- Home
- Volume 27 (2024)
- number 1-2
- Revision of the Lower Cretaceous ovulate cone Pinus belgica Alvin (1960), one of the oldest representatives of Pinus
View(s): 1237 (24 ULiège)
Download(s): 436 (12 ULiège)
Revision of the Lower Cretaceous ovulate cone Pinus belgica Alvin (1960), one of the oldest representatives of Pinus

Abstract
Pinaceous ovulate cones have an abundant fossil record since the middle Lower Cretaceous. Pinus belgica Alvin (1960) is considered the second oldest representative of the genus Pinus. This taxon has thus often been included in phylogenetic and molecular dating analyses although its age and stratigraphy are uncertain. We redescribe this important species and discuss its spatiotemporal occurrence (middle Barremian to late Albian of the Mons Basin, Belgium). Several anatomical features are updated (resin canals abaxial to vascular bundles, bract and ovuliferous scale separating medially first, resin canals distributed at the edge between pith and vascular cylinder). We show that the species does possess the diagnostic characters of Pinus (e.g. thick apophysis, dorsal umbo, resin canals abaxial to vascular tissue, bract and scale traces united at origin) and there are currently no arguments to question its inclusion within the genus Pinus. Yet, this species should be considered with caution in molecular dating analyses, as the broad temporal uncertainty is likely to affect the precision of deep nodes within Pinaceae.
Table of content
1. Introduction
1Fossils of pinaceous ovulate cones are found in abundance since the middle Early Cretaceous. Numerous ovulate cone taxa are described in Aptian strata from North America, Western Europe and Asia, (Miller, 1976a, 1976b; Falder et al., 1998; Ratzel et al., 2001; Rothwell & Mapes, 2001; Smith & Stockey, 2002; Klymiuk & Stockey, 2012; Herrera et al., 2016, 2021; Smith et al., 2017; De Brito & Prestianni, 2021). While the family Pinaceae underwent a diversification phase during the Cretaceous period, the timing of the appearance of its earliest representatives is still debated (Zhang et al., 2011; Leslie et al., 2013; Falcon-Lang et al., 2016a; Domogatskaya & Herman, 2019; Matsunaga et al., 2021), obscuring the tempo of this radiation. The earliest records of the family come from poorly preserved specimens or taxa whose affiliation is under debate, such as the genus Schizolepidopsis Doweld emend. Domogatskaya & Herman, recently assigned to the Pinaceae family (Doweld, 2001; Leslie et al., 2013; Domogatskaya & Herman, 2019; Matsunaga et al., 2021).
2Pinus mundayi Falcon-Lang, Mages, Collinson from the Valanginian of Canada is described as the oldest representative of wood of the genus Pinus Linné (Falcon-Lang et al., 2016a). Its status remains nonetheless debated in the literature, notably the interpretation of its anatomy and dating (Falcon-Lang et al., 2016a, 2016b; Hilton et al., 2016).
3Currently, two ovulate cone taxa are regarded as the oldest representatives of the genus Pinus: Pinus belgica Alvin (Alvin, 1960) and Pinus yorkshirensis Ryberg, Stockey, Hilton, Mapes, Riding et Rothwell (Ryberg et al., 2012). Pinus belgica plays a key role in many phylogenetic and molecular dating analyses despite uncertainties regarding its age, its unknown origin, and gaps in its anatomical description (Eckert & Hall, 2006; Willyard et al., 2007; Gernandt et al., 2011, 2016, 2018; Saladin et al., 2017; Smith et al., 2017). Moreover, until the description of P. yorkshirensis in 2012, it was considered as the oldest ovulate cone belonging to Pinus and has shaped our view of the evolution of the genus and the Pinaceae. For example, Miller (1976a) stated that “the occurrence of P. belgica Alvin, 1960 in the Lowermost Cretaceous not only documents the great antiquity of this modern genus but offers strong support for the hypothesis that the ancestral complex of the Pinaceae is distinctly Pinus centered […] “(Miller, 1976a, p. 114). Alvin (1960) described the species from a single specimen of ovulate cone coming from Wealden sediments, from an unknown locality in the Mons Basin, Belgium. In particular, he stated “Unfortunately, the one specimen upon which this species is based is unlocalized. However, in its practically uncompressed, lignitic state, it resembles most of the other plant remains from the Belgian Wealden, and the adhering particles of fine-grained, grey matrix are similar to the usual kind of matrix; there is thus no good reason to doubt that it comes from the same formation.” (Alvin, 1960, p. 18). The species Pinus bukatkinii Bazhenova & Bazhenov has recently been described from the Middle Jurassic of Russia (?Bathonian, Belgorod Region; Bazhenova et al., 2023). However, its position in the phylogeny of the Pinaceae has not been explored and its Bathonian age is yet unconfirmed. It does, however, possess diagnostic characteristics of the genus Pinus and could prove a more ancient origin of the genus, if its age is confirmed.
4The geographic origin of P. belgica remains unclear. Within the Mons Basin, the western and eastern Wealden facies are diachronous (Yans et al., 2005; Baele et al., 2012). In the western part of the basin, these facies are dated by palynological and geochemical studies as middle Barremian to earliest Aptian (Dejax et al., 2007b, 2007a, 2008; Baele et al., 2012; Spagna et al., 2012). In the eastern part, the sediments are tentatively considered as belonging to the late Albian (Yans et al., 2002, 2005, 2010; Schnyder et al., 2009). It is not known whether the type specimen of P. belgica comes from a western or an eastern locality.
5Since its description, several authors noted the uncertainties surrounding the age of this taxon. In molecular dating studies, Eckert & Hall (2006) included P. belgica to date the sub-genus Pinus. Willyard et al. (2007) included P. belgica with an erroneous age range of Berriasian–Barremian (145–125 Ma) into fossil calibrations of molecular divergence. Their results place P. belgica at the divergence between Pinus and other Pinaceae. However, Saladin et al. (2017) did not use P. belgica to estimate divergence times because of its ambiguous stratigraphy and dating. Several cladistic studies of Pinaceae using morphological and/or molecular data included P. belgica in their analyses (Ryberg et al., 2012; Gernandt et al., 2016, 2018; Smith et al., 2017). It resulted in P. belgica clustered into the Pinus crown group in each analysis, forming a polytomy with other species of Pinus (extant and extinct) and some species of Pityostrobus.
6This work is part of an ongoing revision of pinaceous species recovered in the Belgian Wealden facies. Among these, P. belgica is the only listed species described from a single specimen (Alvin, 1953, 1957, 1960). Considering its evolutionary significance, a revision is therefore particularly needed. Moreover, Wealden sediments are rich in pyrite (Schnyder et al., 2009) and some ovulate cone specimens are affected by it, as are the iguanodons of Bernissart, which come from the same Wealden facies (Godefroit & Leduc, 2008; Leduc, 2012). Pyrite decay appears to have affected the holotype and only specimen of P. belgica, as parts have been degraded since the original description. The purpose of the present study is therefore to review this key species to clarify its taxonomic position but also to preserve some anatomical information that may never be accessible again if the specimen were to be further affected by pyrite decay.
2. Geological settings
7Pinus belgica comes from the Mons Basin (Belgium) that is considered as representing the northeastern part of the Paris Basin (Marlière, 1970; Dejax et al., 2007a; Pirson et al., 2008). The Mons Basin is oriented west–east in Belgium (Fig. 1A) and has accumulated over a thickness of 300 m Mesozoic and Cenozoic deposits (Fig. 1B; Marlière, 1970; Yans et al., 2010; Baele et al., 2012). The deposition of the Wealden facies is largely diachronous, extending from the late Barremian in the west (127.3 Ma), to the late Albian in the east (100.5 Ma; Allen & Wimbledon, 1991; Robaszynski et al., 2001; Yans et al., 2006; Dejax et al., 2007a, 2008; Gernandt et al., 2008). The sediments encountered in the western localities (Baudour, Bernissart, Hautrage and Villerot) belong to the Sainte-Barbe Clay Formation, the Baudour Clay Formation, and the Hautrage Clay Formation. The three formations are dated by palynological and geochemical studies as middle Barremian to earliest Aptian (Dejax et al., 2007a, 2007b, 2008; Baele et al., 2012; Spagna et al., 2012). In the eastern part, the sediments belong to the Saint-Pierre Gravels Formation, which is tentatively considered as belonging to the late Albian (Yans et al., 2005, 2010; Spagna et al., 2012). It includes the localities of La Louvière, Houdeng‐Aimeries, and Soignies. Although this west–east Barremian–Albian age gradient is supported by multiple sources of data (Yans et al., 2005; Yans, 2007), several eastern localities, such as Houdeng‐Aimeries (Roeulx 5) and La Louvière (Roeulx 1), have never been precisely dated. Pinus belgica is thought to come from Wealden sediments (Alvin, 1960) and thus potentially from one of these nine localities (Fig. 1A).
8The Wealden facies is present in the northern part of the Mons Basin and occurs as ‘pockets’ of weakly buried sediments (Pirson et al., 2008). The sediments consist of grey to dark grey clays, often laminated, with thin horizons of wood-bearing sands and silts. They also contain pyrite (often oxidized to jarosite and gypsum), siderite, and goethite (Schnyder et al., 2009). The sedimentary layers from which all the fossilized ovulate cones were collected consist of sandy clay, with proportions varying depending on the locality (Alvin, 1953; Allen, 1955; Allen & Wimbledon, 1991; Robaszynski et al., 2001). In general, the Wealden outcrops are rare and due to their high content in clay minerals and weak diagenesis, their durability and resistance to erosion and weathering are poor (Yans et al., 2011).
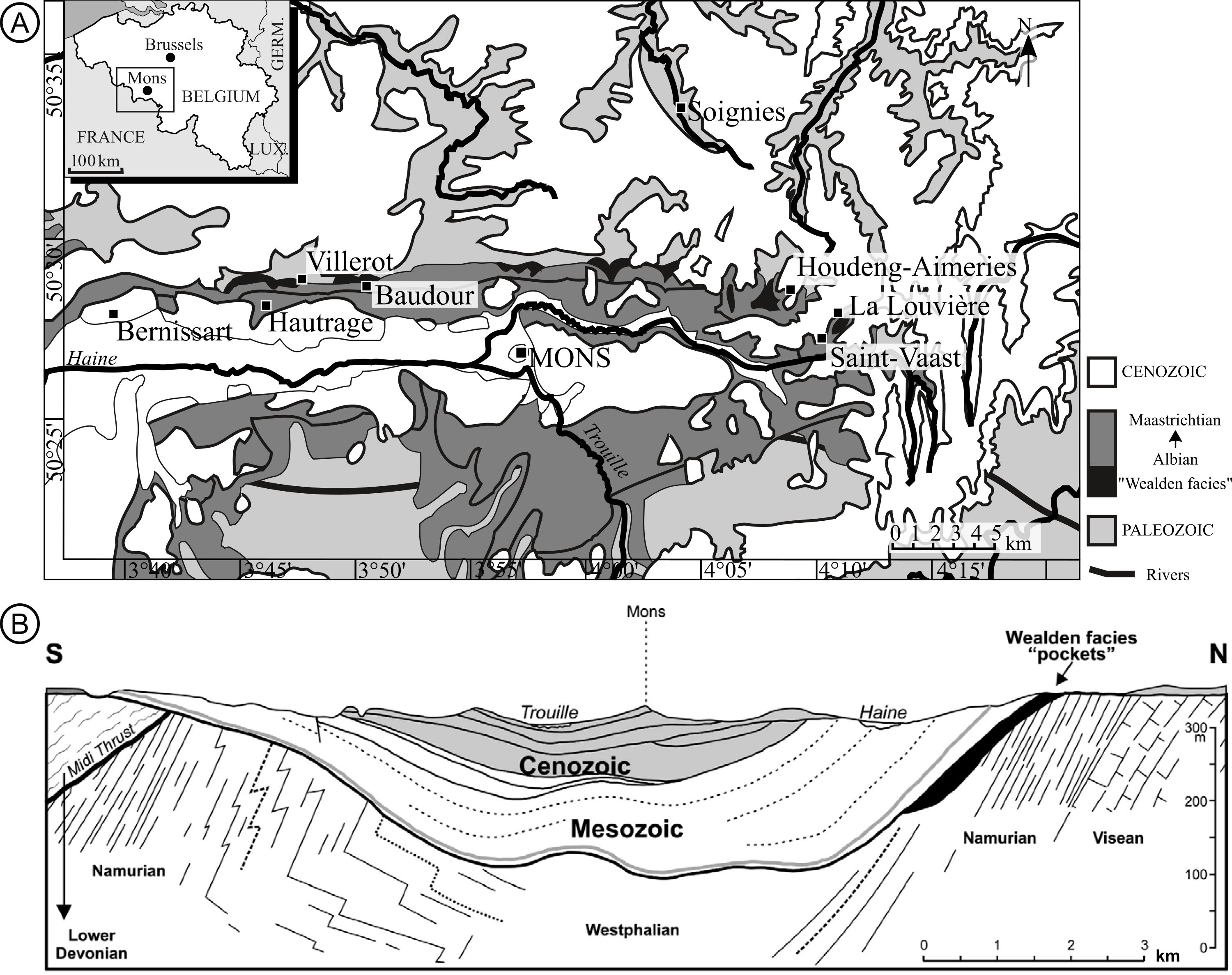
Figure 1. A. Geological map of the Mons Basin and fossil localities, modified from Yans et al. (2010). B. Geological section of the Mons Basin (Marlière, 1970; Yans et al., 2010).
3. Material
9The specimen was collected by Professor Charles Bommer (Bommer, 1892; Harris, 1953; Alvin, 1960). It consists of an ovulate cone broken in several parts (IRSNB b 7690) and three isolated scales, which became dissociated after collection (IRSNB b 7693, IRSNB b 7694, IRSNB b 7697).
10Longitudinal and transverse anatomical thin sections of the axis, scales, and seeds have been prepared by K. L. Alvin for the specimen IRSNB b 7690 (anatomical section numbers: J1, J2, J3, J4, J5, J6). All the material is curated in the Palaeontological collections of the Royal Belgian Institute of Natural Sciences (RBINS) in Brussels, Belgium.
11Additionally, five Pinaceae fossil cone species are included for comparisons, based on publications: Pinus mutoi Saiki, Pinus yorkshirensis, Pityostrobus bommeri Alvin, Pinus cliffwoodensis Miller & Malinky, and Pinus bukatkinii Bazhenova & Bazhenov (Alvin, 1953; Miller & Malinky, 1986; Saiki, 1996; Ryberg et al., 2012; Bazhenova et al., 2023).
4. Methods
12Tri-dimensional observations and anatomical measurements were obtained using the anatomical thin sections of Alvin (1960) and by using nano-Computed Tomography (CT). The three isolated scales were scanned at the nano–CT facility of the RBINS using an EasyTom 130-kV scanner (RX Solutions, 3D X-Ray tomography systems) fitted with a nanofocus X-ray source and a 320#530mm detector. Ovuliferous scale specimens IRSNB b 7693, IRSNB b 7694, IRSNB b 7697 were scanned in their entirety using an iron filter 0.15 mm at a spatial resolution of 10 µm, with an electron acceleration energy of 80 kV and a current of 100 mA. The segmentation software Mimics Innovation Suite 21.0 (Materialise, Belgium) was used to reconstruct the volume renderings of the three isolated scales of P. belgica. Reconstructions are available online (see Supplementary material section).
13The material was also photographed with a Nikon D90 camera equipped with an AF-S DX NIKKOR 18–105mm f/3.5–5.6G ED VR. Measurements on the specimen were performed digitally with ImageJ/Fiji 1.52n software (Schindelin et al., 2012; Schneider et al., 2012; Rueden et al., 2017). Observations and images of anatomical sections were done using a light microscope (Zeiss Axioplan 2 Imaging microscope equipped with and halogen lamp HAL 100) with an Infinity X-32C-Deltap camera. Observations and images of sections were also done with a stereo microscope Zeiss Stemi 2000-C (magnification of 6.5x–50x). The shots were taken with the digiCamControl software.
5. Systematic palaeontology
14Division Pinophyta Cronquist, Takht. & W.Zimm. ex Reveal
15Class Pinopsida Burnett
16Order Pinales Gorozhankin
17Family Pinaceae Spreng. ex Rudolphi
18Genus Pinus L.
19Pinus belgica Alvin emended
20Type specimen. IRSNB b 7690, figured by Alvin (1960, pl. IV, figs 3–13; Pl. V, figs 1–8; text-fig. 4; anatomical section numbers: J1, J2, J3, J4, J5, J6), RBINS Palaeontological collections, Brussels, Belgium.
21Isotypes. IRSNB b 7693, IRSNB b 7694, IRSNB b 7697.
22Type locality and horizon. Unknown locality, Mons Basin, Belgium, Early Cretaceous, middle Barremian–late Albian “Wealden facies”.
23Emended diagnosis. Ovulate cone, ovoid-conic. The cone is 43 mm long and 21 mm wide. Cone-scale complexes helically arranged. Visible peduncle of 3.5 mm long and 4.8 mm wide. Ovuliferous scales are 15–17 mm long, around 7 mm wide in maximum and 2–3 mm thick. Scale base is less than 3.9 mm wide. Scales are thickened into a rhomboid apophysis and have a prominent dorsal umbo, 2–3 mm thick. Scales are persistent (flexer cone). Apophyses are 7 to 10 mm long and 5 to 8 mm wide. Angle formed by the upper margin of the apophyses is 110°. Interseminal ridge present, extending for less than half the diameter of the seed. Axillary complex separating from bract near base. Bract and ovuliferous scale separate laterally first. Bract and scale traces origin is united. Bract resin canals are present, two in number. Vascular cylinder continuous, slightly dissected. Seeds inverted, winged, two per scale.
24Description. The specimen consists of an ovulate cone broken into four parts (Plate 1A–E) and three isolated scales (Plate 1E, G, H). All scales are fragmentary. Several anatomical thin sections were prepared by K. L. Alvin on one of the broken parts of the cone (Plates 2–6). The cone is 43 mm long and 21 mm wide (Plate 1A). The number of gyres is around 6 to 7. The cone is stalked with a visible peduncle of 3.5 mm long and 4.8 mm wide (Plate 1A). Bract-scale complexes are helically arranged (Plate 1A & D).
25Axis. The cone axis diameter was measured on a transversally broken part of the cone (Plate 1C). It measures 5.4 mm in diameter. The available anatomical preparations only display a part of the axis (Plate 2A & B). The pith is incomplete and measures 3.2 mm wide (Plate 2A & B). It is parenchymatous. Cells are variable in size ranging from 25 µm to 99 µm in diameter. Larger cells are situated in the centre of the pith while they progressively get smaller towards the periphery (Plate 2B). There is no resin canal in the pith. Sclerenchymatous cells occur (Plate 2D). They are isolated and dispersed in the centre and get progressively more abundant towards the periphery forming occasional sclerotic nests (Plate 2B). Secretory cells are present (Plate 2E). Few resin canals are present at the margin between the pith and the vascular cylinder (Plate 2A & B).
26The vascular cylinder is 1.6 mm thick, mainly made up of secondary xylem (Plate 2A, B, F, G). The vascular cylinder is continuous, slightly dissected (Plate 2A & B). The vascular cylinder is very dense with small cells. There are no resin canals in the xylem (Plate 2A & B). The secondary xylem is composed of tracheids with circular-bordered pits (Plate 2F) and primary xylem possesses tracheids with helical thickenings (Plate 2G). No growth rings are visible.
27The inner and outer cortex are well delineated (Plate 2A) and measure 3.0–3.5 mm wide together. Resin canals are present in the inner cortex, organized in a circle around the vascular cylinder (Plate 2A & B). They measure 0.2–0.3 mm in diameter and dilate to about three times their size before branching (Plate 3). The inner cortex is slightly sclerified (Plate 2A).
28Bract-scale complexes. Ovuliferous scales are 15–17 mm long, 7.0 mm wide in maximum and 2.7 mm thick (Plate 1E, G, H). Scale bases are less than 3.9 mm wide (Plate 2A). Scales are thickened into a rhomboid apophysis and have a prominent dorsal umbo (Plate 1H; Plate 5E; Plate 6A). Apophyses are 7.3 mm long and 6.3 mm wide (Plate 1E, G, H). The angle formed by the upper margin of the apophyses is 110°. Two resin canals, originating in the cortex, supply each ovuliferous scale. Between cortex and scale bases, the tissue is poorly sclerified with few trichomes (Plate 2A & C). The origination of a bract and a scale is illustrated through serial anatomical thin sections (Plate 3). Bract and scale traces originate from the vascular cylinder. They are united at the base (Plate 3G & H; Fig. 2A). The single trace then divides into three: the central trace (i.e., bract trace - bt) gives that of the bract and the lateral traces (i.e., scale traces - sct) will supply the scale (Plate 3H). Bract and ovuliferous scale separate medially first (Plate 4A & B).
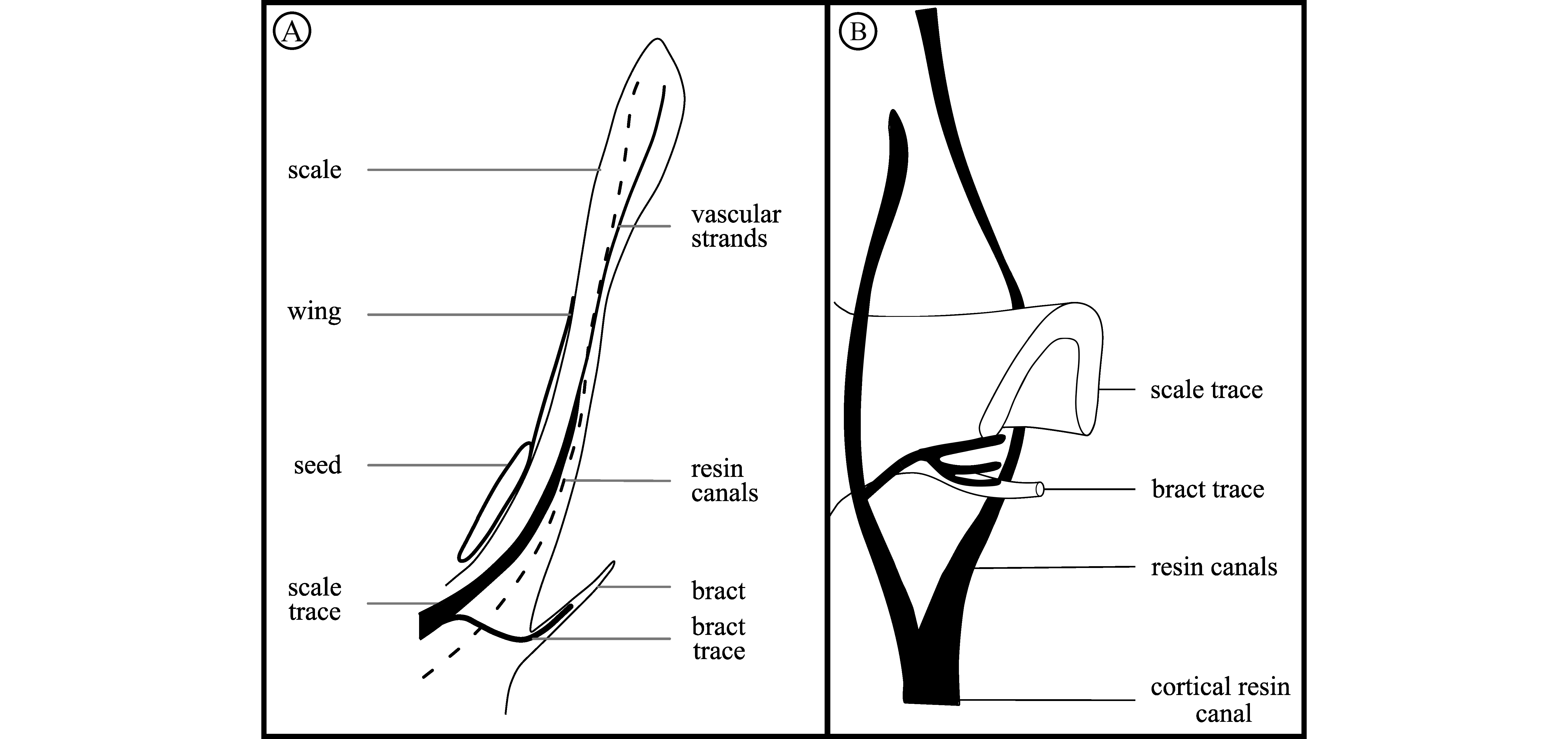
Figure 2. Schematic reconstruction of the bract-scale complex vascularization and distribution of resin canals at the base of the scale. A. Scheme of the scale and bract traces organization. B. Bract and scale traces derivation with associated resin canals. Both reconstructions follow anatomical information given in Miller (1976a) and anatomical observations illustrated on Plates 2–4.
29The axillary complex separates from the bract near the base (Plate 4A–C). Scale trace is abaxially concave (horseshoe shaped) after divergence and tends to form a cylinder with the bract trace (Plate 5B, C, D; Fig. 2B). The scales form an acute angle with the axis (Plate 1A & D). The scale base presents no signs of scale release but is broken (Plate 1A & D). The scale trace derives from the two lateral strands (Plate 2A; Plate 3E–H). At the base, resin canals are situated abaxially to the scales (Plate 2A). The scales are abaxially sclerotic (Plate 2A). Gradually going up toward the middle of the scale, resin canals divide and move to the centre of the scale (Plate 5C, E). Going up, they continue to divide and move to an adaxial position near the top (Plate 5D). At the end at the level of the apophysis, the abaxial part of the scale becomes completely sclerified and the resin ducts are mainly situated on its adaxial side (Plate 5D & E). The level of sclerification varies along the scale (Plate 5). At the base, it is very densely sclerified abaxially and has a thin sclerified layer adaxially (Plate 4B–F). Between the vascular bundles and the adaxial face of the scale, the tissue is parenchymatous (Plate 5A). At the level of the seed, the abaxial part of the scale is more and more sclerified until the lumen of the cells disappears completely (Plate 5A). At the top of the seed level, there is a gradual reduction in the thickness of the densely sclerified part, and it becomes progressively less sclerified abaxially (Plate 5B & C). The lumen of the cells gradually enlarges (Plate 5–B & C), becoming clearly visible at the top of the wing (Plate 5B). The scales are less sclerified thereafter, there is parenchyma abaxially and adaxially to the vascular bundles (Plate 5C). Towards the distal end, the scale undergoes sclerification once again, abaxially forming the apophysis (Plate 5D). Abaxially to the vascular bundles, within the sclerified portion of the apophysis, parenchyma is no longer present. Vascular strands are abaxially convex at the seed level (Plate 6C & E). Towards the top of the scale, the vascular strands bifurcate, forming up to 16 smaller strands (Plate 5D; Plate 6D).
30Bracts. The bract trace is terete (Plate 3A–H). It measures 2–4 mm wide and 0.3–2 mm thick. The bracts can only be seen in cross-section, which means that the length and margins of the bracts cannot be observed (Plate 4). After the divergence with scale trace, the vascular bundle descends before ascending to supply the bract (Plate 3A). No resin canals are present. The bract has one vascular bundle and is parenchymatous (Plate 4). Bract trace extension enters the bract (Plate 2C). Although no bract was cut longitudinally, it was possible to infer its shape by interpreting successive transversal thin sections (Plate 4). The bract is pointed in shape (Plate 4) and becomes thinner distally (Plate 4D). In transversal section, it exhibits a trapezoidal shape at the base (Plate 4A), followed by flattening and thickening in a semi-circular manner in the middle, and further flattening on the sides (Plate 4B–F). At the base of the bract, on the adaxial side, almost in contact with the scale, some rare trichomes were observed (Plate 2C).
31Interseminal ridge and seeds. Scales bear two inverted seeds at their base (Plate 6C & E). Trichomes are present between the scale and the seed. An interseminal ridge is present and extends for less than half the diameter of the seed (Plate 6C). Two of the three isolated scales have immature seeds at their base (Plate 6C & E). Seeds are winged (Plate 1E, G, H). Wings are at least 10.0 mm long and 2.5 mm wide. The wing derives from scale tissue (Plate 1E). Sarcotesta and sclerotesta are 0.012 mm thick. No resin vesicles are visible in the integument. Seed integument is sclerotic (Plate 5A).
32Comparisons. We compare the morphology and geological age of P. belgica to four other species of Cretaceous ovulate cones, belonging to the genera Pinus and Pityostrobus (Table 1; Figure 3). Pinus mutoi is precisely dated from the Coniacian (89.8–86.3 Ma; Saiki, 1996). Pinus yorkshirensis is also relatively well dated from the Valanginian–Hauterivian transition (~132.6 Ma) while P. cliffwoodensis originates during the Santonian–Campanian interval (86.3–72.1 Ma). Pinus belgica and Pit. bommeri are both from the Belgian Wealden facies. Pityostrobus bommeri was found in the Bernissart locality. The eastern Wealden localities of the Mons Basin are probably dated from the late Albian, but further studies are needed to verify this hypothesis (Yans et al., 2005; De Brito & Prestianni, 2021). At the west of the Mons Basin, Wealden deposits of Bernissart and Hautrage localities are dated from middle Barremian to early Aptian (Yans et al., 2005; Dejax et al., 2007a). The species Pit. bommeri is described from the locality of Bernissart (Alvin, 1953). As the type locality of P. belgica is unknown, its stratigraphic repartition is broad and imprecise, between late Barremian and late Albian. It must probably come from a locality of the Mons Basin but it could either come from an eastern or a western locality of the Mons Basin (Alvin, 1960).
33In the original description, the measurements of P. belgica were all higher than ours (Table 1). The cone length is 43 mm instead of 45 mm and the width is 21 mm instead of 30 mm. The pith is not complete in sections. Alvin (1960) measured it at 5 mm. The vascular cylinder is 0.11–0.13 mm thick instead of 2 mm in the original description. Ovuliferous scales are 15–17 mm long instead of 20 mm, and 7 mm wide instead of 9 mm. Immature seeds present in the isolated scale were 1.3–1.5 mm long and 0.6–1 mm wide while in the original description, mature seeds are figured and measured (9 mm long and 4 mm wide (Alvin, 1960, fig. 4L). The seed specimens could not be located in the RBINS Palaeontological collections. Regarding cone length, P. belgica is close to the cone length of P. yorkshirensis, but P. mutoi, Pit. bommeri, and P. cliffwoodensis are much more elongated. Cone widths range from 21 to 31 mm for all species except for P. mutoi, which is 60 mm wide. Pinus belgica exhibits a thickened rhomboid apophysis with a dorsal umbo, similar to those of P. mutoi and P. yorkshirensis (Table 1). Scales of P. belgica are similar in length to those of Pit. bommeri and P. cliffwoodensis (between 10 and 19.9 mm), but they are smaller than those of P. mutoi (35–40 mm) and larger than those of P. yorkshirensis (1.7 mm). The width of the scales is similar in all species, ranging from 5.0 to 11.3 mm (with scales of P. belgica being 7 mm wide). Similar to other species of Pinus and Pityostrobus listed in Table 1, P. belgica has a limited number of resin canals in the cortex, arranged in a ring around the vascular cylinder in the inner cortex. This differs from Alvin’s (1960) interpretation that they were present in the axial cortex (Plate 2A). The sarcotesta and sclerotesta of P. belgica exhibit a thickness ranging from 0.08 to 0.14 mm, similar to that of P. mutoi (0.2 mm) and P. yorkshirensis (0.073–0.087 mm). However, it is significantly thinner than that of P. bommeri, which is 0.41 mm.
34In the original description, Alvin (1960) described resin canals located abaxially and occasionally adaxially at scale base. In our reviewed description, resin canals of the scale base are only abaxial to vascular strands, notably regarding the way the scale trace and resin canals derived from the cone axis (Plate 3H; Fig. 2). Moreover, resin canals were originally described within the vascular cylinder (Alvin, 1960), but our observations reveal that they are actually located at the periphery of the vascular cylinder, along the boundary between the cortex and the vascular cylinder (Plate 2A). In P. yorkshirensis, few resin canals were observed in the secondary xylem of the vascular cylinder, unlike P. belgica, which has no resin canals in the xylem of the vascular cylinder.
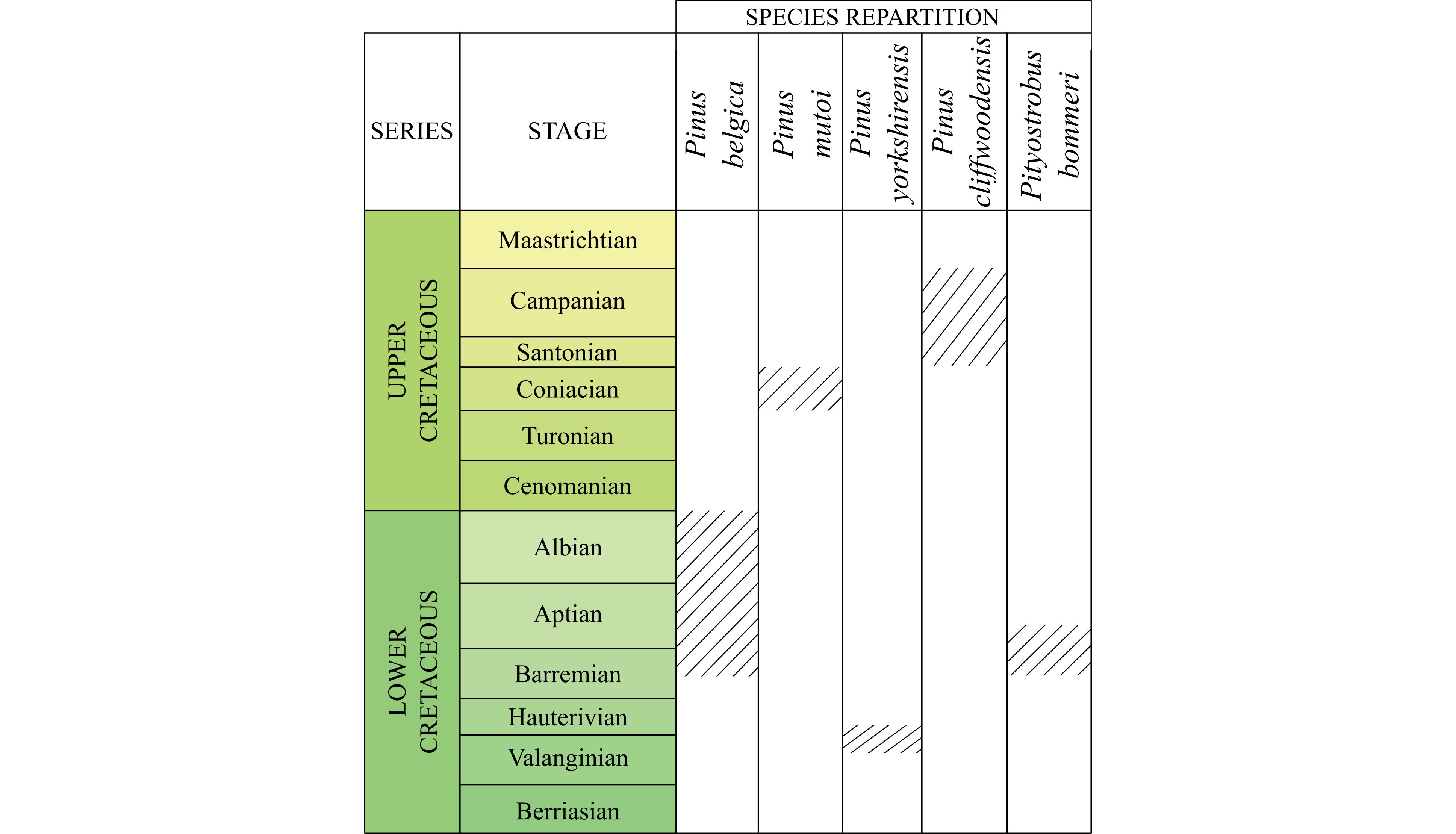
Figure 3. Stratigraphic repartition of Cretaceous Pinus and Pityostrobus species: Pinus belgica Alvin (1960), Pinus mutoi Saiki (1996), Pinus yorkshirensis Ryberg et al. (2012), Pityostrobus cliffwoodensis Miller (1978), and Pityostrobus bommeri Alvin (1953).
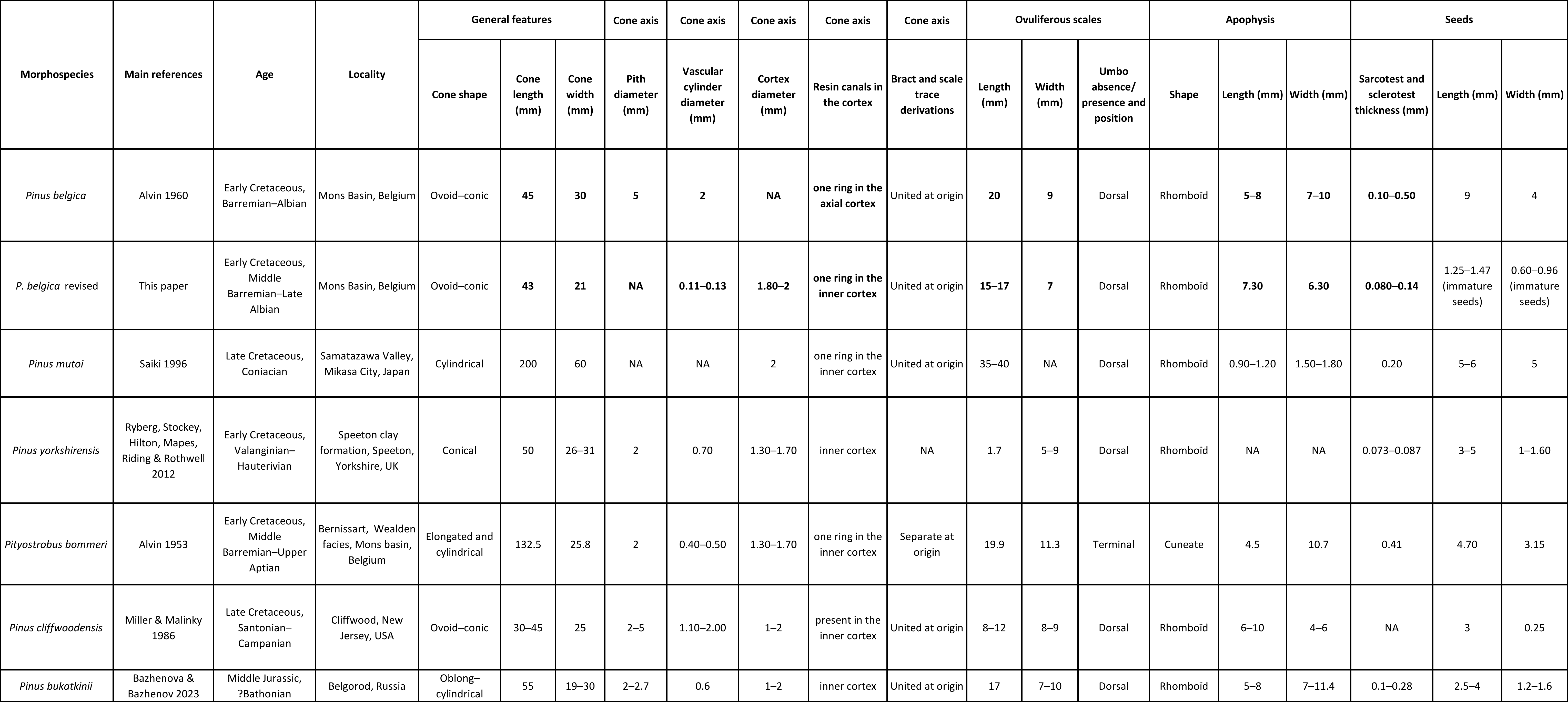
Table 1. Ages, localities and main morphological characters of five Pinus and Pityostrobus fossil ovulate cone species: Pinus belgica Alvin (Alvin, 1960) original description and the revised description of Pinus belgica proposed in this study, Pinus mutoi Saiki (Saiki, 1996), Pinus yorkshirensis Ryberg, Stockey, Hilton, Mapes, Riding et Rothwell (Ryberg et al., 2012), Pityostrobus bommeri Alvin (Alvin, 1953), Pityostrobus cliffwoodensis Miller (Miller, 1978), and Pinus bukatkinii Bazhenova & Bazhenov (Bazhenova et al., 2023). NA = unknown character. Characters in bold are characters of P. belgica modified in the present study.
6. Discussions
35Our revised description of P. belgica confirms the presence of three traits that are diagnostic of the genus Pinus (Miller 1976a): rhomboid thickened apophyses, a pronounced dorsal umbo, bract and scale traces united at the origin. Several traits have been modified from the original description of Alvin (1960), including multiple characters that are usually scored in morphological matrices (Smith et al., 2017; Gernandt et al., 2018). An important reinterpretation deals with the organization of resin canals at scale base. Indeed, Alvin (1960) described the presence of resin canals adaxial and abaxial to the vascular bundles at scale base. However, this was not confirmed by our observations (Plate 5A & Plate 6C): we rather observed resin canals only abaxial to the vascular bundles. This is depicted in the suggested reconstruction illustrating the derivation of the bract-scale from the axis (Fig. 2). It shows that the way the bract and scale derived from the cone axis is only consistent with resin canals abaxial to vascular bundles. Resin canals only abaxial to the vascular bundles is a diagnostic character of the genus Pinus that is not found in ovulate cones of other genera (Miller, 1976a; Shang et al., 2001; Klymiuk & Stockey, 2012). The modification of this trait is important because it was used by Alvin (1960) as an argument for a so-called ‘transitional’ position of P. belgica within the genus Pinus and other genera of Pinaceae. We have described for the first time the way the bract and ovuliferous scale separate as medially first (Plate 4A–B). This character was not described by Alvin (1960), but it was observable on his plates and schemes (Alvin, 1960, p. 19, fig. 4). Usually in phylogenies, this character was indicated to separate laterally first (Gernandt et al., 2018). This feature of the scale and bract separating medially first is known to occur in the genera Abies, Larix, Tsuga, Eathiestrobus, and some species of Pityostrobus. In the other genera of Pinaceae (including the extinct genera Eathiestrobus, Obirastrobus and Pseudoaraucaria) the scale and bract separate laterally first (Miller, 1977; Ryberg et al., 2012). Miller (1977) transferred Pinus lynni into Pityostrobus lynni, arguing that the traces of bract and scale separate medially first and not laterally (and also based on the entrance of bract trace in the free part of the bract). In subsequent phylogenies, P. lynni has always been placed in the Pinus clade (Ryberg et al., 2012; Gernandt et al., 2018). The diagnostic nature of this character should be discussed and explored in further studies. This character may not be all that diagnostic, as Miller (1977) has previously speculated. We therefore assume that this character is maybe not a feature excluding specimens from the genus Pinus at this stage of our knowledge.
36We also report for the first time the presence of a few scale trichomes (Plate 2A, C). Our observations also show that they are present in the periphery of the pith, at the edge between the pith and vascular cylinder (Plate 2A). Overall, the updated description of P. belgica confirms its assignment to the genus Pinus by Alvin (1960). The observation of the diagnostic features of Pinus was made possible by the exceptional preservation of the specimen, typical of the Wealden facies sediments of Belgium. Although the dating of P. yorkshirensis is well constrained, the quality of preservation is lower than in P. belgica and the tissues are less well preserved. To date, this makes P. belgica, one of the oldest representatives of the genus Pinus with such an exceptional preservation.
37Other modifications compared to the original description concern the measurements of the ovulate cone and cone structures made by Alvin (1960). We measured the different structures on the specimen ourselves (Table 1). For most of the structures, we found differences between our measurements and those of Alvin (1960), ranging from 5.5% (for the cone length) to 30% (for the cone width). The length of the cone was measured by Alvin (1960) at 45 mm, and we measured 43 mm (Table 1). Other structures we measured show a larger discrepancy, such as the width of the cone, which we measured at 21 mm against 30 mm for Alvin (1960) description (keeping in mind that the cone has been degraded since its first study). Part of this difference may at least be explained by operator bias. In parallel, in its original publication, Alvin (1960) hypothesized that the size of some specimens was reduced after drying of the matrix and the fossils. He considered that the specimens were probably 20–25% smaller than their original size based on the observation of an ovulate cone specimen from the locality of Villerot. He wrote: “[…] it is striking how much smaller the cone is than the cavity (impression) that it occupies in the now dry matrix. [...] The linear dimensions of the fossil are thus nearly 20 % less than those of the impression.” (Alvin, 1960, p. 3). He suggested that the size of ovulate cone specimens, including the one described as the holotype of P. belgica, was probably reduced after their collection due to drying of the fossil specimens and enclosing sediments. It is worth to note that we have observed first-hand the same phenomenon in recently collected fossil specimens from the locality of Hautrage and the retraction process has been clearly observed (Fig. 4). However, no measurements were made before the process of retraction began and we did not collect specimens showing the same configurations (sediment matrix, external mould, pre- and post-drying measurements) available for comparison. Nonetheless, we were still able to estimate a size reduction of 17% (by measuring the difference between the size of the cone and the imprint left in the dried clay) for the specimen of Pityostrobus sp. collected at Hautrage (Fig. 4), similar to the shrinking estimation of Alvin (1960).
38Importantly, the deformation of ovulate cones after drying is thus attested by the observation of Alvin (1960), on a specimen of P. villerotensis (from the locality of Villerot), and our own, on the specimen we collected from Hautrage (Fig. 4). However, the sediment behaviour was not compared between the different localities (more or less important shrinkage), because the locality of P. belgica is unknown, and Wealden facies are represented by four different formations in the Mons Basin (Sainte-Barbe Clay Formation, the Baudour Clay Formation, the Hautrage Clay Formation and the Saint-Pierre Gravels Formation; Yans et al., 2005, 2010; Dejax et al., 2007a, 2007b; Schnyder et al., 2009; Baele et al., 2012; Spagna et al., 2012). Moreover, Pinaceae material was found in nine different localities where these different formations have been accessible (Baudour, Bernissart, Hautrage, Villerot, Baume, Bracquegnies, La Louvière, Houdeng‐Aimeries and Soignies) (Yans et al., 2005; Yans, 2007). Sedimentological studies have shown differences in sedimentation and depositional environment between Hautrage (fluviatile environment; Spagna et al., 2007, 2008) and Bernissart (lacustrine type environment; Dejax et al., 2007a; Blanco-Moreno & Prestianni, 2021). Since the composition of the sediments between these localities and formations is different, it cannot be assumed that they behave in the same way during drying (unless a comparative study is conducted). At this point, the hypothesis of Alvin (1960) of a possible shrinkage of the type specimen of P. belgica seems to be likely but not certain. This shrinkage might have been continuing over the last 60 years. This hypothesis of cone shrinkage after drying is important because it would mean that the dimensions of the ovulate cones (and maybe other plant remains) in the Wealden sediments could be underestimated and unreliable. This again calls for the importance of conducting a thorough taphonomic study of these fossil plant assemblages.

Figure 4. Example of size modification of a fossil ovulate cone after its collection in Wealden sediments. The specimen collected in June 2019 in Hautrage, Danube-Bouchon quarry. The sediments are dated to middle Barremian to late Albian (Dejax et al., 2008; Yans et al., 2010). This ovulate cone is assigned to Pityostrobus sp. The blue delineation (right) shows the cavity left vacant after the retraction of the specimen. From the measurements of the total length of the cavity initially occupied by the ovulate cone and its present length, we estimate a length reduction of 17%.
7. Conclusions
39The case of Pinus belgica represents a systematic dilemma. The revision points several approximations in the original description (diagnosis, measurements, stratigraphy, age). Its age uncertainty is fairly broad, from middle Barremian to late Albian and it should be thus used carefully in molecular dating studies. We confirm that P. belgica possesses diagnostic characteristics of Pinus (e.g., thickened apophyses, pronounced dorsal umbo, resin canals abaxial to the scale trace, bract and scale traces united at origin) which makes this species the second oldest ovulate cone representative of the genus Pinus. The species P. yorkshirensis is the oldest ovulate cone whose dating and origin are well supported, and it would be advisable to use it in priority in the analyses. We reinterpret several key anatomical features differently than in the original description (resin canals only abaxial to vascular bundles, bract and ovuliferous scale separating medially first, resin canals distributed at the edge between pith and vascular cylinder), thereby modifying our understanding of morphology of the earliest representatives of Pinus.
Acknowledgments
40We thank the Royal Belgian Institute of Natural Sciences for facilitating access to their nano-CT scanner as part of the DIGIT04 project and Camille Locatelli for operating the nano-CT. We also thank Andrew Leslie of Stanford University (USA) and Fabiany Herrera of the Field Museum (Chicago, USA) for their valuable comments, which greatly helped to improve the manuscript.
Author contributions
41Léa De Brito, Valentin Fischer and Cyrille Prestianni conceived the project. L. D.B. processed the nano-X-ray tomography data. L. D.B. and C. P. conducted the observations and description. L. D.B., V. F. and C. P. wrote the manuscript.
Data availability
42All the material is curated in the Palaeontological collections of the Royal Belgian Institute of Natural Sciences (RBINS) in Brussels (Belgium) guaranteeing their long-term safekeeping and availability to other researchers for future studies.
References
43Allen, P., 1955. Age of the Wealden in North-Western Europe. Geological Magazine, 92, 265–281. https://doi.org/10.1017/S0016756800064311
44Allen, P. & Wimbledon, W.A., 1991. Correlation of NW European Purbeck-Wealden (nonmarine Lower Cretaceous) as seen from the English type-areas. Cretaceous Research, 12, 511–526. https://doi.org/10.1016/0195-6671(91)90005-W
45Alvin, K.L., 1953. Three abietaceous cones from the Wealden of Belgium. Mémoires de l’Institut royal des Sciences naturelles de Belgique, 125, 1–42.
46Alvin, K.L., 1957. On the two cones Pseudoaraucaria heeri (Coemans) nov. comb. and Pityostrobus villerotensis nov. sp. from the Wealden of Belgium. Mémoires de l’Institut royal des Sciences naturelles de Belgique, 135, 1–27.
47Alvin, K.L., 1960. Further conifers of the Pinaceae from the Wealden formation of Belgium. Mémoires de l’Institut royal des Sciences naturelles de Belgique, 146, 1–39.
48Baele, J.-M., Godefroit, P., Spagna, P. & Dupuis, C., 2012. A Short introduction to the geology of the Mons Basin and the iguanodon sinkhole, Belgium. In Godefroit, P (ed.), Bernissart Dinosaurs and Early Cretaceous Terrestrial Ecosystems. Indiana University Press, Bloomington, 35–42.
49Bazhenova, N.V., Bazhenov, A.V., Tekleva, M.V. & Resvyi, A.S., 2023. New representative of Pinus L. from Jurassic deposits of Belgorod Region, Russia. Paleontological Journal, 57, 102–119. https://doi.org/10.1134/S0031030123010033
50Blanco-Moreno, C. & Prestianni, C., 2021. Taxonomic revision and palaeoecological interpretation of the plant assemblage of Bernissart (Barremian, Belgium). Cretaceous Research, 124, 104814. https://doi.org/10.1016/j.cretres.2021.104814
51Bommer, C., 1892. Un nouveau gîte à végétaux découvert dans l’argile wealdienne de Bracquegnies. Bulletin de la Société belge de Géologie, de Paléontologie et d’Hydrologie, 6, 160–161.
52De Brito, L. & Prestianni, C., 2021. Pityostrobus andraei (Pinaceae) from the Barremian (Lower Cretaceous) of Belgium: A morphometric revision. International Journal of Plant Sciences, 182, 174–184. https://doi.org/10.1086/712355
53Dejax, J., Dumax, É., Damblon, F. & Yans, J., 2007a. Palynology of Baudour Clays Formation (Mons Basin, Belgium): correlation within the “stratotypic” Wealden. Carnets de Géologie / Notebooks on Geology, Memoir 2007/01, 16–28.
54Dejax, J., Pons, D. & Yans, J., 2007b. Palynology of the dinosaur-bearing Wealden facies in the natural pit of Bernissart (Belgium). Review of Palaeobotany and Palynology, 144, 25–38. https://doi.org/10.1016/j.revpalbo.2005.10.004
55Dejax, J., Pons, D. & Yans, J., 2008. Palynology of the Wealden facies from Hautrage quarry (Mons Basin, Belgium). Memoirs of the Geological Survey of Belgium, 55, 45–51.
56Domogatskaya, K.V. & Herman, A.B., 2019. New species of the genus Schizolepidopsis (conifers) from the Albian of the Russian high Arctic and geological history of the genus. Cretaceous Research, 97, 73–93. https://doi.org/10.1016/j.cretres.2019.01.012
57Doweld, A.B., 2001. Schizolepidopsis, a new substitute generic name for Mesozoic plants. Byulletenʹ Moskovskogo Obshchestva Ispytateleĭ Prirody. Otdel Geologicheskiĭ, 76, 86–88. [In Russian].
58Eckert, A.J. & Hall, B.D., 2006. Phylogeny, historical biogeography, and patterns of diversification for Pinus (Pinaceae): Phylogenetic tests of fossil-based hypotheses. Molecular Phylogenetics and Evolution, 40, 166–182. https://doi.org/10.1016/j.ympev.2006.03.009
59Falcon-Lang, H.J., Mages, V. & Collinson, M., 2016a. The oldest Pinus and its preservation by fire. Geology, 44/4, 303–306. https://doi.org/10.1130/G37526.1
60Falcon-Lang, H.J., Mages, V. & Collinson, M., 2016b. Reaffirming Pinus mundayi as the oldest known pine fossil: REPLY. Geology, 44/8, e402. https://doi.org/10.1130/G38240Y.1
61Falder, A.B., Rothwell, G.W., Mapes, G., Mapes, R.H. & Doguzhaeva, L.A., 1998. Pityostrobus milleri sp. nov., a pinaceous cone from the Lower Cretaceous (Aptian) of southwestern Russia. Review of Palaeobotany and Palynology, 103, 253–261. https://doi.org/10.1016/S0034-6667(98)00041-4
62Gernandt, D.S., Magallón, S., Geada López, G., Zerón Flores, O., Willyard, A. & Liston, A., 2008. Use of simultaneous analyses to guide fossil‐based calibrations of Pinaceae phylogeny. International Journal of Plant Sciences, 169, 1086–1099. https://doi.org/10.1086/590472
63Gernandt, D.S., León-Gómez, C., Hernández-León, S. & Olson, M.E., 2011. Pinus nelsonii and a cladistic analysis of Pinaceae ovulate cone characters. Systematic Botany, 36, 583–594. https://doi.org/10.1600/036364411X583565
64Gernandt, D.S., Holman, G., Campbell, C., Parks, M., Mathews, S., Raubeson, L.A., Liston, A., Stockey, R.A. & Rothwell, G.W., 2016. Phylogenetics of extant and fossil Pinaceae: methods for increasing topological stability. Botany, 94, 863–884. https://doi.org/10.1139/cjb-2016-0064
65Gernandt, D.S., Arias, C.R., Terrazas, T., Dugua, X.A. & Willyard, A., 2018. Incorporating fossils into the Pinaceae tree of life. American Journal of Botany, 105, 1329–1344. https://doi.org/10.1002/ajb2.1139
66Godefroit, P. & Leduc, T., 2008. La conservation des ossements fossiles : le cas des Iguanodons de Bernissart. CeROArt : Conservation, Exposition, Restauration d’Objets d’Art, 2008/2. https://doi.org/10.4000/ceroart.464
67Harris, T.M., 1953. Conifers of the Taxodiaceae from the Wealden Formation of Belgium. Mémoires de l’Institut Royal des Sciences Naturelles de Belgique, 126, 1–43.
68Herrera, F., Leslie, A.B., Shi, G., Knopf, P., Ichinnorov, N., Takahashi, M., Crane, P.R. & Herendeen, P.S., 2016. New fossil Pinaceae from the Early Cretaceous of Mongolia. Botany, 94, 885–915. https://doi.org/10.1139/cjb-2016-0042
69Herrera, F., Shi, G., Bickner, M.A., Ichinnorov, N., Leslie, A.B., Crane, P.R. & Herendeen, P.S., 2021. Early Cretaceous abietoid Pinaceae from Mongolia and the history of seed scale shedding. American Journal of Botany, 108, 1483–1499. https://doi.org/10.1002/ajb2.1713
70Hilton, J., Riding, J.B. & Rothwell, G.W., 2016. Age and identity of the oldest pine fossils: COMMENT. Geology, 44, e400–e401. https://doi.org/10.1130/G38050C.1
71Klymiuk, A.A. & Stockey, R.A., 2012. A Lower Cretaceous (Valanginian) seed cone provides the earliest fossil record for Picea (Pinaceae). American Journal of Botany, 99, 1069–1082. https://doi.org/10.3732/ajb.1100568
72Leduc, T., 2012. Diagenesis of the fossil bones of Iguanodon bernissartensis from the Iguanodon sinkhole. In Godefroit, P (ed.), Bernissart Dinosaurs and Early Cretaceous Terrestrial Ecosystems. Indiana University Press, Bloomington, 111–135.
73Leslie, A.B., Glasspool, I., Herendeen, P.S., Ichinnorov, N., Knopf, P., Takahashi, M. & Crane, P.R., 2013. Pinaceae‐like reproductive morphology in Schizolepidopsis canicularis sp. nov. from the Early Cretaceous (Aptian‐Albian) of Mongolia. American Journal of Botany, 100, 2426–2436. https://doi.org/10.3732/ajb.1300173
74Marlière, 1970. Géologie du bassin de Mons et du Hainaut : un siècle d’histoire. Annales de la Société géologique du Nord, 90, 171–189.
75Matsunaga, K.K.S., Herendeen, P.S., Herrera, F., Ichinnorov, N., Crane, P.R. & Shi, G., 2021. Ovulate cones of Schizolepidopsis ediae sp. nov. provide insights into the evolution of Pinaceae. International Journal of Plant Sciences, 182/6, 490–507. https://doi.org/10.1086/714281
76Miller, C.N., 1976a. Early evolution in the Pinaceae. Review of Palaeobotany and Palynology, 21, 101–117. https://doi.org/10.1016/0034-6667(76)90024-5
77Miller, C.N., 1976b. Two new pinaceous cones from the Early Cretaceous of California. Journal of Paleontology, 50, 821–832.
78Miller, C.N., 1977. Pityostrobus lynni (Berry) comb. nov., a pinaceous seed cone from the Paleocene of Virginia. Bulletin of the Torrey Botanical Club, 104/1, 5–9. https://doi.org/10.2307/2484657
79Miller, C.N., 1978. Pityostrobus cliffwoodensis (Berry) comb. nov., a pinaceous seed cone from the Late Cretaceous of New Jersey. Botanical Gazette, 139/2, 284–287. https://doi.org/10.1086/337002
80Miller, C.N. & Malinky, J.M., 1986. Seed cones of Pinus from the Late Cretaceous of New Jersey, U.S.A. Review of Palaeobotany and Palynology, 46, 257–272. https://doi.org/10.1016/0034-6667(86)90018-7
81Pirson, S., Spagna, P., Baele, J.-M., Damblon, F., Gerrienne, P., Vanbrabant, Y. & Yans, J., 2008. An overview of the geology of Belgium. Memoirs of the Geological Survey of Belgium, 55, 5–25.
82Ratzel, S.R., Rothwell, G.W., Mapes, G., Mapes, R.H. & Doguzhaeva, L.A., 2001. Pityostrobus hokodzensis, a new species of pinaceous cone from the Cretaceous of Russia. Journal of Paleontology, 75, 895–900. https://doi.org/10.1666/0022-3360(2001)075<0895:PHANSO>2.0.CO;2
83Robaszynski, F., Dhondt, A.V. & Jagt, J.W.M., 2001. Cretaceous lithostratigraphic units (Belgium). Geologica Belgica, 4/1-2, 121–134. https://doi.org/10.20341/gb.2014.049
84Rothwell, G.W. & Mapes, G., 2001. Barthelia furcata gen. et sp. nov., with a review of Paleozoic coniferophytes and a discussion of coniferophyte systematics. International Journal of Plant Sciences, 162, 637–667. https://doi.org/10.1086/320129
85Rueden, C.T., Schindelin, J., Hiner, M.C., DeZonia, B.E., Walter, A.E., Arena, E.T. & Eliceiri, K.W., 2017. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics, 18, 529. https://doi.org/10.1186/s12859-017-1934-z
86Ryberg, P.E., Rothwell, G.W., Stockey, R.A., Hilton, J., Mapes, G. & Riding, J.B., 2012. Reconsidering relationships among stem and crown group Pinaceae: oldest record of the genus Pinus from the Early Cretaceous of Yorkshire, United Kingdom. International Journal of Plant Sciences, 173, 917–932. https://doi.org/10.1086/667228
87Saiki, K., 1996. Pinus mutoi (Pinaceae), a new species of permineralized seed cone from the Upper Cretaceous of Hokkaido, Japan. American Journal of Botany, 83, 1630–1636. https://doi.org/10.1002/j.1537-2197.1996.tb12821.x
88Saladin, B., Leslie, A.B., Wüest, R.O., Litsios, G., Conti, E., Salamin, N. & Zimmermann, N.E., 2017. Fossils matter: improved estimates of divergence times in Pinus reveal older diversification. BMC Evolutionary Biology, 17, 95. https://doi.org/10.1186/s12862-017-0941-z
89Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S. Schmid, B., Tinevez, J.-Y., White, D.J., Hartenstein, V., Eliceiri, K., Tomancak, P. & Cardona, A., 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods, 9, 676–682. https://doi.org/10.1038/nmeth.2019
90Schneider, C.A., Rasband, W.S. & Eliceiri, K.W., 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. https://doi.org/10.1038/nmeth.2089
91Schnyder, J., Dejax, J., Keppens, E., Nguyen Tu, T.T., Spagna, P., Boulila, S., Galbrun, B., Riboulleau, A., Tshibangu, J.-P. & Yans, J., 2009. An Early Cretaceous lacustrine record: organic matter and organic carbon isotopes at Bernissart (Mons Basin, Belgium). Palaeogeography, Palaeoclimatology, Palaeoecology, 281, 79–91. https://doi.org/10.1016/j.palaeo.2009.07.014
92Shang, H., Cui, J.-Z. & Li, C.-S., 2001. Pityostrobus yixianensis sp. nov., a pinaceous cone from the Lower Cretaceous of north-east China. Botanical Journal of the Linnean Society, 136, 427–437. https://doi.org/10.1111/j.1095-8339.2001.tb00583.x
93Smith, S.Y. & Stockey, R.A., 2002. Permineralized pine cones from the Cretaceous of Vancouver Island, British Columbia. International Journal of Plant Sciences, 163, 185–196. https://doi.org/10.1086/324553
94Smith, S.Y., Stockey, R.A., Rothwell, G.W. & Little, S.A., 2017. A new species of Pityostrobus (Pinaceae) from the Cretaceous of California: moving towards understanding the Cretaceous radiation of Pinaceae. Journal of Systematic Palaeontology, 15, 69–81. https://doi.org/10.1080/14772019.2016.1143885
95Spagna, P., Vandycke, S., Yans, J. & Dupuis, C., 2007. Hydraulic and brittle extensional faulting in the Wealden facies of Hautrage (Mons Basin, Belgium). Geologica Belgica, 10/3-4, 158–161.
96Spagna, P., Dupuis, C. & Yans, J., 2008. Sedimentology of the Wealden clays in the Hautrage quarry. Memoirs of the Geological Survey of Belgium, 55, 35–44.
97Spagna, P., Yans, J., Schnyder, J. & Dupuis, C., 2012. The paleoenvironment of the Bernissart Iguanodons: Sedimentological analysis of the Lower Cretaceous Wealden facies in the Bernissart area. In Godefroit, P (ed.), Bernissart Dinosaurs and Early Terrestrial Ecosystems. Indiana University Press, Bloomington, 87–95.
98Willyard, A., Syring, J., Gernandt, D.S., Liston, A. & Cronn, R., 2007. Fossil calibration of molecular divergence infers a moderate mutation rate and recent radiations for Pinus. Molecular Biology and Evolution, 24, 90–101. https://doi.org/10.1093/molbev/msl131
99Yans, J., 2007. Lithostratigraphie, minéralogie et diagenèse des sédiments à faciès wealdien du Bassin de Mons (Belgique). Académie royale de Belgique, Bruxelles, Mémoires de la Classe des sciences, 3e série, 9, 179 p.
100Yans, J., Spagna, P., Foucher, J.C., Perruchot, A., Streel, M., Beaunier, P. & Dupuis, C., 2002. Multidisciplinary study of the Wealden deposits of the Mons Basin (Belgium): a progress report. Aardkundige Mededelingen, 12, 39–42.
101Yans, J., Dejax, J., Pons, D., Dupuis, C. & Taquet, P., 2005. Implications paléontologiques et géodynamiques de la datation palynologique des sédiments à faciès wealdien de Bernissart (bassin de Mons, Belgique). Comptes Rendus Palevol, 4, 135–150. https://doi.org/10.1016/j.crpv.2004.12.003
102Yans, J., Dejax, J., Pons, D., Taverne, L. & Bultynck, P., 2006. The iguanodons of Bernissart (Belgium) are middle Barremian to earliest Aptian in age. Bulletin de l’Institut royal des Sciences naturelles de Belgique : Sciences de la Terre, 76, 91–95.
103Yans, J., Gerards, T., Gerrienne, P., Spagna, P., Dejax, J., Schnyder, J., Storme, J.-Y. & Keppens, E., 2010. Carbon-isotope analysis of fossil wood and dispersed organic matter from the terrestrial Wealden facies of Hautrage (Mons Basin, Belgium). Palaeogeography, Palaeoclimatology, Palaeoecology, 291, 85–105. https://doi.org/10.1016/j.palaeo.2010.01.014
104Yans, J., Dejax, J., Schnyder, J., Pons, D., Spagna, P., Dupuis, C., & Gerrienne, P., 2011. Stratigraphie des sédiments à faciès Wealdien dans le Bassin de Mons (Belgique). Bulletin d’Information des Géologues du Bassin de Paris, 48/3, 9–13.
105Zhang, J., D’rozario, A., Yao, J., Wu, Z. & Wang, L., 2011. A new species of the extinct genus Schizolepis from the Jurassic Daohugou Flora, Inner Mongolia, China with special reference to the fossil diversity and evolutionary implications. Acta Geologica Sinica‐English Edition, 85, 471–481. https://doi.org/10.1111/j.1755-6724.2011.00415.x
106Manuscript received 24.01.2024, accepted in revised form 08.08.2024, available on line 29.11.2024.
Supplementary material
107Ovulate cone scale volume renderings are available on Morphosource, Royal Belgian Institute of Natural Sciences RBINS - Scientific Survey of Heritage (SSH) - Paleontology Collections – Paleobotany.
108IRSNB b 7693: https://www.morphosource.org/concern/parent/000480776/media/000480779 and
109https://www.morphosource.org/concern/media/000480789?locale=en;
110IRSNB b 7694: https://www.morphosource.org/concern/media/000480797?locale=en;
111IRSNB b 7697: https://www.morphosource.org/concern/media/000480807?locale=en.

Plate 1. Pinus belgica Alvin 1960 holotype (IRSNB b 7690). A. Complete ovulate cone with the four broken parts reassembled. B. First part of the cone base. C. Second part of the cone base. D. Part of the centre of the cone. E. Cone scale IRSNB b 7693 in abaxial (left) and adaxial (right) views. F. Third part of the cone base. G. Cone scale IRSNB b 7697 in abaxial (left) and adaxial (right) views. H. Cone scale IRSNB b 7694 in lateral (left), abaxial (middle) and adaxial (right) views.
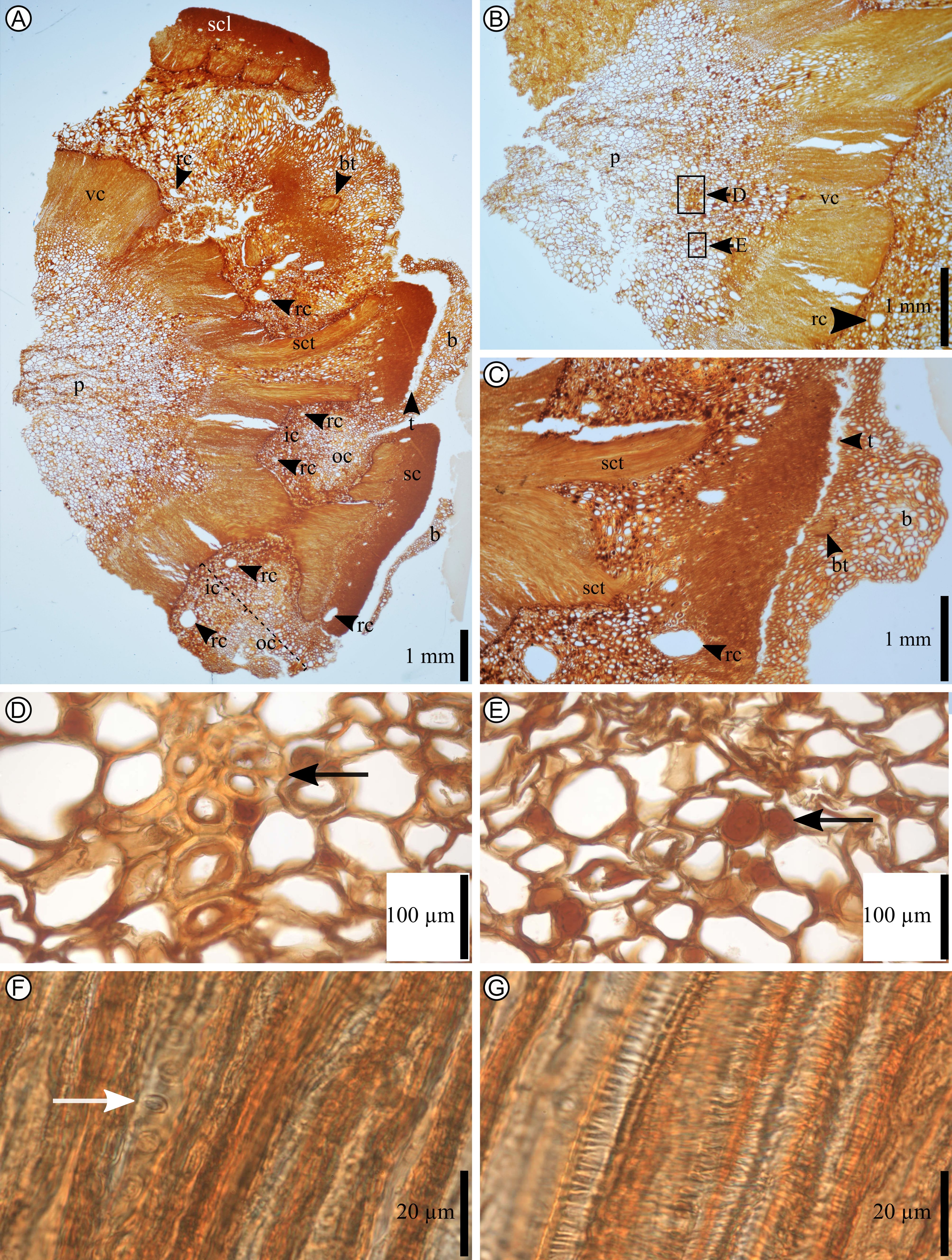
Plate 2. Pinus belgica Alvin (1960) cone axis anatomy of IRSNB b 7690. A. Cone axis in transversal section (section J25C). B. Cone axis in transversal section (section J26A). C. Bract and scale in transversal section (section J22B). D. Sclerotic nest in the pith surrounded by parenchyma (the picture corresponds to the area pointed by the black arrow in B. Section J26a). E. Secretory cells in pith surrounded by parenchyma (black arrow. Section J26A). F. Tracheid’s vascular cylinder with circular-bordered pits in secondary xylem (white arrow). G. Tracheid’s vascular cylinder with helical thickenings in primary xylem. b, bract; bt, bract trace; ic, inner cortex; oc, outer cortex; p, pith; rc, resin canal; sc, scale; scl, sclerenchyma; sct, scale trace; t, trichome; vc, vascular cylinder.
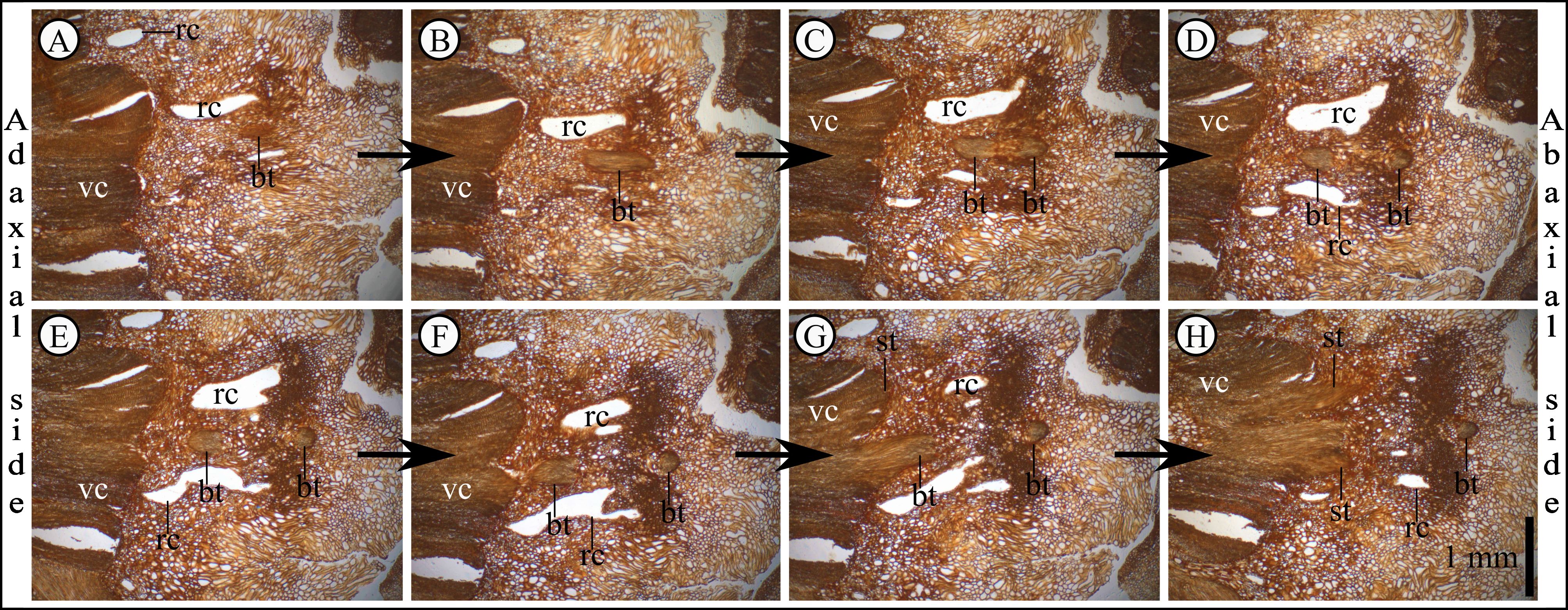
Plate 3. Pinus belgica Alvin (1960) specimen IRSNB b 7690 bract and scale traces derivation. Anatomical thin sections are shown in order from A to H, A being the closest part to the cone base. Bract and scale traces are united at the base. After the divergence with scale trace, the vascular bundle goes down and then goes up to supply the bract. A. Anatomical thin section J29A. B. Anatomical thin section J210A. C. Anatomical thin section J210B. D. Anatomical thin section J210C. E. Anatomical thin section J210D. F. Anatomical thin section J211A. G. Anatomical thin section J211B. H. Anatomical thin section J211C. bt, bract trace; rc, resin canal; sct, scale trace; vc, vascular cylinder. The scale bar in H applies for each panel.
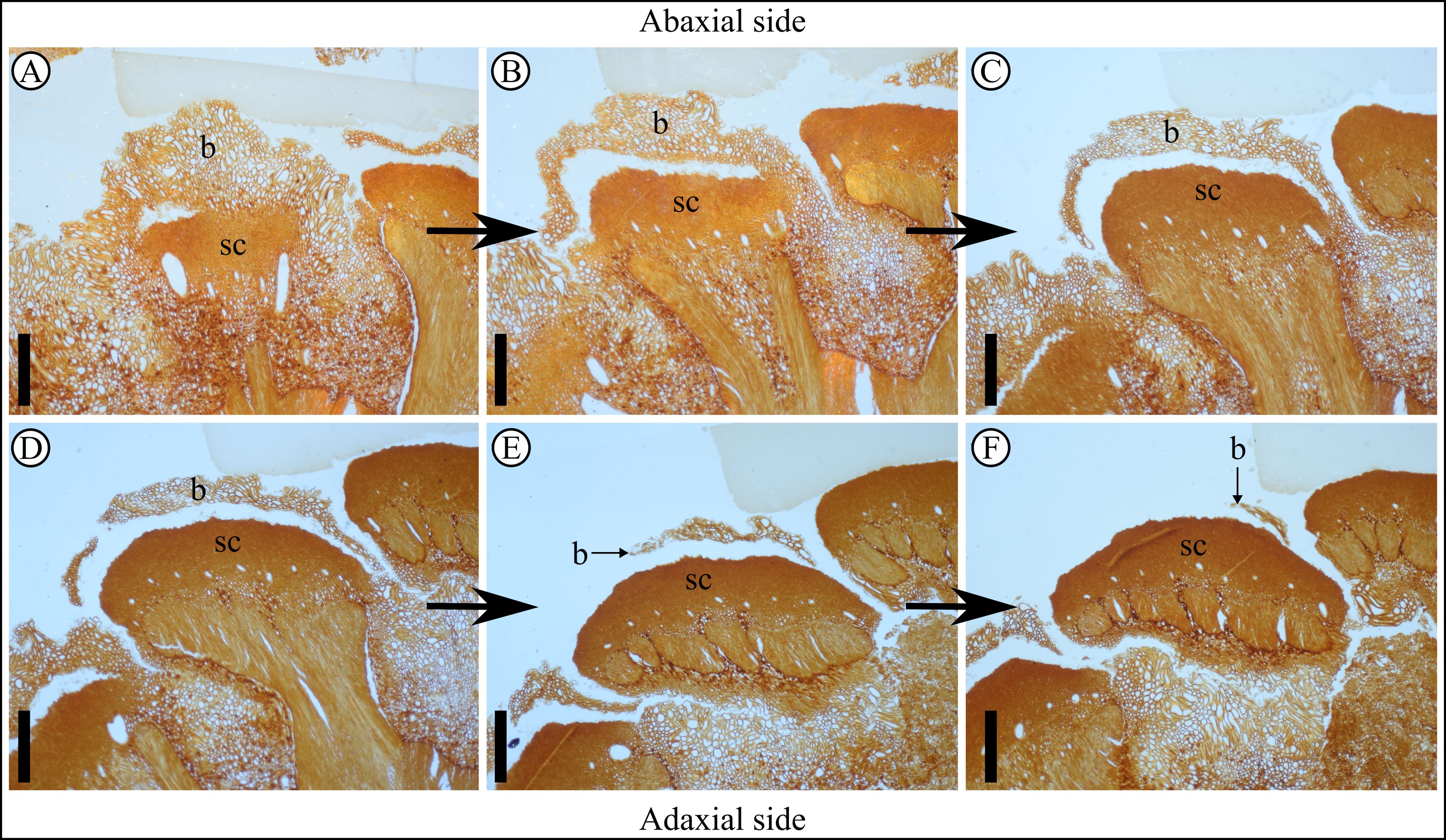
Plate 4. Pinus belgica Alvin (1960) specimen IRSNB b 7690, follow-up of the bract in transversal sections. Anatomical thin sections are shown in order from A to F, A being the base of the bract and F the terminal end of the bract, showing its pointed shape. A–F show the follow-up of the bract that flattened and thickened in a semi-circular way in the middle and flattened on the sides. A. Anatomical thin section J24D, bract base is trapezoidal in shape. B. Anatomical thin section J25D showing bract and scale separating medially first. C. Anatomical thin section J26E. D. Anatomical thin section J26A. E. Anatomical thin section J27B. F. Anatomical thin section J27A. b, bract; sc, scale. Scale bars = 1 mm.
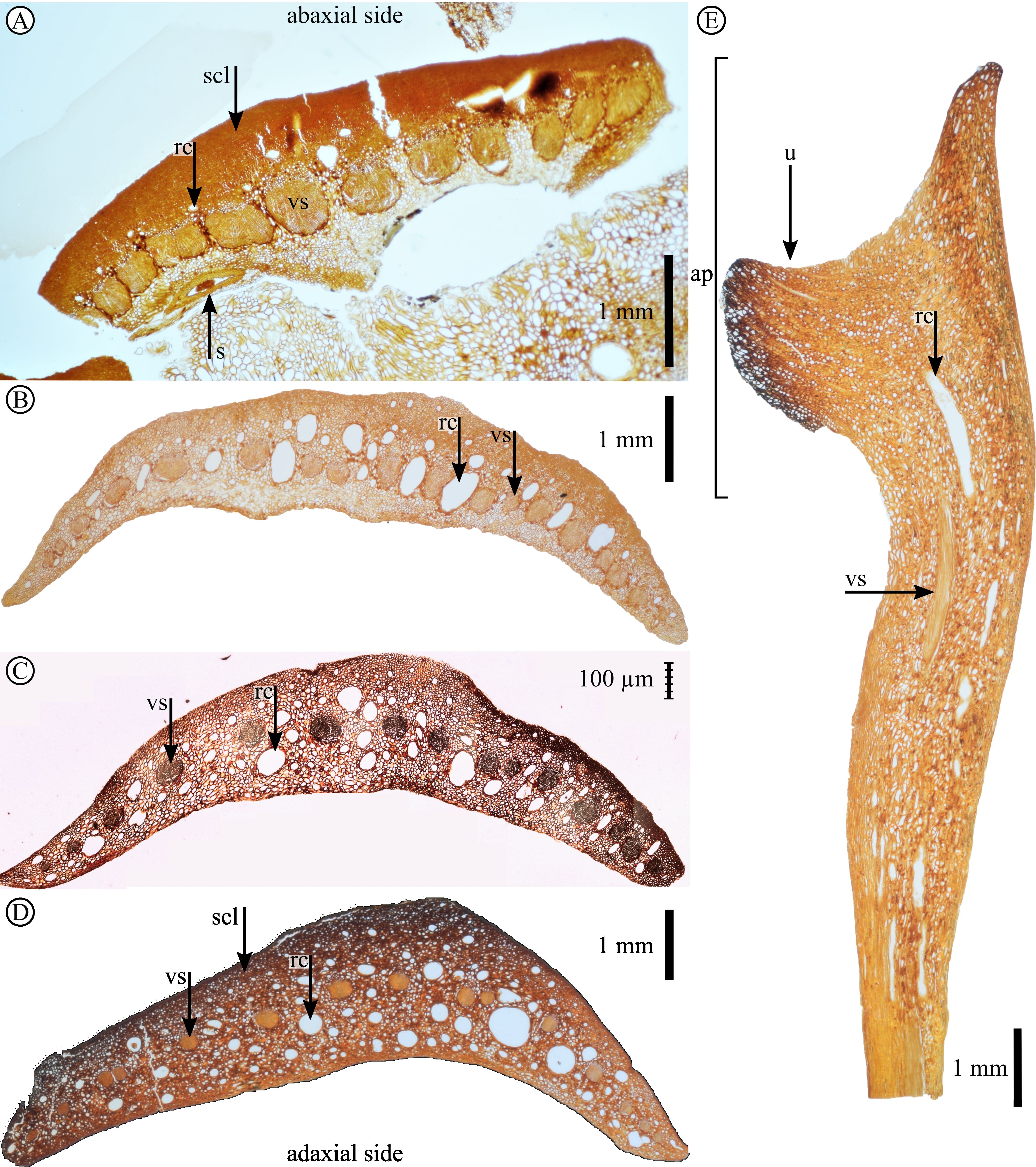
Plate 5. Pinus belgica Alvin (1960) specimen IRSNB b 7690 scale anatomy. A. Scale base in transversal section (anatomical thin section J29A). B. Scale at mid-height in transversal section (anatomical thin section J13F). C. Scale in transversal section (anatomical thin section J14I). D. Scale in transversal section (anatomical thin section J15G). E. Scale in longitudinal section (anatomical thin section J53F). ap, apophysis; rc, resin canal; s, seed; scl, sclerenchyma; u, umbo; vs, vascular strand.
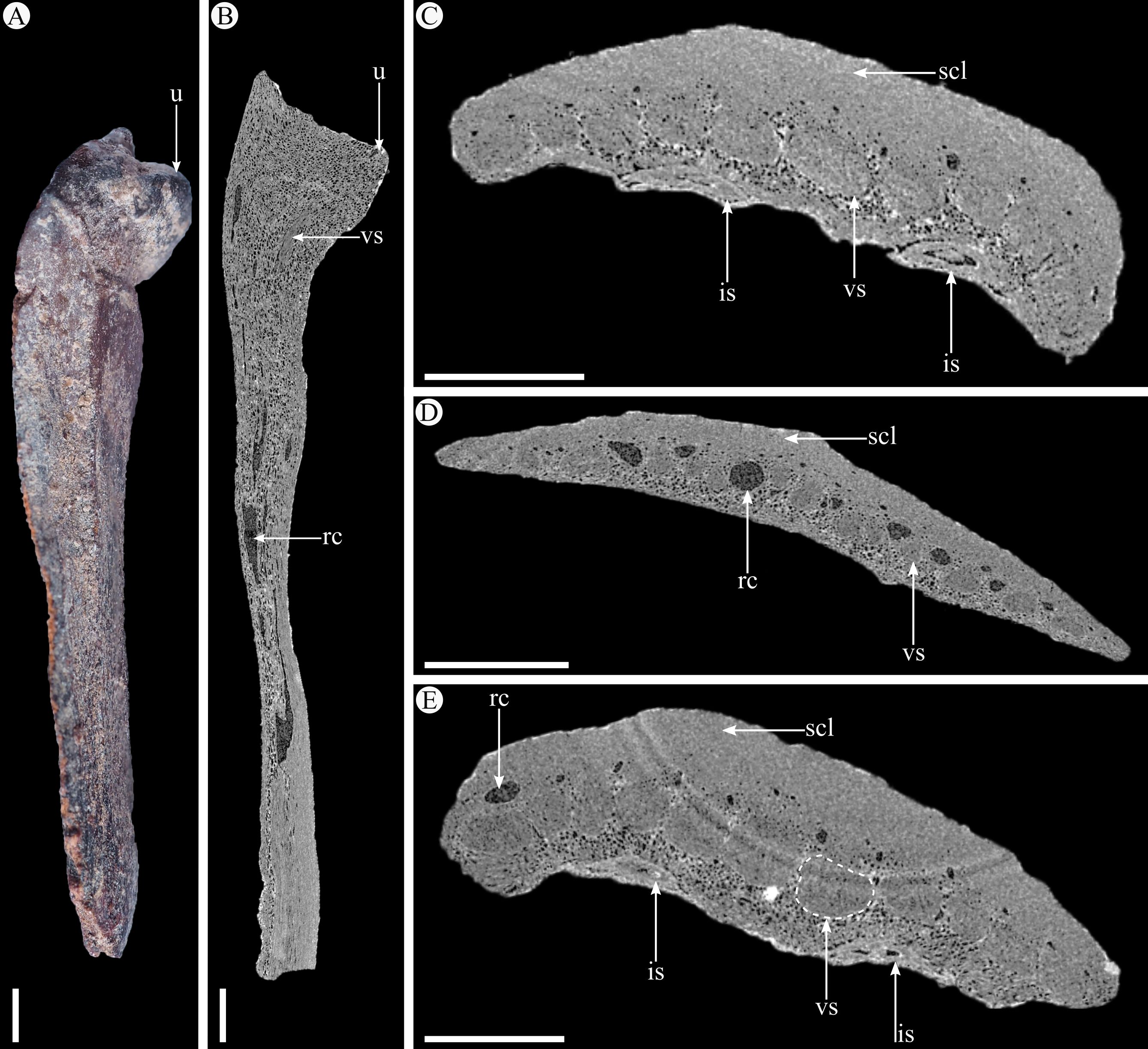
Plate 6. A. Photograph of scale IRSNB b 7694 in lateral view. B. Nano-CT-scanned virtual slice in longitudinal section of scale IRSNB b 7694. C. Nano-CT-scanned virtual slice in transversal section of scale IRSNB b 7694. D. Nano-CT-scanned virtual slice in transversal section of scale IRSNB b 7693. E. Nano-CT-scanned virtual slice in transversal section of scale IRSNB b 7697. is, immature seed; rc, resin canal; scl, sclerenchyma; u, umbo; vs, vascular strand. Scale bars = 1 mm.






