- Portada
- Volume 73 (2020)
- Mosquito biodiversity in Setif region (Algerian High Plains), density and species distribution across two climate zones
Vista(s): 3371 (99 ULiège)
Descargar(s): 87 (0 ULiège)
Mosquito biodiversity in Setif region (Algerian High Plains), density and species distribution across two climate zones

Documento adjunto(s)
Version PDF originaleNotes de la rédaction
Received on May 7th 2019, accepted on January 14th 2020.
Résumé
L’Algérie a reconnu des épidémies liées aux moustiques. De plus, le pays est actuellement exposé à l'installation de l'espèce invasive Aedes albopictus (Theobald 1907). Dans ce contexte, nous avons réalisé un inventaire des moustiques dans la région de Sétif de 2016 à 2018, afin de fournir une liste de moustiques dans la zone d'étude et d'analyser leur biodiversité, densité et répartition dans deux zones climatiques (méditerranéenne Csa et haute plaine BSK) en utilisant des tests biostatistiques. L'identification a été réalisée à l'aide d'une combinaison d'approches morphologiques et moléculaires (COI barcode). Nous avons identifié neuf espèces, dont les vecteurs du paludisme Anopheles labranchiae (Falleroni 1926) et Anopheles cinereus hispaniola (Theobald 1901). Nous signalons aussi la présence d’une nouvelle espèce Culex simpsoni (Theobald 1905). Les séquences COI de six espèces publiées sur Genbank sont fournies (MK047302-MK047315). Culex pipiens s.l (Linnaeus 1758) présente la densité la plus élevée dans la zone BSK, tandis que Culiseta longiareolata (Macquart 1838) présente la densité la plus élevée dans Csa (51,2±63,7). En outre, nous avons révélé une corrélation élevée et positive entre Culex theileri (Theobald 1903) et An labranchiae (rs=0,89, p>0,001). La comparaison par paires et les analyses d'ordination correspondantes ont permis d'établir la présence d'une association significative entre la répartition/densité des espèces et les zones climatiques (KWU=51, p>0,01), ce qui confirme l'effet des changements climatiques sur les populations de moustique. Espérons que les résultats fournis renforceront nos connaissances sur la dynamique des populations de moustique et faciliteront la mise en place d'un programme de contrôle efficace.
Abstract
Algeria has experienced outbreaks related to mosquitoes; additionally, it is exposed at the present to the installation of the invasive species Aedes albopictus (Theobald 1907). In this context, we performed a mosquito inventory in the Algerian high plains (Setif region) from 2016 to 2018, in order to provide the list of mosquitoes in the study area and analyze their biodiversity, density and species distribution across two climate zones (Mediterranean Csa and steppe BSk Zones) using biostatistical tests. The identification of species was done using a combination of morphological and molecular approaches (COI barcoding). The sampling yielded the identification of nine mosquito species including the malaria vectors Anopheles labranchiae (Falleroni 1926) and Anopheles cinereus hispaniola (Theobald 1901). A new species Culex simpsoni (Theobald 1905) is also recorded. The COI sequences of six species are provided in Genbank (MK047302-MK047315). From the total sampled mosquitoes, Culex pipiens s.l (Linnaeus 1758) showed the highest density in BSk zone (34.7±8.9), while Culiseta longiareolata (Macquart 1838) showed the highest density (51.2±28.5) in Csa. Further, we have revealed a high and positive correlation between Culex theileri (Theobald 1903) and An labranchiae (rs=0.89, p>0.001). Moreover, the pairwise comparison and Ordination Corresponding Analyses ascertained the presence of a significant association between species distribution/density and climate zones in the study area (K-W U=51, p>0.01), and confirm the effect of the climate changes on the mosquito population. The results provided will hopefully accentuate our knowledge about mosquito population dynamics and facilitate the installation of an effective control program.
Tabla de contenidos
INTRODUCTION
1The members of the Culicidae or mosquito family are considered from the most important cosmopolitan Diptera insects in the world. Mosquitoes constitute effective and active members when they occupy an ecosystem since they enter into the food chain as prey or likewise as predators; however, many mosquito species can act as vectors of dangerous and deadly diseases and threaten the public health (Schaffner et al., 2013; Guarner & Hale, 2019). Therefore, mosquito-borne-diseases pose a huge impact on human affairs which makes mosquito control a priority (Greisman et al., 2019; Petersen et al., 2019). Thus, effective mosquito control requires a good knowledge of the mosquito population biodiversity in terms of species diversity and ecological characteristics (Manguin & Boëte, 2011; Li et al., 2019).
2Algeria has experienced, in the last decades, fluctuations of mosquito-borne-diseases (Boubidi et al., 2010; Lafri et al., 2017). Anopheles sergentii (Theobald 1907) and Anopheles cinereus hispaniola (Theobald 1901) were involved in malaria transmission (Sinka et al., 2010; Snow et al., 2012); in addition, Aedes albopictus (Skuse 1894), the vector of Zika virus, has invaded lately the North part of the country (Izri et al., 2011; Benallal et al., 2016). Mosquito surveys have been conducted lately in Algeria and a total of 27 mosquito species has been reported (Bouabida et al., 2012; Boudemagh et al., 2013; Lafri et al., 2014). The conducted studies focused more on beta biodiversity, whereas intraspecific interactions, species density, and distribution patterns were poorly explored.
3Furthermore, mosquito inventories conducted previously in Algeria were based on the morphological identification. The invasion of new species (Aedes albopictus) in addition to the existence of members of complexes Culex pipiens (Linnaeus 1758), Anopheles labranchiae (Falleroni 1926) and An c hispaniola, made the morphological identification insufficient because of the difficulty of separating close species (Harbach, 2007; Werblow et al., 2016). The similar morphology of close species, complex species and hybrids led to a major problem particularly to the non-experienced researchers, not only in Algeria but in the entire world. Since the morphological identification is sometimes insufficient or even useless for the separation of mosquito species, the integrative taxonomy approach becomes a more suitable method consisting of the combination of morphological and molecular identification. The remarkable progress in molecular researches served the mosquito identification; the DNA-based identification was adopted as a more accurate identification method to support mosquito inventories using the sequence divergence at cytochrome c oxidase subunit 1 (COI) (Werblow et al., 2016); likewise, COI barcoding is seen as a useful, precise, and time-effective approach in mosquito species separation (Laboudi et al., 2011; Engdahl et al., 2014; Chan et al., 2014; Afizah et al., 2019).
4In this study, we investigated the Setif region which is one of the most populated provinces in Algeria in order to renew our knowledge about mosquito biodiversity. We collected larvae and adult mosquitoes during 2016-2018 and identified them: morphologically, using diagnostic keys; and molecularly by sequencing the COI genes of the harvested specimens using the PCR-PFLP approach. Furthermore, we used the collected data to adopt a better description of mosquito biodiversity in the study area and to analyze the density and species distribution patterns across climate zones. The results will likely provide information crucial for mosquito control in the study area and highlight the effect of global climate change on mosquito populations. The ecological data was analyzed using bio-statistical analyses.
MATERIALS AND METHODS
Study area
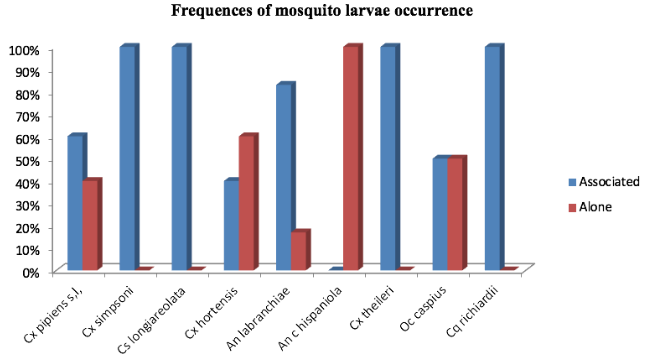
5Setif region of high plains Northeastern Algeria (36°03'N 5°31'E) stretches over a surface of 6504km2, the human population density is approximately 230inhabitants/km2. The human population is distributed in the different landscape structures according to the nature of their life activities. The agriculture constitutes an important sector in the study area due to the availability of farmlands and water surfaces (dams and rivers) (Rouabhi et al., 2012; Rouabhi et al., 2016). Setif region is characterized by heterogeneity of climate according to Köppen climate classification (Köppen et al., 2011). We can differentiate two sectors: a north part of the Csa climate, and a south part of the BSk climate (semi-arid; cold and dry). Therefore, the sampling sites in the study area were regrouped within two groups:
6The first group: includes 16 sites within the region characterized by a Csa climate;
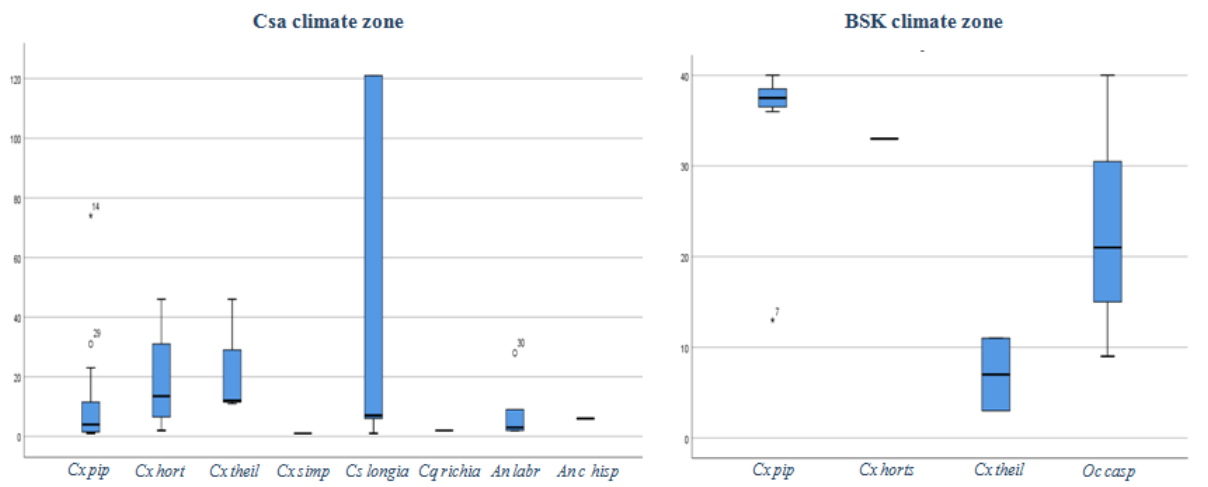
7The second group: regroups 4 sites within the region characterized by a BSk climate.
8The distribution of the sampling sites in the study area and the limitation of the climate zones are illustrated in Figure 1.
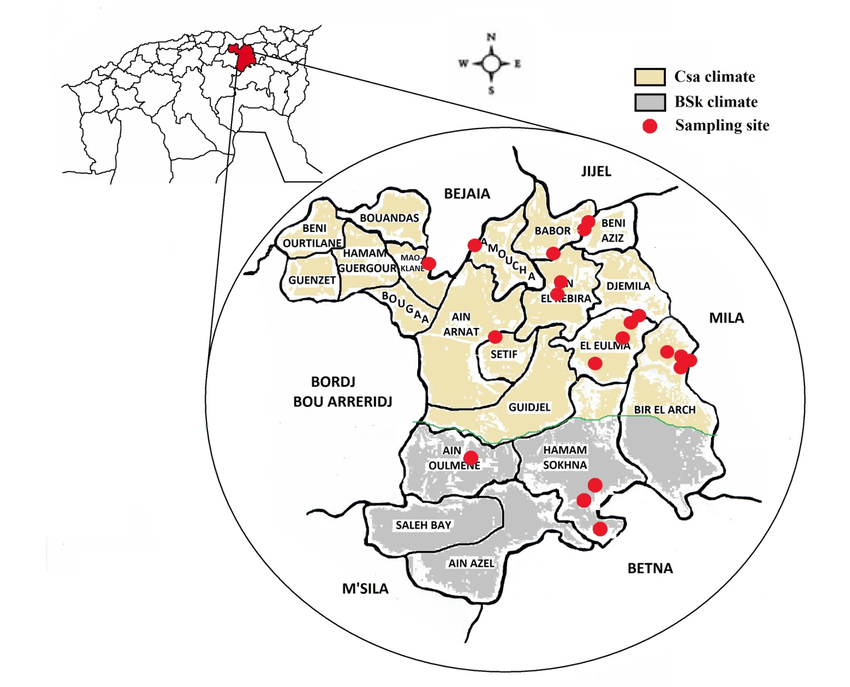
9Figure 1: The geographical localization of Setif region (Algeria) and the distribution of the sampling sites (n=20) in two types of climate zones: Csa (Mediterranean climate) and BSk (steppe climate).
Sampling and morphological identification
10The sampling was conducted during the 2016-2018 and targeted larval and adult forms. The larvae sampling occurred using a standard dipper (1L) while adult sampling was done using simple CDC miniature light traps (Handmade: yellow light lamp and fan "12VDC").
11The sampled specimens were first identified morphologically, 3rd and 4th instar larvae were identified alive (this operation preserve the setae that can be lost easily with the intense manipulation), or after being mounted for permanent preparations (Becker et al., 2003). First and second instar larvae were reared in the breeding site water until they reached the fourth instar. The larvae identification was carried out using a microscope (Browser LCD MICRO 5MP microscope) with a camera built (5MP CNOS 1/2.5", 2560 x 1920 pixel array). Adults were analyzed using binocular microscope loupe. The morphological identification (larvae and adults) was done based on characters described by (Becker et al., 2003) and the last version of interactive keys provided by the French National Research Institute for Sustainable Development (IRD) (Gunay et al., 2018).
Molecular identification
12DNA of the harvested and reared adults (n=24) was extracted from the legs (one of each specimen) using the DNeasy blood & tissue kit (Qiagen, Hilden, Germany) by following the handbook instructions. The PCR amplification of the COI barcode was performed in a total volume of 35 µl consisting of 10x reaction buffer; 2.5 mM MgCl 2; 200 µM of dNTPs; 28 pmol each primer LCO1490 and HCO2191 (Vrijenhoek, 1994); 2.5 U of TaqDNA polymerase. A volume of 3µl of genomic DNA was added to each PCR reaction and samples without DNA were included to exclude carryover contamination. The PCR procedure was as follows: initial denaturation stage and activation of the enzyme at 95°C for 2 minutes; 40 cycles at 94°C for 40 seconds, 50°C for 40 seconds and 72°C for 1 minute, followed by a final extension phase at 72 °C for 7 minutes. PCR products were examined on 1% agarose gel and the band’s intensity was noted using a gel imaging system (ChemiDoc™ XRS+ System with Image Lab™ Software #1708265); both strands of the successful amplifications were sequenced at GATC Biotech (Konstanz, Germany). Sequencing results were analyzed using Geneious 10.2.3 software (Kearse et al., 2012). The data of positive sequences were edited using BioEdit (Hall, 1999) and compared with sequences deposited in GenBank and Bold using BLASTn (Zhao & Chu, 2014). COI sequences were deposited in GenBank with the accession numbers from MK047302 to MK047315.
Data analysis
13We analyzed the total mosquito population by calculating the abundance (the number of species specimens divided by the total number of samples), frequency in percentage (ƒ) and mean density (Mean±Standard deviation). Only identified specimens were included.
14The majority of the sampled larvae co-occurred; thus, we calculated the frequency of the species association and non-association, and the level of correlation between the co-occurred species using the cor.test (method=Spearman’s“ non-normality of data”) and the corrgram package (Wright & Wright, 2018) in R studio Version 2.1.1335 (Team, 2018). The Spearman’s correlation (rs) was considered as weak if 0>rs≤0.4, moderate if 0.4>rs≤0.7 and as strong if 0.7>rs>1. Only species found more than one time was included in the analyses. Species found only one time were excluded for the correlation test.
15Next, the difference in mosquito density between climate zones was analyzed using the non-parametric test Mann-Whitney U (non-normality and heteroscedasticity of data). Further, the mean density of the sampled mosquitoes was calculated by climate zone. The analysis performed using SPSS version 25.
16Simultaneously, Alpha diversity within Csa and BSk climate zones was evaluated using species richness (S), Simpson index (1-D) (Simpson, 1949), Shannon index (H’) (Shannon & Weaver, 1949) and Evenness (E”) indices (Hill, 1973). Moreover, a canonical correspondence analysis (CCA) was performed to evaluate the effect of the variable ‘climate zone’ on mosquito distribution in the study area where sites and species constituted the axes 1 and 2. The diversity and multivariate analyses were conducted using PAST3 (Hammer et al., 2001).
RESULTS
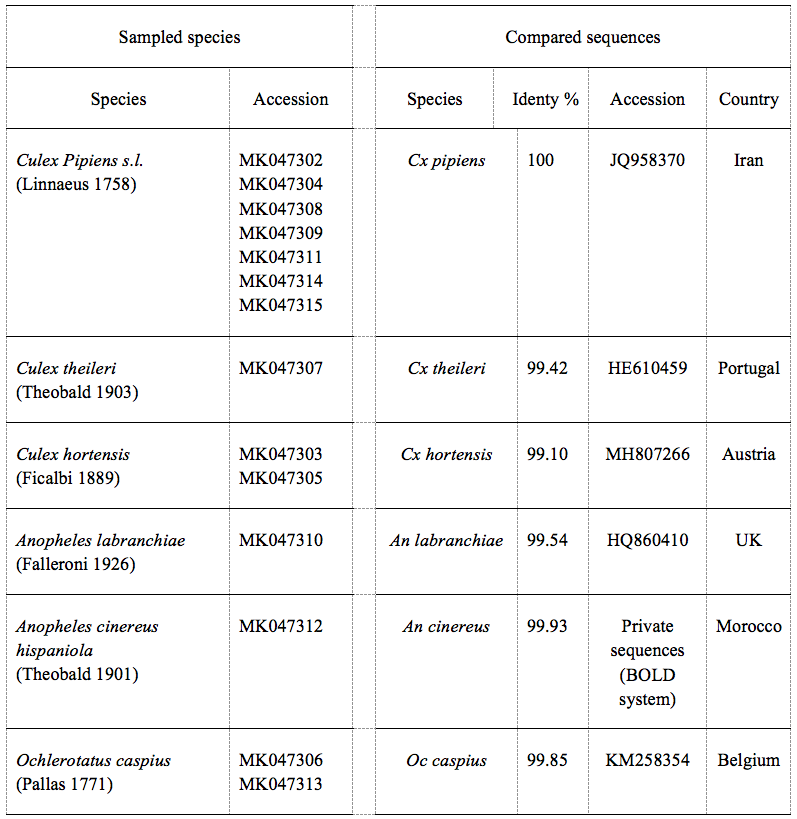
17From 38 samples distributed in 20 sites, a total of 1144 specimens were harvested (921 larvae and 223 adults), of which 94.5% of specimens were identified. The sampling yielded nine mosquito species of which six were confirmed by molecular analysis. BLAST analysis of the COI gene of our samples displayed an identity of 99% and 100% on the nucleotide level with a null error value (Table 1). The rest three species were confirmed by morphological identification using diagnostic keys. Culiseta longiareolata (Macquart 1838) was easy to distinguish in its larval stage, the siphon was short and the saddle was incomplete (Figure 2). Coquillettidia richiardii (Ficalbi 1889) was identified in the larval stage; the saddle was without tuffs (Figure 2). Finally, a new record of Culex simpsoni (Theobald 1905) (n=2) was noted, the larvae had a long siphon (siphon index=9.6), the sub-apical spine S-2 was short, and the siphonal seta 1a-S was longer than the diameter of the siphon (Figure 2).
18The majority of the sampled species showed a tendency to co-occur (Figure 3). However, the spearman’s rho test revealed only one significant high and positive correlation between Culex theileri (Theobald 1903) and An labranchiae (rs=0.89, p>0.001). Along similar lines, the test rejects the presence of a real correlation in the other association cases (Figure 4).
19Table : Comparison of BLASTn results of the sampled species’ sequences with sequences published in Genbank and BOLD displayed an identity between 99% and 100% on the nucleotide level with a null error value.
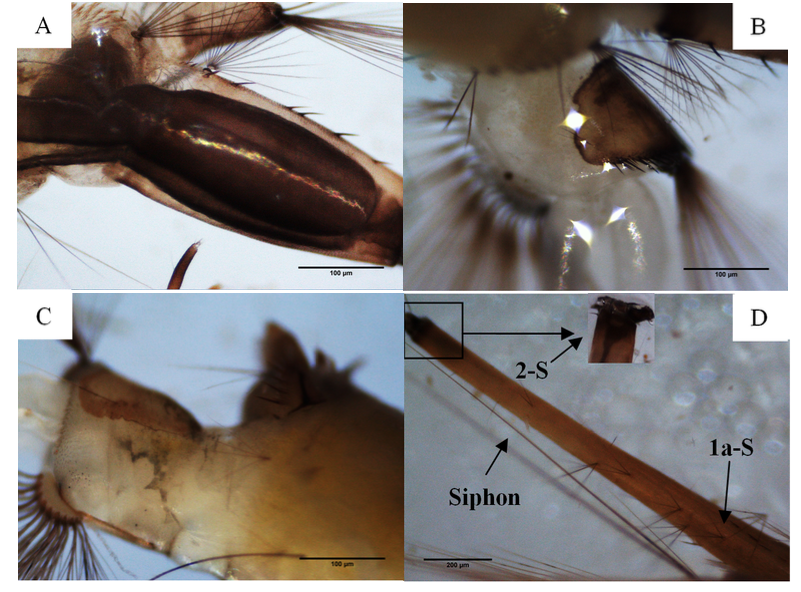
20Figure 2: Morphological characters of three mosquito species: (A) siphon of Culiseta longiareolata (Macquart 1838) larvae. (B) Incomplete saddle in Cs longiareolata larvae. (C) Saddle without tuffs in Coquillettidia richiardii (Ficalbi 1889). (D) Long siphon in Culex simpsoni (Theobald 1905) with 1a-S longer than the siphon diameter and 2-S short.
21Figure 3: Frequencies of association and non-association in larvae occurrence for the sampled mosquito species (Culex pipiens (Linnaeus 1758), Culex hortensis (Ficalbi 1889), Culex theileri (Theobald 1903), Culex simpsoni (Theobald 1905), Culiseta longiareolata (Macquart 1838), Ochlerotatus caspius (Pallas 1771), Coquillettidia richiardii (Ficalbi 1889), Anopheles labranchiae (Falleroni 1926), Anopheles cinereus hispaniola (Theobald 1901)).
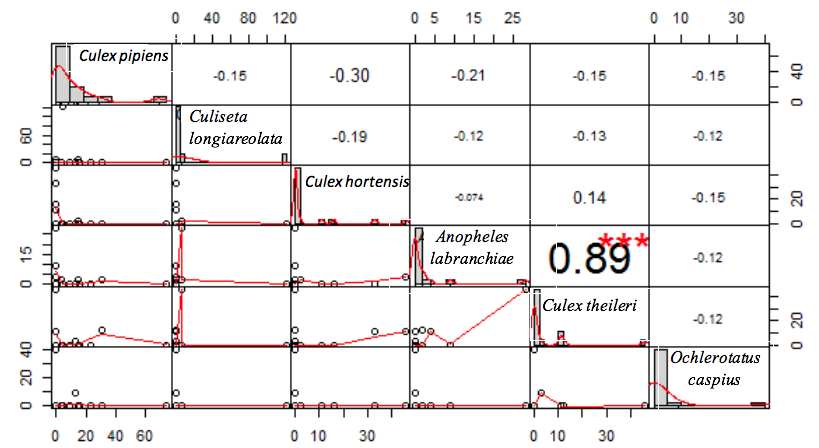
22Figure 4: Correlogram of mosquito larvae co-occurrence within Setif region, Algeria Culex pipiens (Linnaeus 1758), Culex hortensis (Ficalbi 1889), Culex theileri (Theobald 1903), Culiseta longiareolata (Macquart 1838), Ochlerotatus caspius (Pallas 1771), Anopheles labranchiae (Falleroni 1926). Upper are the correlation coefficients (rho) with the significance level (***=p-value>0.001). Lower are scatter plots with tendency curves. Values with no significant level refer to the absence of correlation.
23As the study area is characterized by heterogeneity of climate, we have analyzed the distribution and the density of mosquito species by climate zones (Csa and BSk). The mean mosquito density has varied across climate zones (K-W U=108, p>0.01). The highest density was observed in the BSk (28.2±13.6) comparing to Csa (20.1±42.3). Further, the difference in the mean density of Culex pipiens s.l between Csa and BSk zones was statistically highly significant (K-W U=13, p>0.001); the density of Cx pipiens s.l was higher in BSK zone (34.7±8.9), it was followed by Ochlerotatus caspius (Pallas 1771) (23.3±15.9). Further, Cs longiareolata showed the higher mean density in Csa zone (51.2±28.5), it was followed by Culex hortensis (Ficalbi 1889) (18.7±19); while Cx pipiens s.l (9.7±15.7) and An labranchiae (7.8±10.2) showed the lowest mean density (Figure 5). The ecological data noted during the sampling including density, abundance, and positive sites are provided in (Table 2).
24Figure 5: Box and whisker plot showing mean density of the mosquito species Culex pipiens (Linnaeus 1758), Culex hortensis (Ficalbi 1889), Culex theileri (Theobald 1903), Culex simpsoni (Theobald 1905), Culiseta longiareolata (Macquart 1838), Ochlerotatus caspius (Pallas 1771), Coquillettidia richiardii (Ficalbi 1889), Anopheles labranchiae (Falleroni 1926), Anopheles cinereus hispaniola (Theobald 1901) sampled from Setif province, from 2016 to 2018, in two different climate zones (BSk and Csa).
25Table 2: The total sampled mosquitoes by climate zones within the Setif region, Algeria (2016-2018); with species abundance and positive sites.
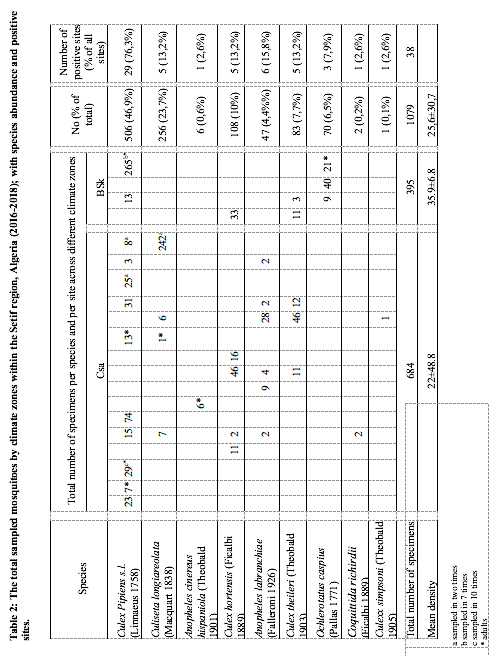
26Likewise, we noted higher biodiversity indices in the Csa climate zone (1-D=0.7, H’=1.5) comparing to BSk (1-D=0.5, H’=0.9). However, the species frequencies in the BSk zone were more similar (E”=0.6). On the other hand, the species abundance was not the same across climate zones. In Csa sites, Culiseta longiareolata (38.1%) was the most abundant, followed by Cx pipiens s.l (33.3%), Cx hortensis (11%), Cx theileri (10.1%) and An labranchiae (6.9%); while, Cx simpsoni (0.1%), Cq richiardii (0.3%) and An c hispaniola (0.9%) was noted as sporadic. In BSk sites, Cx pipiens s.l (70.4%) was the most abundant, followed by Oc caspius (17.7%). Moreover, the CCA analysis displayed ‘climate zone’ as a variable that may explain the species distribution in the study area. The results indicated the existence of two separate species/climate zone clusters: An labranchiae, An c hispaniola, Cs logiareolata, Cx simpsoni, Cq richiardii /Csa and Oc caspius/BSk. Another cluster constituted by three species Cx pipiens s.l, Cx hortensis and Cx theileri was noted, it appeared less associated to a specific climate zone (Figure 6)
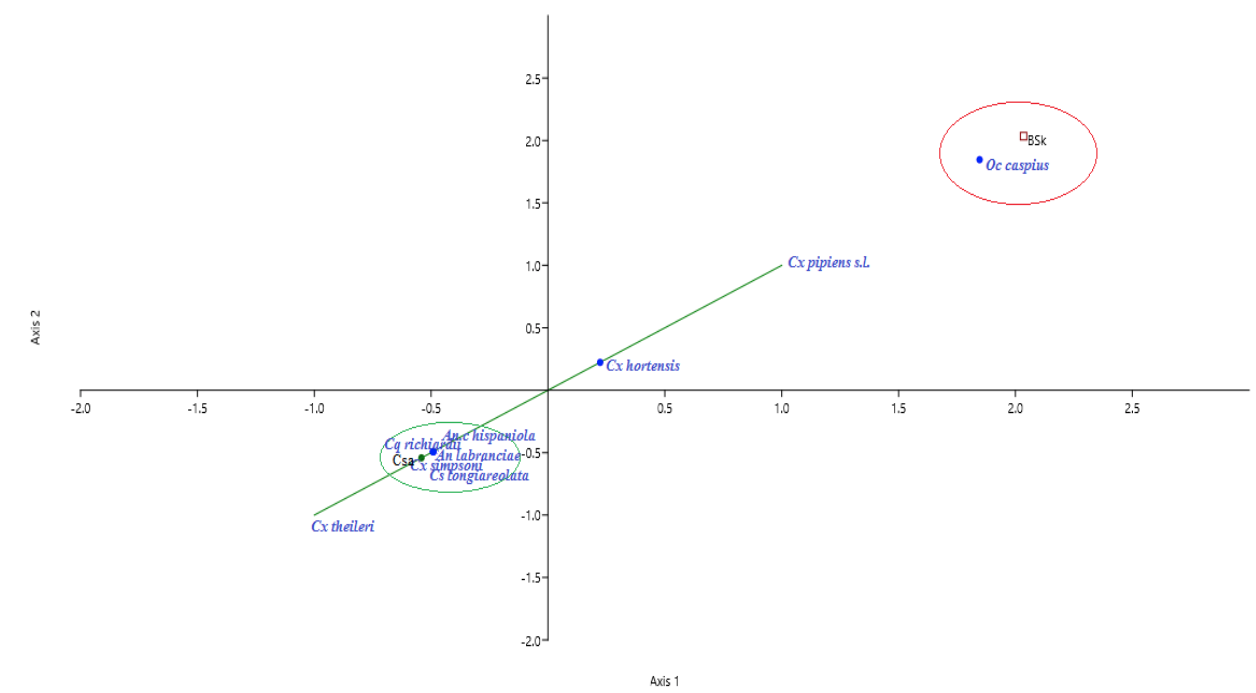
27Figure 6: Canonical Correspondence Analysis (CCA) ordination biplot of species distribution in Setif region by climate zones (Csa, BSk). Csa and BSk climate zone with species constituted two distinct clusters. Culex pipiens (Linnaeus 1758), Culex hortensis (Ficalbi 1889) and Culex theileri (Theobald 1903) are widely distributed.
DISCUSSION
28The invasion of Aedes albopictus in the Algerian territory requires a renovation of the list of mosquito species; the current work was carried out in order to inspect a new region Northeastern Algeria. The inventory of Setif province has yielded the identification of nine mosquito species. As in the rest of the inspected Algerian regions (Lafri et al., 2014), Cx pipiens s.l was the most abundant and the most frequent species in the study area; this species is a competent vector that can transmit West Nile Virus (Andreadis et al., 2001; Hamer et al., 2008), a human and animal neurophathogen worldwide disease that can range in severity from uncomplicated West Nile fever to a fatal meningoencephalitis (Campbell et al., 2002; Kaleemullah & Sill, 2019). Cx pipiens s.l. is also a vector of Rift Valley fever virus (Moutailler et al., 2008), an emerging disease that can cause important livestock industry losses, and moderate human morbidity and mortality (Pepin et al., 2010; Hartman et al., 2019). On the other hand, two important members of the Anopheles subfamily, An labranchiae and An c hispaniola, were identified molecularly to ensure the morphological identification results. For An labranchiae, the comparison of our sequences revealed a 99.54% of similarity with An labranchiae sequences from UK (it was the higher matching for the maculipennis complex species sequences provided in Genbank and BOLD). Likewise, an inventory conducted by Laboudi et al., (2011) confirmed that An labranchiae is the only representative member of An maculipennis s.l. Meigen complex in North Africa. An labranchiae constituted with An sergentii and Anopheles gambiae (Giles 1902) the malaria vectors in Algeria (Boubidi et al., 2010; Snow et al., 2012; WHO-Algeria, 2014 ), the frequent presence of this species in the study area, even at a low density, poses the risk of outbreaks in the region. The other Anopheles species An c hispaniola was not recorded in Algeria since 1983 (Ramsdale, 1983). The comparison with sequences from the BOLD platform revealed a 99.93% of similarity with An c hispaniola sequences from Morocco. An c hispaniola is a member of the complex An cinereus; it is usually distributed in the Arab Maghreb and other Mediterranean regions (Samanidou-Voyadjoglou & Darsie Jr, 1993; Trari et al., 2002; Bueno Marí & Jiménez Peydró, 2010; Tabbabi & Daaboub, 2017); while the other An cinereus member An c cinereus is distributed in Arabian Peninsula, and Eastern, South and Central Africa (Amr et al., 1997; Alahmed, 2012; Animut et al., 2012). An c hispaniola is as well considered as a potential malaria vector, it was found infected by Plasmodium falciparum (Trager and Jensen 1976) in Eritrea (Shililu et al., 2003). However, An c hispaniola was found one time during the sampling, thus, it could be considered as a sporadic species. The sampling yielded likewise the identification of Cx simpsoni; as far as we know, this species is reported for the first time in Algeria. Cx simpsoni was identified in Morocco and it is usually distributed in south Africa and southwestern Asia (Army Public Health Center, 2019). The larvae of this species are close to those of Cx antennatus, Cx sinaiticus and Cx theileri (Gunay et al., 2018); for this reason, we have adopted pictorial keys for its discrimination; Seta 5-C was 2 branched while it is more branched in Cx theileri (3-4 branches), seta 1a-S was 3 branched and longer than the siphon diameter while it is shorter than the diameter of the siphon in Cx antennatus and the pecten was on less than one third of the siphon, while the pecten is longer in Cx sinaiticus (Harbach, 1985). However, the morphological identification was not sufficient to ensure the species; especially that only two specimens were sampled. Further, the sampling results confirmed the absence of the invasion species Ae albopictus in the Algerian high plains and limit its presence in the far North of the country.
29The presence of vector species in the study area makes a reason for the importance of the evaluation of the species interactions, habitat preferences and distribution patterns. The mosquito population stability is important to control the popular health situation; this stability is significantly associated with the relationships between conspecific individuals (Porretta et al., 2016). The species sampled in the study area showed a tendency to co-occur with other mosquito species. However, the spearman’s rho test confirmed only one real correlation. Cx theileri and An labranchiae larvae were strongly correlated, the level of the correlation purpose the possibility of considering Cx theileri as a species indicator for An labranchiae, however, the measurement of species co-occurrence for choosing indicator species needs detailed studies (De Cáceres et al., 2012; Neeson & Mandelik, 2014) and this subject is not well developed in the entomological field.
30Better control of mosquito population stability is related as well to the knowledge of the density and the distribution patterns in the study area. The comparative statistical analyses of the mosquito densities between climate zones showed a significant difference between BSk and Csa. The mosquito mean density was higher in BSk sites, this climate zone features hot and dry summer and cold wet winter; the temperature in this zone tend to feature major swings between day and night. Simultaneously, the biodiversity indices and CCA analyses confirmed that the Csa sites are more diversified and the majority of the sampled species are related to the Csa climate zone. This zone is characterized by a more humid climate and longer wet season with hot and dry summer. The majority of the mosquito-born-diseases are related to mosquito density (Churcher et al., 2015; Bradley et al., 2018) and are sensitive to climate features (Reiter, 2001; Li et al., 2019). Further, there is a direct and clear association between mosquito dynamics and climate variations (Beck-Johnson et al., 2013; Wilke et al., 2017). Therefore, the significant difference noted in the mosquito mean densities between climate zones, and the density and distribution patterns that were related to a particular zone, explain the importance of surveying mosquito populations according to a defined climate zone as the best strategy to control outbreaks.
CONCLUSION
31Overall, the current study has provided a list of mosquito species occurred in the study area with COI sequences of six species provided in Genbank under the accession numbers from MK047302 to MK047315. Likewise, the study analyzed the data collected during the sampling and provided information about the density and distribution patterns of mosquito populations. Further, the existence of a high and positive correlation between two species poses the possibility of using mosquito species as species indicators. Moreover, a strong relationship between mosquito population and climate zones was confirmed, thus, the climate changes can affect the mosquito population density and distribution. Finally, the obtained results will hopefully constitute a database for the installation of an effective mosquito control program.
ACKNOWLEDGEMENT
32The authors are grateful to Dr. Bruno Mathieu for the help in the realization of Molecular analyses and the Institute of Parasitology and Tropical Pathology, Strasbourg, France (IPPTS) for funding the PCR tests.
Bibliographie
References
Afizah A.N. et al., 2019. DNA barcoding complementing morphological taxonomic identification of mosquitoes in Peninsular Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health. 50(1), 36-46.
Alahmed A.M., 2012. Mosquito fauna (Diptera: Culicidae) of the Eastern Region of Saudi Arabia and their seasonal abundance. Journal of King Saud University-Science. 24(1), 55-62.
Amr Z.S. , Al-Khalili Y. & Arbaji A., 1997. Larval mosquitoes collected from northern Jordan and the Jordan Valley. Journal of the American Mosquito Control Association-Mosquito News. 13(4), 375-378.
Andreadis T.G. , Anderson J.F. & Vossbrinck C.R., 2001. Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, and Culiseta melanura. Emerging Infectious Diseases. 7(4), 670.
Animut A. et al., 2012. Abundance and dynamics of Anopheline larvae in a highland malarious area of south-central Ethiopia. Parasites & vectors. 5(1), 117.
Army Public Health Center, 2019. Culex (Culex) simpsoni Theobald, WRBU specimen CXssi, Character descriptions: Edwards,1941:309,ttps://phc.amedd.army.mil/PHC%20Resource%20Library/Culex(Culex)simpsoniTheobaldWRBUspecimenCXssi_TA-319-0116.pdf, (31/01/2019).
Beck-Johnson L.M. et al., 2013. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PloS One. 8(11), e79276.
Becker N. et al., 2003. Mosquitoes and their control: Springer.
Benallal K.E. et al., 2016. First report of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Oran, West of Algeria. Acta Tropica. 164, 411-413.
Bouabida H. , Djebbar F. & Soltani N., 2012. Etude systématique et écologique des Moustiques (Diptera: Culicidae) dans la région de Tébessa (Algérie). Entomologie faunistique-Faunistic Entomology. 65, 99-103.
Boubidi S.C. et al., 2010. Plasmodium falciparum malaria, southern Algeria, 2007. Emerging Infectious Diseases. 16(2), 301-303.
Boudemagh N. , Bendali-Saoudi F. & Soltani N., 2013. Inventory of Culicidae (Diptera: Nematocera) in the region of Collo (North-East Algeria). Annals of Biological Research. 4(3), 1-6.
Bradley J. et al., 2018. Predicting the likelihood and intensity of mosquito infection from sex specific Plasmodium falciparum gametocyte density. Elife. 7, e34463.
Bueno Marí R. & Jiménez Peydró R., 2010. New Anopheline records from the Valencian autonomous region of eastern Spain (Diptera: Culicidae: Anophelinae). European Mosquito Bulletin. 28, 148-156.
Campbell G.L. et al., 2002. West Nile virus. The Lancet infectious diseases. 2(9), 519-529.
Chan A. et al., 2014. DNA barcoding: complementing morphological identification of mosquito species in Singapore. Parasit Vectors. 7(1), 569.
Churcher T.S. , Trape J.-F. & Cohuet A., 2015. Human-to-mosquito transmission efficiency increases as malaria is controlled. Nature communications. 6 (2015), 6054.
De Cáceres M. et al., 2012. Using species combinations in indicator value analyses. Methods in Ecology and Evolution. 3(6), 973-982.
Engdahl C. et al., 2014. Identification of Swedish mosquitoes based on molecular barcoding of the COI gene and SNP analysis. Molecular Ecology Resources. 14(3), 478-488.
Greisman L. , Koenig B. & Barry M., 2019. Control of Mosquito-Borne Illnesses: A Challenge to Public Health Ethics. The Oxford Handbook of Public Health Ethics. 459.
Guarner J. & Hale G. 2019. Four human diseases with significant public health impact caused by mosquito-borne flaviviruses: West Nile, Zika, dengue and yellow fever. In Seminars in diagnostic pathology. WB Saunders.
Gunay F. , Picard M. & Robert V., 2018. MosKeyTool, an interactive identification key for mosquitoes of Euro-Mediterranean. Version 2. , http://medilabsecure.com/moskeytool, (10/06/2018).
Hall T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT; [London]: Information Retrieval Ltd., c1979-c2000. In Nucleic acids symposium series. 41, p 95-98.
Hamer G.L. et al., 2008. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. Journal of Medical Entomology. 45(1), 125-128.
Hammer Ø. , Harper D.A. & Ryan P.D., 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia electronica. 4(1), 9.
Harbach R.E., 1985. Pictorial keys to the genera of mosquitoes, subgenera of Culex and the species of Culex (Culex) occurring in southwestern Asia and Egypt, with a note on the subgeneric placement of Culex deserticola (Diptera: Culicidae). Mosquito systematics. 17(2).
Harbach R.E., 2007. The Culicidae (Diptera): a review of taxonomy, classification and phylogeny. Zootaxa. 1668(1), 591-638.
Hartman D.A. et al., 2019. Entomological risk factors for potential transmission of Rift Valley fever virus around concentrations of livestock in Colorado. Transboundary and Emerging Diseases.
Hill M.O., 1973. Diversity and evenness: a unifying notation and its consequences. Ecology. 54(2), 427-432.
Izri A. , Bitam I. & Charrel R.N., 2011. First entomological documentation of Aedes (Stegomyia) albopictus (Skuse, 1894) in Algeria. Clinical Microbiology and Infection. 17(7), 1116-1118.
Kaleemullah F. & Sill J., 2019. The Wild West Nile Virus. D48 Critical care case reports: infection and sepsis II: American Thoracic Society. p A6598-A6598.
Kearse M. et al., 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12), 1647-1649.
Köppen W. , Volken E. & Brönnimann S., 2011. The thermal zones of the earth according to the duration of hot, moderate and cold periods and to the impact of heat on the organic world (Translated from: Die Wärmezonen der Erde, nach der Dauer der heissen, gemässigten und kalten Zeit und nach der Wirkung der Wärme auf die organische Welt betrachtet, Meteorol Z 1884, 1, (215-226). Meteorologische Zeitschrift. 20(3), 351-360.
Laboudi M. et al., 2011. DNA barcodes confirm the presence of a single member of the Anopheles maculipennis group in Morocco and Algeria: An. sicaulti is conspecific with An. labranchiae. Acta Tropica. 118(1), 6-13.
Lafri I. et al., 2014. An inventory of mosquitoes (Diptera: Culicidae) in Algeria. Bulletin de la Société Zoologique de France. 139(1-4), 255-261.
Lafri I. et al., 2017. Seroprevalence of West Nile virus antibodies in equids in the North-East of Algeria and detection of virus circulation in 2014. Comparative Immunology, Microbiology and Infectious Diseases. 50, 8-12.
Li R. et al., 2019. Climate-driven variation in mosquito density predicts the spatiotemporal dynamics of dengue. Proceedings of the National Academy of Sciences. 116(9), 3624-3629.
Manguin S. & Boëte C., 2011. Global impact of mosquito biodiversity, human vector-borne diseases and environmental change, The importance of biological interactions in the study of biodiversity. 27-50.
Moutailler S. et al., 2008. Potential vectors of Rift Valley fever virus in the Mediterranean region. Vector-borne and zoonotic Diseases. 8(6), 749-754.
Neeson T.M. & Mandelik Y., 2014. Pairwise measures of species co-occurrence for choosing indicator species and quantifying overlap. Ecological Indicators. 45, 721-727.
Pepin M. et al., 2010. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Veterinary Research. 41(6), 61.
Petersen L.R. , Beard C.B. & Visser S.N., 2019. Combatting the increasing threat of vector-borne disease in the United States with a national vector-borne disease prevention and control system. The American journal of tropical medicine and hygiene. 100(2), 242-245.
Porretta D. et al., 2016. Intra-instar larval cannibalism in Anopheles gambiae (ss.) and Anopheles stephensi (Diptera: Culicidae). Parasites & vectors. 9(1), 566.
Ramsdale C., 1983. Anophelism in the Algerian Sahara and some implications of the construction of a trans-Saharan highway. The Journal of tropical medicine and hygiene. 86(2), 51-58.
Reiter P., 2001. Climate change and mosquito-borne disease. Environmental Health Perspectives. 109(suppl 1), 141-161.
Rouabhi A. , Hafsi M. & Kebiche M., 2012. Assessment of the relationship between the typology and economic performance of farms: A case study for a rural area of province Setif, Algeria. Advances in Environmental Biology. 6(8), 2259-2268.
Rouabhi A. et al., 2016. Farming transitions under socioeconomic and climatic constraints in the southern part of Setif, Algeria. Journal of Agriculture and Environment for International Development (JAEID). 110(1), 139-153.
Samanidou-Voyadjoglou A. & Darsie Jr R.F., 1993. An annotated checklist and bibliography of the mosquitoes of Greece (Diptera: Culicidae). Mosquito systematics. 25(3), 177-185.
Schaffner F. , Medlock J. & Van Bortel, 2013. Public health significance of invasive mosquitoes in Europe. Clinical Microbiology and Infection. 19(8), 685-692.
Shannon C.E. & Weaver W., 1949. The mathematical theory of communication–University of Illinois Press. Urbana. 117.
Shililu J. et al., 2003. Distribution of Anopheline mosquitoes in Eritrea. The American journal of tropical medicine and hygiene. 69(3), 295-302.
Simpson E.H., 1949. Measurement of diversity. Nature. 163(4148), 688.
Sinka M.E. et al., 2010. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasites & vectors. 3(1), 117.
Snow R.W. et al., 2012. The changing limits and incidence of malaria in Africa: 1939–2009. Advances in Parasitology. 78, 169-262.
Tabbabi A. & Daaboub J., 2017. Mosquitoes (Diptera: Culicidae) in Tunisia, with Particular Attention to Proven and Potential Vectors: A Review. Journal of Tropical Diseases & Public Health. 5, 249.
Team R. 2018. RStudio: Integrated Development for R. RStudio, Inc., Boston, MA Version Version 2.1.1335
Trari B. et al., 2002. Les moustiques (Diptera Culicidae) du Maroc: Revue bibliographique (1916-2001) et inventaire des espèces. . Bulletin de la Société de Pathologie Exotique. 95(4), 329-334.
Vrijenhoek R., 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology. 3(5), 294-299.
Werblow A. et al., 2016. Direct PCR of indigenous and invasive mosquito species: a time- and cost-effective technique of mosquito barcoding. Medical and Veterinary Entomology. 30(1), 8-13.
WHO-Algeria, 2014. World Health Organization-Algeria. World Malaria Report 2014. Geneva: World Health Organization.
Wilke A.B.B. et al., 2017. Mosquito populations dynamics associated with climate variations. Acta Tropica. 166, 343-350.
Wright K. & Wright M.K., 2018. Package ‘corrgram’.
Zhao K. & Chu X., 2014. G-BLASTN: accelerating nucleotide alignment by graphics processors. Bioinformatics. 30(10), 1384-1391.