- Startpagina tijdschrift
- Volume 25 (2022)
- number 1-2
- First fossil record of Mustelus aff. punctulatus Risso, 1826: new evidence for a smooth-hound shark population in the Late Miocene North Sea Basin
Weergave(s): 9175 (49 ULiège)
Download(s): 2504 (9 ULiège)
First fossil record of Mustelus aff. punctulatus Risso, 1826: new evidence for a smooth-hound shark population in the Late Miocene North Sea Basin

Abstract
Mustelus is a problematic genus in palaeoichthyology. Due to the scarcity of fossil teeth, relative homogeneity in tooth morphology and the lack of published extant dentitions, the early history of smooth-hound sharks remains poorly understood. Recently, two teeth were collected in the Tortonian Deurne Member (Diest Formation) near Antwerp (Belgium). Surprisingly, a detailed SEM-based comparison with the extant North-Eastern Atlantic species (Mustelus mustelus, Mustelus asterias, Mustelus punctulatus) allowed assigning the fossil teeth to Mustelus aff. punctulatus. Today, this species is largely restricted to the Mediterranean and lies at the very base of the placental Mustelus clade evolution. Until now, this species remained unrecognized in the existing fossil record. By (re)evaluating isolated teeth from other upper Miocene localities in the southern North Sea Basin, the existence of a widely distributed population of Mustelus aff. punctulatus for the late Serravallian and the Tortonian can now be postulated. Thereafter, the species disappeared from the North Sea. Until today, no single record of Mustelus punctulatus is known from the Mediterranean predating the Messinian Salinity Crisis. Therefore, it is hypothesized that the current populations have their origin in southward migration from northern, Atlantic populations, and this probably after the “Zanclean Flooding”.
Inhoudstafel
1. Introduction
1In 2014, large roadworks occurred along the R11 between Borsbeek and Mortsel (Province of Antwerp, Belgium), south of Antwerp International Airport (AIA) (Fig. 1). A tunnel was excavated, allowing in situ sampling of an extensive Neogene succession, dominated by the Tortonian Diest Formation (Goolaerts et al., 2020). Especially the highly fossiliferous basal gravel of its upper unit, the Deurne Member, could be sampled in detail. The upper Miocene Deurne Member is of particular interest since it is rarely exposed and yields a diverse, transitional elasmobranch fauna intermediary between the well-known early–middle Miocene and Pliocene faunas in and around the city of Antwerp. A preliminary inventory of the elasmobranch fauna was given by Hoedemakers & Dufraing (2015). The recorded elasmobranch remains of the Deurne Member yielded two teeth belonging to the genus Mustelus Linck, 1790 (Plates 1–2). The fossil record of this genus is not as established as for most other species in the North Sea Basin. This may be partly due to sampling bias, as smaller-sized teeth are lacking in most collections, and thus Mustelus teeth can easily be overlooked. However, multiple fossiliferous Neogene formations in the North Sea Basin have been extensively sampled over the years, mostly without yielding any teeth of Mustelus (e.g. Herman et al., 1974; Reinecke et al., 2011; Bor et al., 2012). Therefore, a general scarcity of this genus can be assumed. In addition, Mustelus is known as a problematic genus in palaeontology, because it is very difficult to separate species solely based on their teeth (Herman et al., 1988). Moreover, until now, ontogenetic variation in Mustelus tooth morphology has been poorly studied. In this study, the Mustelus teeth from the Deurne Member are described and discussed, and their morphology is compared in detail with teeth of recent relatives from North-Eastern Atlantic waters. Palaeoichthyology strongly relies on the morphological comparison of fossil teeth with extant dentitions, as other cartilaginous skeletal remains are very rare in the fossil record. However, even though more than 27 extant species of Mustelus have been currently identified (Ebert et al., 2021), detailed illustrations and descriptions of tooth morphology are generally unavailable. This strongly hampers palaeoichthyological research (Guinot et al., 2018; Mollen, 2019). Currently, illustrations of extant dentitions only exist of a female Mustelus mustelus (Linnaeus, 1758) caught off the Senegalese coast, and a male specimen of Mustelus henlei (Gill, 1863) from California (Herman et al., 1988). Unfortunately, detailed descriptions and illustrations of the other two North-Eastern Atlantic species, Mustelus asterias Cloquet, 1819 and Mustelus punctulatus Risso, 1826 (see Ebert et al., 2021; Ebert & Dando, 2021), are non-existent in the available literature and are described for the first time in this paper. Scanning electron microscope (SEM) pictures of Mustelus teeth are provided to allow comparison with the fossil specimens (Plates 3–7). A review of all European fossil Mustelus records is provided, and a hypothesis on the palaeobiogeography of Mustelus during the European Neogene is proposed.
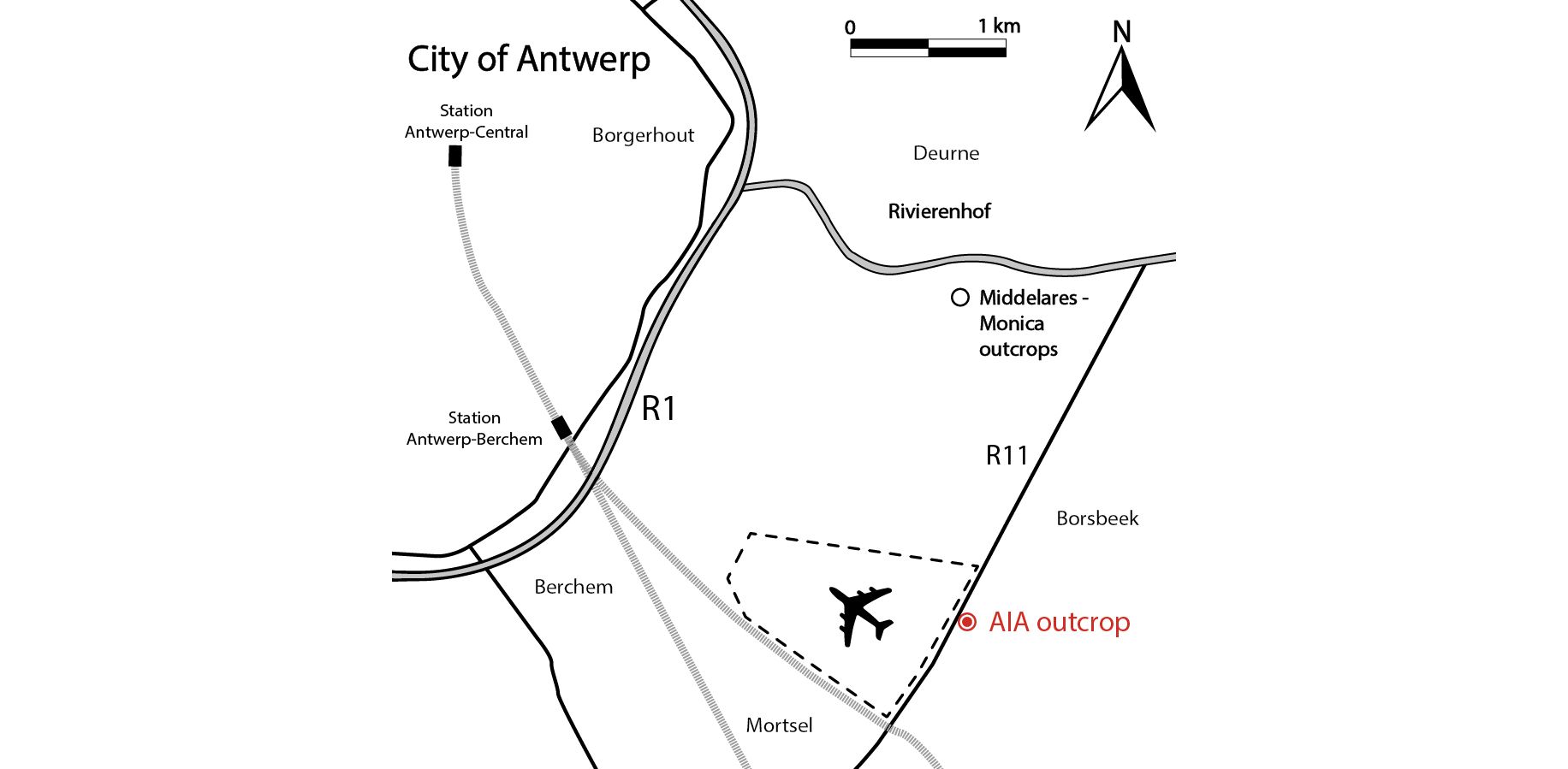
Figure 1. Map of the Antwerp area in northern Belgium. The Antwerp International Airport (AIA) outcrop is located along the R11 between Borsbeek and Mortsel (see Goolaerts et al., 2020), ca 2 km south of the Middelares-Monica outcrops (Bosselaers et al., 2004; Lambert & Goolaerts, 2021).
2. Locality, stratigraphy and palaeoenvironment
2The temporary Antwerp International Airport (AIA) exposure (Fig. 1; DOV TO_20140101C) presented the opportunity to sample an extensive stratigraphic succession covering a broad age range of sediments from the Miocene Berchem and Diest formations up to the Quaternary. The stratigraphy of this large temporary outcrop was studied in detail by Goolaerts et al. (2020) and Wesselingh et al. (2020).
3A twofold lithological structure was recognized in the Diest Formation, with the newly proposed Borsbeek member covered by the foresets of the redefined Deurne Member (Goolaerts et al., 2020). The palaeoenvironment of the Borsbeek member was suggested to be a confined marine tidal inlet, close to the shore, while the Deurne Member was deposited in shallower (30–50 m) and higher energetic environments, resulting from a system of eastward migrating, large hydraulic sand dunes (Goolaerts et al., 2020). The contact between both members is represented by a thin (max. 10 cm) basal gravel (layer 4) with quartz pebbles and (bio)clasts, as the large throughs of the Deurne Member have eroded the top of the underlying Borsbeek member. This locally resulted in high concentrations of chondrichthyan and teleostean remains, including the studied Mustelus teeth. Notable amounts of these fossils might in fact be reworked from the underlying sediments: the elasmobranch fauna in the basal gravel (31 species) is much more diversified than the poor fauna (9 species) scattered throughout the overlying foresets (Hoedemakers & Dufraing, 2015; Goolaerts et al., 2020). Contrary to the comparative table in Hoedemakers & Dufraing (2015), Mustelus specimen IRSNB P 10267 was found in layer 4 (Deurne Member). Additionally, the occurrence of isolated Mustelus teeth in the underlying Borsbeek member (layer 5b) was reported by Goolaerts et al. (2020) and Mollen (pers. comm, 2021). In recent years, both units were extensively sampled in the AZ Monica (Fig. 1) and Aquafin-Boechout outcrops, in the vicinity of the former AIA outcrop (see Lambert & Goolaerts, 2021). Nevertheless, it can be expected that future residue picking might yield additional material.
4The dinoflagellate cyst assemblages of both the Borsbeek and Deurne members indicate a middle Tortonian age, as the Amiculosphaera umbraculum zone of Dybkjær & Piasecki (2010) and the DN8 zone of de Verteuil & Norris (1996) were recognized (Louwye, 2002; Goolaerts et al., 2020). Hooyberghs & Moorkens (2005) came to a similar conclusion for the Deurne Member, based on foraminifera.
3. Material and methods
5Fossiliferous sediment of the basal gravel of the Deurne Member (layer 4) was collected in large bags at the AIA locality and sieved off-site using water and a 0.5 mm mesh. About 500 kg of sediment was processed by the first author, its residue screened with the aid of a binocular microscope, leading to the discovery of IRSNB P 10267 (Plate 1). In the same way, another specimen (IRSNB P 10268, Plate 2) was collected from the same level by Pieter De Schutter (RBINS).
6To allow comparison with extant taxa, Dirk Hovestadt (Terneuzen) provided access to multiple jaws for this study. Three jaws of Mustelus asterias Cloquet, 1819 were selected based on size, sex and provenance. Then, to consider sexual dimorphism and ontogenetic variation in tooth morphology and ornamentation, three teeth were carefully extracted at the outer edges of each upper and lower jaw of these three specimens: one tooth in the proximity of the median tooth file, one anterior or anterolateral tooth and one lateral or posterior tooth. All teeth were added to the recent vertebrate collections of the RBINS:
-
KBIN 26722: Mustelus asterias, juvenile female: 500 mm TL; obtained in Ostend (Belgium) in 1980.
-
KBIN 26723: Mustelus asterias, adult female: 880 mm TL; obtained in Terneuzen (the Netherlands) in 1987.
-
KBIN 26724: Mustelus asterias, adult male: 900 mm TL; obtained in Ostend (Belgium) in 1980.
7Based on the findings of Farrell et al. (2009), Brevé et al. (2016) and the positive morphological identification by Dirk Hovestadt (derived from the characteristic white spots in M. asterias), any identification other than M. asterias can be excluded for the specimens collected at Ostend and Terneuzen.
8For Mustelus mustelus (Linnaeus, 1758), a selection of teeth of a female specimen (890 mm TL, Senegal) is refigured in greater detail after Herman et al. (1988).
9Additionally, Frederik Mollen (ERB) provided one jaw of Mustelus punctulatus Risso, 1826 for study. This specimen (ERB 1047) represents an adult male (874 mm TL), obtained off the coast of Algeria (research vessel ‘Belkacem Grine’) in 2014. The jaw was collected following the protocol outlined by Mollen (2019). Due to the scarcity of M. punctulatus jaws accessible for study in both private and public collections, and the destructive process of tooth extraction, only a single anterolateral tooth from the right upper jaw was extracted and added to the RBINS collection (KBIN 27206). Other tooth positions were only observed and photographed using a binocular microscope.
10To clean the extracted teeth from remaining organic material before imaging, the teeth were soaked for 24 hours at approximately 30 °C in water with tryplase powder, a multienzyme combination of amylase, lipase and protease. This method for removing organic residues on chondrichthyan teeth was developed by Dirk and Maria Hovestadt-Euler. Afterwards, the teeth were rinsed with water and treated in an ultrasonic cleaner for 5 minutes. Finally, SEM pictures were taken of the cleaned teeth at the RBINS.
11Specimens figured on Plates 1–3 & 5–7 were photographed with the RBINS FEI Environmental Scanning Electron microscope (ESEM) in uncoated state, except for the Nettetal specimen.
12Terminology. The elasmobranch tooth morphology terminology outlined by the review of Bor (2013) is used.
13Abbreviations. RBINS, Royal Belgian Institute of Natural Sciences; IRSNB, Palaeontology collection numbers of the RBINS; KBIN, Biology collection numbers of the RBINS; ERB, Elasmobranch Research, Belgium; TL, total length of the shark.
4. Extant Mustelus in North-Eastern Atlantic waters
14Currently, three species of smooth-hound sharks (Mustelus) are known from North-Eastern Atlantic waters: the common smooth-hound shark Mustelus mustelus, the starry smooth-hound Mustelus asterias and the blackspot smooth-hound Mustelus punctulatus (Ebert & Dando, 2021). Generally, these sharks can attain 100 cm TL, with females usually being slightly larger than males (Ebert & Dando, 2021). Mustelus asterias can have white dorsal spots, and M. punctulatus dark dorsal spots. However, the use of these visual features to identify live catch is often misleading, as also non-spotted specimens are observed, rendering a given geographic distribution rather dubious. Even though the genus has a circumglobal distribution, species are mostly endemic, mainly situated in coastal areas (Ebert et al., 2021). Although Ebert & Dando (2021) still insist on the presence of M. mustelus in the Channel and around the British Isles, molecular identification methods suggest that only M. asterias occurs in these waters (Farrell et al., 2009, 2010; Brevé et al., 2016). Mustelus mustelus seems to occur further south in the Atlantic, along the West coast of Africa and throughout the Mediterranean, whereas M. punctulatus seems limited to the Mediterranean (Ebert & Dando, 2021). These sharks are generally fast-growing and viviparous, yet M. asterias is known to be an aplacental species whereas M. mustelus and M. punctulatus are placental (Farrell et al., 2010; Ebert & Dando, 2021; Ebert et al., 2021). The latter two are also known to interbreed, having hybrid pups, making positive morphological identification even more difficult (Marino et al., 2015). Species of Mustelus are benthic bottom feeders whose diet mainly consists of small crustaceans, but it is suggested that M. mustelus also feeds on bony fish and molluscs (Ebert & Dando, 2021).
4.1. Dentition of Mustelus punctulatus Risso, 1826 (Fig. 2 & Plate 3)
General observations and characteristics
15Overall, dignathic heterodonty can be noted, in that upper jaw teeth are generally smaller in size compared to their lower jaw counterparts, and that upper jaw teeth have a distinct convex main cusp whereas this feature is reduced in lower jaw teeth. Monognathic heterodonty is present since median teeth are symmetrical, and asymmetry increases towards the posterior and commissural tooth files. Lateral and posterior teeth are more elongated and often lose the presence of a distinct uvula. Furthermore, median, anterior, and anterolateral tooth files show lingually inclined distal and mesial crown faces (Plate 3.4C/E), whereas more posterior and commissural teeth are more elongated and lose this feature (Fig. 2).
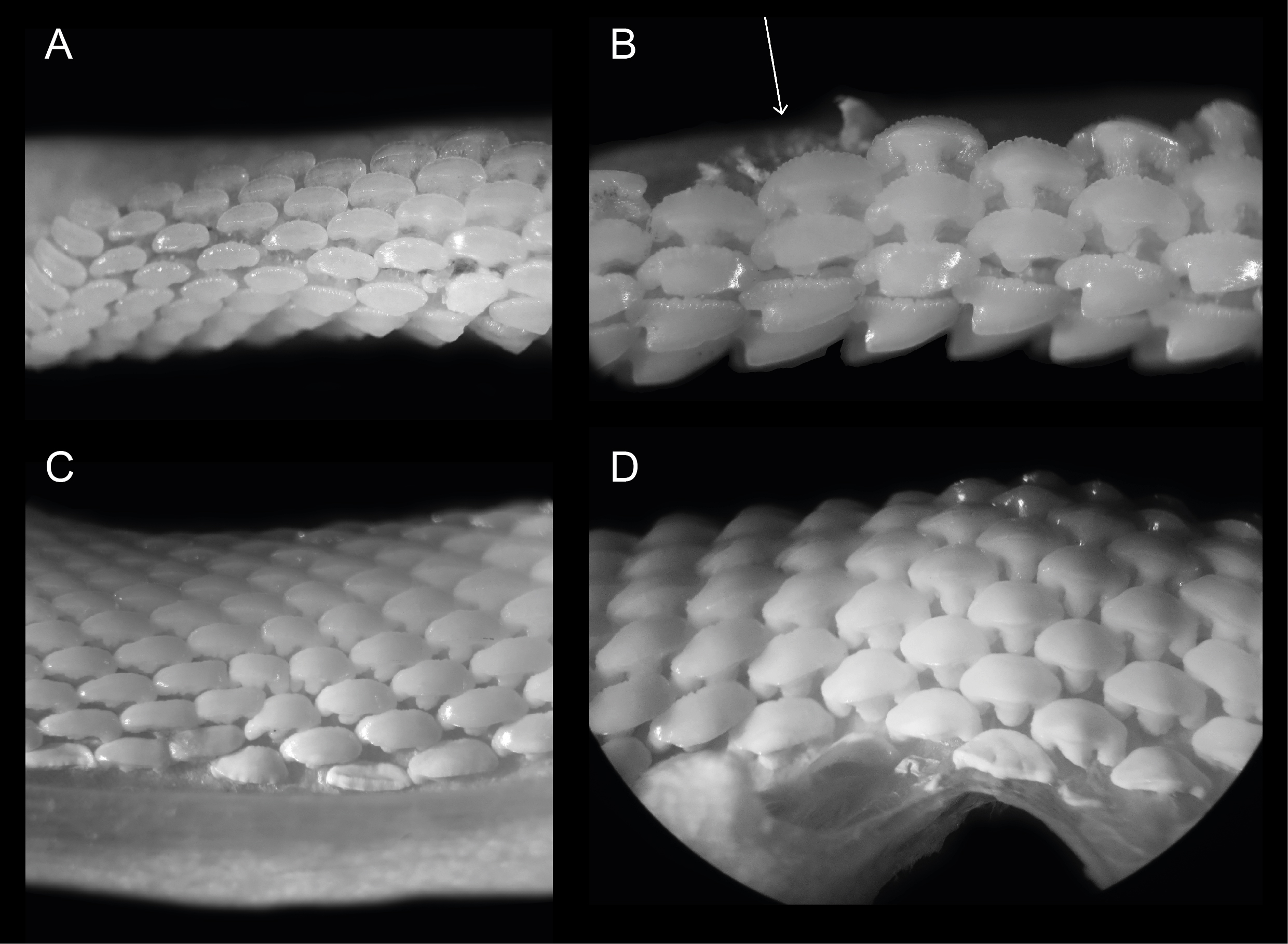
Figure 2. Close ups of ERB 1047, Mustelus punctulatus Risso, 1826. (A) Right upper lateral and posterior files. (B) Right upper anterior and anterolateral files. The arrow indicates the provenance of specimen KBIN 27206. (C) Left lower lateral files. (D) Lower median and anterior files. All teeth are between 0.5 and 1.5 mm wide.
Median and anterior teeth
16Median teeth in the upper- and lower jaw are symmetrical in appearance. Lower jaw teeth are generally larger in size compared to those of the upper jaw. These teeth have a well-developed median uvula on the lingual crown face. In this tooth position, the uvula is rounded and bulbous. From an apical point of view, the distal parts of the crown are lingually inclined, hence these teeth are somewhat comparable to female rhinobatoid teeth. The crown has a scalloped appearance from the same viewpoint. A transverse crest is present and develops in a distinct main cusp in upper jaw teeth. The cusp is less pronounced in lower jaw teeth. In anterior tooth files, the asymmetry increases. In upper jaw anterior teeth, the main cusp is well established and distally inclined. A distal heel is present.
Anterolateral, lateral and posterior teeth
17In these tooth positions, asymmetry increases even further and teeth become more elongated towards the distal part of the jaw. In upper anterolateral teeth, the crown develops a distinct main cusp, as well as a distal heel. The main cusp has a convex appearance mesio-distally. The transition of the apex into the distal heel is marked by an acute angle. In lower jaw teeth the cusp is less pronounced. A median uvula is present on the lingual crown face in teeth of both the upper- and lower jaw. This becomes less pronounced in more lateral and posterior tooth files. Furthermore, KBIN 27206, the only Mustelus punctulatus tooth available for SEM photography (Plate 3.4F), shows two types of crown ornamentation. Distinct, widely spaced vertical ridges are present on the lingual crown face, where they reach halfway towards the apex. These ridges develop irregularities on the mesial and distal crown extremities. On the labial side, these ridges are only present on the basal part of the crown. Reticulate pitting is omnipresent on the basal lingual crown face and less pronounced on the labial side. The basal parts of the marginal lingual crown faces are lobed, due to the presence of several deep notches, caused by the distal and mesial extremities of the tooth being lingually inclined. This gives these teeth a bent appearance, especially visible on the root from a basal viewpoint (Plate 3.4C). The root is holaulacorhizid and shows a distinct nutrient groove. A central foramen could not be observed. Multiple foramina are present on the lingual, labial and basal faces of both root lobes.
4.2. Dentition of Mustelus mustelus (Linnaeus, 1758) (Plate 4)
18The teeth on Plate 4 are re-figured after Herman et al. (1988), for a discussion on general observations and characteristics, we also refer to the latter.
Median and anterior teeth
19Both upper and lower median tooth files show a high degree of symmetry. Rather thin, narrowly spaced vertical ridges are present on both the lingual and labial crown face. On the labial side they are more pronounced but remain largely restricted to the base of the crown. Lingually they are thinner but extend halfway up the crown face. Pronounced reticulate pitting is present on most teeth, especially visible on the lingual crown face where it can extend high upon the crown. A slightly convex transverse crest is present, forming a reduced symmetrical cusp. Lingually, a distinct median uvula is present. The crown overhangs the root, forming a labial and lingual visor. The labial side of the crown has a scalloped appearance in apical view. The holaulacorhizid root presents a distinct nutrient groove. Multiple foramina are present on the lingual, labial, and basal faces of both root lobes.
Anterolateral, lateral and posterior teeth
20These teeth are generally more asymmetrical in overall appearance. This is visible both on the crown and the root, where the mesial side is usually more prolonged than the distal side. Anterolateral teeth in the upper jaw show a main cusp, which is distally inclined. A clear distal heel is not present. In the lower jaw, anterolateral teeth seem to lack a main cusp. Here, the transverse crest is smoothly convex in appearance. The crown overhangs the root, and apically, a scalloped appearance can be observed.
21A distinct median uvula is present. In more lateral and posterior tooth files, the median uvula on the lingual tooth face seems to disappear completely. Both types of crown ornamentation are present, in accordance with median and anterior teeth. The roots of these tooth positions share the same characteristics with median and anterior teeth, though less symmetrical in appearance.
4.3. Dentition of Mustelus asterias Cloquet, 1819 (Plates 5–7)
General observations and characteristics
22The observed teeth of the adult male and female M. asterias (KBIN 26724, Plate 5; KBIN 26723, Plate 7) as well as the juvenile female (KBIN 26722, Plate 6) possess multiple similar characteristics. Hence, these teeth are both sexually and ontogenetically rather homodont. Adult teeth often show deep scratches and grooves on the occlusal crown face, suggesting harder shelled prey. Furthermore, the jaws have a crushing type design, with teeth arranged in a pavement-like pattern. Both upper and lower jaws show a gradual monognathic heterodonty. Teeth in the proximity of the median tooth file are more symmetrical and often show a width-height ratio that is close to ca 1:1. This changes in anterolateral and lateral teeth, which are often asymmetrical and display a width-height ratio in excess of ca 2:1. The roots are holaulacorhizid in nature. A slight dignathic heterodonty can be observed in that upper jaw teeth are generally slightly smaller in size compared to their lower jaw counterparts. The transverse crest in upper jaw teeth seems slightly more inclined to form a main cusp, which is never fully present, whereas in lower jaw teeth the transverse crest is more elongated and flattened.
23An important observation is the presence of two forms of crown ornamentation on all teeth. Firstly, the crowns show faint vertical ridges on both the lingual and labial basal crown faces, with a more regular spacing on the labial crown face. These vertical ridges never extend much further than halfway up the crown. The second type of ornamentation consists of reticulate pitting which is restricted to the basal crown face. It is marginally present on the labial side, but often omnipresent on the lingual side.
Median and anterior teeth
24These teeth are symmetrical in appearance. Lower jaw teeth are slightly wider than they are high, whereas in upper jaw teeth this ratio is more proportionate. The occlusal crown face is smooth. A narrow lingual median uvula is present, accompanied by basal notches on the mesial and distal sides of the uvula. The lingual and labial crown faces show both types of ornamentation, but less pronounced compared to other tooth positions. A transverse crest is present but never develops into a distinct main cusp. A basal depression forms a clear distinction between crown and root, especially visible on the lingual side, forming a clear transition where the two neighbouring teeth interlock. On the labial side this is less visible due to the distinct labial visor of the crown which overhangs the root. A lingual visor is also present but less distinct. The root shows a clear nutrient groove with a large central pore. Small foramina are present on the basal face of the root. On the lingual root face, one to three foramina can be observed whereas on the labial root face foramina can be present but are less pronounced.
Anterolateral, lateral and posterior teeth
25These teeth are more asymmetrical with the mesial side often being more prolonged compared to the distal side. This distinction is most visible on the lingual crown face. Mesio-distally, these teeth are elongated whereas labio-lingually they are compressed, resulting in teeth that are wider than they are high. The occlusal crown face is smooth. A wide, bulbous and robust median uvula is present, which is often distally inclined but becomes more variable and less pronounced in posterior positions. Both types of basal ornamentation are present. The vertical ridges are more pronounced on the labial crown face, whereas the reticulate pitting is most visible on the lingual crown face. The ornamentation extends higher towards the apex compared to more median teeth—especially on the lingual side—, but never reaches the transverse crest. The latter does not develop into a clear cusp, resulting in a broad, slightly curved appearance of the apex. A basal depression beneath the crown is also present. A visor is present, both on the labial and lingual crown face but it is more developed on the labial side where the crown clearly overhangs the root. Two root lobes are clearly defined by a deep nutrient groove containing a central foramen. The root lobes are distally inclined in more anterolateral and lateral positions, but seem more symmetrical in posterior teeth. Multiple large foramina are present on both sides of the nutrient groove. In contrast to more median teeth, these foramina are pronounced on the labial side of the root as well. The basal face of the root shows small foramina.
5. Systematic palaeontology
26Subclass Elasmobranchii Bonaparte, 1838
27Order Carcharhiniformes Compagno, 1973
28Family Triakidae Gray, 1851
29Genus Mustelus Linck, 1790
30Type species. Squalus mustelus Linnaeus, 1758
31Mustelus aff. punctulatus Risso, 1826
32(Plates 1–2)
33aff. 1826 Mustelus punctulatus Risso, p. 128.
341987 Mustelus sp. a-b; Lienau, p. 30, pl. 6, figs 1–5.
351998 Mustelus cf. mustelus; Moths, p. 57, pl. 12, fig. 1a-c.
361999 Mustelus cf. mustelus; De Jong, p. 47, fig. 18.
372015 Mustelus sp.; Hoedemakers & Dufraing, p. 15, pl. 1, fig. 7 (= IRSNB P 10267).
38Locality. Antwerp International Airport (AIA) temporary outcrop, Borsbeek, Province of Antwerp, Belgium.
39Stratum. Base of the Deurne Member, Diest Formation (layer 4 in Goolaerts et al., 2020).
40Age. Late Miocene, middle Tortonian (A. umbraculum Zone of Dybkjær & Piasecki, 2010).
41Material. Two anterolateral teeth IRSNB P 10267 and P 10268.
5.1. Preliminary remark
42Mustelus punctulatus was described in the third volume (p. 128) of Risso’s “Histoire naturelle des principales productions de l’Europe méridionale et particulièrement de celles des environs de Nice et des Alpes Maritimes”. Although several authors refer to Risso (1827), the original date of publication seems to be 1826, as can be seen on the front page of the third volume. Therefore, in our opinion, the correct year of publication should be 1826.
5.2. Description
43IRSNB P 10267 (Plate 1). This tooth most likely originates from an upper anterolateral position in the left jaw. It measures 1.5 mm mesio-distally (width) and 0.99 mm in height. Hence, the tooth is elongated mesio-distally and compressed labio-lingually. The crown is slightly higher than the root. It is characterized by strong widely interspaced vertical ridges near the base of the crown. Further towards the apex, the presence of the vertical ridges decreases, resulting in a smooth labial crown surface halfway through the length of the cusp. Faint remnants of reticulate pitting seem present at the labial crown base (Plate 1.1B detail). The lingual crown ornamentation almost reaches the crown’s transverse crest which is irregular. The main cusp is well pronounced and is distally inclined. It has a straight, upward sloping appearance mesio-distally. The apex makes an acute angle and develops a distinct distal heel which shows more rugose vertical ridges, which can be described as fine tubercles (Plate 1.1A detail). The labial crown face is longer than the lingual surface of the tooth. On the lingual side, there is a profound median uvula above the U-shaped nutrient groove. The uvula is V-shaped and lingually inclined. A basal depression is present and marks the border between the crown and the root. The crown overhangs the root on all sides. Combined with the well-defined vertical ridges, this gives a scalloped appearance of the visor in occlusal and basal views. The root is clearly holaulacorhizid in nature and has a pronounced, deep nutrient groove with a central foramen. The lingual root face shows two distinct foramina on both root lobes whereas the labial root face has one distinct foramen. From a basal viewpoint, the root is straight mesio-distally (Plate 1.1C).
44IRSNB P 10268 (Plate 2.2A-D). The tooth probably originates from an upper anterolateral position in the left jaw. It is asymmetrical, elongated mesio-distally (width 1.73 mm) and compressed labio-lingually. The tooth is 1.1 mm in height. The occlusal crown face is smooth. Strongly pronounced and widely interspaced vertical ridges are present, extending halfway up the labial crown face and even reaching the apex on the lingual crown face, giving the lingual crown face a wrinkled appearance. These distinct vertical ridges give the visor a scalloped appearance in occlusal and basal viewpoints, especially on the labial visor. A transverse crest develops into a clear main cusp, which is strongly angled. Mesio-distally the crown has a concave appearance. Distally, a well-defined raised heel is present. Here, the vertical ridges become rugose and develop into irregular tubercles on the lingual crown face (Plate 2.2A detail). The lingual mesial crown face is poorly preserved. The median uvula on the lingual crown face is present but shows signs of damage. A basal depression beneath the crown is present. The crown clearly overhangs the root, both on the labial and lingual sides. The root is badly damaged and shows signs of bioerosion. The labial root face is best preserved and shows a nutrient groove, one foramen on the mesial root lobe and two foramina on the distal root lobe. The basal root face reveals a large central pore in the nutrient groove and multiple foramina throughout the basal root face. From a basal viewpoint, the root is straight mesio-distally (Plate 2.2C).
5.3. Morphological comparison with extant North-Eastern Atlantic taxa
45Both IRSNB P 10267 and 10268 (Plates 1–2) can be distinguished from extant M. mustelus (Plate 4) and M. asterias (Plates 5–7) by the presence as well as lack of several important characteristics. The well-defined main cusp makes them stand out from M. mustelus which shows only a weakly defined main cusp in similar tooth positions, as well as M. asterias where the transverse crest only shows a shallow ridge. Neither M. mustelus nor M. asterias display a distinct distal heel in any of their tooth positions, while being a prominent feature of both IRSNB P 10267 and 10268.
46A striking difference between IRSNB P 10267 and 10268 and both M. asterias and M. mustelus is the crown ornamentation. Both extant species show very ornate reticulate pitting, especially on the basal lingual crown face. This is congruent between male, female as well as adult and juvenile specimens. This form of ornamentation is generally absent in both fossil specimens, except for a faint remnant on the basal labial crown face in IRSNB P 10267. The vertical ridges are deeper and more widely spaced in both IRSNB P 10267 and 10268 compared to M. mustelus, where they can be more densely packed and often branch off in multiple smaller ridges. In M. asterias these vertical ridges are often fainter, do not extend as far up the crown and are more irregular on the lingual crown face, often being more present around the uvula and becoming fainter towards the distal and mesial sides of the crown.
47The roots of both the extant species and fossil specimens seem similar, with foramina being variable in number depending on tooth position.
48The general appearance of IRSNB P 10267 and 10268 is very comparable to that of upper anterolateral teeth of extant M. punctulatus (Plate 3). A distinct main cusp that acutely transitions into a distinct distal heel is clearly visible. Somewhat different in appearance is the slope of the main cusp, being erect or concave in the fossil specimens, yet convex in M. punctulatus. The extant teeth display rugose vertical ridges, that reach more than halfway up the crown on the lingual crown face, similar to the fossil specimens. Furthermore, in contrast to M. mustelus the vertical ridges are more widely spaced. Additionally, they are more pronounced on the lingual side of the crown than in M. asterias. Moreover, this ornamentation can become highly irregular (developing into tubercles), reaching the outer edges of the crown in both the fossil specimens and recent M. punctulatus, which is not the case in both M. mustelus and M. asterias. In the fossil specimens, these tubercles are even more pronounced than in the recent M. punctulatus, with the formation of a flattened, overhanging plateau at the crown base.
49Similar to M. mustelus and M. asterias, extant M. punctulatus (KBIN 27206) displays a complex network of reticulate pitting, especially on the lingual crown face. Only one fossil tooth (IRSNB P 10267) displays this faintly, and solely on the labial side. Erosion of this type of ornamentation is less likely because of the omnipresence of this type of ornamentation in all three extant North-Eastern Atlantic species. If—after erosion—this type of ornamentation would remain somewhat visible, it would most likely survive on the lingual crown face which is where the reticulate pitting is most dominant in the studied extant species. Besides, the recent tooth also differs from the fossil material by displaying lobes on the crown face, divided by multiple notches. Also, the recent tooth displays a more bent appearance. Furthermore, the SEM figured tooth of M. punctulatus displays multiple small foramina on the root lobes, whereas in both IRSNB P 10267 and 10268 very few foramina are present. However, the latter characteristic can be very variable.
50In conclusion, due to the obvious morphological similarities between IRSNB P 10267, 10268 and extant Mustelus punctulatus, but considering the limited amount of material for study as well as some differences in ornamentation, we tentatively attribute both fossil specimens to Mustelus aff. punctulatus. In this way, we recognise the probable evolutionary relationship, but take into account the existing morphological differences.
6. Discussion
6.1. Crown ornamentation as a descriptive feature (Fig. 3)
51One of the descriptive features used in the identification of (fossil) elasmobranchs can be the crown ornamentation (Herman et al., 1988). Our observations allow to document that at least two discernible types of crown ornamentation are present in extant Mustelus.
52Type 1 (primary ornamentation): vertical ridges on the crown. These ridges start basally and extend further towards the apex. Usually, they are more prominent on the lingual crown face, whereas they remain a basal phenomenon on the labial crown face. The comparison between extant M. punctulatus, M. mustelus and M. asterias has shown variation in the extension towards the apex, prominence as well as interspacing (Fig. 3A-C).
53Type 2 (secondary ornamentation): reticulate pitting on the crown. Several species of Mustelus display an ornate webbing of honeycomb-like pitting. This type of ornamentation is mostly restricted to the basal parts of the crown and is most visible on the lingual crown face. Additionally, placoid scales of extant Mustelus can also show this feature, as is the case in Mustelus schmitti Springer, 1939 (Fig. 3E). Since shark teeth and dermal denticles share a common evolutionary origin, the presence of this type of secondary ornamentation in both structures probably indicates a deep embedding of this trait in the Mustelus genome.
54Strikingly, the three studied North-Eastern Atlantic species all display the second type of ornamentation whereas it is only very faintly present in one of the fossil specimens from AIA. Similarly, Laurito Mora (1999), studying Mustelus from the Costa Rican Messinian, found the second type ornamentation on only one of six specimens. Bor et al. (2012) considered similar pitted ornamentation to be diagnostic in separating Plesiobatis from Dasyatis. Additionally, changes in ornamentation in successive stratigraphical contexts allowed Noubhani & Cappetta (1992) to hypothesize a diet change in Burnhamia. These authors erected several new batoid species in which crown ornamentation served as a helpful diagnostic feature. However, in Mustelus, the secondary ornamentation occurs around the lingual and labial crown base. The exposed occlusal crown face is often smooth. Hence, it is hard to relate changes in secondary ornamentation to dietary changes in Mustelus. Furthermore, as demonstrated in M. asterias (Plates 5–7), the secondary ornamentation does not seem sex or age related. Unfortunately, since few extant dentitions of Mustelus are studied, the significance of reticulate pitting remains poorly understood. It is striking that in fossil Mustelus teeth from the North Sea Basin, secondary ornamentation is mostly absent. Vague remains of pitting on IRSNB P 10267 (Plate 1.1B detail) indicate that the genes behind this ornamentation were at least present in the fossil specimens. However, the question remains whether the degree and extent of this ornamentation are diagnostic at the species level in Mustelus, or whether its phenotypic expression is influenced by yet unknown factors (season, temperature, hormonal fluctuations, etc.). Although we consider abrasion (see 5.3.) less likely for the near absence of pitting in the fossils, the influence of microbial decay during the early diagenesis remains unknown.
55For all these reasons, further research into the occurrence of secondary ornamentation in extant Mustelus and other taxa is required.
56
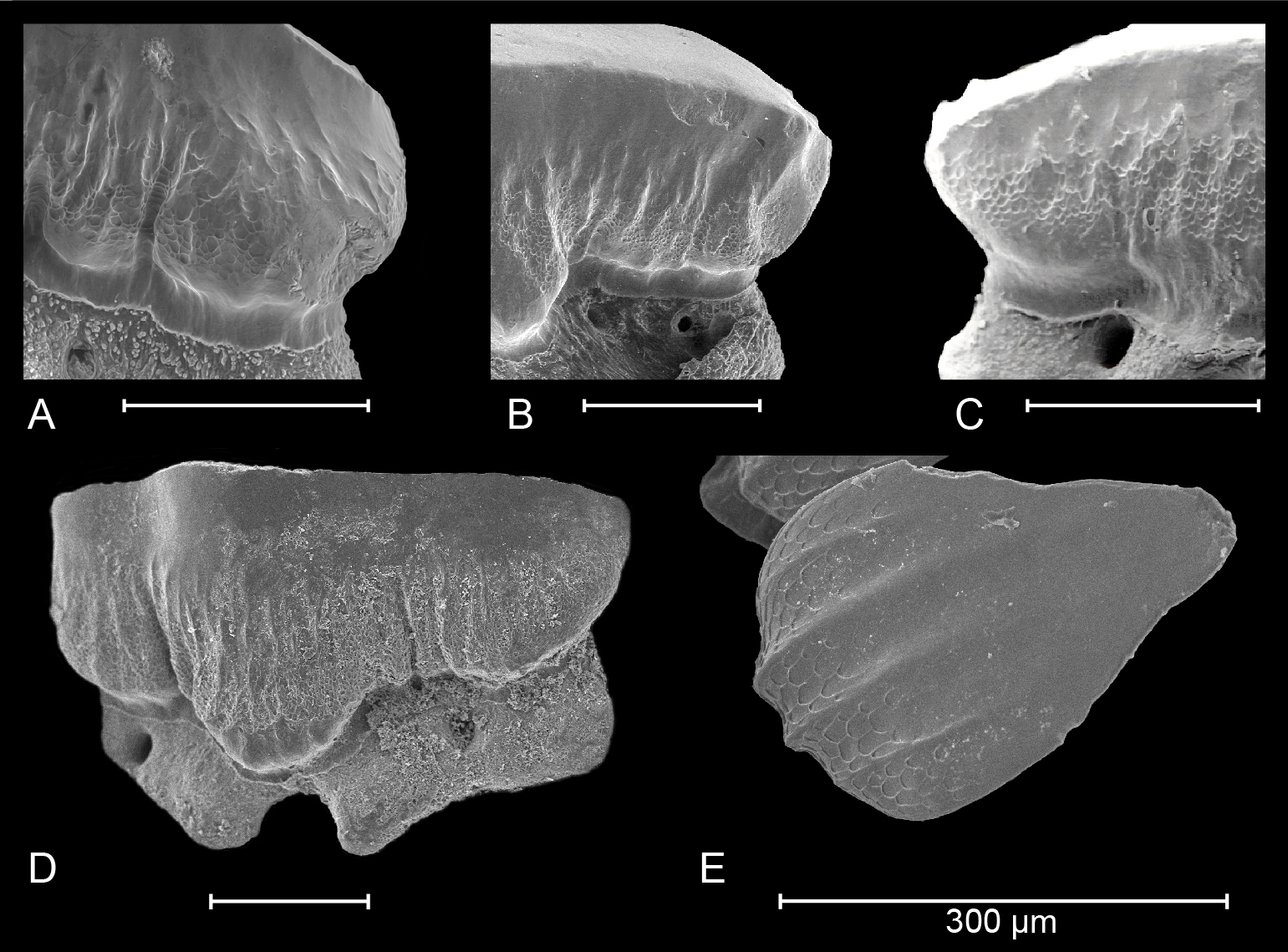
Figure 3. Details of crown ornamentation in extant (A) Mustelus punctulatus Risso, 1826; (B) Mustelus asterias Cloquet, 1819; (C) Mustelus mustelus (Linnaeus, 1758) (after Herman et al., 1988) and South American (D) Mustelus schmitti Springer, 1939 (refigured after Belleggia et al., 2014). (E) Honeycomb-like, reticulate pitting can also be observed on dermal denticles of M. schmitti (picture provided by M. Belleggia).
57
6.2. Mustelus in the Miocene of the North Sea Basin (Fig. 4)
58In the North Sea Basin, the fossil record of Mustelus is scarce, strongly contrasting their extant abundance: Mustelus is ranked as the third most common shark genus in the North Sea (Daan et al., 2005). Small teeth can be overlooked when larger screen meshes are used for sediment sieving. However, sampling bias cannot be the only explanation as multiple formations have been studied very thoroughly. Herman (1982) already questioned the scarcity of fossil Mustelus in Western Europe considering that extant Mustelus (1) are cosmopolitan and common, and (2) have high tooth replacement rates (e.g. Mustelus schmitti replace more than 5300 during their lifetime, Belleggia et al., 2014). Hence, the scarcity in the North Sea Basin is even more striking as the genus is slightly more common in the Miocene of North America (Herman, 1982; Müller, 1999; Kent, 2018). Records from the early and middle Miocene are completely lacking in the North Sea Basin (Reinecke et al., 2008; Reinecke & Wienrich, 2009; Reinecke et al., 2011; Bor et al., 2012; Everaert et al., 2019; Hoedemakers & Dufraing, 2021). Their occurrence seems limited to the upper Miocene, which is noteworthy, as early–middle Miocene faunas are more extensively studied. This is largely because accessible outcrops with fossiliferous upper Miocene sediments are less common. Moreover, the international chronostratigraphy for these outcrops is not always clear, as poorly calibrated regional stages were often used.
59Germany, Sylt – Lienau (1987)
60The first records of Mustelus in the upper Miocene of the North Sea Basin were made in the ‘Glimmerton’ at Morsum Kliff in Sylt (Lienau, 1987). A general overview of the stratigraphy of this section was given by Bossau & Klockenhoff (1977). The “Syltian” (regional stage) sediments belong to the German-Danish Gram Formation and can be correlated with the Tortonian (King, 2016). Lienau (1987) recognized four types of Mustelus in his recorded material, but left them in open nomenclature. However, the morphology of Mustelus sp. a (Lienau, 1987, plate 6, figs 1-3) is very close to our specimens IRSNB P 10267 and P 10268. All figured specimens of Sylt show coarse, widely interspaced vertical ridges nearing the transverse crest. These ridges give the visor a scalloped appearance. Furthermore, any secondary ornamentation (reticulate pitting) seems completely absent, in contrast to recent M. mustelus, M. asterias and M. punctulatus. Specimens 2 and 3 of Lienau (1987) show a distinct main cusp, with a noticeable distal heel showing small tubercles. From a basal point of view, the root of specimen 3 is straight mesio-distally. In specimen 1, the main cusp is not as pronounced, since it most likely originates from the lower jaw. Based on these characteristics, we interpret these teeth as belonging to M. aff. punctulatus. It should also be noticed that these teeth are relatively common in the elasmobranch record of Sylt, contrasting the very rare occurrences in all other outcrops. Mustelus sp. b (Lienau, 1987, plate 6, figs 4-5), especially the tooth on fig. 4, appears more symmetrical with less pronounced vertical ridges. This most likely indicates a more median tooth position. Indeed, Lienau (1987) did not exclude that these teeth belonged to a certain tooth position of his Mustelus sp. a. Since median tooth positions are outnumbered by more distally inclined teeth (i.e. anterior, anterolateral, lateral positions), this might explain the low number (3 specimens) of Mustelus sp. b compared to Mustelus sp. a (14 specimens) (Lienau, 1987). The specimens identified as Mustelus sp. c. (9 specimens) strongly differ from Mustelus sp. a and b. They are mesio-distally almost twice as large, and are characterized by a smooth, strongly flattened, almost concave crown face. The crown displays no ornamentation at all. Additionally, the visor is completely smooth from an occlusal point of view. Therefore, in our opinion, these teeth should rather be assigned to a batoid (probably Rajiformes). The same may be the case for the single tooth of the intermediate size described as Mustelus sp. d., but this specimen is unidentifiable based on the provided pictures. Therefore, in our opinion, only one Mustelus can be recognized in the Tortonian of Sylt, being M. aff. punctulatus.
61Germany, Groß Pampau – Moths (1998)
62Mustelus is also known from the upper Miocene ‘Glimmerton’ mica clay in the quarry of Groß Pampau. Moths (1998) described five teeth from Groß Pampau III in a layer 6 m below the surface (“schicht mit Plinthicus”). Mustelus is also mentioned from a shelly concentration with Murex spinicosta and Conus antediluvianus 8 m below the surface in Groß Pampau II (Moths, 1998). The ‘Glimmerton’ of Groß Pampau belongs to the Langenfeldian regional stage, which corresponds to the late Serravallian and early–middle Tortonian (King, 2016). However, the exposed mica-clay sequence lacks easily recognisable fossil marker horizons, making the stratigraphy and age often unclear. Therefore, mollusc biostratigraphy is often used (e.g. Hinsch, 1990), as these assemblages are correlated to well-calibrated Bolboforma zones (Spiegler & Gürs, 1996). The presence of Astarte anus points to a latest Serravallian age (“Lüneburgian”, middle Langenfeldian) for the Mustelus teeth from Moths’ (1998) “Schicht mit Plinthicus” (Spiegler & Gürs, 1996; King, 2016). Besides, the absence of A. anus in the shell concentration with Murex and Conus (Moths, 1994, table b) might suggest an early Tortonian age for the other Mustelus mentioned by Moths (1998). This was confirmed by Reinecke (pers. comm., 2021), who did identify Mustelus in the A. gleuei-biozone, the youngest part of the section. The three latest-Serravallian teeth were tentatively attributed to Mustelus cf. mustelus (Moths, 1998, plate 12, fig. 1a-c). Unfortunately, poor picture resolution does not allow distinguishing the presence or absence of reticulate pitting on the crown face. Type 1 ornamentation is present, widely interspaced and forming small tubercles on the distal lingual crown face. A distinct heel is not present as such, since this tooth originates from the lower jaw. Furthermore, in a basal viewpoint, the root is straight mesio-distally. This compares well to the observed diagnostic features of M. aff. punctulatus from AIA, and makes identification as M. mustelus or M. asterias less likely. Two other teeth were tentatively assigned to the genus Mustelus, but were left in open nomenclature (Moths, 1998, plate 12, fig. 3). The figured tooth stands out by its larger size (length 4.3 mm compared to 1.5 mm), smooth crown surface, seeming absence of ornamentation and a smooth visor from the occlusal viewpoint. This is very similar to the earlier discussed large specimens of Lienau (1987), probably also representing a Rajiform rather than Mustelus.
63Germany, borehole Nettetal-Haak (Plate 2.3A)
64Other unpublished records from multiple boreholes in North Rhine-Westphalia were brought to our attention by F. Von der Hocht (pers. comm., 2021). Several teeth were found in ‘Gramian’ (middle–late Tortonian) strata in Keppeln, and Lüneburgian (latest Serravallian) strata in Alst, Haak and Lobberich. An SEM picture of an upper Serravallian specimen of the Nettetal-Haak borehole (depth 149–159 m) is provided (Plate 2.3A). Although the specimen represents a median tooth position, the distinct main cusp can be distinguished at first sight. Moreover, a very rough, somewhat irregular ornamentation is observed at both distinct, raised heels, with deep vertical ridges, even developing small tubercles. Based on the provided image, no reticulate pitting seems present. Therefore, also this specimen can be assigned to M. aff. punctulatus.
65Germany, Twistringen – Menzel (1979)
66Besides teeth, Menzel (1979) tentatively assigned one dermal denticle from Twistringen (Lower Saxony) to Mustelus sp. The ‘Twistringer Schichten’ are ‘lower Reinbekian’ in age, which correlates to the upper Langhian (Gürs & Janssen, 2002; Spiegler, 2002). However, the identification as Mustelus sp. is highly questionable (Reinecke & Wienrich, 2009), and is disregarded here.
67The Netherlands, Liessel – e.g. De Jong (1999)
68In the Netherlands, Van den Bosch et al. (1975) did not record Mustelus in both in the upper Miocene Zenderen and Delden Members. However, more recently, the genus Mustelus was reported from the Hoogdonk sandpit in Liessel (De Jong, 1999; Peters, 2009; Mollen, 2010; Hoedemakers & Dufraing, 2015) where upper Miocene sediments are dredged from the Diessen Formation (the upper part of the recently abandoned Breda Formation, see Munsterman et al., 2019). While dinoflagellate cyst biostratigraphy revealed that the Liessel succession includes Tortonian and even Messinian sediments (Munsterman, 2007; Peters, 2009), a Tortonian age is usually given to almost all published (in)vertebrate remains (e.g. Lammers & Hoedemakers, 2005; Jagt et al., 2009; Marx et al., 2016; Bisconti et al., 2020). Mustelus appears very rare in Liessel. De Jong (1999) recorded only a single tooth, tentatively assigned to Mustelus cf. mustelus. Unfortunately, this specimen is lost (N. Peters, pers. comm., 2021). The tooth crown was characterized by coarse, widely interspaced vertical ridges extending close towards the transverse crest. Reticulate pitting seems absent, however tentative, due to the poor resolution of the surviving images. In occlusal view, the visor has a strongly scalloped appearance. The curved transverse crest shows an obtuse apex. The reduced height of the apex suggests a lower jaw tooth position. Based on these characteristics, we revise De Jong’s (1999) identification to M. aff. punctulatus. A similar, poorly preserved tooth from the same locality was observed by the authors in the ERB collection.
69The occurrence of morphologically similar Mustelus teeth at multiple upper Miocene localities along a 500 km path following the southern edge of the North Sea Basin, points to the existence of a continuous population in a restricted area during a constrained time frame (Fig. 4). The introduction and continuity of this population can be placed within the palaeogeographical history of the North Sea Basin. In the earliest Miocene (‘Vierlandian’), the Channel was closed and the only entrance to the North Sea Basin was the cold/temperate northern route along Scandinavia and the northern edge of the British Isles (Reinecke et al., 2008, 2011; Schwarzhans, 2010). Later, a substantial increase in elasmobranch diversity was noted during the middle Burdigalian, indicating the existence of a new marine passage along the Channel (Reinecke et al., 2011). This southern connection allowed an intensive exchange of warm, oceanic elasmobranch species from the eastern Atlantic Ocean to the southern North Sea Basin (Reinecke et al., 2011). Additionally, also the otolith records suggest an exchange between the eastern Atlantic Aquitaine Basin and the North Sea Basin during the Hemmoorian (middle Burdigalian–early Langhian) and Reinbekian (late Langhian–early Serravallian) (Schwarzhans, 2010). However, changes in tectonics occurred in the late middle Miocene and resulted in accelerated uplift along the Weald-Artois axis, causing the closure of the Channel seaway by the late Miocene (Gibbard & Lewin, 2016), a situation that would last until the early Pliocene (see also Van Vliet-Lanoë et al., 2002). Therefore, given its absence in older strata, it seems that M. aff. punctulatus reached the North Sea Basin during a dispersal event in the late Serravallian, prior to the closure of this hypothesized marine connection. Subsequently, Mustelus populations probably became isolated within the semi-enclosed Tortonian North Sea Basin. As almost all extant Mustelus species (except M. mustelus and M. asterias) are restricted to relatively (warm) temperate to (sub)tropical areas (Ebert et al., 2021), migration of Atlantic species in and out the North Sea Basin via the cooler northern route seems unlikely.
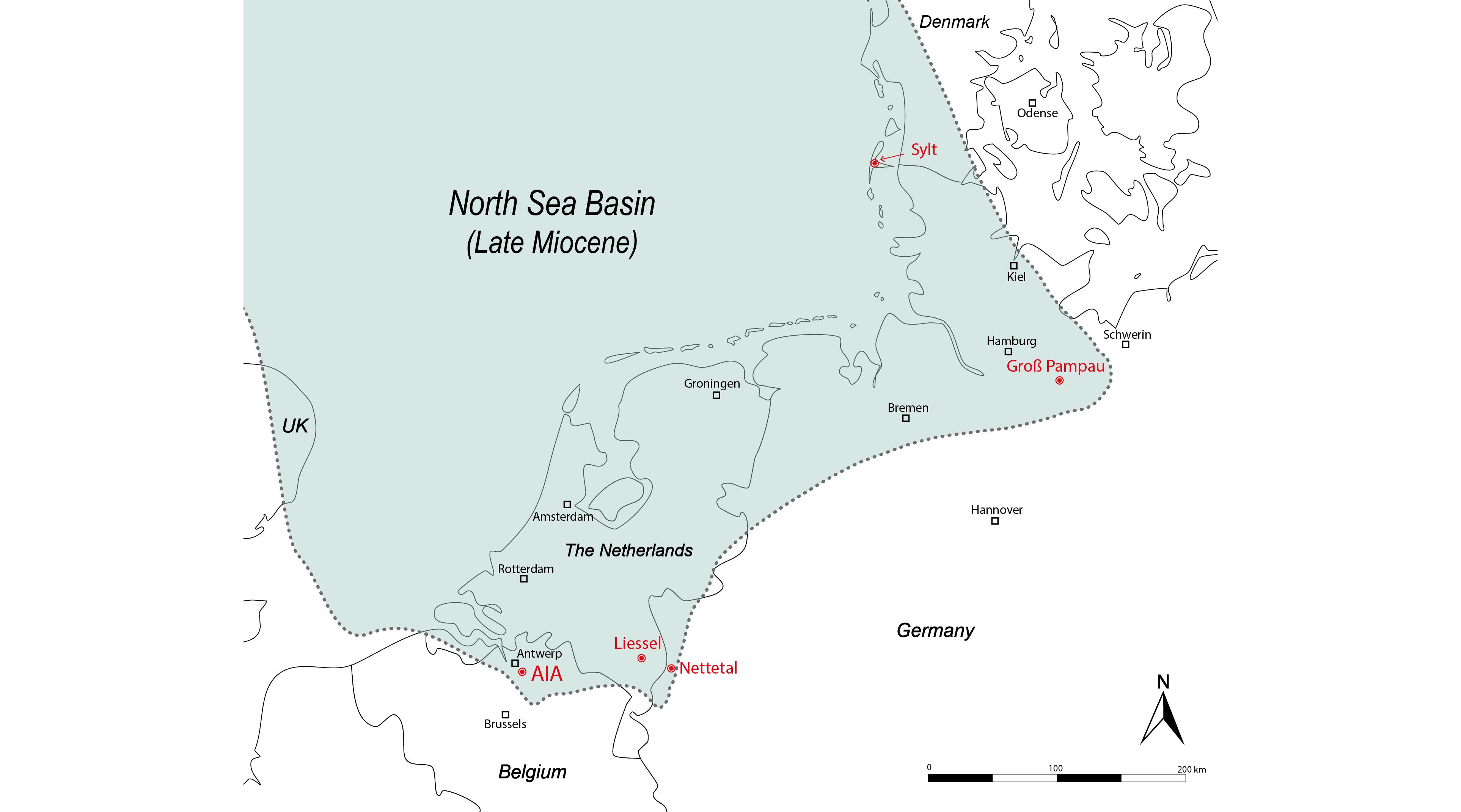
Figure 4. Palaeogeographical reconstruction of the southern North Sea Basin during the Late Miocene (Tortonian), based on the distribution of upper Miocene strata (after Vinken, 1988, map 7; Schwarzhans, 2010, text-fig. 13). The localities yielding Mustelus teeth (indicated in red) were located in shallow marine coastal environments.
6.3. Mustelus in the European fossil record
6.3.1. Paleocene
70In general, literature on fossil Mustelus in Western Europe is scarce and often limited to Paleogene sediments (e.g. Cappetta, 1976; Herman, 1982; Baut & Génault, 1995; Adnet & Cappetta, 2008; Cappetta, 2012). The geologically oldest records date to the Paleocene, with Mustelus biddlei Baut & Génault, 1995 described from the late Thanetian Bracheux Formation of Ressons-sur-Matz (NP9) (France). This species is easily distinguishable from our late Miocene material by its much larger size (towards 3 mm), and its nearly multi-cusped teeth with less pronounced ornamentation, especially on the labial crown base. Also, the middle Thanetian (NP8) ‘Tuffeau de St-Omer’ yielded some very rare M. biddlei (Moreau & Mathis, 2000). In addition, this species is also present in the Châlons-sur-Vesle Formation (NP8) (De Schutter, pers. comm., 2021). From southern France, Goret et al. (2013) figured a single specimen from the Thanetian of the ‘Petites Pyrénées’. In Belgium, M. biddlei is recorded from the Dormaal Member, which was deposited around the Paleocene–Eocene Thermal Maximum (Smith et al., 1999). In Germany, M. biddlei is known from the Thanetian ‘Lellinge Greensand Formation’ (Reinecke & Engelhard, 1997).
6.3.2. Eocene
71Two Mustelus species appear in the early Eocene (Ypresian) of Europe, Mustelus vanderhoefti Herman, 1982 and Mustelus whitei Cappetta, 1976. Compared to our Tortonian material, the teeth of Mustelus vanderhoefti are characterized by their larger size and their more pronounced crown ornamentation (Herman, 1982; Baut & Génault, 1995; Adnet & Cappetta, 2008). In contrast, the holotype of M. whitei seems much less ornamented than M. vanderhoefti and is labio-lingually more compressed. However, the damaged root of the holotype makes further evaluation difficult. Mustelus vanderhoefti was described by Herman (1982) from the Ypresian Egem Member (horizon with Venericor planicosta), in Belgium. It was also found in the Eocene Bracklesham Group near Southampton (UK) (Bone et al., 1991). In France, Adnet & Cappetta (2008) assigned teeth from the late Ypresian of Prémontré to Mustelus aff. M. vanderhoefti. It seems that this species and its relatives probably ranged at least till the middle Lutetian, as very similar teeth were found in the Lutetian–Bartonian of Landes (France), although left in open nomenclature (Adnet, 2006). Mustelus whitei on its behalf was described by Cappetta (1976) from the Ypresian London Clay and was later also found in the British middle Eocene (Kemp et al., 1990). The youngest record of M. whitei is mentioned from the Lutetian (sensu Steurbaut & Nolf, 2021) Oedelem Member of the Aalter Formation in West-Flanders (Belgium) (Hovestadt & Hovestadt-Euler, 2010). Finally, an occurrence of Mustelus sp. is mentioned by Adnet et al. (2008) from the Bartonian of Peyrehorade (south-western France). As Mustelus teeth from the European Priabonian seem absent in the literature, it is likely that Mustelus disappeared from European waters in the late Eocene. South of Europe, only a single tooth was found in the Priabonian Gehannam Formation in Egypt (Underwood et al., 2011).
6.3.3. Oligocene
72No evidence exists for the presence of Mustelus in the European Oligocene. Only one fossil from the Rupelian of the Alsace (Bouxwiller, France) was assigned to Mustelus sp. by Théobald (1934). Unfortunately, this anterior part of a skeleton with a (partial?) dentition was destroyed by a fire (Cappetta, 1987, 2012), which makes it impossible to verify its identification. Herman (1982) mentions the presence of Mustelus in the Belgian Oligocene (and Neogene), but no descriptions or figures were provided. Moreover, we could not find Mustelus in any collection of the Belgian Rupelian (pers. obs.). Therefore, leaving aside Théobald (1934), Mustelus seems absent in Europe during the Oligocene. Sampling bias cannot be considered the main reason, as multiple chondrichthyan faunas of this time interval have been studied in great detail, both from the Rupelian (e.g. Van den Bosch, 1978; Steurbaut & Herman, 1978; Van den Bosch, 1980; Baut & Génault, 1999; Reinecke et al., 2001; Génault, 2012; Herman et al., 2013; Baut et al., 2021) and the Chattian (e.g. Reinecke et al., 2005, 2014; Haye et al., 2008). The Eocene–Oligocene transition was characterized by global cooling and Antarctic glaciation (Hutchinson et al., 2020). As Mustelus populations disappeared in the latest Eocene, cooler temperatures during the Rupelian may have prevented a return to European waters during the Oligocene. However, this cannot be the only reason, as the climate in the Chattian became considerably warmer again (De Man & Van Simaeys, 2004).
6.3.4. Miocene and Pliocene
73The first record of Mustelus postdating the Paleogene is mentioned from the Langhian in Brielas (Portugal) (Fialho et al., 2016). Mustelus only reached the North Sea by the late Serravallian and stayed during the Tortonian. Also in the upper Miocene of Portugal, records of Mustelus are well known, especially from the Messinian Esbarrondadoiro Formation (Balbino, 1995; Antunes et al., 1999; Antunes & Balbino, 2004). Balbino (1995) noted very close similarities between this material and M. mustelus. Indeed, multiple specimens (Balbino, 1995, plate 10, figs 7a-b; plate 11, figs 2a-b, 3a-b) are very close to M. mustelus, i.e. abundant, narrow, closely interspaced vertical ridges, the often (very) broad uvula and a not outspoken or absent main cusp. Characteristic reticulate pitting can be observed on plate 10, fig. 7a and plate 11, fig. 3a of Balbino (1995). Some teeth also share characteristics with M. asterias, e.g. Balbino (1995, plate 10, figs 5a-b, 6; plate 11, fig. 1), by their much less pronounced ornamentation, almost absent on the distal and mesial crown face. In addition, two specimens resemble M. punctulatus (Balbino, 1995, plate 11, figs 4a-b, 5). Especially the specimen on their plate 11, fig. 4, by its more pronounced main cusp, its narrow uvula and its particular type 1 ornamentation, developing into irregular tubercles at the base of the distal and mesial crown heels. Because of these different characteristics within the fossil assemblage and strong similarities with extant dentitions, Balbino (1995) left all fossil specimens in open nomenclature, which was probably the most suitable decision at that time. However, as shown above, we believe that this Portuguese Messinian fauna already shows notable similarities with the extant North-Eastern Atlantic species.
74At the same time, a dramatic event took place in the Mediterranean due to the closure of the Atlantic connection: the Messinian Salinity Crisis (MSC) (e.g. Roveri et al., 2014). As the Mediterranean became largely desiccated, these extreme conditions resulted in massive extinction within multiple marine biotic groups. Also, the elasmobranch fauna was strongly affected by these events (e.g. Vialle et al., 2011). However, the extent of the extinction remains debated, as some thermophilic “Miocene Survivors” have recently been discovered in the Italian Pliocene (e.g. Di Cencio et al., 2021; Collareta et al., 2021b). Finally, the “Zanclean flooding” brought the MSC to an end, with the Mediterranean Sea rapidly refilling due to a renewed marine connection with the Atlantic (Roveri et al., 2014). Given that many niches were vacant, recolonization by species from the Atlantic and dispersal of survivors from intrabasinal refugia led to the introduction of multiple new species and a notable radiation in the early Pliocene Mediterranean (e.g. Tsigenopoulos et al., 2003; Triest & Sierens, 2014; Collareta et al., 2021a). Curiously, Mustelus is completely absent in the elasmobranch record of the Miocene Mediterranean (e.g. Cappetta, 1970, 1973; Vialle et al., 2011; Martínez-Pérez et al., 2017). Only after the MSC, Mustelus appears sporadically in the lower Pliocene strata (Cappetta & Cavallo, 2006; Cappetta, 2012). In our opinion, the specimens described as Mustelus sp. by Cappetta & Cavallo (2006) from Alba (Piémont, Italy) show similarities with extant M. mustelus; i.e. less pronounced main cusp, abundant fine, densely vertical ridges on the lingual crown face and a relatively wide uvula. This might also be the case for the specimen of Bellegarde in Southern France, drawn in Cappetta (1987, 2012), although speculative. It is possible that extensive sampling on smaller mesh sizes (<1 mm) in the Mediterranean could yield more Mustelus teeth in the future, including M. punctulatus postdating the MSC.
75In the Atlantic, Mustelus sp. is known from the lower Pliocene Huelva Formation in the Spanish Guadalquivir basin (García et al., 2011). Unfortunately, the illustrated material still contains matrix residues and is poorly preserved. The rather symmetrical specimens in fig. 5a, b, e in García et al. (2011) show superficial resemblance to M. asterias by the quasi absence of ornamentation. The specimen on their fig. 5d is closer to M. punctulatus, by the coarse ornamentation (especially on the mesial and distal heel) and the pointed appearance of the uvula. Additionally, also the specimen in fig. 5g-h displays distinct widely interspaced vertical ridges, approaching the transverse crest, congruent with extant M. punctulatus.
76In contrast to the Atlantic, Mustelus seems to disappear after the Tortonian in the North Sea Basin. No specimens are known from the Messinian. However, this may be caused by the scarcity of Messinian outcrops, mostly devoid of fossils (e.g. the Belgian Kasterlee Formation, Vandenberghe et al., 2020). In the Pliocene, Mustelus is absent in the Zanclean Kattendijk Formation in northern Belgium (Herman et al., 1974) and the Pliocene Delden and Lievelde Members of the Netherlands (Van den Bosch, 1980). Although Herman (1982) mentions Mustelus from the Belgian Pliocene, no evidence or figures have been provided, and no teeth are known from private collections.
6.4. Palaeobiogeography and migration of Mustelus punctulatus
77The evolution and palaeobiogeographical distribution of Mustelus are poorly understood. In recent years, however, several hypotheses about Mustelus evolution were made by comparing multiple protein-coding gene sequences, mainly from mitochondrial DNA (López et al., 2006; Boomer et al., 2012; Maduna et al., 2020). These phylogenetic analyses and Bayesian molecular clock datings have provided valuable insights on the worldwide dispersal and radiation of Mustelus species. For example, Mustelus appears to be a paraphyletic assemblage, which can only be monophyletic when some Triakis species and Scylliogaleus quecketti are included (López et al., 2006). Moreover, two clades exist within the expanded Mustelus genus, consisting of placental, non-spotted species (‘M. mustelus’ clade) and white spotted, ovoviviparous aplacental species (‘M. asterias’ clade). Both clades were separated during a major cladogenic event, estimated around the Thanetian, based on the NADH-2 phylogeny (Maduna et al., 2020, fig. 2). The global high diversity of extant species can be explained by radiation after multiple long-distance dispersal events, especially during the Miocene, up to the Pleistocene (Boomer et al., 2012; Maduna et al., 2020). The species in the southern hemisphere are believed to be descendants of migrated populations from the northern hemisphere.
78The dark to black-spotted M. punctulatus appears to be one of the oldest Mustelus representatives, as it is placed at the very base of the placental, non-spotted clade (Maduna et al., 2020). Therefore, these findings suggest the secondary loss of black spots within this clade. In their phylogeny of the NADH-2 gene sequences, Maduna et al. (2020) estimated the split-up between the ancestors of M. punctulatus and the other, non-spotted species around the Ypresian–Lutetian boundary. However, no fossil specimens have been identified as M. punctulatus to date, which hinders the verification of the proposed hypotheses. Therefore, the presence of M. aff. punctulatus in the late Miocene southern North Sea Basin sheds new light on the early distribution of this nowadays predominantly Mediterranean species. After the absence of Mustelus in the North Sea Basin since the late Eocene, M. aff. punctulatus appears for the first time at the end of the middle Miocene, during the late Serravallian in Germany. The species probably migrated to the North Sea via a temporary southern connection with the Atlantic Ocean. During the late Serravallian–Tortonian interval, M. aff. punctulatus remained the dominant, and probably only Mustelus present in the North Sea. Despite some differences, the fossil specimens show a stronger affinity with extant teeth of M. punctulatus due to their general morphology, and much less with contemporary M. mustelus and M. asterias. During the late Miocene, the North Sea population probably became isolated, as the Channel was closed until the early Pliocene. Although sampling bias cannot be fully excluded, the absence of fossil teeth in the lower Pliocene deposits of the southern North Sea (e.g. Kattendijk Formation, Herman et al., 1974) might indicate that M. aff. punctulatus disappeared from the North Sea Basin around the Miocene–Pliocene boundary. Possibly, this could be linked to gradual decreasing temperatures, due to global cooling and further glaciation (Zachos et al., 2001). In younger Pliocene sediments, towards the mid-Pliocene Warm Period (e.g. Lillo Formation), no records of Mustelus in private collections are known to the authors. In any case, M. punctulatus disappeared from the North Sea, which is still the case today.
79In the authors’ opinion, the ancestors of extant M. punctulatus, M. mustelus and M. asterias are also present in the Messinian of the Atlantic (southern Portugal), based on teeth depicted by Balbino (1995). In addition, in the Spanish Pliocene (García et al., 2011), teeth similar to M. punctulatus can be recognized. Therefore, given the absence of Mustelus records in the Mediterranean predating the MSC, the likelihood of the ancestors of M. punctulatus only having entered the Mediterranean during or after the Zanclean flooding can be considered. This would suggest that the origin of this nowadays largely Mediterranean species actually lies more to the north (the Atlantic Ocean and the North Sea Basin).
7. Conclusion
80Mustelus is a problematic genus in the fossil record, as its teeth are rarely found and have a relatively homogeneous morphology at first sight. Its crushing-type dentition remained largely unchanged since the Paleocene. Moreover, only a few dentitions of the 27 recent species (Ebert et al., 2021) have been described and figured (Herman et al., 1988; Belleggia et al., 2014). Therefore, most fossil specimens are left in open nomenclature (e.g. Lienau, 1987; Balbino, 1995; Cappetta & Cavallo, 2006).
81The present study reveals morphological differences between teeth of different species of Mustelus. These differences are often subtle and only identifiable with SEM imagery. It can be concluded that in addition to tooth size, other characteristics such as the shape of the uvula, the presence/absence of a distinct main cusp, the density, extent and size of vertical ridges and the presence/absence of secondary ornamentation (reticulate pitting) are important descriptive features. Moreover, the study of extant dentitions of M. asterias suggests little sexual and ontogenetic heterodonty. Examination of the three extant North-Eastern Atlantic species proved that all Miocene teeth of the North Sea Basin could be attributed to M. aff. punctulatus, a species nowadays largely restricted to the Mediterranean. Geographical dispersal since the Neogene is taken into account. To achieve a better understanding of the evolution of Mustelus, an important task for both biologists and palaeontologists remains (see also Guinot et al., 2018; Mollen, 2019). It is our conviction that molecular evolutionary studies can be further corroborated by fossil material, but this requires cautious and broad interdisciplinary study.
82From the current case study, one can conclude that the extant Mediterranean population of M. punctulatus most likely had its origin from migration out of the North-Eastern Atlantic, postdating the Messinian Salinity Crisis. Moreover, the hotspot for the fossil record of this species lies more northern, in the North Sea region. Despite some noted differences, the general morphology of the fossil teeth points to a plausible affinity with extant M. punctulatus, instead of M. mustelus and M. asterias. Therefore, the fossils of the Belgian Tortonian were attributed to M. aff. punctulatus. Re-evaluation of the published record indicates the presence of a widely distributed population along the southern edge of the late Miocene North Sea Basin.
8. Acknowledgements
83We would like to express our gratitude to Gunther Cleemput (Ternat) for his extensive efforts in illustrating this article. Dirk Hovestadt (Terneuzen) for giving full access to his recent comparison dentitions and donating multiple teeth of Mustelus asterias. Taco Bor (Sliedrecht), Pieter De Schutter (RBINS), Stijn Goolaerts (RBINS), Dirk Hovestadt and Frederik Mollen (Elasmobranch Research Belgium, Bonheiden) for proofreading the manuscript and giving suggestions during this research. Pieter De Schutter (RBINS) is also thanked for kindly donating IRSNB P 10268, and providing additional photographic material together with Jacques Herman (Beigem). Frederik Mollen (ERB) gave access to his extant dentition of M. punctulatus and donated specimen KBIN 27206. Fritz Von der Hocht (Kerpen) is kindly thanked for providing information about fossil Mustelus in Germany, and allowing the publication of the Nettetal specimen. Thomas Reinecke (Bochum) for sharing data on the German Miocene. Stijn Goolaerts (RBINS) for sharing valuable ideas about the Deurne Member and providing access to the RBINS facilities, together with Thierry Smith (RBINS), which made this study possible. Julien Cillis and Laetitia Despontin (RBINS) for their time and efforts in making high-quality ESEM images. Mauro Belleggia (National Institute for Fisheries Research and Development, Buenos Aires) for sharing and allowing the use of multiple unpublished photographs of M. schmitti. Noud Peters (Oertijdmuseum De Groene Poort, Boxtel) for providing information on the Liessel Mustelus. Russell Soon for improving grammar. Theo Lambrechts (Heist-op-den-Berg) is thanked for his assistance in screening the residues of the Deurne Member. Kristiaan Hoedemakers (RBINS) and Jürgen Pollerspöck (Shark References) for additional literature suggestions. Finally, we thank the reviewers David J. Ward (Natural History Museum, London) and Prof. Jürgen Kriwet (University of Vienna) for their helpful suggestions, improving our paper.
9. Author contribution
84Both authors contributed equally to the data acquisition, their interpretation and the writing of this article.
10. Data availability
85Most studied specimens are housed in official repositories guaranteeing their long-term safekeeping and availability to other researchers for future studies.
11. References
86Adnet, S. 2006. Nouvelles faunes de Sélaciens (Elasmobranchii, Neoselachii) de l’Éocène moyen des Landes (Sud−Ouest, France). Implication dans la connaissance des communautés de sélaciens d’eaux profondes. Palaeo Ichthyologica, 10, 5–128.
87Adnet, S. & Cappetta, H., 2008. New fossil triakid sharks from the early Eocene of Prémontré, France, and comments on fossil record of the family. Acta Palaeontologica Polonica, 53, 433–448. https://doi.org/10.4202/app.2008.0306
88Adnet, S., Cappetta, H. & Reynders, J., 2008. Contribution of Eocene sharks and rays from southern France to the history of deep-sea selachians. Acta Geologica Polonica, 58, 261–264.
89Antunes, M.T. & Balbino, A.C., 2004. Os carcharhiniformes (Chondrichthyes, Neoselachii) da Bacia de Alvalade (Portugal). Revista Española de Paleontología, 19, 73–92. https://doi.org/10.7203/sjp.19.1.20523
90Antunes, M.T., Balbino, A.C. & Cappetta, H., 1999. Sélaciens du Miocène terminal du bassin d’Alvalade (Portugal). Essai de synthèse. Ciências da Terra (UNL), 13, 115–129.Balbino, A.C., 1995. Seláceos (Pisces) do Miocénico terminal da Bacia de Alvalade (Portugal): Sistemática, ecologia, paleoambientes, comparação com faunas actuais. Unpublished Ph.D. Thesis, University of Évora, Évora, 200 p.
91Baut, J.-P. & Génault, B., 1995. Contribution à l'étude des Elasmobranches du Thanétien (Paléocène) du Bassin de Paris. 1. Découverte d'une faune d'Elasmobranches dans la partie supérieure des Sables de Bracheux (Thanétien, Paléocène du Bassin de Paris) des régions de Compiègne (Oise) et de Montdidier (Somme). In Herman, J. & Van Waes, H. (eds), Elasmobranches et Stratigraphie. Service géologique de Belgique, Professional Papers, 278, 185–259.
92Baut, J.-P. & Génault, B., 1999. Les Elasmobranches des Sables de Kerniel (Rupélien), à Gellik, Nord Est de la Belgique. Memoirs of the Geological Survey of Belgium, 45, 1–61.
93Baut, J.-P., Merle, D., de Lapparent de Broin, F., Brisswalter, G. & Profichet, P., 2021. Stratigraphie, vertébrés marins et peuplements associés de gisements disparus du Stampien (Rupélien) du Nord du Hurepoix (Essonne, France). Bulletin d’Information des Géologues du Bassin de Paris, 58/2, 2–77.
94Belleggia, M., Figueroa D.E. & Bremec, C., 2014. The dentition of the narrownose smooth-hound shark, Mustelus schmitti. Marine and Freshwater Research, 65, 688–696. https://doi.org/10.1071/MF13122
95Bisconti, M., Munsterman, D.K., Fraaije, R.H.B., Bosselaers, M.E.J. & Post, K., 2020. A new species of rorqual whale (Cetacea, Mysticeti, Balaenopteridae) from the Late Miocene of the Southern North Sea Basin and the role of the North Atlantic in the paleobiogeography of Archaebalaenoptera. PeerJ 8, e8315. http://doi.org/10.7717/peerj.8315
96Bone, D.A., Todd, J.A. & Tracey, S., 1991. Fossils from the Bracklesham Group exposed in the M27 Motorway excavations, Southampton, Hampshire. Tertiary Research, 12, 131–137.
97Boomer, J.J., Harcourt, R.G., Francis, M.P. & Stow, A.J., 2012. Genetic divergence, speciation and biogeography of Mustelus (sharks) in the central Indo-Pacific and Australasia. Molecular Phylogenetics and Evolution, 64, 697–703. http://dx.doi.org/10.1016/j.ympev.2012.05.024
98Bor, T., 2013. Terminologie en determinatie van haaien- en roggentanden. Afzettingen WTKG, 24, 116–137.
99Bor, T., Reinecke, T. & Verschueren, S., 2012. Miocene Chondrichthyes from Winterswijk-Miste, the Netherlands. Palaeontos, 21, 136 p.
100Bossau, K.U. & Klockenhoff, R., 1977. Neues zur Paläontologie und Stratigraphie der Sylt-Stufe am Morsumkliff/Sylt. Schriften des Naturwissenschaftlichen Vereins für Schleswig-Holstein, 47, 25–38.
101Bosselaers, M., Herman, J., Hoedemakers, K., Lambert, O., Marquet, R. & Wouters, K., 2004. Geology and palaeontology of a temporary exposure of the Late Miocene Deurne Sand Member in Antwerpen (N. Belgium). Geologica Belgica, 7, 27–39.
102Brevé, N.W.P., Winter, H.V., Van Overzee, H.M.J., Farrell, E.D. & Walker, P.A., 2016. Seasonal migration of the starry smooth-hound shark Mustelus asterias as revealed from tag-recapture data of an angler-led tagging programme. Journal of Fish Biology, 89, 1158–1177.https://doi.org/10.1111/jfb.12994
103Cappetta, H., 1970. Les sélaciens du Miocène de la région de Montpellier. Palaeovertebrata, 3 (ext.), 1–139. http://dx.doi.org/10.18563/pv.3.ext.1-139
104Cappetta, H., 1973. Les sélaciens du Burdigalien de Lespignan (Hérault). Geobios, 6, 211–223. https://doi.org/10.1016/S0016-6995(73)80016-6
105Cappetta, H., 1976. Sélaciens nouveaux du London Clay de l’Essex (Yprésien du Bassin de Londres). Géobios, 9/5, 551–557. https://doi.org/10.1016/S0016-6995(76)80024-1
106Cappetta, H., 1987. Chondrichthyes II: Mesozoic and Cenozoic Elasmobranchii. In Schultze, H.-P. (ed.), Handbook of Paleoichthyology, Vol. 3B. Gustav Fischer Verlag, Stuttgart, 1–193.
107Cappetta, H., 2012. Chondrichthyes: Mesozoic and Cenozoic Elasmobranchii: teeth. In Schultze, H.-P. (ed.), Handbook of Paleoichthyology, Vol. 3E. Friedrich Pfeil, München, 1–512.
108Cappetta, H. & Cavallo, O., 2006. Les sélaciens du Pliocène de la région d’Alba (Piémont, Italie Nord-Ouest). Rivista Piemontese di Storia Naturale, 27, 33–76.
109Collareta, A., Mollen, F.H., Merella, M., Casati, S. & Cencio, A.D., 2021a. Remarkable multicuspid teeth in a new elusive skate (Chondrichthyes, Rajiformes) from the Mediterranean Pliocene. PalZ Paläontologische Zeitschrift, 95, 117–128. https://doi.org/10.1007/s12542-020-00542-7
110Collareta, A., Merella, M., Casati, S., Coletti, G. & Di Cencio, A., 2021b. Another thermophilic “Miocene survivor” from the Italian Pliocene: A geologically young occurrence of the pelagic eagle ray Aetobatus in the Euro-Mediterranean region. Carnets de Géologie, 21, 203–214. https://dx.doi.org/10.2110/carnets.2021.2110
111Compagno, L.J.V., 1973. Interrelationships of living elasmobranchs. Zoological Journal of the Linnean Society, 53 (supplement 1), 15–61.
112Daan, N., Heessen, H. & ter Hofstede, R., 2005. North Sea Elasmobranchs: distribution, abundance and biodiversity. International Council for the Exploration of the Sea, ICES CM 2005/N:06, 1–15.
113De Jong, A., 1999. Haaien- en Roggentanden uit Leissel (N.-B.), een ‘Paleozoölogisch’ onderzoek. Unpublished dissertation, Vakgroep Biologie, Faculteit Educatie, Fontys Hogescholen Tilburg, 69 p.
114De Man, E. & Van Simaeys, S., 2004. Late Oligocene Warming Event in the southern North Sea Basin: benthic foraminifera as paleotemperature proxies. Netherlands Journal of Geosciences / Geologie en Mijnbouw, 83, 227–239. https://doi.org/10.1017/S0016774600023520
115de Verteuil, L. & Norris, G., 1996. Miocene dinoflagellate stratigraphy and systematics of Maryland and Virginia. Micropaleontology, 42, Supplement, 1–172. https://doi.org/10.2307/1485926
116Di Cencio, A., Dulai, A., Catanzariti, R., Casati, S. & Collareta, A., 2021. First record of the brachiopod Lingula? from the Pliocene of Tuscany (Italy): The youngest occurrence of lingulides in the Mediterranean Basin. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 299, 237–249. http://dx.doi.org/10.1127/njgpa/2021/0966
117Dybkjær, K. & Piasecki, S., 2010. Neogene dinocyst zonation for the eastern North Sea Basin, Denmark. Review of Palaeobotany and Palynology, 161, 1–29. https://doi.org/10.1016/j.revpalbo.2010.02.005
118Ebert, D.A. & Dando, M., 2021. Field Guide to Sharks, Rays & Chimaeras of Europe and the Mediterranean. Princeton University Press, Princeton, 383 p.
119Ebert, D.A., Dando, M & Fowler, S., 2021. Sharks of the World: A Complete Guide. Princeton University Press, Princeton, 608 p.
120Everaert, S., De Schutter, P., Mariën, G., Cleemput, G., Van Boeckel, J., Rondelez, D. & Bor, T., 2019. Een vroeg-miocene fauna uit het Zand van Kiel (Formatie van Berchem) bij Post X in Berchem (Antwerpen). Afzettingen WTKG, 40, 83–100.
121Farrell, E.D., Clarke, M.W. & Mariani, S., 2009. A simple genetic identification method for Northeast Atlantic smoothhound sharks (Mustelus spp.). ICES Journal of Marine Science, 66, 561–565. https://doi.org/10.1093/icesjms/fsn218
122Farrell, E.D., Mariani, S. & Clarke, M.W., 2010. Age and growth estimates for the starry smoothhound (Mustelus asterias) in the Northeast Atlantic Ocean. ICES Journal of Marine Science, 67, 931–939. https://doi.org/10.1093/icesjms/fsp295
123Fialho, P.R., Balbino, A. & Antunes, M.T., 2016. Seláceos do Langhiano da Bacia do Baixo Tejo (Brielas, Portugal). Simpósio Novapaleo, Departamento de Ciências da Terra, FCT-UNL, 47–50.
124García E.X.M., Balbino A., Antunes M., Ruiz F., Civis, J., Abad, M. & Toscano-Grande, A., 2011. Los tiburones Carcharhiniformes (Chondrichthyes, Galeomorphii) del Plioceno inferior de la Formación arenas de Huelva, suroeste de la cuenca del Guadalquivir, España. Revista Mexicana de Ciencias Geológicas, 28, 474–492
125Génault, B. 2012. Vertébrés. Requins et raies (chondrichtyens). In Lozouet, P. (ed.), Stratotype Stampien. Biotope, Mèze, Muséum national d’Histoire naturelle, Paris, Patrimoine géologique, 4, 299–308.
126Gibbard, P.L. & Lewin, J., 2016. Filling the North Sea Basin: Cenozoic sediment sources and river styles. Geologica Belgica, 19, 201–2017. http://dx.doi.org/10.20341/gb.2015.017
127Goolaerts, S., De Ceuster, J., Mollen, F.H., Gijsen, B., Bosselaers, M., Lambert, O., Uchman, A., Van Herck, M., Adriaens, R., Houthuys, R., Louwye, S., Bruneel, Y., Elsen, J. & Hoedemakers, K. 2020. The upper Miocene Deurne Member of the Diest Formation revisited: unexpected results from the study of a large temporary outcrop near Antwerp International Airport, Belgium. Geologica Belgica, 23, 219–252. https://doi.org/10.20341/gb.2020.011
128Goret, B., Téodori, D. & Lebrun, P., 2013. Le Crétacé-Paléogène des Petites Pyrénées, des fossiles en Haute-Garonne et Ariège. Fossiles, 16, 44–58.
129Guinot, G., Adnet, S., Shimada, K., Underwood, C.J., Siversson, M., Ward, D.J., Kriwet, J. & Cappetta, H., 2018. On the need of providing tooth morphology in descriptions of extant elasmobranch species. Zootaxa, 4461, 118–126. https://doi.org/10.11646/zootaxa.4461.1.8
130Gürs, K. & Janssen, A.W., 2002. Revised pteropod biostratigraphy for the Miocene of the North Sea Basin. In Gürs, K. (ed.), Northern European Cenozoic Stratigraphy, Proceedings of the 8th Biannual Joint Meeting of the RCNNS/RCNPS. Landesamt für Natur und Umwelt des landes Schleswig-Holstein, Flintbek, 117–131.
131Haye, T., Reinecke, T., Gürs, K. & Piehl, A., 2008. Die Elasmobranchier des Neochattiums (Oberoligozän) von Johannistal, Ostholstein, und Ergänzungen zu deren Vorkommen in der Ratzeburg-Formation (Neochattium) des Sudöstlichen Nordseebeckens. Palaeontos, 14, 55–95.
132Herman, J., 1982. Additions to the Eocene fish fauna of Belgium. 5. The discovery of Mustelus teeth in Ypresian, Paniselian and Wemmelian strata. Tertiary Research, 3, 189–193.
133Herman, J., Crochard, M. & Girardot, M., 1974. Quelques restes de sélaciens récoltés dans les Sables du Kattendijk à Kallo. Bulletin de la Société belge de Géologie, 83, 15–31.
134Herman, J., Hovestadt-Euler, M. & Hovestadt, D.C., 1988. Part A: Selachii. N2a. Order: Carcharhiniformes. Family: Triakidae. In Stehmann, M. (ed.), Contributions to the Study of the Comparative Morphology of Teeth and Other Relevant Ichthyodorulites in Living Supraspecific Taxa of Chondrichthyan Fishes. Bulletin de l’Institut Royal des Sciences naturelles de Belgique, 58, 99–126.
135Herman, J., Van Waes, H., Doutrelepont, H., Kenis, L., Van Nuffel, J., Cloetens, J., Vanderhoeft, E. & Vervoenen, M., 2013. Additional Contributions to the Knowledge of the Sediments, Taphonomy, Ichnofossils, Bacteria, Invertebrata, Vertebrata, Algae and Plantae of the Sint Niklaas Phosphorite Bed in its type locality: Sint Niklaas (Eastern Flanders, Belgium). Part Three: Vertebrata. Géominpal Belgica, 5.3, 248 p.
136Hinsch, H., 1990. Biostratigraphy of Reinbekian/Levensauian/Lüneburgian/Langenfeldian boundary stratotypes in Pampau area (SE-Holstein). Veröffentlichungen aus dem Übersee-Museum Bremen, A10, 55–79.
137Hoedemakers, K. & Dufraing, L., 2015. Elasmobranchii in de ontsluiting aan de luchthaven te Borsbeek (prov. Antwerpen, België). Afzettingen, 36, 12–19
138Hoedemakers, K. & Dufraing, L., 2021. Elasmobranchii uit het Zand van Edegem (Mioceen van België). Afzettingen WTKG, 42, 48–61.
139Hooyberghs, H.J.F. & Moorkens, T.L.A., 2005. Biostratigraphic study of the “Middelares Hospital” outcrop section in the Deurne Sand Member (Upper Miocene, Belgium) as based on foraminifera. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 237, 5–28. https://doi.org/10.1127/njgpa/237/2005/5
140Hovestadt, D. & Hovestadt-Euler, M., 2010. Urobatis molleni nov. sp. (Chondrichthyes, Myliobatiformes, Urolophidae) in the Eocene of Belgium. Geominpal Belgica, 1/3, 66–69.
141Hutchinson, D.K., Coxall, H.K., Lunt, D.J., Steinthorsdottir, M., De Boer, A.M., Baatsen, M., von der Heydt, A., Huber, M., Kennedy-Asser, A.T., Kunzmann, L., Ladant, J.B., Lear, C.H., Moraweck, K., Pearson, P.N., Piga, E., Pound, M.J., Salzmann, U., Scher, H.D., Sijp, W.P., Sliwinska, K.K., Wilson P.A. & Zhang, Z., 2020. The Eocene-Oligocene transition: a review of marine and terrestrial proxy data, models and model-data comparisons. Climate of the Past, 17, 269–315. https://doi.org/10.5194/cp-17-269-2021
142Jagt, J.W.M., Fraaije, R.H.B. & van Bakel, B.W.M., 2009. A late Miocene astropectinid (Echinodermata, Asteroidea) and associated ichnofossils from Liessel, province of Noord-Brabant, the Netherlands. Netherlands Journal of Geosciences – Geologie en Mijnbouw, 88, 127–131. https://doi.org/10.1017/S0016774600000937
143Kemp, D.J., Kemp, L. & Ward, D.J., 1990. An illustrated Guide to the British Middle Eocene Vertebrates. Privately published, London, 59 p.
144Kent, B.W., 2018. The cartilaginous fishes (chimaeras, sharks, and rays) of Calvert Cliffs, Maryland, USA. In Godfrey, S.J. (ed.), The Geology and Vertebrate Paleontology of Calvert Cliffs, Maryland, USA. Smithsonian Contributions to Paleobiology, 100, 45–156.
145King, C., 2016. A revised Correlation of Tertiary Rocks in the British Isles and adjacent Areas of NW Europe. Gale, A.S. & Barry, T.L. (eds). The Geological Society, London, Special Reports, 27, 719 p. https://doi.org/10.1144/SR27
146Lambert, O. & Goolaerts, S., 2021. Late Miocene survival of a hyper-longirostrine dolphin and the Neogene to recent evolution of rostrum proportions among odontocetes. Journal of Mammalian Evolution, 29, 99–111. https://doi.org/10.1007/s10914-021-09573-6
147Lammers, T.H. & Hoedemakers, K., 2005. Otolieten van Beenvissen (Teleostei) uit boring 165 te Liessel (Noord-Brabant, Nederland). Grondboor & Hamer, 5/6, 116–123.
148Laurito Mora, C.A., 1999. Los seláceos fósiles de la localidad de Alto Guayacán (y otros ictiolitos asociados), Mioceno Superior-Plioceno Inferior de la Formación Uscari, provincia de Limón, Costa Rica. Privately published (Texto Comunicación), San José, 168 p.
149Lienau, H-W., 1987. Haie und Rochen aus dem Sylter Ober-Miozän. In von Hacht, U. (ed.), Fossillien von Sylt II. Verlag Inge-Maria von Hacht, Hamburg, 19–75.
150López, J.A., Ryburn, J.A., Fedrigo, O. & Naylor, G.J P., 2006. Phylogeny of sharks of the family Triakidae (Carcharhiniformes) and its implications for the evolution of carcharhiniform placental viviparity. Molecular Phylogenetics and Evolution, 40, 50–60. https://doi.org/10.1016/j.ympev.2006.02.011
151Louwye, S., 2002. Dinoflagellate cyst biostratigraphy of the Upper Miocene Deurne Sands (Diest Formation) of northern Belgium, southern North Sea Basin. Geological Journal, 37, 55–67. https://doi.org/10.1002/gj.900
152Maduna, S.N., Hull, K.L., Farrell, E.D., Boomer, J.J., Veríssimo, A.N.A., Marion, I.A.M., Mazzoldi, C., Zane, L., Wintner, S.P., Chesalin, M.V., da Silva, C., Gubili, C., Mariani, S. & Bester-Van der Merwe, A.E., 2020. Historical biogeography of smoothhound sharks (genus Mustelus) of Southern Africa reveals dispersal events from the Northern Hemisphere. Systematics and Biodiversity, 18, 633–645. https://doi.org/10.1080/14772000.2020.1787550
153Marino, I.A.M., Riginella, E., Gristina, M., Rasotto, M.B., Zane, L. & Mazzoldi, C., 2015. Multiple paternity and hybridization in two smooth-hound sharks. Scientific Reports, 5, 12919, https://doi.org/10.1038/srep12919
154Martínez-Pérez, C., Carrillo-Briceño, J.D., Esparza, C., Ferrón, H.G., Manzanares, E., Hammann, C. & Botella, H., 2017. A Serravallian (Middle Miocene) shark fauna from Southeastern Spain and its palaeoenvironmental significance. Historical Biology, 30, 422–232. https://doi.org/10.1080/08912963.2017.1326111
155Marx, F.G., Bosselers, M.E.J. & Louwye, S., 2016. A new species of Metopocetus (Cetacea, Mysticeti, Cetotheriidae) from the Late Miocene of the Netherlands. PeerJ, 4, e1572. https://doi.org/10.7717/peerj.1572
156Menzel, H., 1979. Die Fischfauna aus dem Mittelmiozän von Twistringen. Abhandlungen des Naturwissenschaftlichen Vereins zu Bremen, 39, 83–127.
157Mollen, F.H., 2010. A partial rostrum of the porbeagle shark Lamna nasus (Lamniformes, Lamnidae) from the Miocene of the North Sea Basin and the taxonomic importance of rostral morphology in extinct sharks. Geologica Belgica, 13, 61–76.
158Mollen, F.H., 2019. Making Louis Agassiz’s wish come true: combining forces and a new protocol for collecting comparative skeletal material of sharks, skates and rays, as a comment and an addition to ‘The need of providing tooth morphology in descriptions of extant elasmobranch species’ by Guinot et al. (2018). Zootaxa, 4571, 295–300. https://doi.org/10.11646/zootaxa.4571.2.13
159Moreau, F. & S. Mathis, 2000. Les élasmobranches du Thanétien (Paléogène) du Nord de la France, des carrières de Templeuve et de Leforest. Cossmanniana, 7, 1–18.
160Moths, H., 1994. Der Glimmerton-Aufschluß Groß Pampau (Langenfeldium, Obermiozän), seine Entwicklung und Fossilführung. Der Geschiebesammler, 27, 143–183.
161Moths, H., 1998. Die Hai- und Rochenfauna aus dem Miozän (Langenfeldium) von Groß Pampau. Der Geschiebesammler, 31, 51–113.
162Müller, A., 1999. Ichthyofaunen aus dem atlantischen Tertiär der USA. Leipziger Geowissenschaften, 9/10, 1–360.
163Munsterman, D.K., 2007. De resultaten van het dinoflagellaatcystenonderzoek naar de ouderdom van de pulsboring Hoogdonk (B52C1978; Liessel, Peelhorst, Noord Brabant): traject 9.2-44.5 m. Intern TNO rapport 2007-U-R0860/A.
164Munsterman, D.K., ten Veen, J.H, Menkovic, A., Deckers, J., Witmans, N., Verhaegen, J., Kerstholt-Boegehold S.J., van de Ven, T & Busschers, F.S., 2019. An updated and revised stratigraphic framework for the Miocene and earliest Pliocene strata of the Roer Valley Graben and adjacent blocks. Netherlands Journal of Geosciences, 98, e8. https://doi.org/10.1017/njg.2019.10
165Noubhani, A. & Cappetta, H., 1992. Evolution de la taille et de la morphologie des dents dans deux lignées de sélaciens: application biostratigraphique. Tertiary Research, 14/1, 1–18
166Peters, N., 2009. Brabant tussen walvissen en mastodonten: fossielen uit Liessel. Nationaal Beiaard- en Natuurmuseum Asten & Oertijdmuseum De Groene Poort Boxtel, 110 p.
167Reinecke, T. & Engelhard, P., 1997. The selachian fauna from Geschiebe of the Lower Selandian basal conglomerate (Thanetian, Late Paleocene) in the Danish subbasin (Sealand, Scania, Western Baltic Sea). Erratica, 2, 3–45.
168Reinecke, T. & Wienrich, G., 2009. Elasmobranchii. In Wienrich G., Die Fauna des marinen Miozäns von Kevelaer (Niederrhein), Band 5. Backhuys Publishers, Leiden, Margraf Publishers, Weikersheim, 972–993.
169Reinecke, T., Stapf, H. & Raisch, M., 2001. Die Selachier und Chimären des Unteren Meeressandes und Schleichsandes im Mainzer Becken (Alzey- und Stadecken Formation, Rupelium, Unteres Oligozän). Palaeontos, 1, 1–73.
170Reinecke, T., Moths, H., Grant, A., & Breitkreutz, H., 2005. Die Elasmobranchier des Norddeutschen Chattiums insbesondere des Sternberger Gesteins (Eochattium, Oberes Oligozän). Palaeontos, 8, 1–135.
171Reinecke, T, Von der Hocht, F. & Gürs, K., 2008. Die Elasmobranchier des Vierlandiums, unteres Miozän, im Nordwestdeutschen Becken aus Bohrungen und glaziofluviatilen Geröllen (“Hollsteiner Gestein”) der Vierlande-Feinsande (Holstein) und der Kakert-Schichten (Niederrhein). Palaeontos, 14, 1–54.
172Reinecke, T., Louwye, S., Havekost, U. & Moths, H., 2011. The elasmobranch fauna of the Late Burdigalian, Miocene, at Werder-Uesen, Lower Saxony, Germany and its relationships with Early Miocene Faunas in the North Atlantic, central Paratethys and Mediterranean. Palaeontos, 20, 170 p.
173Reinecke, T., Balsberger, M., Beaury, B. & Pollerspöck, J., 2014. The elasmobranch fauna of the Thalberg Beds, early Egerian (Chattian, Oligocene), in the Subalpine Molasse Basin near Siegsdorf, Bavaria, Germany. Palaeontos, 26, 127 p.
174Risso, A., 1826. Histoire naturelle des principales productions de l’Europe méridionale et particulièrement de celles des environs de Nice et des Alpes maritimes. Vol. 3. F.-G. Levrault, Paris, 480 p.
175Roveri, M., Flecker, R., Krijgsman, W., Lofi, J., Lugli, S., Manzi, V., Sierro, F.J., Bertini, A., Camerlenghi, A., De Lange, G., Govers, R., Hilgen, F.J., Hübscher, C., Meijer, P.T. & Soica, M., 2014. The Messinian Salinity Crisis: Past and future of a great challenge for marine sciences. Marine Geology, 352, 25–58. https://doi.org/10.1016/j.margeo.2014.02.002
176Schwarzhans, W., 2010. The Otoliths from the Miocene of the North Sea Basin. Backhuys Publishers, Leiden, Margraf Publishers, Weikersheim, 352 p.
177Smith, R., Smith, T. & Steurbaut, E., 1999. Les élasmobranches de la transition Paléocène-Éocène de Dormaal (Belgique) : implications biostratigraphiques et paléobiogeographiques. Bulletin de la Société géologique de France, 170/3, 327–334.
178Spiegler, D., 2002. Correlation of marine Miocene Bolboforma zonation and Uvigerina zonation in northern Germany. In Gürs, K. (ed.), Northern European Cenozoic Stratigraphy, Proceedings of the 8th Biannual Joint Meeting of the RCNNS/RCNPS. Landesamt für Natur und Umwelt des landes Schleswig-Holstein, Flintbek, 133–141.
179Spiegler, D. & Gürs, K., 1996. Der miozäne Glimmerton von Groß Pampau, Schleswig-Holstein (Mollusken, Foraminiferen und Bolboformen). Meyniana, 48, 135–164.
180Steurbaut, E. & Herman, J., 1978. Biostratigraphie et poisons fossiles de la Formation de l’Argile de Boom (Oligocène moyen du Bassin Belge). Geobios, 11/3, 297–325. https://doi.org/10.1016/S0016-6995(78)80033-3
181Steurbaut, E. & Nolf, D., 2021. The Mont-des-Récollets section (N France): a key site for the Ypresian-Lutetian transition at mid-latitudes – reassessment of the boundary criterion for the base-Lutetian GSSP. Geodiversitas, 43/11, 311–363. https://doi.org/10.5252/geodiversitas2021v43a11
182Théobald, N., 1934. Contribution à la paléontologie du basin oligocène du Haut-Rhin et du Territoire de Belfort. Les poisons Oligocènes. Bulletin du Service de la Carte géologique d’Alsace et de Lorraine, 2, 117–162.
183Triest, L. & Sierens, T., 2014. Seagrass radiation after Messinian Salinity Crisis reflected by strong genetic structuring and out-of-Africa Scenario (Ruppiaceae). PLoS ONE, 9, e104264. https://doi.org/10.1371/journal.pone.0104264
184Tsigenopoulos, C.S., Durand, J.D., Ünlü, E. & Berrebi, P., 2003. Rapid radiation of the Mediterranean Luciobarbus species (Cyprinidae) after the Messinian salinity crisis of the Mediterranean Sea, inferred from mitochondrial phylogenetic analysis. Biological Journal of the Linnean Society, 80, 207–222. https://doi.org/10.1046/j.1095-8312.2003.00237.x
185Underwood, C.J., Ward, D.J., King, C., Antar, S.M., Zalmout, I.S. & Gingerich, P.D., 2011. Shark and ray faunas in the Middle and Late Eocene of the Fayum Area, Egypt. Proceedings of the Geologist’s Association, 122, 47–66. https://doi.org/10.1016/j.pgeola.2010.09.004
186Vandenberghe, N., Wouters, L., Schiltz, M., Beerten, K., Berwouts, I., Vos, K., Houthuys, R., Deckers, J., Louwye, S., Laga, P., Verhaegen, J., Adriaens, R. & Dusar, M., 2020. The Kasterlee Formation and its relation with the Diest and Mol Formations in the Belgian Campine. Geologica Belgica, 23, 265–287. https://doi.org/10.20341/gb.2020.014
187Van den Bosch, M., 1978. On shark teeth and scales from the Netherlands and the biostratigraphy of the Tertiary of the eastern part of the country. Mededelingen van de Werkgroep voor Tertiaire en Kwartaire Geologie, 15, 129–136.
188Van den Bosch, M., 1980. Elasmobranch associations in Tertiary and Quaternary deposits of the Netherlands (Vertebrata, Pisces), 2. Paleogene of the eastern and northern part of the Netherlands, Neogene in the eastern part of the Netherlands. Mededelingen van de Werkgroep voor Tertiaire en Kwartaire Geologie, 17, 65–70.
189Van den Bosch, M., Cadée, M.C. & Janssen, A.W., 1975. Lithostratigraphical and biostratigraphical subdivision of Tertiary deposits (Oligocene-Pliocene) in the Winterswijk-Almelo region (eastern part of the Netherlands). Scripta Geologica, 29, 1–167.
190Van Vliet-Lanoë, B., Vandenberghe, N., Laurent, M., Laignel, B., Lauriat-Rage, A., Louwye, S., Mansy, J.L., Mecier, D., Hallégouët, B., Laga, P., Laquement, F., Meilliez, F., Michel, Y., Moguedet, G. & Vidier, J.P., 2002. Palaeogeographic evolution of northwestern Europe during the Upper Cenozoic. Geodiversitas, 24, 511–541.
191Vialle, N., Adnet, S. & Cappetta, H., 2011. A new shark and ray fauna from the Middle Miocene of Mazan, Vaucluse (southern France) and its importance in interpreting the paleoenvironment of marine deposits in the southern Rhodanian Basin. Swiss Journal of Palaeontology, 130, 241–258. https://doi.org/10.1007/s13358-011-0025-4
192Vinken, R. (ed.), 1988. The Northwest European Tertiary Basin. Geologisches Jahrbuch, Reihe A, 100, 508 p
193Wesselingh, F.P., Busschers, F.S. & Goolaerts, S., 2020. Observations on the Pliocene sediments exposed at Antwerp International Airport (northern Belgium) constrain the stratigraphic position of the Broechem Fauna. Geologica Belgica, 23, 315–321. https://doi.org/10.20341/gb.2020.026
194Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K., 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292, 686–693. https://doi.org/10.1126/science.1059412
195Manuscript received 15.12.2021, accepted in revised form 03.03.2022, available online 30.04.2022.
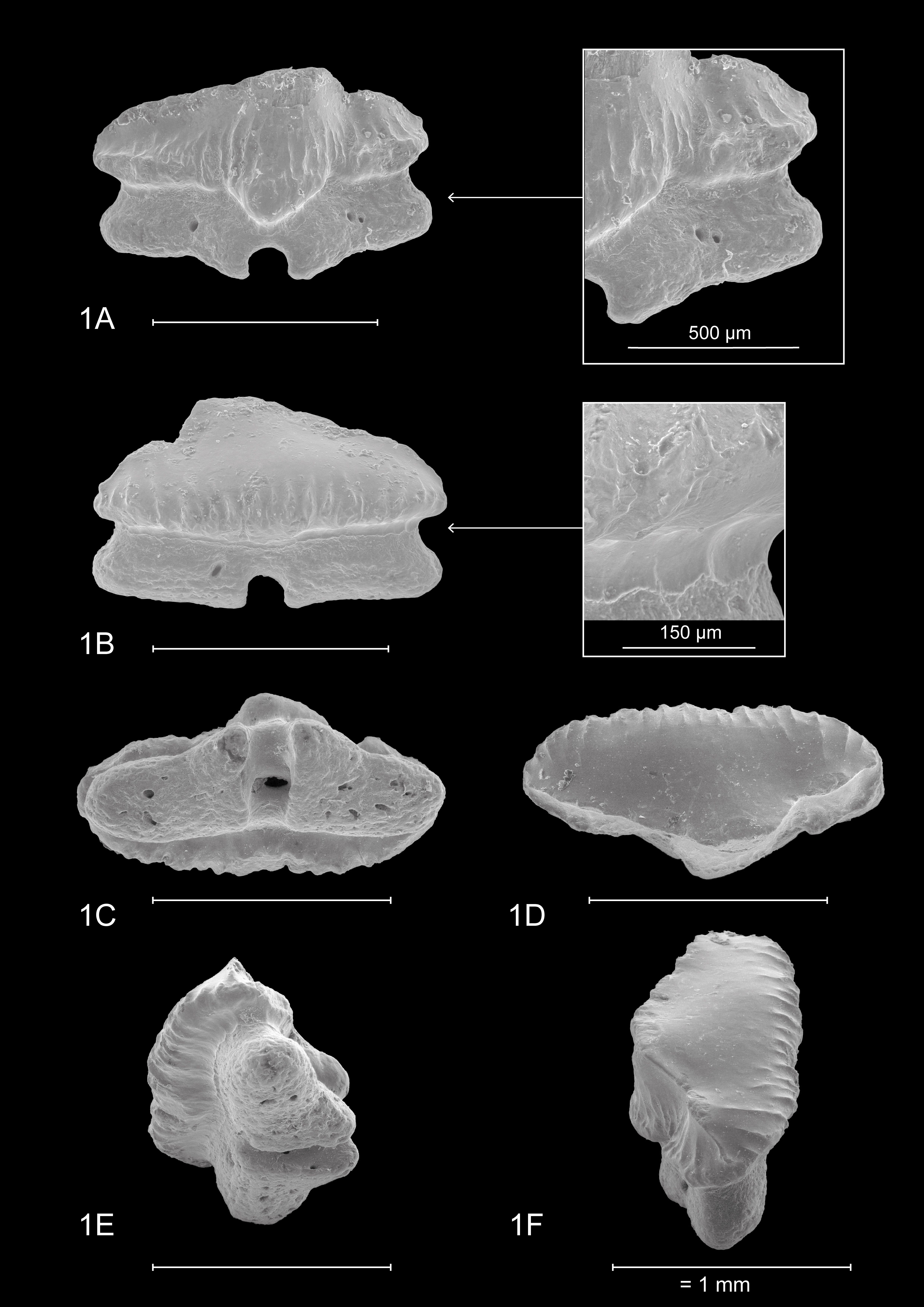
Plate 1
Mustelus aff. punctulatus Risso, 1826; IRSNB P 10267. Base Deurne Member (Diest Formation), AIA outcrop, Borsbeek (Belgium). Miocene, middle Tortonian. Left upper jaw: anterolateral tooth, scale bar 1 mm
1A – Lingual view & detail of the raised distal heel with tubercular vertical ridges
1B – Labial view & detail remnants Type 2 ornamentation (reticulate pitting)
1C – Basal view
1D – Occlusal view
1E – Mesio-distal basal view, with a detailed view of the labial visor
1F – Distallo-mesial occlusal view, showing the distal heel and the occlusal crown face

Plate 2
Mustelus aff. punctulatus Risso, 1826; IRSNB P 10268. Base Deurne Member (Diest Formation), AIA outcrop, Borsbeek (Belgium). Miocene, middle Tortonian. Left upper jaw: anterolateral tooth, scale bar 1 mm
2A – Lingual view & detail of the raised distal heel with tubercular vertical ridges
2B – Labial view
2C – Basal view
2D – Occlusal view
Mustelus aff. punctulatus Risso, 1826. Haak borehole, Nettetal (Germany). Miocene, upper Serravallian. Collection and picture F. Von Der Hocht. Median tooth (lower jaw?), scale bar 1 mm
3A – Apical view
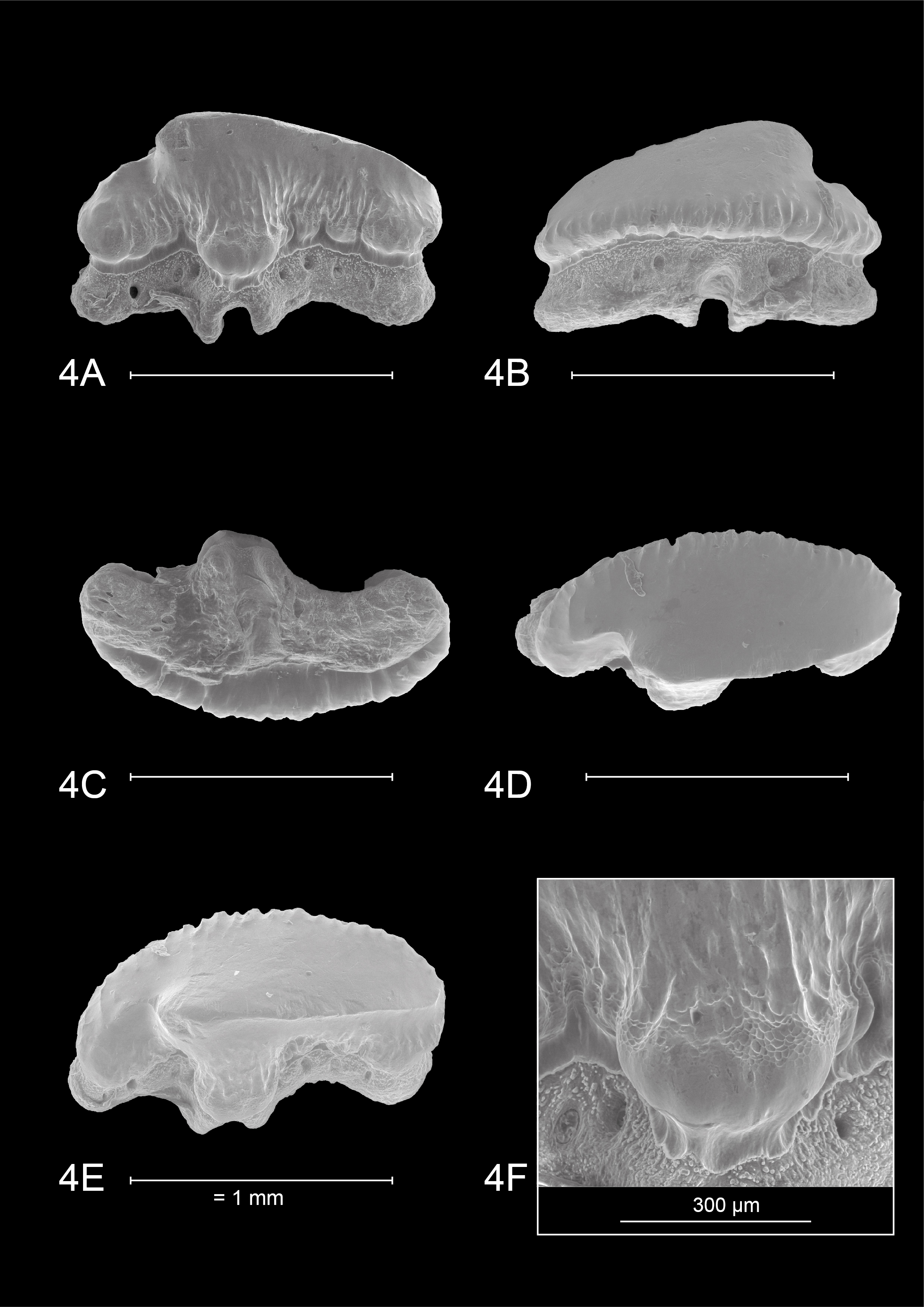
Plate 3
Mustelus punctulatus Risso, 1826; KBIN 27206. Adult male (TL 874 mm). Mediterranean Sea: off the coast of Algeria. Right upper jaw: anterolateral tooth, scale bar 1 mm
4A – Lingual view
4B – Labial view
4C – Basal view
4D – Occlusal view
4E – Apical view
4F – Detail uvula with Type 1 and 2 ornamentation
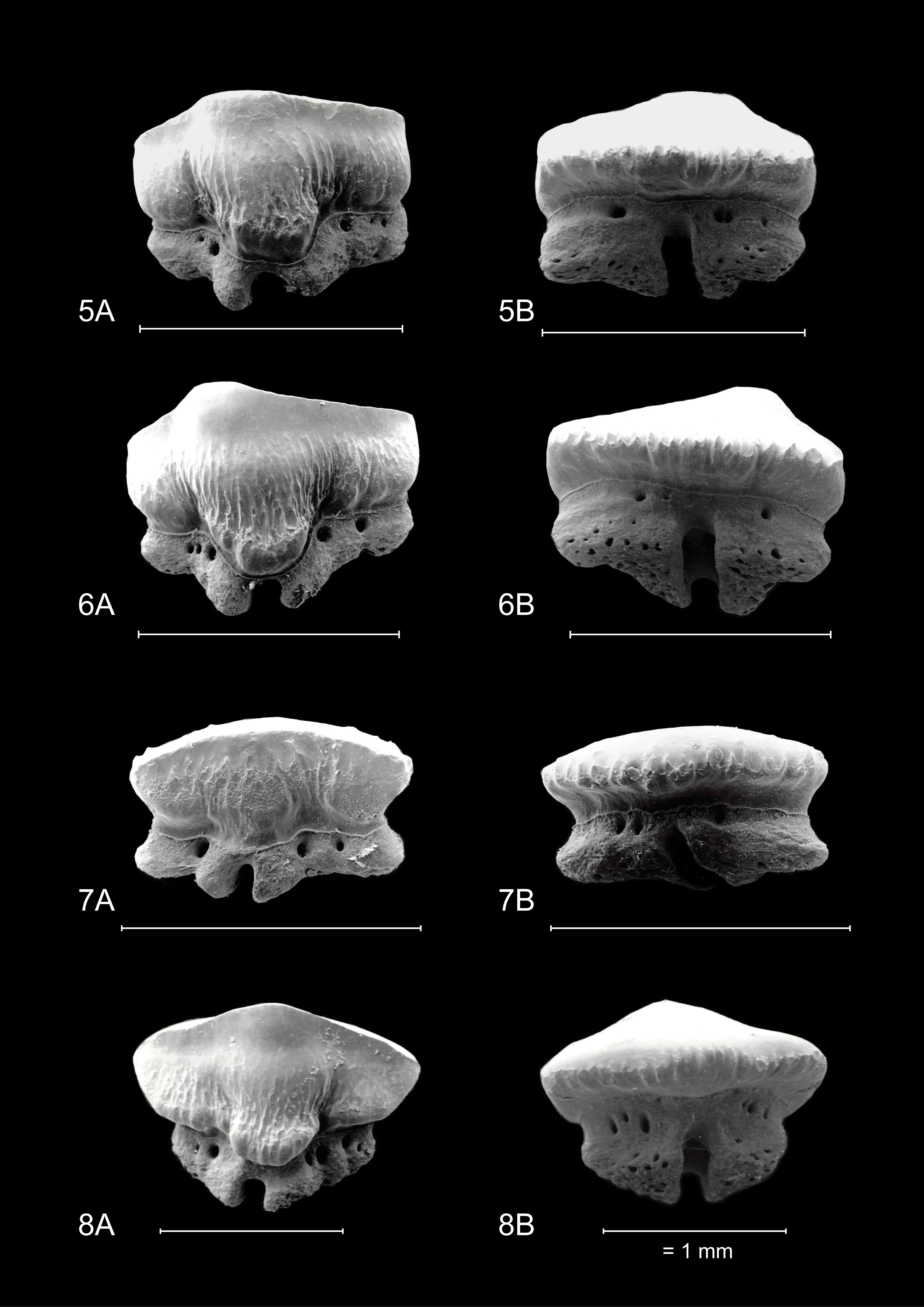
Plate 4
Mustelus mustelus (Linnaeus, 1758), refigured after Herman et al. (1988). Teeth of adult female (TL 890 mm). Atlantic Ocean, Senegal, scale bar 1 mm
5A – Right upper jaw: lateral tooth, lingual view
5B – Right upper jaw: lateral tooth, labial view
6A – Right upper jaw: anterior tooth, lingual view
6B – Right upper jaw: anterior tooth, labial view
7A – Left lower jaw: lateral tooth, lingual view
7B – Left lower jaw: lateral tooth, labial view
8A – Lower jaw: median tooth, lingual view
8B – Lower jaw: median tooth, labial view
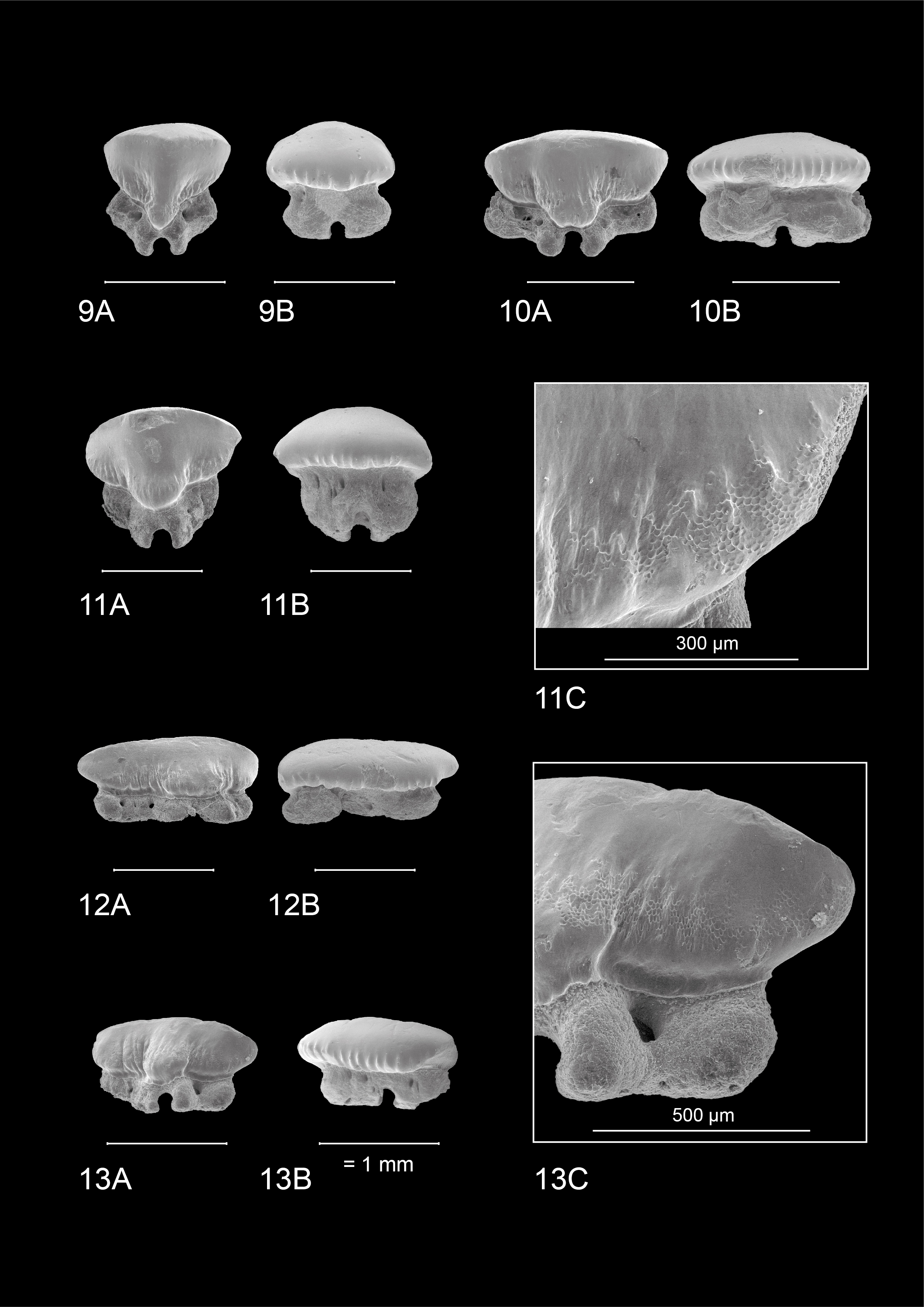
Plate 5
Mustelus asterias Cloquet, 1819; KBIN 26724. Teeth of adult male (TL 900 mm). Ostend harbour (Belgium), scale bar 1 mm
9A – Upper jaw: median tooth, lingual view - KBIN 26724a
9B – Upper jaw: median tooth, labial view - KBIN 26724a
10A – Right upper jaw: lateral tooth, lingual view - KBIN 26724b
10B – Right upper jaw: lateral tooth, labial view - KBIN 26724b
11A – Lower jaw: median tooth, lingual view - KBIN 26724c
11B – Lower jaw: median tooth, labial view - KBIN 26724c
11C – Detail of the right distal side of Fig. 11A: mostly Type 2 ornamentation
12A – Right lower jaw: anterolateral tooth, lingual view - KBIN 26724d
12B – Right lower jaw: anterolateral tooth, labial view - KBIN 26724d
13A – Right lower jaw: lateral tooth, lingual view - KBIN 26724e
13B – Right lower jaw: lateral tooth, labial view - KBIN 26724e
13C – Detail of the distal side of Pl.5.13A: Faint Type 1 and omnipresent Type 2 ornamentation, absence of distal heel
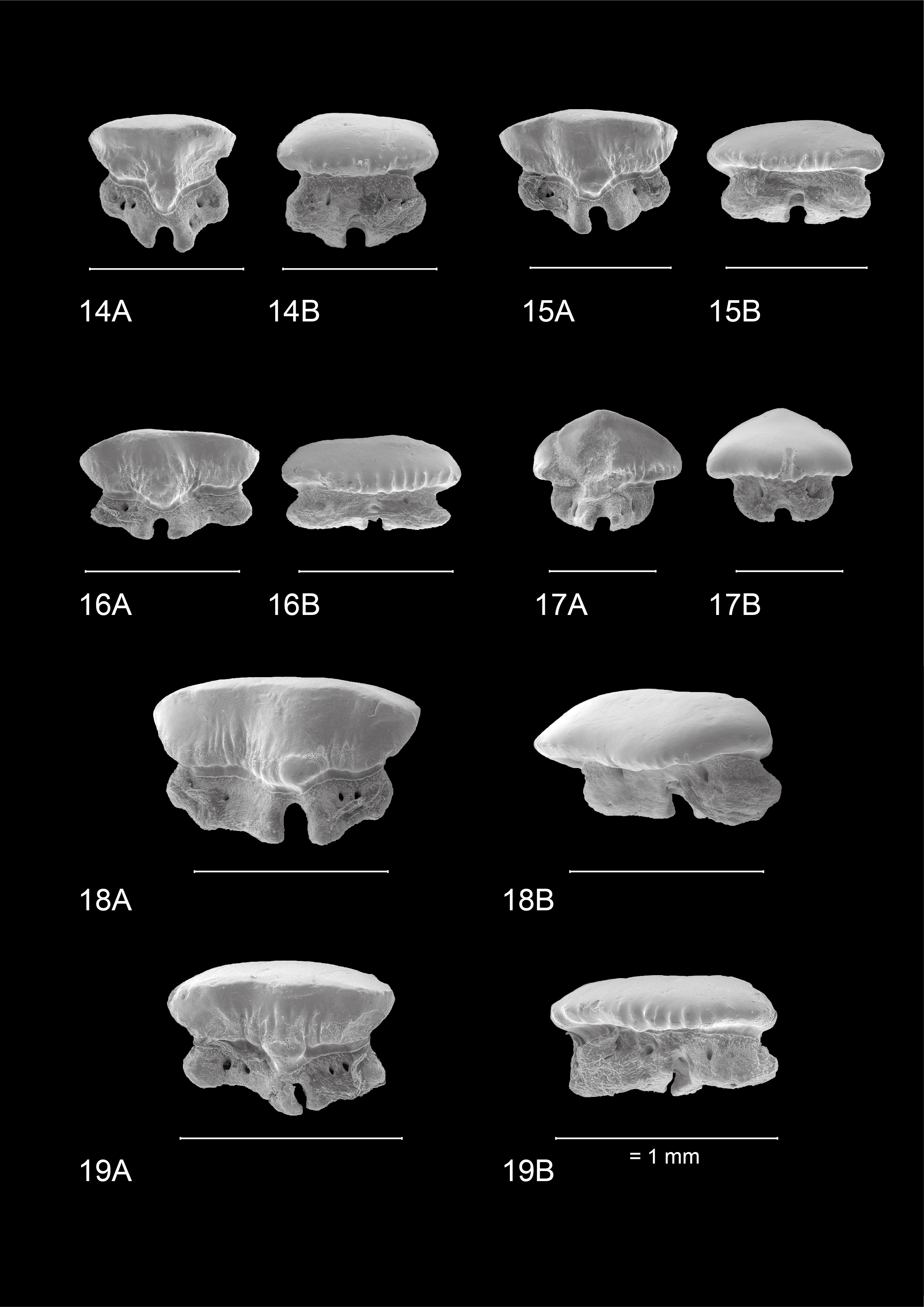
Plate 6
Mustelus asterias Cloquet, 1819; KBIN 26722. Teeth of juvenile female (TL 500 mm). Ostend harbour (Belgium), scale bar 1 mm
14A – Upper jaw: median tooth, lingual view - KBIN 26722a
14B – Upper jaw: median tooth, labial view - KBIN 26722a
15A – Right upper jaw: anterior tooth, lingual view - KBIN 26722b
15B – Right upper jaw: anterior tooth, labial view - KBIN 26722b
16A – Right upper jaw: lateral tooth, lingual view - KBIN 26722c
16B – Right upper jaw: lateral tooth, labial view - KBIN 26722c
17A – Lower jaw: median tooth, lingual view - KBIN 26722d
17B – Lower jaw: median tooth, labial view - KBIN 26722d
18A – Right lower jaw: anterior tooth, lingual view - KBIN 26722e
18B – Right lower jaw: anterior tooth, labial view - KBIN 26722e
19A – Right lower jaw: lateral tooth, lingual view - KBIN 26722f
19B – Right lower jaw: lateral tooth, labial view - KBIN 26722f
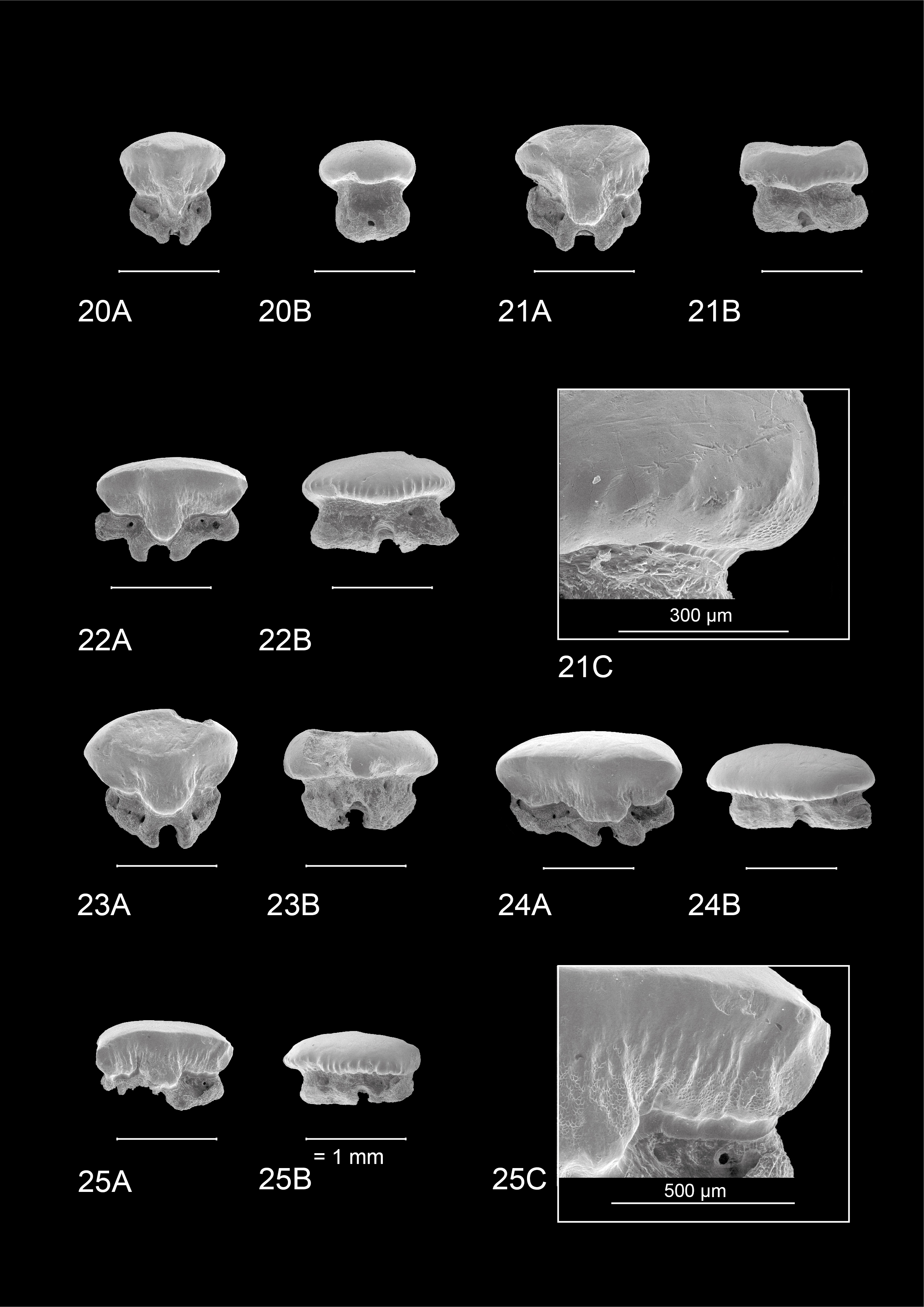
Plate 7
Mustelus asterias Cloquet, 1819; KBIN 26723. Teeth of adult female (TL 880 mm). Terneuzen harbour (the Netherlands), scale bar 1 mm
20A – Upper jaw: median tooth, lingual view - KBIN 26723a
20B – Upper jaw: median tooth, labial view - KBIN 26723a
21A – Right upper jaw: anterior tooth, lingual view - KBIN 26723b
21B – Right upper jaw: anterior tooth, labial view - KBIN 26723b
21C – Detail of distal side of Pl.7.21B, faint Type 1 and Type 2 ornamentation
22A – Right upper jaw: lateral tooth, lingual view - KBIN 26723c
22B – Right upper jaw: lateral tooth, labial view - KBIN 26723c
23A – Lower jaw: median tooth, lingual view - KBIN 26723d
23B – Lower jaw: median tooth, labial view - KBIN 26723d
24A – Right lower jaw: anterolateral tooth, lingual view - KBIN 26723e
24B – Right lower jaw: anterolateral tooth, labial view - KBIN 26723e
25A – Right lower jaw: lateral tooth, lingual view - KBIN 26723f
25B – Right lower jaw: lateral tooth, labial view - KBIN 26723f
25C – Detail of distal side of Pl.7.25A: Type 1 and Type 2 ornamentation






