- Portada
- Volume 21 (2018)
- number 3-4
- Bacterial origin of the red pigmentation of Phanerozoic carbonate rocks: an integrated study of geology-biology-chemistry (Ernest Van den Broeck medallist lecture 2017)
Vista(s): 2676 (58 ULiège)
Descargar(s): 598 (8 ULiège)
Bacterial origin of the red pigmentation of Phanerozoic carbonate rocks: an integrated study of geology-biology-chemistry (Ernest Van den Broeck medallist lecture 2017)

Abstract
Explaining the color of rocks is still a complex problem. This question was raised long ago in the community of geologists, particularly for the pigmentation of the ‘red marbles’ of the Frasnian of Belgium at the beginning of the last century, with many unsatisfactory hypotheses. Our recent analysis of different red carbonate rocks in Europe and North Africa (Morocco) may provide an alternative explanation for the color of these rocks. For this it was necessary to bring together diverse and complementary skills involving geologists, microbiologists and chemists. We present here a synthesis of these works. It is suggested that the red pigmentation of our studied Phanerozoic carbonate rocks, encompassing a time range from Pragian to Oxfordian, may be related to the activity of iron bacteria living in microaerophilic environments. A major conclusion is that this red color is only related to particular microenvironments and has no paleogeographic or climatic significance. All red carbonates have not necessarily acquired their pigmentation through the process established in this review. Each geological series must be analyzed in the light of a possible contribution of iron bacteria and Fungi.
Tabla de contenidos
1. Introduction
1The conference presented by one of us (A.P.) in September 2017 in honor of the Van den Broeck medal is the result of recurrent questions asked by many geologists, especially Belgians, about the origin of the red color of the Frasnian ‘marbles’ of our Ardennes. These marbles have been known throughout the world for several centuries both for their use as decorative material and also for the geological interpretation of their depositional setting and, naturally, of their red color.
2They are also known at a world scale by Dupont’s description (in 1881) who reported particular cavities, the ‘stromatactis’, later defined by Bathurst (1982). Since then an infinite literature has been devoted to them and the term ‘stromatactis’, originally described in the Belgian Frasnian, is commonly used in the Precambrian and global Phanerozoic carbonates.
3Finally, following numerous questions on the origin of these marbles, they were considered as ‘reefs’ as soon as they were discovered, Boulvain (1990) definitively established the depositional environment of these mud mounds showing how these ‘reefs’ were built in relation to relative sea level variations. The red color was also discussed in his work, and attributed to iron-bacterial activity in dysaerobic media.
4This study was initiated at the Université Libre de Bruxelles, at the initiative of Professors A. Herbosch and B. Mamet. It is with the latter that one of us (A.P.) decided to extend this theme to other ‘marbles’ and red limestones in various geological series of Europe and North Africa (Morocco). Very quickly it turned out that the Jurassic series of Rosso Ammonitico from Italy had the most interesting study potential. A sustained collaboration with Prof. Luca Martire from the University of Turin was then initiated.
5It also logically appeared that collaboration with microbiologists could help to clarify and validate the geological interpretation of microenvironmental conditions. This collaboration started during the PhD thesis of D. Gillan under the supervision of C. De Ridder (Laboratoire de Biologie marine, Université Libre de Bruxelles). As a result, geological interpretations and biological observations on Recent organisms (an irregular echinoid Echinocardium cordatum and a bivalve Tellimya (Montacuta) ferruginosa) were at the origin of a paleoecological model integrating activities of iron-bacteria and Fungi, and redox conditions in the ancient and Recent sediments.
6Finally, the third and last phase of this multidisciplinary research was devoted to the chemistry of iron-bacterial activities. With the collaboration of N. Mattielli and J. de Jong (G-Time Laboratory, ULB), the study of iron isotopes was conducted on carbonate red- (with iron-bacteria and Fungi) or gray- (without iron-bacteria and Fungi) matrices of the Rosso Ammonitico from Italy and also on iron deposits produced by iron bacteria in the biofilms of the echinoid E. cordatum and the bivalve T. ferruginosa. A clear isotopic biofractionation of iron isotopes was demonstrated in both cases, with the same order of magnitude of fractionation as observed in the Jurassic rocks. The origin of the red pigmentation of the rocks was therefore possibly linked to the activity of microorganisms.
7This research of nearly 15 years is the result of a continuous collaboration between geologists (sedimentologists and geochemists) and microbiologists. The results have been the subject of numerous reports to conferences, key lectures in universities or scientific institutes and congresses, Belgian academies or abroad, and of course many publications in journals of international rank. This is a particularly attractive subject, because it mixes geology and microbiology intimately and especially because it addresses one logical question in geology, namely how to explain the color of a rock! To answer this seemingly simple question is not easy. As proof, one of us (A.P) was invited to the Geoitalia Congress in 2011 to introduce a special session devoted to the origin of the color of sedimentary rocks (Préat et al., 2011a). Finally, it was clear that no one was able to explain the reason for the black, brown, white, pink color of the sedimentary rocks. On the other hand, the red color of our studied limestones may be explained by the activity of bacteria.
8This color problem is much deeper than we may think: why are almost all Paleozoic limestones much darker than almost all of the limestones in the Mesozoic and Cenozoic? Some hypotheses exist of course (compaction?, organic matter? ...) but on this subject no certainty exists. The problem is much more complicated than it appears, because many Precambrian limestones have intermediate colors between those of Mesozoic and Paleozoic limestones, they may be darker or lighter than those of the Phanerozoic. And finally, another particular question with no clear answer is the color of the dolomitic rocks!
9At the end of this introduction it's important to remember two points:
10(i) the first is that a question of very simple appearance (the color of the rocks) may require a long-term study and the collaboration of scientists from different disciplines (here especially geologists, biologists and chemists),
11(ii) the second is that the result of our study is counterintuitive, the red color of the limestones and marbles studied is related to dysoxic (i.e. with very low oxygen levels) microenvironments and the gray/blue limestones (for example those present near the top of the Belgian Frasnian mud mounds (Boulvain, 1990) or those interstratified in the Jurassic Rosso Ammonitico of Italy, Mamet & Préat, 2006) are well oxygenated. Préat et al. (2006) have shown that the presence of different colored facies in the Jurassic Rosso Ammonitico is related to the variation of oxygenation gradients. Bottom oxygenated conditions favored the spread of burrowers and prevented the development of iron-oxidizing bacterial biofilms (ultimately responsible for hematite) which require a poorly oxygenated environment. Among the different consequences (coloration, petrography, chemistry including the Fe-isotopes, see below) the structure (or ‘petrofabric’) of the micritic matrix will be pertained to the different colored samples and influenced by the bacterial filaments. In the case of occurrence of iron bacterial mats (in the red matrices), the coalescence among the micronic crystals was prevented leading to subhedral micritic structure producing microspar, while in the absence of the iron bacteria (in the gray facies), the fusion of the crystals was the rule and produced coalescent structures (see SEM figs 7 and 8 in Préat et al., 2006).
12Finally we must not conclude by making us assert what we have not said: many red limestones have also formed in the presence of oxygen, and, in these environments, iron-bacteria are eliminated, as high levels of oxygen are toxic to them (Fenchel & Finlay, 1995). Biological mediation is therefore replaced by ‘pure’ chemical activity. A single example can fix ideas: on many beaches of the world a worm called Arenicola marina lives in a burrow formed in fine sands. These sands can be few oxygenated and turn black when bacterial sulfate reduction produces H2S that combines to Fe to form black FeS. The wall of the burrow can be lined by red Fe(III) oxyhydroxides because seawater entering the burrow provides oxygen that chemically oxidizes the FeS of the sediment. The burrow wall shows a sedimentary discontinuity on a very small scale. Here, although iron-bacteria may be present, they cannot compete with the fast chemical oxidation of FeS.
13To be complete, let us quote red sedimentary rocks formed in oxygenated medium: this is the case of most of the very well-known CORB carbonates (or Cretaceous Oceanic Red Beds; Hu et al., 2009) and all the red sandstones of the Triassic of numerous geological series, of which the most famous are those of the Vosges (these sandstones served in the construction of Strasbourg Cathedral and many other monuments). Many other carbonates and red sandstones have also formed in oxygenated media.
14This multidisciplinary study has integrated many techniques from the field (geology) to the laboratory (biology and chemistry): for example, petrography and cathodoluminescence, SEM, XRD, energy dispersive XRD (EDAX), MC-ICP-MS, spectrophotometry, trace elements, isotopic analysis (carbon, oxygen, iron), DNA sequencing, etc.
15As the main part of our work on the subject has been published and presented at the conference of Geologica Belgica on September 28th, 2017, the present article is therefore a detailed summary with related references.
2. The question of the origin of the red pigmentation
16Different pathways can be proposed to explain the origin of the red pigmentation present in many Phanerozoic limestones. We investigated three possible models: (i) telogenetic alteration; (ii) detrital input and (iii) the role of iron bacteria. The third hypothesis may be supported by our data for a number of Phanerozoic series. With such a hypothesis, not only can we highlight the importance of the microaerophilic environments at the dysoxic-anoxic interfaces for the bacterial Fe-oxidation, but we can also conclude that the red limestones have no particular paleogeographic meaning. Whatever may be the relation with iron, the following three scenarios are possible:
-
telogenetic (post-sedimentary) superficial alteration;
-
detrital origin of the iron derived from continental weathering during sedimentation;
-
presence of iron-bacteria in the sedimentation site.
17In their ‘State of the Art’ article concerning this subject, Mamet & Préat (2006) concluded that the microbial (iron-bacteria and Fungi) hypothesis has the merit to be compatible with most observed pigmentation pattern. They gave seven major arguments synthesized as discussion in their conclusions.
18The scope of these results is of general interest because the 30 studied geological sections were obtained from 12 localities encompassing both a wide range of paleoenvironments (from littoral/coastal settings to pelagic basinal sedimentation) and of geological time (from Pragian – Lower Devonian – to Oxfordian – Upper Jurassic) in Europe and North Africa (Morocco).
19The studied series can be grouped as follows from the shallowest to the deepest environments:
-
‘Oolite ferrugineuse de Bayeux’, Middle Jurassic of Normandy; littoral in very shallow waters with red stromatolites (Préat et al., 2000);
-
‘Red marbles’, in the Czech Pragian; inner ramp setting (Mamet et al., 1997);
-
‘Griottes’ facies, in the Devonian of South France (Mamet & Perret, 1995, Préat et al., 1999), Viséan of North Spain (Mamet & Boulvain, 1990), shallow carbonate platform and outer ramp setting;
-
Red marbles in the Belgian Frasnian mud mounds (Boulvain, 1990, Boulvain et al., 2001), outer/distal ramp setting;
-
Red lenses in the Bashkirian (Carboniferous) of North Spain (Della Porta et al., 2007), slope setting;
-
Rosso Ammonitico, in the Jurassic of Italy (Martire, 1996; Mamet & Préat, 2003; Préat et al., 2006, 2008a), Jurassic of Sicily (Préat et al., 2011b), Jurassic of South of Spain (Mamet & Préat, 2007), hemipelagic basinal sedimentation;
-
Red condensed marbles, Upper Devonian of Anti-Atlas (Morocco) (Préat et al., 2008b), pelagic basinal sedimentation.
3. The results
3.1. Carbonate petrography
20The dispersion of the omnipresent but rare hematite or goethite in the micritic matrix caused the pigmentation, grading from pink to deep red. Hematite (or goethite, Baele et al., 2008) is submicronic. Despite some sedimentary characteristics are particular to each locality, eight major types of hematite/goethite concentrations have been recognized in nearly all the studied cases and related to filamentous iron bacteria forming various biofilms and microstromatolites filling original fossil cavities (foraminiferal chambers, bryozoan zooecia, gastropod chambers …) and developed on various firm surfaces (ostracod valves, crinoid ossicles, echinoid spines, hardgrounds …) (Mamet & Préat, 2006). Various types of stromatolites are present (crenulated stromatolites, microstromatolites, endolithic stromatolites and oncolites), biofilms are single or multiple, regular or irregular.
21Taking together all these observations common to all the studied series, we can summarize the petrographic analysis as follows:
22(1) Iron is not dispersed at random but is concentrated in a number of specific sedimentary features. It is not linked to tectonic fissures;
23(2) The only post-sedimentary reworking of the iron is observed in stylolites or in pressure solution seams;
24(3) Iron hydroxides closely fill tiny protoglobigerinid or fusuline chambers, a process which must have occurred early in the sedimentation. Thus, the iron is not related to late infiltration or later contamination. This is confirmed by the fact that exclusively the lattice of echinoderm plates is replaced;
25(4) Various stromatolites and oncolites are an indication of biological activity. Moreover endostromatolites grow in dark cavities emphasizing that there was no light control of their growth;
26(5) The most pertinent indication that a biological control is at the base of the phenomenon is the systematic presence of filaments. Omnipresent micronic regular filaments with Fe-rich sheaths can be reasonably attributed to iron-bacteria. They are normally destroyed during diagenesis but the presence of hardgrounds seems to be favorable for their preservation, and they could also be preserved in early cemented cavities. These hardgrounds indicate extremely slow sedimentation and no compaction. All these successions are condensed and did not suffer deep burial with a minimum of tectonic or diagenetic overprint, except in the Frasnian mounds where late burial cement was observed (Boulvain, 1990). Scattered sections of Paleozoic tectonized red limestones (for example Late Visean of Playa la Huelga, Asturias, Mamet pers. obs.) preserved ghosts of stromatolites but not filaments.
3.2. Biologic evidence
27How do these observations relate with the three pathways defined in the introduction? The first pathway related to near-surface has been proposed by Hofmann & Farmer (2000). They emphasized the possible existence and role of iron-bacteria in the alteration of various pre-existing rocks. There are still some doubts concerning the age of the observed iron structures in the >140 reported localities (Hofmann & Farmer, 2000). Many outcrops are fresh and could have been contaminated by modern living iron-bacteria (Gorbushina & Krumbein, 2000). Since our series are fully marine and do not exhibit any alteration or karstic imprints, e.g., no internal vadose sediments or vadose cementation, this near surface or subsurface pathway is not applicable to any of our cases. Moreover our samples never underwent low temperature mineralization. Present-day iron-bacteria have usually been observed in poorly oxygenated microenvironments, at dysoxic–anoxic interfaces near the water–sediment contact (Cowen, 1992). These microorganisms produce anionic exopolymeric substances (EPS) forming cellular coatings and bind a variety of metals such as iron and manganese (McLean et al., 1996). Fossilized ferric networks engulfing various carbonate particles are common in our series (example of Coumiac, Upper Devonian, Montagne Noire, southern France where they are abundant, Préat et al., 1999; or in the Italian Rosso Ammonitico, Mamet & Préat, 2003). These regular networks are probably organic material of bacterial origin, like EPS. Such networks are frequently observed around bacteria studied under SEM or TEM (Cowen, 1992), and have been observed on the surface of ferromanganese nodules along with bacterial microcolonies (LaRock & Ehrlich, 1975). They result from the collapse of the polysaccharides forming the EPS due to the dehydration of the sample (Costerton, 1995).
28These observations and the fact that abundant iron sheaths have been observed on the outer wall of the micronic filamentous fabrics suggest a simple mechanism for the accumulation of the (hydro)oxides (now hematite) in the studied series. Steps in the proposed mechanism are as follows:
29(1) ferric iron mineral precipitation in the EPS of the microorganisms which formed a benthic community at the water/micrite interface or within the sediment;
30(2) strong bacterial upward growth as small microtufts or unoriented biofilms around various carbonate particles with degradation of their lower parts supplying the micritic sediment with sub-micrometric iron-hydroxides;
31(3) progressive burial of the mineral-encrusted microorganisms;
32(4) lysis of these microorganisms and EPS degradation further liberating the submicrometric iron-hydroxides (now hematite) in the sediment.
33This hypothetical early diagenetic mechanism is not based on hazardous taxonomic comparisons and does not infer a particular metabolic mechanism. It is compatible with most of the observed pigmentation patterns and paleoenvironments. It suggests relatively deep calm marine water and a depth well below the fairweather wave-base, i.e. near or below the storm wave-base, and below the photic zone (Mamet & Préat, 2006). Anoxic to dysoxic conditions where iron (and manganese) would have been in its soluble reduced state (Fe2+) are proposed. It is also compatible with shallow and very shallow water paleoenvironments with dysoxic/anoxic gradients colonized at a microscale by iron bacteria and Fungi.
34The preservation of bacterial filaments will then depend on the lithification conditions; in case of cementation the filaments are fixed ‘instantaneously’ and preserved from later compaction while they are destroyed during microsparitization of the micritic matrix (Boulvain, 1990). Indeed being submicronic (<1 µm in diameter), the filaments cannot withstand the calcitic microspar whose crystals are generally larger than 5 microns (5–10 µm). Note that the filaments are only well preserved in the cavities that volumetrically represent less than 1% in the mud of the mounds. They were surely very abundant in the mud, as they are at the origin of the red pigmentation, but not preserved, or very rarely observed as broken fragments (Figs 1–3).
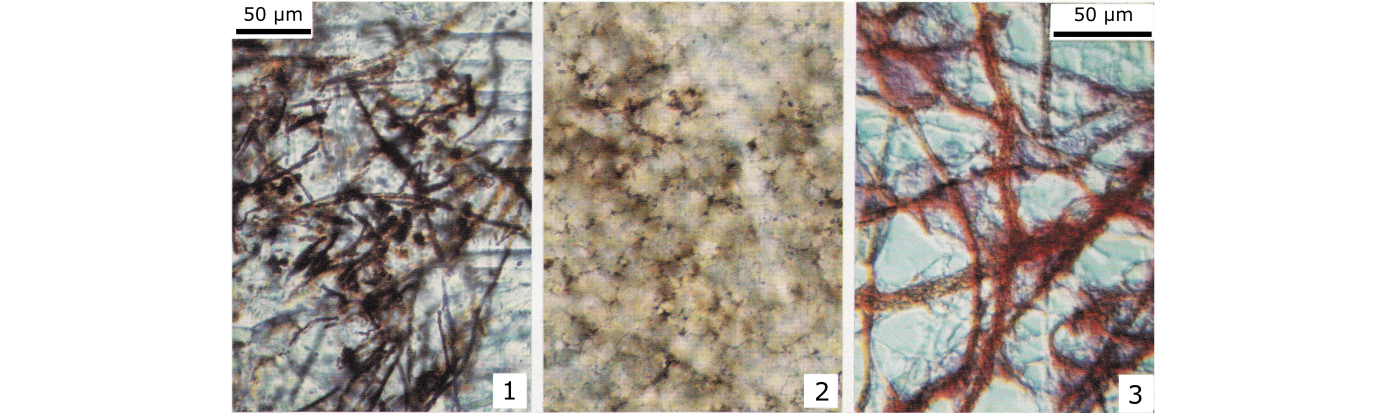
35Figures 1–3. (1) Well fossilized iron-bacteria and iron coccoids (hematite in the sheaths) in a stromatactis cavity with granular sparitic calcite cement. (2) Relics of iron-bacteria in a slightly microsparitized matrix (griottes facies). The iron-bacteria were strongly altered and broken during the microsparitization (same scale as 1). Figures 1 and 2, from F2j mud mounds. (3) Present day iron-bacteria (Sphaerotilus-Leptothrix group) from the dysoxic–anoxic sediment from a small stream in Brussels (Lab. Marine Biology, ULB). The red color is due to ruthenium (interferential contrast). (Boulvain, 1990 and 1993).
3.3. Recent organisms
36In order to better understand the ferruginization related to the iron bacteria, evidenced from the studied geological samples, we tried to compare our geological data with current biological systems involving ferruginous biofilms. Two marine invertebrates, the sea urchin Echinocardium cordatum and its symbiotic bivalve Tellimya (Montacuta) ferruginosa, were particularly studied by two of us (De Ridder, 1986, 1994; De Ridder & Jangoux, 1993; Gillan & De Ridder, 1995, 1997; Brigmon & De Ridder, 1998; De Ridder et al., 1985a; Gillan et al., 2000; De Ridder & Brigmon, 2003). They live burrowed in fine-grained silty quartz sands in coastal subtidal and tidal environments. T. ferruginosa is a commensal symbiont that shares the burrow of its echinoid host E. cordatum.
37E. cordatum commonly burrows below or at the level of the oxidized-reduced interface (Temara et al., 1993). It ingests deep and surface sediments (De Ridder & Jangoux, 1993). Surface sediment reaches the echinoid through a vertical tube that connects the burrow to the surface and provides the detritus on which it feeds (De Ridder et al., 1985a, b). As a result, deep reduced sediment not only occurs in habitat but also within the digestive tube of the echinoid. Symbiotic filamentous bacteria form laminated mats around detrital particles and build multilayered nodules (diameters usually ranging from 3 to 7 mm) in the hindgut caecum in E. cordatum (Fig. 4). The symbiotic bacteria thrive in the outer layers of the nodules (Fig. 5), and empty bacterial sheaths accumulate in the inner layers, they are piled and tightly packed forming an internal layer of exopolymeric substances (EPS) around the nucleus. These filamentous symbiotic bacteria belong to the genus Desulfonema, a sulfate-reducing anaerobic bacterium belonging to the δ-Proteobacteria (Thorsen et al., 2003; Gomes da Silva et al., 2006). These bacteria usually produce H2S when degrading the organic matter, and as the ingested sediments are Fe-rich, black FeS may then precipitate in the vicinity of the nodule (frequently in its center). When oxygen levels increase in the vicinity of the nodules, these FeS minerals are then oxidized in Fe(III) oxyhydroxides (occasionally nodules can thus be red). Each filament of Desulfonema consists of tens of rods placed end to end. The diameter of the rods ranges from 1.5 to 2 µm, and their length from 8 to 10 µm (Temara et al., 1993; De Ridder, 1986).
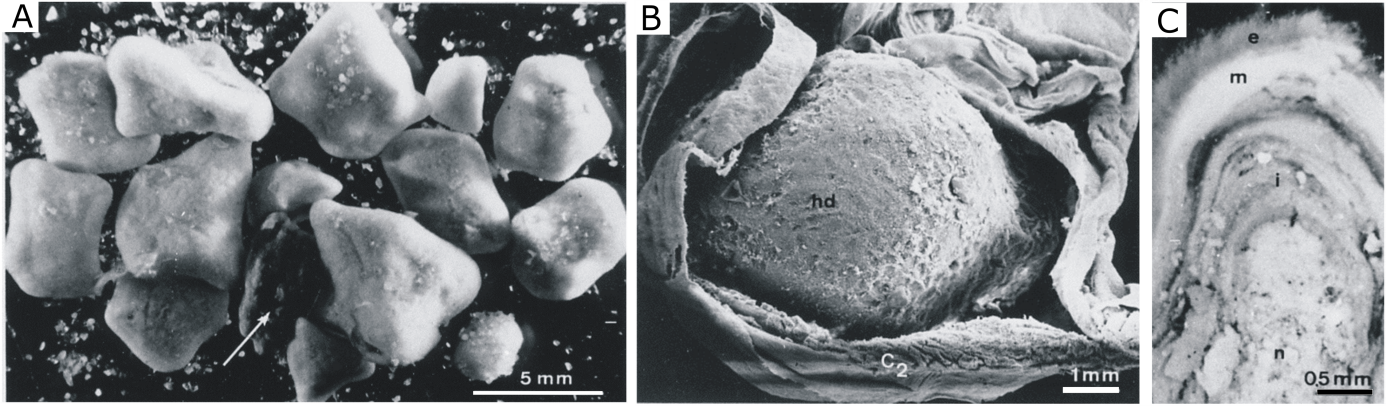
38Figure 4. (A) Set of nodules isolated from the intestinal caecum of E. cordatum. Note the occurrence of an almost uncoated nodule core (arrow). (B) Nodule in dissected caecum, nd: nodule, C2: caecum intestinal. (C) Cross-sectioned isolated nodule showing the three-layered bacterial biofilm, n: nucleus, i: coat inner layer, m: coat middle layer, e: coat external layer (SEM observation) (De Ridder et al., 1985b).

39Figure 5. General view (SEM) of the nodule external surface showing entangled filamentous bacteria occurring in the outer layer of the mat that forms the coat (De Ridder, 1986).
40The shell of the bivalve T. ferruginosa is covered with a rust-colored biofilm including filamentous bacteria and protozoa encrusted with a mineral, rich in ferric ion and phosphate (Gillan & De Ridder, 1995, 1997). Under the scanning electron microscope the biofilm of T. ferruginosa appears as a structured microbial mat with three separate layers (Fig. 6). The inner layer is relatively thick (about 50 µm) and made of densely packed mineral granules that adhere to the shell. Remains of filamentous bacteria (ferric iron-encrusted empty sheaths and extracellular material) are occasionally found in this layer (Gillan, 2003). The intermediary layer is relatively thin (up to 10 µm) with various types of ferric iron-encrusted microorganisms. Filamentous bacteria are predominant and correspond to 2 morpho-physiological types that resemble the sulfide-oxidizing Beggiatoa and Thiothrix. However, these filamentous bacteria were never identified with certainty. Remains of lysed filaments frequently occur and are always heavily encrusted with ferric iron. The outer layer consists of living microorganisms that are similar to those of the intermediary layer (Fig. 6). The filamentous bacteria are particularly abundant, they extend up to 3 mm from the bivalve, they are partly encrusted with ferric minerals. Gillan et al. (2000) and Gillan & De Ridder (2001) showed that ferric iron precipitation in the biofilm may have proceeded by way of microbial Fe(II) oxidation as well as microbial degradation of organic Fe(III) complexes. In the first case oxidation occurred at the marine anoxic–oxic transition zone and microorganisms adsorbed colloidal Fe oxyhydroxides or precipitated Fe oxyhydroxides in their exopolymeric substances (EPS) or passively (Gillan et al., 2000).
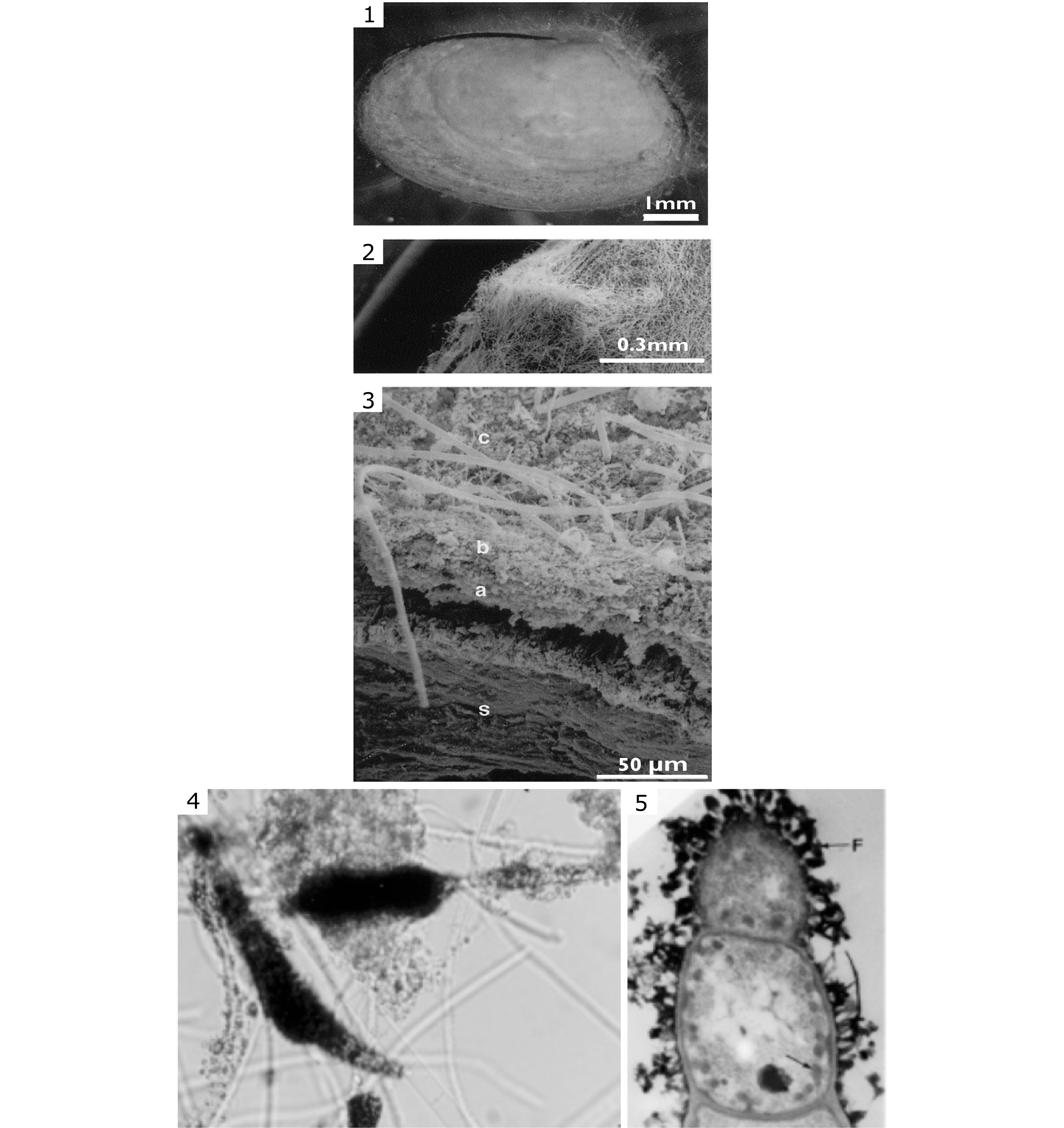
41Figure 6. (1-3) Microbial mat on T. ferruginosa shell (SEM observation); the layered biofilm is detailed in (3) showing the shell (s), the inner layer (a, about 50 µm-thick with densely packed mineral granules), the intermediary layer (b, up to 10 µm with various types of ferric iron-encrusted microorganisms – mainly Beggiatoaceae), the outer layer (c, with living filamentous bacteria similar to those of the intermediary layer). The filamentous bacteria extend up to 3 mm from the bivalve. (4-5) Ferric iron encrustation in the sheath of filamentous Beggiatoaceae (diameter 1–5 µm) at the base of the outer layer where (4) is a group of filaments seen in photonic microscopy and (5) are details of a bacterial filament (diameter 1 µm) with iron deposits (<1 µm) in the sheath (see F horizontal arrow in the upper part) and intracellular iron nanogranules (see oblique arrow in the lower right part). (TEM observation in Gillan & De Ridder, 1997).
42E. cordatum and T. ferruginosa highlighted the importance of filamentous bacteria in the formation of iron minerals (FeS during anaerobiosis and Fe(III) oxyhydroxides when oxygen levels are higher). These minerals are always intimately associated to the bacterial filaments. However, the exact process of mineral precipitation remains unknown. It may have been brought about indirectly as a consequence of metabolism that changed the environmental conditions (e.g., pH) or the degree of complexation (Gerke, 1997). Precipitation may also occur directly as a consequence of enzymatic degradation of small organic Fe complexes such as citrate, known to complex Fe ions (Gillan et al. 2000).
43In all geological samples examined (red marbles, etc.), the presence of ferruginous morphs was noted, e.g., filaments, coccoids, and microstromatolites. This suggests the omnipresence of bacteria, mostly in the filamentous form (Fig. 7). We can therefore infer a similar bacterial mediation, as observed for the echinoid and the bivalve. The pigmentation of the rock may simply be explained by the dispersion of submicrometric oxyhydroxides (now hematite) formed by bacterial mediation during early diagenesis in various microaerophilic environments. The limiting factor is the oxygen content which was probably low in these very quiet and relatively deep environments (Mamet & Préat, 2006). By this hypothesis not only can we highlight the importance of microenvironments at the dysoxic–anoxic water sediment interfaces for the bacterial Fe-oxidation but also can conclude that the red limestones have no particular paleogeographic meaning, only a microenvironmental meaning.
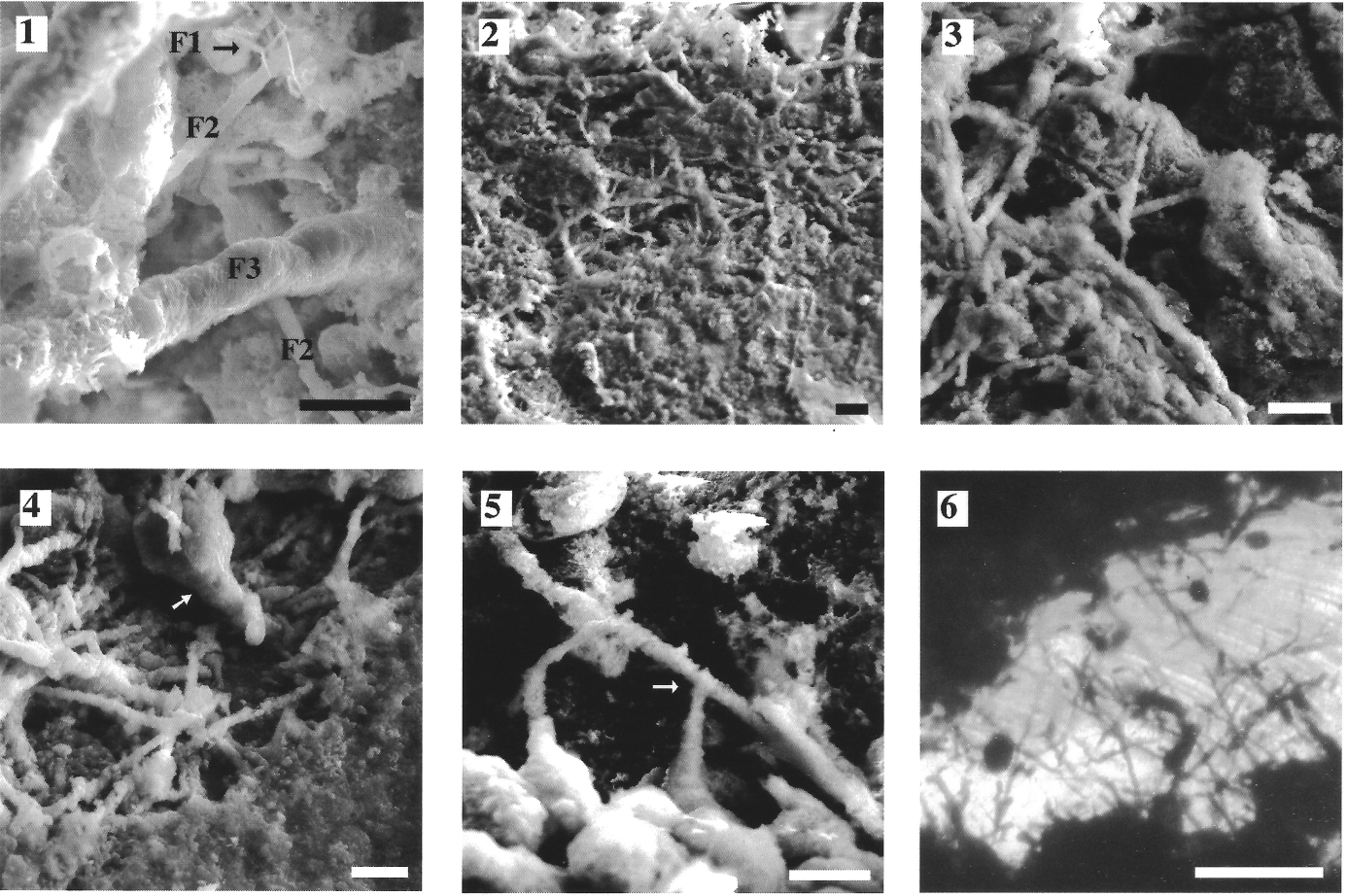
44Figure 7. Samples of the Bajocian stratotype, Mid-Jurassic, northern Normandy, France. (1-5) Acid-leached sections (SEM observations); three types of iron-encrusted filaments, F1, F2, F3 (0.2-0.7 µm and about 10 µm long) are illustrated. (6) Thin section seen in photonic microscopy with presumed endolithic filaments. Scale bars are respectively 10 µm in 1-5, and 50 µm in 6. Arrow indicates a small stalked body (in 4), and a dichotomy (in 5). (Préat et al., 2000).
3.4. Iron isotope compositions
45Since bacterial mediation was established for the formation of iron deposits in the studied geological series and in the biofilms of E. cordatum and T. ferruginosa, a final step was to check if the activity of iron bacteria could have generated biofractionation of iron natural isotopes. If this was the case, an additional argument for the activity of the iron bacteria causing the red pigmentation of the samples would be provided.
46This isotopic study was difficult because natural mass-dependent variation in isotopic composition of iron is very small, varying from +0.9‰ to -1.6‰ in δ56Fe in sedimentary rocks (Anbar, 2004). To achieve our goal, the carbonate matrices of the Italian Rosso Ammonitico were chosen among all the studied cases, as the activity of iron bacteria was the most pronounced in this condensed formation. Indeed, these rocks feature abundant and rich hardgrounds with filamentous bacteria and ferruginous microstromatolites (Martire, 1996; Mamet & Préat, 2003). Moreover, this formation is not homogeneous: it is interstratified with gray facies and also gives an opportunity to compare the isotopic composition of the iron at the contacts of red facies (with bacterial mediation) and gray (without bacterial mediation), at an infra-centimetric scale (Préat et al., 2006). As a result 16 Jurassic limestones from four coeval stratigraphic sections in the lower part of the Rosso Ammonitico Veronese (Italy) containing red and gray hemipelagic facies and one sample in the upper part of the Calcari Grigi (see below) were selected and analyzed by Multiple-Collector Inductively Coupled Plasma Mass Spectrometry (MC-ICP-MS) (Préat et al., 2008a). Analytical procedures have been described in more detail elsewhere (De Jong et al., 2007, 2008; Préat et al., 2008a) and will not be presented here.
47Such analysis allowed for the recognition of a clear iron isotopic fractionation (mean -0.8‰, ranging between -1.52 to -0.06‰) on a millimeter–centimeter scale between the red and gray facies of the studied formation (Préat et al., 2006; 2011c). All the analyzed red and gray samples belong to the same stratigraphic unit (Lower Rosso Ammonitico) except for one sample (sample AR100 in Préat et al., 2011c) taken at the top of the Calcari Grigi (Pliensbachian) at the contact with the Lower Rosso Ammonitico (Bajocian-Callovian; Martire, 1996), where an important discontinuity associated with neptunian dykes is present. After gentle acid leaching, measurements of the Fe isotopic compositions gave δ56Fe values that were systematically lower in the red facies residues (median: -0.84‰, range: -1.46 to +0.26‰) compared to the gray facies residues (median: -0.08‰, range: -0.34 to +0.23‰). In addition, the red facies residues were characterized by a lighter δ56Fe signal relative to their corresponding leachates. These Fe isotopic fractionations could be a sensitive fingerprint of a biotic process; systematic isotopic differences between the red and gray facies residues, which consist of hematite and X-ray amorphous iron hydroxides, respectively, are hypothesized to have resulted from the oxidizing activity of iron bacteria and Fungi in the red facies. The gray Fe isotopic data match the Fe isotopic signature of the terrestrial baseline established for igneous rocks and low-Corg clastic sedimentary rocks (Beard & Johnson, 2004). The Fe isotopic compositions of the gray laminations are consistent with the influx of detrital iron minerals and lack of microbial redox processes at the water-interface during deposition (Préat et al., 2006).
48These analyses showed that a pronounced Fe isotope fractionation is observed in the Jurassic red hardground levels and in the more condensed red facies where bacteria and Fungi lived and have accumulated, with values typically lighter by -1‰ than the gray facies where microorganisms were absent (Fig. 9, red vertical bars 1 and 2). This fractionation probably involved the passive accumulation of originally light pore water Fe in the exopolymeric substances (EPS) produced by filamentous bacteria, thereby favoring heavier Fe isotopes. Alternating stages of oxidation Fe(II)/Fe(III) occurred near the sediment/water interfaces as a consequence of microenvironmental changes in the marine pore waters and caused the red/gray facies interlayering.
49In the meantime, iron isotopic compositions of iron deposits in biofilms (E. cordatum and T. ferruginosa; Figs 4 and 6) and in tissue (connective tissue of E. cordatum) (Fig. 8) were selected from 8 samples (Préat et al., 2011c). Fe isotopic compositions (δ56Fe) from Fetot solid phase (amorphous minerals) trapped in the microbial mats of E. cordatum and T. ferruginosa are negative and range between -1.78‰ and -0.74‰. Solid phase Fetot concentrations range from 10.10% to 0.75% as determined by isotope dilution. The pristine and the lightest δ56Fe (Figs 8 and 9‘G’) is associated with the iron granules occurring in the connective tissue of the intestinal wall of E. cordatum (Fig. 8), which were probably related to the chemical oxidation of Fe(II) into Fe(III) without the activity of iron microbial communities. This lightest Fe isotopic composition could have resulted of former fractionation in the interstitial fluids present in the pore waters of the silty quartzose sediment through dissimilatory Fe(III) reduction or other processes as those invoked by Severmann et al. (2006) in the marine pore waters along the California Coast (Fig. 9, green vertical bar).

50Figure 8. Connective tissue layer (C) in the intestinal wall (caecum) of E. cordatum, with very abundant iron nanograins (small-sized black dots) (TEM observation, De Ridder, 1986). These nanograins were analyzed for iron isotopes (see text).
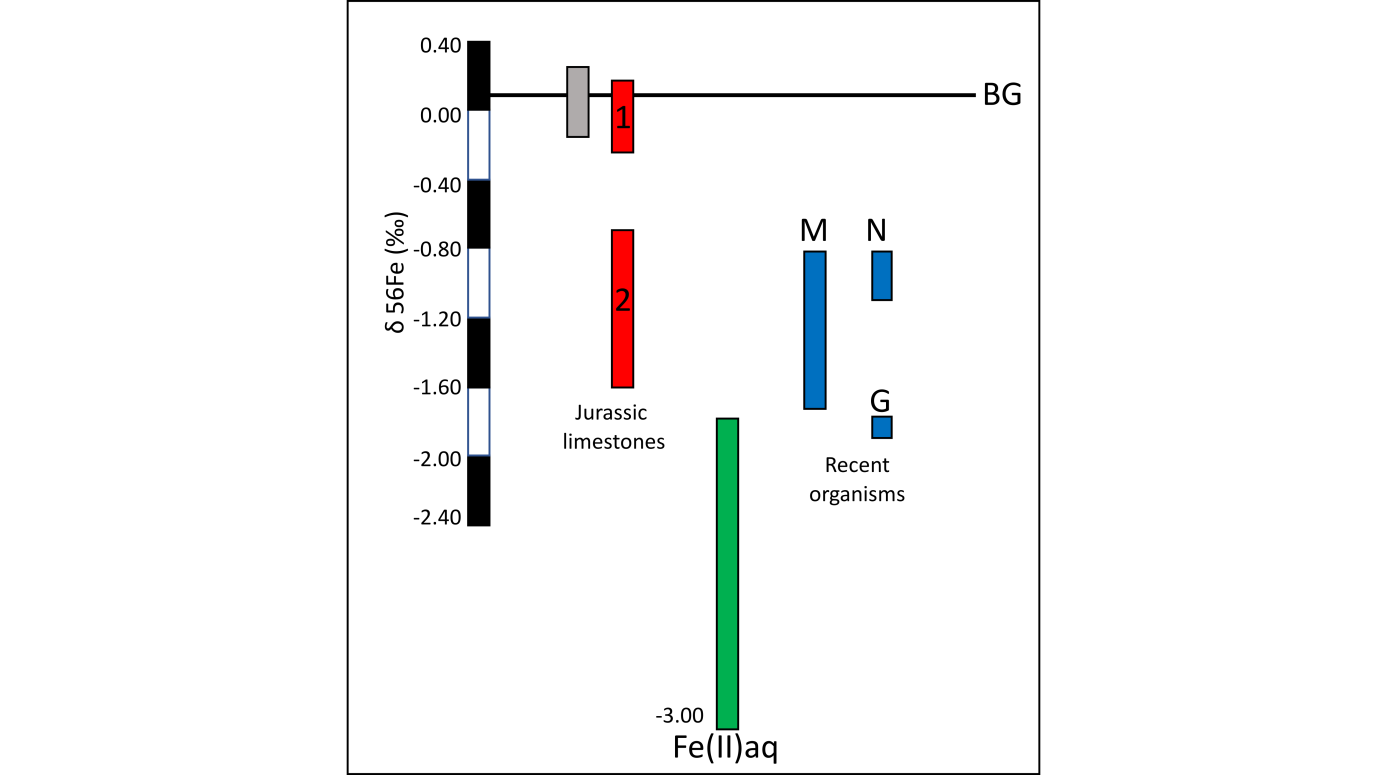
51Figure 9. The colored bars show iron isotopic compositions (δ56Fe) of the analyzed iron minerals from our geological and biological samples, and also of the reduced iron in the pore water. Abbreviations: gray vertical bar = gray facies (in the Italian Rosso Ammonitico), red vertical bars = red facies (idem, Jurassic samples), ‘M’ blue vertical bar = iron deposits in T. (Montacuta) ferruginosa, N and G blue vertical bars = iron deposits in the nodules (N) and iron nanograins (G) in the connective tissue of E. cordatum, green vertical bar = reduced iron in the pore water (from Severmann et al., 2006). BG line represents the background baseline for igneous rocks and low-Corg clastic sedimentary rocks (Beard & Johnson, 2004). Red bar ‘1’ is for burrowed sediments and red bar ‘2’ is for hardgrounds and condensed levels (Italian Rosso Ammonitico). (Préat et al., 2011c).
52The δ56Fe isotopic compositions of the amorphous minerals related to the bacterial mats associated with the echinoid and the bivalve are similar to the compositions of the Fe-oxide (hematite) of the red facies in the Rosso Ammonitico of Verona (median -0.84‰, range: -1.46 to +0.26‰) (Préat et al., 2008a). As for the results of the Rosso Ammonitico, the observed Fe fractionations are not correlated with the total iron concentrations (not discussed here, Préat et al., 2006) in the analyzed fractions.
53Ferrous iron in the nodules of E. cordatum, ferric iron in the amorphous minerals of T. ferruginosa and in the hematite of the red facies of the Rosso Ammonitico Veronese show an iron isotopic fractionation (at least up to +1‰, Fig. 9) on a local scale (in the same burrow for the recent organisms and on a millimeter–centimeter scale in the red facies relative to the gray ones) through the activity of iron bacteria (red limestones and T. ferruginosa) and the activity of a complex mixed bacterial consortia (E. cordatum; Gomes da Silva et al., 2006) . In these cases, iron accumulated passively more specifically in the EPS produced by the filamentous bacteria. Alternating stages of oxidation Fe(II)/Fe(III) occurred near the sediment/water interfaces as a consequence of microenvironmental changes in the marine pore waters. The resulting Fe isotope fractionation is more pronounced in the Jurassic red facies with values lighter than -0.7‰ observed in the hardground levels and in the more condensed facies where bacteria and Fungi lived and have accumulated (Préat et al., 2008a) and less pronounced (between 0% and -0.20%) in the red burrowed facies (Fig. 9). The oxygen content is probably one of the main controlling factors (Fenchel & Finlay, 1995). Both modern organisms (the echinoid and the bivalve) studied here thrive in similar microenvironmental conditions as those of the condensed red facies. Their Fe isotope compositions are the same (Fig. 9), as is the range of the probable fractionation (Préat et al., 2011c).
4. Conclusions
54Our study mixing geological, biological and chemical approaches offers the preliminary results of a fascinating geomicrobiological investigation of modern biological and ancient geological samples. The following conclusions are proposed on the basis of consistent ecological (biology) and sedimentological observations (geology), and on Fe isotopic analyses of amorphous minerals present in E. cordatum and in T. ferruginosa, and iron minerals associated with the red and gray laminar limestones from the Middle Jurassic of the Trento Plateau (Lower Rosso Ammonitico Veronese).
55The Fe isotopic compositions of the gray facies display values in a narrow range in δ56Fe (around 0‰) and reflect the conservative behavior of Fe during weathering under oxygenated surface conditions (Fig. 9; Beard & Johnson, 2004). All other Fe isotope compositions are negative and display significant Fe isotope variations. This is particularly the case for the red facies of the Rosso Ammonitico and it seems also the case for the studied recent marine organisms.
56The pristine and the lightest δ56Fe (Figs 8 and 9 ‘G’) is associated with the iron granules in the intestinal wall of E. cordatum, which were probably related to the chemical oxidation of Fe(II) into Fe(III) without the activity of iron microbial communities (i.e. only metabolic activity of the echinoid). This lightest Fe isotopic composition could have resulted of former fractionation in the interstitial fluids present in the pore waters of the silty quartzose sediment through dissimilatory Fe(III) reduction or other processes as those invoked by Severmann et al. (2006) in the marine pore waters along the California Coast.
57This contribution highlights the role of the EPS in microbial biofilms. They potentially may provoke a biosignature through the Fe isotope composition of the minerals they induce. This is of particular importance in studying microbial communities and stromatolites in the geologic record. Our study also shows that the iron communities have no paleogeographic and depth significances: the intertidal sandy beach (E. cordatum and T. ferruginosa) and the hemipelagic carbonate (Rosso Ammonitico) environments display a similar Fe isotopic cycling during early diagenesis despite completely opposite contexts (different lithologies, different depths). We believe the key is EPS.
5. Acknowledgments
58We are grateful to Prof. F. Boulvain (University of Liège) and Prof. Luca Martire (University of Torino) for their constructive suggestions.
6. References
59Anbar, A.D., 2004. Iron stable isotopes: beyond biosignatures. Earth and Planetary Science Letters, 217, 223–236. https://doi.org/10.1016/S0012-821X(03)00572-7
60Baele, J.M., Boulvain, F., de Jong, J., Mattielli, N., Papier, S. & Préat, A. (2008) Iron microbial mats in Modern and Phanerozoic environments. In Hoover, R.B., Levin, G.V., Rozanov, A.Y. & Davies, P.C.W (eds), Instruments, Methods, and Missions for Astrobiology XI. Proceedings of SPIE, vol. 7097, 12 p. https://doi.org/10.1117/12.801597
61Bathurst, R.G.C., 1982. Genesis of stromatactis cavities between submarine crusts in Palaeozoic carbonate mud buildups. Journal of the Geological Society of London, 139, 165–181. https://doi.org/10.1144/gsjgs.139.2.0165
62Beard, B.L. & Johnson, C.M., 2004. Fe isotopes variations in the Modern and Ancient earth and other planetary bodies. Reviews in Mineralogy and Geochemistry, 55, 319–357. https://doi.org/10.2138/gsrmg.55.1.319
63Boulvain, F., 1990. Sédimentologie et diagenèse des monticules micritiques frasniens « F2j » de Belgique. Unpublished PhD thesis. Université libre de Bruxelles, Belgium, 535 p.
64Boulvain, F., 1993. Sédimentologie et diagenèse des monticules micritiques « F2j » du Frasnien de l’Ardenne. Service géologique de Belgique, Professional Paper, 260, 436 p.
65Boulvain, F., De Ridder C., Mamet, B., Préat, A. & Gillan, D., 2001. Iron microbial communities in Belgian Frasnian carbonate mounds. Facies, 44, 47–60. https://doi.org/10.1007/BF02668166
66Brigmon, R.L & De Ridder, C., 1998. Symbiotic relationship of Thiothrix spp. with an echinoderm. Applied and Environmental Microbiology, 64, 3491–3495.
67Costerton, J.W., 1995. Overview of microbial biofilms. Journal of Industrial Microbiology, 15, 137–140. https://doi.org/10.1007/BF01569816
68Cowen, J.P., 1992. Morphological study of marine bacterial capsules: implications for marine aggregates. Marine Biology, 114, 85–95. https://doi.org/10.1007/BF00350858
69de Jong, J.T.M., Schoemann, V., Tison, J.-L., Becquevort, S., Masson, F., Lannuzel, D., Petit, J., Chou, L., Weis, D. & Mattielli, N., 2007. Precise measurement of Fe isotopes in marine samples by multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS). Analytica Chimica Acta, 589, 105–119. https://doi.org/10.1016/j.aca.2007.02.055
70de Jong, J., Schoemann, V., Lannuzel, D., Tison, J.-L. & Mattielli, N., 2008. High-accuracy determination of iron in seawater by isotope dilution multiple collector inductively coupled plasma mass spectrometry (ID-MC-ICP-MS) using nitrilotriacetic acid chelating resin for pre-concentration and matrix separation. Analytica Chimica Acta, 623, 126–139. https://doi.org/10.1016/j.aca.2008.06.013
71Della Porta, G., Mamet, B. & Préat, A., 2007.Microbial mediation in the formation of red limestones, Upper Carboniferous, Cantabrian Mountains, Spain. In Wong, Th. (ed.), Proceedings of the XVth International Congress on Carboniferous and Permian Stratigraphy. Utrecht, 10-16 August 2003. Royal Netherlands Academy of Arts and Sciences, Amsterdam, 243–250.
72De Ridder, C., 1986. La nutrition chez les Echinodermes psammivores. Etude particulière du spatangide fouisseur Echinocardium cordatum (Pennant) (Echinodermata, Echinoidea). Unpublished PhD thesis. Université Libre de Bruxelles (Faculté des Sciences), Belgium, 270 p.
73De Ridder, C., 1994. Symbioses between spatangoids (Echinoidea) and Thiothrix-like bacteria (Beggiatoales). In David, B., Guille, A., Féral, J.P. & Roux, M. (eds), Echinoderms Through Time: Proceedings of the 8th International Echinoderm Conference, Dijon 1993. Balkema, Rotterdam, 619–625.
74De Ridder, C. & Brigmon, R.L., 2003. “Farming” of microbial mats in the hindgut of echinoids. In Krumbein, W.E., Paterson, D.M. & Zavarzin, G.A. (eds), Fossil and Recent Biofilms: A Natural History of Life on Earth. Kluwer Academic, Boston, 217–225.
75De Ridder, C. & Jangoux, M., 1993. The digestive tract of the spatangoid echinoid Echinocardium cordatum (Echinodermata): morphofunctional study. Acta Zoologica, 74, 337–351. https://doi.org/10.1111/j.1463-6395.1993.tb01248.x
76De Ridder, C., Jangoux, M. & Van Impe, E., 1985a. Food selection and absorption efficiency in the spatangoid echinoid, Echinocardium cordatum (Echinodermata). In Keegan, B.E. & O'Connor, B.D. (eds), Echinodermata: Proceedings of the 5th International Echinoderm Conference, Galway 1984. Balkema, Rotterdam, 507–512.
77De Ridder, C., Jangoux, M. & De Vos L., 1985b. Description and significance of a peculiar intradigestive symbiosis between bacteria and a deposit-feeding echnoid.Journal of Experimental Marine Biology and Ecology, 91, 65–76. https://doi.org/10.1016/0022-0981(85)90221-7
78Dupont, E., 1881. Sur l’origine des calcaires dévoniens de la Belgique. Bulletin de l’Académie royale de Belgique, 3ème série, 2, 264–280.
79Fenchel , T. & Finlay, B.J., 1995. Ecology and Evolution in Anoxic Worlds. Oxford University Press, Oxford, Oxford Series in Ecology and Evolution, 276 p.
80Gerke, J., 1997. Aluminium and iron(III) species in the soil solution including organic complexes with citrate and humic substances. Zeitschrift für Pflanzenernährung und Bodenkunde, 160, 427–432. https://doi.org/10.1002/jpln.19971600313
81Gillan, D., 2003. The study of a Recent iron-encrusted biofilm in the marine environment. In Krumbein, W.E., Paterson, D.M. & Zavarzin, G.A. (eds), Fossil and Recent Biofilms: A Natural History of Life on Earth. Kluwer Academic, Boston, 241–248.
82Gillan, D. & De Ridder, C., 1995. The microbial community associated with Montacuta ferruginosa, a commensal bivalve of the echinoid Echinocardium cordatum. In Emson, R.H., Smith, A.B. & Campbell, A.C. (eds), Echinoderm Research 1995: Proceedings of the 4th European Echinoderms Colloquim, London, 10-13 April 1995. Balkema, Rotterdam, 71–76.
83Gillan, D. & De Ridder, C., 1997. Morphology of a ferric iron-encrusted biofilm forming of the shell of a burrowing bivalve (Mollusca). Aquatic Microbiology Ecology, 12, 1–10. https://doi.org/10.3354/ame012001
84Gillan, D. & De Ridder, C., 2001. Accumulation of a ferric mineral in the biofilm of Montacuta ferruginosa bivalve (Mollusca, Bivalvia). Biomineralization, bioaccumulation, and inference of paleoenvironments. Chemical Geology, 177, 371–379. https://doi.org/10.1016/S0009-2541(00)00420-4
85Gillan, D., Warnau, M., De Vrind-De Jong, E.W., Boulvain, F., Préat, A. & De Ridder, C., 2000.Iron oxidation and deposition in the biofilm covering Montacuta ferruginosa (Mollusca, Bivalvia). Geomicrobiology Journal, 17, 141–150. https://doi.org/10.1080/01490450050023818
86Gomes da Silva, S., Gillan, D., Dubilier, N. & De Ridder, C., 2006. Characterization by 16S rRNA gene analysis and in situ hybridization of bacterial living in the hindgut of a deposit-feeding echinoid (Echinodermata). Journal of the Marine Biological Association of the United Kingdom, 86, 1209–1213. https://doi.org/10.1017/S0025315406014202
87Gorbushina, A.A. & Krumbein, W.E., 2000. Subaerial microbial mats and their effects on soil and rock. In Riding, R.E. & Awramik, S.M. (eds), Microbial Sediments. Springer Verlag, Berlin, 161–170.
88Hofmann, B.A. & Farmer, J.D., 2000. Filamentous fabrics in low-temperature mineral assemblages: are they fossil biomarkers? Implications for the search for a subsurface fossil record on the early Earth and Mars. Planetary and Space Science, 48, 1077–1086. https://doi.org/10.1016/S0032-0633(00)00081-7
89Hu, X., Wang, C., Scott, R.W., Wagreich, M. & Jansa, L. (eds), 2009. Cretaceous Oceanic Red Beds: Stratigraphy, Composition, Origins, and Paleoceanographic significance. SEPM Special Publication, 91, 276 p. https://doi.org/10.2110/sepmsp.091
90LaRock, P.A. & Ehrlich, H.L., 1975. Observations of bacterial microcolonies on the surface of ferromanganese nodules from Blake plateau by scanning electron microscopy. Microbial Ecology, 2, 84–96. https://doi.org/10.1007/BF02010383
91Mamet, B. & Boulvain, F., 1990. Constructions hématitiques des Griottes carbonifères (Asturies, Espagne). Bulletin de la Société belge de Géologie, 99, 229–239.
92Mamet, B. & Perret, M.-F., 1995. Bioconstructions hématitiques de Griottes dévoniennes (Pyrénées centrales). Géobios, 28, 655–661. https://doi.org/10.1016/S0016-6995(95)80055-7
93Mamet, B. & Préat, A., 2003. Sur l’origine bactérienne et fongique de la pigmentation de l’Ammonitico Rosso (Jurassique, région de Vérone, Italie du Nord). Revue de Micropaléontologie, 46/1, 35–46. https://doi.org/10.1016/S0035-1598(03)00006-0
94Mamet, B. & Préat, A., 2006. Iron-bacterial mediation in Phanerozoic red limestones: State of the art. Sedimentary Geology, 185, 147–157. https://doi.org/10.1016/j.sedgeo.2005.12.009
95Mamet, B. & Préat, A., 2007. Why is ‘red marble’ red? Revista Espanola de Micropaleontologia, 37/1, 13–21.
96Mamet, B., Préat, A. & De Ridder C., 1997. Bacterial origin of the red pigmentation in the Devonian Slivenec Limestone, Czech Republic. Facies, 36,173–188. https://doi.org/10.1007/BF02536883
97Martire, L., 1996. Stratigraphy, facies and synsedimentary tectonics in the Jurassic Rosso Ammonitico Veronese (Altopiano di Asiago, NE Italy). Facies, 35, 209–236. https://doi.org/10.1007/BF02536963
98McLean, R.J.C., Fortin, D. & Brown, D.A., 1996. Microbial metal-binding mechanisms and their relation to nuclear waste disposal. Canadian Journal of Microbiology, 42, 392–400. https://doi.org/10.1139/m96-055
99Préat, A., Mamet, B., Bernard, A. & Gillan, D., 1999. Bacterial mediation, red matrices diagenesis, Devonian, Montagne Noire (southern France). Sedimentary Geology, 126, 223–242. https://doi.org/10.1016/S0037-0738(99)00042-1
100Préat, A., Mamet, B., De Ridder, C., Boulvain, F. & Gillan, D., 2000. Iron bacterial and fungal mats, Bajocian stratotype (Mid-Jurassic, northern Normandy, France). Sedimentary Geology, 137/3-4,107–126. https://doi.org/10.1016/S0037-0738(00)00101-9
101Préat, A., Morano, S., Loreau, J.P., Durlet, C. & Mamet, B., 2006. Petrography and biosedimentology of the Rosso Ammonitico Veronese (Middle-Upper Jurassic), Northeastern Italy. Facies, 52, 265–278. https://doi.org/10.1007/s10347-005-0032-2
102Préat, A., de Jong, J., Mamet, B. & Mattielli, N., 2008a. Stable iron isotopes and microbial mediation in red pigmentation of the Rosso Ammonitico (Mid-Late Jurassic, Verona area, Italy). Astrobiology, 8/4, 841–857. https://doi.org/10.1089/ast.2006.0035
103Préat, A., El Hassani, A. & Mamet, B., 2008b. Iron bacteria in Devonian carbonates (Tafilalt, Anti-Atlas, Morocco). Facies, 54/1, 107–120. https://doi.org/10.1007/s10347-007-0124-2
104Préat, A., de Jong, J., De Ridder, C., Gillan, D. & Martire, L., 2011a. Why is ‘red marble’ red: could Fe-isotopes shed light on this question through the study of the Italian Ammonitico Rosso? Sessione M4, Il colore degli eventi nel tempo, Epitome 2011, Geoitalia VIII Forum Italiano di Science della Terre, 19-23 September, vol.4., p. 236. (Key lecture).
105Préat, A., Mamet, B., Di Stefano, P., Martire, L. & Kolo, K., 2011b. Microbially-induced Fe and Mn oxides in condensed pelagic sediments (Middle-Upper Jurassic, Western Sicily). Sedimentary Geology, 237, 179–188. https://doi.org/10.1016/j.sedgeo.2011.03.001
106Préat, A., de Jong, J., De Ridder, C. & Gillan, D., 2011c. Possible Fe fractionation during microbiological processing in ancient and modern marine environments. In Tewari, V.C. & Seckbach, J. (eds), Stromatolites: Interaction of Microbes with Sediments. Springer, Dordrecht, Cellular Origin, Life in Extreme Habitats and Astrobiology, 18, 651–673. https://doi.org/10.1007/978-94-007-0397-1_29
107Severmann, S., Johnson, C.M., Beard, B.L. & McManus, J., 2006. The effect of early diagenesis on the Fe isotope compositions of porewaters and authigenic minerals in continental margin sediments. Geochimica et Cosmochimica Acta, 70, 2006–2022. https://doi.org/10.1016/j.gca.2006.01.007
108Temara, A., De Ridder C., Kuenen, J.G. & Robertson, L.A., 1993. Sulfide-oxidizing bacteria in the burrowing echinoid, Echinocardium cordatum (Echinodermata). Marine Biology, 115, 179–185. https://doi.org/10.1007/BF00346333
109Thorsen, M.S., Wieland, A., Ploug, H., Kragelund, C. & Nielsen, P.H., 2003. Distribution, identity and activity of symbiotic bacteria in anoxic aggregates from the hindgut of the sea urchin Echinocardium cordatum. Ophelia, 57, 1–12. https://doi.org/10.1080/00785236.2003.10409501
110Manuscript received 28.06.2018, accepted in revised form 03.10.2018, available on line 10.12.2018.






