- Accueil
- volume 38 (2020)
- Numéro 2
- Microbiological evaluation of minimally processed and marketed fish in popular market of the city of Tepic Nayarit, Mexico
Visualisation(s): 20887 (1094 ULiège)
Téléchargement(s): 0 (0 ULiège)
Microbiological evaluation of minimally processed and marketed fish in popular market of the city of Tepic Nayarit, Mexico
Sanitary quality of tilapia (Oreochromis niloticus)

Notes de la rédaction
Received September 18, 2019 and accepted July 18, 2020
Abstract
Foodborne diseases are considered a public health challenge worldwide, due to their high morbidity and mortality. Fish is a highly nutritious food and consumed around the world; however, fish is also a perishable and an easy-to-contaminate food. The presence of pathogenic microorganisms in fish for human consumption is of high risk to public health. The objective of the present investigation was to carry out the microbiological evaluation of fresh Nile tilapia (Oreochromis niloticus) marketed in fillet presentation in the “Juan Escutia” market in Tepic Nayarit, Mexico. The samples collected consisted of fresh fillet from different fish shop in the market. The microbiological analysis was of Aerobic Mesophylls (AM), Mold and Yeasts, Total Coliforms (TC) and Fecal Coliforms (FC), Salmonella spp., and S. aureus. In 100% of the samples they presented AM accounts below what was established in the sanitary regulations, regarding mold the maximum value was 490 CFU / g and yeasts of 540 CFU / g, TC and FC were present in the 16.6% of the samples values above the microbiological limits established in sanitary regulations, while 33.3% of the samples were also outside the microbiological limit for Salmonella spp., and S. aureus. The study showed evidence of the potential health risk in the consumption of these foods sold in this market primarily if it is in raw state.
Table des matières
Introduction
1Foodborne Diseases (FD) are considered a syndrome caused by the ingestion of contaminated food or water with etiological agents in amounts that affect the health of the consumer. These diseases are characterized by the appearance of a variety of gastrointestinal symptoms, ranging from nausea, vomiting, diarrhea, abdominal pain and fever; and in some cases severe complications, such as sepsis, meningitis, abortions, Reiter's syndrome, Guillan Barré syndrome or death (51).
2Foodborne diseases are established as a major challenge in public health worldwide, due to their high morbidity and mortality. These diseases have, as main population target, groups affecting children, pregnant women and the elderly, leading to economic losses, high costs in health services, implementation and monitoring of food safety policies (2,42,47).
3Approximately 250 agents causing foodborne diseases have been described, including bacteria, viruses, fungi, parasites, prions, toxins, chemicals and metals (27,42). The most frequent FD are those of biological origin, having mainly as causative agents to bacteria: Salmonella spp., Campylobacter spp., Shigella spp., Vibrio spp., Yersinia spp., Escherichia coli, S. aureus, Aeromonas hydrofila, Bacillus cereus, Clostridium perfringens, Listeria monocytogenes, among others (27,47,51). In recent years, the increase in these diseases has as contributing factors the commercial globalization of food, urbanization, changes in the eating habits of society, which include the consumption of packaged foods, meals outside home, stands of prepared foods and fast food (42,47,52).
4The World Health Organization (WHO) estimates that, worldwide, the FD affect 1 in 10 people annually, and cause approximately 42,000 deaths, mainly in children under 5 years old (56).
5In Mexico, according to the National Epidemiological Surveillance System (SINAVE Spanish acronym), during the corresponding week 27 from June 30 to July 6, 2019, reported approximately 6,000,000 cases of intestinal infectious diseases (typhoid fever, paratyphoid, other salmonellosis, shigellosis, bacterial food poisoning, amebiasis, intestinal infections due to protozoa, giardiasis, helminths, ascaris, and intestinal infection by other microorganisms and poorly defined), where also the largest proportion of these diseases occurred in women (14).
6Fishing and aquaculture are important sources of food, nutrition, income and livelihoods for hundreds of millions of people worldwide; only in year 2014 the global supply of fish per inhabitant reached 20 kg (17). Fish is a highly commercialized and nutritious food because it is a source of protein, lipids (ω-3), vitamins and minerals (17,18,30). Fish is currently seen as a substitute for beef, pork and poultry, because consumers nowadays demand meat with a lower fat content, in order to comply with a healthy lifestyle (49). However, and regarding its nutritional properties, is a highly perishable food and susceptible to contamination (17,18,30). After the capture and death of the fish, it gradually undergoes deterioration processes due to autolysis, lipid degradation and microbial activity (18, 30).
7The fish can house various microorganisms derived from its initial load which will depend on the time of the year, species, food, physicochemical and biological characteristics of the environment from which it is captured and cultivated, as well as the capture system; or in post-harvest activities (storage and handling) it can be contaminated by modifying its microbiological quality (11, 18, 47).
8Among the pathogenic bacteria transmitted by fish are those indigenous (C. botulinum, Aeromonas hydrophila, Listeria monocytogenes, V. cholerae, V. parahaemolyticus) distributed in aquatic environments around the world where water temperatures have a selective effect and form part of the fish's natural microbiota; and non-indigenous bacteria (Salmonella spp., Shigella spp., E. coli, and S. aureus) present in fish as a result of fecal contamination of water where it is captured, cultivated or derived from poor handling practices becoming a potential carrier of diseases and a risk to the health of consumers (23,46,48,49).
9In Europe countries such as Spain, through the National Network of Epidemiological Surveillance (RENAVE Spanish acronym) and for the period 2008-2011, they reported a total of 2,342 outbreaks of FD with 30,219 cases, being the main causative agents, related to the outbreaks, bacteria such as Salmonella spp., Staphylococcus aureus, Bacillus cereus, E. coli, among others; further identifying that in 69.5% of the outbreaks the food involved was mainly eggs, egg products and mayonnaise (24.6%), seafood (7.4%), fish and fish products (6.5%) (16).
10Meanwhile in countries of South America such as Chile, the Ministry of Health reported 1,164 outbreaks of FD with 7,841 cases for 2013, where from the total outbreaks in 10 % of them it was possible to identify the causative agent, being Salmonella spp., the responsible for 54 % of them; likewise, it was found that the main foods involved corresponded to prepared meals and dishes (40 %), fish and fishery products (32%) and eggs and ovo-products (10%) (27).
11Food safety is defined as the guarantee of the foods to not cause any harm to the health of the consumer (27,52). Food safety is a fundamental attribute of quality and has an impact on society, involving consumers, government, food industry and academia, so around the world various technologies, hygiene practices, preventive and management systems have been implemented of safety in food production (27). Within hygiene practices in food production, health legislations around the world have established limits to the presence of pathogenic microorganisms or those responsible for deterioration, in order to guarantee the quality and safety of these foods and protect public health (18,47).
12The microbiological or sanitary quality of food can determine that these can cause infections and food poisoning, as well as alterations or deterioration that affect the natural characteristics of the food (8,21).
13Sanitary control in food production is essential to reduce risk factors in the transmission of foodborne illness and protect consumer health. Microbiological criteria in the food industry and regulatory agencies contribute to the guidelines in the control of food safety systems. Microbiological criteria can be used as: 1. Indicator microorganisms, which is defined as those microbial groups that share some morphological, physiological or ecological characteristics whose presence in large quantities correlates with poor hygienic quality and contamination of food, and 2. The presence of pathogenic microorganisms (19,21).
14Among the indicator microorganisms used to determine the sanitary quality of food in microbiological aspects are: aerobic mesophylls, osmophilic, psychotrophs, molds, yeasts, enterococci, total coliforms, fecal coliforms, pathogens such as Salmonella spp., S. aureus, Listeria monocytogenes, among others (7,8,19,21).
15Since there are no reports available of the microbiological analysis of fish and products marketed in the city of Tepic, Nayarit México and specifically of tilapia, in order to determine the potential health risks of this food in consumers. The objective of the present investigation is the microbiological evaluation of fresh Nile tilapia (Oreochromis niloticus) marketed in its fillet presentation in fish shops from the popular market “Juan Escutia”, in the city of Tepic Nayarit, Mexico.
Materials and methods
Study zone
16The study was conducted in the city of Tepic, capital of the state of Nayarit in Mexico, which is located in western Mexico (21º 30 59 and 104º 53 39) (5) (figure 1).
Sample collection
17The place to obtain the fish sample was the popular “Juan Escutia” market that is located in the downtown area of the capital city, Tepic. The sample consisted of fresh fish as Nile tilapia (Oreochromis niloticus), which was collected from 4 different fish shops inside the “Juan Escutia” market (A, B, C, D) for 4 weeks in the period between the months of February and March, 2019. Every week 3 samples were collected from a different fish shop, with a total of 12 samples for the study. Fresh fish prior to its collection was subjected to minimal processing through operations such as eviscerated and filleted by personnel from fish shop; the resulting fish fillets were then collected and transported in cold and aseptic conditions to the laboratory of the technological university of Nayarit, and following the methodology of the official Mexican standard (35) for subsequent preparation and microbiological analysis.
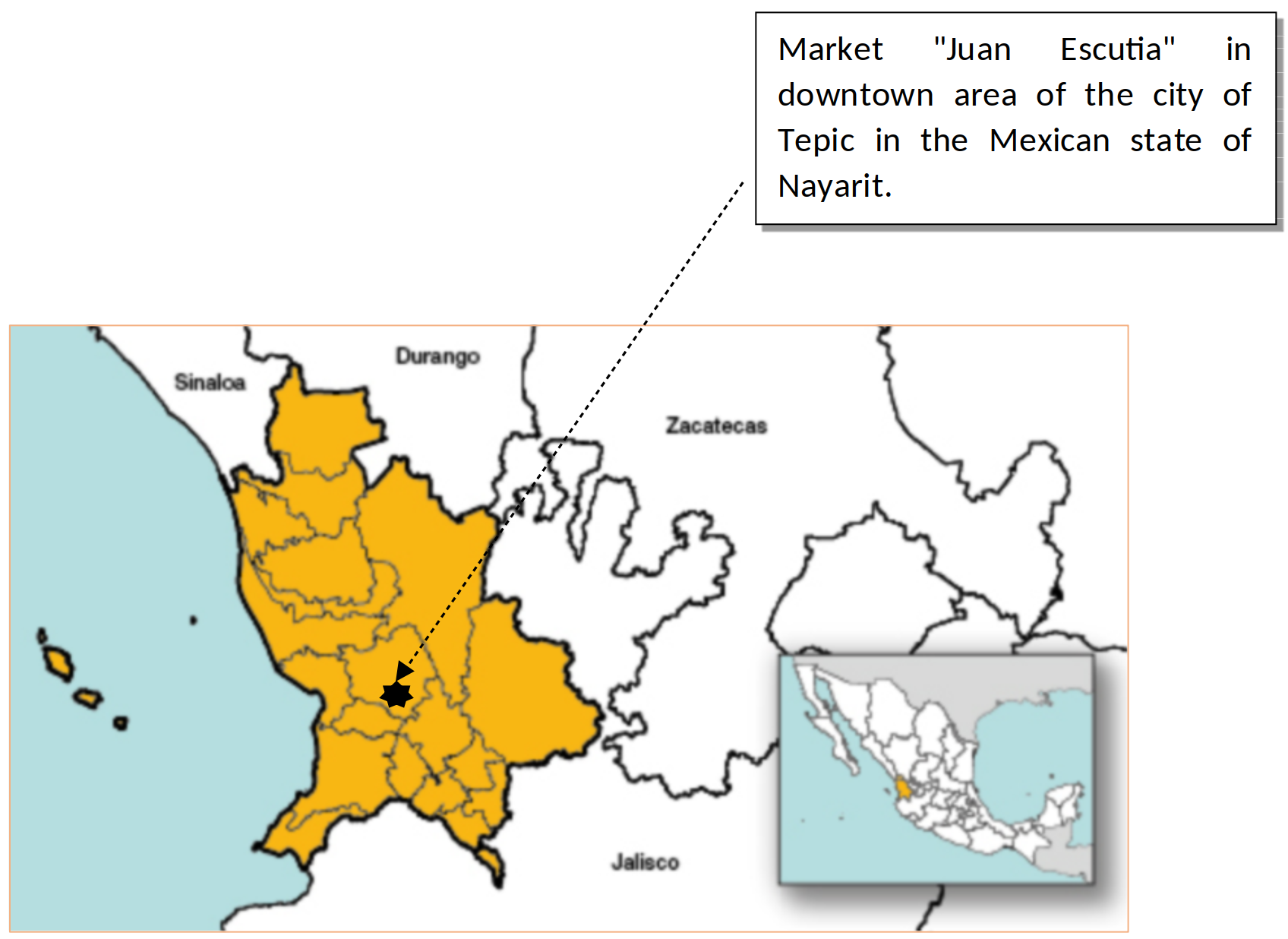
Figure 1: Geographical location of the city of Tepic, Nayarit in Mexico.
The geographical coordinates of the state of Nayarit are Longitude 106° 41'16.8"W at 103°43'15.6" W, Latitude 20°36'11.52 "N at 23°5'4.2" N (https://www.inegi.org.mx/app/areasgeograficas/?ag=18) (25). The city of Tepic is located in the central part of the state of Nayarit, at the extreme geographical coordinates 21° 51' and 21° 24' of latitude N and 104° 34' and 105° 05' of longitude W (5). The “Juan Escutia” market is located in the downtown area of the city of Tepic, the geographical coordinates are: 21° 30´47”N 104 ° 53´34” W (22)
Sample preparation
18Prior to the microbiological analysis, the sample was prepared and followed the methodology of the official Mexican standard (36), where 10g of the sample were weighed, liquefied and homogenized within 90 ml of diluent (Buffer phosphate saline or peptonated water) to generate a primary dilution and, subsequently, the additional decimal dilutions (10-1,10-2) were carried out for later microbiological analysis.
Microbiologic analysis
Aerobic mesophylls
19The analysis was carried out by duplicate, which consisted on placing 1 ml of sample in a Petri dish and subsequently emptying the standard account agar (DIBICO®), homogenized, solidified and then incubated the plates to 48 h and at 35 ± 2 °C; after this time the developed colonies were counted, the results were reported as CFU / ml or g of sample (29,34).
Molds and yeasts
20The analysis was carried out by duplicate, which consisted on placing 1 ml of sample in a Petri dish and subsequently emptying the potato dextrose agar (PDA) (BD Bioxon®) acidified with 10% tartaric acid, homogenized and solidified; after that, the plates were incubated for 5 days at 25 ± 1 °C, after this time the developed colonies were counted, and the results were reported as colony-forming unit CFU / ml or g of sample (37).
Total and fecal coliforms
21It consisted on presumptive tests (lauryl sulfate tryptose broth (DIBICO®)), and confirmatory (bright green bile lactose broth (DIFCO®)) through multiple lactose fermentation tubes (tube dilution) of the Most Probable Number (MPN), which provides a statistical estimate of the microbial density present based on the probability of obtaining tubes with positive growth that decreases as the inoculated sample volume is smaller. Coliform bacteria ferment lactose when incubated at 35 ± 1°C (total coliforms) and 44.5 °C (fecal coliforms) for 24 to 48 hours, resulting in the production of acid and gas (7,19,29,32,38).
Pathogen analysis
Staphylococcus aureus
22The analysis of presence of S. aureus in food was carried out, using the selective and differential culture medium (Baird-Parker) (DIBICO®), and confirmation by means of the biochemical tests of catalase, coagulase and thermonuclease (19,29,40).
231.1 ml of sample was deposited on the surface of the Baird-Parker agar plates and the inoculum was distributed on the surface of the agar. The plates were held in position until the inoculum was absorbed by the agar. The plates were inverted and incubated 48 h at 35 °C. Plaques that had typical colonies of Staphylococcus aureus were selected. Typical colonies of S. aureus were considered to be black, circular, bright, convex, smooth, 1 to 2 mm in diameter showing an opaque and light halo around the colony. Typical colonies were selected for testing catalase, coagulase and thermonuclease for confirmation (19,29,40).
Salmonella spp
For the analysis, different stages were used, such as pre-enrichment (lactose broth) (DIBICO®), selective enrichment (selenite cystine broth (DIBICO®), tetrathionate broth (DIBICO®) or Vassiliadis-Rappaport), isolation in selective and differential culture media (Bismuth Sulphite Agar (SB) Difco®), Xylose Lysine Deoxycholate Agar (XLD) (BD®), Salmonella and Shigella Agar (SS) (BD Bioxon®)), and biochemical identification (Triple Sugar Iron Agar (TSI), Lysine Iron Agar (LIA), Sulfide Indole Motility Medium (SIM), citrate (DIBICO®)). 25 g of the sample was aseptically weighed, homogenized and 225 ml of the sterile pre-enrichment medium (lactose broth) was added and homogenized for 1 minute. The homogenate was aseptically transferred to a sterile container or bottle, its pH was measured and, if necessary, pH was adjusted to 6.8 it was put for 60 minutes at room temperature.
24The bottle was then incubated for 24h at 35 °C. Once this time was over, 1 ml of the mixture was transferred to a tube with 10 ml of tetrathionate broth and to another with 10 ml of selenite cystine broth. The tubes were incubated from 18 to 24 h at 35 °C. After this time, the contents of the tubes were homogenized with culture broth and each was striated in Xylose Lysine Deoxycholate Agar (XLD), Bismuth Sulfite Agar (BS) and Salmonella-Shigella Agar (SS). The plates were incubated for 24h at 35 °C. After that, it was observed if there was growth of characteristic colonies to Salmonella spp., in the selective and differential agars. In XLD agar: the colonies are pink or red, can be transparent with or without black center and, in some cases, they may appear completely black. In BS agar: typical colonies can be brown, gray or black; with or without metallic brightness, and some strains produce green colonies without the formation of the dark halo. In SS agar: the colonies of Salmonella spp., are translucent colonies, occasionally opaque (some colonies give black center); typical Salmonella colonies were selected and inoculated in tubes with triple iron sugar agar (TSI) and lysine iron agar (LIA), incubated 24 h at 35 °C for biochemical identification. Characteristic reactions and growth to Salmonella spp., were observed, and complementary biochemical identification was performed. Results are reported as: presence or absence of Salmonella spp., in 25 g or ml of sample (29,39).
Analysis of data
25The data analysis was performed using the Microsoft Excel spreadsheet software for Windows version 15 (2013).
Results
Microbiological indicators: aerobic mesophylls, total and fecal coliforms
26Table 1 shows the results obtained in the count of Aerobic Mesophylls (AM), Total Coliforms (TC) and Fecal Coliforms (FC). Of the results obtained, the fish shop that obtained the highest counts was “A” with 79500 CFU / g followed by “B”, with 83500 CFU / g, “D” 47000 CFU / g and finally “C” with 13900 CFU / g.
Table 1: Count of Aerobic Mesophylls (AM), Total Coliforms (TC) and Fecal Coliforms (FC) in tilapia fillet (Oreochromis niloticus) sold in different fish shop of the “Juan Escutia” market in the city of Tepic Nayarit, Mexico.
|
Fish shop |
Sample |
AM CFU/g |
TC MPN/g |
FC MPN/g |
Microbiological Criteria CFU/g*+ |
Microbiological Criteria MPN/g* |
|
A |
1 |
57500 ± 7778 |
460 |
460 |
1 x 107 |
400 |
|
2 |
78000 ± 7071 |
120 |
120 |
|||
|
3 |
79500 ± 9192 |
460 |
460 |
|||
|
B |
1 |
83500 ± 6393 |
240 |
7 |
||
|
2 |
54000 ± 11313 |
21 |
4 |
|||
|
3 |
49000 ± 7071 |
150 |
15 |
|||
|
C |
1 |
13900 ± 3252 |
16 |
16 |
||
|
2 |
9500 ± 282 |
16 |
11 |
|||
|
3 |
10850 ± 636 |
64 |
64 |
|||
|
D |
1 |
38500 ± 7077 |
<3 |
<3 |
||
|
2 |
47000 ± 7071 |
9 |
<3 |
|||
|
3 |
46000 ± 2828 |
3 |
<3 |
TC: total coliforms. FC: fecal coliforms. CFU: Colony-Forming Unit. MPN: Most Probable Number. References:* (33,41). +(24).
27For the total coliforms (TC) and fecal coliforms (FC), all fresh fillet samples showed signs of contamination by these microorganisms, where only the counts in the fillet samples from the “A” fish shop exceeded the microbiological criteria of official Mexican standards with 460 MPN / g, not being suitable for human consumption due to the health risk, and even its presence may indicate the existence of enteric pathogens such as Salmonella spp., Low levels of fecal coliforms may indicate good post-capture and storage manipulation at temperatures that prevent the proliferation of these microorganisms. It should be noted that in fish the detection of a high proportion of FC indicates a contamination by wastewater or bad handling practices since their natural habitat is the gastrointestinal tract of humans and other mammals (30). In this region of Mexico it is common to consume this type of fish in the form of ceviche, which involves in its preparation the use of raw fish. Although ceviche is a typical dish in different regions of Latin America and that it’s prepared based on onion, spices and pH below 4 whose conditions will inhibit FC and different food pathogens, its presence has been reported being therefore a risk that the final consumer is running when acquiring a product with bad hygiene practices in its handling and conservation conditions (8).
Molds and yeasts
28Table 2 shows the count of fungi and yeasts in fresh tilapia fillet from different fish shop in the “Juan Escutia” market.
29The highest values for filamentous fungi were located in the samples from fish shop “D” with a maximum value of 490 CFU / g followed by 470 CFU / g in the samples from fish shop “C”. While in yeast, the highest values were those corresponding to samples from fish shop “A” with 540 CFU/g.
30Table 2. Count of molds and yeasts in tilapia fillet (Oreochromis niloticus) sold in different fish shop of the “Juan Escutia” market in the city of Tepic Nayarit, Mexico.
|
Fish shop |
Sample |
Molds CFU/g |
Yeasts CFU/g |
|
A |
1 |
10±1 |
170±3 |
|
2 |
60±1 |
540±2 |
|
|
3 |
80±1 |
330±1 |
|
|
B |
1 |
60±1 |
85±1 |
|
2 |
45±1 |
60±1 |
|
|
3 |
65±1 |
10±1 |
|
|
C |
1 |
470±1 |
20±1 |
|
2 |
390±1 |
50±1 |
|
|
3 |
170±1 |
10±1 |
|
|
D |
1 |
280±1 |
25±1 |
|
2 |
490±1 |
10±1 |
|
|
3 |
430±1 |
10±1 |
CFU: Colony-Forming Unit.
Pathogens: Salmonella spp., and S. aureus.
31Table 3 shows the result of the qualitative analysis of Salmonella spp., and the quantitative analysis of Staphylococcus aureus in tilapia fillets (Oreochromis niloticus) from several fish shop of the “Juan Escutia” market in the city of Tepic, Nayarit.
32For Salmonella spp., only the fish shop “C” of the 4 under study the presence of Salmonella spp., was negative, while in 66.6% of samples from fish shop “A” and “D” and 33.6% in samples from fish shop “B” were positive for Salmonella spp., and therefore outside the limits or microbiological criteria of different regulations for fish and meat products around the world, such as the official Mexican standards (NOM-027 and NOM-242), the commission regulation (EC) No 2073/2005 (10) and the Resolution RDC No. 12 of the National Health Surveillance Agency of Brazil (ANVISA portuguese acronym) (1) placing consumers at risk of salmonellosis.
Table 3: Analysis of Salmonella spp., and Staphylococcus aureus in tilapia fillet (Oreochromis niloticus) marketed in different fish shop of the “Juan Escutia” market in the city of Tepic, Nayarit.
|
Fish shop |
Sample |
(Ausence / presence) of Salmonella spp., in 25g |
S. aureus CFU/g° |
Microbiological criteria for Salmonella spp.*+” |
Microbiological criteria for S. aureus CFU/g*” |
|
A |
1 |
Absence |
Absence |
Absence in 25g of sample |
1 x 103 |
|
2 |
Presence |
Absence |
|||
|
3 |
Presence |
Abscense |
|||
|
B |
1 |
Absence |
Absence |
||
|
2 |
Presence |
Absence |
|||
|
3 |
Absence |
25000 |
|||
|
C |
1 |
Absence |
5200 |
||
|
2 |
Absence |
8500 |
|||
|
3 |
Absence |
Absence |
|||
|
D |
1 |
Absence |
Absence |
||
|
2 |
Presence |
25000 |
|||
|
3 |
Presence |
Absence |
References: * (33,41). + (10). ”(1). °102 CFU / g (sensitivity method). CFU: Colony-Forming Unit.
33On the other hand, the analysis of Staphylococcus aureus in tilapia fillets was not detected in samples from fish shop “A”, while for fish shop “B” it was detected in 33.33% of samples, for fish shop “C” (66.66%) and fish shop “D” (33.33%) with a maximum level of 25,000 CFU / g, exceeding the microbiological criteria of sanitary regulations for these products and placing them as a potential health risk for consumers.
Discussion
Microbiological indicators: aerobic mesophylls, total and fecal coliforms
34The results obtained from the analysis of aerobic mesophylls in the fresh fish fillets of the different commercial premises (table 1) demonstrate that these are below the microbiological criteria allowed in the official health standard in Mexico, of the microbiological criteria established by the American Public Health Association (APHA) of 100,000 CFU / g and microbiological limits established by the International Commission on Microbiological Specifications for Food (ICMSF) (1000000 to 10000000 CFU/g) (30)
35Differences in AM value can be related to fish handling and storage conditions. The AM as microbiological indicators in food indicate that a high proportion is due to inadequate storage and handling in the sales establishment, where in many occasions these commercial places do not present good sanitary conditions for the sale of this product (7,30).
36For the results obtained in the analysis of total coliforms (TC) and fecal coliforms (FC) (table 1), all the samples of fresh fillet showed signs of contamination by these microorganisms, where only the counts in the fillet samples from room “A” exceeded the microbiological criteria of the official Mexican sanitary standard with 460 MPN / g, thus this food not being suitable for human consumption due to the health risk, where even its presence may indicate the presence of various enteric pathogens such as Salmonella spp. Low levels of fecal coliforms may indicate good post-capture handling and storage at temperatures that prevent the proliferation of these microorganisms, it should be noted that the detection of a high proportion of FC in fish is an indication of contamination by wastewater or null or poor hygiene in handling, since the natural habitat of these microorganisms is the gastrointestinal tract of humans and other mammals. (30). In this region of Mexico it is common to consume this type of fish in the form of ceviche, which involves raw fish in its preparation as the main ingredient. Although ceviche is typical food in different regions of Latin America and is prepared from onions and spices with a pH lower than 4, whose conditions can inhibit FC and different food pathogens. However, it has been reported that the detection of FC is a potential risk to the health of consumers when acquiring a food subject to poor hygiene practices under conditions of handling and conservation (8).
Molds and yeasts
37Fungal microorganisms are generally aerobic, from nature cosmopolitan, they are found as part of the normal microbiota of food or as contamination. They can intervene in food alteration processes leading to deterioration and putrefaction due to the metabolism of carbohydrates, proteins and lipids, leading to deterioration of nutritional quality, negative sensory characteristics (bad odor, alteration of taste and color) on the surface of contaminated products, in addition to allowing the growth of pathogenic bacteria (7,9,44).
38The presence of fungi and yeasts in food can be an indicator of contamination derived from procedures in inadequate hygienic and sanitary conditions (29). The sanitary regulation of Mexico does not have microbiological limits or criteria for fungi and yeasts in fresh fish and products. The importance of the presence of fungal organisms in fresh fish subjected to refrigeration and freezing conditions is that they can intervene in deterioration processes affecting their quality (9,15). Centeno and Rodriguez (9) reported for frozen fish filamentous fungus and yeast counts of 1.9 x 103 CFU / g in samples of fish (Scomberomorus spp.) and 2.0 x 102 CFU / g in samples of Merluza (Merluccius spp.), where the main fungal isolates were Geotrichum candidum, Aspergillus niger, Penicillium expansum and yeasts such as Rhodotorula spp., microorganisms resistant to adverse environmental conditions and where their presence is related to the contamination of these products during processing, compromising their shelf life and safety for consumption.
Pathogens: Salmonella spp., and S. aureus.
39Salmonella spp., is not part of the native microbiota of fish. The presence of Salmonella spp. In fish has been related to contamination with fecal matter from natural waters or from the aquatic environments from which it comes, in addition these microorganisms can survive for long periods of time, and their presence may also be due to the application of poor hygiene practices for fish farming and post-harvest, such as transport, processing, marketing and conservation activities. (23,30,49). The detection of Salmonella spp., For sanitary regulation in food and public health is of importance because it is one of the different causal agents frequently related to outbreaks of foodborne illness. (2,16,23,30,49).
40On the other hand, the analysis of Staphylococcus aureus in tilapia fillets was not detected in 100% of samples from fish shop “A”, while for fish shop “B” it was detected in 33.33% of samples, for fish shop “C” in 66.66% of samples and local “D” in 33.33% of samples with a maximum level of 25,000 CFU / g, exceeding the microbiological criterion of sanitary regulation for these products and placing them as a potential health risk for consumers (table 3). Staphylococcus aureus is responsible for outbreaks of foodborne illness called food poisoning, derived from the consumption of food contaminated by this microorganism where it has multiplied and produced enterotoxins (7,45). This microorganism is not part of the fish microbiota, its main reservoir is the be human (skin and mucosa); thus, its presence in this food is indicative of post-capture contamination, derived from handling by carriers and the use of contaminated materials and equipment (30,45).
41In related studies on the sanitary quality of these foods, Junior et al.,(28) they reported that the analysis of coliforms, Staphylococcus sp., and Salmonella spp., in samples of tilapia fillet from 10 supermarkets located in the northwestern region of the state of São Paulo, in Brazil, presented a proportion of thermotolerant coliforms of between <3-100 MNP / g, Staphylococcus sp., <1.0 × 102 to 9.4 × 103 CFU / g and absence of Salmonella spp., the author noted that qualitative and quantitative results are indicators of inadequate hygiene practices in handling, preserving and fish processing which must be supervised for the production and marketing of this type of food in order to reduce the risks to public health. Ramirez et al.,(46) they performed a microbiological analysis of red tilapia (Oreochromis sp.) Commercialized in the Municipality of El Colegio, Cundinamarca in Colombia, reporting the absence of Salmonella spp., in 100% of samples, presence in 20% of samples of Staphylococcus coagulase positive ( 9 x 105 CFU / 25g) and various members of the Enterobacteriaceae family mostly related to the water from which the fish comes, such as K. oxytoca, K. ozaenae, E. tarda, P. mirabilis and V. metschnikovii concluding the need to implement periodic controls on good manufacturing practices and personnel hygiene that contribute to reducing the degree of contamination in fresh fish shops and reducing the risk to public health. Teka et al., (53) they carried out a microbiological analysis of raw frozen tilapia fillets (O. niloticus) and undercooked in a hotel zone in Ethiopia, as well as an analysis of the implementation of food safety practices for handlers. The author reported that 25% of the total samples had E. coli, 3.75% Salmonella spp., and 41.25% S. aureus; furthermore, a considerable percentage of handlers did not implement 100% basic hygiene practices in food handling and preparation. Therefore, the researcher suggests a strengthening of training in hygienic practices for food handlers, in addition to the fact that public health authorities in the state must guarantee adequate supervision and surveillance of the handling and sale of fish, especially of ready-to-eat products to in order to reduce risks to public health.
42And finally, Vazquez et al., (54) they carried out the microbiological evaluation (E. coli, Salmonella spp., and S. aureus) of fish and shellfish sold in four markets in the city of Huánuco, Peru. The author reported the absence of Salmonella spp., And the presence of E. coli and S. aureus in all samples collected in all markets in a total average proportion of 2,063,265 and 2,631,020 CFU / g respectively, exceeding levels of the technical food standard, considering it a risk to the health of the consumer, also indicates that a percentage greater than 50% of food handlers in their marketing do not know the basic rules and regulations of hygiene which are a determining factor in food safety.
43The diversity of the microorganisms present in the fish will depend on various factors ranging from the type of fish, capture habitat, water quality, among others (9.28,48). For live tilapia the microbiota will depend on the existing one in the waters where it lives and presents a variety according to the species, temperature, depth, and degree of contamination of the waters (28,31). Among the microorganisms reported present are 1.- Gram negatives that include Pseudomonas, Shewanella, Moraxella, Acinetobacter, Flavobacterium, Aeromonadaceae, and Enterobacteria (6,31). In the case of members of the Enterobacteriaceae family, their presence is typical in the intestine of animals, they cause serious pathologies and can contaminate food, especially tilapia meat; these microorganisms can be transmitted to humans through: consumption, handling of contaminated meat and traumatic wounds that frequently occur when in contact with the eviscerations of the slaughtered animal. The most frequent microorganisms in tilapia that can be transmitted to humans are: Escherichia coli, Klebsiella oxytoca, Citrobacter spp., Salmonella spp., Proteus spp., Vibrio spp., Enterobacter spp. Shigella spp., among others (6,28). And 2.- Gram positives in variable proportion such as: Bacillus, Micrococcus, Clostridium, Lactobacillus, Corynebacterium, among others. Pathogenic or contamination-indicating bacteria are rarely found in fresh fish, unless it comes from water excessively contaminated with fecal matter and / or poor hygiene practices in handling (31,49). Therefore, with the microbial presence and if the inadequate treatment of the food exists and the time to be subjected to preservation processes is prolonged, the product represents a health risk (9).
44Tilapia is considered a fish of good acceptance as food and an attractive option in its consumption nationally and internationally, so its production is increasing, being its typical commercialization in its fresh form and fillets (26,31). Fish is considered an important food from a nutritional, economic and public health perspective, the latter for its association with foodborne illness (3,12,50).
45Food safety is the guarantee that they will not generate illnesses for the consumer (20). Food safety is an important public health issue at the global level, in addition to being one of the basic characteristics that together with nutritional, sensory and commercial characteristics constitute the total quality of food (13,20). In order to generate the control and prevention of hazards in fish that lead to diseases transmitted by its consumption, this control must cover all phases of the food chain (from primary production to the consumer's table) (27,28,43,49).
46In order to reduce and control the hazards to fish safety throughout the food chain, the implementation of different risk reduction tools is involved, such as good practices in aquaculture, fishing, and manufacturing hygiene, as well as Sanitation Standard Operating Procedures (SSOP) which are the basis for the implementation of food safety assurance systems such as the Hazard Analysis and Critical Control Points (HACCP) system, the latter is widely requested to guarantee food safety, and which is currently part of sanitary regulations, food safety standards and certifications (Safe Quality Food SQF, British Retail Consortium BRC, ISO 22000, Food Safety System Certification FSSC 22000, GLOBAL GAP among others) for the production, acceptance and marketing global of food (4,20,27,28,43,49,55). Lastly, constant training in food hygiene for end handlers and consumers is required, particularly in that this type of food must not be consumed in the raw state, it must be subjected to heat treatment and maintained at adequate temperatures prior to consumption, in the preparation avoid contact of raw and cooked food (cross contamination), as well as the use of good quality water and harmless raw materials in its production, in order to avoid contamination of this product and generate a potential risk to consumer health (27,28,43).
Conclusions
47The microbiological analysis shows evidence that in the samples of freshly processed fish (O. niloticus) minimally processed in its fillet presentation sold in the “Juan Escutia” market in the city of Tepic, Nayarit, microbial growth was presented.
48The microbiological indicators (aerobic mesophylls) in the samples of fresh fish (O. niloticus) in their fillet presentation were within the limits or microbiological criteria in the official Mexican standards for this food product.
49Microbiological indicators (total and fecal coliforms) were presented in 16.6% of freshly fish samples (O. niloticus) minimally processed in their fillet presentation, being those above the limits or microbiological criteria established in the official Mexican standards for this product food.
50In the analysis of pathogens such as Salmonella spp., and S. aureus, 33.3% of the samples of fresh fish (O. niloticus) minimally processed in their fillet presentation were outside the limits or microbiological criteria for these microorganisms established in the standards Mexican and international officers for this food product.
51Most of the tilapia (O. niloticus) minimally processed in its fillet presentation marketed in the “Juan Escutia” market fish shop in the city of Tepic Nayarit, Mexico, met the requirements or microbiological criteria established by the sanitary regulations for fish.
52Fish is considered a nutritious food and is widely consumed around the world. However, it is also very perishable and of easy microbiological contamination, being related to outbreaks of foodborne illnesses, so it is essential to consider the control and prevention of these diseases through fish, in addition to monitoring the sanitary quality will be not to consume this product raw, maintain hygienic conditions in handling, processing and preservation, including cooking time and temperature controls until its previous consumption in order to guarantee its safety.
53As a recommendation derived from this study in order to reduce the presence of contamination by pathogens and microbiological indicators outside the microbiological specification of the corresponding food legislation for this food marketed in fish shops; it is suggested the permanent implementation of training programs in good hygiene practices for production, processing, conservation, distribution and sale, which is aimed at producers, processors, marketers and general consumers. It is also necessary to install a system of Regular surveillance through sanitary inspections and the microbiological analysis of the product by the corresponding sanitary authorities in order to ensure the sanitary quality of this type of commercialized food and prevent foodborne illnesses to the population that is supplied in the commercial zone.
Acknowledgments
54To the Technological University of Nayarit in its division of engineering in bio-food technologies for providing of facilities, equipment, chemical and biological materials for the realization of the microbiological analysis.
Conflict of interest
55The authors declare no conflict of interest in the development and publication of this manuscript.
References
-
Agência Nacional de Vigilância Sanitária (ANVISA). 2001. Nº 12, de 02 de Janeiro de 2001. Dispõe sobre os principios gerais para o estabelecimento de critérios e padrões microbiológicos para alimentos. Brasil. http://portal.anvisa.gov.br/documents/33880/2568070/RDC_12_2001.pdf/15ffddf6-3767-4527-bfac-740a0400829b
-
Alerte, V., Cortés, A. S., Díaz, T. J., Vollaire, Z. J., Espinoza, M. M. E., Solari G, V., Cerda, L. J., & Torres, H. M. 2012, Foodborne disease outbreaks around the urban Chilean areas from 2005 to 2010. Revista chilena de infectología, 29(1), 26-31. https://dx.doi.org/10.4067/S0716-10182012000100004
-
Arias Echandi, M. L., & Chaves Ulate, E. C. 2012, Microbiological quality of raw material and final product of tilapia and shrimp ceviche expended in the Metropolitan Area of San José, Costa Rica. Cuadernos de Investigación, 4(1), 85-92.
-
Badui Dergal S. 2015, Inocuidad en la industria alimentaria. Industria alimentaria.37(3):14-25.
-
Bueno Durán, A. Y., Barcelos García, R. G., Ventura Ramón, G. H., Toledo Ibarra, G. A., Navidad Murrieta, M. S., Zambrano Soria, M., Robles Pérez, A. G., Girón Pérez, M. I. 2019, Coliforms bacteria, fungi and aflatoxins detection in medicinal herbs marketed in Nayarit, Mexico. Revista Bio Ciencias 6(2), e636. doi: https://doi.org/10.15741/revbio.06.02.04
-
Cagua Montaño L., Rodas Pazmiño K., Rodas Pazmiño J., Pazmiño Gómez, B., Rodas Neira E., González Quinde G., Rodas Pazmiño A. 2017, Gram negative bacteria study on tilapia sold in the canton Milagro, October-November 2013. Cumbres, 3(2), 9-16. http://investigacion.utmachala.edu.ec/revistas/index.php/Cumbres
-
Campuzano, S., Flórez, D. M., Ibarra, C. M., & Sánchez, P. P. 2015, Determination of microbiological and sanitary quality of prepared food sold in the street of the city of Bogota, DC. Nova, 13(23), 81-92. http://hemeroteca.unad.edu.co/index.php/nova/article/view/1708/1961
-
Carbajal Mendoza, María T., Rabelo Salva, P., Sebastián Gonzales, C., & Ayala Galdós, María E. 2003, Evaluación microbiológica de productos adquiridos en el mercado mayorista pesquero de ventanilla - Perú. Revista Cubana de Salud Pública, 29(2), 121-123. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-34662003000200005&lng=es&tlng=es.
-
Centeno, S., & Rodríguez, R. 2005, Microbiological Evaluation of Frozen Fish Produced in Cumana, Sucre State, Venezuela. Revista Científica, XV (2), 168-175.
-
Commission regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Official Journal of the European Union. https://eur-lex.europa.eu/legal-content/ES/TXT/?uri=celex:32005R2073
-
Corrales Ramírez, L. C., Alvarado Ospina, M. A., Castillo Fonseca, L. A., Camacho Beltran, Y. C. 2011, Bacteriological quality of fresh fish, Catfish (Pseudoplatystoma sp.) and Red Tilapia (Oreochromis sp.), as marketed in the municipality of El Colegio, Cundinamarca (Colombia). Nova, 9(15).149-157.
-
Cortés Sánchez, A. 2020. Food, Fish and Diseases Transmitted to the Consumer. Journal of Advanced Laboratory Research in Biology, 11(2), 16-23.
-
De la Fuente Salcido, N. M. & Barboza Corona, J. E. 2010. Inocuidad y bioconservación de alimentos. Acta Universitaria, 20(1),43-52. https://www.redalyc.org/articulo.oa?id=416/41613084005
-
DGE, 2019, Boletín Epidemiológico Sistema Nacional de Vigilancia Epidemiológica Sistema Único de Información. Número 27, Volumen 36, Semana 27, Del 30 de junio al 6 julio del 2019. Dirección General de Epidemiología. Secretaria de salud. México. https://www.gob.mx/cms/uploads/attachment/file/476788/sem27.pdf
-
El-Shemy, M. G. Y., Yasin, N., Gadallah, M. G. E., & Hanafi, E. K. 2016, Microbiological Quality and Enzymes Activity of Refrigerated Bolti Fish (Tilapia nilotica) Pretreated with Organic Acids. Journal of Agricultural and Veterinary Sciences, 267(3732), 1-14.
-
Espinosa, L., Varela, C., Martínez, E. V., & Cano, R. 2015, Brotes de enfermedades transmitidas por alimentos. España, 2008-2011 (excluye brotes hídricos). Boletín epidemiológico semanal, 22(11), 130-136.
-
FAO 2016, El estado mundial de la pesca y la acuicultura 2016. Contribución a la seguridad alimentaria y la nutrición para todos. Roma. 224 pp. http://www.fao.org/3/a-i5555s.pdf
-
Farias, Maria do Carmo A., & Freitas, José de A. 2008, Qualidade microbiológica de pescado beneficiado em indústrias paraenses. Revista do Instituto Adolfo Lutz (Impresso), 67(2), 113-117. http://periodicos.ses.sp.bvs.br/scielo.php?script=sci_arttext&pid=S0073-98552008000200005&lng=pt&tlng=pt.
-
Félix Fuentes, A., Campas Baypoli, O. N., & Meza Montenegro, M. 2005, Calidad sanitaria de alimentos disponibles al público de Ciudad Obregón, Sonora, México. RESPYN Revista de Salud Pública y Nutrición, 6(3). http://respyn.uanl.mx/index.php/respyn/article/view/149/131
-
Fuertes Vicente H.G., Paredes López F., Saavedra Gálvez D. I. 2014, Good practice manufacturing and preservation on board: fish safe. Revista big ban faustiniano.3(4):40-45. http://revistas.unjfsc.edu.pe/index.php/BIGBANG/article/view/234/233
-
Hernández Urzua, M. A. 2016, Microbiología de los alimentos: fundamentos y aplicaciones en ciencias de la salud. México, D.F. Editorial Medica Panamericana. 232p. ISBN 978-607-9356-84-2.
-
Https://www.google.com/intl/es/earth/
-
Huss, H.H. 1997, Aseguramiento de la calidad de los productos pesqueros. FAO Documento Técnico de Pesca. No. 334. Roma, Food and Agriculture Organization of the United Nations (FAO). 1997. 174p. http://www.fao.org/3/t1768s/T1768S00.htm#TOC
-
ICMSF (International Commission on Microbial Specifications for Foods) 1986, Microorganisms in Foods. 2. Sampling for microbiological analysis: Principles and specific aplications. 2nd ed. Blackwell Scientific Publications.
-
INEGI 2019, Instituto Nacional de Estadística, Geografía e Informática (INEGI). México en Cifras. Nayarit. https://www.inegi.org.mx/app/areasgeograficas/?ag=18
-
Jácome J., C. Quezada Abad, O. Sánchez Romero, J.E. Pérez & M. N. 2019, Tilapia in Ecuador: paradox between aquaculture production and the protection of Ecuadorian biodiversity. Revista peruana de biología 26(4): 543 – 550. Doi: http://dx.doi.org/10.15381/rpb.v26i4.16343
-
Jorquera, D., Galarce, N., & Borie, C. 2015, The challenge of controlling foodborne diseases: bacteriophages as a new biotechnological tool. Revista chilena de infectología, 32(6), 678-688.
-
Junior, P. G., Assunção, A. W., Baldin, J. C., & Amaral, L. A. 2014, Microbiological quality of whole and filleted shelf-tilapia. Aquaculture, 433, 196-200. https://doi.org/10.1016/j.aquaculture.2014.06.015
-
Machado Pinto-e-Silva M. E., Batista Campos von Atzingen M. C., Mascaretti Dias G. 2015, Microbiological quality of home-made fish hydrolysate. Vigilância Sanitária em Debate: Sociedade, Ciência & Tecnologia, 3(1), 43-47. DOI: 10.3395/2317-269x.00273
-
Montiel, N. J. M., Romero, M. D. F., Leal, K. J. V., & Colina, A. E. S. 2005, Bacteriological and organoleptic evaluation of two fish species from the Maracaibo lake, Venezuela. Veterinaria Trop. 29-30 (1 y 2): 61-82. 2004-2005.
-
Morales, G., Blanco, L., Arias, M. L., & Chaves, C. 2004, Evaluación de la calidad bacteriológica de tilapia fresca (Oreochromis niloticus) proveniente de la Zona Norte de Costa Rica. Archivos Latinoamericanos de Nutrición, 54(4), 433-437. http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0004-06222004000400010&lng=es&tlng=es.
-
NMX-F-308-1992. Alimentos - Cuenta de organismos coliformes fecales. Foods - fecals coliform organisms count. Normas mexicanas. Dirección general de normas. https://www.colpos.mx/bancodenormas/nmexicanas/NMX-F-308-1992.PDF
-
NOM-027-SSA1-1993. Bienes y servicios. productos de la pesca. pescados frescos-refrigerados y congelados. especificaciones sanitarias. http://www.salud.gob.mx/unidades/cdi/nom/027ssa13.html
-
NOM-092-SSA1-1994. Bienes y servicios. Método para la cuenta de bacterias aerobias en placa. Norma oficial mexicana. http://www.salud.gob.mx/unidades/cdi/nom/092ssa14.html
-
NOM-109-SSA1-1994. Procedimientos para la Toma, Manejo y Transporte de Muestras de Alimentos para su Análisis Microbiológico. Proyecto de norma oficial mexicana. http://www.economia-noms.gob.mx/normas/noms/1994/p109ss194.pdf
-
NOM-110-SSA1-1994. Bienes y servicios. Preparación y dilución de muestras de alimentos para su análisis microbiológico. Norma oficial mexicana. http://www.salud.gob.mx/unidades/cdi/nom/110ssa14.html
-
NOM-111-SSA1-1994. Bienes y servicios. Método para la cuenta de mohos y levaduras en alimentos. Norma oficial mexicana. http://www.salud.gob.mx/unidades/cdi/nom/111ssa14.html
-
NOM-112-SSA1-1994. Bienes y servicios. Determinación de bacterias coliformes. Técnica del número más probable. Norma oficial mexicana. http://www.salud.gob.mx/unidades/cdi/nom/112ssa14.html
-
NOM-114-SSA1-1994. Bienes y servicios. Método para la determinación de Salmonella en alimentos. Norma oficial mexicana. http://www.salud.gob.mx/unidades/cdi/nom/114ssa14.html
-
NOM-115-SSA1-1994. Bienes y servicios. Método para la determinación de Staphylococcus aureus en alimentos. Norma oficial mexicana. http://www.salud.gob.mx/unidades/cdi/nom/115ssa14.html
-
NOM-242-SSA1-2009. Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba. Norma Oficial Mexicana. http://dof.gob.mx/nota_detalle.php?codigo=5177531&fecha=10/02/2011
-
Olea, A., Díaz, J., Fuentes, R., Vaquero, A., & García, M. 2012, Foodborne disease outbreaks surveillance in Chile. Revista chilena de infectología, 29(5), 504-510. https://dx.doi.org/10.4067/S0716-10182012000600004
-
OMS 2007, Manual sobre las cinco claves para la inocuidad de los alimentos. Organización Mundial de la Salud. World Health Organization. https://www.who.int/foodsafety/publications/consumer/manual_keys_es.pdf?ua=1
-
Orberá Ratón, T M. 2004, Acción perjudicial de las levaduras sobre los alimentos. Revista Cubana de Salud Pública, 30(3) http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-34662004000300016&lng=es&tlng=es.
-
Puig Peña, Y., Espino Hernández, M., Leyva Castillo, V., Apórtela López, N., Pérez Muñoz, Y., & Soto Rodríguez, P. 2015, Resistencia antimicrobiana en cepas de estafilococos coagulasa positiva aisladas en alimentos y manipuladores. Revista Cubana de Alimentación y Nutrición, 25(2), 245-260. https://www.medigraphic.com/pdfs/revcubalnut/can-2015/can152c.pdf
-
Ramírez, L. C. C., Ospina, M. A. Á., Fonseca, L. A. C., & Beltran, Y. C. C. 2011, Estudio bacteriológico de la calidad del pescado fresco, Bagre (Pseudoplatystoma sp.) y Mojarra Roja (Oreochromis sp.) comercializado en el municipio de El Colegio, Cundinamarca (Colombia). Nova, 9(16).149-157.
-
Rodríguez Torrens, H., & Barreto Argilagos, G., & Sedrés Cabrera, M., & Bertot Valdés, J., & Martínez Sáez, S., & Guevara Viera, G. 2015, The foodborne diseases, a health problem inherited and increased in the new millennium. REDVET. Revista Electrónica de Veterinaria, 16 (8), 1-27.
-
Romero Jarero, J. M., & Negrete Redondo, M. D. P. 2011, Presence of Gram negative bacteria in fish muscle of commercial importance in the Mexican Caribbean zone. Revista mexicana de biodiversidad, 82(2), 599-606. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532011000200019&lng=es&tlng=es.
-
Semedo Fernandes, Dandara Virginia G., Castro Silva, V., Cunha Neto, Adelino da., & Figueiredo, Eduardo Eustáquio de S. 2018, Salmonella spp. in the fish production chain: a review. Ciência Rural, 48(8), e20180141. https://dx.doi.org/10.1590/0103-8478cr20180141
-
Soares, K. M. D. P., & Gonçalves, A. A. 2012, Qualidade e segurança do pescado. Revista do Instituto Adolfo Lutz (Impresso), 71(1), 1-10.
-
Soto Varela, Z., Pérez Lavalle, L., & Estrada Alvarado, D. 2016, Bacteria causing of foodborne diseases: an overview at Colombia. Revista Salud Uninorte, 32(1), 105-122.
-
Tafur Garzón, M. 2009, La inocuidad de alimentos y el comercio internacional. Revista Colombiana de Ciencias Pecuarias, 22(3), 330-338. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-06902009000300009&lng=en&tlng=en.
-
Teka, W., Nölkes, D., Getachew, Y., & Mulachew, M. 2017, Microbiological quality of frozen raw and undercooked Nile tilapia (Oreochromis niloticus) fillets and food safety practices of fish handlers in Arba Minch town, SNNPR, Ethiopia. Journal of Veterinary Medicine and Animal Health, 9, 55-62.
-
Vásquez Ampuero J.M., Tasayco Alcántara W.R., Chuquiyauri Talenas M.Á., Apac Sotil S., 2018, Evaluación microbiológica de pescados y mariscos expendidos en mercados de la ciudad de Huánuco. Investigación Valdizana, 12(2), 75-82.
-
WHO 2007. Manual sobre las cinco claves para la inocuidad de los alimentos. World Health Organization (WHO). https://www.who.int/foodsafety/publications/consumer/manual_keys_es.pdf
-
WHO 2019. Food safety. Infographics: Estimates of the global burden of foodborne diseases. World Health Organization (WHO). https://www.who.int/foodsafety/areas_work/foodborne-diseases/ferg_infographics/en/
Pour citer cet article
A propos de : Ana Guadalupe Lerma-Fierro
Engineering student. Universidad Tecnológica de Nayarit
A propos de : María Karla Flores-López
M. Sc. Professor. Universidad Tecnológica de Nayarit
A propos de : Martha Lorena Guzmán-Robles
M.Sc. Professor. Universidad Tecnológica de Nayarit. 1Universidad Tecnológica de Nayarit. Carretera Federal 200 Km 9. C.P. 63780. Xalisco, Nayarit, México.
A propos de : Alejandro De Jesús Cortés-Sánchez
Ph. D. Professor. Consejo Nacional de Ciencia y Tecnología (CONACYT). Unidad Nayarit del Centro de Investigaciones Biológicas del Noroeste (UNCIBNOR+). Calle Dos No. 23. Cd. del Conocimiento. Av. Emilio M. González. Cd. Industrial. C.P. 63173. Tepic, Nayarit, México: alecortes_1@hotmail.com