- Accueil
- volume 38 (2020)
- Numéro 2
- Viability and germination capacities of Saba senegalensis (A. DC.) Pichon seeds, a multi-purpose agroforestry species in West Africa
Visualisation(s): 2672 (70 ULiège)
Téléchargement(s): 0 (0 ULiège)
Viability and germination capacities of Saba senegalensis (A. DC.) Pichon seeds, a multi-purpose agroforestry species in West Africa

Notes de la rédaction
received November 13, 2019 and accepted July 20, 2020
Résumé
Viabilité et capacités de germination des graines de Saba senegalensis (A. DC.) Pichon, une espèce agroforestière à multiple usages en Afrique de l’Ouest
Saba senegalensis (A. DC.) Pichon est une liane ligneuse de la famille des Apocynaceae, originaire d’Afrique subsaharienne. L’espèce est connue comme une plante alimentaire à multiple usages avec un fort potentiel de contribution à la conservation des sols et de l’eau. Cependant, il existe peu d’études sur la variation entre les provenances de l’espèce et les capacités de germination des graines de l'espèce. De telles informations sont pourtant nécessaires aux initiatives de domestication ainsi que pour les programmes de conservation. La présente étude vise à évaluer les variations inter-provenances, l’effet de la durée de conservation et les conditions de conservation sur la viabilité des graines de S. senegalensis. Les facteurs expérimentaux étaient le dépulpage et le non dépulpage de semences de neuf provenances et le stockage des semences en conditions ambiantes et au réfrigérateur à 5°C. Les paramètres de germination ont été calculés et les valeurs soumises à une ANOVA. Les graines non dépulpées n’ont pas germé alors que celles dépulpées ont donné des taux de germination variant de 59% à 92% en fonction des provenances avec une valeur moyenne de 77 ± 16.87 %. La provenance n’a pas eu d’effet significatif ni sur la capacité de germination ni sur le temps moyen de germination. Cependant, des différences significatives ont été observées entre certaines provenances pour la durée (p= 0.023) et la vitesse (p= 0.037) de germination. Par ailleurs, le stockage des fruits au réfrigérateur conserve la capacité de germination des graines jusqu’à 3 mois. En complément des résultats de la présente étude, des recherches futures sont nécessaires pour comprendre les interactions potentielles entre la durée de stockage des graines et les provenances..
Abstract
Saba senegalensis (A. DC.) Pichon is a woody liana of the Apocynaceae family native to sub-Saharan Africa. The species is known as a useful food wild crop with a high potential to contribute to soil and water conservation. However, little is documented about the variation among the species’ provenances and seeds germination. Such baseline information is needed to initiate S. senegalensis breeding, domestication and conservation programs. The present study aimed to evaluate the inter-provenance variation and effects of storage duration and conditions on seeds viability. The experimental trial used de-pulped and not de-pulped seeds from nine provenances stored in both ambient room and refrigeration at 5°C conditions. Germination parameters were calculated and subjected to analysis of variance. Seeds sown with pulp did not germinate at all, while de-pulped seed germination rates ranged from 59% to 92% according to provenances with a mean value of 77 ± 16.87 %. Moreover, storing fruits in a refrigerator helped to maintain the seeds germination capacity and viability, until 3 months. Further investigations are needed to understand the potential interaction between length of seeds storage time and provenances with regard to germination parameters.
Table des matières
Introduction
1Seed germination is the first step in a plant’s life cycle (41). It is the key step for successful establishment, growth and further expansion of plant populations (18). Seed germination can be influenced by various intrinsic factors (e.g. dormancy in its various forms, seed quality, maturity, tolerance to desiccation, age) and environmental conditions (e.g. water, oxygen, temperature, light) (12, 42).
2To germinate, a plant’s seeds also need to be well preserved to conserve their viability for as long as possible, because the storage conditions can strongly affect seed germination (15, 25). It is known that all seeds lose viability with age, but this loss of viability is even more pronounced and rapid when they are stored in the open air and at high temperatures (39). In fact, drying and low temperatures can promote the preservation of seeds of many plant species (45). But there are seeds of tree species from certain dry climatic zones for which such storage conditions are harmful. These seeds have a short viability, are not dormant and can have rapid germination. Many authors have addressed the deterioration of seeds during their storage (6, 22). In general, the deterioration process is accompanied by a decrease of germination capacity (sometimes even a total loss of viability) and production of non-vigorous seedlings (4, 33). The main factors affecting the viability of seeds during storage are their moisture content, the storage temperature and relative humidity, and seed maturity and quality at harvest time (22, 44).
3In terms of seed desiccation tolerance and storage behavior, seeds are divided into three categories; orthodox or recalcitrant (13, 35, 43) or intermediate (16). Orthodox seeds are dehydration-tolerant and can be stored at negative temperatures if their moisture content is less than 10%. As for recalcitrant seeds, they are dehydration-intolerant and must avoid dry and low temperature storage conditions. These seeds have a high moisture content, estimated to be in the range of 30-70% at maturity (10). They are able to germinate immediately after shedding, without a quiescent phase, and continuously retain a high metabolic activity (9, 31). They germinate rapidly when sown fresh but are sensitive to desiccation and freezing. It is therefore important for all species’ seeds to be stored in their optimal conditions, as this will maximize their viability (34). Intermediate seeds are those not included either in the orthodox or recalcitrant class. These seeds are sensitive to sub-zero temperatures and tolerate partial desiccation. Their longevity in dry storage conditions is decreased by reducing the temperature below about 10°C and/or the moisture content value below that of equilibrium with about 40%-50% relative humidity (16). Depending on whether seeds are orthodox or recalcitrant or intermediate, they will age and deteriorate differently during different storage conditions. The phenomenon of ageing is manifested by a reduction in germination rate, which depends on the species, genotypes, time and storage conditions. Desiccation tolerance and seed dormancy are known to be acquired traits.
4To date, studies on the storage and viability of seeds of woody species of Sudanian savannas are rare (15, 30). This is even true for recalcitrant seeds (11, 30, 39). The present study focuses on Saba senegalensis (A. DC.) Pichon, due to its high socioeconomic values (i.e. household food and income potential) and increasing market interest (2, 24). This liana is a medicinal and food plant (2, 39) that produces egg-shaped berries whose sweet-acid pulp is rich in vitamins A, K and C (24, 29). Trade of the fruit contributes to the income generation and improvements in livelihood conditions of rural populations of Sudanian savannas (24). The species begins to bear fruit around 3-4 years after planting (2). Monkeys and humans are the main disseminators of its seeds. The seeds lose completely their viability when their moisture content drops to below 6% during storage at room temperature (27-37 °C). During storage, they retain their germination capacity for 4 months when packaged in well-sealed, fungicide-treated containers stored at 15 °C with a seed moisture content ranging from 30 to 37%. These traits mean S. senegalensis can be classified as a recalcitrant seed species (11).
5Previous studies in other contexts have reported that it is difficult to preserve the germination capacity of S. senegalensis seeds over time (11). This difficulty requires further investigation as this may help to improve the germination of the species’ seeds for its cultivation. Indeed, very little research has been carried out on the effects of provenance and pre-treatment on seed germination (8, 17, 46). Yet, provenance studies of forest trees are very important as they contribute to identifying the best and most adaptable provenances (17). Often, local provenances are advisable because they are expected to be better adapted to the local site conditions that facilitate vegetation establishment (7). The main objective of this study is to help extend the short-longevity of S. senegalensis seeds. Specifically, the aims are to (i) assess seed germination abilities at harvest according to different provenances, (ii) test two pre-treatments (i.e. seed de-pulping and no de-pulping), and (iii) determine the viability of the seeds as a function of time (i.e. 0-5 months) and storage conditions (i.e. refrigeration at 5°C and ambient room conditions).
Materials and methods
Seed sources and storage conditions
6Mature fruits of S. senegalensis were collected in July 2018 at nine sites (or provenances) across a climatic gradient in Burkina Faso (Figure 1). All the provenances were in different altitudes, longitudes and latitudes (Table 1). Fruits were collected from 30 randomly selected parent trees at each provenance. To ensure genetic diversity, the parent trees were located at distances of approximately 100 m apart (28). Ten mature fruits were manually collected from each individual tree. The different individuals were selected based on their vegetative state (i.e. absence of pest attacks, presence of mature fruits). A total of 300 fruits were collected per provenance. Mean seeds number per fruit was 18 ± 9.
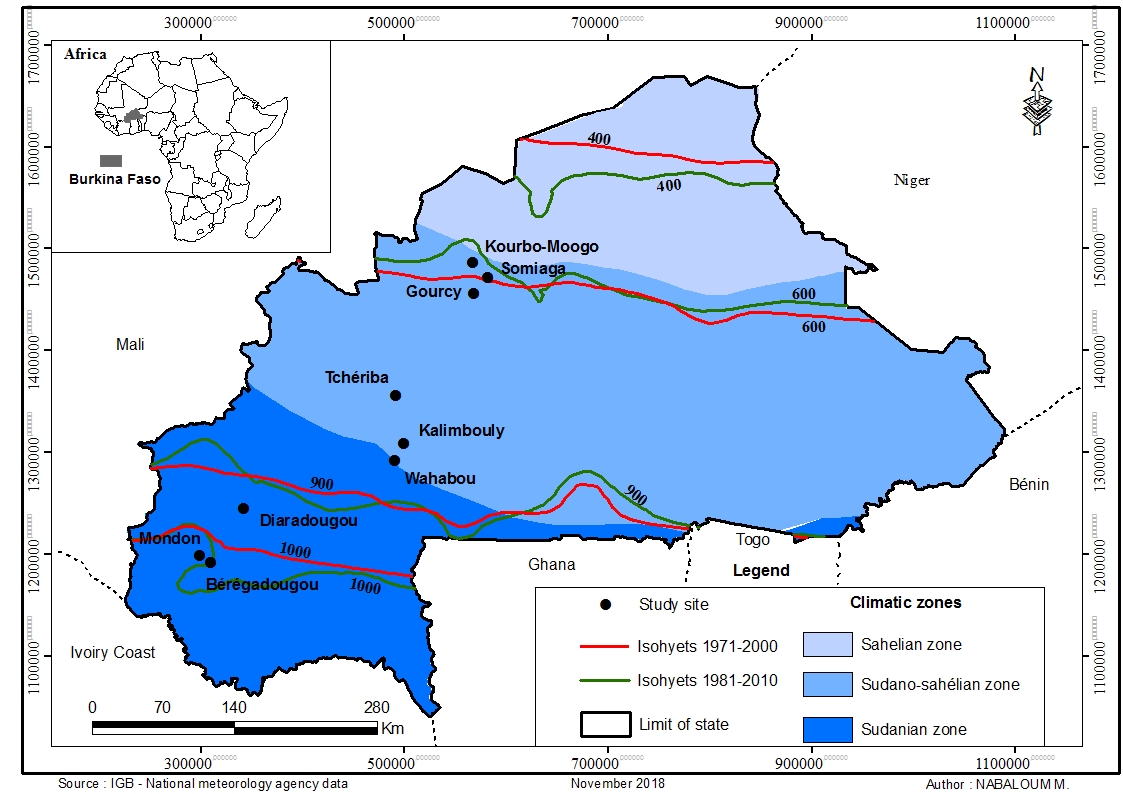
Figure 1: Locations of seed provenances
Table 1: Location, altitude and climate data of the selected study sites
|
Provenance |
|
|
Latitude (°) |
Longitude (°) |
Altitude (m) |
Mean annual air temperature (°C) |
Annual rainfall (mm) |
Humidity (%) |
|
Kourbo-Moogo |
13.44 |
2.37 |
330 |
29.4 |
733 |
38.9 |
||
|
Somiaga |
13.30 |
2.24 |
300 |
29.4 |
733 |
38.9 |
||
|
Gourcy |
13.17 |
2.36 |
315 |
29.4 |
733 |
38.9 |
||
|
Tchériba |
12.26 |
3.06 |
294 |
29.2 |
814 |
46.1 |
||
|
Kalimbouly |
11.83 |
3.00 |
286 |
28.8 |
1,608 |
49.2 |
||
|
Wahabou |
11.69 |
3.08 |
278 |
28.8 |
1,608 |
49.2 |
||
|
Diaradougou |
11.26 |
4.43 |
346 |
27.8 |
1,704 |
53.9 |
||
|
Mondon |
10.84 |
4.83 |
480 |
27.8 |
1,704 |
53.6 |
||
|
Bérégadougou |
|
10.78 |
4.73 |
349 |
27.8 |
1,704 |
53.9 |
|
Climate data obtained from Burkina Faso weather station. Climate data of the study sites are those of the nearest weather station
7From the 300 fruits collected per provenance, 120 fruits were randomly selected and subdivided into three batches. The first batch was composed of fruits of which the seeds were used for germination immediately after harvest to understand variation among provenances and effects of de-pulping. The second batch of fruits was stored in air-tight containers in the refrigerator (temperature = 5°C and humidity = 24%) and the fruits of third batch were stored in ambient room conditions until needed for the monthly germination trials. For the 2nd and 3rd batches, all provenances fruits were mixed as, based on the first tests, there was no significant difference between provenances. Mean temperatures in the ambient conditions during fruit storage were 26.4 ± 1.3 °C in the morning, 30.2 ± 1.2°C in the noon and 29 ± 1.7°C in the evening. With these last two fruit batches, germination tests were conducted monthly up to 5 months (when no germination was recorded) to determine the effects of storage conditions and duration on the germination parameters of the species.
Determination of seeds water content
8Before germination tests, seeds moisture content of fruit batches was determined. An overall sample of 30 seeds per fruit batches divided into 6 replicates of 5 seeds was used to determine their water content (WC) (23). All seeds were weighed individually to determine their initial fresh weight (Pf). Then, the seeds were dried in an oven at the temperature set at 103 ± 2 °C for 3 h and reweighed to determine dry weight (Ps) and WC. The WC based on the average of 6 replicates, was calculated in relation to fresh weight according to the formula: WC = 100 x (Pf - Ps) / Pf (20).
Germination tests
9Intact seeds and de-pulped seeds soaked in cold water were considered for the germination tests. The de-pulping consisted in kneading the seeds in fine sand to remove the pulp. Pre-sowing treatments applied to the seeds were: control - intact seeds without any treatment, and de-pulped - seeds soaked in cold water for 24 hours following the conventional treatment as recommended by the National Tree Seed Center (CNSF).
10A randomized block design was used for the germination tests. For the first batch of seeds, 4 × 25 seeds were sampled per pre-treatment for each provenance to investigate the variation in germination among provenances. To understand how storage duration and conditions impact on seed longevity, each month, 10 fruits were randomly selected, and seeds were extracted and de-pulped for germination tests. Seeds with pulp were no more included in the test as they showed nil germination during the first test. For each germination test, 4 × 25 seeds were placed in Petri dishes on two layers of filter paper moistened with tap water. Petri dishes were subsequently arranged on the germination table and watering was done once or twice daily with tap water to avoid drying of the medium. The criterion for germination was radicle growth more than 2 mm (19). The experiment lasted 30 days for each germination test and germinated seeds were counted daily and then removed from Petri dishes. The germination table was adjusted to 30 °C from 6.00 a.m. to 6.00 p.m. and 25 °C from 6.00 p.m. to 6.00 a.m. The germination room was illuminated from 6.00 a.m. to 6.00 p.m. with 20 μEm-2s-1 light from a fluorescent lamp (F40 W/33 RS cool white light) mounted on the germination table to simulate conditions of day and night. After the end of each germination test, the viability of ungerminated seeds was estimated by seed dissection.
Data Analysis
11Single value germination indices across all nine provenances and storage durations were computed using the package “germinationmetrics” in R (1). The germinated seeds were counted daily over the incubation period and time to germination was recorded for calculating germination duration (GD). This is the difference between time for the last germination (FGT) and time for the first germination (IGT). At the end of each germination test, the seed germination percentage (GP) was calculated by dividing the number of germinated seeds against the total number of seeds sown and multiplying by one hundred. Also, other germination parameters including mean germination time (MGT), speed of germination (GS) and time at maximum germination rate (TMGR) were calculated (1).
12MGT is the average length of time required for maximum germination of a seed lot and is estimated according to the following formula:
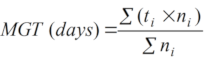
13where ni is the number of seeds germinated at each day, N is total number of seeds sown and ti is the number of days starting from the date of sowing.
14GS is the rate of germination in terms of the total number of seeds that germinate in a time interval. It is estimated as follows:
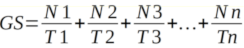
15Where N1, N2, N3, · · ·, Nn are the number of germinated seeds observed at time (days) T1, T2, T3, · · ·, Tn after sowing.
16TMGR represents the point in time when the instantaneous rate of germination starts to decline.
17The GP for each provenance and pre-treatment were analyzed using the Generalized Linear Model (GLM) with Logit as the link function and Binomial as the probability distribution. The GLM was also used to test the GD, the MGT and the TMGR for each storage condition and storage time using Inverse as the link function and Gamma as the probability distribution. The GS for each storage condition and storage time was analyzed using GLM with a Poisson distribution. Before the analysis, data exploration was performed following the protocol described by Zuur et al. (47). Data were analyzed using the package “Rcmdr” in R (37). All reported values in this paper were mean ± standard deviation (SD) for four repetitions in each batch of seed. For all tests, differences were considered significant at P < 0.05.
Results
Variation in seeds germination parameters among provenances
18In our experiment, S. senegalensis seeds sown with pulp did not germinate at all. On average, the result shows that de-pulped seeds gave a germination percentage of 77 ± 16.87 % in 10.53 ± 6.18 days after sowing. The mean germination speed was 4.36 ± 1.12 days during the test and mean germination time had remained 5.45 ± 0.64 days. Seeds WC at the time of harvest ranged from 38% to 39% for all provenances. Among the provenances, there were no significant differences with respect to germination percentage and mean germination time. However, significant differences were observed between provenances for the germination duration (F = 2.765, p = 0.023) and germination speed (F = 2.473, p = 0.037) (Figure 2). The longest germination duration was observed for seeds collected at the Tchériba site (17.75 ± 4.50) in the Sudano-Sahelian zone while the shortest was recorded for seeds from Diaradougou site (3.25 ± 1.31) in the Sudanian zone. The greatest speed of germination was obtained for seeds collected at the Wahabou site (5.73 ± 0.6 germinated seeds/day) and the lowest was recorded for seeds from Diaradougou site (3.40 ± 1.60 germinated seeds/day).
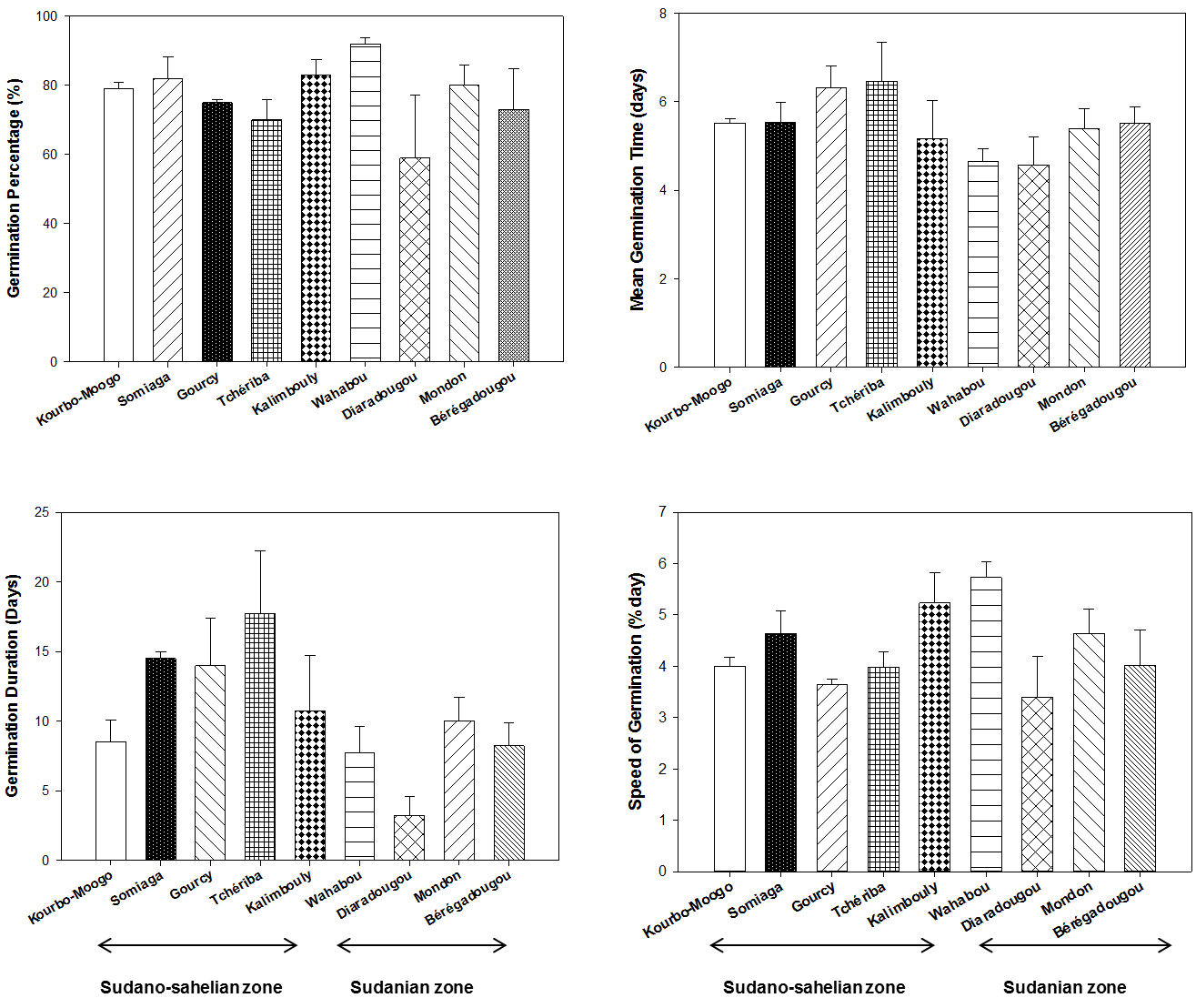
Figure 2: The effect of provenance on germination parameters of Saba senegalensis seeds
19For the 9 provenances, the same germination trend was observed between days 0 and 5 (Figure 3), then the provenances began to differentiate. At 10 days, almost all provenances had reached their maximum germination and the germination curves began to flatten, with the Wahabou site having the highest germination percentage at the end of the experiment and Diaradougou the lowest.
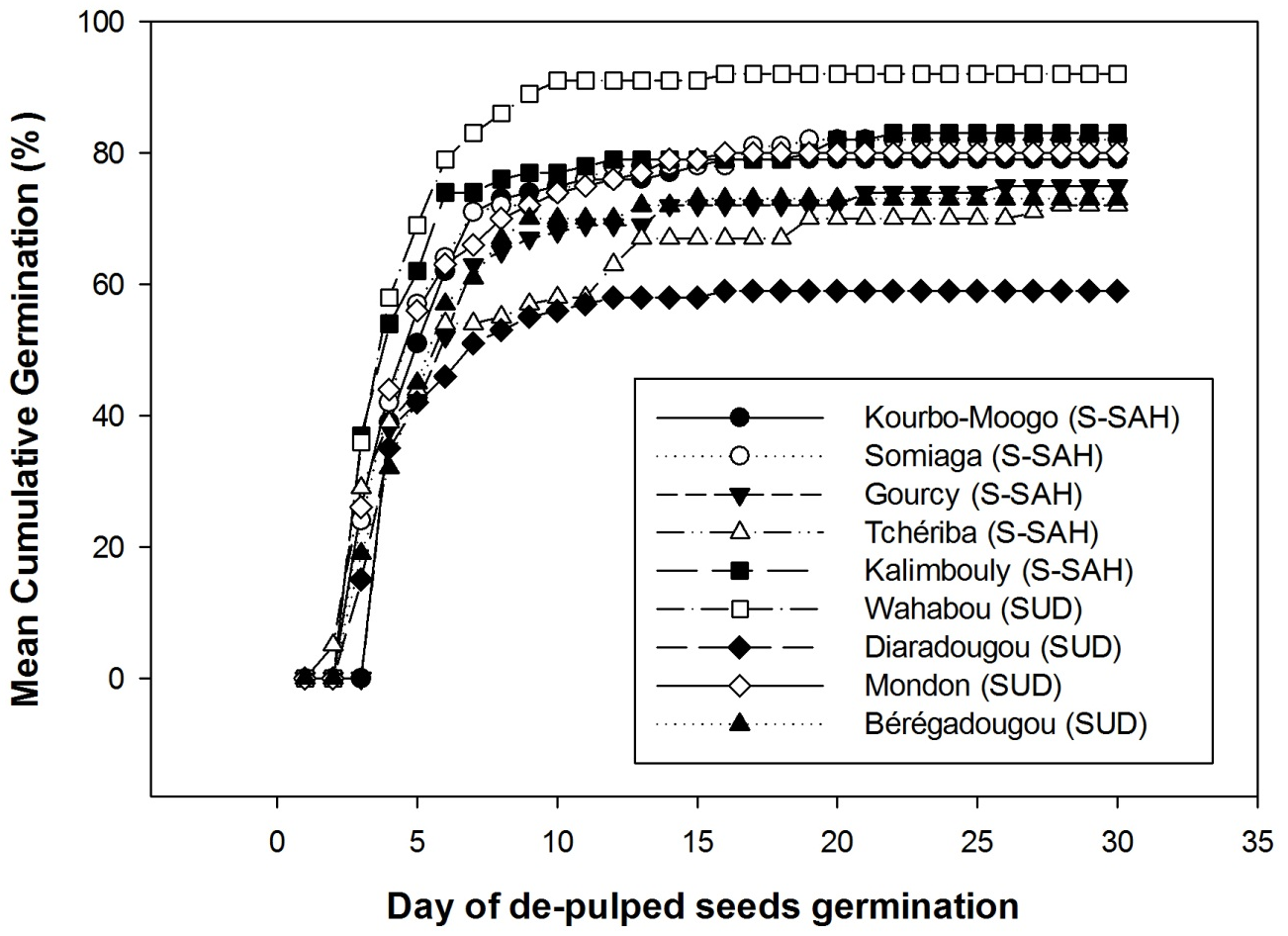
Figure 3: Cumulative germination percentages for Saba senegalensis seeds from different provenances in two climatic zones, Sudano-sahelian (S-SAH) and Sudanian zone (SUD)
Effect of storage conditions on seed germination parameters
20After one month of storage, the germination percentage decreased from 77% to 38% for seeds stored in the refrigerator and from 77% to 34% for the seeds stored in the ambient conditions. During the same period of storage, in ambient conditions, fruits were dried and seeds WC was 27%. At two months of storage, there remained no germination for seeds extracted in fruits stored in the ambient conditions up to the end of the experiment (seeds WC was 18%). However, for fruits stored in the refrigerator, seeds germination was observed at one (38%), two (about 4%) and three (about 10%) month (s) of storage before dropping to zero at 4 and 5 months of storage. Seeds WC of fruits stored in refrigerator was 39% for all months of storage. In general, the storage time had influenced germination percentage (F = 123.372, p < 0.01), germination duration (F = 31.094, p < 0.01), mean germination time (F = 13.08, p < 0.01) and germination speed (F = 12.563, p < 0.01). In both conditions of storage, there were significant differences between the four germination parameters after one month of storage (P < 0.05). No statistical test was made between storage conditions after one month (i.e., starting from 2 months) as germination at ambient room conditions was nil.
21The mean germination duration for seeds extracted from fruits stored in ambient conditions, increased from 10.53 days at the time of seed collection to 12 days after one month storage then there was no germination in this storage condition. In contrast, for seeds stored in the refrigerator, mean germination duration decreased to about 5.75, 2 and 1.5 days for one, two and three month (s) storage period, respectively. In general, germination parameters were better for seeds stored in the refrigerator compared to seeds stored in the ambient conditions (Figure 4).
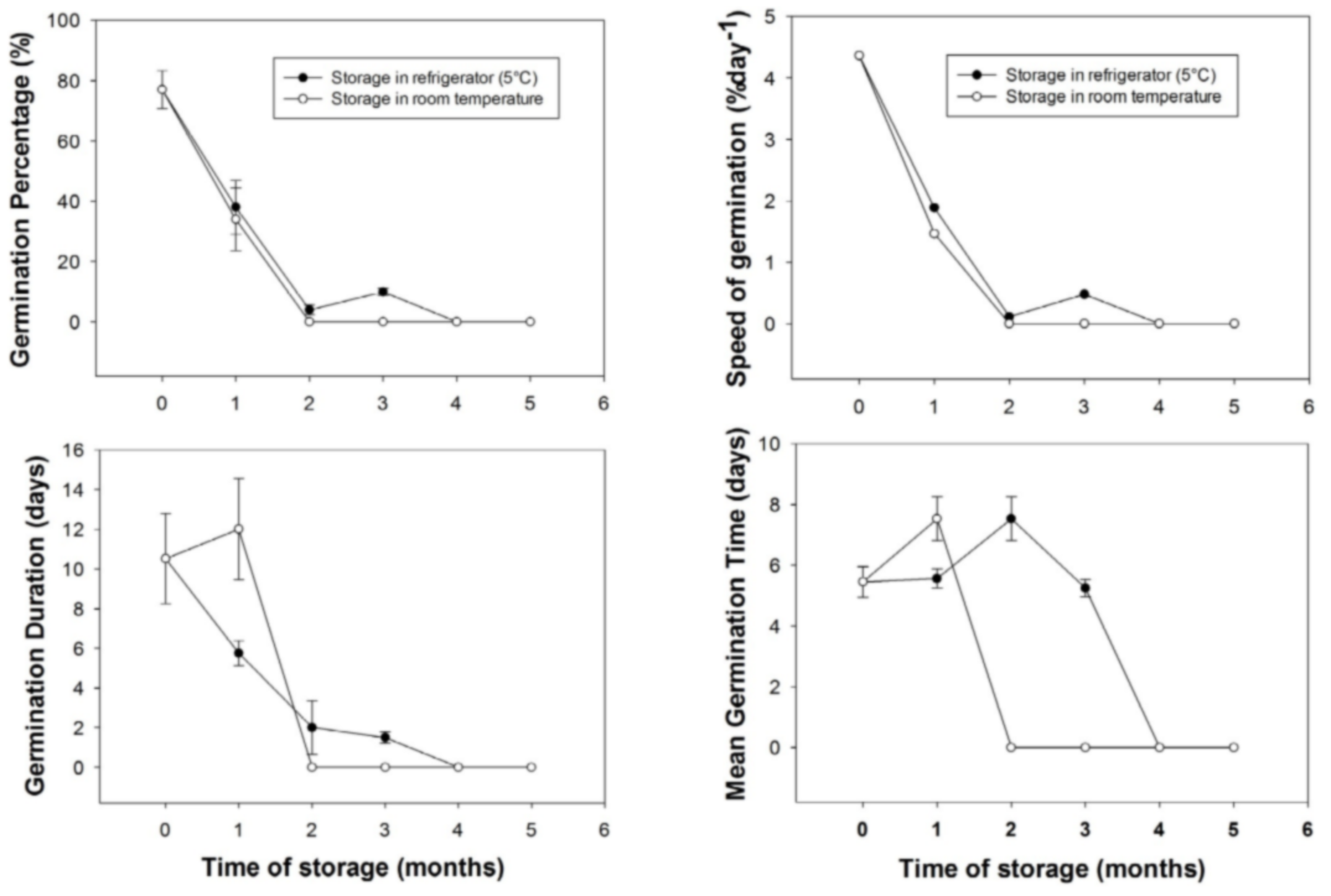
Figure 4: Effect of storage conditions and time on germination parameters of Saba senegalensis seeds
Discussion
22The results of the experiment revealed that de-pulping of S. senegalensis seeds before sowing improved the germination percentage and that germination began at 3 days after sowing. Considering the germination period, these seeds could be described as “non-dormant seeds”, because they germinate in less than 7 days. Rapid germination of seeds is an advantage in natural stands conditions because it may reduce the duration of seed exposure to predation (12). This would mean that in the wild, when favorable germination conditions are in place, S. senegalensis seeds will have the opportunity to germinate quickly before being attacked by seed predators or burned by bushfires, provided that de-pulping occurs during the dispersal process. In S. senegalensis natural environment, monkeys are primarily responsible for the dispersal of its seeds. Indeed, fruit of this species is most often consumed by monkeys and seeds are swallowed and defecated intact. In general, small sprouts of germinating S. senegalensis are found in monkeys faeces (39). The digestive process yields de-pulped seeds (already good for germination as shown in our study). Also, the passage of seeds through the digestive tract simulates acidic seed scarification which could improve their germination rate. Furthermore, urine and faecal deposits are a source of P, N, K, and other micronutrients for seedlings (26). Seeds with pulp did not germinate, probably due to fungal attack. Indeed, after the germination tests, two fungi (i.e. Aspergillus niger and A. flavus) known to increase seed deterioration through the production of toxins that degrade seeds (27), were identified on the seeds sown with the pulp. Also, the fermentation of the pulp of seeds during experimentation may have damaged the embryo. Indeed, the pulp of S. senegalensis fruits is sweet and tart and could be a favorable environment for the development of micro-organisms such as bacteria and fungi that could make it problematic to preserve seeds viability (27).
23Differences in the germination duration and speed of germination were observed between the different provenances. These results are in accordance with those observed by certain authors regarding different germination characteristics among different provenances of widely distributed plant species such as Pinus densata (46), Adansonia digitata (3) and Combretum aculeatum (8). These authors have found that provenance can be a parameter that influences germination of seeds. The obtained variation within S. senegalensis populations by provenance could be used to guide breeding, domestication (e.g. afforestation) and conservation (e.g. regeneration) programs for this species. The variation among provenance can be of genetic origin or caused by the local environmental conditions under which the fruits matured (8, 14).
24Storage conditions had a significant influence on cumulative germination of the S. senegalensis seeds. The results indicated that germination decreased with increasing storage duration; this reduction was different between the two different storage conditions. The refrigerated storage reduced the initial germination percentage from 77% to 38%, 4%, 10%, 0% and 0% after 1, 2, 3, 4 and 5 storage months, respectively. The storage in ambient conditions diminished the initial germination percentage from 77% to 34 % after 1 month of storage, then (starting from 2 months) no more germination was observed because, seeds were dried in the fruits. These results indicate that S. senegalensis seeds germinate rapidly when sown fresh, but they do not tolerate desiccation and completely loose viability if fruits are stored for more than 3 months in refrigerated storage, despite their high-water content (39%) during storage.
25When investigating seed storage for a species, it is essential to evaluate the germination capacity of the seeds over time. In fact, whether they are orthodox, recalcitrant or intermediate, the seeds age and deteriorate differently during variable periods of storage. This induces a reduction in the germination rate, which will vary according to the species, genotypes, time and storage conditions (21). In general, storage of recalcitrant seeds presents a greater challenge than orthodox seeds that tolerate desiccation and low temperatures (5, 32). This is due to their sensitivity to water loss, which makes it necessary to retain their high-water content. However, this internal moisture can favor the attack of micro-organisms but also the early commencement of germination. In our study, after one month of storage, fruits stored in the ambient conditions were attacked by Aspergillus fungi (A. niger and A. flavus) and became dry. This sensitivity to drying indicates that the seeds of S. senegalensis are recalcitrant. The death of recalcitrant seeds due to the loss of moisture is principally assigned to the loss of membrane integrity and nuclear disintegration (10). Several studies have found that reasons for the loss of viability in recalcitrant seeds were highly related to the damage of the antioxidant system (17, 38). This could partly explain the absence of germination and the attack by fungi of the S. senegalensis seeds after one month of storage in the ambient conditions. The same fungi were present in the fruits stored in the refrigerator for three months and probably damaged the seed membranes, which resulted in their suppressed germination.
Conclusion
26In this study, S. senegalensis showed good germination capacity at the time of seed collection. Storage in ambient room conditions depresses germination while cold storage (i.e. in a refrigerator) maintains relatively better germination behavior, at least for a short period. Our results suggest that for ensuring good establishment of S. senegalensis, necessary provisions should be taken for immediate sowing of newly-collected seeds or for their cold storage for a very short period (3 months maximum). Provenance tests did not reveal significant differences in the parameters such as germination capacity and mean germination time, although seeds of certain sites performed relatively better for germination duration and speed. In this experiment, a provenance comparison was conducted only once, at the time of seed collection. It would therefore be interesting to investigate whether the behavior of the different provenances changes with different lengths of seed storage. Moreover, other experiments testing a wide range of storage temperatures and different seed moisture contents will be useful in elaborating more on optimum storage conditions for S. senegalensis seeds.
Acknowledgements
27We are indebted to the INERA for their technical and logistical support during field work. We also thank the CGIAR Research Program on Forests, Trees and Agroforestry (FTA) for their assistance during the writing of the manuscript. We thank the TREEFOOD research project (n° 1507-143) for the financial support for this work. Thanks are due to Dr. John A. Meadows for proofreading and editing the paper. The authors also thank Mr Koudous Kaboré and Alain Bambara for their assistance on field to collect data.
References
-
Aravind J., Vimala Devi S., Radhamani J., Jacob S.R. & Kalyani S, 2019, germinationmetrics: Seed Germination Indices and Curve Fitting. R package version 0.1.3.9000.
-
Arbonnier M., 2009, Arbres, arbustes et lianes des zones sèches d’Afrique de l’Ouest - Paris, France : MNHN, éd. QUAE.
-
Assogbadjo A. E. Glèglè-Kakai, R., Edon, S., Kyndt, T. & Sinsin, B., 2011, Natural variation in fruit characteristics, seed germination and seedling growth of Adansonia digitata L. in Benin, New Forests, 41, 1, 113-125.
-
Ballesteros D. & Pence V.C., 2017, Survival and death of seeds during liquid nitrogen storage: a case study on seeds with short lifespans, Cryo letters, 38, 4, 278-289.
-
Barbedo C. J., 2018, A new approach towards the so-called recalcitrant seeds, J. Seed Sci., 40, 3, 221-236.
-
Basra A. M. S., Rehman U. K. & Iqbal S., 2000, Cotton Seed Deterioration: Assessment of some Physiological and Biochemical Aspects, Int. J. Agric. Biol., 2, 3, 195-198.
-
Bischoff A., Vonlanthen B., Steinger T. & Muller-Scharer H., 2006, Seed provenance matters - Effects on germination of four plant species used for ecological restoration, Basic Appl. Ecol., 7, 347-359.
-
Bognounou F., Thiombiano A., Oden P.C. & Guinko S., 2010, Seed provenance and latitudinal gradient effects on seed germination capacity and seedling establishment of five indigenous species in Burkina Faso, Trop. Ecol., 51, 2, 207-220, ISSN 0564-3295.
-
Caccere R., Teixeira, S.P., Centeno, D.C., Figueiredo-Ribeiro R.C. & Braga, M.R., 2013, Metabolic and structural changes during early maturation of Inga vera seeds are consistent with the lack of a desiccation phase, J. Plant Physiol., 170, 791-800, doi:10.1016/j.jplph.2013.01.002.
-
Chin H.F., 1995, Storage of recalcitrant seeds. In Basra A.S., « Seed Quality Basic Mechanisms and Agriculture Implications», The Haworth Press, Inc. NY, USA, pp. 209-222.
-
Danthu P., Gueye, A., Boye, A., Bauwens, D. & Sarr, A., 2000, Seed storage behaviour of four Sahelian and Sudanian tree species (Boschia senegalensis, Butyrospermum parkii, Cordyla pinnata and Saba senegalensis), Seed Sci. Res., 10, 183-187.
-
Daws M.I., Gaméné, C.S., Sacandé M., Pritchard H.W., Groot S.P.C. & Hoekstra F., 2005, Desiccation and storage of Lannea microcarpa seeds from Burkina Faso. In Sacandé M., Jøker D., Dulloo M.E., Thomsen K.A., «Comparative storage biology of tropical tree seeds», pp. 32-39, ISBN: 92-9043-641-7.
-
Daws M.I., Garwood N.C. & Pritchard H.W., 2006, Prediction of Desiccation Sensitivity in Seeds of Woody Species: A Probabilistic Model Based on Two Seed Traits and 104 Species, Ann. Bot., 97, 4, 667-674, doi:10.1093/aob/mcl022.
-
Dayamba S.D., Tigabu M., Sawadogo L. & Oden P.C., 2008, Seed germination of herbaceous and woody species of the Sudanian savanna-woodland in response to heat shock and smoke, Forest Ecol. Manag., 256, 462-470.
-
Dayamba S.D., Savadogo P., Diawara S. & Sawadogo L., 2016, Perspectives in restoration: storage and pretreatments of seeds for better germination of Sudanian savanna-woodland species, J. Forest. Res., 27, 4, 773-778, doi:10.1007/s11676-016-0220-7.
-
Ellis R.H., Hong T.D. & Roberts E.H., 1991, An intermediate category of seed storage behavior? Effects of provenance, immaturity and imbibition on desiccation-tolerance in coffee. J. Exp. Bot., 42, 65–657.
-
Fornah Y., Mattia S.B., Otesile A.A. & Kamara E.G., 2017, Effects of provenance and seed size on germination, seedling growth and physiological traits of Gmelina arborea, Roxb, Int. J. Agric. For., 7, 1, 28-34.
-
Hao J-H., Lv S.-S., Bhattacharya S. & Fu J.-G., 2017, Germination Response of Four Alien Congeneric Amaranthus Species to Environmental Factors, PLoS ONE, 12, 1, e0170297. https://doi.org/10.1371/journal.pone.0170297.
-
ISTA, 1999, International rules for seed testing, Seed Sci. Technol., 27, 1–333.
-
ISTA, 2009, Règles internationales pour les essais de semences. Bassersdorf, Suisse: Association internationale d'essais de semences (AIES).
-
Jain N., Koopar R. & Saxena S., 2006, Effect of accelerated ageing on seed of radish (Raphanus sativus), Asian J. Plant Sci., 5, 3, 461-464.
-
Kooshki M.M., Moradi A., Balouchi H. & Fahliani R.A., 2018, Evaluation of the germination performance and biochemical indices of faba bean (Vicia faba L.) seeds stored at different temperatures and moisture contents, J. Plant Proc. Func., 7, 25, 18-28.
-
Krishnapillary B. & Marzalina M., 1994, Méthode statistique de détermination de la taille d'échantillon pour la mésure de la teneur en humidité des graines récalcitrantes d'arbres forestiers (Etude de cas sur Shorea leprosula et Shorea parvifolia). Informations sur les Ressources Génétiques Forestières, 21. Rome: FAO.
-
Lamien N., Traoré S. & Kini F., 2010, Potentialités productive, nutritive et économique de la liane goïne (Saba senegalensis A.DC. Pichon) dans le Sahel Burkinabé, Etudes et Recherches Sahéliennes, n° 14-15, pp. 115-127.
-
Liu K., Baskin J.M., Baskin C.C., Bu H., Liu M., Liu W. & Du G., 2011, Effect of storage conditions on germination of seeds of 489 species from high elevation grasslands of the eastern Tibet Plateau and some implications for climate change, Am. J. Bot., 98, 1, 12-19.
-
Mills A.J. & Fey M.V., 2005, Interactive response of herbivores, soils and vegetation to annual burning in a South African savanna, Austral Ecol., 30, 435-444.
-
Mng'omba S. A., Du Toit E. S. & Akinnifesi F. K., 2007, Germination characteristics of tree seeds: spotlight on Southern africain tree species, Tree For. Sci. Biotech., 1, 1, 81-88.
-
Mulawarman, Roshetko J. M., Sasongko S. M. & Irianto D., 2003, Tree seed management - Seed sources, seed collection and seed handling: A field manual for field workers and farmers. International Centre for Research in Agroforestry (ICRAF) and Winrock International. Bogor, Indonesia. 54 p.
-
Nafan G., Jesus F.I., Souleymane S., Lenifere S.C., Emmanuel I.A. & Abdourahamane S.A., 2013. Genetic variation of Saba senegalensis Pychon (Apocynaceae) and few nutritionnal value, International Journal of Biotechnology and Allied Fields, 1, 121-135.
-
Neya T., Daboué E., Neya O. & Ouédraogo I., 2017, Germination characteristics of Parinari curatellifolia Planch. Ex Benth, Vitex doniana Sweet and Zanthoxylum zanthoxyloides (Lam) Watermann Seeds, Annu. Res. Rev. Biol. ,12, 3, 1-12.
-
Parkhey S., Naithani S.C. & Keshavkant S., 2014, Protein metabolism during natural ageing in desiccating recalcitrant seeds of Shorea robusta, Acta Physiol. Plant., 36: 1649-1659, doi:10.1007/s11738-014-1540-x.
-
Plitta-Michalak B.P., Naskret-Barciszewska M.Z., Kotlarski S., Tomaszewski D., Tylkowski T., Barciszewski J., Chmielarz P. & Michalak M., 2018, Changes in genomic 5-methylcytosine level mirror the response of orthodox (Acer platanoides L.) and recalcitrant (Acer pseudoplatanus L.) seeds to severe desiccation, Tree Physiol., 38, 4, 617-629.
-
Popova E.V., Kim D.H., Han S.H., Moltchanova E., Pritchard H.W. & Hong Y.P., 2013 Systematic over-estimation of Salicaceae seed survival using radicle emergence in response to drying and storage: implications for ex situ seed banking, Acta Physiol. Plant, 35, 3015-3025.
-
Pradhan B. K. & Badola K., 2012, Effect of storage conditions and storage periods on seed germination in eleven populations of Swertia chirayita: a critically endangered medicinal herb in Himalaya, Sci. World J., article ID 128105, 9 p, doi:10.1100/2012/128105.
-
Pritchard H.W., Daws M.I., Fletcher B.J., Gaméné C.S., Msanga H.P. & Omondi W., 2004. Ecological correlates of seed desiccation tolerance in tropical African dryland trees, Am. J. Bot., 91, 863-870.
-
R Core Team, 2015, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
-
Sahu A.K., Sahu B., Soni A. & Naithani S.C., 2017, Active oxygen species metabolism in neem (Azadirachta indica) seeds exposed to natural ageing and controlled deterioration, Acta Physiol. Plant., 39, 197, doi 10.1007/s11738-017-2494-6.
-
Sanogo S., Sacandé M., Van Damme P. & Ndiaye I., 2013, Caractérisation, germination et conservation des graines de Carapa procera DC. (Meliaceae), une espèce utile en santé humaine et animale, Biotechnol. Agron. Soc. Environ. 17, 2, 321-331.
-
Sarr M.G., Ndiaye N.D., Ayessou N.C., Faye P.G., Cisse M., Sakho M. & Diop C.M., 2018, Saba senegalensis: Key Features and Uses, Food Nutr. Sci., 9, 1099-1111.https://doi.org/10.4236/fns.2018.99080.
-
Schlaepfer D.R., Glattli M., Fischer M. & Van Kleunen M., 2010, A multi-species experiment in their native range indicates pre-adaptation of invasive alien plant species, New Phytol., 185, 1087-1099, doi: 10.1111/j.1469-8137.2009.03114.x PMID: 19968796.
-
Simão E. & Takaki M., 2008. Effect of light and temperature on seed germination in Tibouchina mutabilis (Vell.) Cogn. (Melastomataceae), Biota Neotrop., 8, 2 , 64-68.
-
Tweddle J.C., Dickie J.B., Baskin C.C. & Baskin J.M., 2003, Ecological aspects of seed desiccation sensitivity, J. Ecol., 91, 294-304.
-
Vargas-Figueroa J.A. & Torres-Gonzalez A.M., 2018, Germination and seed conservation of a pioneer species, Tecoma stans (Bignoniaceae), from tropical dry forest of Colombia, Rev. Biol. Trop. (Int. J. Trop. Biol.), 66, 2, 918-936.
-
Whitehouse K.J., Owoborode O.F., Adebayo O.O., Oyatomi O.A., Olaniyan A.B., Abberton M.T. & Hay F.R., 2018, Further evidence that the genebank standards for drying orthodox seeds may not be optimal for subsequent seed longevity, Biopreserv. biobank., 16, 5, 327-336, doi: 10.1089/bio.2018.0026.
-
Xu Y., Cai N., He B., Zhang R., Zhao W., Mao J., Duan A., Li Y. & Woeste, K., 2016, Germination and early seedling growth of Pinus densata Mast. provenances, J. Forest. Res., 27, 2, 283-294.
-
Zuur A.F., Ieno E.N. & Elphick C.S., 2010, A protocol for data exploration to avoid common statistical problems, Methods in Ecology & Evolution 1, 3 -14.
Pour citer cet article
A propos de : Sata Diawara
Burkinabè, Doctorante, Université JKZ, Ingénieure de Recherche, INERA, diawara.sata@gmail.com, Telephone: 00226 76 49 94 75. Centre National de la Recherche Scientifique et Technologique, Institut de l’Environnement et de Recherches Agricoles (INERA), Département Environnement et Forêts, 03 BP 7047, Ouagadougou 03, Burkina Faso
A propos de : Didier Zida
Burkinabè, PhD, Chargé de Recherches, INERA, Email: didierzida@hotmail.com. Centre National de la Recherche Scientifique et Technologique, Institut de l’Environnement et de Recherches Agricoles (INERA), Département Environnement et Forêts, 03 BP 7047, Ouagadougou 03, Burkina Faso
A propos de : Sidzabda Djibril Dayamba
Burkinabé, PhD, Attaché de Recherches, INERA, Email: djibril.dayamba@yahoo.fr. Centre National de la Recherche Scientifique et Technologique, Institut de l’Environnement et de Recherches Agricoles (INERA), Département Productions Animales, 03 BP 7047, Ouagadougou 03, Burkina Faso
A propos de : Patrice Savadogo
Burkinabè, PhD, Maître de Recherches, INERA, Email: savadogo.patrice@gmail.com. Centre National de la Recherche Scientifique et Technologique, Institut de l’Environnement et de Recherches Agricoles (INERA), Département Environnement et Forêts, 03 BP 7047, Ouagadougou 03, Burkina Faso
A propos de : Amadé Ouedraogo
Burkinabè, PhD, Professeur Titulaire, Université JKZ, Email: amadeouedraogo@gmail.com. Unité de Formation et de Recherche en Sciences de la Vie et de la Terre (UFR/SVT)/Laboratoire de Biologie et Ecologie Végétales, Université Joseph KI-ZERBO, 03 BP 7021, Ouagadougou 03, Burkina Faso