Diversity and distribution of the Palaearctic Cryptocephalini and Pachybrachini (Coleoptera: Chrysomelidae)
Résumé
Parmi le total de 987 espèces de Cryptocephalini et Pachybrachini listées dans l'édition actuelle du catalogue des Coléoptères paléarctiques, d'Afrique du Nord, d'Asie et d'Europe, 79, 666 et 214 espèces ont été enregistrées respectivement. Du Paléarctique occidental et du Paléarctique oriental, 723 et 479 espèces ont été enregistrées respectivement. La diversité des Cryptocephalini et Pachybrachini diffère considérablement entre les subdivisions de la région paléarctique. La diversité est la plus faible dans l'Arctique avec 26 espèces. La richesse spécifique la plus élevée a été trouvée dans la région séthienne (désert) et un total de 507 espèces de Cryptocephalini (419 espèces) et de Pachybrachini (88 espèces) a été enregistré. Plus précisément, la sous-région méditerranéenne de la région hespérienne (forêt à feuilles persistantes) et la sous-région irano-turanienne de la région séthienne (désert) sont riches en espèces avec respectivement 297 et 291 espèces. Le pays le plus riche en espèces est la Chine avec un total de 289 suivie de la Russie (avec des parties européennes et asiatiques) avec un total de 166 espèces et de la France et de l'Italie avec respectivement 143 et 142 espèces. Le pays avec le pourcentage le plus élevé (34%) d'espèces endémiques est le Népal et le plus grand nombre d'espèces endémiques se trouve au Kazakhstan (27 espèces) suivi par l'Espagne et l'Iran avec respectivement 26 et 25 espèces. La répartition géographique des genres et sous-genres de Cryptocephalini et Pachybrachini est compilée. Les espèces appartenant à la région Orientale sont discutées. 27 espèces sont considérées comme Orientales. Sur cette base, un total de 915 espèces paléarctiques de Cryptocephalini et Pachybrachini peut être estimé. Les espèces insulaires sont listées et la biogéographie insulaire est discutée.
Abstract
Among the total of 987 species of Cryptocephalini and Pachybrachini listed in the actual edition of the catalogue of Palaearctic Coleoptera, from North Africa, Asia and Europe 79, 666 and 214 species were recorded, respectively. From the Western Palaearctic and Eastern Palaearctic, 723 and 479 species were recorded, respectively. The diversity of Cryptocephalini and Pachybrachini differs considerably among the subdivisions of the Palaearctic region. Diversity is lowest in the Arctic, with 26 species. The highest species richness was found in the Sethian (desert) region, a total of 507 species of Cryptocephalini (419 species) and Pachybrachini (88 species) were recorded. Specifically, the Mediterranean subregion of the Hesperian (evergreen forest) region and the Irano-Turanian subregion of the Sethian (desert) region are species-rich with 297 and 291 species, respectively. The most species-rich country is China with a total of 289 species, followed by Russia (with both European and Asian parts) with a total of 166 species and France and Italy with 143 and 142 species, respectively. The country with the highest percentage (34%) of endemic species is Nepal. The highest number of endemic species is found in Kazakhstan (27 species) followed by Spain and Iran with 26 and 25 species, respectively. The geographical distribution of the genera and subgenera of Cryptocephalini and Pachybrachini is compiled. Species belonging to the Oriental region are discussed. Seventy-two species are considered Oriental, based on this a total of 915 species of Palaearctic Cryptocephalini and Pachybrachini can be estimated. Species on islands are listed, and island biogeography is discussed.
1The Cryptocephalini and the Pachybrachini are two out of four tribes in the subfamily Cryptocephalinae (Gómez-Zurita & Cardoso, 2021). The adults are feeding on annual herbs, trees, or flowers, while the larvae of most species are feeding on leaf litter. The larvae carry a self-constructed case, for this reason the Cryptocephalinae are also called case-bearing leaf beetles (Erber, 1988).
2The Region proposed by Wallace (1876) is widely accepted as a natural subdivision of the biosphere and was adopted in the respective chapters of the catalogue of Palaearctic Chrysomeloidea (Lopatin et al., 2010; Schöller et al., 2010) which included information on the distribution of the species. In the forthcoming new edition of the catalogue of Palaearctic Chrysomelidae (Bezděk & Sekerka, 2023, in press), a total of 988 species of Cryptocephalini and Pachybrachini are listed. However, the diversity and distribution of the Palaearctic Cryptocephalini and Pachybrachini was not reviewed previously. In the present publication, the information on geographical distribution in the new edition of the catalogue is analysed. Boundaries towards the Oriental and Afrotropical regions are discussed. Species on islands are listed, and island biogeography is treated for the first time for this group of leaf beetles.
3The Palaearctic region includes Africa north of the Sahara, Asia except for the part that is arbitrarily defined as belonging to the Oriental region, and Europe. For the catalogue of Palaearctic Coleoptera, the Oriental region was defined as areas south of the People’s Republic of China and Taiwan, areas south of the Himalaya in India, the Philippines, Malaysia and Indonesia. Europe includes Russia west of the main ridge of the Ural Mountains, the Permsk Oblast, Bashkortostan Republic and Orenburskaya Oblast, and the small part of Kazakhstan west of the Ural River. The south-eastern boundaries are the political boundaries of Georgia, Azerbaijan, Kazakhstan, and the Caspian and Black seas. North Africa includes Morocco, Algeria, Tunisia, Libya and Egypt west of the Suez Canal, and the Canary Islands and Madeira. The boundaries of the Palaearctic region usually followed national boundaries for practical reasons (Löbl & Smetana, 2010). This concept was continued in the 2023 edition of the catalogue with little modifications in detail. China and India were subdivided according to Provinces. Russia was subdivided according to biogeographical criteria (Bezděk & Sekerka, 2023). A total of 140 areas (states, provinces, biogeographical areas) were distinguished (North Africa: 8; Asia: 81; Europe: 51). Additionally, the occurrence on islands was listed separately.
4A total of 988 species of Cryptocephalini and Pachybrachini are recorded in the catalogue. The numbers of species recorded from North Africa, Asia and Europe are 79, 666 and 214, respectively (Figure 1).
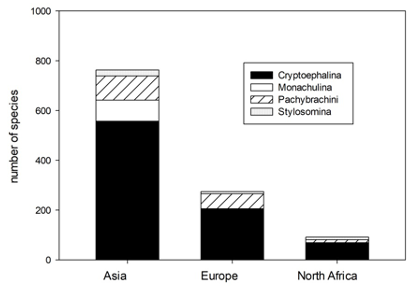
Fig. 1: Number of species of Pachybrachini and Cryptocephalini and its subtribes in the catalogue of Palaearctic Chrysomelidae in Asia, Europe and North Africa.
5The three main parts of the Palaearctic Region differ considerably by their surface area, i.e. North Africa, Asia and Europe have 4,750,000 km2, 44,580,000 km2 and 10,530,000 km2, respectively. If the number of species per m² is calculated, the numbers are 26.02 x 10-6 species per m2, 19.36 x 10-6 species per m2 and 20.63 x 10-6 species per m2 for North Africa, Asia and Europe, respectively.
6The most species-rich country is China with a total of 289 species, followed by Russia (with both European and Asian parts) with a total of 166 species (Table 1).
7These are followed by two European countries, i.e., France and Italy with 143 and 142 species, respectively. All countries with more than 49 species are given in Table 1.
8While the ranking is identical for Cryptocephalini and Cryptocephalina for the top four countries, the subtribe Stylosomina is most species-rich in Kazakhstan followed by Spain and Uzbekistan. The Monachulina were only recorded from China (68 species) and Nepal (10 species). The Pachybrachini are most species-rich in Spain followed by Italy, Kazakhstan, and Turkey.
Table 1: Diversity of Palaearctic Cryptocephalini and Pachybrachini per country (countries with more than 49 species only).
Oriental species
9The conventionally accepted Palaearctic-Oriental boundary inside China has an east-west orientation (see He et al., 2017 and references therein). Potentially, the fauna of the following Chinese provinces belongs to the Oriental region: Anhui, Chongqing, Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hubei, Hunan, Jiangxi, Yunnan, and Zhejiang. The number of species of Cryptocephalini in the catalogue occuring exclusively in one or more of these provinces is 72. Specifically, these are in the genera Adiscus (20 species), Coenobius (4 species), Cryptocephalus (Burlinius) (5 species), Cryptocephalus (Cryptocephalus) (37 species), Cryptocephalus (Sopidus) (1 species), Isnus (1 species) and Melixanthus (4 species). Among these, 36 species are distributed in Yunnan only. No species of Pachybrachini was found yet in this area.
Afrotropical species
10The Palaearctic-Afrotropical boundary is delimited by the south of the Sahara Desert, and eastwards including the South of the Arabian Peninsula (Kreft & Jetz, 2010). Most species found on the Arabian Peninsula are either endemic, or the distribution is extended towards the Middle East (Schöller, 2021a). Few species are also found in Western Africa. An exception is Cryptocephalus barkeri Jacobi 1901 from South Africa, which is known from a single specimen from Saudi Arabia but not from the areas in between and might have been translocated.
Palaearctic species
11The catalogue lists eight genera in Cryptocephalini (with four, three and one genera in Cryptocephalina, Monachulina and Stylosomina, respectively), and one genus in Pachybrachini. The genera Isnus and Dichachus are not Palaearctic, therefore the Palaearctic region has six genera in Cryptocephalini (with three, two and one genera in Cryptocephalina, Monachulina and Stylosomina, respectively). Peculiar to the Palaearctic region is the genus Jaxartiolus.
12If the 72 Oriental species and one Afrotropical species are excluded from the species listed in the catalogue, a total of 915 species of Palaearctic Cryptocephalini and Pachybrachini can be estimated. Based on the above, the numbers of Palaearctic species are 59, 666, 34 and 156 in Monachulina, Cryptocephalina, Stylosomina and Pachybrachini, respectively. The number of Palaearctic species in Asia calculated is 594, with 59, 511, 24 and 97 in Monachulina, Cryptocephalina, Stylosomina and Pachybrachini, respectively.
13Most Palaearctic species do not occur outside the region. Specifically, this applies to 77 species (7.8 %). Diachus auratus (Fabricius 1801) can also be found in the Neotropical, Nearctic, Afrotropical and Oriental regions. Six species of Cryptocephalus and Isnus biseriatus (Chapuis 1877) are shared with the Afrotropical region. Cryptocephalus moraei (Linné 1758) was imported to the Nearctic region. A total of 67 species (6.8 % of the 988 species in the catalogue) do also occur in the Oriental region.
14The Palaearctic region can be divided into the Eastern Palaearctic and the Western Palaearctic with the Yenisey River as a natural boundary between the two halves, which approximates to 90 degrees (de Lattin, 1967.
Western Palaearctic
15From the Western Palaearctic, 723 species were recorded. The top most widely distributed species, i.e. with the largest number of areas are Cryptocephalus (Cryptocephalus) bipunctatus bipunctatus (Linné 1758) (51 areas), C. (Burlinius) ocellatus (Drapiez 1819) (51 areas), C. (Cryptocephalus) moraei (50 areas), C. (Cryptocephalus) sericeus (Linné 1758) (48 areas), C. (Burlinius) fulvus fulvus Goeze 1777 (47 areas), C. (Cryptocephalus) parvulus Müller 1776 (46 areas), C. (Burlinius) labiatus (Linné 1761) (46 areas), C. (Homalopus) coryli (Linné 1758) (44 areas), C. (Burlinius) bilineatus (Linné 1767) (43 areas), and C. (Cryptocephalus) nitidus (Linné 1758), C. (Cryptocephalus) sexpunctatus sexpunctatus (Linné 1758), C. (Burlinius) elegantulus Gravenhorst 1807 and C. (Cryptocephalus) anticus Suffrian 1848 (40 areas each.
Eastern Palaearctic
16From the Eastern Palaearctic, 479 species were recorded. The most widely distributed species, i.e. with more than 15 areas are Cryptocephalus (Burlinius) exiguus amiculus Baly 1873 and C. (Burlinius) confusus Suffrian 1854 (19 areas each), C. (Sopidus) koltzei Weise 1887 (17 areas), C. (Cryptocephalus) parvulus (16 areas), C. (Burlinius) nigrofasciatus Jacoby 1885 (16 areas), C. (Cryptocephalus) regalis regalis Gebler 1830 (15 areas) and C. (Cryptocephalus) hyacinthinus Suffrian 1860 (15 areas).
17In the following, the biodiversity of Cryptocephalini and Pachybrachini in the main divisions of the Palaearctic region (after Emeljanov, 1974, Figure 2) is given.
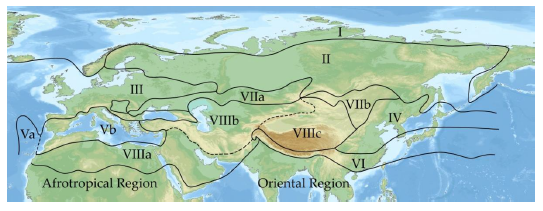
Fig. 2: Main divisions of the Palaearctic region (after Emeljanov, 1974, simplified).
I = Arctic (Circumpolar Tundra) region, II = Taiga (Euro-Siberian) region, III = European (nemoral) region, IV = Stenopean (nemoral) region, V = Hesperian (evergreen forest) region [Va = Macaronesian subregion. Vb = Mediterranean subregion], VI = Orthrian (evergreen forest) region, VII = Scythian (steppe) region [VIIa = West Scythian subregion, VIIb = East Scythian subregion], VIII = Sethian (desert) region [VIIIa = Saharo-Arabian subregion, VIIIb = Irano-Turanian subregion, VIIIc = Central Asian subregion].
Arctic (Circumpolar Tundra) region
18In the northernmost and coldest part of the Palaearctic, Cryptocephalini and Pachybrachini can be found in the tundra areas of Chukotka (Chernov et al., 2014) and the Southern part of Norway. These are 25 species of Cryptocephalus and one species of Pachybrachis. Few species of Chrysomelidae are truly arctic, among them no Cryptocephalini and Pachybrachini (Silfverberg, 1994). However, some Cryptocephalini reach the arctic although their main range lies further south. Cryptocephalus (Cryptocephalus) krutovskyi Jakobson 1900 is distributed from North-West Siberia and Altai to the Ussuri river, Sachalin and Japan, and reaches the Arctic at the lower Ob River. Pachybrachis amurensis Medvedev 1973 ranges from Western Mongolia to the Amur River and to Chukotka, where it reaches the Arctic (Medvedev & Korotyaev, 1980). The species reaching the arctic represent 2.5% of the diversity of the two tribes.
Taiga (Euro-Siberian) region
19From the taiga, 122 and 13 species of Cryptocephalini and Pachybrachini were recorded, respectively (Table 2). The species most widely distributed in the Taiga are Cryptocephalus (Burlinius) bilineatus, C. (Burlinius) elegantulus, C. (Burlinius) frontalis Marsham 1802, C. (Burlinius) pallifrons Gyllenhal 1813, C. (Cryptocephalus) parvulus, C. (Cryptocephalus) nitidulus Fabricius 1787, C. (Cryptocephalus) sericeus, C. (Cryptocephalus) sexpunctatus sexpunctatus, Cryptocephalus (Homalopus) coryli and Pachybrachis hieroglyphicus Laicharting 1781.
European (nemoral) region
20In the European (nemoral) region a total of 199 species of Cryptocephalini (168 species) and Pachybrachini (31 species) were recorded. The most widely distributed species here are C. (Burlinius) fulvus fulvus (40 areas), C. (Cryptocephalus) moraei (36 areas), C. (Cryptocephalus) bipunctatus bipunctatus (34 areas), C. (Cryptocephalus) nitidus (32 areas), C. (Cryptocephalus) sericeus (31 areas), Cryptocephalus (Homalopus) coryli, C. (Cryptocephalus) parvulus and C. (Burlinius) labiatus (30 areas, respectively), C. (Cryptocephalus) hypochaeridis (Linné 1758) and C. (Burlinius) ocellatus (28 areas each), and C. (Cryptocephalus) sexpunctatus sexpunctatus and C. (Burlinius) bilineatus (27 areas each).
Stenopean (nemoral) region
21In the Stenopean (nemoral) region, a total of 101 species of Cryptocephalini (92 species) and Pachybrachini (9 species) were recorded.
22The most widely distributed species here are Cryptocephalus (Burlinius) exiguus amiculus (11 areas), C. (Burlinius) confusus (10 areas), C. (Cryptocephalus) parvulus (9 areas), C. (Sopidus) koltzei (8 areas), C. (Sopidus) semenowi Weise 1889, C. (Cryptocephalus) regalis regalis, C. (Burlinius) nigrofasciatus (7 areas, respectively), C. (Cryptocephalus) hyacinthinus, C. (Cryptocephalus) ochroloma Gebler 1830, C. (Cryptocephalus) sexpunctatus sexpunctatus and Pachybrachis (Pachybrachis) ochropygus Solsky 1871 (6 areas each).
Hesperian (evergreen forest) region
23The Hesperian region is subdivided into the Mediterranean subregion (297 species of Cryptocephalini and Pachybrachini) and the Macaronesian subregion (5 species of Cryptocephalini).
Mediterranean subregion
24Among the most widely distributed species in the Mediterranean subregion are some which are widespread in the whole western Palaearctic region, like Cryptocephalus (Burlinius) ocellatus and C. (Burlinius) fulvus fulvus (13 areas in the Mediterranean subregion each), C. (Burlinius) pygmaeus vittula Suffrian 1848, C. (Cryptocephalus) anticus Suffrian 1848, C. (Cryptocephalus) bipunctatus bipunctatus, C. (Cryptocephalus) parvulus and C. (Cryptocephalus) moraei (10 areas each).
25More characteristics for the Mediterranean subregion are Cryptocephalus (Burlinius) macellus Suffrian 1860 (13 areas), Stylosomus (Stylosomus) tamarisci Herrich-Schäffer 1836 (11 areas), C. (Cryptocephalus) rugicollis rugicollis Olivier 1792, C. (Cryptocephalus) trimaculatus Rossi 1790 and C. (Burlinius) connexus Olivier 1808 (10 areas each).
26Some species form subspecies in the Western and Eastern Mediterranean, respectively. Examples are C. (Cryptocephalus) rugicollis rugicollis and C. (Cryptocephalus) rugicollis sexnotatus Fabricius 1792 or C. (Cryptocephalus) violaceus scaffaiolus Burlini 1961 and C. (Cryptocephalus) violaceus violaceus Laicharting 1781.
Macaronesian subregion
27The diversity is low with 5 species only. All four Cryptocephalus species are in the subgenus Burlinius. The only species which is present on all islands is C. gounellei Pic 1922. The fifth species is Stylosomus (Stylosomus) biplagiatus Wollaston, 1864.
Orthrian (evergreen forest) region
28The estimated number of species for the Orthrian (evergreen forest) region is 201. The species are in the genera Adiscus (29 species), Coenobius (18 species), Cryptocephalus (Burlinius) (16 species), Cryptocephalus (Cryptocephalus) (122 species), Cryptocephalus (Sopidus) (1 species), Isnus (1 species), Melixanthus (11 species) and Stylosomus (Stylosomus) (3 species). The most widely distributed species here are Cryptocephalus (Cryptocephalus) guttifer Suffrian 1854 (6 areas), C. (Cryptocephalus) suavis Duvivier 1892 and C. (Cryptocephalus) trifasciatus Fabricius 1787 (5 areas each), C. (Cryptocephalus) deficiens Suffrian 1854, C. (Cryptocephalus) exsulans Suffrian 1854, C. (Cryptocephalus) luteosignatus Pic 1922, C. (Cryptocephalus) sannio Kollar & Redtenbacher 1844, C. (Cryptocephalus) triangularis triangularis Hope 1831, C. (Cryptocephalus) tricinctus Kollar & Redtenbacher 1844 and Coenobius fulvipes Baly 1877 (Figure 3q) (with 4 areas, respectively).
Scythian (steppe) region
29In the Scythian region, a total of 237 species of Cryptocephalini (190 species) and Pachybrachini (47 species) were recorded. The most widely distributed species here are Cryptocephalus (Homalopus) coryli and C. (Burlinius) bilineatus (7 areas each), P. (Pachybrachis) scriptidorsum Marseul 1874, Cryptocephalus (Sopidus) bohemius Drapiez 1819, Cryptocephalus (Sopidus) apicalis Gebler 1830, C. (Cryptocephalus) virens Suffrian 1847, C. (Cryptocephalus) sericeus, C. (Cryptocephalus) laetus Fabricius 1792, C. (Cryptocephalus) flavipes Fabricius 1781, C. (Cryptocephalus) cordiger (Linné 1758), C. (Burlinius) ocellatus, C. (Burlinius) labiatus and C. (Burlinius) elegantulus (with 6 areas, respectively).
30In the West Scythian subregion, a total of 175 species of Cryptocephalini (142 species) and Pachybrachini (33 species) were recorded. The most widely distributed species here are Cryptocephalus (Burlinius) ocellatus, C. (Sopidus) apicalis and (Homalopus) coryli (with 6 areas, respectively).
31In the East Scythian subregion, a total of 97 species of Cryptocephalini (79 species) and Pachybrachini (18 species) were recorded. The most widely distributed species here are Cryptocephalus (Sopidus) altaicus Harold 1872 and C. (Sopidus) koltzei (with 3 areas, respectively).
Sethian (desert) region
32In the Sethian (desert) region, a total of 507 species of Cryptocephalini (419 species) and Pachybrachini (88 species) were recorded. The most widely distributed species here are Melixanthus melanocephalus Suffrian 1857 (12 areas), Pachybrachis (Pachybrachis) glycirrhizae Olivier 1808 (11 areas), P. (Pachybrachis) scripticollis Faldermann 1837, P. (Pachybrachis) nigropunctatus Suffrian 1854 and Cryptocephalus (Sopidus) tamaricis Solsky 1867 (8 areas each), C. (Sopidus) undulatus Suffrian 1854, C. (Cryptocephalus) anticus, C. (Burlinius) connexus (7 areas each), C. (Burlinius) dilutellus Jacobson 1901, C. (Cryptocephalus) petraeus Suffrian 1854, C. (Cryptocephalus) semiargenteus Reitter 1894, C. (Homalopus) tarsalis tarsalis Weise 1887, P. (Pachybrachis) scriptidorsum and Stylosomus (Microsomus) weberi Reitter 1905 (with 6 areas, respectively).
33In the Saharo-Arabian subregion, a total of 140 species of Cryptocephalini (119 species) and Pachybrachini (21 species) were recorded. The most widely distributed species here are Melixanthus melanocephalus (9 areas), C. (Cryptocephalus) petraeus (6 areas) and Pachybrachis (Pachybrachis) glycirrhizae, Stylosomus (Stylosomus) tamarisci and C. (Cryptocephalus) dumonti Peyerimhoff 1924 (with 5 areas, respectively).
34In the Irano-Turanian subregion, a total of 291 species of Cryptocephalini (225 species) and Pachybrachini (66 species) were recorded. The most widely distributed species here are Cryptocephalus (Sopidus) undulatus (7 areas), C. (Sopidus) tamaricis, C. (Cryptocephalus) semiargenteus, C. (Burlinius) dilutellus, Stylosomus (Microsomus) weberi, Pachybrachis (Pachybrachis) glycirrhizae and P. (Pachybrachis) nigropunctatus (with 6 areas, respectively).
35In the Central Asian subregion, a total of 132 species of Cryptocephalini (121 species) and Pachybrachini (11 species) were recorded. The most widely distributed species here are Cryptocephalus (Cryptocephalus) regalis regalis (5 areas), Pachybrachis (Pachybrachis) scriptidorsum, Stylosomus (Stylosomus) submetallicus Chen 1941 and Adiscus variabilis (Jacoby 1890) (with 4 areas, respectively).
36Species richness of Cryptocephalini is highest in the Sethian region and the Hesperian region with 419 and 228 species, respectively. The Pachybrachini are most species-rich in the Sethian region and the Mediterranean subregion with 88 and 74 species, respectively (Table 2).
Table 2: Diversity of Cryptocephalini and Pachybrachini in the main divisions of the Palaearctic region (see Fig. 2).

Geographical distribution of the genera and subgenera of Cryptocephalini and Pachybrachini
37Genus Cryptocephalus Geoffroy 1762
38subgenus Bertiellus Lopatin 1977
39The subgenus Bertiellus was erected for four species from Afghanistan. Beside the original descriptions, no information on these species is available. In Figure 3a external morphological characters are illustrated for the first time, i.e., the relatively short antenna, elytra with sparse setae and the expanded tibiae with an apical row of spines.
40subgenus Burlinius Lopatin 1965
41The subgenus comprises 141 species and subspecies. Almost all (134 species) are Palaearctic in distribution. One species was found in Yemen (Sana’a, Wadi Dhar, 29.V.1987, specimen not determinable) and another in Ethiopia. Five species are in the Oriental area of China (Figures 3b, c).

Fig. 3: Representatives of Palaearctic Cryptocephalini and Pachybrachini
a) Cryptocephalus (Bertiellus) lopatini Medvedev 1978, b) C. (Burlinius) presuturalis Pic 1907, c) C. (Burlinius) flabellatus Schöller 2009, d) C. (Cryptocephalus) sericeus (Linné 1758), e) C. (Cryptocephalus) nigronotatus Bryant 1954, f) C. (Cryptocephalus) distinguendus Schneider 1792, g) C. (Cryptocephalus) pexicollis Suffrian 1847, h) C. (Disopus) pini pini (Linné 1758), i) C. (Homalopus) tarsalis tarsalis Weise 1887, j) C. (Lamellosus) laevicollis Gebler 1830, k) Diachus auratus (Fabricius 1801), l) C. (Sopidus) manchuricus Gressit & Kimoto 1961, m) C. (Protophysus) euchirus Kraatz 1879, n) Melixanthus assamensis (Jacoby 1908), o) Adiscus mouhoti (Baly 1877), p) Adiscus lewisii (Baly 1873), q) Coenobius fulvipes Baly 1877, r) Isnus yunnanus Schöller 2021, s) Stylosomus (Stylosomus) tamarisci Herrich-Schäffer 1836, t) Pachybrachis (Pachybrachis) pallidulus Suffrian 1852.
42subgenus Cryptocephalus Geoffroy 1762
43For the nominate subgenus, 385 species are listed in the catalogue. The number of species restricted to the Oriental part of China is 37, i.e. the number of Palaearctic species and subspecies of Cryptocephalus is 348. These are distributed from Portugal to Japan. Within the nominate subgenus, a number of species-groups were suggested (e.g. Weise, 1882; Schöller, 2021a). However, much more studies are required on species-groups and their distribution (Figures 3d-g).
44subgenus Disopus Chevrolat 1836
45Five species and subspecies only are in this subgenus, these are distributed from Portugal to Japan. Cryptocephalus pini pini Linnaeus 1758 is of economic importance as a pest of pine plantations in some areas of its areal (Figure 3h).
46subgenus Homalopus Chevrolat 1837
47In this subgenus, 27 species and subspecies are placed. In Asia and Europe, 12 and 16 species were recorded, respectively. One species, C. coryli Linnaeus 1758, is widely distributed and the only species in the Taiga. Otherwise the subgenus is especially species-rich in the European (nemoral) and Mediterranean regions with 12 and 15 species, respectively (Figure 3i).
48subgenus Lamellosus Tomov 1979
49The subgenus Lamellosus was erected for two species only, i.e., C. angorensis Pic 1908 from Turkey and C. laevicollis Gebler 1830 (Figure 3j) distributed from Germany in the West to Southern Siberia in the East.
50subgenus Protophysus Chevrolat 1836
51The subgenus Protophysus was erected for five species and subspecies from Europe and Asia, distributed from Spain via Iran to Mongolia (Figure 3m).
52subgenus Sopidus Jacobson 1901
53In the subgenus Sopidus (= Asionus Lopatin 1988), 119 species are placed. In Asia, Europe and North Africa, 113, 20 and 3 species were recorded, respectively. The subgenus is especially diverse in Central Asia, Russia and China (Figure 3l). Most species-rich is the Sethian (desert) region (82 species), followed by the Scythian (steppe) region (66 species), European (nemoral) region (21 species), Taiga (20 species), Stenopean (nemoral) region (11 species), Mediterranean subregion (7 species), and Orthrian (evergreen forest) region (1 species).
54Genus Diachus LeConte 1881
55The genus Diachus is originally Neotropical. The species Diachus auratus (Fabricius 1801), a pest of sweet potato and other agricultural crops, was introduced to Asia and other biogeographical regions (Figure 3k).
56Genus Jaxartiolus Jacobson 1922
57The genus Jaxartiolus was erected for three species from Central Asia and China (Gansu). Beside the original descriptions, little information is available.
58Genus Melixanthus Suffrian 1854
59In the catalogue, 21 species of Melixanthus are listed. Four species are Oriental, i.e., there are 17 Palaearctic species. Melixanthus melanocephalus Suffrian 1857 and similar species are widely distributed in Northern Africa and the Middle East, and another 13 species were recorded from China (Figure 3n).
60The genus Melixanthus was originally described by monotypy for a species from Borneo. Already in his original description, Suffrian (1854) discussed the lack of specific characters to distinguish Cryptocephalus from Melixanthus. Some following authors placed all species with appendiculate or toothed tarsal claws in Melixanthus based on this character alone and included species in the subgenus Anteriscus Weise of Cryptocephalus as well. However, this leads most likely to a polyphyletic grouping. Anteriscus Weise was synonymised with Cryptocephalus (Schöller, 2021a). The second frequently mentioned character for Melixanthus are short and widened antennae. However, there is a continuum between short and widened and long and slender antennae in various Cryptocephalina. Future studies are needed to solve the question of generic affiliation of the species currently listed in Melixanthus.
61Genus Adiscus Gistel 1857
62For the genus Adiscus, 53 species are listed in the catalogue, 20 of which are Oriental. Most of the 33 Palaearctic species are distributed in China (Duan & Zhou, 2022), the others in Northern India and Nepal (Figures 3o, p). The Orthrian (evergreen forest), Sethian (desert) and Stenopean (nemoral) regions have 29, 13 and 4 species, respectively.
63Genus Coenobius Suffrian 1857
64The genus Coenobius is species-rich both in the Afrotropical and the Oriental regions. In the catalogue, 30 species are listed, four of which are Oriental. Again, most of the 26 Palaearctic species are distributed in China and the others in Northern India and Nepal, with six species extending to Japan (Figure 3q). The Orthrian (evergreen forest) region has 18 species.
65Genus Isnus Weise 1898
66The genus Isnus is similar to Coenobius (with upper eye lobes separated, not touching as in Coenobius), and most species-rich in Africa. In the catalogue, there is the Afrotropical species C. biseriatus Chapuis 1877 which is extending its range to the Arabian Peninsula, and the Oriental species C. yunnanus Schöller 2021 (Figure 3r).
67Genus Stylosomus Suffrian 1848
68The genus Stylosomus is restricted to the Palaearctic region, 34 species and subspecies are listed in the catalogue. The subgenus Microsomus Burlini 1957 is distributed in Asia and Europe, the subgenus Stylomicrus Petitpierre & Alonso-Zarazaga 2000 and the nominate subgenus are distributed in Asia, Europe and Northern Africa (Figure 3s). The Sethian (desert) region, Mediterranean subregion, Scythian (steppe) region and European (nemoral) region have 22, 14, 7 and 7 species, respectively.
69Genus Pachybrachis Chevrolat 1836
70The genus Pachybrachis is distributed in the Neotropical, Nearctic and Palaearctic regions. In the catalogue, 156 Palaearctic species are listed. The subgenus Chloropachys Rey 1883 is distributed in the Iberian Peninsula and Northern Africa. The nominate subgenus is distributed from Portugal to Japan. The areal of some species is very small, e.g. P. fraudolentus Müller 1955 in a few alpine valleys, P. chafarinensis on the small islands of Isla del Congreso or P. sassii Montagna 2011 on Giglio island (Figure 3t).
71The Sethian (desert) region, Mediterranean subregion, Scythian (steppe) region, European (nemoral) region and Stenopean (nemoral) region have 88, 74, 47, 31 and 9 species, respectively.
72Genus Acolastus Gerstaecker 1855
73In the new catalogue, the genus Acolastus was included in the tribe Clytrini. It is especially diverse in the Afrotropical region and in Central Asia, 105 species are listed. The nominate subgenus and the subgenus Lapsionus are distributed all over North Africa and Asia. The subgenus Anodontelytrus Jacobson 1917 is distributed in the Middle East and North Africa. There are three Oriental species from Southern India and Sri Lanka (subgenus Pachylanka Medvedev 1989).
Endemism
74The number of endemic species (= in one territory only) in the catalogue is 273, out of 988 species, i.e. 27.6%. There are 29 endemic species (36.71%) in North Africa (8 areas) and 48 endemic species (22.43%) in Europe (51 areas). In Asia (81 areas), a total of 319 species out of 666 species are endemic (47.90%). More than one third of the fauna is endemic in Nepal, Afghanistan and Kirgistan. More than 27% of the species are endemic in Spain, Tajikistan and Morocco (Table 3).
Table 3: Number of endemic species of Cryptocephalini and Pachybrachini per country
Island biogeography
75Eighty-five species are endemic to islands, i.e. 20 European species (Table 4), 59 Asian species (Table 5) and six North African species (Table 6). In Northern Africa, all endemic island species in the genus Cryptocephalus belong to the subgenus Burlinius. Fifty-nine species were recorded from one island only, and out of these, 39 from Taiwan (Tables 7, 8). Taiwan is the island with most species, followed by Sicily, Honshu, Corsica, Sardinia, Great Britain and Kyushu (Table 9). An example for an extremely local species is Cryptocephalus (Burlinius) chafarinensis which is known so far only from Isla del Congreso (Spain, Islas Chafarinas close to the coast of Morocco) which has an area of 0.256 km2 only or Pachybrachis (Pachybrachis) sassii Montagna 2011 living only on Giglio island, the latter with a surface of 23.80 km2. Among the species with the most remote island distributions are Cryptocephalus (Burlinius) crenatus endemic to Madeira, C. (Cryptocephalus) sokotrensis Schöller 2014 endemic to Sokotra and C. (Burlinius) kurentzovi Medvedev 1966 endemic to the Kuriles islands (Tables 5, 6).
Table 4: European species of Cryptocephalini and Pachybrachini endemic to islands
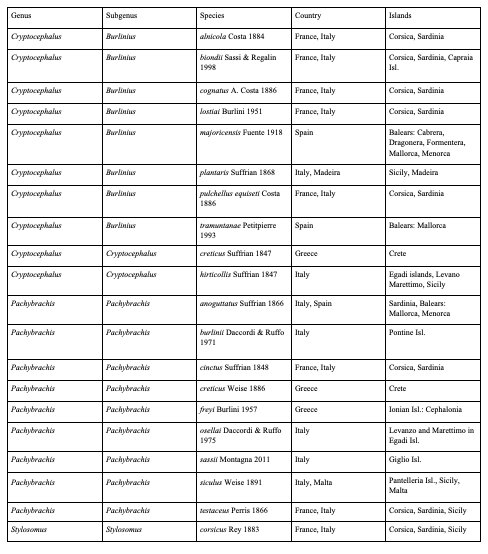
Table 5: Asian species of Palaearctic Cryptocephalini and Pachybrachini endemic to islands (except for Taiwan)
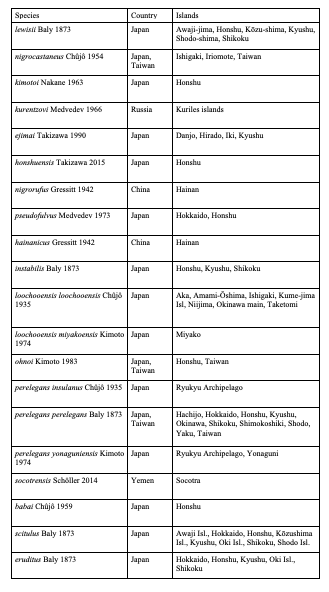
Table 6: North African species of Cryptocephalini endemic to islands
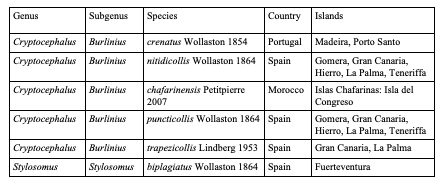
Table 7: Species of Cryptocephalini endemic to Taiwan: Cryptocephalina
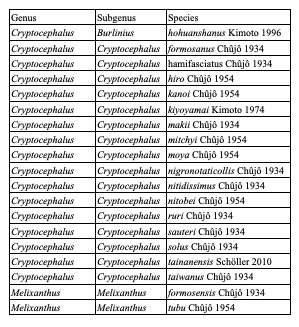
Table 8: Species of Cryptocephalini endemic to Taiwan: Monachulina
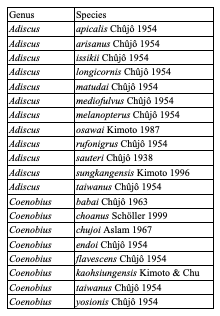
Table 9: Diversity of Palaearctic Cryptocephalini and Pachybrachini on islands (islands with more than 4 species only)
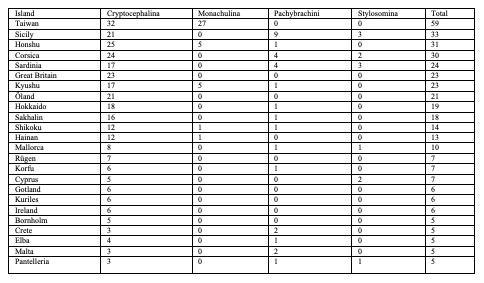
Table 10: Species of Crytocephalini recorded from six islands or more
|
Species |
No. islands |
Islands |
|
Cryptocephalus (Burlinius) fulvus fulvus Goeze 1777 |
35 |
Korčula, Rab (CR) Anholt, Bornholm, Læsø (DE) Corsica, L'Île de Groix, L'Île-d'Yeu (FR) Isle of Wight (GB) Borkum, Fehmarn, Föhr, Hiddensee, Juist, Langeoog, Norderney, Rügen, Spiekeroog, Vilm (GE) Cephalonia, Korfu, Psathoura, Rhodes, Samos, Skiathos (GR) Aeolian Isl., Elba, Pantelleria, Sardinia, Sicily (IT) Ibiza, Formentera, Illas Cíes (SP) Gotland, Öland (SV) |
|
Coenobius obscuripennis Chûjô 1935 |
15 |
Amami-oshima, Honshu, Iriomote, Ishigaki-jima, Kouri-jima, Kyushu, Miyako-shima, Okinawa-jima, Okinoerabu-jima, Ryukyu Archipelago, Shikoku, Tokara-Retto, Toku-no-shima, Yabuchi-shima, Yaku-shima, Yonaguni (JA) |
|
Cryptocephalus (Cryptocephalus) moraei Linnaeus 1758 |
14 |
Bornholm (DE) Corsica (FR) Hiddensee, Langeoog, Rügen (GE) Alonnisos, Euboea, Korfu, Skiathos, Skopelos (GR) Sicily (IT) Ibiza (SP) Gotland, Öland (SV) |
|
Diachus auratus Fabricius 1801 |
13 |
Aguni-jima, Ikei-jima, Kourishima, Minami-daito, Miyako, Okinawa main Isl., Ryukyu Archipelago, Senaga-shima, Sesoko-jima, Tonaki-jima, Tsuken-jima, Yoronshima (JA) Taiwan |
|
Cryptocephalus (Burlinius) macellus Suffrian 1860 |
13 |
Corsica (FR) Crete, Cephalonia, Euboea, Naxos, Rhodes, Skiathos (GR) Aeolian Isl., Pontine Isl., Pantelleria Isl., Sardinia, Sicily (IT) Mallorca (SP) |
|
Cryptocephalus (Cryptocephalus) parvulus O. F. Müller 1776 |
12 |
Corsica (FR) Rügen (GE) Sardinia (IT) Gotland, Öland (SV) Awaji Isl., Hokkaido, Honshu, Kyushu, Shikoku, Kurile Isl. (JA) Sakhalin (RU) |
|
Cryptocephalus (Cryptocephalus) hyacinthinus Suffrian 1860 |
11 |
Zhoushan Isl. (CH) Awaji Isl., Goto Isl., Honshu, Iki-No Isl., Kyushu, Oki Isl., Shikoku, Shodo Isl., Tsushima Isl. (JA) Yeongheungdo Isl. (SC) |
|
Cryptocephalus (Burlinius) exiguus amiculus Baly 1873 |
11 |
Hokkaido, Honshu, Kyushu, Oki Isl., Rishiri Isl., Shikoku (JA) Jeju-Do (SC) Furugelm, Kuriles Isl., Popov, Sakhalin (RU) |
|
Cryptocephalus (Burlinius) rufipes Goeze 1777 |
10 |
Grand Cavallo, Petit Cavallo, Tazrout (AG) L'Île de Groix, L'Île-d'Yeu (FR) Jersey (GB) Borkum, Langeoog, Spiekeroog (GE) Illas Cíes (SP) |
|
Cryptocephalus (Cryptocephalus) luridipennis signaticeps Baly 1873 |
9 |
Awaji Isl., Hokkaido, Honshu, Kōzu-shima Isl., Kyushu, Oki Isl., Shikoku, Shodo Isl., Tobi Isl., Tsushima Isl. (JA) |
|
Cryptocephalus (Cryptocephalus) perelegans perelegans Baly 1873 |
9 |
Hachijo, Hokkaido, Honshu, Kyushu, Okinawa, Shikoku, Shimokoshiki, Shodo, Yaku (JA) |
|
Coenobius piceus Baly 1873 |
9 |
Hachijo-shima, Honshu, Kyushu, Miyake-shima, Oki-no, Oshima, Shikoku, Tane-ga-jima, Tsushima (JA) |
|
Cryptocephalus (Burlinius) labiatus Linnaeus 1761 |
9 |
Rab (CR) Bornholm (DE) Rügen (GE) Korfu (GR) Sardinia, Sicilia (IT) Gotland, Öland (SV) Sakhalin (RU) |
|
Cryptocephalus (Burlinius) pusillus Fabricius 1777 |
9 |
Bornholm (DE) Isle of Wight (GB) Hiddensee, Langeoog, Norderney, Rügen, Sylt, Vilm (GE) Öland (SV) |
|
Cryptocephalus (Burlinius) confusus Suffrian 1854 |
8 |
Honshu, Awaji, Kōzu, Kyushu, Shôdo, Shikoku, Tsushima (JA) Sakhalin (RU) |
|
Melixanthus scitulus Baly 1873 |
8 |
Awaji, Hokkaido, Honshu, Kōzushima, Kyushu, Oki, Shikoku, Shodo (JA) |
|
Cryptocephalus (Burlinius) gounellei Pic 1922 |
7 |
Fuerteventura, Gomera, Gran Canaria, Hierro, La Palma, Lanzarote, Teneriffa (SP) |
|
Cryptocephalus (Cryptocephalus) loochooensis loochooensis Chûjô 1935 |
7 |
Aka, Amami-Ōshima, Ishigaki, Kume-jima, Niijima, Okinawa main Isl., Taketomi (JA) |
|
Cryptocephalus (Cryptocephalus) sexpunctatus sexpunctatus (Linnaeus 1758) |
7 |
Gotland, Öland (SV) Hokkaido, Honshu, Kuriles, Shikoku (JA) Sakhalin (RU) |
|
Cryptocephalus (Cryptocephalus) bipunctatus bipunctatus (Linnaeus 1758) |
7 |
Mljet (CR) Bornolm (DE) Rügen (GR) Korfu (GR) Elba (IT) Gotland, Öland (SV) |
|
Cryptocephalus (Burlinius) fulvus fuscolineatus Chûjô 1940 |
7 |
Honshu, Kyushu, Shikoku, Tsuno (JA) Kuriles Isl., Popov, Sakhalin (RU) |
|
Cryptocephalus (Cryptocephalus) nobilis Kraatz 1879 |
6 |
Honshu, Kōzu-shima, Kyushu, Oki, Shikoku, Shodo (JA) |
|
Cryptocephalus (Cryptocephalus) nitidulus Fabricius 1787 |
6 |
Öland (SV) Hokkaido, Rebun-to, Rishiri (JA) Sakhalin (RU) |
|
Cryptocephalus (Cryptocephalus) tetradecaspilotus Baly 1873 |
6 |
Awaji-jima, Honshu, Kyushu, Oki, Shikoku (JA) Jeju-Do (SC) |
|
Adiscus lewisii Baly 1873 |
6 |
Awaji-jima, Honshu, Kōzu-shima, Kyushu, Shodo-shima, Shikoku (JA) |
|
Coenobius piceipes Gressitt 1942 |
6 |
Hachijo-shima, Honshu, Kyushu, Oki, Shikoku, Tsushima (JA) |
|
Coenobius sulcicollis Baly 1873 |
6 |
Hirado, Honshu, Kyushu, Shikoku, Shodo-jima, Tsushima (JA) |
CH=China, CR=Croatia, DE=Denmark, FR=France, GB=Great Britain, GE=Germany, GR=Greece, IT=Italy, JA=Japan, RU=Russia, SC=South Korea, SP=Spain, SV=Sweden
76Among the species which are not endemic to islands, few occur on many islands (Table 10). The most widely distributed species on islands is Cryptocephalus (Burlinius) fulvus fulvus which was recorded from 35 islands (Figure 4). Another example is C. (Burlinius) macellus which was recorded, beside its mainland distribution, on Mediterranean islands only (Figure 5). This species is more thermophilous compared to C. (Burlinius) fulvus fulvus. In Germany, its northernmost distribution is the state of Saxony-Anhalt. It does not reach the islands of the Baltic Sea.
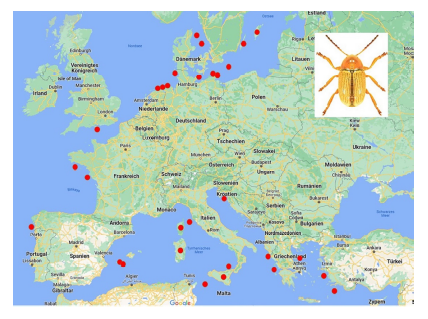
Fig. 4: Island distribution of Cryptocephalus (Burlinius) fulvus fulvus (mainland distribution not shown).
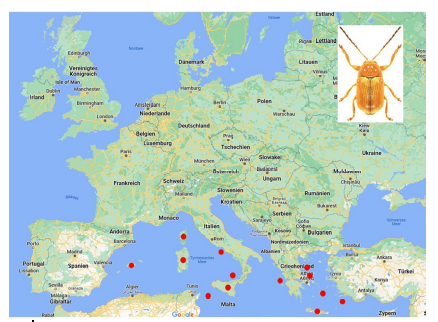
Fig. 5: Island distribution of Cryptocephalus (Burlinius) macellus (mainland distribution not shown).
77There are a number of assumptions in island biogeography which were tested with the data on Cryptocephalini and Pachybrachini. Generally, more animals live on large islands compared to small islands. In this study, a significant correlation of number of species and island surface area was found (nonlinear regression, P<0.005, R=0.458) (Figure 6).
78The general pattern is for species richness to increase from polar to tropical regions (Willig & Presley, 2013). There is a tendency of decrease of number of species of Cryptocephalini and Pachybrachini on islands with increase in latitude (Figure 7). However, this correlation is not significant (nonlinear regression, P<0.170, R=0.311).
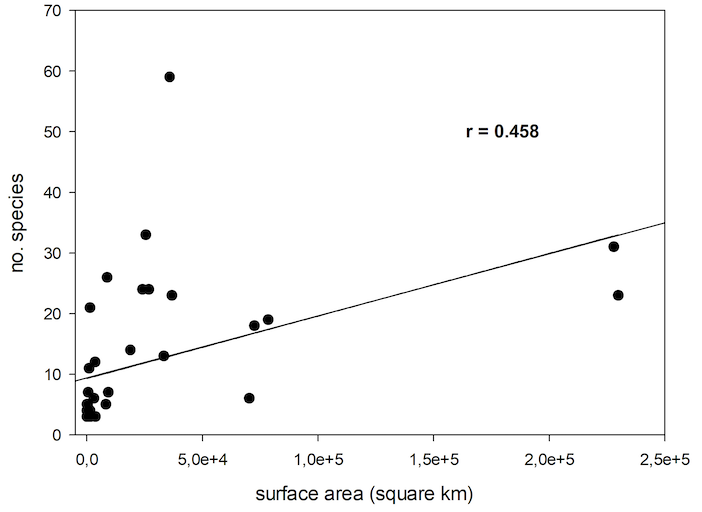
Fig. 6: Number of species of Cryptocephalini and Pachybrachini depending on Palaearctic Island surface area.
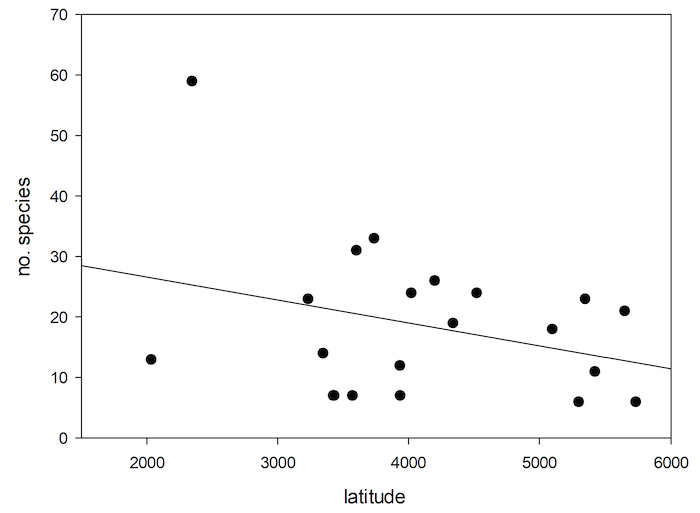
Fig.7: Number of Palaearctic species of Cryptocephalini and Pachybrachini depending on island latitude.
79How precisely the current distribution of a species is known obviously depends on the existence of studies in the various territories in the Palaearctic. Fortunately, the knowledge on Cryptocephalinae diversity and distribution is comparatively good. For major geographical areas, the fauna was reviewed. Examples are Afghanistan (Medvedev, 1978), the Arabian peninsula (Medvedev, 1996), Central Asia and Kazakhstan (Lopatin, 1977), Central Europe (Schmitt et al., 2014), China (Gressitt & Kimoto, 1961), Eastern Europe (Lopatin et al., 2004), the Iberian Peninsula (Petitpierre, 2000), Iran (Schöller & Nasserzadeh, 2010), Korea (Cho & An, 2020), Mongolia (Medvedev, 1982), Morocco (Kocher, 1958), Nepal (Medvedev & Sprecher-Uebersax, 1997), Northern Europe (Silfverberg, 2010), Balkan (Gruev, 2005), Siberia and the Russian Far East (Medvedev, 1973) and Turkey (Sassi & Kismali, 2000).
80Warchalowski (2000) published identification keys for most of the area treated here and additional information on China is available in Gressitt & Kimoto (1961). However, these works were published over a long period of time. Even though several more recent publications are available (e.g., Duan & Zhou, 2022; Schöller, 2021b), the fact a total of about 100 new country records were published in the course of the preparation of the catalogue (Schöller, 2020; Bezděk & Sekerka, 2023) shows there are still gaps in knowledge. Especially for China, many new findings can be expected.
81The similar values for the number of species per square meter for North Africa, Asia and Europe despite the considerable differences in size of these areas may be a result of an increase of diversity from the temperate regions to the subtropical regions in the Palaearctic. In a large part of Northern Europe and especially the north of Asia, few or even no records of Cryptocephalini exist. Species numbers are declining from the most southern main divisions of the Palaearctic region towards the Arctic region, i.e. from 507 species to 26 species. This is in accordance with the general pattern of species richness of animals depending on latitude with an increase from polar to tropical regions (Willig & Presley, 2013). Continental, wet winter and dry winter climates vegetation types are less favourable for the establishment of Cryptocephalini and Pachybrachini populations. Cryptocephalini and Pachybrachini are typically absent in polar vegetation types like tundra and perpetual frost. Even though ecological factors like e.g. polyphagy and case-bearing in larve are probable causes for the observed pattern in Cryptocephalini and Pachybrachini, evolutionary, historical and stochastic processes have to be considered in future studies as well. The following factors were hypothesised to enable the Cryptocephalini to colonise semi-desert and desert environments and diversify there: the rectal apparatus enables adults to retain water from faeces, and the egg- and larval cases protect eggs and larvae physically from dessication. Moreover, the latter protect the immatures from general predators like ants which are predominant in semi-desert and desert environments (Schöller, 1995).
82A great belt of Northern hemisphere deserts occurs from North Africa to North Western China. In the eastern parts of Asian deserts, Cryptocephalini and Pachybrachini are the most species-rich group among the leaf beetles. Cryptocephalini, Clytrini, Pachybrachini and Eumolpinae dominate this fauna because they are better adapted to xeric environments because their larvae are case-bearing or soil-dwelling. They are mesophilous insects, however, some species of Cryptocephalus and Pachybrachis are active not only in spring but also in summer (C. curtissimus, C. kiritschenkiellus, C. rubi, C. rufofasciatus, C. semiargenteus) (Lopatin, 1999). The genus Jaxartiolus is endemic here. On the species-level, diversity is declining from West to East in deserts of Central and Middle Asia: 32 and 16 species of Cryptocephalini and Pachybrachini, respectively, in the Western part versus 9 and 6 species in the Eastern part (Lopatin, 1999).
83Recent studies suggested that the Palaearctic-Oriental boundary inside China has a stronger south-north orientation rather than the conventionally accepted east-west orientation (see He et al. (2017) and references therein). Konstantinov et al. (2009) stated the southern border of the Palaearctic in East Asia is nearly impossible to define, particularly in China. As the fauna of all the Chinese provinces was considered in the catalogue, the composition of Palaearctic and Oriental species can be analysed again in the future in case new findings on the Palaearctic-Oriental boundary emerge.
Islands
84Both ecological and geographical factors must be considered in analysing species distribution (Rossaro, 1995). Even though Cryptocephalini are phytophagous beetles, the geographical distribution of host plants is not necessarily a major factor for the present distribution. The larvae of most species are phyto-saprophagous and the adults of most species are oligophagous or polyphagous (Schöller, 1996).
85Cryptocephalus (Burlinius) species are comparatively small, which might ease distribution by air currents. However, North-African species endemic to islands might also be relicts of formerly more widely distributed species. One species within this subgenus, C. (Burlinius) fulvus fulvus is the species colonizing the highest number of islands, i.e. 35 (Table 10). This species is developing in dune vegetation (Schöller, pers. obs.), which is an important pre-adaptation for building up stable populations in the new environments and the colonization of islands.
86In this study, a correlation of species number and island surface, but no correlation with distance from the closest continental mass was found for Cryptocephalini and Pachybrachini. One reason might be, most Palaearctic islands are only five to 10 km away from the closest continental mass and few are more than 100 km away. Muscarella & Baragona (2017) did find a correlation of species number and island surface, too, but no correlation with distance from the closest continental mass as well when studying all organisms from the Sicilian islands.
87Only 15 species were recorded from Taiwan and other areas, compared to 39 endemic Taiwanese species. There are only two species which occur in Taiwan as well as in Nepal (Cryptocephalus trifasciatus) or the far east of Russia (C. tetradecaspilotus) and can be considered as Palaearctic. The remaining 13 species being common with Japan or continental China are distributed there in the subtropical parts of the countries, e.g. the Ryukyu Archipelago of Japan or Yunnan and other southern provinces of China. This data suggests a radiation of Cryptocephalini species on Taiwan complemented by a number of Oriental mainland species. Consequently, the fauna of Taiwan has to be considered oriental.
88The identification of hotspots of diversity and information on extinction threat, endemicity or rarity are important for conservation strategies (Biondi et al., 2013). Concerning the responsibility for conservation, Table 2 provides an overview on countries with a high percentage of endemic species. Cryptocephalini and Pachybrachini should be included in conservation projects in these areas.
Acknowledgments : I would like to thank Cora Schöller for designing the map with the main divisions of the Palaearctic region, Jan Bezděk for his patience during the work on the Catalogue of Palaearctic Coleoptera and Jean Fagot for critically reviewing this manuscript.
Bibliographie
Bezděk J. & Sekerka L. (eds), 2023. Catalogue of Palaearctic Coleoptera Volume 6/2. Chrysomeloidea 2 (Orsodacnidae, Megalopodidae, Chrysomelidae). Revised and updated second edition - Brill, Leiden, Boston (in press).
Biondi M., Urbani F. & D’Alessandro P., 2013. Endemism patterns in the Italian leaf beetle fauna (Coleoptera, Chrysomelidae). ZooKeys 332, 177-205.
Chernov Y.I., Makarova O.L., Penev L.D. & Khruleva O.A., 2014. The beetles (Insecta Coleoptera) in the Arctic Fauna. Communication 1. Faunal composition. Zoologichesky Zhurnal 93, 7-44.
Cho H.-W. & An S.L., 2020. An annotated checklist of leaf beetles (Coleoptera: Chrysomelidae) of Korea, with comments and new records. Far Eastern Entomologist 404, 1-36.
de Lattin G., 1967. Grundriss der Zoogeographie. G. Fischer, Stuttgart, 602 pp.
Duan W.-Y. & Zhou H.-Z., 2022. Revision of the genus Adiscus Gistel, 1857 (Coleoptera: Chrysomelidae, Cryptocephalinae) from mainland China. Zootaxa 5096(1), 1-80.
Emeljanov A.F., 1974. Proposals on the classification and nomenclature of ranges. Entomologicheskoe Obozrenie 53, 497-522.
Erber D., 1988. Biology of Camptostomata Clytrinae–Cryptocephalinae–Chlamisinae–Lamprostomatinae, In: Jolivet P.A., Petitpierre E. & Hsiao T.H. (eds) Biology of Chrysomelidae, 513–552. Kluwer Academic Publishers, Dordrecht/Boston/London. xxiv + 615 pp.
Gómez-Zurita J. & Cardoso A., 2021. Molecular systematics, higher-rank classification and Gondwanan origins of Cryptocephalinae leaf beetles. Zoologica Scripta 50(5), 529-688.
Gressitt J.L. & Kimoto S., 1961. The Chrysomelidae (Coleopt.) of China and Korea, part 1. Pacific Insects Monograph 1A, 1-299.
Gruev B.A., 2005. A comparative list of the leaf beetles of the Balkan countries (Coleoptera: Chrysomelidae). Animalia 41, 23-46.
He J., Kreft H., Gao E., Wang Z. & Jiang H., 2017. Patterns and drivers of terrestrial vertebrates in China. Journal of biogeography 44, 1172-1184.
Kocher L., 1958. Catalogue commenté des coléoptères du Maroc. Fascicule VIII. Phytophages. Travaux de l’Institut Scientifique Chérifien, Série Zoologie 19, 1-172.
Konstantinov A., Korotyaev B.A. & Volkovitsh M.G., 2009. Insect Biodiversity in the Palearctic Region. In: Foottit R. & Adler P. (eds) Insect Biodiversity: Science and Society, 1st edition, 107-162. Blackwell Publishing, Hoboken, New Jersey, DOI: 10.1002/9781444308211.ch7 ISBN 978-1-4051-5142-9
Kreft, H. & Jetz, W. 2010: A framework for delineating biogeographical regions based on species distribution. Journal of biogeography 37(11), 2029-2053.
Löbl I. & Smetana A., 2010. Catalogue of Palearctic Coleoptera. Volume 6. Chrysomeloidea. Apollo Books, Stenstrup. 924 pp.
Lopatin I.K., 1977. Zhuki-listoedy (Chrysomelidae) Sredney Azii i Kazakhstana. [Leaf-beetles (Chrysomelidae) of Central Asia and Kazakhstan]. Leningrad: Nauka, 268 pp.
Lopatin I.K., 1999. Biology and biogeography of desert leaf beetles of Central Asia. In: Cox M.L. (ed.) Advances in Chrsomelidae biology I, 159-168. Backhuys Publishers, Leiden.
Lopatin I.K., Aleksandrovitch O.R. & Konstantinov A.S., 2004. Check list of leaf-beetles (Coleoptera, Chrysomelidae) of the Eastern Europe and Northern Asia. Wydawnicto Mantis, Olsztyn. 265 pp.
Lopatin I.K., Smetana A. & Schöller M., 2010. Tribe Cryptocephalini Gyllenhal, 1813, genus Cryptocephalus Geoffroy, 1762, In: Löbl I. & Smetana A. (eds) Catalogue of Palaearctic Coleoptera, Volume 6, Chrysomeloidea, 580-606. Apollo Books, Stenstrup, Denmark. 924 pp.
Medvedev L.N., 1973. Obzor roda Cryptocephalus Geoffr. (Chrysomelidae) Sibiri i Dal’nego Vostoka. [Review of the genus Cryptocephalus Geoffr. (Chrysomelidae) of Siberia and Far East]. Trudy Biologo-pochvennogo Instituta, Novaya Seriya 9, 104-127.
Medvedev L.N., 1978. K faune listoedov (Coleoptera, Chrysomelidae) Afganistana. (On the fauna of leaf-beetles (Coleoptera, Chrysomelidae) of Afghanistan). Entomologicheskoe Obozrenie 57 (4): 859-869.
Medvedev L.N., 1982. Listoedy MNR. Opredelitel. [Leaf-beetles of Mongolia. Key to identification]. Moskva: Nauka, 304 pp.
Medvedev L.N., 1996. The Chrysomelidae of Arabia. Fauna of Saudi Arabia 15, 211-263.
Medvedev L.N. & Korotyaev B.A., 1980. Notices on the fauna of chrysomelid beetles (Coleoptera, Chrysomelidae) of Arctic Asia and Kamchatka. In: Medvedev G.S. & Matis E.G. (eds) Issledovaniya po Entomofaune Severo-Vostoka SSSR, 77-95. Vladivostok.
Medvedev L.N. & Sprecher-Uebersax E., 1997. Chrysomelidae of Nepal and neighbouring regions (Coleoptera: Chrysomelidae). Coleoptera 1, 203-247.
Muscarella C. & Baragona A., 2017. The endemic fauna of the sicilian islands. Biodiversity journal 8(1), 249-278.
Petitpierre E., 2000. Coleoptera, Chrysomelidae I. Fauna Ibérica, vol. 13. Madrid: Museo Nacional de Ciencias Naturales, CSIS, 521 pp.
Rossaro B., 1995. The distribution of Palaearctic Diamesinae (Insecta, Diptera, Chironomidae). Spixiana 18(2), 177-186.
Sassi D. & Kismali S., 2000. The Cryptocephalinae of Turkey, with information on their distribution and ecology (Coleoptera Chrysomelidae). Memorie della Società Entomologica Italiana 78(1), 71-129.
Schmitt M., Bäse W., Beenen R., Drovenik B., Fritzlar F., Geiser E., Jäckel R., Langer M., Mauser J., Ringel H., Schöller M. & Siede D., 2014. Das Projekt CHRYFAUN – Faunistik der mitteleuropäischen Blatt- und Samenkäfer (Chrysomelidae s.l.). Entomologische Blätter und Coleoptera 110, 33-38.
Schöller M., 1995. Zur Evolution der Camptosoma (Coleoptera: Chrysomelidae). Entomologische Blätter 91(1/2), 53-61.
Schöller M., 1996. Ökologie mitteleuropäischer Blattkäfer, Samenkäfer und Breitrüssler (Coleoptera: Chrysomelidae einschließlich Bruchinae, Anthribidae). EVCV, Bürs, ISBN 3-9500146-6-7; 65 pp.
Schöller M., 2020. New country records and a new synonym in the subgenus Burlinius of Cryptocephalus (Coleoptera: Chrysomelidae). Mitteilungen des Internationalen Entomologischen Vereins 43(3-4), 189-192
Schöller M., 2021a. Cryptocephalus from Oman and Yemen, with description of a new species and notes on subgenera (Coleoptera: Chrysomelidae). Mitteilungen des Internationalen Entomologischen Vereins 44(1-2), 13-32.
Schöller M., 2021b. Three new species of Cryptocephalini from China (Coleoptera: Chrysomelidae: Cryptocephalinae). Mitteilungen des Internationalen Entomologischen Vereins 44(3-4), 135-145.
Schöller M., Löbl I. & Lopatin I., 2010. Tribe Cryptocephalini Gyllenhal, 1813, remaining Cryptocephalini. – In: Löbl I. & Smetana A. (eds) Catalogue of Palaearctic Coleoptera, Volume 6, Chrysomeloidea, 606-617. Apollo Books, Stenstrup. 924 pp.
Schöller M. & Nasserzadeh H., 2010. The distribution of leaf beetles of the tribe Cryptocephalini (Chrysomelidae: Cryptocephalinae) in Iran. Mitteilungen internationaler entomologischer Verein 35, 55-87.
Silfverberg H., 1994. Chrysomelidae in the Arctic. In: Jolivet P.H., Cox M.L. & Petitpierre E. (eds) Novel aspects of the biology of Chrysomelidae, 503-510. Kluwer Academic Publishers, The Netherlands.
Silfverberg H., 2010. Enumeratio renovata Coleopterorum Fennoscandiae, Daniae et Baltiae. Sahlbergia 16(2), 1-144.
Suffrian E. 1854. Verzeichniss der bis jetzt bekannt gewordenen asiatischen Cryptocephalen. Linnaea Entomologica 9, 1-169.
Wallace A.R, 1876. The geographical distribution of animals, Vol. 1. 503 pp., Vol. 2. 607 pp., Harper, New York.
Warchałowski A. 2010. The Palaearctic Chrysomelidae. Identification keys. Volume 1 & 2. 1212 pp., Natura Optima Dux Foundation, Warszawa.
Weise J., 1882. Chrysomelidae. In: Naturgeschichte der Insekten Deutschlands. Erste Abtheilung. Coleoptera. Sechster Band. [1881–1893], 193-368 [Lieferung 2]. Nicolaische Verlags-Buchhandlung R. Stricker, Berlin xiv + 1161 pp.
Willig M.R. & Presley S.J., 2013. Latitudinal gradients of biodiversity. In: Levin S.A. (ed.) Encyclopedia of biodiversity, second edition, Volume 4, 612-626. Elsevier, Academic Press, Waltham, Massachusetts.
(44 Réf.)

