- Home
- Volume 93 - Année 2024
- No 1
- Behavioural Contexts of Sound Emission in Holocentrids: Insights
View(s): 1071 (32 ULiège)
Download(s): 46 (7 ULiège)
Behavioural Contexts of Sound Emission in Holocentrids: Insights

Attached document(s)
original pdf fileRésumé
Cette synthèse aborde la communication acoustique chez les poissons Holocentridae, s’appuyant sur la combinaison de plusieurs découvertes récentes issues de la thèse de doctorat de l’autrice et de la littérature existante. Pour aider les lecteurs qui ne sont pas familiers avec le domaine de la communication acoustique, l’article commence par fournir un aperçu de cette discipline fascinante, accompagné d’une brève introduction au groupe des Holocentridae. Le manuscrit développe ensuite les connaissances acquises sur l’éthologie de la communication acoustique de ce taxon et démontre comment les résultats de ces travaux enrichissent ce domaine de recherche. Ces résultats sont le fruit de plusieurs mois de recherche menée dans diverses régions du monde. Une collecte de données extensive (enregistrements de sons et de vidéos) a été entreprise à travers l’océan Indo-Pacifique (Seychelles, Philippines, Guam, Polynésie française). Ces données recensent les contextes comportementaux de l’émission sonore de plusieurs espèces d’Holocentridae, basées sur environ 77 heures d’enregistrements vidéo dans le milieu naturel. De plus, une expérience a été réalisée en aquarium pour étudier la réponse comportementale, incluant la production de sons, d’une espèce d’Holocentridae à l’introduction d’un prédateur (p.ex., une murène). Le très grand nombre d’espèces étudiées dans cette recherche, en plus de la combinaison de données issues du milieu naturel et d’expériences menées en laboratoire, constitue à ma connaissance, la plus grande base de données relatives à l’éthologie de la production de sons jamais collectée pour comprendre la communication acoustique chez des téléostéens.
Abstract
This synthesis addresses acoustic communication in holocentrid fishes, based on the combination of several recent findings from the author’s doctoral thesis and existing literature. To assist readers unfamiliar with the field of acoustic communication, the article begins with an overview of this fascinating discipline, accompanied by a brief introduction to the family Holocentridae. The manuscript then delves into the knowledge gained about the ethology of acoustic communication in this taxon and demonstrates how these research findings enrich this field of study. These results are the product of several months of research conducted in various regions around the world. An extensive data collection (sound and video recordings) was undertaken across the Indo-Pacific Ocean (Seychelles, Philippines, Guam, French Polynesia). These data document the behavioural contexts of sound production in several holocentrid species, based on about 77 hours of video recordings in the natural environment. Additionally, an experiment was conducted in aquariums to study the behavioural response, including sound production, of a holocentrid species to the introduction of a predator (e.g., a moray eel). The large number of species investigated in this research, along with the combination of data from both the natural environment and laboratory experiments, constitutes to my knowledge, the largest dataset on the ethology of sound production ever collected to understand acoustic communication in teleosts.
Table of content
Manuscrit reçu le 26 mai 2024 et accepté le 10 novembre 2024
This work is distributed under the Creative Commons CC BY 4.0 Licence.
Cet article a reçu un des Prix Annuels 2024 de la Société Royale des Sciences de Liège.
This paper was awarded one of the Annual Prizes 2024 of the Société Royale des Sciences de Liège.
1. Ethological Aspects of Acoustic Communication in Fishes
1Communication can be defined as an exchange of information between two individuals, following which the recipient should alter its behaviour in a way that is advantageous to the sender. According to Myrberg [1], this interaction should confer an adaptive advantage for the sender or enhance its fitness, while any benefits to the receiver are considered incidental. Many animal groups such as mammals, sauropterygians, amphibians, birds, insects and fishes [2–7] can communicate by the use of sounds, calls or vocalizations, highlighting the significance and essential character of these signals in their way of life. In fish, acoustic communication can be used in different behavioural contexts. It plays a crucial role in several activities, enabling species to warn others of danger [8–11], coordinate within groups for defence [12] or hunting [13], attract mates [14, 15], and establish territory [16]. The production of sounds in social interaction contexts constitutes a low-energy communication channel, especially during agonistic encounters, where the use of sounds can repel an opponent [17] or reduce the risk of physical injuries [18]. Acoustic communication constitutes also an important and conspicuous part of the breeding biology in many teleosts. For instance, sounds serve in the establishment and maintenance of territories [19], in facilitating the attraction of conspecific mates during courtship [20–23] and in the synchronization of reproductive behaviours [23–26]. Additionally, acoustic communication can be used in negotiating social hierarchies [27] or in schooling [28].
2While a lot of information is available regarding acoustic communication in terrestrial organisms and despite the extremely high diversity of teleosts, which account for approximately half the biodiversity of vertebrates [29] and their first recognition as sound producers by Aristotle more than 2000 years ago [30, 31], scientific interest in fish acoustic communication has emerged quite recently [32]. Among the 34900 extant fish species listed on FishBase [33], about 4% have been examined for effective sound production [34]. This observation highlights that in the animal kingdom, the study of acoustic behaviour in fish has been somewhat neglected, mainly for technical reasons. Lately, fish bioacoustics has switched from anecdotal reports to long-term, large-scale monitoring studies, capable of providing high resolution information on fish populations’ composition and dynamics [35]. The emergence of this discipline in teleosts over these last few decades is due to the advances in acoustic-recording technology [34, 36]. For field studies, the necessary equipment to conduct in situ observations underwater is still being developed and, so far, does not allow long-lasting recordings of both videos and sounds. There are also additional limitations due to light conditions underwater or at night, as well as challenges posed by depth and/or currents. Besides, studies in aquariums can alter not only the behaviour but also the features of emitted acoustic signals [37–40], and playback experiments are complicated due to the limited capabilities of underwater speakers [41].
3Despite the small number of recorded species, various indicators however highlight the importance of acoustic communication in the way of life of fish. First, the ability to produce sounds would have appeared at least 33 times throughout the evolution of Actinopterygii [42] and has resulted in the highest diversity of sound-producing mechanisms among vertebrates [43], which indicates that this part of the behaviour is well supported by natural selection. Second, although nearly 1000 fish species have been formally identified as vocal [44], this is likely an underestimation. For instance, only about 50 species of Pomacentridae have been recorded although the mechanism responsible for sound production is present in all members of the family, encompassing about 433 species [45, 46]. Similarly, in the fish community associated with deep-sea environments, taxa such as Ophidiiformes [47–49] and Gadiformes [50] for example, have been studied for their sound-producing mechanism, but their calls have not been extensively investigated due to limited access to their deep-sea habitats [51]. Finally, it is worth noting that many vocal teleost species are mostly nocturnal or evolve in turbid, low-light or deep-water environments where the efficiency of visual cues dramatically decreases, while sound propagates quickly and in all directions, providing an ideal way of communication [17, 52].
4Although the importance of voluntary sound production in social communication in teleosts was recognized decades ago [7], many studies have been limited to mere observations whose primary goal was to simply demonstrate a vocal capacity in precise taxa, supported by a brief description of sounds. Too often, with a few exceptions [22, 53–56], studies are incomplete in the sense that, once a species or a small group of species from a same taxon is recorded and some sounds are described, researchers shift their focus to another taxon. The simplest and least expensive way to obtain such results in acoustics remains to disturb the fish [57–59] or to hold it in hand [60–62], which will elicit the fish to produce sounds. In the field, the use of cameras coupled with hydrophones now allows for the investigation of other acoustical behaviours, provided the species are territorial [19, 21, 22, 63]. Unfortunately, this restricts the observations to diurnal behaviours only, although we know that acoustic communication takes place at night in several taxa [64]. It is important to consider that the literature has reported a continuum of capabilities between two extremes regarding the production of voluntary sounds. On one end are fish that are capable of emitting only one type of sound, or at least for which only one type of sound has been described [65–69] regardless of behaviour. Modifications in acoustic parameters are then inherent to size, motivation, individual, or season [27, 70–72]. The primary purpose of the sound would then be to complement a behaviour [27, 73]. On the other hand, some species (Gobiidae, Gadidae) are capable of emitting different types of sounds during the same behavioural sequence, such as different phases of courtship, or transitioning from courtship to spawning [20, 74, 75]. There are also fish that can emit multiple types of sounds in different behavioural contexts [19, 22, 76]. One of the best known example are the Dascyllus (Pomacentridae) species [19, 22]. These species produce six different sound types, each being associated with different behaviours (i.e., signal jump, mating/visiting, conspecific and heterospecific chases, and conspecific and heterospecific fighting behaviours). In clownfishes Amphiprion, sounds associated with threat postures (charge and chase) differ from those produced while exhibiting a submissive posture [27]. A last category includes species for which multiple sound types have been described but the corresponding behavioural contexts have not yet been formally identified [77–79]. These studies highlight the need to clarify the terminology used. Stereotyped sounds provide reliable and predictable signals, with minimal variation in their acoustic structure regardless of the individual. Some species can produce different types of stereotyped sounds. In the Dascyllus example above, the six different sound types described for these species are stereotyped. Since each can be associated with a specific behavioural context, they are also behaviourally specific. In other species, such as in Oreochromis niloticus for example, the same stereotyped sound can be associated with different behaviours, indicating that it is not behaviourally specific.
5In scholarly discourse, communication is frequently perceived as restricted to interactions within the same species. Yet, the literature provides numerous examples where signals are integral to interspecific relationships. Although the motivations for acoustic communication typically fall into two main categories, reproductive and aggressive interactions, it is obvious that the former necessitates a species-specific code, while in the latter, messages may serve both conspecific and heterospecific exchanges. It results that the codes used to convey messages can vary depending on the intended recipients, those involved in interspecific communication being generally more concise than those used in intraspecific scenarios [22, 80].
2. Holocentridae
6Fishes of the family Holocentridae Bonaparte 1833 are worldwide marine tropical and subtropical reef fishes [81, 82]. The family name comes from “holos” and “kentron” in Greek, meaning “whole” and “sting,” respectively, and refers to the numerous spines on their body. Holocentridae are divided into two subfamilies: the Myripristinae (soldierfishes) and the Holocentrinae (squirrelfishes) [83]. The vernacular name “soldierfish” could be related to their swimming behaviour in nicely organized schools that are said to resemble military formations. Holocentrinae are commonly called “squirrelfish,” a name that derives from their large and bright eyes, characteristic of nocturnal species, allowing them to see in low light conditions similar to squirrels, which also have relatively large eyes for their size, well-suited for nocturnal or crepuscular vision.
7Another main difference between the two subfamilies consists in the shape of the swim bladder and its position with respect to the auditory bulla [83]. The swim bladder of the Holocentrinae is characterised by a single cavity extending all across the body cavity while the swim bladder of the Myripristinae consists of a main chamber and two anterior diverticula that insert directly next to the auditory bullae, at the posterior end of the neurocranium. This connection between the swim bladder and the inner ear would be responsible for enhanced hearing abilities (better sensitivity and larger frequency spectrum) in the Myripristinae [84, 85].
8Holocentrids are mainly nocturnal [86, 87]. They generally spend the day hidden in crevices, caves or beneath ledges of reefs and wait for the night fall to go and feed [86, 88, 89]. Their visual system is well adapted to this nocturnal lifestyle [90]. Despite these shared characteristics, Myripristinae and Holocentrinae differ in their behavioural ecology. Myripristinae species mainly live in non-territorial schools that can reach several dozens of individuals, whereas Holocentrinae species are considered more solitary and territorial [91, 92] Feeding behaviour also diverges between the two subfamilies: Myripristinae would mainly feed on zooplankton in the water column, whereas the diet of the Holocentrinae would mainly be composed of invertebrates and small fishes on the seabed [86]. Surprisingly, the only information available on the reproduction behaviours of holocentrids indicates that Myripristis would spawn in open water, apparently a few days after full moon. However, the original source of this information is unknown.
3. Acoustic Communication in Holocentridae
3.1. Sound production in Holocentridae: state of the art and update
9The American oceanographer and marine biologist Marie Poland Fish [93] was the first to evoke the ability of Holocentridae to produce sounds. Among the 91 recognized species of the family, 14 species from four genera are known to be vocal [57, 58, 88, 91, 92, 94]. Those species had been recorded either in the wild or under laboratory conditions. In the wild, holocentrids produce sounds both during the day and at night, with an increase calling rate after sunset [95] and peaks at dawn and dusk [91]. Spontaneous sound production has been reported for a wide variety of behaviours: when startled or handled [57, 94], during territory defence [91], predator signalling and alarm calls [53, 88, 91, 92]. Five sound types (growl, staccato, grunt, knock, thump) have been described for several species. However, whether the different sound types are associated with specific behaviours is unclear. It is indeed uncertain whether the different authors consistently used the same terms for different sound types, or if they used different terms to describe potentially identical sounds, the various terms being mixed. Moreover, in addition to a lack of physical descriptions and oscillograms, few quantitative data are provided, preventing statistical comparisons.
10The sound-production mechanism of holocentrids relies on the simultaneous contraction of paired bilateral sonic muscles [92], innervated by occipital nerves [96, 97], that originate on the skull and insert on ribs that are in tight connection with the swim bladder (Fig. 1), causing its vibration, which produces sounds [57, 94, 98]. In Holocentrus rufus, Gainer et al. [97] showed that the contraction rhythm of the sonic muscles determines the fundamental frequency (ca. 75–85 Hz). The general sonic mechanism is consistent across four investigated holocentrid genera (Holocentrus, Neoniphon, Sargocentron, Myripristis) [94]. The authors however reported variations in the insertions of the sonic muscles, tendons and ligaments, and the number of ribs involved in the mechanism and suggested that there was a relationship between this number of ribs and the number of peaks composing a pulse.
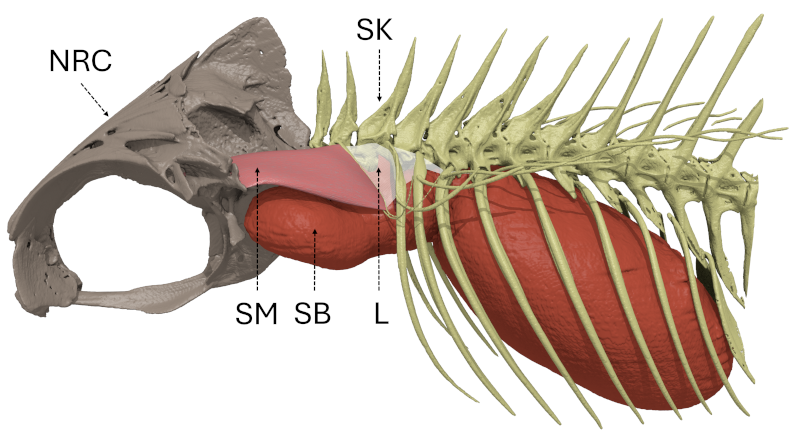
Figure 1: General organization of the sound-production mechanism of Myripristis vittata (Myripristinae). Lateral view of the left side. L: ligament; NRC: neurocranium; SB: swim bladder; SK: skeleton; SM: sonic muscle.
11The next part of this paper aims at providing a reflection issued from our data collected on the acoustic behaviour of holocentrids through three original approaches based on observations made in situ or in aquariums.
-
We have first provided the description of a new kind of acoustic communication in the symbiotic relationship between cleaner wrasses (Labridae) and their holocentrid clients [99]. While these cleaners can cooperate by removing ectoparasites from their clients, they can also deceive by feeding on the client’s mucus, a behaviour usually referred to as “cheating behaviour” that often leads to a discernible jolt from the client fish. In this first study, we have shown, using video cameras coupled with hydrophones, that nine different species of holocentrids can use acoustic communication throughout their interactions with cleaner fish, especially in moments of ending cooperation or rejection of the interaction. Sounds were predominantly observed during agonistic behaviours and seem to support visual cues from the client. This study provides a novel example of acoustic communication during a symbiotic relationship in teleosts. Interestingly, it also shows that acoustic communication in such a context is based on acoustic events that are made of one to several sounds, themselves composed of one to several pulses (Fig. 2a). The production of different sound types during the interaction indicates that there is no fixed acoustic signal that consistently corresponds to this kind of behaviour. However, the differences in the structure and composition of the acoustic events could be related to the severity of the client’s response.
-
We have then investigated the acoustic mobbing behaviour of a common holocentrid species, Sargocentron caudimaculatum, in response to a predator (i.e., moray eel) [100]. When a moray eel was introduced in the mesocosm occupied by S. caudimaculatum at night, several specimens swam towards the predator with their heads pointed in its direction and their dorsal fins erected while increasing their calling rate. Staccatos were the only sounds produced in this context. These stereotyped sounds consist of a variable number of grunts rapidly repeated (Fig. 2d). This observation supports a mobbing behaviour with specimens shifting from an escape behaviour to an aggressive response in the presence of predators. In the same way, responses of H. rufus to the approach or intrusion of their territories by large heterospecific fishes, as also often exhibited towards human observers, mainly consisted in the production of staccato calls accompanied with dorsal fin erection [91]. According to Winn et al. [91], staccatos could be alarm calls, that are produced as a result of the sudden presentation of any strange object, in particular large fish.
-
Finally, we have described the behavioural contexts of sound emission and related sounds for nine species in their natural environment [101]. This last study shows that all holocentrid species are able to produce sounds in at least six behavioural contexts of both agonistic (conspecific and heterospecific chases, competition) and social signalling (acceleration, broadcasting, body quivering) types. Similarly to the acoustic communication between holocentrids and cleaner wrasses, this study shows that acoustic communication in these behaviours is based on acoustic events whose variability (number, types and order of sounds composing the events) prevents consistent association with any specific behaviour. They can indeed be made of a single sound or a series of sounds of different types that are arranged randomly (Fig. 2b,c).
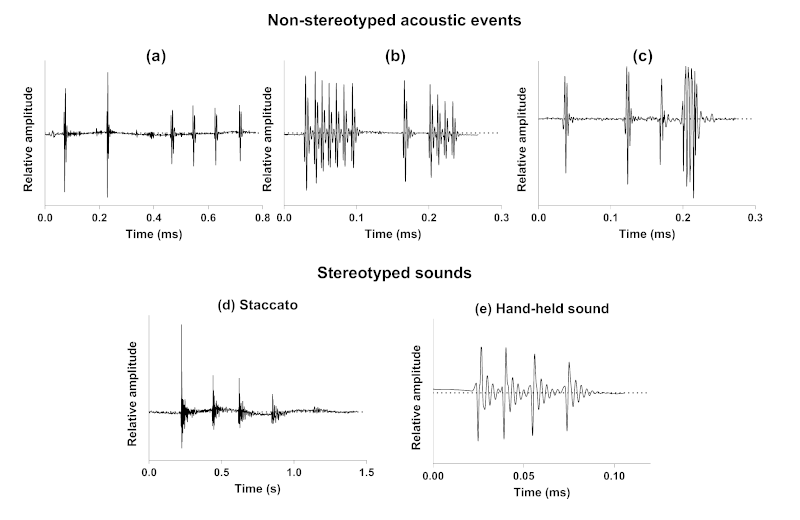
Figure 2: Example of non-stereotyped acoustic events produced by Neoniphon sammara during symbiotic (a), agonistic (b) and social signalling (c) interactions, and of stereotyped staccato (d) and hand-held (e) sounds produced by Sargocentron caudimaculatum.
3.2. Diversity of sounds and contexts of sound production in Holocentridae
12Our contribution to the understanding of acoustic communication in social interaction contexts in Holocentridae is notable for being based on a large number of species investigated in the wild across the Indo-Pacific Ocean (about 77 hours of diurnal video recordings from 64 refuges used by Holocentridae in French Polynesia, Guam, Seychelles and Philippines), in addition to the response experiment of S. caudimaculatum to the introduction of a moray eel in experimental conditions at night. It also provides new insights into the use of sounds in different behaviours.
13The results of this broad scale study reveal that holocentrids produce sounds at least in both agonistic (conspecific and heterospecific chases, competition) and social signalling (acceleration, broadcasting, body quivering) contexts [101], but also as a way to refuse or terminate their symbiotic relationship with cleaner fishes Labroides spp [99]. No spawning behaviour was unfortunately observed, which is in accordance with the literature where there is virtually no information on the mating of Holocentridae [89]. Holocentrids are mainly nocturnal, as suggested by their large eyes [86, 87]. Moreover, while they may occasionally be caught on hook and line, their stomachs are almost always empty, except at night and early in the morning [102]. During the day, Holocentrinae (squirrelfishes) are likely territorial, hiding in holes and cracks in the reef. However, this behavioural trait probably diminishes at night when they leave their refuges to hunt [89, 91]. In various species from the West Indies, stomachs sometimes contain fragments of seagrass along with prey, indicating that the fish had been foraging in grass beds away from their home reef [102]. Although no observations concerning spawning were made, large seasonal aggregations have been observed for H. rufus in Florida [103]. All these behavioural observations support that these species are primarily active at night, particularly in terms of foraging, which involves migration away from their shelters. This suggests that the full range of their behavioural repertoire, including reproductive activities, may not have been captured during our daytime recordings in the wild. Furthermore, if daytime reproductive activities were taking place on the reefs, they would likely have been observed.
14For over five decades, the sounds of Holocentridae have primarily been known through their onomatopoeia (growl, staccato, grunt, knock, thump). Our field observations have now provided both quantitative and qualitative physical descriptions for each of these sounds, and confirmed their stereotypy [101]. Surprisingly, our study also reveals that these different sound types are not consistently associated to any particular behaviour but can be emitted in various behavioural contexts. Additional studies are therefore required to understand the coexistence of different kinds of sounds within holocentrids. Within the framework of current knowledge, and given that all these sounds appear to be produced using the same mechanism, we suggest that differences could be related to the motivational state of the sender or the intended recipient.
3.3. Association between sounds and behavioural contexts
15In Holocentridae, acoustic events emitted during agonistic and social signalling interactions, regardless of the behaviour or species, are composed of stereotyped sounds. However, the number of sounds, their type and the arrangement of these units are not fixed (Fig. 2b,c). Therefore, they do not uniquely indicate any particular behaviour [101]. Similarly, acoustic signals frequently involved, additionally to visual cues, in the mediation of their interaction with cleaner wrasses, consist of variable sequences of sounds (Fig. 2a; [99]). In the latter context, sounds are produced by the holocentrids to refuse the interaction or to express their desire to end the cooperation. This absence of stereotypy in sound production (i.e., acoustic events with variable composition and structure) is highly surprising, but could be related to the limitations of our observations. For instance, the relationship between the holocentrid and the cleaner wrasse is not instantaneous; it may be conditioned by the history of interactions between the participants. An holocentrid client that has been deceived by the cheating behaviour of the cleaner wrasse (i.e., feeding on the client’s mucus instead of its ectoparasites) might recognize it, just as the cleaner wrasse may adopt different behaviours based on previous encounters with the holocentrid client or depending on specific competitors present in the environment [104–106]. Our observations did not consider histories between protagonists but merely allowed us to observe an acoustic behaviour at a specific moment. It could thus be considered that the type of acoustic message from the holocentrid depends on the history between the participants. Moreover, the number of sounds produced during the interaction and their type could also be related to the severity of the punishment (i.e., the motivation) that the holocentrid wishes to impose on the cheating cleaner wrasse. The apparent lack of stereotypy in sound production could also be related to emotional or motivational aspects that we were unable to assess in our studies. Although there may be some minor differences [94], the sound production mechanism in Holocentridae is based on a common principle where a pair of sonic muscles contracts regularly to move the first ribs, which are closely linked to the swim bladder, thereby generating sound. This mechanical aspect of sound production easily suggests that the various types of sounds described for Holocentridae [99, 101] could result from simple modifications in the rhythm and number of contractions [107–109]. The basic principle is that one contraction–relaxation cycle produces a pulse. Variations in the number of contractions therefore define the number of pulses composing a sound, whereas variations in the generation of the nerve impulses would affect the pulse period. In case of rapid muscle stimulation, nerve impulses could generate a summation preventing the different pulses to be fully expressed in the sound because they are followed by the initiation of the next pulse, the resulting sound consisting in several close pulses rather than well distinct pulses. These modifications in the firing rate easily explain the production of different kinds of sounds. If our behavioural study allows us to describe the different types of sounds, it does not enable us to gauge the sender’s intention and thus link it to the type of sound produced. At this stage, we can only specify that the type of sound does not necessarily correspond to a specific behaviour as such. Also, a lack of stereotypy in sound production could be related to the communicative aspect, which is not solely directed towards conspecifics but must also be understood by heterospecifics.
16While different sound types can be produced in the same behavioural contexts, we have identified certain behaviours in Holocentridae that are exclusively associated with one stereotyped sound. When confronted by a predator (i.e., moray eel), holocentrids shift from an escape behaviour to an aggressive response referred to as mobbing behaviour [100]. This behaviour would consist in a strategy to reduce predation risk [110]. During such mobbing behaviour, holocentrids only emit staccato sounds, supporting that these calls consist in alarm signals (Fig. 2d; [100]). It is important to note that staccato sounds were produced before the introduction of the predator into the aquarium in our experiment. However, their calling rate significantly increased upon the predator introduction, which also suggests that isolated sounds might have a different meaning when emitted alone or in a group. In another distress behavioural context, hand-held fish always produced the same kind of stereotyped sounds (Fig. 2e). The consistency and predictability of these sounds are such that they were used to conduct a comparative analysis aimed at determining whether they encode a species-specific character [111].
17The use of two different types of distress calls (hand-held and staccato sounds) suggest the ability of holocentrids to provide graded information based on the perceived risk. Such ability has also been reported in mammals such as primates [8] or mongooses that modify the structure of their calls based on both the type of predator and the level of urgency [9]. In birds (i.e., Siberian jays), anti-predator calls can also inform on the predator behaviour (perched, searching and attacking hawks) [10], in addition to both the predator category and the risk posed by a predator as observed from mobbing calls [11]. In such stressful contexts, acoustic signals could convey information on the location and type of danger faced by the caller, whereas more frequent harmless situations would not need to carry such information. In this case, the non-stereotyped acoustic signals produced would strengthen the visual behaviours, such as in O. niloticus [112].
Acknowledgments
18This research was made possible through grants from the Fonds de la Recherche Scientifique–FNRS, Grant/Award (T.0192.20).
Further Information
Author’s ORCID identifier
190000-0001-9689-1079 (Marine Banse)
Conflicts of interest
20The author declares no conflict of interest.
Bibliographie
[1] Myrberg, A. A. (1981) Sound communication and interception in fishes. In Hearing and Sound Communication in Fishes, pages 395–426. Springer, New York. https://dx.doi.org/10.1007/978-1-4615-7186-5_20.
[2] Sueur, J. (2023) Histoire naturelle du silence. Actes Sud, 272 pages.
[3] Busnel, R. G. (editor) (1963) Acoustic Behaviour of Animals. Elsevier, Amsterdam (NL).
[4] Mathevon, N. (2021) Les animaux parlent, sachons les écouter. Humensciences Editions, 513 pages.
[5] Danchin, É., Giraldeau, L.-A., and Cézilly, F. (2005) Écologie comportementale – Cours et questions de réflexion. Dunod, Malakoff (FR), 637 pages.
[6] Chen, Z. and Wiens, J. J. (2020) The origins of acoustic communication in vertebrates. Nature Communications, 11, 369. https://dx.doi.org/10.1038/s41467-020-14356-3.
[7] Ladich, F. (editor) (2015) Sound Communication in Fishes. Springer, Wien (AT), 244 pages. https://dx.doi.org/10.1007/978-3-7091-1846-7.
[8] Seyfarth, R. M., Cheney, D. L., and Marler, P. (1980) Monkey responses to three different alarm calls: Evidence of predator classification and semantic communication. Science, 210, 801–803. https://dx.doi.org/10.1126/science.7433999.
[9] Manser, M. B. (2001) The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proceedings of the Royal Society of London. Series B: Biological Sciences, 268, 2315–2324. https://dx.doi.org/10.1098/rspb.2001.1773.
[10] Griesser, M. (2008) Referential calls signal predator behavior in a group-living bird species. Current Biology, 18(1), 69–73. https://dx.doi.org/10.1016/j.cub.2007.11.069.
[11] Griesser, M. (2009) Mobbing calls signal predator category in a kin group-living bird species. Proceedings of the Royal Society B: Biological Sciences, 276, 2887–2892. https://dx.doi.org/10.1098/rspb.2009.0551.
[12] Mahamoud-Issa, M. (2023) Functions and mechanisms of intra-group communication and song coordination during territorial defense in a group breeding bird species, the Yellow-breasted Barbet (Trachyphonus margaritatus). Ph.D. thesis, Adam Mickiewicz University of Poznań, Poznań (PL). https://bip.amu.edu.pl/__data/assets/pdf_file/0017/522413/Mahamoud-Issa-Mathieu_rozprawa-doktorska.pdf.
[13] Mine, J. G., Slocombe, K. E., Willems, E. P., Gilby, I. C., Yu, M., Thompson, M. E., Muller, M. N., Wrangham, R. W., Townsend, S. W., and Machanda, Z. P. (2022) Vocal signals facilitate cooperative hunting in wild chimpanzees. Science Advances, 8(30), eabo5553. https://dx.doi.org/10.1126/sciadv.abo5553.
[14] Amorim, M. C. P., Vasconcelos, R. O., and Fonseca, P. J. (2015) Fish sounds and mate choice. In Sound Communication in Fishes, edited by Ladich, F., pages 1–33. Springer-Verlag, Wien (AT). https://dx.doi.org/10.1007/978-3-7091-1846-7_1.
[15] Amorim, M. C. P. (2006) Diversity of sound production in fish. In Communication in Fishes, edited by Ladich, F., Collin, S. P., Moller, P., and Kapoor, B. G., volume 1, pages 71–105. Science Publishers.
[16] Hawkins, A. D. (1986) Underwater sound and fish behaviour. In The Behaviour of Teleost Fishes, edited by Pitcher, T. J., pages 114–151. Springer, Boston (US-MA). https://dx.doi.org/10.1007/978-1-4684-8261-4_5.
[17] Ladich, F. and Myrberg, A. A., Jr. (2006) Agonistic behavior and acoustic communication. In Communication in Fishes, edited by Ladich, F., Collin, S. P., Moller, P., and Kapoor, B. G., volume 1, chapter 5, pages 121–148. Science Publishers.
[18] Schwarz, A. (2010) The inhibition of aggressive behavior by sound in the cichlid fish, Cichlasoma centrarchus. Zeitschrift für Tierpsychologie, 35(5), 508–517. https://dx.doi.org/10.1111/j.1439-0310.1974.tb00464.x.
[19] Parmentier, E., Kéver, L., Casadevall, M., and Lecchini, D. (2010) Diversity and complexity in the acoustic behaviour of Dacyllus flavicaudus (Pomacentridae). Marine Biology, 157(10), 2317–2327. https://dx.doi.org/10.1007/s00227-010-1498-1.
[20] Lugli, M., Torricelli, P., Pavan, G., and Mainardi, D. (1997) Sound production during courtship and spawning among freshwater gobiids (pisces, gobiidae). Marine and Freshwater Behaviour and Physiology, 29(1-4), 109–126. https://dx.doi.org/10.1080/10236249709379003.
[21] Boyle, K. S. and Cox, T. E. (2009) Courtship and spawning sounds in bird wrasse Gomphosus varius and saddle wrasse Thalassoma duperrey. Journal of Fish Biology, 75(10), 2670–2681. https://dx.doi.org/10.1111/j.1095-8649.2009.02459.x.
[22] Mann, D. A. and Lobel, P. S. (1998) Acoustic behavior of the damselfish dascyllus albisella: behavioral and geographic variation. Environmental Biology of Fishes, 51(4), 421–428. https://dx.doi.org/10.1023/A:1007410429942.
[23] Lobel, P. S. and Mann, D. A. (1995) Spawning sounds of the damselfish, Dascyllus Albisella (Pomacentridae), and relationship to male size. Bioacoustics, 6(3), 187–198. https://dx.doi.org/10.1080/09524622.1995.9753289.
[24] Casaretto, L., Picciulin, M., Olsen, K., and Hawkins, A. D. (2014) Locating spawning haddock (Melanogrammus aeglefinus, linnaeus, 1758) at sea by means of sound. Fisheries Research, 154, 127–134. https://dx.doi.org/10.1016/j.fishres.2014.02.010.
[25] Bolgan, M., Crucianelli, A., Mylonas, C. C., Henry, S., Falguière, J. C., and Parmentier, E. (2020) Calling activity and calls’ temporal features inform about fish reproductive condition and spawning in three cultured sciaenidae species. Aquaculture, 524, 735243. https://dx.doi.org/10.1016/j.aquaculture.2020.735243.
[26] Lobel, P. S. (1992) Sounds produced by spawning fishes. Environmental Biology of Fishes, 33(4), 351–358. https://dx.doi.org/10.1007/BF00010947.
[27] Colleye, O. and Parmentier, E. (2012) Overview on the diversity of sounds produced by clownfishes (pomacentridae): Importance of acoustic signals in their peculiar way of life. PLoS ONE, 7(11), e49179. https://dx.doi.org/10.1371/journal.pone.0049179.
[28] Wahlberg, M. and Westerberg, H. (2003) Sounds produced by herring (Clupea harengus) bubble release. Aquatic Living Resources, 16(3), 271–275. https://dx.doi.org/10.1016/S0990-7440(03)00017-2.
[29] Hastings, P. A., Walker, H. J., and Galland, G. R. (2014) Fishes: A Guide to Their Diversity. University of California Press, Oakland (US-CA), 1. edition, 345 pages.
[30] Lobel, P. S., Kaatz, I. M., and Rice, A. N. (2010) Acoustical behavior of coral reef fishes. In Reproduction and Sexuality in Marine Fishes: Patterns and Processes, edited by Cole, K., chapter 10, pages 307–386. University of California Press, Berkeley (US-CA). https://dx.doi.org/10.1525/california/9780520264335.003.0010.
[31] Aristotle (2016) The History of Animals. Kypros Press.
[32] Popper, A. N., Amorim, C., Fine, M. L., Higgs, D. M., Mensinger, A. F., and Sisneros, J. A. (2024) Introduction to the special issue on fish bioacoustics: Hearing and sound communication. The Journal of the Acoustical Society of America, 155(4), 2385–2391. https://dx.doi.org/10.1121/10.0025553.
[33] Froese, R. and Pauly, D. (2024). FishBase. World Wide Web electronic publication. https://www.fishbase.org. Last access: 15 May 2024.
[34] Looby, A., Cox, K., Bravo, S., Rountree, R., Juanes, F., Riera, A., Vela, S., Davies, H. L., Reynolds, L. K., and Martin, C. W. (2023) Fish sound production research: Historical practices and ongoing challenges. In The Effects of Noise on Aquatic Life, edited by Popper, A. N., Sisneros, J., Hawkins, A. D., and Thomsen, F., pages 1–20. Springer, Cham (CH). https://dx.doi.org/10.1007/978-3-031-10417-6_92-1.
[35] Parmentier, E., Bertucci, F., Bolgan, M., and Lecchini, D. (2021) How many fish could be vocal? An estimation from a coral reef (Moorea Island). Belgian Journal of Zoology, 151, 1–29. https://dx.doi.org/10.26496/bjz.2021.82.
[36] Parsons, M. J. G. (2009) An investigation into active and passive acoustic techniques to study aggregating fish species. Ph.D. thesis, Curtin University, Perth (AU). https://espace.curtin.edu.au/bitstream/20.500.11937/2133/2/147448_Parsons%20M%202009%20Full.pdf.
[37] Akamatsu, T., Okumura, T., Novarini, N., and Yan, H. Y. (2002) Empirical refinements applicable to the recording of fish sounds in small tanks. The Journal of the Acoustical Society of America, 112(6), 3073–3082. https://dx.doi.org/10.1121/1.1515799.
[38] Okumura, T., Akamatsu, T., and Yan, H. Y. (2002) Analyses of small tank acoustics: Empirical and theoretical approaches. Bioacoustics, 12(2-3), 330–332. https://dx.doi.org/10.1080/09524622.2002.9753738.
[39] Banse, M., Lecchini, D., Bertucci, F., and Parmentier, E. (2023) Reliable characterization of sound features in fishes begins in open-water environments. The Journal of the Acoustical Society of America, 154(1), 270–278. https://dx.doi.org/10.1121/10.0020149.
[40] Parmentier, E., Tock, J., Falguière, J.-C., and Beauchaud, M. (2014) Sound production in Sciaenops ocellatus: Preliminary study for the development of acoustic cues in aquaculture. Aquaculture, 432, 204–211. https://dx.doi.org/10.1016/j.aquaculture.2014.05.017.
[41] Mosharo, K. K. and Lobel, P. S. (2023) A comparison of underwater speakers for fish playback studies. The Journal of the Acoustical Society of America, 154(4), 2365–2382. https://dx.doi.org/10.1121/10.0021307.
[42] Rice, A. N., Farina, S. C., Makowski, A. J., Kaatz, I. M., Lobel, P. S., Bemis, W. E., and Bass, A. H. (2022) Evolutionary patterns in sound production across fishes. Ichthyology & Herpetology, 110(1), 1–12. https://dx.doi.org/10.1643/i2020172.
[43] Ladich, F. and Fine, M. L. (2006) Sound-generating mechanisms in fishes: A unique diversity in vertebrates. In Communication in Fishes, edited by Ladich, F., Collin, S. P., Moller, P., and Kapoor, B. G., volume 1, chapter 1, pages 1–41. Science Publishers.
[44] Looby, A., Cox, K., Bravo, S., Rountree, R., Juanes, F., Reynolds, L. K., and Martin, C. W. (2022) A quantitative inventory of global soniferous fish diversity. Reviews in Fish Biology and Fisheries, 32(2), 581–595. https://dx.doi.org/10.1007/s11160-022-09702-1.
[45] Eschmeyer W., N., Fricke, R., and Fong, J. D. Eschmeyer’s catalog of fishes. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. Electronic version accessed 18 April 2024.
[46] Tang, K. L., Stiassny, M. L. J., Mayden, R. L., and DeSalle, R. (2021) Systematics of damselfishes. Ichthyology & Herpetology, 109(1), 258–318. https://dx.doi.org/10.1643/i2020105.
[47] Fine, M. L., Lin, H., Nguyen, B. B., Rountree, R. A., Cameron, T. M., and Parmentier, E. (2007) Functional morphology of the sonic apparatus in the fawn cusk-eel Lepophidium profundorum (Gill, 1863). Journal of Morphology, 268(11), 953–966. https://dx.doi.org/10.1002/jmor.10551.
[48] Parmentier, E., Fontenelle, N., Fine, M., Vandewalle, P., and Henrist, C. (2006) Functional morphology of the sonic apparatus in ophidion barbatum (teleostei, ophidiidae). Journal of Morphology, 267(12), 1461–1468. https://dx.doi.org/10.1002/jmor.10496.
[49] Howes, G. J. (1992) Notes on the anatomy and classification of ophidiiform fishes with particular reference to the abyssal genus Acanthonus Günther, 1878. Bulletin of the British Museum (Natural History) Zoology, 58(2), 95–131. https://biostor.org/reference/114566.
[50] Marshall, N. B. (1967) Sound-producing mechanisms and the biology of deep-sea fishes. In Marine Bio-Acoustics: Volume 2, edited by Tavolga, W. N., volume 2, pages 123–133. Pergamon Press, Oxford (UK).
[51] Bolgan, M. and Parmentier, E. (2020) The unexploited potential of listening to deep-sea fish. Fish and Fisheries, 21(6), 1238–1252. https://dx.doi.org/10.1111/faf.12493.
[52] Fine, M. L. and Parmentier, E. (2015) Mechanisms of fish sound production. In Sound Communication in Fishes, edited by Ladich, F., pages 77–126. Springer-Verlag, Wien (AT). https://dx.doi.org/10.1007/978-3-7091-1846-7_3.
[53] Mélotte, G., Vigouroux, R., Michel, C., and Parmentier, E. (2016) Interspecific variation of warning calls in piranhas: a comparative analysis. Scientific Reports, 6(1), 36127. https://dx.doi.org/10.1038/srep36127.
[54] Colleye, O., Vandewalle, P., Lanterbecq, D., Lecchini, D., and Parmentier, E. (2011) Interspecific variation of calls in clownfishes: degree of similarity in closely related species. BMC Evolutionary Biology, 11, 365. https://dx.doi.org/10.1186/1471-2148-11-365.
[55] Horvatić, S., Malavasi, S., Vukić, J., Šanda, R., Marčić, Z., Ćaleta, M., Lorenzoni, M., Mustafić, P., Buj, I., Onorato, L., Ivić, L., Cavraro, F., and Zanella, D. (2021) Correlation between acoustic divergence and phylogenetic distance in soniferous european gobiids (Gobiidae; Gobius lineage). PLoS ONE, 16(12), e0260810. https://dx.doi.org/10.1371/journal.pone.0260810.
[56] Kaatz, I. M. and Stewart, D. J. (2012) Bioacoustic variation of swimbladder disturbance sounds in neotropical doradoid catfishes (Siluriformes: Doradidae, Auchenipteridae): Potential morphological correlates. Current Zoology, 58(1), 171–188. https://dx.doi.org/10.1093/czoolo/58.1.171.
[57] Moulton, J. M. (1958) The acoustical behavior of some fishes in the Bimini area. The Biological Bulletin, 114(3), 357–374. https://dx.doi.org/10.2307/1538991.
[58] Tricas, T. C. and Boyle, K. S. (2014) Acoustic behaviors in Hawaiian coral reef fish communities. Marine Ecology Progress Series, 511, 1–16. https://dx.doi.org/10.3354/meps10930.
[59] Parmentier, E., Herrel, A., Banse, M., Hornstra, H., Bertucci, F., and Lecchini, D. (2023) Diving into dual functionality: Swim bladder muscles in lionfish for buoyancy and sonic capabilities. Journal of Anatomy, 244(2), 249–259. https://dx.doi.org/10.1111/joa.13963.
[60] Raick, X., Lecchini, D., Kéver, L., Colleye, O., Bertucci, F., and Parmentier, É. (2018) Sound production mechanism in triggerfish (Balistidae): a synapomorphy. Journal of Experimental Biology, 221(1), jeb168948. https://dx.doi.org/10.1242/jeb.168948.
[61] Parmentier, E., Boyle, K. S., Berten, L., Brié, C., and Lecchini, D. (2011) Sound production and mechanism in Heniochus chrysostomus (Chaetodontidae). Journal of Experimental Biology, 214(16), 2702–2708. https://dx.doi.org/10.1242/jeb.056903.
[62] Bertucci, F., Parmentier, E., Hillion, A., Cordonnier, S., Lecchini, D., and René-Trouillefou, M. (2021) First highlight of sound production in the glassy sweeper Pempheris schomburgkii (Pempheridae). Marine Biology, 168(3), 32. https://dx.doi.org/10.1007/s00227-021-03829-8.
[63] Parmentier, E., Donaldson, T., and Banse, M. (2022) Cleaning of coral reef fishes by the humbug damselfish Dascyllus aruanus. Marine Biodiversity, 52(4), 39. https://dx.doi.org/10.1007/s12526-022-01275-3.
[64] Ruppé, L., Clément, G., Herrel, A., Ballesta, L., Décamps, T., Kéver, L., and Parmentier, E. (2015) Environmental constraints drive the partitioning of the soundscape in fishes. Proceedings of the National Academy of Sciences, 112(19), 6092–6097. https://dx.doi.org/10.1073/pnas.1424667112.
[65] Di Iorio, L., Raick, X., Parmentier, E., Boissery, P., Valentini-Poirier, C.-A., and Gervaise, C. (2018) ‘Posidonia meadows calling’: a ubiquitous fish sound with monitoring potential. Remote Sensing in Ecology and Conservation, 4(3), 248–263. https://dx.doi.org/10.1002/rse2.72.
[66] Parmentier, E., Fine, M. L., and Mok, H.-K. (2016) Sound production by a recoiling system in the pempheridae and terapontidae. Journal of Morphology, 277(6), 717–724. https://dx.doi.org/10.1002/jmor.20529.
[67] Ladich, F. and Schleinzer, G. (2018) Sound production in female Trichopsis schalleri (Labyrinth fishes): comparison to males and evolutionary considerations. Bioacoustics, 29(2), 123–139. https://dx.doi.org/10.1080/09524622.2018.1555773.
[68] Johnston, C. E. and Vives, S. P. (2003) Sound production in Codoma ornata (Girard) (Cyprinidae). Environmental Biology of Fishes, 68(1), 81–85. https://dx.doi.org/10.1023/A:1026067913329.
[69] Parmentier, E., Lanterbecq, D., and Eeckhaut, I. (2016) From commensalism to parasitism in Carapidae (Ophidiiformes): heterochronic modes of development? PeerJ, 4, e1786. https://dx.doi.org/10.7717/peerj.1786.
[70] Parmentier, E. and Fine, M. L. (2016) Fish sound production: Insights. In Vertebrate Sound Production and Acoustic Communication, edited by Suthers, R. A., Fitch, W. T., Fay, R. R., and Popper, A. N., Springer Handbook of Auditory Research, volume 53, chapter 19–49, pages 19–49. Springer, Cham (CH). https://dx.doi.org/10.1007/978-3-319-27721-9_2.
[71] Ladich, F. (2018) Acoustic communication in fishes: Temperature plays a role. Fish and Fisheries, 19(4), 598–612. https://dx.doi.org/10.1111/faf.12277.
[72] Myrberg, A. A. and Riggio, R. J. (1985) Acoustically mediated individual recognition by a coral reef fish (Pacentrus partitus). Animal Behaviour, 33(2), 411–416. https://dx.doi.org/10.1016/S0003-3472(85)80065-8.
[73] Bertucci, F., Beauchaud, M., Attia, J., and Mathevon, N. (2010) Sounds modulate males’ aggressiveness in a cichlid fish. Ethology, 116(12), 1179–1188. https://dx.doi.org/10.1111/j.1439-0310.2010.01841.x.
[74] Hawkins, A. D. and Amorim, M. C. P. (2000) Spawning sounds of the male haddock, melanogrammus aeglefinus. Environmental Biology of Fishes, 59(1), 29–41. https://dx.doi.org/10.1023/A:1007615517287.
[75] Rowe, S. and Hutchings, J. A. (2006) Sound production by atlantic cod during spawning. Transactions of the American Fisheries Society, 135(2), 529–538. https://dx.doi.org/10.1577/t04-061.1.
[76] Hyacinthe, C., Attia, J., and Rétaux, S. (2019) Evolution of acoustic communication in blind cavefish. Nature Communications, 10, 4231. https://dx.doi.org/10.1038/s41467-019-12078-9.
[77] Parmentier, E., Fine, M. L., Berthe, C., and Lecchini, D. (2018) Taxonomic validation of Encheliophis chardewalli with description of calling abilities. Journal of Morphology, 279(7), 864–870. https://dx.doi.org/10.1002/jmor.20816.
[78] Parmentier, E., Colleye, O., and Lecchini, D. (2016) New insights into sound production in Carapus mourlani (Carapidae). Bulletin of Marine Science, 92(3), 335–342. https://dx.doi.org/10.5343/bms.2016.1014.
[79] Bussmann, K., Utne-Palm, A. C., and de Jong, K. (2020) Sound production in male and female corkwing wrasses and its relation to visual behaviour. Bioacoustics, 30(6), 629–651. https://dx.doi.org/10.1080/09524622.2020.1838324.
[80] Lagardère, J. P., Millot, S., and Parmentier, E. (2005) Aspects of sound communication in the pearlfish Carapus boraborensis and Carapus homei (Carapidae). Journal of Experimental Zoology Part A: Comparative Experimental Biology, 303A(12), 1066–1074. https://dx.doi.org/10.1002/jez.a.230.
[81] Nair, R. J. and Dineshkumar, S. (2016) New distributional records of three soldier fishes (Pisces: Holocentridae: Myripristis) from Indian waters. Marine Biodiversity Records, 9, 89. https://dx.doi.org/10.1186/s41200-016-0092-8.
[82] Nelson, J. S. (2006) Fishes of the World. John Wiley & Sons, Hoboken (US-NJ), 4. edition, xix+601 pages.
[83] Nelson, E. M. (1955) The morphology of the swim bladder and auditory bulla in the Holocentridae. Fieldiana Zoology, 37(5), 121–130. https://dx.doi.org/10.5962/bhl.title.2938.
[84] Coombs, S. and Popper, A. N. (1979) Hearing differences among Hawaiian squirrelfish (family Holocentridae) related to differences in the peripheral auditory system. Journal of Comparative Physiology · A, 132(3), 203–207. https://dx.doi.org/10.1007/BF00614491.
[85] Tavolga, W. N. and Wodinsky, J. (1963) Auditory capacities in fishes: pure tone thresholds in nine species of marine teleosts. Bulletin of the American Museum of Natural History, 126(2), 179–239. http://hdl.handle.net/2246/1122.
[86] Nelson, J. S. (1994) Fishes of the world. John Wiley & Sons, Hoboken (US-NJ), 3. edition, 600 pages.
[87] Randall, J. E. (1955) Fishes of the Gilbert Islands. Atoll Research Bulletin, 47, 1–243. https://dx.doi.org/10.5479/si.00775630.47.1.
[88] Horch, K. and Salmon, M. (1973) Adaptations to the acoustic environment by the squirrelfishes Myripristis violaceus and M. Pralinius. Marine Behaviour and Physiology, 2(1-4), 121–139. https://dx.doi.org/10.1080/10236247309386920.
[89] Wyatt, J. (1983) The biology, ecology and bionomics of the squirrelfishes, holocentridae. In Caribbean coral reef fishery resources, edited by Munro, J. L., pages 50–58. International Center for Living Aquatic Resources Management, Manila (PH). https://pdf.usaid.gov/pdf_docs/PNAAN957.pdf.
[90] de Busserolles, F., Cortesi, F., Fogg, L., Stieb, S. M., Luehrmann, M., and Marshall, N. J. (2020) The visual ecology of Holocentridae, a nocturnal coral reef fish family with a deep-sea-like multibank retina. Journal of Experimental Biology, 224(1), jeb233098. https://dx.doi.org/10.1242/jeb.233098.
[91] Winn, H. E., Marshall, J. A., and Hazlett, B. (1964) Behavior, diel activities, and stimuli that elicit sound production and reactions to sounds in the longspine squirrelfish. Copeia, 1964(2), 413–425. https://dx.doi.org/10.2307/1441036.
[92] Salmon, M. (1967) Acoustical behavior of the Menpachi, Myripristis berndti, in Hawaii. Pacific Science, 21, 364–381. https://www.biodiversitylibrary.org/part/243617.
[93] Fish, M. P. (1948) Sonic fishes of the Pacific. Woods Hole Oceanographic Institution. https://dx.doi.org/10.1575/1912/2767.
[94] Parmentier, E., Vandewalle, P., Brié, C., Dinraths, L., and Lecchini, D. (2011) Comparative study on sound production in different Holocentridae species. Frontiers in Zoology, 8(1), 12. https://dx.doi.org/10.1186/1742-9994-8-12.
[95] Luczkovich, J., Stewart, C., and Sprague, M. (2002) Nocturnal sound production by longspine squirrelfish (holocentrus rufus) with notes on sound production by fishes on the Turneffe Atoll coral reef in Belize. The Journal of the Acoustical Society of America, 112(5_Supplement), 2202. https://dx.doi.org/10.1121/1.4778661.
[96] Carlson, B. A. and Bass, A. H. (2000) Sonic/vocal motor pathways in squirrelfish (Teleostei, Holocentridae). Brain, Behavior and Evolution, 56(1), 14–28. https://dx.doi.org/10.1159/000006674.
[97] Gainer, H., Kusano, K., and Mathewson, R. F. (1965) Electrophysiological and mechanical properties of squirrelfish sound-producing muscle. Comparative Biochemistry and Physiology, 14(4), 661–671. https://dx.doi.org/10.1016/0010-406X(65)90253-7.
[98] Winn, H. E. and Marshall, J. A. (1963) Sound-producing organ of the squirrelfish, Holocentrus rufus. Physiological Zoology, 36(1), 34–44. https://www.jstor.org/stable/30152736.
[99] Banse, M., Lecchini, D., Sabbe, J., Hanssen, N., Donaldson, T., Iwankow, G., Lagant, A., and Parmentier, E. (2024) Production of sounds by squirrelfish during symbiotic relationships with cleaner wrasses. Scientific Reports, 14, 11158. https://dx.doi.org/10.1038/s41598-024-61990-8.
[100] Banse, M., Minier, L., Lecchini, D., and Parmentier, E. (2024) Acoustic mobbing behaviour: vocal fish responses to predation risk through sound communication. Marine Biology, 171(7), 141. https://dx.doi.org/10.1007/s00227-024-04455-w.
[101] Banse, M., Hanssen, N., Sabbe, J., Lecchini, D., Donaldson, T. J., Iwankow, G., Lagant, A., and Parmentier, E. (2024) Same calls, different meanings: Acoustic communication of Holocentridae. PLoS ONE, 19(11), e0312191. https://dx.doi.org/10.1371/journal.pone.0312191.
[102] Randall, J. E. (1967) Food Habits of Reef Fishes of the West Indies, Studies in tropical oceanography, volume 5, pages 665–847. Institute of Marine Sciences, University of Miami. https://www.aoml.noaa.gov/general/lib/CREWS/Cleo/PuertoRico/prpdfs/randall-habits.pdf.
[103] Domeier, M. and Colin, P. (1997) Tropical reef fish spawning aggregations: Defined and reviewed. Bulletin of Marine Science, 60(3), 698–726. https://www.ingentaconnect.com/contentone/umrsmas/bullmar/1997/00000060/00000003/art00006.
[104] Roche, D. G., Jornod, M., Douet, V., Grutter, A. S., and Bshary, R. (2021) Client fish traits underlying variation in service quality in a marine cleaning mutualism. Animal Behaviour, 175, 137–151. https://dx.doi.org/10.1016/j.anbehav.2021.03.005.
[105] Bshary, R. and Grutter, A. S. (2006) Image scoring and cooperation in a cleaner fish mutualism. Nature, 441, 975–978. https://dx.doi.org/10.1038/nature04755.
[106] McAuliffe, K., Drayton, L. A., Royka, A., Aellen, M., Santos, L. R., and Bshary, R. (2021) Cleaner fish are sensitive to what their partners can and cannot see. Communications Biology, 4, 1127. https://dx.doi.org/10.1038/s42003-021-02584-2.
[107] Bass, A. H. and McKibben, J. R. (2003) Neural mechanisms and behaviors for acoustic communication in teleost fish. Progress in Neurobiology, 69(1), 1–26. https://dx.doi.org/10.1016/S0301-0082(03)00004-2.
[108] Banse, M., Chagnaud, B. P., Huby, A., Parmentier, E., and Kéver, L. (2021) Sound production in piranhas is associated with modifications of the spinal locomotor pattern. Journal of Experimental Biology, 224(9), jeb242336. https://dx.doi.org/10.1242/jeb.242336.
[109] Millot, S., Vandewalle, P., and Parmentier, E. (2011) Sound production in red-bellied piranhas (Pygocentrus nattereri, Kner): an acoustical, behavioural and morphofunctional study. Journal of Experimental Biology, 214(21), 3613–3618. https://dx.doi.org/10.1242/jeb.061218.
[110] Caro, T. (2005) Antipredator Defenses in Birds and Mammals. University of Chicago Press, Chicago (US-IL), xv + 592 pages.
[111] Banse, M., Bertimes, E., Lecchini, D., Donaldson, T. J., Bertucci, F., and Parmentier, E. (2024) Sounds as taxonomic indicators in holocentrid fishes. npj Biodiversity, 3, 33. https://dx.doi.org/10.1038/s44185-024-00064-4.
[112] Longrie, N., Poncin, P., Denoël, M., Gennotte, V., Delcourt, J., and Parmentier, E. (2013) Behaviours associated with acoustic communication in Nile tilapia (Oreochromis niloticus). PLoS ONE, 8(4), e61467. https://dx.doi.org/10.1371/journal.pone.0061467.
To cite this article
About: Marine Banse
eMail: mbanse@uliege.be