’ and LA4 = ‘first lateral’ in Leriche, 1910). Figure 7B represents IRSNB P 678f rotated by 90° buccally. Interestingly, there is an upper intermediate tooth on this lower jaw fragment, oriented in the opposite direction as the lower anterior teeth, which is in contradiction with Leriche (1910, p. 265), who explicitly indicated no intermediate teeth were found. Figure 7C (IRSNB P 678g, Leriche 1910, pl. XIV, fig. 7) represents a partial right lower jaw, detached from IRSNB P 678f (Fig. 7A–B) (Leriche, 1910, p. 266), showing teeth belonging to the second to fourth lower tooth files (LA2 to LA4). Finally, Figure 7D (IRSNB P 678a, Leriche 1910, pl. XIV, figs 1, 1a) represents the upper symphysis, consisting of the left jaw half including three anterior tooth files (UA1 to UA3) and the right jaw half showing the first two anterior tooth files (UA1-UA2) (UA1 = ‘symphyseal’ in Leriche, 1910). Leriche (1910, p. 267) also observed that, while the majority of the teeth possess folds on the lingual crown face, some teeth have a smooth lingual crown surface, strongly limiting the taxonomic value of this character.

Figure 7. Carcharias contortidens (Agassiz, 1843) articulated tooth set. A. IRSNB P 678f – lower symphysis (Meckel’s cartilage). B. IRSNB P 678f, rotated by 90° buccally. The upper intermediate tooth is encircled in white. C. IRSNB P 678g - partial right lower jaw half, detached from IRSNB P 678f. D. IRSNB P 678a - upper symphysis (palatoquadrate cartilage).
214Family Lamnidae Bonaparte, 1835
215Genus Isurolamna Cappetta, 1976
216Type species. Isurolamna affinis (Casier, 1946)
217Isurolamna gracilis (Le Hon, 1871)
218Plate 6.1 (IRSNB P 10311)
2191871 Oxyrhina gracilis Le Hon, p. 11, text-fig.
2201871 Otodus rupeliensis Le Hon, p. 11, text-fig.
2211910 Lamna rupeliensis, Le Hon, 1871; Leriche, p. 271, pl. 15, figs 22–47.
2221910 Oxyrhina Desori (L. Agassiz) Sismonda, 1849; Leriche, p. 275, pl. 16, figs 16–31.
2231999 Isurus desori (Sismonda, 1849); Baut & Génault, p. 17, pl. 3, figs 7–8, ?9.
2241999 Rhizoquadrangulus rupeliensis (Le Hon, 1871); Baut & Génault, p. 21, text-fig. 10, pl. 4, figs 1–3.
2252001 Isurolamna gracilis (Le Hon, 1871); Reinecke et al., p. 21, pl. 31, figs a–g; pl. 32, fig. b; pl. 33, figs a–f; pl. 34, figs a–g.
2262012 Isurus desori (Sismonda, 1849); Génault, pl. 193, figs 2–3, ?4.
2272012 Isurolamna rupeliensis (Le Hon, 1871); Génault, pl. 193, figs 7–8.
228Material. A single (?upper) anterior tooth.
229Description. This anterior tooth measures 29 mm in height, but the root is strongly abraded. The large crown is distally directed and slightly sigmoidal in profile. The cutting edges are smooth and stop just before the crown base. There are no visible lateral cusplets.
230Remarks. Isurolamna gracilis is one of the most common species of large-sized sharks in the Oligocene of the North Sea Basin (e.g. Baut & Génault, 1999; Reinecke et al., 2001; Génault, 2012). For a good iconography, see Leriche 1910, plates 15 (lateral teeth) and 16 (anterior teeth reported as Oxyrhina desori Sismonda, 1849).
231Family Cetorhinidae Gill, 1862
232Genus Keasius Welton, 2013
233Type species. Keasius taylori Welton, 2013
234Keasius parvus (Leriche, 1908)
235Plate 6.2 (IRSNB P 10312)
2361908 Cetorhinus parvus Leriche, p. 877 (gill rakers).
2371910 Cetorhinus parvus, Leriche, 1908; Leriche, p. 294, text-figs 91–94 (gill rakers).
2381978 Cetorhinus parvus Leriche, M., 1908; Steurbaut & Herman, p. 303 (table only).
2391979 Cetorhinus parvus Leriche, 1908; Herman, p. 366, text-fig. 5; pl. 3, figs 1–2.
2402001 Cetorhinus parvus Leriche, 1908; Reinecke et al., p. 24, pl. 36; pl. 37, figs a–c; pl. 38, figs a–d.
2412012 Cetorhinus parvus Leriche, 1910; Hovestadt & Hovestadt-Euler, p. 72, fig. 3.
2422013 Keasius parvus (Leriche, 1908); Welton, p. 39.
2432015 Keasius parvus (Leriche, 1908); Reinecke et al., p. 54, figs 12–16, 22A, 22B, 24.
244Material. Several fragmentary gill rakers. No teeth were found.
245Description. See Welton (2013) and Reinecke et al. (2015) for gill raker terminology.
246The genus Keasius is represented by some fragments of gill rakers only. They are strongly abraded and often lack diagnostic characters. However, one specimen (IRSNB P 10312 - Plate 6.2) is suitable for comparison, with the raker base and lower part of the filament preserved. The filament is narrow and moderately curved. The axe-shaped raker base is moderately long, with a large basal height. The attachment surface exhibits numerous small foramina. There is a slight distal protuberance. The mesial edge is rounded and convex. The basal edge is rounded to subangular. The medial process is relatively long and narrow. The bight shape is subangular.
247Remarks. This specimen fits well within the diagnosis of Keasius parvus (Leriche, 1908) (see Welton, 2013; Reinecke et al., 2015), the only cetorhinid species to have been reported from the Belgian Oligocene (e.g. Leriche, 1910; Herman, 1979; Hovestadt & Hovestadt-Euler, 2012, as Cetorhinus parvus; Reinecke et al., 2015). For a long time, it was included within the genus Cetorhinus, along with the extant basking shark C. maximus (Gunnerus, 1765); however, based on teeth, gill rakers and vertebrae, Welton (2013) created the genus Keasius for his newly erected species K. taylori and included the species parvus.
248Order Squaliformes Goodrich, 1909
249Family Squalidae Bonaparte, 1838
250Genus Squalus Linnaeus, 1758
251Type species. Squalus acanthias Linnaeus, 1758
252Squalus alsaticus (Andreae, 1890)
253Plate 6.3-4 (IRSNB P 10313–10314)
2541890 Acanthias alsaticus Andreae, p. 108, text-fig. 2.
255? 1910 Acanthias, sp.; Leriche, p. 250, text-fig. 65. (spine)
2561978 Squalus alsaticus (Andreae, 1892); Steurbaut & Herman, p. 304, pl. 1, figs 1–2.
2572001 Squalus alsaticus (Andreae, 1892); Reinecke et al., p. 8, pl. 6, figs a–d; pl. 7, figs a–g.
258Material. 113 teeth.
259Description. A description of the teeth of S. alsaticus and a comparison with those of the extant S. acanthias were provided by Steurbaut & Herman (1978). Some teeth in our sample are strongly mesio-distally elongated (Plate 6.4), representing (?lower) posterior teeth (Herman et al., 1989).
260Remarks. The presence of Squalus alsaticus (Andreae, 1890) can be expected as it is the most common elasmobranch species across the Boom Formation (e.g. Steurbaut & Herman, 1978; Hovestadt & Hovestadt-Euler, 1995). Extant species of the genus Squalus are known to form large schools (e.g. Compagno, 1984; Ebert et al., 2021) which could explain this large concentration of teeth. Squalus is an opportunistic scavenger, known to feed on carcasses (e.g. Auster et al., 2020).
261Order Rajiformes Berg, 1937
262Family Arhynchobatidae Fowler, 1934
263Genus Atlantoraja Menni, 1972
264Type species. Atlantoraja cyclophora (Regan, 1903)
265Atlantoraja cecilae (Steurbaut & Herman, 1978)
2661978 Raja cecilae Steurbaut & Herman, p. 306, pl. 2, fig. 4.
2671978 Raja heinzelini Steurbaut & Herman, p. 306, pl. 2, fig. 2.
2681978 Raja terhagenensis Steurbaut & Herman, p. 307, pl. 2, fig. 3.
2691995 Raja cecilae Steurbaut & Herman, 1978; Hovestadt & Hovestadt-Euler, p. 265, pl. 3, figs la–1d; pl. 4, figs la–1d; pl. 5, figs la–1d; pl. 6, figs 1a–lc; pl. 7, figs 1a–lc; pl. 8, figs 1a–lc.
2702015 Atlantoraja cecilae (Steurbaut & Herman, 1978); Reinecke, p. 3., figs 3a & 6b.
271Material. 4 abraded teeth
272Description. See Hovestadt & Hovestadt-Euler (1995) for a review and description of these teeth.
273Remarks. Steurbaut & Herman (1978) described three rajoid species from the Boom Formation, Raja terhagenensis, Raja heinzelini and Raja cecilae, which were later considered to be different morphs of a single valid species, R. cecilae (Hovestadt & Hovestadt-Euler, 1995). Reinecke (2015) confirmed these observations and reassigned R. cecilae to the extant genus Atlantoraja Menni, 1972.
5.5. Teleostean otoliths (KH)
274The otoliths generally are of good preservation. Ten different species have been identified, figured on Plates 9 to 12 and listed in Table 1. Since most species discovered in the present study are well known, comments will only be provided for the new species as well as for Myoxocephalus primas (Koken, 1891) and Lophius gibbosus Nolf, 1977. Capros siccus Schwarzhans, 2008 (Plate 11.7) is new for the Belgian Rupelian. Systematics follows Nelson et al. (2016).
275Class Osteichthyes Huxley, 1880
276Subclass Actinopterygii Klein, 1885
277Division Teleostei Müller, 1846
278Order Carangiformes Jordan, 1923
279Family Carangidae Rafinesque, 1815
280Genus Trachurus Gronow in Gray, 1854
281Trachurus reineckei Hoedemakers sp. nov.
282https://zoobank.org/urn:lsid:zoobank.org:act:C6F25691-62E2-4A82-B204-5B6AC92764A6
283Plate 10.1–8
2842000 Erythrocles cf. ohei Schwarzhans; Müller & Rozenberg, p. 109–110, pl. 6, figs 7, ?9.
285Diagnosis. Elongated otoliths characterized by a postdorsal angle of ca 90°, a coarse lobation on the posterior rim, posterior dorsal rim and posterior ventral rim, a large, rounded posterior extension at the height of the posterior part of the cauda and a lobation in the centre of the outer face only.
286Types. Holotype: left otolith (IRSNB P 10334), 7 paratypes (IRSNB P 10329–10333, 10335–10336).
287Material. 149, of which 8 are type specimens.
288Locus typicus. Ceulemans clay pit at Schelle, 51°07'01.22'' N, 4°21'21.30'' E.
289Stratum typicum. Silty lens associated with a tree trunk on top of pink R-horizon, Terhagen Member, Boom Formation, Rupelian (NP23), Oligocene.
290Derivatio nominis. Named after Dr Thomas Reinecke (Bochum, Germany), in recognition of his contributions to palaeoichthyology, his much-appreciated help with photography and his friendship.
291Description. Dimensions of holotype: L = 5.4 mm, H = 2.9 mm, thickness (T) = 0.9 mm, L/H = 1.9; L/T = 6.0. All other specimens are damaged at the rostrum and cannot be reliably measured. Outer face slightly convex and lobated at centre; lobes mostly not reaching rims but sometimes reaching to the dorsal rim in specimens about 4 mm or larger; smaller specimens strongly lobated at rims but not in centre. Inner face smooth, convex in antero-posterior direction; shallow ventral furrow present along ventral rim; distinct dorsal depression extending along entire crista superior. Dorsal and posterior rims as well as posterior part of ventral rim lobated, sometimes remnants (due to erosion) of fine crenulation present on anterior part of ventral rim; posterior lobation decreasing in largest specimen (Plate 10.1); ventral rim regularly convex, with distinct postventral angle; rostrum pointed and elongated; excisura mostly rounded and connecting to small antirostrum, dorsal rim with a few large lobes and gently sloping to postdorsal angle which generally is the highest point of the otolith and connecting to posterior rim at an angle of ca 90°; posterior rim straight in upper part, then oblique, mostly lobated (sometimes coarsely), with a rounded extension at the height of the posterior part of the cauda. Sulcus median, elongated, deep, with distinct cristae, divided in ostium (ca 40% of sulcus length) and cauda; posterior part of cauda turning toward ventral rim at angle of ca 45°, but not connecting to it. Specimens under 4 mm in length (Plate 10.8) have more crenulated rims and a less sloping dorsal rim with sometimes a less distinct postdorsal angle in very small specimens but with an elongated sulcus readily fitting them in an ontogenetic series of T. reineckei.
292Discussion. Otoliths of species of Trachurus can be encountered in many associations of the Oligocene and Neogene, but mostly damaged and in small numbers, so that are usually left in open nomenclature. One otolith of Carangidae indet. is known from the Boom Formation (Steurbaut & Herman, 1978). We inspected it and found it to be a small and very eroded specimen that might nevertheless belong to the new species. Its state of preservation, however, does not allow any definite conclusion on the identity. One species based on a skull, Belgocaranx luypaertsi Taverne et al., 2006, could not be assigned to any of the extant genera of Carangidae. Leriche (1910, p. 305) mentioned findings of carangid vertebrae, but without further identification. Carangid otoliths discovered in Neogene associations often represent extant species (see Nolf, 2013, p. 99, pls 241–242). An exception is the Miocene Trachurus miosensis Lafond-Grellety in Nolf & Steurbaut, 1979, which can be found in large numbers in Serravallian deposits in SW France (Nolf & Steurbaut, 1979; Steurbaut, 1984). These otoliths differ from those of Trachurus reineckei in the smaller and less rounded to pointed posterior extension, the entirely crenulated ventral rim, the less distinct dorsal depression, a different postdorsal angle and the absence of a ventral furrow and lobation on the outer face. Another early Miocene species well represented in the North Sea Basin is Trachurus elegans Jonet, 1973 (Schwarzhans, 2010, p. 198, 200, pl. 77.1–7), whose otoliths differ from those of T. reineckei in the less sloping dorsal rim, a different postdorsal angle, more and finer crenulation on all rims, and the absence of a marked posterior extension.
293Otoliths of the extant Trachurus picturatus, T. trachurus and T. mediterraneaus were studied in the collection of Recent otoliths as well as from illustrations on the AFORO website (Lombarte et al., 2006) and in Nolf et al. (2009) for T. trachurus. Otoliths of all three species differ from those of T. reineckei in the crenulation of the rims, the absence of an extended posterior rim and in many individuals by the distinct postdorsal angle as well.
294One specimen of Erythrocles cf. ohei in Müller & Rozenberg (2000, pl. 6, fig. 7) shows the characteristics of Trachurus reineckei sp. nov. (including the lobation on the outer face, reaching the dorsal rim) and is herein synonymized with it. The other specimen figured by Müller & Rozenberg (2000, pl. 6, fig. 9) is larger and less high than the previous one, but still within the range of otoliths of T. reineckei, and is tentatively synonymized with it; the uncertainty is due to the almost unlobated dorsal rim (vs strongly lobated in T. reineckei) and the lobation on the outer face is invisible due to attached sediment.
295Schwarzhans (1994) described and figured otoliths of Erythrocles ohei from the Chattian of Germany. We inspected the three type specimens kept at the Gutenberg University (Mainz, Germany) and compared them directly with our material; they are in many aspects similar with our specimens: elongated cauda, all cristae, distance cauda to ventral rim, ventral rim regularly convex, notched posterior rim and lobated outer face (still visible despite erosion). They differ, however, from the new species in the more convex dorsal rim which expands the dorsal portion (vs dorsal rim gently sloping posteriorly in T. reineckei giving the otoliths a smaller dorsal portion), the more oblique posterior rim caused by of the more massive posterior extension, the less lobated rims, a different postdorsal angle and a rounded rostrum (vs pointed rostrum in T. reineckei). An important difference is observed in ventral view: the ventral rim is concave on the outer face in the Chattian otoliths versus convex in those of T. reineckei. Otoliths of T. reineckei also differ from the Chattian Trachurus opprimatus Schwarzhans, 1994, which has a more crenulated ventral rim, a more pointed posterior rim, a more rounded dorsal rim, a posterior widening of the cauda and a different postdorsal rim.
296Otoliths of Erythrocles Jordan, 1919 lack the posterior extension, generally have less convex rims and a more crenulated ventral rim, as opposed to otoliths of the genus Trachurus Rafinesque, 1810 (compare iconography in Lombarte et al., 2006; Nolf et al., 2009; Lin & Chang, 2012). For these reasons, we transfer the Chattian specimens to the genus Trachurus as Trachurus ohei (Schwarzhans, 1994).
297Order Scorpaeniformes Garman, 1899
298Family Cottidae Bonaparte, 1831
299Genus Myoxocephalus Tilesius, 1811
300Myoxocephalus primas (Koken, 1891)
301Plate 12.1–9
3021891 Otolithus (?Agonus) primas Koken, p. 131–132 (not figured).
3031977 Liparis minusculus Nolf, p. 45–46, pl. xiii; figs 14–16.
3041978 Congridarum trapezioides n. sp.; Gaemers & van Hinsbergh, p. 8–9, pl. 2, fig. 3 (based on an eroded specimen).
3052013 “Agonida” minuscula Nolf; Nolf, p. 86, pl. 189.
3062016 “Agonida” minuscula Nolf; Hoedemakers & Schneider, p. 128 (table), pl. 8, fig. 1.
307See Schwarzhans (2008) for a more detailed synonymy list regarding M. primas.
308Our specimens compare well with the type and other specimen described and figured by Weiler (1942, p. 66–67, pl. 4, figs 22–23) and refigured by Schwarzhans (1994, 135, figs 329–330) based on the summary description by Koken (1891, not figured). They show a cauda which is ca ¾ times as long as the ostium, with a large posterior portion behind the cauda and a high dorsal area. In these aspects, they differ from those figured by Schwarzhans (2008, p. 23, fig. 5d–e) which appear more elongate, less high and somewhat more thickset. We figure a growth series of otoliths (Plate 12.1–8) as well as the holotype of Liparis minusculus (Plate 12.9). The sulcus of all specimens shows a long ostium, widely opening on the anterior rim, and a shorter cauda. The outline is very similar in all specimens, with a notched ventral rim in the smaller specimens, the ventral rim gently sloping towards the anterior rim which has a large rostrum but no anti-rostrum. The anterior rim gently connects to the convex dorsal rim which has a straight posterior part with a rounded postdorsal angle connecting with a convex posterior rim. The posterior rim passes into the ventral rim without postventral angle (compare Plate 12.5–6 with Plate 12.9). The small specimens are more thickset in ventral view than the larger ones. The holotype of L. minusculus perfectly fits in the growth series of M. primas and is therefore synonymized with the latter.
309Order Lophiiformes Garman, 1899
310Family Lophiidae Rafinesque, 1810
311Genus Lophius Linnaeus, 1758
312Lophius gibbosus Nolf, 1977
313Plate 11.8
3141977 “genus Lophiidarum” gibbosus Nolf, p. 20–21, pl. xi, fig. 29.
3152013 “Lophiida” gibbosa Nolf; Nolf, p. 71, pl. 145.
316One left otolith, L = 6.4 mm, H = 4.9 mm, T = 1.4 mm (IRSNB P 10380). Our specimen is slightly more than twice as large as the holotype of Lophius gibbosus Nolf, 1977 from the Rupelian of Kruibeke. It is much thinner in ventral view and the posterior rim is rounded versus pointed. Its dorsal rim is more lobated than that of the holotype. Specimens of similar size of the extant species Lophius piscatorius indicate that the fossil specimen from Schelle may have derived from a fish of ca 40 cm total length (TL).
317A comparison with otoliths of the L. piscatorius Linnaeus, 1758 from the Gulf of Biscay was made to understand the growth trend. These modern otoliths show an ontogenetical evolution: small otoliths (L=3–4 mm) derived from fish of 18–20 cm TL are quite thickset overall in ventral view, whereas larger otoliths are thickset only in the central part in ventral view. The posterior rim is acuminated in many otoliths, but sometimes it is rounded, so this character cannot be used with confidence to characterize otolith-based species, even more so as it is independent of ontogeny. Generally, adult otoliths of L. piscatorius become larger anteroposteriorly, but not ventrodorsally. Large otoliths of L. piscatorius become strongly lobated on the dorsal rim. The ventral rim can be slightly convex to almost straight in otoliths of any length. Summarizing, otoliths of L. piscatorius tend to become thinner, more lobated dorsally and larger anteroposteriorly with ontogeny.
318The holotype of L. gibbosus appears quite high and has an L/H ratio of 1.4, whereas this ratio in our specimen is 1.3. Because very few otoliths of L. gibbosus are known, the ontogeny cannot be reconstructed with certainty. The holotype of L. gibbosus and our specimen have a comparable L/H ratio. The holotype is thickset with an unlobated dorsal rim, whereas the specimen in our sample is thinner and has a lobated dorsal rim. After comparison with a growth series of otoliths of L. piscatorius, we conclude that the specimen from the wood-fall assemblage putatively fits in a growth series of L. gibbosus, whereby the holotype is interpreted as having derived from a juvenile fish.
5.6. Miscellanea
319A number of additional fossils were found, including several large benthic foraminifera. Furthermore, a fragment of a portunoid dactylus was encountered (Plate 6.5 - IRSNB 7735), most probably belonging to Coeloma rupeliense Stainier, 1887 (Van Bakel, pers. comm., 2021). This species dominates the crab fauna of the Boom Formation, but is mostly preserved in nodules (Verheyden, 2002).
Table 1. List of taxa found in the silty lens associated with a tree trunk on top of pink R-horizon, Terhagen Member, Boom Formation, Rupelian (NP23), Oligocene.
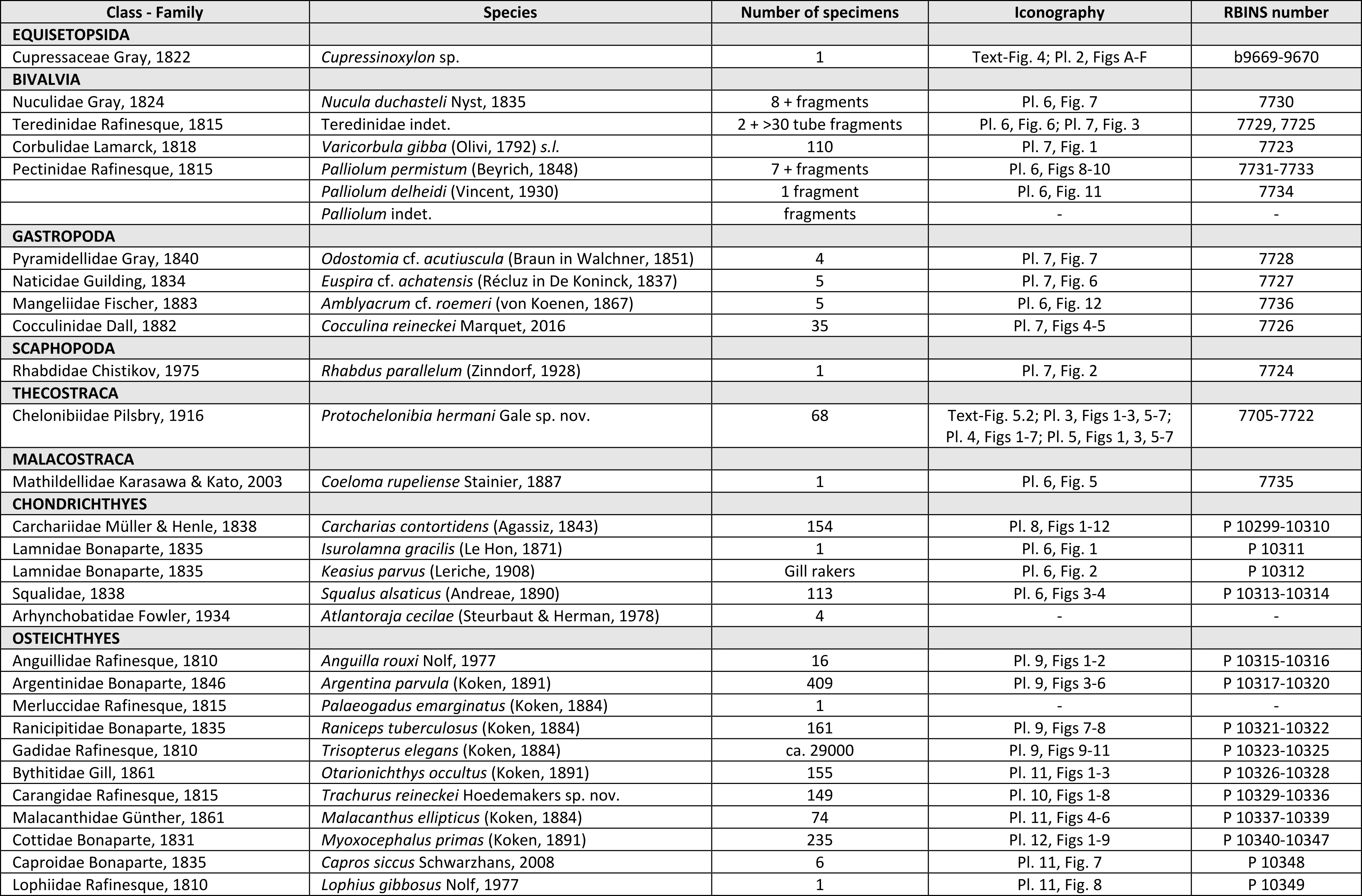
6. Palaeoecology
Driftwood as wood-fall in Rupelian clay deposits in the North Sea Basin
320All fossils described herein were discovered in connection with a fragment of sunken driftwood in a 5 to 10 cm thick layer of silty sediment trapped between the trunk and a side branch. The presence of driftwood in the Boom Formation was reported before by Van den Broeck (1887) and in bed 28 of the Terhagen Member by Vandenberghe (1978, 2017, fig. 9). Terrestrial phytoclasts in the clay are well known on a microscopic scale: black stained layers in the Boom Formation are dark coloured due to the influx of detrital terrestrial organic matter (total organic carbon content of 1–5 wt.%) (Vandenberghe, 1976; Vandenberghe et al., 2014). This model invokes the periodic destruction of coastal vegetation due to eustatic sea-level rise. The vegetation cover of that submerged land is destroyed and the plants are transported into the basin where they are mostly deposited as silt-sized phytoclast particles, and this periodically from the top of an individual silt horizon till the middle of the overlying clay horizon (Vandenberghe, 1976; Vandenberghe et al., 2014). The driftwood has a similar origin as the phytoclasts and only differs from the latter by its size and therefore by its settling history.
321Driftwood in the Boom Formation is occasionally associated with a specific fossil assemblage, with taxa usually different from those occurring in the surrounding soft-bottom environments, as was discovered near a tree trunk in the S30 level at Niel (Marquet & Herman, 2012; Marquet, 2016). According to the explanation of Marquet & Herman (2012), these floating and subsequently submerged wood fragments form a small local slack water environment, assembling a much richer fossil assemblage than usual at any level. A similar phenomenon has been described by Hoedemakers & Schneider (2016) from Bad Freienwalde (Germany), where an unusually high number of otoliths, representing 26 taxa, was concentrated against a piece of driftwood in a similar ‘Rupel Clay/Septarienton’ deposit. This association is dominated by otoliths of Trisopterus elegans (as Palimphemus parvus), Argentina parvula, Palaeogadus compactus and Hoplobrotula acutangula, which together constitute more than 95.5% of all otoliths discovered. The association of fossils and driftwood at Schelle is therefore another rare occasion to further investigate this remarkable phenomenon. The large quantity of macrofossils associated with the studied trunk in Schelle warrants an explanation as macrofossils in the Rupelian clay usually are very dispersed. After sinking to the bottom, the trunk formed a large bottom irregularity, which probably increased local wave-induced turbulence on the seafloor, contributing to the local concentration of relatively coarser sediments and macrofossils near the trunk. Given the low sedimentation rates (the Boom Formation banding pattern is primarily driven by the 41 ka obliquity cycle; Abels et al., 2007), concentrating effects due to turbulence can be substantial after a long time. This process may have taken several hundreds to perhaps a couple of thousand years, until the driftwood was entirely covered by sedimentation. However, such a simple concentration by turbulence cannot be the only explanation for the large size of the encountered fossil assemblage, as the volume of clay that would have to be concentrated would be excessively large in view of the low number of macrofossils usually present in the Boom Clay. The presence of a large number of species unique or very rare to the Boom Formation indicates that the driftwood may have been a particular substrate that allowed colonization by multiple organisms, i.e., a case of wood-fall. In order to assess this special habitat, we compared with cases of wood-fall in past and present as well as with present-day man-made obstacles on the seafloor.
Past and present-day wood-falls and man-made obstacles on the seafloor
322Data from Late Eocene to Miocene wood- and whale-falls from bathyal depths in the NE Pacific are available (Kiel & Goedert, 2006). This wood was heavily bored by teredinid or xylophagain bivalves. All other associated molluscs (among others an unidentified cocculinid gastropod, three species of Nuculana and a pectinid), including predators (e.g., naticids, turrids, Dentalium laneensis), were found in close proximity. No data on elasmobranch or teleost fishes were given.
323The diversity of organisms associated with present-day wood-fall depends on the type of wood and depth of deposition (Bienhold et al., 2013; Saulsbury, 2014; Judge & Barry, 2016). Most present-day experiments (i.e. wood dropped on the sea bottom) are executed at greater depths and involve tree species different from the one at Schelle, but generally develop largely endemic and highly diverse communities with predators upon them within a few years (Voight, 2007; McClain & Barry, 2014; McClain et al., 2016; Webb et al., 2016). These communities are distinct from those in the surrounding sediment (McClain et al., 2016). Early colonizers are wood-boring bivalves (Voight, 2007; Bienhold et al., 2013; McClain & Barry, 2014), but a large diversity of invertebrates was also observed (Judge & Barry, 2016; McClain et al., 2016). No data on fishes are available from these studies.
324Man-made obstacles of all sorts on the seafloor of shallow seas (e.g., oil rigs, platforms for windmills, shipwrecks) constitute artificial reefs and provide hard substrates on soft bottoms, attracting substrate-associated species as well as demersal fish species that live on or near them. Studies show that the density and diversity of this fauna and flora increase over the years (see e.g. Santos et al., 2012; Coolen et al., 2020; De Backer et al., 2021), with higher levels of species richness and abundance than on the soft bottoms at a short distance from them (Zintzen, 2007; Consoli et al., 2015).
325An experimental artificial reef composed of blocks of stabilized coal-fired power station waste, consisting of eight conical units (each ca 1 m high and ca 8 m across), was established in Poole Bay (English Channel, UK) at a depth of ca 10 m to observe the colonization by living organisms (Jensen et al., 1994). The first settlers were tube worms, followed by species of Tunicata, algae, bryozoans and hydroids. Decapoda came and went as mobile species living on site on the artificial reef but also moving away at other times. Worms and sponges moved in as space became available, seasonal colonizers were barnacles. All these species were observed preying on each other. After one year, colonization was complete, meaning that the faunal composition on the artificial reef was comparable with that on natural reefs in the area. The most important fish species associated with different parts of the reef was Trisopterus luscus which preyed extensively on the invertebrates.
326Present-day shipwrecks at shallow depths (<60 m) in the southern North Sea provide protection from predators, currents and sand scouring, and change the hydrodynamic regime in the surrounding sediment (Zintzen, 2007; Lengkeek et al., 2013). Zintzen (2007) observed a shift from a habitat dominated by bivalves and polychaetes on soft sediments to shipwrecks dominated by crustaceans, polychaetes and cnidarians. On shipwrecks, crustaceans are often very abundant (Jensen et al., 1994; Lengkeek et al., 2013); in one case, amphipods and nematodes were the most abundant groups (Mallefet et al., 2008).
327It can be concluded that hard-substrate obstacles on the seafloor are quickly colonized by various groups of invertebrates, which prey and are preyed upon among themselves.
7. Discussion and conclusion
328Based on the sedimentological, taphonomic and faunistic data, combined with the abovementioned studies, a reconstruction can be made of the development of the fossil concentration associated with the driftwood at Schelle. First, the trunk of Cupressinoxylon sp. was transported from the hinterland into the basin by rivers or directly during inundation and destruction of coastal forests by the rising sea. During its transport on the water surface, the driftwood became colonized by xylophagous Teredinidae (see Romano et al., 2020), which was already noted by Van den Broeck (1887). This may even have started in a brackish water area with continuing colonization in the open marine environment, probably also after sinking. The deepest burrow in the wood measured 6 cm. Based on growth rates reported for Teredo navalis (1.8 mm d-1), Teredo gregoryi (0.54 mm d-1) and Lyrodus pedicellatus (0.59 mm d-1) (Edmondson, 1962; Gallager et al., 1981; Paalvast & van der Velde, 2011), colonization by teredinids was probably complete within a few months (at least 30 days, possibly exceeding 100 days). Finally, when the wood became waterlogged, the trunk started to sink to the bottom. After having sunk, the water-logged trunk was also colonized by Cocculina reineckei, using the wood as a substrate to consume its associated wood-degrading bacteria. The trunk provided a natural irregularity on the sea bottom, until it became covered by sediment. The large number of macrofossils associated with the trunk and the fact that it survived at all may indicate that it was at some time accessible for colonization by demersal species, but the exact duration remains unknown. Remains of preserved invertebrates are quite rare, but most of these species were not fossilized and their presence can only be inferred from the presence of predators based on comparison with modern artificial reefs. For example, pyramidellid snails like Odostomia are ectoparasites on polychaetes and molluscs (Cole & Hancock, 1955; Høisæter, 2014). While they are very rare elsewhere in the Boom Formation, the presence of these snails may indicate the presence of polychaetes near the sunken trunk. Our sample also contained abundant otoliths of Trisopterus elegans, a small gadoid species, which probably preyed on the invertebrates on and near the trunk as the present-day Trisopterus luscus does. It is accepted that otoliths commonly enter the sediment through excrements of predators (Nolf, 2013), implying that ‘larger’ predators were also present. Remains of large elasmobranchs are rare, except for Carcharias contortidens and Squalus alsaticus, the latter living benthically. The teeth of Carcharias contortidens were largely derived from a single dead specimen, which may have floated against the tree trunk prior to or during rotting, whereupon its teeth were concentrated, as was the case with the teleost otoliths, probably due to long-term effects of turbulence. Interestingly, no shark vertebrae have been preserved. Some mollusc species are probably present simply because they were common in the palaeoenvironment of the Boom Formation (e.g. Nucula duchasteli, Euspira achatensis). For some other rare species (e.g. Palliolum permistum, Amlyacrum cf. roemeri), it is less clear whether their presence can be linked to the unique environment of the sunken trunk or merely due to the coincidental proximity of their populations.
329The studied fossil association has a unique composition. For the first time since Vincent (1930), complete specimens and fragments of Palliolum permistum have been found, while the gastropod Amblyacrum cf. roemeri is even completely new to the Belgian Rupelian. The same applies to a unique set of well-preserved turtle barnacle compartmental plates (Protochelonibia), never reported before from the Boom Formation, representing the largest known concentration from any location of Rupelian age (see e.g. Collareta et al., 2021; Perreault et al., 2022). These can indirectly attest to the presence of sea turtles, reported before from the Rupelian (e.g. Smets, 1886a, 1886b, 1887a, 1887b, 1888). The mollusc fauna studied here differs considerably from another mollusc assemblage associated with driftwood, found in the septaria level S30 of the Terhagen Member in Niel (Marquet, 2010). For example, the latter fauna contained the bivalve genus Thyasira Leach, 1818. Thyasira is also reported from the Mediterranean in present-day wood-falls (Bienhold et al., 2013), a Miocene whale-fall (Danise et al., 2016) as well as from Cenozoic wood- and whale-falls in the NE Pacific (Kiel & Goedert, 2006). In contrast to our assemblage, large genera like Arctica and Glycymeris were also found associated with the S30 driftwood (Marquet, 2010). Normally, both genera are very rare in the clays of the Boom Formation and are more common in shallow, sandy deposits of the Kerniel and Berg members (Bilzen Formation) and the Grimmertingen Member (Sint-Huibrechts-Hern Formation) in Limburg (Vervoenen, 1995; Marquet et al., 2012). Marquet (2010) mentions two juvenile specimens of Glycymeris lunulata lunulata auct. non. Nyst, 1836 from Niel. Like Glibert’s (1957) specimens, these are eroded. Marquet (2010) therefore suggested that they may have been transported over a long distance, which is quite unusual for the Boom Formation. Besides, also several dozens of Callucina (Callucina) thierensi (Hébert, 1849) were encountered near the S30 driftwood, a species previously not reported from the Boom Formation and absent in our fauna.
330Interestingly, there are also some similarities in molluscs between the Cenozoic wood-falls in the NE Pacific (Kiel & Goedert, 2006) and our association: both contain teredinids, cocculinids, pectinids, naticids and a scaphopod. Another similarity is with present-day shallow-water anomalies (shipwrecks and experimental artificial reefs) where the teleost associations are dominated by a species of Trisopterus. The teleosts show more similarities with a contemporaneous piece of driftwood from Bad Freienwalde (Hoedemakers & Schneider, 2016), with nine species in common, including the same two dominating species (Tristoperus elegans, then described as Palimphemus parvus, and Argentina parvula). Most species indicate open-marine shelf conditions (<100 m). The concentration of otoliths was hypothesized to have occurred through accumulation near an obstruction on the seafloor or by burrowing sedentary predators (Hoedemakers & Schneider, 2016). It seems more likely, however, that the driftwood at Bad Freienwalde provided the same type of artificial reef with associated fauna as the tree trunk at Schelle, combined with long-term concentration due to wave-driven turbulences.
331In conclusion, the presence of a large tree trunk on top of the R-horizon (base bed 22) of the Terhagen Member represents a wood-fall, providing a snapshot of the surrounding biological communities in Schelle during the Rupelian. A mix of common and fairly unique species was preserved in an exceptionally large concentration for the Boom Formation. Since wood-fall is a relatively rare phenomenon in the Boom Formation, future finds could also include the study of microfossils to investigate whether they are concentrated in the same way as macrofossils and which of these, if any, are dominant. Moreover, each trunk seems to have its own unique assemblage of macrofossils, so new finds of driftwood could possibly lead to the discovery of unknown taxa of various groups in the European Rupelian.
Author contributions
332Geert De Borger and Walter Van Remoortel discovered and excavated the driftwood and collected and picked-out the fossils. Pieter De Schutter conceptualised this paper and described the elasmobranch remains. Stijn Everaert wrote the "Geological background” chapter and studied the molluscs. Andy Gale described the turtle barnacles. Kristiaan Hoedemakers studied the otoliths and co-edited the paper. Jakub Sakala and Vít Koutecký identified the woody remains. Stijn Everaert, Kristiaan Hoedemakers and Pieter De Schutter contributed to the “Palaeoecology” and “Discussion and conclusion” chapters.
Acknowledgements
333We would like to express our gratitude to the company Ceulemans (Schelle) for allowing access to the clay pit; to Noël Vandenberghe (KU Leuven) for providing suggestions and corrections on the geological background; to Ronald Janssen (Senckenberg Institut, Germany) for his help with the molluscs; to Barry Van Bakel (Oertijdmuseum, the Netherlands) for identifying the crustacean dactylus; to Annelise Folie (RBINS, Brussels) for the possibility to study material in the collections under her care; to Laetitia Despontin (RBINS) for making the SEM images; to Theo Lambrechts for the photographs of the Squalus and Julien Lalanne (RBINS) for the photographs of the holotype of Liparis minusculus; to Aad Bastemeijer (Den Haag) for providing literature on molluscs; to K. Grimm and L.M. Ebert (both J. Gutenberg University, Mainz) for allowing access to the otolith collection of the Lower Rhine Embayment under their care; to Gunther Cleemput (Ternat) for his technical support; to Annick Anceau (ULiège) and Etienne Steurbaut (RBINS) for their suggestions; and finally to the four reviewers, Alberto Collareta (Università di Pisa, Italy), Noël Vandenberghe (KU Leuven), Chien-Hsiang Lin (Academica Sinica, Taipei, Taiwan) and Ronald Janssen (Senckenberg Institut, Germany) for their helpful comments and suggestions, which greatly improved the quality of this manuscript. The research of JS and VK was supported by the Cooperatio Programme Research Area GEOL and SVV (both Charles University).
Data availability
334All studied specimens are housed in official repositories guaranteeing their long-term safekeeping and availability to other researchers for future studies.
References
335Abels, H.A., Van Simaeys, S., Hilgen, F.J., De Man, E. & Vandenberghe, N., 2007. Obliquity-dominated glacio-eustatic sea level change in the early Oligocene: evidence from the shallow marine siliciclastic Rupelian stratotype (Boom Formation, Belgium). Terra Nova, 19, 65–73. https://doi.org/10.1111/j.1365-3121.2006.00716.x
336Adolfssen, J.S. & Ward, D.J., 2015. Neoselachians from the Danian (early Paleocene) of Denmark. Acta Palaeontologica Polonica, 60, 313–338. https://doi.org/10.4202/app.2012.0123
337Agassiz, L., 1833–1843. Recherches sur les poissons fossiles. Vol. 3. Imprimerie de Petitpierre, Neuchâtel, 390 p.; Atlas, 83 pls. https://doi.org/10.5962/bhl.title.4275
338Albrecht, J.C.H. & Valk, W., 1943. Oligocäne invertebraten von Süd-Limburg. Mededeelingen van de Geologische Stichting, Serie C-IV-1-N°3, 163 p.
339Andreae, A., 1890. Weitere Beiträge zur Kenntniss des Oligocäns im Elsass. Mitteilungen der Geologischen Landesanstalt von Elsass–Lothringen, 3, 105–122.
340Applegate, S.P., 1965. Tooth terminology and variation in sharks with special reference to the sand shark, Carcharias taurus Rafinesque. Los Angeles County Museum Contributions in Science, 86, 3–18.
341Ardila, N.E. & Harasewych, M.G., 2005. Cocculinid and pseudococculunid limpets (Gastropoda: Cocculiniformia) from off the Caribbean coast of Colombia. Proceedings of the Biological Society of Washington, 118, 344–366. https://doi.org/10.2988/0006-324X(2005)118[344:CAPLGC]2.0.CO;2
342Auster, P.J., Cantwell, K., Grubbs, R.D. & Hoy, S., 2020. Observations of deep-sea sharks and associated species at a large food fall on the continental margin off South Carolina, USA (NW Atlantic). Journal of the Ocean Science Foundation, 35, 48–53.
343Baut, J.-P. & Genault, B., 1999. Les Elasmobranches des Sables de Kerniel (Rupélien), à Gellik, Nord Est de la Belgique. Memoirs of the Geological Survey of Belgium, 45, 1–61.
344Beyrich, E., 1848. Zur Kenntniss des tertiären Bodens der Mark Brandenburg. Archiv für Mineralogie, Geognosie, Bergbau und Hüttenkunde, 22, 3–102.
345Bienhold, C., Ristova, P.P., Wenzhöfer, F., Dittmar, T. & Boetius, A., 2013. How deep-sea wood falls sustain chemosynthetic life. PLoS ONE, 8/1, e53590. https://doi.org/10.1371/journal.pone.0053590
346Bohaty, S.M., Zachos, J.C. & Delaney, M.L., 2012. Foraminiferal Mg/Ca evidence for the Southern Ocean cooling across the Eocene–Oligocene transition. Earth and Planetary Science Letters, 317-318, 251–261. https://doi.org/10.1016/j.epsl.2011.11.037
347Bor, T., Verschuere, S. & Reinecke, T., 2012. Miocene Chondrichthyes from Winterswijk-Miste, the Netherlands. Palaeontos, 21, 1–136.
348Bouchet, P., Rocroi, J-P., Hausdorf, B., Kaim, A., Kano, Y., Nützel, A., Parkhaev, P., Schrödl, M. & Strong, E., 2017. Revised classification, nomenclator and typification of Gastropod and Monoplacophoran families. Malacologia, 61, 1–526. https://doi.org/10.4002/040.061.0201
349Braun, A., 1851. Die fossile Fauna des Mainzer Beckens. Wirbellose Thiere. In Walchner, F.A., 1846–1851, Handbuch der Geognosie: zum Gebrauche bei seinen Vorlesungen und zum Selbststudium mit besonderer Berücksichtigung der Geognostischen Verhältnisse des Grossherzogthums Baden; Zweite Auflage. Karlsruhe, Groos, 1112–1142.
350Cappetta, H., 2012. Chondrichthyes: Mesozoic and Cenozoic Elasmobranchii: Teeth. Verlag Dr. Friedrich Pfeil, Munich, Handbook of Paleoichthyology, Vol. 3E., 512 p.
351Cappetta, H. & Nolf, D., 1991. Les sélaciens du Pliocène inférieur de Le-Puget-sur-Argens (Sud-Est de la France). Palaeontographica A, 218, 49–67.
352Cappetta, H. & Nolf, D., 2005. Révision de quelques Odontaspididae (Neoselachii : Lamniformes) du Paléocène et de l’Eocène du Bassin de la Mer du Nord (Revision of some Odontaspididae (Neoselachii: Lamniformes) from the Paleocene and Eocene of the North Sea Basin). Bulletin de l’Institut royal des Sciences naturelles de Belgique, Sciences de la Terre, 75, 237–266.
353Christenhusz, M.J.M., Reveal, J.L., Farjon, A., Gardner, M.F., Mill, R.R. & Chase, M.W., 2011. A new classification and linear sequence of extant gymnosperms. Phytotaxa, 19, 55–70. https://doi.org/10.11646/phytotaxa.19.1.3
354Cole, H.A. & Hancock, D.A., 1955. Odostomia as a pest of oysters and mussels. Journal of the Marine Biological Association of the United Kingdom, 34, 25–31. https://doi.org/10.1017/S0025315400008584
355Collareta, A. & Bianucci, G., 2021. The occurrence of the coronuloid barnacle Chelonibia Leach, 1817 as an encruster on mammalian bone in the central Mediterranean Sea. Acta Adriatica, 62, 83–92. https://doi.org/10.32582/aa.62.1.6
356Collareta, A. & Newman, W.A., 2020. Protochelonibia melleni (Zullo, 1982) comb. nov., an archaic barnacle from the lower Oligocene of Mississippi (USA), and its impact on the stratigraphic and geographic distribution of the early coronuloids of Western Tethys. Bollettino della Società Paleontologica Italiana, 59, 179–181.
357Collareta, A., Harzhauser, M. & Rasser, M.W., 2021. New and overlooked occurrences of the rarely reported protochelonibiine “turtle” barnacles from the Oligocene and Miocene of Europe. PalZ, 96, 197–206. https://doi.org/10.1007/s12542-021-00576-5. [Published online 4 October 2021, printed June 2022].
358Collareta, A., Newman, W.A., Bosio, G. & Coletti, G., 2022a. A new chelonibiid from the Miocene of Zanzibar (East Africa) sheds light on the evolution of shell architecture in turtle and whale barnacles (Cirripedia: Coronuloidea). Integrative Zoology, 17, 24–43. https://doi.org/10.1111/1749-4877.12554
359Collareta, A., Rasser, M.W., Frey, E. & Harzhauser, M., 2022b. Turtle barnacles have been turtle riders for more than 30 million years. PalZ, 97, 353–363. https://doi.org/10.1007/s12542-022-00641-7. [Published online 17 October 2022, printed June 2023].
360Compagno, L.J.V., 1984. FAO Species Catalogue. Sharks of the world: an annotated and illustrated catalogue of shark species known to date. Vol. 4, part 1. United Nations Development Programme, Rome, FAO Fisheries Synopsis, 125, 249 p.
361Consoli, P., Martino, A., Romeo, T., Sinopoli, M., Perzia, P., Canese, S., Vivona, P. & Andaloro, F., 2015. The effect of shipwrecks on associated fish assemblages in the central Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom, 95/1, 17–24. https://doi.org/10.1017/S0025315414000940
362Coolen, J.W., van der Weide, B., Cuperus, J., Blomberg, M., Van Moorsel, G.W., Faasse, M.A., Bos, O.G., Degraer, S. & Lindeboom, H.J., 2020. Benthic biodiversity on old platforms, young wind farms, and rocky reefs. ICES Journal of Marine Science, 77/3, 1250–1265. https://doi.org/10.1093/icesjms/fsy092
363Cunningham, S., 2000. A comparison of isolated teeth of early Eocene Striatolamia macrota (Chondrichthyes, Lamniformes), with those of a Recent sand shark, Carcharias taurus. Tertiary Research, 20/1-4, 17–31.
364Danise, S., Bertolaso, L. & Dominici, S., 2016. Bathymodioline mussel dominated Miocene whale fall from Italy. Bollettino della Società Paleontologica Italiana, 55/1, 47–53. https://doi.org/10.4435/BSPI.2016.05
365De Alessandri, G., 1895. Contribuzione allo studio dei Cirripedi fossili d’Italia. Bolletino della Società Geologica d’Italia, 13, 234–314.
366De Alessandri, G., 1906. Studi monografici sui Cirripedi fossili d’Italia. Palaeontographica Italia, 12, 207–324.
367De Backer, A., Wyns, L. & Hostens, K., 2021. Continued expansion of the artificial reef effect in soft-sediment epibenthos and demersal fish assemblages in two established (10 years) Belgian offshore wind farms. In Degraer S., Brabant R., Rumes B. & Vigin L. (eds), Environmental Impacts of Offshore Wind Farms in the Belgian Part of the North Sea: Attraction, avoidance and habitat use at various spatial scales. Royal Belgian Institute of Natural Sciences, Brussels, Memoirs on the Marine Environment, 2021, 61–68.
368De Koninck, L., 1838. Description des coquilles fossiles de l’argile de Basele, Boom, Schelle etc. Nouveaux Mémoires de l’Académie royale des Sciences et Belles Lettres de Bruxelles, 11, 1–37.
369De Man, E. & Van Simaeys, S., 2004. Late Oligocene Warming Event in the southern North Sea Basin: benthic foraminifera as paleotemperature proxies. Netherlands Journal of Geosciences / Geologie en Mijnbouw, 83, 227–239. https://doi.org/10.1017/S0016774600023520
370Dolezych, M. & Schneider, W., 2006. Inkohlte Hölzer und Cuticulae dispersae aus dem 2. Miozänen Flözhorizont im Tagebau Welzow (Lausitz) – Taxonomie und vergleichende feinstratigraphisch-fazielle Zuordnung. Zeitschrift für Geologische Wissenschaften, 34, 165–259.
371Ebert, D.A., Dando, M., & Fowler, S. 2021. Sharks of the World. A complete guide. 2nd ed. Princeton University Press, Princeton, New Jersey, 607 p.
372Edmondson, C.H., 1962. Teredinidae, ocean travelers. Occasional Papers of Bernice P. Bishop Museum, Honolulu, Hawaii, 23, 45–59.
373Esteban, L.G., de Palacios, P., Guindeo, A. & García, F., 2004. Characterisation of the xylem of 352 conifers. Investigación agraria. Sistemas y recursos forestales, 13, 452–478.
374Everaert, S., De Schutter, P., Mariën, G., Cleemput, G., Van Boeckel, J., Rondelez, D. & Bor, T., 2019. Een vroeg-miocene fauna uit het Zand van Kiel (Formatie van Berchem) bij Post X in Berchem (Antwerpen). Afzettingen WTKG, 40, 83–100.
375Fischer, P., 1886. Description d’un nouveau genre de Cirrhipèdes (Stephanolepas) parasite des tortues marines. Actes de la Société linnéenne de Bordeaux, 4, 193–196.
376Gaemers, P.A.M. & van Hinsbergh, V.W.M., 1978. Rupelian (Middle Oligocene) fish otoliths from the clay pit ‘De Vlijt’ near Winterswijk, The Netherlands. Scripta Geologica, 46, 1–77.
377Gallager, S.M., Turner, R.D. & Berg Jr, C.J., 1981. Physiological aspects of wood consumption, growth, and reproduction in the shipworm Lyrodus pedicellatus Quatrefages (Bivalvia: Teredinidae). Journal of Experimental Marine Biology and Ecology, 52, 63–77. https://doi.org/10.1016/0022-0981(81)90171-4
378Génault, B., 2012. Le contenu paléontologique. Vertébrés. Requins et raies (chondrichtyens). In Lozouet, P. (ed.), Stratotype Stampien. Muséum national d’Histoire naturelle, Paris; Biotope, Mèze, Patrimoine géologique, 4, 299–308.
379Glibert, M., 1955. Quelques espèces nouvelles ou mal connues de l’Oligocène moyen et supérieur de la Belgique. Bulletin de l’Institut royal des Sciences naturelles de Belgique, 31/86, 1–7.
380Glibert, M., 1957. Pélécypodes et gastropodes du Rupélien et du Chattien de la Belgique. Institut royal des Sciences naturelles de Belgique, Mémoire, 137, 1–9.
381Glibert, M. & de Heinzelin de Braucourt, J., 1954. L’Oligocène inférieur belge. In Volume jubilaire. Victor Van Straelen, directeur de l’Institut royal des Sciences naturelles de Belgique, 1925-1954. F. Hayez, Bruxelles, 279–438.
382Gürs, K. & Janssen, A.W., 2004. Sea-level related molluscan plankton events (Gastropoda, Euthecosomata) during the Rupelian (Early Oligocene) of the North Sea Basin. Netherlands Journal of Geosciences / Geologie en Mijnbouw, 83, 199–208. https://doi.org/10.1017/S0016774600020278
383Harzhauser, M., Newman, W. A. & Grunert, P., 2011. A new Early Miocene barnacle lineage and the roots of sea- turtle fouling Chelonibiidae (Cirripedia, Balanomorpha). Journal of Systematic Palaeontology, 9, 473–480. https://doi.org/10.1080/14772019.2010.528053
384Hayashi, R., 2013. A checklist of turtle and whale barnacles (Cirripedia: Thoracica: Coronuloidea). Journal of the Marine Biological Association of the United Kingdom, 93, 143–182. https://doi.org/10.1017/S0025315412000847
385Herman, J., 1979. Réflexions sur la systématique des Galeoidei et sur les affinités du genre Cetorhinus à l’occasion de la découverte d’éléments de la denture d’un exemplaire fossile dans les sables du Kattendijk à Kallo (Pliocène inférieur, Belgique). Annales de la Société géologique de Belgique, 102, 357–377.
386Herman, J. & Marquet, R., 2012. Reinvestigation of the invertebrate fauna of the Boom Clay Formation and the Ruisbroek Sand Member (Oligocene, Rupelian) of Belgium, with the description of a new lithostratigraphic unit: the Sint Niklaas Phosphorite Bed. Cainozoic Research, 9, 101–120.
387Herman, J., Hovestadt-Euler, M. & Hovestadt, D.C., 1989. Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living superspecific taxa of Chondrichthyan fishes. Part A: Selachii. No. 3: Order: Squaliformes - Families: Echinorhinidae, Oxynotidae and Squalidae. Bulletin de l’Institut royal des Sciences naturelles de Belgique, Biologie, 59, 101–158.
388Herman, J., Van Waes, H., Doutrelepont, H., Kenis, L., Van Nuffel, J., Cloetens, J., Vanderhoeft, E. & Vervoenen, M., 2013. Additional Contributions to the Knowledge of the Sediments, Taphonomy, Ichnofossils, Bacteria, Invertebrata, Vertebrata, Algae and Plantae of the Sint Niklaas Phosphorite Bed in its type locality: Sint Niklaas (Eastern Flanders, Belgium). Part Three: Vertebrata. Géominpal Belgica 5.3.
389Høisæter, T., 2014. The Pyramidellidae (Gastropoda, Heterobranchia) of Norway and adjacent waters. A taxonomic review. Fauna norvegica, 34, 7–78. https://doi.org/10.5324/fn.v34i0.1672
390Hrs-Brenko, M., 2006. The basket shell, Corbula gibba Olivi, 1792 (Bivalve Mollusks) as a species resistant to environmental disturbances: a review. Acta Adriatica: International Journal of Marine Sciences, 47, 49–64.
391Hoedemakers, K. & Schneider, S., 2016. Fish otoliths from the Rupelian (Early Oligocene) of Bad Freienwalde (NE Germany). Paläontologische Zeitschrift, 90, 125–144. https://doi.org/10.1007/s12542-015-0278-0
392Höltke, O., Unger, E., Pollerspöck, J. & Rasser, M.W., 2020. The elasmobranch fauna from the Upper Marine Molasse (Lower Miocene, Burdigalian) of Ursendorf (SW-Germany). Palaeontos, 33, 1–53.
393Hovestadt, D.C. & Hovestadt-Euler, M., 1995. Additions to the fauna of the Boom Clay Formation of Belgium (Rupelian, Oligocene). Taxonomic adjustments on the Scyliorhinidae and Rajoidae, discovery of a dasyatid species (Pisces, Chondrichthyes) and of a curculionid species (Insecta, Coleoptera). In Herman, J. & Van Waes, H. (eds), Elasmobranches et Stratigraphie. Belgian Geological Survey, Professional Paper, 278, 261–282.
394Hovestadt, D.C. & Hovestadt-Euler, M., 2012. A partial skeleton of Cetorhinus parvus Leriche, 1910 (Chondrichthyes, Cetorhinidae) from the Oligocene of Germany. Paläontologische Zeitschrift, 86/1, 71–83. https://doi.org/10.1007/s12542-011-0118-9
395Hovestadt, D. & Steurbaut, E., 2023. Annotated iconography of the type specimens of fossil chondrichthyan fishes in the collection of the Royal Belgian Institute of Natural Sciences, Brussels. Royal Belgian Institute of Natural Sciences, Brussels, Monographs in Natural Sciences, 1, 122 p.
396Hovestadt, D.C., Hovestadt-Euler, M., & Micklich, N., 2010. A review of the chondrichthyan fauna of Grube Unterfeld (Frauenweiler) clay pit. Kaupia. Darmstädter Beiträge zur Naturgeschichte, 17, 57–71.
397Hutchinson, D.K., Coxall, H.K., Lunt, D.J., Steinthorsdottir, M., de Boer, A.M., Baatsen, M., von der Heydt A., Huber, M., Kennedy-Asser, A.T., Kunzmann, L., Ladant, J.-B., Lear, C.H., Moraweck, K., Pearson, P.N., Piga, E., Pound, M.J., Salzmann, U., Scher, H.D., Sijp, W.P., Sliwinska, K.K., Wilson, P.A. & Zhang, Z., 2021. The Eocene–Oligocene transition: a review of marine and terrestrial proxy data, models and model-data comparisons. Climate of the Past, 17/1, 269–315. https://doi.org/10.5194/cp-17-269-2021
398Janssen, A.W., 1984. Mollusken uit het Mioceen van Winterswijk-Miste: een inventaris, met beschrijving en afbeelding van alle aangetroffen soorten. Koninklijke Nederlandse Natuurhistorische Vereniging, Nederlandse Geologische Vereniging, Rijksmuseum van Geologie en Mineralogie, Leiden, 451 p.
399Janssen, A.W., 1989. Some new Pteropod species from the North Sea Basin Cainozoic (Mollusca: Gastropoda, Euthecosomata). Mededelingen van de Werkgroep voor Tertiaire en Kwartaire Geologie, 26, 91–133.
400Janssen, A.W. & Dijkstra, H.H., 1996. Morphological differences between two species of Palliolum (Bivalvia: Pecinidae). Basteria, 59, 107–113.
401Janssen, R., 1978. Die Mollusken des Oberoligozäns (Chattium) im Nordsee-Becken. 1. Scaphopoda, Archaeogastropoda, Mesogastropoda. Archiv für Molluskenkunde, 109, 137–227.
402Janssen, R., 1979. Die Mollusken des Oberoligozäns (Chattium) im Nordsee-Becken. 2. Neogastropoda, Euthyneura, Cephalopoda. Archiv für Molluskenkunde, 109, 277–376.
403Jensen, A.C., Collins, K.J., Lockwood, A.P.M., Mallinson, J.J. & Turnpenny, W.H., 1994. Colonization and fishery potential of a coal-ash artificial reef, Poole Bay, United Kingdom. Bulletin of Marine Science, 55/2-3, 1263–1276.
404Judge J. & Barry J.P., 2016. Macroinvertebrate community assembly on deep-sea wood falls in Monterey Bay is strongly influenced by wood type. Ecology, 97/11, 3031–3043. https://doi.org/10.1002/ecy.1546
405Kiel, S. & Goedert J.L., 2006. Deep-sea food bonanzas: early Cenozoic whale-fall communities resemble wood-fall rather than seep communities. Proceedings of the Royal Society B, 273, 2625–2631. https://doi.org/10.1098/rspb.2006.3620
406Kłusek, M., 2014. Miocene coniferous woods of the Polish Carpathian Foredeep. Acta Palaeontologica Polonica, 59, 697–708. https://doi.org/10.4202/app.2011.0158
407Koken, E., 1891. Neue Untersuchungen an tertiären Fisch-Otolithen. II. Zeitschrift der Deutschen Geologischen Gesellschaft, 43, 77–170.
408Lagrou, D., Vandenberghe, N., Van Simaeys, S. & Hus, J., 2004. Magnetostratigraphy and rock magnetism of the Boom Clay (Rupelian stratotype) in Belgium. Netherlands Journal of Geosciences / Geologie en Mijnbouw, 83/3, 209–225. https://doi.org/10.1017/S001677460002028X
409Le Hon, H., 1871. Préliminaires d’un mémoire sur les poissons tertiaires de Belgique. H. Merzbach, Bruxelles, 15 p.
410Lengkeek, W., Coolen, J., Gittenberger, A. & Schrieken, N., 2013. Ecological relevance of shipwrecks in the North Sea. Nederlandse faunistische Mededelingen, 41, 49–57.
411Leriche, M., 1908. Sur un appareil fanonculaire de Cetorhinus trouvé à l’état fossile dans le Pliocène d’Anvers. Comptes rendus hebdomadaires des séances de l’Académie des Sciences, Paris, 146, 875–878.
412Leriche, M., 1910. Les poissons tertiaires de la Belgique. III. Les poissons oligocènes. Mémoires du Musée royal d’Histoire naturelle de Belgique, 5, 229–363.
413Lin, C.-H. & Chang, C.-W., 2012. Otolith Atlas of Taiwan Fishes. National Museum of Marine Biology and Aquarium, NMMBA Atlas Series, 12, 415 p.
414Linnaeus, C., 1758. Systema Naturae, Edition 10. Holmiae. Reformata, 1, 824 p.
415Lombarte, A., Chic, Ò., Parisi-Baradad, V., Olivella, R., Piera, J. & García-Ladona, E., 2006. A web-based environment from shape analysis of fish otoliths. The AFORO database. Scientia Marina, 70, 147–152. Available from http://aforo.cmima.csic.es, accessed 04/07/2023.
416Lozouet, P. & Maestrati, P., 2012. Le contenu paléontologique. Mollusques. In Lozouet, P. (ed.), Stratotype Stampien. Muséum national d’Histoire naturelle, Paris; Biotope, Mèze, Patrimoine géologique, 4, 239–297.
417Mallefet, J., Vanden Berghe, E., Vincx, M., Massin, C. & Norro, A., 2008. Belgian Shipwreck: Hotspots for Marine Biodiversity (BEWREMABI). Part 2. Global Change, Ecosystems and Biodiversity. Belspo, Brussels, 154 p.
418Marquet, R., 2005. The Neogene Bivalvia (Heterodonta and Anomalodesmata) and Scaphopoda from Kallo and Doel (Oost-Vlaanderen, Belgium). Palaeontos, 6, 1–142.
419Marquet, R., 2010. Reassessment of the Bivalvia (Mollusca) from the Boom Formation (Rupelian, Oligocene) of Belgium, with description of new species. Bulletin de l’Institut royal des Sciences naturelles de Belgique, Sciences de la Terre, 80, 253–282.
420Marquet, R., 2016. A revision of the gastropod fauna of the Boom Clay Formation (Rupelian, Early Oligocene) in Belgium, 1. Vetigastropoda and Caenogastropoda to Eulimoidea. Cainozoic Research, 16, 13–33.
421Marquet, R. & Herman, J., 2012. Reinvestigation of the invertebrate fauna of the Boom Clay Formation and the Ruisbroek Sand Member (Oligocene, Rupelian) of Belgium, with the description of a new lithostratigraphic unit: the Sint Niklaas Phosphorite Bed. Cainozoic Research, 9, 101–120.
422Marquet, R., Lenaerts, J. & Laporte, J., 2012. A systematic study of the Bivalvia (Mollusca) from the Grimmertingen Sand Member and from the Klimmen Member (Early Oligocene) in Belgium and the Netherlands. Palaeontos, 22, 1–156.
423Marquet, R., Lenaerts, J. & Laporte, J., 2016. A systematic study of the Gastropoda (Mollusca) of the Grimmertingen Sand Member (Early Oligocene) in Belgium. Palaeontos, 29, 1–159.
424Marshall, B.A., 1985. Recent and Tertiary Cocculinidae and Pseudococculinidae (Mollusca: Gastropoda) from New Zealand and New South Wales. New Zealand Journal of Zoology, 12, 505–546. https://doi.org/10.1080/03014223.1985.10428301
425McArthur, A.G., & Harasewych, M.G., 2003. Molecular systematics of the major lineages of the Gastropoda. In Lydeard, C. & Lindberg, D.R. (eds), Molecular Systematics and Phylogeography of Mollusks. Smithsonian Books, Washington, 140–160.
426McClain, C. & Barry, J., 2014. Beta-diversity on deep-sea wood falls reflects gradients in energy availability. Biology Letters, 10, e20140129. https://doi.org/10.1098/rsbl.2014.0129
427McClain, C., Barry, J., Eernisse, D., Horton, T., Judge, J., Kakui, K., Mah, C. & Warén, A., 2016. Multiple processes generate productivity-diversity relationships in experimental wood-fall communities. Ecology, 97/4, 885–898. https://doi.org/10.1890/15-1669.1
428McLean, J.H., & Harasewych, M.G., 1995. Review of Western Atlantic Species of Cocculinid and Pseudococculinid Limpets, with Descriptions of New Species (Gastropoda: Cocculiniformia). Contributions in Science, 453, 1–33. https://doi.org/10.5962/p.208088
429Miller, K.G., Wright, J.D. & Fairbanks, R.G., 1991. Unlocking the ice house: Oligocene-Miocene oxygen isotopes, eustasy, and margin erosion. Journal of Geophysical Research, 96/B4, 6829–6848. https://doi.org/10.1029/90JB02015
430Miller, K.G., Kominz, M.A., Browning, J.V., Wright, J.D., Mountain, G.S., Katz, M.E., Sugarman, P.J., Cramer, B.S., Christie-Blick, N. & Pekar, S.F., 2005. The Phanerozoic record of global sea-level change. Science, 310, 1293–1298. https://doi.org/10.1126/science.1116412
431Moerdijk, P.W., Janssen, A.W., Wesselingh, F.P., Peeters, G.A., Pouwer, R., Van Nieulande, F.A.D., Janse, A.C., Van der Slik, L., Meijer, T., Rijken, R., Cadée, G.C., Hoeksema, D., Doeksen, G., Bastemeijer, A., Strack, H., Vervoenen, M. & Ter Poorten, J.J., 2010. De fossiele schelpen van de Nederlandse kust. Nederlands Centrum voor Biodiversiteit Naturalis, Leiden, 332 p.
432Moths, H., 2000. Die Molluskenfauna im Rupelton der Ziegeleitongrube Malliß im Wanzeberg (südwestl. Mecklenburg-Vorpommern). Regionalmuseum des Amtes Malliß (Mecklenburg-Vorpommern), 103 p.
433Moths, H., Montag, A. & Grant, A., 1996. Die Molluskenfauan des oberoligozänen “Sternberger Gesteins” (Chattium) von Norddeutschland. 1. Teil: Scaphopoda, Archaeogastropoda, Mesogastropoda. Erratica, 1, 3–62.
434Müller, A., 2011. Der Steinbruch Mammendorf NW Magdeburg – ein Felslitoral der unteroligozänen Nordsee. Geologica Saxonica, 57, 3–120.
435Müller, A. & Rozenberg, A., 2000. Fischotolithen (Pisces: Teleostei) aus dem Unteroligozän Mitteldeutschlands. Leipziger Geowissenschaften, 12, 71–141.
436Naylor, G.J.P., Martin, A.P., Matisson, E.G. & Brown, W.M., 1997. Interrelationships of lamniform sharks: Testing phylogenetic hypotheses with sequence data. In Kocher, T.D & Stepien, C.A. (eds), Molecular Systematics of Fishes. Academic Press, London, 199–218. https://doi.org/10.1016/B978-012417540-2/50014-2
437Nelson, J., Grande, T.C. & Wilson, M.V.H., 2016. Fishes of the World. 5th ed. Wiley, Hoboken, NJ, 707 p. https://doi.org/10.1002/9781119174844
438Neuffer, F.O., 1973. Die Bivalven des Unteren Meeressandes (Rupelium) im Mainzer Becken. Abhandlungen des Hessischen Landesamtes für Bodenforschung, 68, 1–113.
439Nolf, D., 1977. Les otolithes des téléostéens de l’Oligo-Miocène belge. Annales de la Société royale zoologique de Belgique, 106, 3–119.
440Nolf, D., 1988. Haaie- en Roggentanden uit het Tertiair van Belgie. Koninklijk Belgisch Instituut voor Natuurwetenschappen, Brussels, 171 p.
441Nolf, D., 2013. The Diversity of Fish Otoliths, Past and Present. Royal Belgian Institute of Natural Sciences, Brussels, 222 p.
442Nolf, D., & Steurbaut, E., 1979. Les otolithes de téléostéens des faluns sallomaciens d’Orthez et de Sallespisse (Miocène moyen d’Aquitaine méridionale, France). Palaeontographica A, 164, 1–23.
443Nolf, D., De Potter, H. & Lafond-Grellety, J., 2009. Hommage à Joseph Chaine et Jean Duvergier. Diversité et variabilité des otolithes de poissons. Palaeo Publishing & Library vzw, Antwerpen, 59 p.
444Nyst, H., 1835. Recherches sur les coquilles fossiles de la province d’Anvers. Perichon, Bruxelles, 1–36.
445Nyst, H., 1836. Recherches sur les coquilles fossiles de Kleyn-Spauwen et Housselt (province du Limbourg). Messager des Sciences et des Arts de la Belgique, 4, 139–180.
446Nyst, P.-H., 1845. Description des coquilles et des polypiers fossiles des terrains tertiaires de la Belgique. Mémoires couronnés et Mémoires des Savants étrangers publiés par l’Académie royale des Sciences et Belles-Lettres de Bruxelles, 17, 1–697.
447Paalvast, P. & van der Velde, G., 2011. Distribution, settlement, and growth of first-year individuals of the shipworm Teredo navalis L. (Bivalvia: Teredinidae) in the Port of Rotterdam area, the Netherlands. International Biodeterioration & Biodegradation, 65, 379–388. https://doi.org/10.1016/j.ibiod.2010.11.016
448Pérez-Losada, M., Høeg J.T., Simon-Blecher, N., Achituv, Y., Jones, D. & Crandall, K.A., 2014. Molecular phylogeny, systematics and morphological evolution of the acorn barnacles (Thoracica: Sessilia: Balanomorpha). Molecular Phylogenetics and Evolution, 81, 147–158. https://doi.org/10.1016/j.ympev.2014.09.013
449Perreault, R.T., Collareta, A. & Buckeridge, J.S., 2022. A new species of the archaic “turtle barnacle” Protochelonibia (Coronuloidea, Chelonibiidae) from the upper Rupelian Chickasawhay Formation of Mississippi (USA). Neues Jahrbuch für Geologie und Paläontologie, 305, 225–235. https://doi.org/10.1127/njgpa/2022/1087
450Poppe, G.T. & Goto, Y., 1993. European Seashells, volume II (Scaphopoda, Bivalvia, Cephalopoda). Verlag Crista Hemmen, Wiesbaden, 221 p.
451Prothero, D.R., Ivany, L. & Nesbitt, E., 2003. From Greenhouse to Icehouse: The Marine Eocene-Oligocene Transition. New York, Columbia University Press, 541 p.
452Reinecke, T., 2015. Batoids (Rajiformes, Torpediniformes, Myliobatiformes) from the Sülstorf Beds (Chattian, Late Oligocene) of Mecklenburg, northeastern Germany: a revision and description of three new species. Palaeovertebrata, 39, e2, 1–32. https://doi.org/10.18563/pv.39.2.e2
453Reinecke, T., Stapf, H. & Raisch, M., 2001. Die Selachier und Chimären des Unteren Meeressandes und Schleichsandes im Mainzer Becken (Rupelium, Unteres Oligozän). Palaeontos, 1, 1–73.
454Reinecke, T., Louwye, S., Havekost, U. & Moths, H., 2011. The elasmobranch fauna of the late Burdigalian, Miocene, at Werder-Uesen, Lower Saxony, Germany and its relationships with Early Miocene Faunas in the North Atlantic, central Paratethys and Mediterranean. Palaeontos, 20, 1–170.
455Reinecke, T., von der Hocht, F. & Dufraing, L., 2015. Fossil basking shark of the genus Keasius (Lamniformes, Cetorhinidae) from the boreal North Sea Basin and Upper Rhine Graben: evolution of dental characteristics from the Oligocene to late Middle Miocene and description of two new species. Palaeontos, 28, 39–98.
456Robin, N., Velasquez, M., Boura, A., Garcia, G., Jauvion, C., Boiteau, J-M., Gomez, B., Daviero-Gomez, V. & Valentin, X., 2018. The oldest shipworms (Bivalvia, Pholadoidea, Teredinidae) preserved with soft parts (Western France): insights into the fossil record and evolution of Pholadoidea. Palaeontology, 61, 905–918. https://doi.org/10.1111/pala.12376
457Román-Jordán, E., Esteban, L.G., de Palacios, P. & Fernández, F.G., 2016. Wood anatomy of Cupressus and its relation to geographical distribution. IAWA Journal, 37, 48–68. https://doi.org/10.1163/22941932-20160120
458Romano C., Nunes-Jorge A., Le Bris N., Rouse G.W., Martin D. & Borowski C., 2020. Wooden stepping stones: diversity and biogeography of deep-sea wood boring Xylophagaidae (Mollusca: Bivalvia) in the North-East Atlantic Ocean, with the description of a new genus. Frontiers in Marine Sciences, 7, e579959. https://doi.org/10.3389/fmars.2020.579959
459Ross, A. & Frick, M.G., 2007. Systematics of the coronuloid barnacles. In Epibiont Research Cooperative (ed.), A Synopsis of the Literature on the Turtle Barnacles (Cirripedia: Balanomorpha: Coronuloidea), 1758–2007. Epibiont Research Cooperative Special Publication, 1, 23–38.
460Sadowsky, V., 1970. On the dentition of the sand shark, Odontaspis taurus, from the vicinity of Cananéia, Brazil. Boletim do Instituto Oceanografico, Sao Paulo, 18/1, 37–44. https://doi.org/10.1590/S0373-55241969000100005
461Sakala, J., 2003. Podocarpoxylon helmstedtianum Gottwald from Kučlín (Late Eocene, Czech Republic) reinterpreted as Tetraclinoxylon vulcanense Privé. Feddes Repertorium, 114, 25–29. https://doi.org/10.1002/fedr.200390015
462Sandberger, F., 1858–1863. Die Conchylien des Mainzer Tertiärbeckens. Kreidel, Wiesbaden, (1-2): 1–72 (1858), (3): 73–112 (1859), (4): 113–152 (1860), (5-6): 153–232 (1861), (7): 233–272 (1862), (8): 273–458 (1863).
463Santos, M.N., Oliveira, M.T. & Cúrdia, J., 2012. A comparison of the fish assemblages on natural and artificial reefs off Sal Island (Cape Verde). Journal of the Marine Biological Association of the United Kingdom, 93/2, 437 452. https://doi.org/10.1017/S0025315412001051
464Saulsbury, J.G., 2014. Allochthonous woody input into a tropical shallow marine lagoon and its effect on biodiversity. Moorea Research Symposium 2014. http://www.moorea-ucb.org/uploads/6/6/8/3/6683664/saulsbury_final_paper.pdf, accessed 01/29/2023.
465Schnetler, K.I. & Beyer, C., 1987. A Late Oligocene (Chattian B) mollusc fauna from the clay-pit of Galten Brickworks at Nørre Vissing, Jylland, Denmark. Mededelingen van de Werkgroep voor Tertaire en Kwartaire Geologie, 24, 193–224.
466Schwarzhans, W., 1994. Die Fisch-Otolithen aus dem Oberoligozän der Niederrheinischen Bucht. Systematik, Palökologie, Paläobiogeographie, Biostratigraphie und Otolithen-Zonierung. Geologisches Jahrbuch A, 140, 3–248.
467Schwarzhans, W., 2008. Otolithen aus küstennahen Sedimenten des Ober-Oligozän der Niederrheinischen Bucht (Norddeutschland). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 248/1, 11–44. https://doi.org/10.1127/0077-7749/2008/0248-0011
468Schwarzhans, W., 2010. The Otoliths from the Miocene of the North Sea Basin. Backhuys Publishers, Leiden; Margraf Publishers, Weikersheim, 352 p.
469Siverson, M., 1999. A new large lamniform shark from the uppermost Gearle Siltstone (Cenomanian, Late Cretaceous) of Western Australia. Transactions of the Royal Society of Edinburgh: Earth Sciences, 90, 49–66. https://doi.org/10.1017/S0263593300002509
470Smets, G., 1886a. Les Tortues Rupéliennes. Annales de la Société scientifique de Bruxelles, 10, 12–14.
471Smets, G., 1886b. Chelone Van Benedenii. Annales de la Société scientifique de Bruxelles, 10, 109–128.
472Smets, G., 1887a. Chelone (Bryochelys) waterkeynii, Van Ben. Annales de la Société scientifique de Bruxelles, 11, 291–302.
473Smets, G., 1887b. Chelyopsis littoreus, Van Ben. Annales de la Société scientifique de Bruxelles, 11, 303–307.
474Smets, G., 1888. Les Chélonées Rupéliennes. Annales de la Société scientifique de Bruxelles, 12, 193–214.
475Speijer, R.P., Pälike, H., Hollis, C.J., Hooker, J.J. & Ogg, J.G., 2020. The Paleogene period. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D. & Ogg, G.M. (eds), Geologic Time Scale 2020. Elsevier, Amsterdam, 1087–1140. https://doi.org/10.1016/B978-0-12-824360-2.00028-0
476Stein, G., Moths, H., Albrecht, F., Havekost, U. & Fehse, D., 2016. Revision der Miozänen Molluskenfauna (Hemmoorium) von Werder bei Achim (Nordwest-Niedersachsen). Palaeofocus, 5, 289 p.
477Steurbaut, E. 1984. Les otolithes de téléostéens de l’Oligo-Miocène d’Aquitaine (sud-ouest de la France). Palaeontographica A, 186, 1–126.
478Steurbaut, E., 1992. Integrated stratigraphic analysis of lower Rupelian deposits (Oligocene) in the Belgian Basin. Annales de la Société géologique de Belgique, 115/1, 287–306.
479Steurbaut, E., & Herman, J., 1978. Biostratigraphie et poissons fossiles de la Formation de l’Argile de Boom (Oligocène moyen du Bassin Belge). Geobios, 11/3, 297–325. https://doi.org/10.1016/S0016-6995(78)80033-3
480Stone, N.R. & Shimada, K., 2019. Skeletal anatomy of the Bigeye Sand Tiger shark, Odontaspis noronhai (Lamniformes: Odontaspididae), and its implications for lamniform phylogeny, taxonomy, and conservation biology. Copeia, 107, 632–652. https://doi.org/10.1643/CG-18-160
481Taverne, L., Van Simaeys, S. & Steurbaut, E., 2006. Belgocaranx luypaertsi gen. and sp. nov., a new skeleton-based carangid fish from the Boom Clay (Rupelian, early Oligocene) at Kallo (N. Belgium). Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen, 76, 119–130.
482Teodoridis, V. & Sakala, J., 2008. Early Miocene conifer macrofossils from the Most Basin (Czech Republic). Neues Jahrbuch für Geologie und Paläontologie, 250, 287–312. https://doi.org/10.1127/0077-7749/2008/0250-0287
483van Aartsen, J.J., Gittenberger, E. & Goud, J., 1998. Pyramidellidae (Mollusca, Gastropoda, Heterobranchia) collected during the Dutch CANCAP and MAURITANIA expeditions in the south-eastern part of the North Atlantic Ocean (part 1). Zoologische Verhandelingen Leiden, 321, 1–57.
484Van Beneden, P.-J., 1860. Rapport. Bulletin de l’Académie royale des Sciences, des Lettres et des Beaux-Arts de Belgique, 29/2, 403–410.
485Vandenberghe, N., 1976. Phytoclasts as provenance indicators in the Belgian septaria clay of Boom (Rupelian age). Sedimentology, 23, 141–145. https://doi.org/10.1111/j.1365-3091.1976.tb00044.x
486Vandenberghe, N., 1978. Sedimentology of the Boom Clay (Rupelian) in Belgium. Verhandelingen Koninklijke Academie voor Wetenschappen, Letteren en Schone Kunsten van België., Klasse Wetenschappen, 40, 1–137.
487Vandenberghe, N., 2017. Tectonic and climatic signals in the Oligocene sediments of the Southern North-Sea Basin. Geologica Belgica, 20, 105–123. https://doi.org/10.20341/gb.2017.007
488Vandenberghe, N. & Mertens, J., 2013. Differentiating between tectonic and eustasy signals in the Rupelian Boom Clay cycles (lower Oligocene, Southern North Sea Basin). Newsletters on Stratigraphy, 46/3, 319–337. https://doi.org/10.1127/0078-0421/2013/0034
489Vandenberghe, N., Laenen, B., Van Echelpoel, E. & Lagrou, D., 1997. Cyclostratigraphy and climatic eustasy. Example of the Rupelian stratotype. Comptes Rendus de l’Académie des Sciences de Paris, Earth and Planetary Science, 325, 305–315. https://doi.org/10.1016/S1251-8050(97)81377-8
490Vandenberghe, N., Laga, P., Steurbaut, E., Hardenbol, J. & Vail, P.R., 1998. Tertiary sequence stratigraphy at the southern border of the North Sea Basin in Belgium. In de Graciansky, P.-C., Hardenbol, J., Jacquin, T. & Vail, P.R. (eds), Mesozoic and Cenozoic Sequence Stratigraphy of European Basins. SEPM Society for Sedimentary Geology, Special Publication, 60, 119–154. https://doi.org/10.2110/pec.98.02.0119
491Vandenberghe, N., Hager, H., van den Bosch, M., Verstraelen, A., Leroi, S., Steurbaut, E., Prüfert, J., & Laga, P., 2001. Stratigraphical correlation by calibrated well logs in the Rupel Group between North Belgium, the Lower-Rhine area in Germany and Southern Limburg and the Achterhoek, in The Netherlands. Aardkundige Mededelingen, 11, 69–84.
492Vandenberghe, N., Herman, J. & Steurbaut, E., 2002. Detailed analysis of the Rupelian Ru-1 transgressive surface in the type area (Belgium). In Gürs, K. (ed.), Northern European Cenozoic stratigraphy. Proceedings of the 8th Biannual Meeting RCNNS/RCNPS. Landesamt für Natur und Umwelt des Landes Schleswig-Holstein. Flintbek 2002, 67–83.
493Vandenberghe, N., Brinkhuis, H. & Steurbaut, E., 2003. The Eocene/Oligocene boundary in the North Sea area: A sequence stratigraphic approach. In Prothero, D.R., Ivany, L.C. & Nesbitt, E.A. (eds), From Greenhouse to Icehouse: the Marine Eocene-Oligocene Transition. Columbia University Press, New York, 419–437.
494Vandenberghe, N., Van Simaeys, S., Steurbaut, E., Jagt, J.W.M. & Felder, P.J., 2004. Stratigraphic architecture of the Upper Cretaceous and Cenozoic along the southern border of the North Sea Basin in Belgium. Netherlands Journal of Geosciences, 83, 155–171. https://doi.org/10.1017/S0016774600020229
495Vandenberghe, N., De Craen, M. & Wouters, L., 2014. The Boom Clay geology from sedimentation to present-day occurrence: a review. Memoirs of the Geological survey of Belgium, 60, 1–76.
496Van den Broeck, M., 1887. L’argile de Boom. Causerie géologique faite à l’occasion de l’excursion à la fabrique de ciment de la Société de Niel-on-Rupel. Société belge des Ingénieurs et des Industriels, Bruxelles, 1–11.
497Van Simaeys, S., Munsterman, D. & Brinkhuis, H., 2005. Oligocene dinoflagellate cyst biostratigraphy of the southern North Sea Basin. Review of Palaeobotany and Palynology, 134, 105–128. https://doi.org/10.1016/j.revpalbo.2004.12.003
498Van Simaeys, S. & Vandenberghe, N., 2006. Rupelian. Geologica Belgica, 9, 95–101.
499Vaudois, N. & Privé, C., 1971. Révision des bois fossiles de Cupressaceae. Palaeontographica B: Palaeophytologie, 134, 61–86.
500Vélez-Zuazo, X. & Agnarsson, I., 2011. Shark tales: A molecular species-level phylogeny of sharks (Selachimorpha, Chondrichthyes). Molecular Phylogenetics and Evolution, 58, 207–217. https://doi.org/10.1016/j.ympev.2010.11.018
501Verheyden, T. 2002. Decapods from the Boom Clay Formation (Rupelian, Oligocene) in Belgium. Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen, Aardwetenschappen, 72, 171–191.
502Vervoenen, M., 1995. Taphonomy of some Cenozoic Seabeds from the Flemish Region, Belgium. Belgische Geologische Dienst, Professional paper, 1994/5, 272, 115 p.
503Vincent, E., 1930. Observations sur les Pectens de l’Argile de Boom (Rupélien Supérieur). Bulletin du Musée royal d’Histoire naturelle de Belgique, 6, 1–9.
504Vis, G.-J., Verweij, H. & Koenen, M., 2016. The Rupel Clay Member in the Netherlands: towards a comprehensive understanding of its geometry and depositional environment. Netherlands Journal of Geosciences / Geologie en Mijnbouw, 95, 221–251. https://doi.org/10.1017/njg.2016.25
505Voight, J.R., 2007. Experimental deep-sea deployments reveal diverse northeast Pacific wood-boring bivalves of Xylophagainae (Myoida: Pholadidae). Journal of Molluscan Studies, 73, 377–391. https://doi.org/10.1093/mollus/eym034
506von Koenen, A., 1867. Das marine Mittel-Oligocän Nord-Deutschlands und seine Mollusken-Fauna. Erster Theil. Einleitung, Geognostische Beschreibung, Paläontologische Beschreibung der Gastropoden. Palaeontographica, 16, 53–128
507von Koenen, A., 1868. Das marine Mittel-Oligocän Nord-Deutschlands und seine Mollusken-Fauna. Zweiter Theil. Paleontologische Beschreibung der Pteropoden, Brachiopoden, Pelecypoden und Schlussbemerkungen. Palaeontographica, 16, 223–296.
508Ward, D.J. & Bonavia, C.G., 2001. Additions to, and a review of, the Miocene Shark and Ray fauna of Malta. The Central Mediterranean Naturalist, 3, 131–146.
509Webb, T.J., Barry, J.P. & McClain, C.R., 2016. Abundance-occupancy relationships in deep sea wood fall communities. Ecography, 40, 1339–1347. https://doi.org/10.1111/ecog.02618
510Weiler, W., 1942. Die Otolithen des rheinischen und nordwestdeutschen Tertiärs. Abhandlungen des Reichsamts für Bodenforschung, N.F., 206, 9–140.
511Welle, J., 1998. Oligozäne Mollusken aus dem Schacht 8 der Bergwerksgesellschaft Sophia Jacoba bei Erkelenz (Niederrheinische Bucht). Teil 2: Gastropoda. Leipziger Geowissenschaften, 6, 1–197.
512Welton, B.J., 2013. A New Archaic Basking Shark (Lamniformes: Cetorhinidae) from the Late Eocene of Western Oregon, U.S.A., and Description of the Dentition, Gill Rakers and Vertebrae of the Recent Basking Shark Cetorhinus maximus (Gunnerus). New Mexico Museum of Natural History and Science, Bulletin, 58, 48 p.
513Zardus, J.D., Lake, D.T., Frick, M.G. & Rawson, P.D., 2014. Deconstructing an assemblage of “turtle” barnacles: species assignments and fickle fidelity in Chelonibia. Marine Biology, 161, 45–59. https://doi.org/10.1007/s00227-013-2312-7
514Zinndorf, J., 1928. Die Versteinerungen aus den Tertiär-Ablagerungen von Offenbach a.M. – 1. Die Conchylien des Rupeltones (Septarientones). Berichte des Offenbacher Vereins für Naturkunde, 66-68, 1–65.
515Zintzen, V., 2007. Biodiversity of Shipwrecks from the Southern Bight of the North Sea. Unpublished Ph.D. Thesis, Université Catholique de Louvain, Louvain-la-Neuve and Royal Belgian Institute of Natural Sciences, Brussels, 336 p.
516Zonneveld, J.-P., Zonneveld, Z.E.E., Bartels, W.S., Gingras, M.K. & Head, J.J., 2022. Bone modification features resulting from barnacle attachment on the bones of loggerhead sea turtles (Caretta caretta), Cumberland Island, Georgia, USA: Implications for the paleoecological and taphonomic analyses of fossil sea turtles. Palaios, 37, 650–670. https://doi.org/10.2110/palo.2022.021
517Zullo, V.A., 1982. A new species of the turtle barnacle Chelonibia Leach, 1817, (Cirripedia, Thoracica) from the Oligocene Mint Spring and Byram Formations of Mississippi. Mississippi Geology, 2, 1–6.
518Manuscript received 08.11.2022, accepted in revised form 30.03.2023, available online 01.08.2023.

Plate 1. View of the excavation. A–B. General view. C. Lower lateral tooth of C. contortidens in situ. D. Detail of the lens. The black spots are wood fragments, while the white spots represent the remains of calcareous fossils.
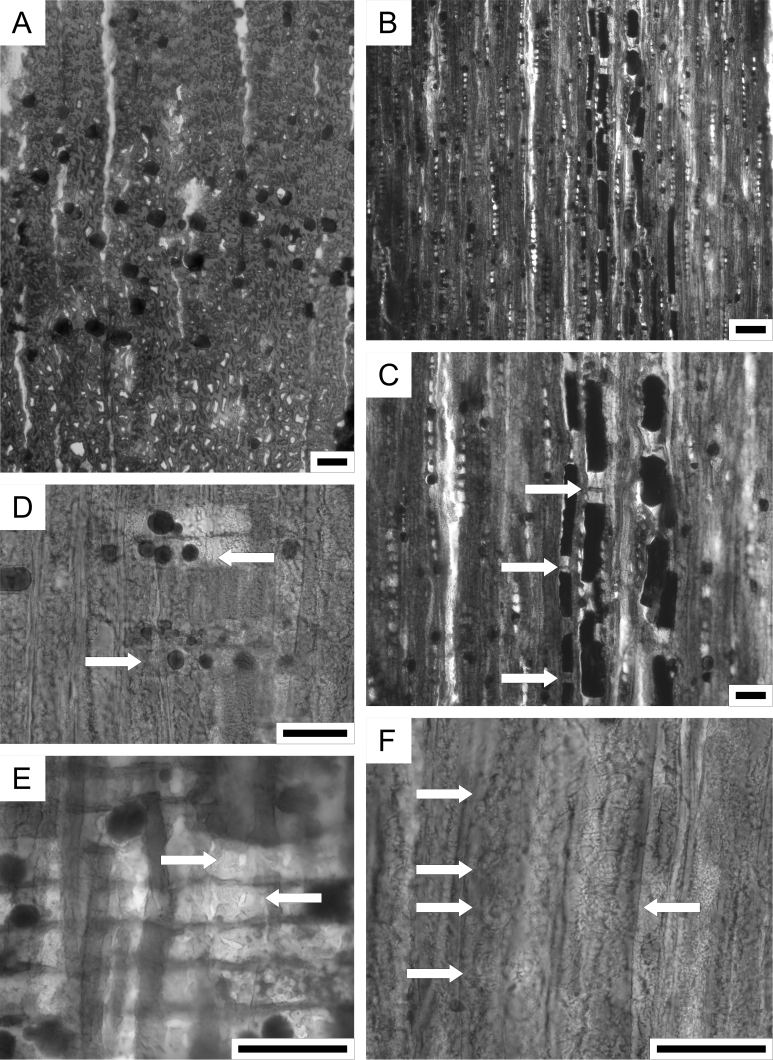
Plate 2. Cupressinoxylon sp., sample: IRSNB b9669. A. Early- and latewood tracheids destructed by compression, axial parenchyma diffuse and abundant, transverse section. B. Uniseriate rays medium in average high, axial parenchyma with thin and smooth to beaded transverse end walls, tangential longitudinal section. C. Axial parenchyma with thin and smooth to beaded transverse end walls (arrows), tangential longitudinal section. D. Ray parenchyma cells with thin and smooth horizontal walls and resiniferous infill (arrows), radial longitudinal section. E. One cupressoid pit per cross-field (arrows), radial longitudinal section. F. Rounded bordered pits in radial tracheid wall (arrows) arranged in one vertical row, radial longitudinal section. Scale bars: A, C, D, E, F = 50 μm, B = 100 μm.
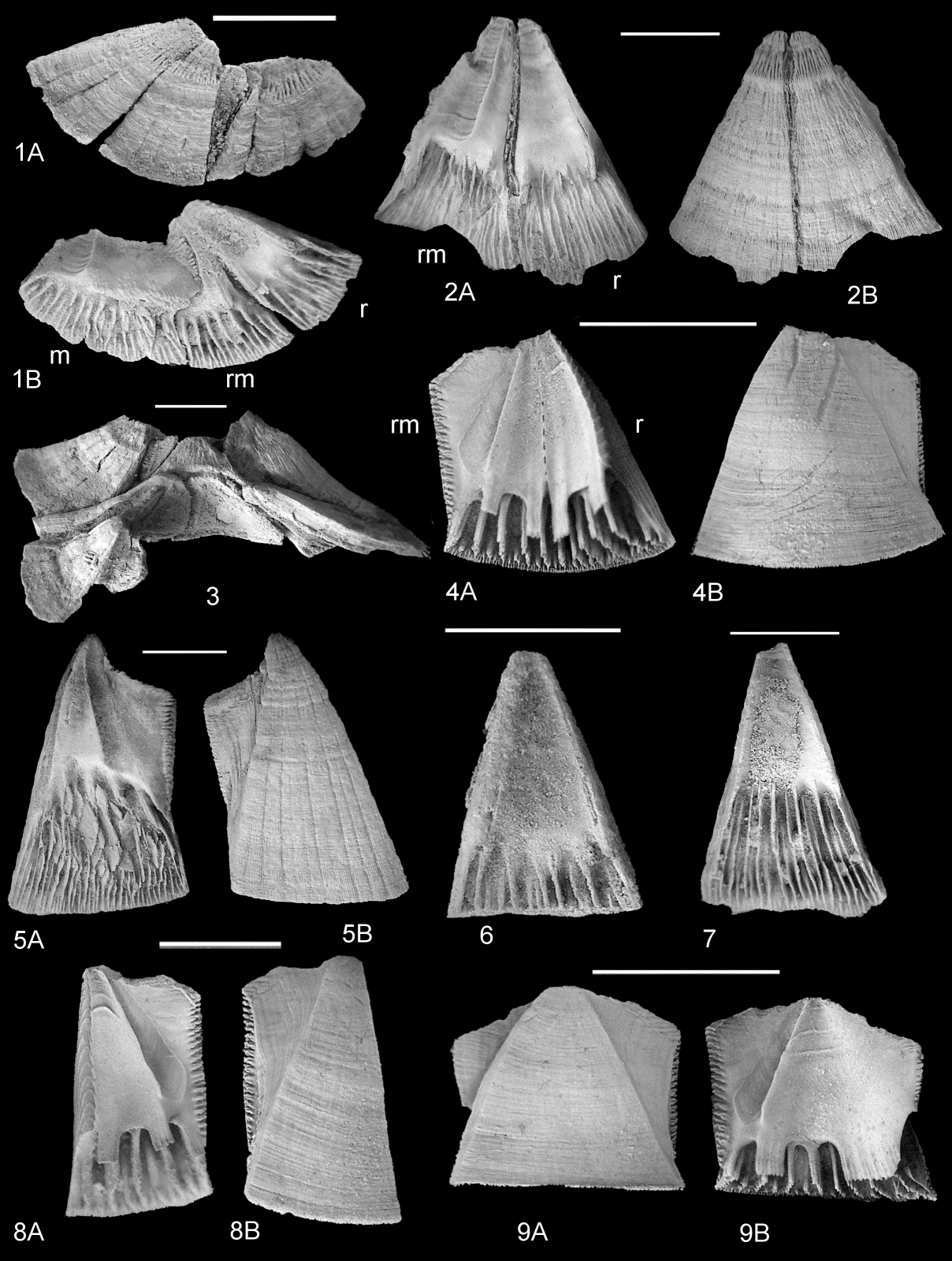
Plate 3. Cirripedia. 1–3, 5–7. Protochelonibia hermani sp. nov. 1A (external), 1B (internal) views of articulated rostrum, rostromarginal and marginal of small specimen (IRSNB 7705). 2A (internal), 2B (external) views of articulated rostrum and rostromarginal (IRSNB 7706). 3, partially articulated group of specimens (IRSNB 7707). 5A (internal) and 5B (external) views of large rostromarginal (IRSNB 7708). 6, 7, internal views of rostra (IRSNB 7709, 7710). 4, 8, 9. Chelonibia testudinaria (Linnaeus, 1858), forma patula (Ranzani, 1817), present day, Florida, USA. 4A (internal), 4B (external) views of articulated rostrum and rostromarginal. 8A (internal), 8B (external) views of rostromarginal. 9A (external), 9B (internal) views of marginal. Scale bars = 5 mm.

Plate 4. Cirripedia. 1–7. Protochelonibia hermani sp. nov. 1A (internal), 1B (external) and 1C (lateral) views of carinomarginal (IRSNB 7711). 2A (internal) and 2B (external) views of elongated marginal (IRSNB 7712). 3A (external) and 3B (internal) views of articulated rostrum and rostromarginals (IRSNB 7713). 4–6, internal views of rostromarginals, showing variation in angle of superior radial margin, from strongly (4) to less (5, 6) inclined (IRSNB 7714-7716). 7, internal view of carina with weakly dependant sheath overlying ridged processes (IRSNB 7717). Scale bars = 5 mm.
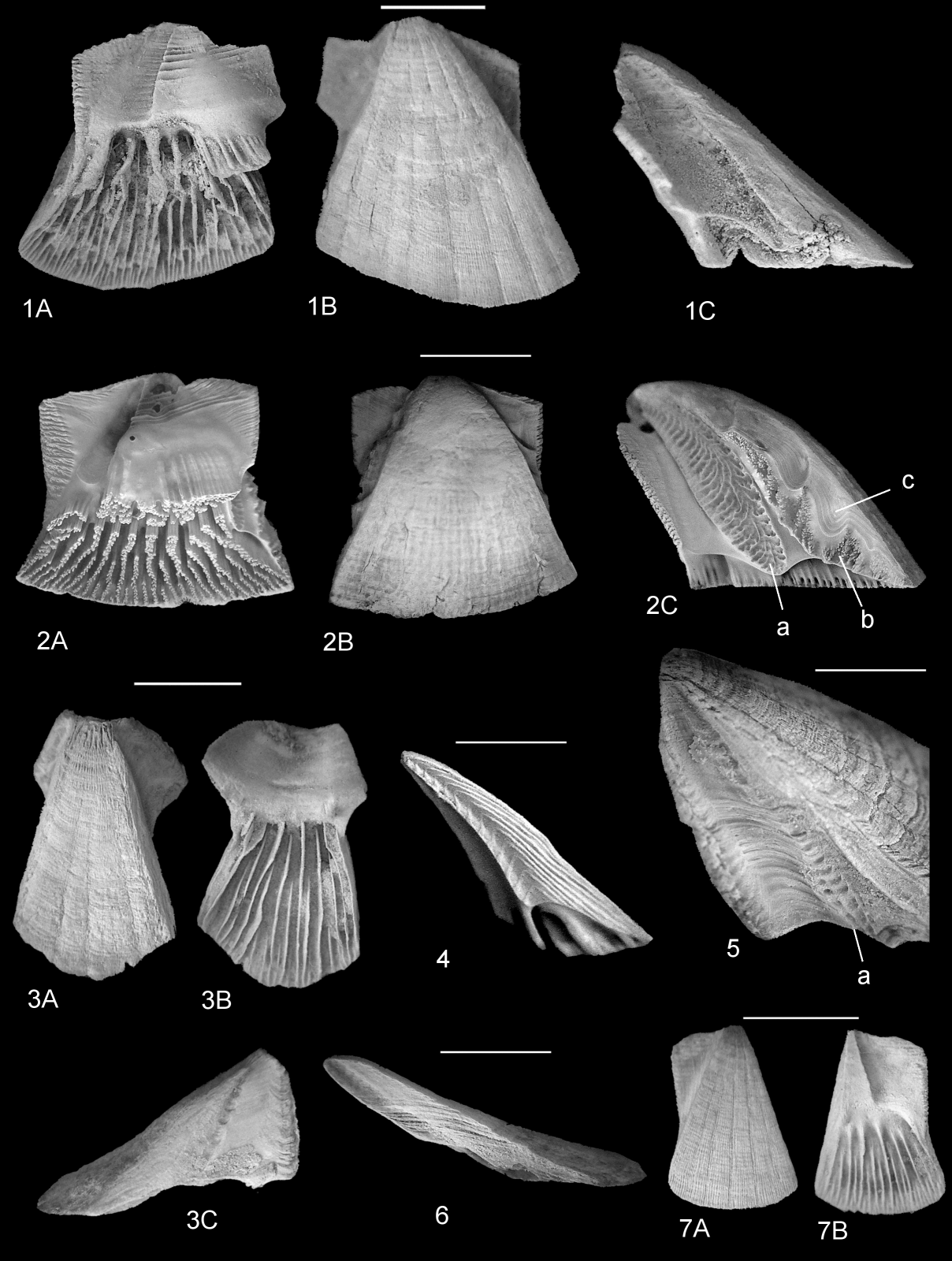
Plate 5. Cirripedia. 1, 3, 5–7. Protochelonibia hermani sp. nov. 1A (internal), 1B (external) and 1C (lateral) views of holotype marginal (IRSNB 7718). 3A (external), 3B (internal), 3C (lateral) views of carina (IRSNB 7719). 5, enlarged image of ala of marginal plate (IRSNB 7721) to show narrow, pitted surface for articulation with radius of adjacent plate (a); note absence of denticulate surface (b) present in C. testudinaria (2C). 6, lateral view of articular surface of rostrum (IRSNB 7720) for comparison with C. testudinaria (4); note concave profile and lack of articular ridges. 7A (external), 7B (internal) views of small rostromarginal (IRSNB 7722). 2, 4. Chelonibia testudinaria (Linnaeus, 1858), present day, North Carolina, USA. 2A (internal), 2B (external), 2C (lateral) views of marginal of Chelonibia testudinaria forma testudinaria (Linnaeus, 1758). Note strongly developed articulation surfaces. 4, Chelonibia testudinaria forma patula, articulation surface of rostrum. Note narrow ridges and grooves. Abbreviations: a, specialised pitted articular surface on ala for denticulate ridge on radius of adjacent plate; b, articulation surface between adjacent parietes comprising columns of small denticles; c, depressions between parietes. Scale bars = 5 mm.

Plate 6. Shark teeth, decapod dactylus and molluscs. Scale bars = 5 mm, unless specified otherwise. 1A–B. Isurolamna gracilis (Le Hon, 1871) - IRSNB P 10311 - anterior tooth; lingual (A) and labial (B) views. 2. Keasius parvus (Leriche, 1908) - IRSNB P 10312 - gill raker. Scale bar = 1 mm. 3. Squalus alsaticus (Andreae, 1890) - IRSNB P 10313. Scale bar = 1 mm. 4. Squalus alsaticus (Andreae, 1890) - IRSNB P 10314. Scale bar = 1 mm. 5. Coeloma rupeliense Stainier, 1887, dactylus - IRSNB 7735. Scale bar = 1 mm. 6. Teredinidae indet. – calcareous tube - IRSNB 7729. 7A–B. Nucula duchasteli Nyst, 1835 - IRSNB 7730. 8. Palliolum permistum (Beyrich, 1848) - IRSNB 7731. 9. Palliolum permistum (Beyrich, 1848) - IRSNB 7732. 10A–C. Palliolum permistum (Beyrich, 1848) - IRSNB 7733. Detail of ornamentation (C). 11. Palliolum delheidi (Vincent, 1930) - IRSNB 7734. 12A–D. Amblyacrum cf. roemeri (von Koenen, 1867) - IRSNB 7736. Abapertural (A), apertural (B) & apical (C) views & detail of the protoconch (D). Scale bars = 1 mm (A–C) and 100 µm (D).
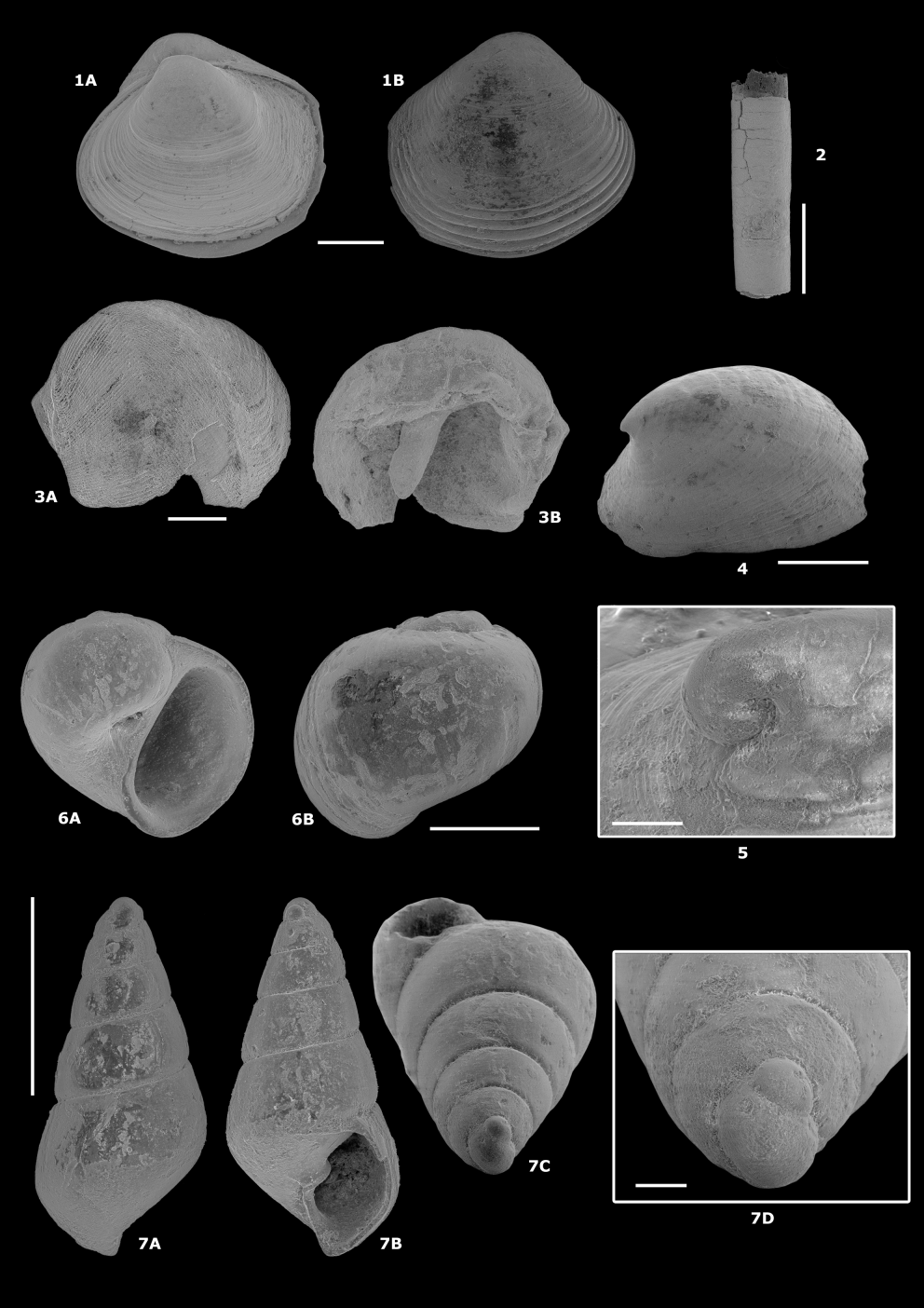
Plate 7. Molluscs. Scale bars = 1 mm, unless specified otherwise. 1A–B. Varicorbula gibba (Olivi, 1792) s.l. - IRSNB 7723. Left valve (A), right valve (B). 2. Rhabdus parallelus (Zinndorf, 1928) - IRSNB 7724. 3A–B. Teredinidae indet. - IRSNB 7725. Left valve, exterior (A) and interior (B) views. 4. Cocculina reineckei Marquet, 2016 - IRSNB 7726. 5. Cocculina reineckei Marquet, 2016 - specimen lost during scanning. Scale bar = 100 µm. 6A–B. Euspira cf. achatensis (De Koninck, 1838) - IRSNB 7727. Apertural (A) & abapertural (B) views. 7A–D. Odostomia cf. acutiuscula (Braun in Walchner, 1851) - IRSNB 7728. Abapertural (A), apertural (B) & apical (C) views & detail of the protoconch (D). Scale bars = 1 mm (A–C) and 100 µm (D).
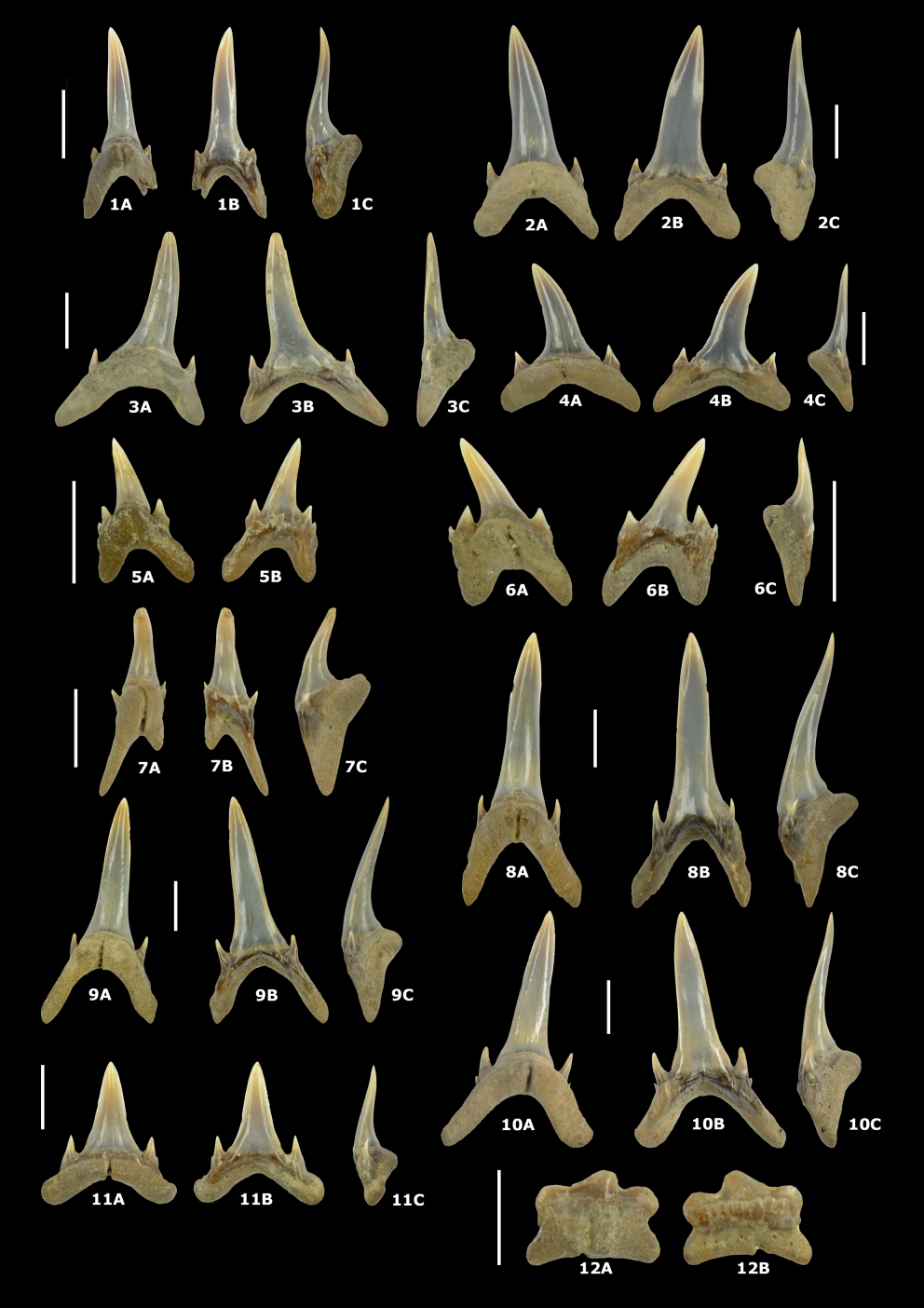
Plate 8. Carcharias contortidens (Agassiz, 1843); lingual (A), labial (B) and mesial/distal (C) views. Scale bars = 5 mm, unless specified otherwise. 1A–C. IRSNB P 10299 - First upper anterior tooth (UA1). 2A–C. IRSNB P 10300 - Second upper anterior tooth (UA2). 3A–C. IRSNB P 10301 - Third upper anterior tooth (UA3). 4A–C. IRSNB P 10302 - Upper lateral tooth (UL). 5A–B. IRSNB P 10303 - Intermediate tooth. 6A–C. IRSNB P 10304 - Intermediate tooth. 7A–C. IRSNB P 10305 - First lower anterior tooth (LA1). 8A–C. IRSNB P 10306 - Second lower anterior tooth (LA2). 9A–C. IRSNB P 10307 - Third lower anterior tooth (LA3). 10A–C. IRSNB P 10308 - Fourth lower anterior tooth (LA4). 11A–C. IRSNB P 10309 - Lower lateral tooth (LL). 12A–B. IRSNB P 10310 - Lower posterior tooth. Scale bar = 2 mm.
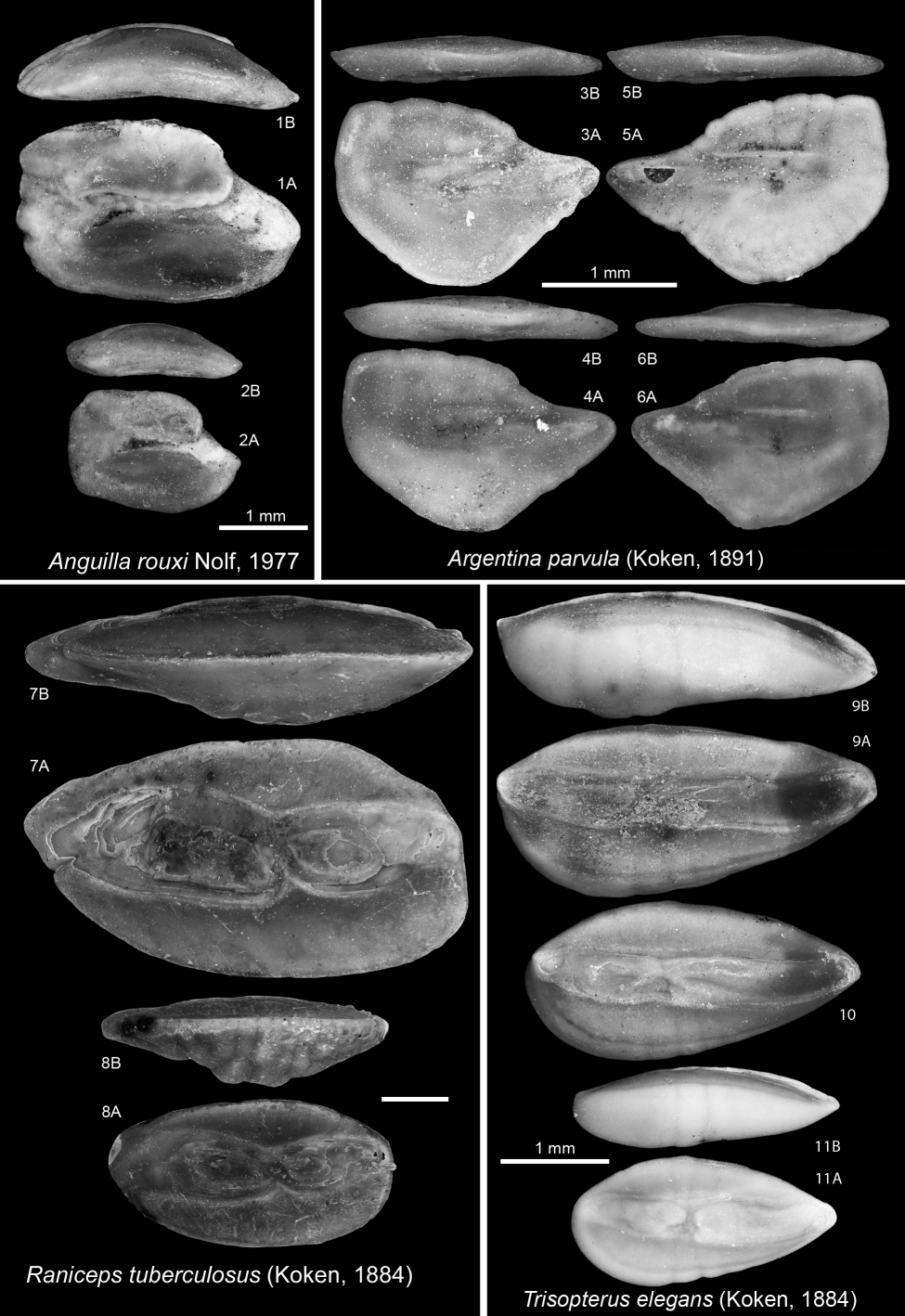
Plate 9. 1–2. Anguilla rouxi Nolf, 1977. 1. IRSNB P 10315. 2. IRSNB P 10316. 3–6. Argentina parvula (Koken, 1891). 3. IRSNB P 10317. 4. IRSNB P 10318. 5. IRSNB P 10319. 6. IRSNB P 10320. 7–8. Raniceps tuberculosus (Koken, 1884). 7. IRSNB P 10321. 8. IRSNB P 10322. 9–11. Trisopterus elegans (Koken, 1884). 9. IRSNB P 10323. 10. IRSNB P 10324. 11. IRSNB P 10325. A = inner view; B = ventral view.

Plate 10. 1–8. Trachurus reineckei sp. nov. 1. Paratype IRSNB P 10329. 2. Paratype IRSNB P 10330. 3. Paratype IRSNB P 10331. 4. Paratype IRSNB P 10332. 5. Paratype IRSNB P 10333. 6. Holotype IRSNB P 10334. 7. Paratype IRSNB P 10335. 8. Paratype IRSNB P 10336. A = inner view; B = outer view; C = ventral view. Abbreviation: htp = holotype.

Plate 11. 1–3. Otarionichthys occultus (Koken, 1891). 1. IRSNB P 10326. 2. IRSNB P 10327. 3. IRSNB P 10328. 4–6. Malacanthus ellipticus (Koken, 1884). 4. IRSNB P 10337. 5. IRSNB P 10338. 6. IRSNB P 10339. 7. Capros siccus Schwarzhans, 2008, IRSNB P 10348. 8. Lophius gibbosus Nolf, 1977, IRSNB P 10349. A = inner view; B = ventral view.
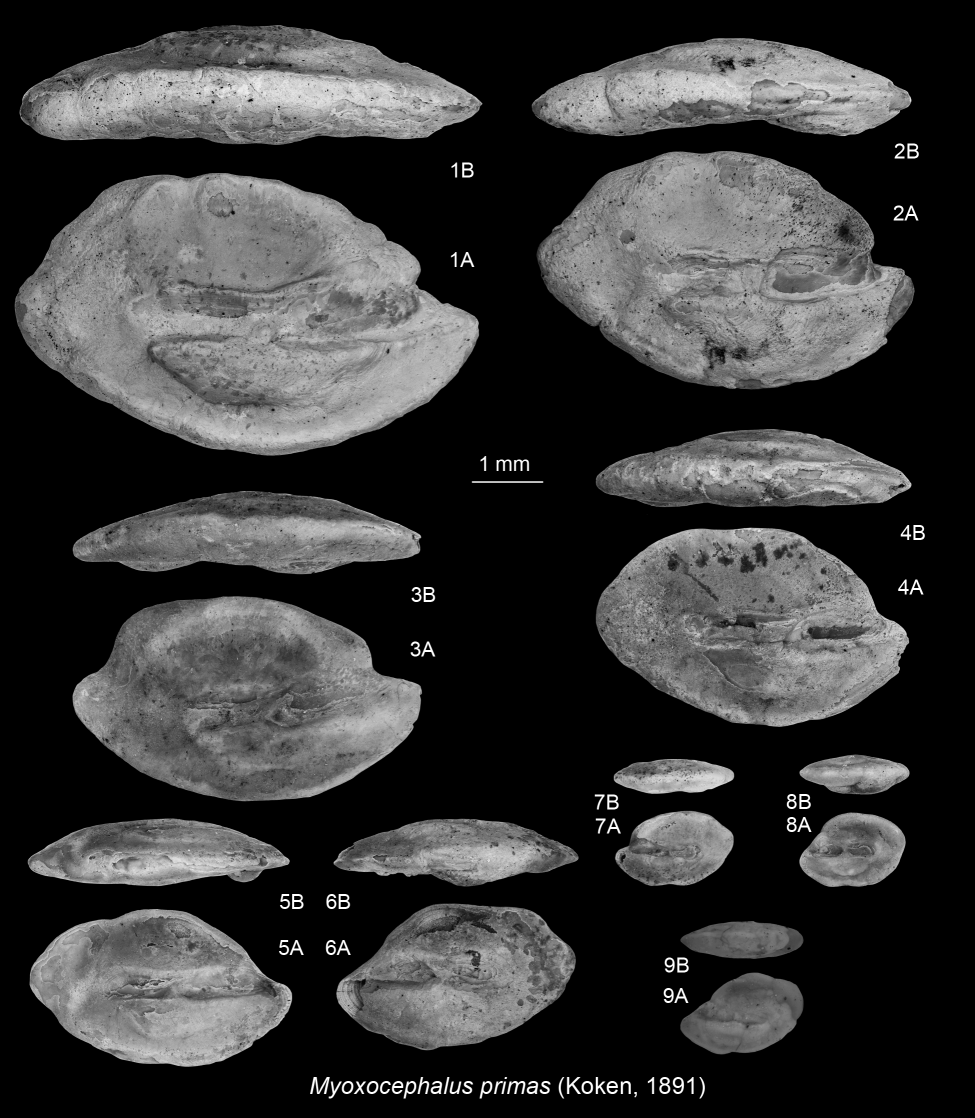
Plate 12. 1–9. Myoxocephalus primas (Koken, 1891). 1. IRSNB P 10340. 2. IRSNB P 10341. 3. IRSNB P 10342. 4. IRSNB P 10343. 5. IRSNB P 10344. 6. IRSNB P 10345. 7. IRSNB P 10346. 8. IRSNB P 10347. 9. Holotype of Liparis minusculus Nolf, 1977, Rupelian of Kruibeke, IRSNB P 02569. A = inner view; B = ventral view.






