- Accueil
- Volume 27 (2024)
- number 1-2
- Postcephalic segmentation and spines of the Siluro-Devonian odontopleurine trilobites Leonaspis Richter & Richter and Kettneraspis Prantl & Přibyl, with description of Bruthansovaspis gen. nov. from the Wenlock to Ludlow of the Prague Basin
Visualisation(s): 3194 (21 ULiège)
Téléchargement(s): 1614 (18 ULiège)
Postcephalic segmentation and spines of the Siluro-Devonian odontopleurine trilobites Leonaspis Richter & Richter and Kettneraspis Prantl & Přibyl, with description of Bruthansovaspis gen. nov. from the Wenlock to Ludlow of the Prague Basin

Abstract
Species of the odontopleurine trilobites Leonaspis Richter & Richter, 1917 and Kettneraspis Prantl & Přibyl, 1949 from the Silurian and the Devonian have been studied. Dorsal tubercle and spine patterns are highly variable and deemed of restricted use. The pygidial segmentation of Leonaspis is consistent among species in the development of a rudimentary inner pleural ridge or wide space between the pleural ridge and the axis. In Kettneraspis, an inner pleural ridge is never developed but the primitive condition is to have a space here. The formation of this ridge and an auxiliary interior border spine pair was arrested prematurely during ontogeny in species assigned to Kettneraspis at present. The type species of the monotypic Rupbachella Alberti, 2021 is regarded as a juvenile Leonaspis. Bruthansovaspis gen. nov. (type species: Acidaspis roemeri Barrande, 1852) is proposed for a Silurian clade of former Kettneraspis species from peri-Gondwana, that are principally distinct in lacking the combination of stunted anterior and macropleural posterior, thoracic segments synapomorphic of Kettneraspis and Leonaspis. The types of Bruthansovaspis roemeri, Bruthansovaspis dormitzeri (Hawle & Corda, 1847), Bruthansovaspis dumortieri (Hawle & Corda, 1847), Kettneraspis propinqua (Barrande, 1852), Kettneraspis lindackeri (Hawle & Corda, 1847) and Bruthansovaspis zenkeri (Hawle & Corda, 1847) (= B. dormitzeri) from the Silurian of the Barrandian, all of which had previously been lumped as dormitzeri, are figured and their classifications revisited. Exceptionally well-preserved odontopleurine material from the Devonian in the Tafilalt (southern Morocco) is described as Kettneraspis freitagi sp. nov. Leonaspis? strix Lütke, 1965 from the Devonian of the Harz Mountains is in need of revision; a previously tentatively assigned pygidium does not belong in that genus.
Table des matières
1. Introduction
1The phylogeny of Siluro-Devonian odontopleurines traditionally associated with Leonaspis and Kettneraspis has been much debated. In a pivotal study aiming to subdivide the polyphyletic Leonaspis, Ramsköld & Chatterton (1991) proposed synapomorphies and revised diagnoses for both genera. The majority of species were reassigned to Kettneraspis and several others to the acidaspidine Exallaspis Ramsköld & Chatterton, 1991, but a necessary investigation of the ingroup structure of Kettneraspis was beyond the scope of that paper. The most widely accepted feature to discriminate Kettneraspis and Leonaspis since, has been the number of pygidial interior border spines (= “medial secondary spines” of Ramsköld, 1991). Van Viersen & Heising (2015) demonstrated that in some Kettneraspis and Leonaspis species, the number of interior border spines as a key distinguishing feature, conflicts with putative other synapomorphies. Holloway (2021) reviewed diagnostic characters and concluded that these genera may reliably be distinguished only by the number of interior border spines. The use of the interior border spine numbers as a single discriminating character, however, is not without controversy, as will be argued herein.
2This work is an increment towards natural Kettneraspis and Leonaspis, aiming to 1) appraise the nature and taxonomic relevance of postcephalic dorsal and border spine patterns, 2) identify and remove some of the paraphyly, and 3) document a new Kettneraspis species from the Devonian of Morocco.
2. Postcephalic segmentation and spines of Leonaspis and Kettneraspis
3The major border spines encompass a principal landmark on the pygidia of Leonaspis and Kettneraspis; they are comparatively long, posteriorly directed projections linked to the first axial ring by the pleural ridges (Fig. 1). Anterior and posterior to the major border spines are the lateral and interior border spines, respectively. In species with four interior border spines, the abaxial spine pair may be connected to rudimentary “inner” pleural ridges (Fig. 1A). If developed, these ridges are proximally separated from the axis by axial furrows that are usually firmly impressed.
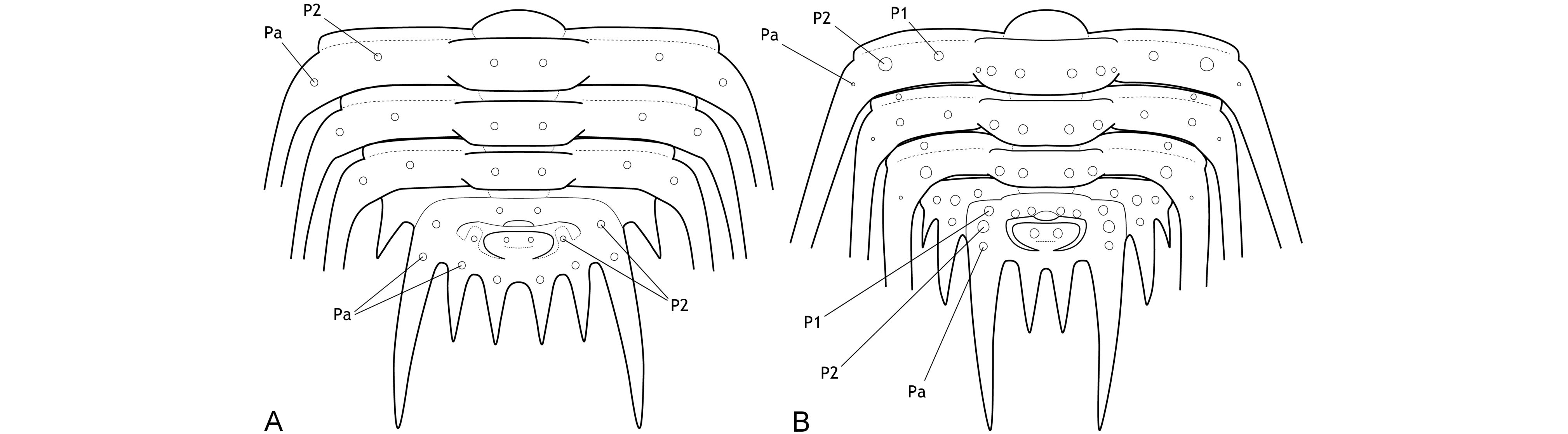
Figure 1. Theoretic pygidium and posterior thoracic segments of Leonaspis (A) and Kettneraspis (B).
4Transitory pygidia are known from several Leonaspis and Kettneraspis species (e.g., Chatterton, 1971; Ramsköld & Chatterton, 1991; Chatterton & Speyer, 1997; Chatterton et al., 2006; Basse & Müller, 2016; Alberti, 2021, 2023; Basse, 2022). These all share well defined pleural ridges carrying a single dorsal spine, and long, weakly to strongly dorsally projected, major border spines. As segments were released into the thorax during ontogeny, the major border spines assumed a subhorizontal direction and the dorsal spines were variably retained. The latter were commonly also reduced to tubercles during late ontogeny, but not always (e.g., Whittington & Campbell, 1967, pl. 17, figs 1, 10; Haas, 1969, pl. 84, fig. 6; Chatterton, 1971, pl. 16, fig. 8; van Viersen & Heising, 2015, pl. 1, figs A–F; Basse & Müller, 2016, pl. 24, figs 224, 232, 233). Thus, the thoracic dorsal tubercles and spines of Kettneraspis and Leonaspis species offer a crude log of their ontogenetic development. The resulting patterns reveal general trends associated with each genus, but they are highly variable, probably even intraspecifically, and convergence has led to exceptions to every potential rule. Yet they may sometimes offer valuable clues that help to reveal the true interior border spines, as will be argued below.
5Intraspecific variation of interior border spine numbers is rare but not exceptional in Silurian odontopleurines, nor is it exclusive to the subfamily (e.g., Whittington, 1956). Irregular spine tallies have been reported for a number of odontopleurine species: e.g., the Silurian Edgecombeaspis longstaffei (Chatterton & Perry, 1983) (Chatterton & Perry, 1983, pl. 14, figs 21, 22, 24, 29; Fig. 2R), Odontopleura brevigena Chatterton & Perry, 1983 (Chatterton & Perry, 1983, pl. 1, figs 27, 29, 31–34), Sinespinaspis nehedensis (Chatterton & Perry, 1983) (Chatterton & Perry, 1983, pl. 3, figs 5–7, 10, 11, 13, 14, 18, 21, 22, 25–27; Fig. 2S), Sinespinaspis greenwoodi (Chatterton & Perry, 1983) (Chatterton & Perry, 1983, pl. 3, figs 34–43) and K. jaanussoni (Chatterton & Perry, 1983) (Ramsköld & Chatterton, 1991, p. 337, fig. 3a, d, e; Fig. 2H). Exceeding variation, however, occurs in the type species of Leonaspis, L. leonhardi (Barrande, 1846) from the Ludlow, which may have two to five interior border spines (e.g., Vokáč et al., 2021; Fig. 2A). The holotype pygidium of Odontopleura zenonis Hawle & Corda, 1847 (Prantl & Přibyl, 1949, pl. 1, fig. 24; Šnajdr, 1984b, pl. 16, fig. 3; Fig. 2A5) has three interior spines and has been tentatively referred to by Prantl & Přibyl (1949) as a L. leonhardi with the adaxial interior spine pair fused (or failed to separate fully). In Devonian species such variations are exceedingly rare, hence epitomising stable concepts of two- and four-spined forms referrable to their appropriate genus.
6In the following reviews the two-spined forms shall be referred to as Kettneraspis and the four-spined forms as Leonaspis. Some species previously assigned to either genus are excluded. The numbering of dorsal tubercles or spines is intended to facilitate discussions of their distribution in the studied genera and should not be applied categorically within the family.
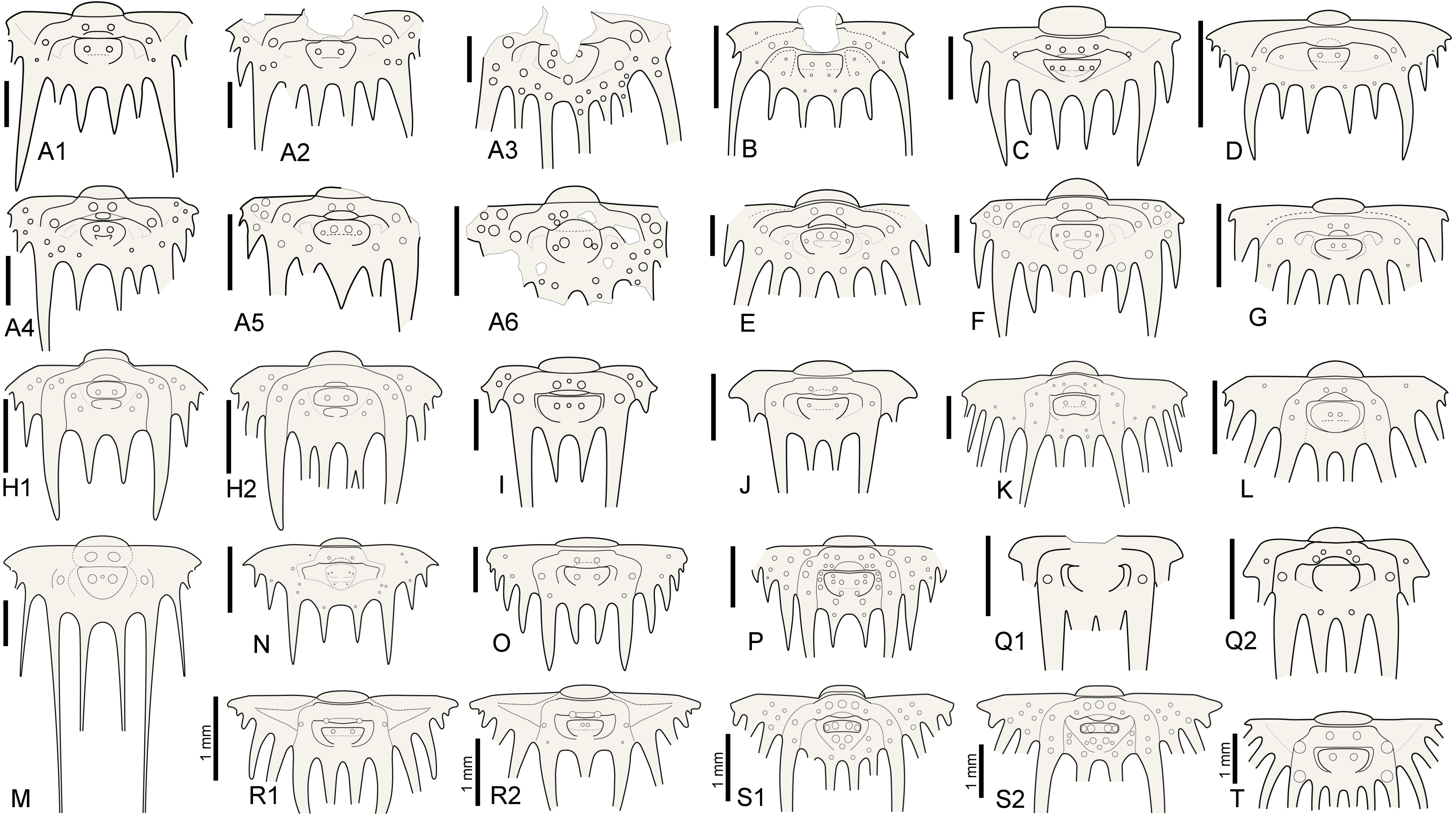
Figure 2. Reconstructions of selected odontopleurine pygidia. A. Normal and teratological pygidia of Leonaspis leonhardi (Barrande, 1846); A1–Vokáč et al. (2021, pl. 6, fig. 4), A2–ibid., fig. 5, A3–ibid., fig. 8, A4–ibid., fig. 7, A5–Šnajdr, 1984b, pl. 16, fig. 3 [=holotype of Odontopleura zenonis Hawle & Corda, 1847], A6–Vokáč et al. (2021, pl. 6, fig. 6). B. Leonaspis? expilata Lütke, 1965. C. Leonaspis kleini Basse in Basse & Müller, 2004. D. Leonaspis nuratensis Ivanova et al., 2009. E. Leonaspis maura Alberti, 1969. F. Leonaspis hastata Alberti, 1967. G. Leonaspis haddanei Chatterton et al., 2006. H. Normal and teratological pygidium of Kettneraspis jaanussoni (Chatterton & Perry, 1983); H1–Chatterton & Perry (1983, pl. 6, fig. 19), H2–Ramsköld & Chatterton (1991, p. 337, fig. 3e). I. Kettneraspis angelini (Prantl & Přibyl, 1949). J. Kettneraspis wrightae Adrain & Ramsköld, 1997. K. Kettneraspis pigra (Barrande, 1872). L. Kettneraspis knoppi Basse & Müller, 2017. M. Bruthansovaspis dormitzeri (Hawle & Corda, 1847). N. Kettneraspis geinitziana (Hawle & Corda, 1847). O. Kettneraspis prescheri van Viersen & Heising, 2015. P. Kettneraspis eftychia van Viersen et al., 2019. Q. Variable pygidia of Kettneraspis lenzi (Chatterton & Perry, 1983); Q1–Adrain & Ramsköld (1997, fig. 6.25), Q2–Chatterton & Perry (1983, pl. 8, fig. 22). R. Normal and teratological pygidium of Edgecombeaspis longstaffei (Chatterton & Perry, 1983); R1–Chatterton & Perry (1983, pl. 14, fig. 21), R2–Chatterton & Perry (1983, pl. 14, fig. 22). S. Normal and teratological pygidium of Sinespinaspis nehedensis (Chatterton & Perry, 1983); S1–Chatterton & Perry (1983, pl. 3, fig. 10), S2–Chatterton & Perry (1983, pl. 3, fig. 6). T. Leonaspis? strix Lütke, 1965? Scale bars are 2 mm except where indicated otherwise in the figure.
2.1. Patterns in Kettneraspis
7The primitive condition of Kettneraspis is to carry three metamerically repeated tubercle pairs dorsally on the thoracic posterior pleural bands: a small auxiliary tubercle (Pa) near the fulcrum and two larger tubercles (an abaxial P2 and adaxial P1) evenly spaced between Pa and the axial furrow (Fig. 1B). This basic pattern is revealed by the oldest known Kettneraspis species, K. jaanussoni, from the lower Llandovery. It is furthermore shared with various Ordovician and Silurian odontopleurines (e.g., Acanthalomina Prantl & Přibyl, 1949, Diacanthaspis Whittington, 1941, Edgecombeaspis Adrain & Ramsköld, 1997), hence likely to be symplesiomorphic. Other Kettneraspis species may show various combinations of Pa, P1 and P2 tubercles on some or all segments, except that P2 is invariably present. In derived species, assessing the nature of the tubercles is not always straightforward without the availability of articulated material. In K. seiberti Basse in Basse & Müller, 2004 (Basse & Müller, 2016, pl. 24, figs 222–226, 230–233) the positionings of the P2 spines are gradational, migrating progressively adaxially on thoracic segments three to one where P1 is lacking. In exceptional cases, a tubercle or spine is developed between the P1 and P2 spines on some segments of specimens of this same species (Basse & Müller, 2016, pl. 23, figs 218, 219, 221), but also on the ninth segment of the holotype of K. freitagi sp. nov. (Fig. 3E, F), similarly to abnormal spines reported on the thorax of Sinespinaspis markhami (Edgecombe & Sherwin, 2001) by Bicknell & Smith (2022). Some species have P1 tubercles positioned close to the axial furrow (e.g., K. elliptica (Burmeister, 1843) (Ramsköld & Chatterton, 1991, p. 360, fig. 9)). In such species, P1 may be absent on the anterior or posterior few segments (e.g., K. bayarti van Viersen, 2007, K. propinqua (Barrande, 1852) (Fig. 6A), K. seiberti), on the middle segments (e.g., K. freitagi (Fig. 3E, F)) or, perhaps abnormally, only one tubercle in the same pair is developed (e.g., K. williamsi (Whittington, 1956), K. tuberculata (Hall, 1859) (Whiteley et al., 2002, pl. 34; specimen misnamed K. callicera due to a print error [G. Kloc, pers. comm. in 2005])).
8The pygidial tubercle pattern is often different from the thorax. Many species only have P2 developed. Pa is present in some species (e.g., K. prescheri van Viersen & Heising, 2015 (Fig. 2O), K. tuberculata (Whittington, 1956, pl. 57, fig. 4)) and variably so in others (e.g., K. williamsi (Whittington, 1956) (Whittington, 1956, pl. 57, fig. 12, pl. 58, fig. 2), K. rojanensis Feist & Clarkson, 2023 (Feist & Clarkson, 2023, fig. 3O–Q)). The tubercle pairs are rarely all three present, with few exceptions (e.g., K. eftychia van Viersen et al., 2019 (Fig. 2P), K. favonia (Haas, 1969), K. pigra (Barrande, 1872) (Alberti, 1969, pl. 41, figs 2, 5; Fig. 2K)).
9In K. jaanussoni there is sufficient space (tr.) between the pleural ridge and the second axial ring for a small inner pleural ridge; its presence is hinted on by P1 and P2 tubercles similarly arranged (and probably serially homologous) to those on the pleural ridge. Other Silurian species often show this same space although P1 and P2 tubercles are normally lacking (e.g., K. centrina (Dalman, 1828) (Bruton, 1967, p. 234, fig. 1), K. geinitziana (Hawle & Corda, 1847) (Vokáč et al., 2018, pl. 2, fig. 7; Fig. 2N), K. wrightae Adrain & Ramsköld, 1997) (Fig. 2J)). Sometimes the space is variably present intraspecifically (e.g., K. lenzi (Chatterton & Perry, 1983) (Adrain & Ramsköld, 1997, p. 243, figs 6.25, 6.28, 6.30; Fig. 2Q)). Yet another group of Silurian species lack the space entirely and this is often coupled with a small overall pygidium size (e.g., K. angelini (Prantl & Přibyl, 1949) (Fig. 2I), K. crenata (Emmrich, 1844) (Ramsköld, 1984, p. 255, fig. 4), K. lindoei Adrain & Ramsköld, 1997, K. parkini (Siveter, 1989)). A narrow space between the second axial ring and the major pleural ridge is common in Devonian species yet variably coupled with a small pygidium size.
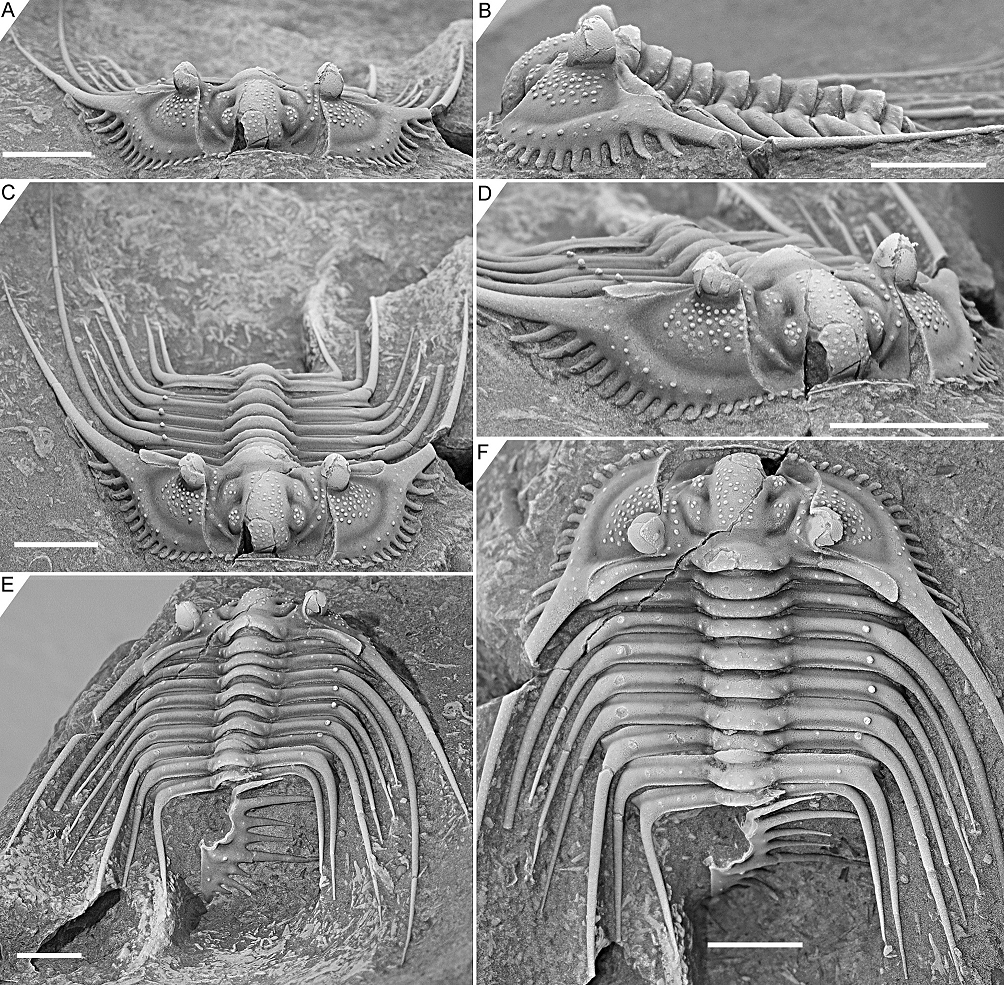
Figure 3. Kettneraspis freitagi sp. nov., Emsian–Eifelian transition, Hamar Laghdad, Tafilalt, Morocco. Holotype, IRSNB a13909, in anterior (A), lateral (B), oblique anterior (C), anterolateral (D), oblique posterior (E) and dorsal (F) views. Scale bars are 5 mm.
2.2. Patterns in Leonaspis
10The combination of a central (tr.) P2 and (often well-developed) Pa on the thoracic pleurae is ubiquitous in Leonaspis so far as species are known from complete specimens. P1 is rarely developed. A specimen of Leonaspis lochkovensis (Prantl & Přibyl, 1949) figured by Chlupáč (1993, pl. 7, fig. 7) bears P1 tubercles on the anterior six thoracic segments, the lectotype of L. leonhardi (Basse & Müller, 2016, pl. 31, fig. 326) carries them on the first three segments, and the lectotype of L. hoernesi (Barrande, 1846) (Basse & Müller, 2016, pl. 31, fig. 325) on the anterior two segments. Exceeding variability of tubercles on the thoracic pleurae of L. jenkinsi (Etheridge & Mitchell, 1896) is apparent in the specimens recorded by Chatterton (1971).
11The pygidial pattern is similar to the thorax, with P1 generally lacking except, e.g., in L. expilata Lütke, 1965 (Fig. 2B). P2 is always present except in L. bassei Alberti, 2018 (Alberti, 2021, p. 59, fig. 3a; Basse & Müller, 2023, pl. 35, fig. 7) and L. haddanei Chatterton et al., 2006 (Fig. 2G) which only have Pa developed. Pa, if present, is a tubercle so far as known lacking in transitory pygidia. Significant intraspecific variability of Pa was documented in L. leonhardi by Vokáč et al. (2021).
12The inner pleural ridge of Leonaspis is often rudimentary or imperceptible (e.g., L. glabrata (Roemer, 1843) (Alberti, 1969, pl. 41, figs 7, 8), L. kleini Basse in Basse & Müller, 2004 (Fig. 2C), L. lochkovensis (Bruton, 1968, pl. 2, fig. 9; Přibyl & Vaněk, 1966, pl. 1, fig. 8), L. nuratensis Ivanova et al., 2009 (Fig. 2D)). In some species it forms a distinctive, raised band (e.g., L. hastata Alberti, 1967 (Fig. 2F), L. maura Alberti, 1969 (Fig. 2E)). Species lacking the inner pleural ridge always show a large (tr.), depressed plain here; its former position may sometimes be inferred from a Pa tubercle (e.g., L. belisarius Haas, 1968; L. ezellina Šnajdr, 1983) or, in rare cases, a P2 tubercle (e.g., the L. leonhardi specimen figured by Horný & Bastl, 1970, pl. 20, fig. 2). The presence of these tubercles is subject to intraspecific variation.
2.3. Nature of the pygidial interior border spines
2.3.1. Rejection of Rupbachella
13Basse & Müller (2016) illustrated partially and fully articulated exoskeletons of Leonaspis kleini and Kettneraspis seiberti from the upper Emsian Rupbach Shales in the Rhenish Mountains. Their material includes small individuals with two interior border spines (Basse & Müller, 2016, pl. 21, figs 199–202, 205; Basse, 2022, p. 68, fig. 1) that were identified by Basse & Müller (2016) as juvenile K. seiberti. Alberti (2021) reassigned these to a new species in a monotypic genus, Rupbachella paedomorpha. However, there are four principle reasons to refer them to degree 8 meraspides of L. kleini instead: (1) the small overall body size combined with what appear to be eight thoracic segments in one specimen which is nearly complete, although this argument alone may be unviable (cf., e.g., eight-segmented large holaspides of K. williamsi discussed by Ramsköld & Chatterton, 1991), (2) the absence of spines dorsally on the genal spine (character state 17:0 of Ramsköld & Chatterton, 1991) similarly to large holaspides of L. kleini––K. seiberti carries two large spines here, (3) the occurrence of single (P2) spine pairs abaxially on the thoracic posterior pleural bands and pygidial pleural ridge, similar to large holaspides of L. kleini––K. seiberti has double tubercle pairs (P1 and P2) on most thoracic segments, and (4) the single spine distally on the posterior cranidial border (spine B of Whittington, 1956), serially homologous with the postcephalic P2 spines.
2.3.2. Ontogeny of the two- and four-spined states
14If correctly assigned, the meraspides of Leonaspis kleini offer new insights into the development of the dorsal and border spines of Leonaspis. The postaxial spine pair of these specimens might be taken as posteriorly directed dorsal spines. In that case they conceal the more ventrally lying interior border spines and likewise, the posterodorsally directed major border spines overhang a pair of interior border spines (cf., e.g., this condition in Acanthalomina churkini (Chatterton & Perry, 1979) (Chatterton & Perry, 1979, pl. 4, figs 5, 8) and Acanthalomina thorsteinssoni (Perry & Chatterton, 1979) (Perry & Chatterton, 1979, pl. 75, fig. 8)). The similar posterodorsal orientations of the major border spines and the postaxial spines, however, suggest that the latter are true border spines. The pygidial P2 and major border spines are serially homologous with the thoracic dorsal P2 and posterior pleural spines as is apparent from their corresponding positions relative to one another. Theoretically, no further changes would be required at this point to reach the two-spined Kettneraspis state. Thus, the four-spined state of Leonaspis must have been attained (early) in the holaspid phase. This leads to the postulation here, that the formation of the inner pleural ridges and second interior border spine pair was arrested precipitately in species assigned to Kettneraspis, primitively leaving a space (sometimes marked by P1 and P2 tubercles), and that control over this process was fully accomplished in derived species, resulting in constant expressions of the two-spined condition without the inner pleural space. The absence of an inner pleural space is exclusive to, but not characteristic of, Kettneraspis. The formation of a rudimentary inner pleural ridge or wide space, combined with four interior border spines characterises Leonaspis. The only exceptions to these rules are anomalous pygidia.
2.3.3. Outlook
15We may now consider the exclusion of some problematic species from either genus as shown in the case of Leonaspis? strix Lütke, 1965 below. But the designations here of two- and four-spined groups as Kettneraspis and Leonaspis, though convenient for their discrimination at present, offer far from convincing evidence to uphold their monophyly. They do not invalidate the hypothesis posited by van Viersen & Heising (2015) that some Leonaspis species are actually Kettneraspis with an odd pygidium. Clearly a lot of work remains ahead, beginning with the collection of more data. Many species have been described on the basis of disarticulated sclerites, and the ontogeny of the vast majority of species remains fully unknown.
2.4. Thoracic macropleural spines
16Adrain & Ramsköld (1997) transferred a number of Kettneraspis species to their new genus Edgecombeaspis and discussed four potential apomorphies of Kettneraspis sensu stricto. Three of these, the smooth pygidial doublure, the relative lengths of the anterior and posterior branches of the facial sutures, and the anterior “pinching out” of the eye ridge, are all variable within Kettneraspis and Leonaspis. The fourth character involves the macropleural spines of Kettneraspis. Adrain & Ramsköld (1997) argued that many species of which the thorax is known have macropleural posterior spines on all of segments 4 to 9 and that this pattern may be synapomorphous with Leonaspis. However, macropleural spine patterns are highly variable within Kettneraspis and Leonaspis. Many Kettneraspis species have macropleural spines on segments 3 to 9 (e.g., K. angelini, K. centrina, K. elliptica, K. rattei (Etheridge & Mitchell, 1896), K. seiberti, K. tuberculata). Others have exceedingly long pleural spines on segment 3 followed by shorter spines on segments 4 to 9 (e.g., K. prescheri, K. williamsi). Leonaspis may have macropleural spines on segments 2 to 9 (e.g., L. hoernesi), on segments 4 to 9 (e.g., L. kleini, L. leonhardi), and on segments 5 to 9 (e.g., L. haddanei) but, as to my knowledge, never exclusively on segments 3 to 9 (L. spinicurva Chatterton et al., 2006 and L. jenkinsi are potential examples and better-preserved material is required to corroborate this, but clearly, the fourth segment has much longer spines than the third). A Wenlock to Ludlow clade of Kettneraspis species from the Barrandian (Prague Basin) that are commonly assigned to K. dormitzeri (Hawle & Corda, 1847) lack the combination of stunted anterior segments and macropleural remaining segments. The group is recognised as Bruthansovaspis gen. nov. below.
3. Systematic palaeontology
17Materials and methods. Specimens were treated with ammonium chloride sublimate prior to photography unless stated otherwise. The material is housed by the National Museum, Prague (prefixes NM L, IT, ČE), the Czech Geological Survey (prefix CGS JV) and the Institut royal des Sciences naturelles de Belgique (prefix IRSNB).
18Family Odontopleuridae Burmeister, 1843
19Subfamily Odontopleurinae Burmeister, 1843
20Genus Kettneraspis Prantl & Přibyl, 1949
21Type species. Acidaspis pigra Barrande, 1872, from the Acanthopyge Limestone (Eifelian) in the Barrandian.
22Kettneraspis freitagi sp. nov.
23(Figs 3–5)
24cf. 2018 Kettneraspis sp.; Lebrun, p. 285, fig. c, p. 287, unnumb. fig.
25Etymology. Named after Paul Freitag, who prepared and donated the types for study.
26Holotype. Incomplete specimen, IRSNB a13909 (Fig. 3).
27Paratypes. Slightly disarticulated specimen lacking the pygidium, IRSNB a13910; incomplete specimen, IRSNB a13911.
28Type locality and horizon. Hamar Laghdad “red cliff” locality (Klug, 2002), Tafilalt, southern Morocco; reddish limestones of late Emsian or lower Eifelian age, with Austerops and Proetopeltis.
29Diagnosis. Anterior border dorsally convex (tr.), bearing 18 laterally expanded tubercles. Longitudinal glabellar furrow fully effaced near posterior half of L1. Anterior two segments with moderately short, slender posterior pleural spines. Segments 3 to 9 macropleural, the first pleural spine pair of which is the longest and projects far beyond the pygidium. Thoracic axial rings carrying two tubercle pairs centrally (exsag.), the first of which is markedly more anteriorly positioned.
30Description. Border furrow in front of median glabellar lobe shallow medially, widening (exsag.) and deepening abaxially. Occipital ring with convexly outlined median part and weakly inflated lateral occipital lobes. S0 slightly deeper posterior to L1 than to median glabellar lobe. Axial furrow rudimentary posteriorly near occipital ring; only developed anterior to junction with S0. Subrectangular median glabellar lobe, widest points near lateral glabellar lobes L2 and anteriorly; anterolaterally separated from eye ridge by comparatively deep, longitudinal furrow. Longitudinal glabellar furrow deep in junctions with S1 and S2; weakly impressed near central to posterior part of L2. Subtriangular fixigena; as broad as L1 posteriorly, narrow near anterolateral corner of L1, and then dying out, reaching halfway (exsag.) L2. Eye ridge runs exsagittally proximally, flexes adaxially near L2 and runs towards median glabellar lobe from here. Posterior border progressively posterolaterally curved. Posterior border furrow is a transverse groove, interrupted by postocular sutural ridge. Ocular sutures divergent from ω before flexing inward and running towards ε; path ε–δ–γ depicts a high parabola (tr.). Small palpebral lobe, bearing a small pit close to δ. Sculpture on glabella anterior to S0 consists of moderately widely spaced, randomly scattered pustules, alternated on median glabellar lobe by closely spaced granules. Fixigena and eye ridge each bear a row of tubercles. Occipital ring carries densely spaced granules, a large median tubercle, and several tubercles posteriorly that verge to forming a single row. Posterior border covered with granules; two evenly spaced tubercles on abaxial half that are serially homologous with the thoracic P1 and P2 tubercles.
31Librigenal border broad, slightly tapering anteriorly. There are 13–15 border spines with laterally expanded tips; towards posterior each spine is longer and more acuminate; posterior two pairs of spines can be positioned slightly posteriorly (relics of their distinct ontogenetic origin). Border furrow is shallow throughout, generally recognisable by a change of slope between border and librigenal field. Angle between lateral border furrow and posterior border furrow ±80°. Librigenal field is moderately weakly inclined. Eye is tall, conical, higher than occipital ring, posterior half placed opposite S0. Exceedingly long, thin genal spines that diverge at a close to 80° angle proximally and subsequently very gently converge; no dorsal spines on the base of the genal spine (character state 17:0). Sculpture consists of densely spaced pustules proximally on the librigenal field, few randomly scattered tubercles on lateral border and on genal spine; otherwise genal spine is covered with dense granulation that verges to forming longitudinal terrace lines (Fig. 5I). Epiborder tubercles above border spines 3, 5, 7 and 8; possibly also 10 and 11.
32Thorax consists of nine segments: each comprising low convex ring (tr.) carrying tubercles posteriorly and fine granules similarly organised to occipital ring but without a median tubercle; weakly defined lateral lobes carrying single tubercles; straight (tr.) anterior pleural band with distally about three or four (dichotomous) spines; articulating flange developed between axial furrow and fulcrum, about as long (exsag.) as anterior pleural band; posterior pleural band carries P1 and P2 tubercles but irregularly.
33Pygidium broadly rounded, about 3.5 times wider than long, carrying one or two tubercle pairs positioned anteriorly in pleural region. First axial ring carrying two tubercle pairs; medially incised posteriorly by a small pseudo-articulating half ring. Second axial ring bears a single tubercle pair. Terminal axial piece of similar size to second axial ring and not well separated. Pleural ridge is proximally posterolaterally oriented, then flexed posteriorly, bears a single P2 tubercle just posterior to flexure; distally slightly outward curved. Two interior border spines between slightly divergent major border spines. Few, unpaired tubercles on pygidium; more on border spines.
34Discussion. The apparent erratic P1 and P2 patterns on the thorax of the three types are partially caused by inadequate preservation. One of the paratypes, IRSNB a13911 (Fig. 5), carries a large P2 and small P1 tubercle on the right side of the third thoracic segment, whilst on the left side only a small P2 tubercle is visible. This inconsistency suggests that the left pleura is less well-preserved or that it has sustained more abrasive damage during the preparation. However, in the holotype, P1 tubercles are evidently not expressed on segments three to six. Paratype IRSNB a13911 was collected from a yellowish level slightly below the reddish horizon that yielded the holotype and the other paratype, and this could account for the small variations, although this is hard to tell based on so few specimens. Dense granulation is reported in specific dorsal areas of the exoskeleton but it is also present on small, exceedingly well preserved patches, e.g., on the librigenal field and border, and the thoracic posterior pleural spines. This suggests that granules may have been ubiquitous and that they were partially lost during preparation or not always preserved in the first place.
35Kettneraspis knoppi Basse & Müller, 2017 from the upper Emsian in the Rhenish Mountains has a similar cephalon with in particular the sculpture on the glabella, librigenal field and lateral cephalic border, and the shape of the fixigena. It is principally different from the new species in having 14 tubercles on the anterior border, longitudinal glabellar furrow moderately firmly incised near posterior part of L1, posteriorly positioned median node on occipital ring added to by two tubercle pairs on its posterior margin. The pygidium of K. knoppi (Fig. 2L) has P1 and P2 tubercles on the more swollen pleural ridge and there are three lateral border spine pairs.
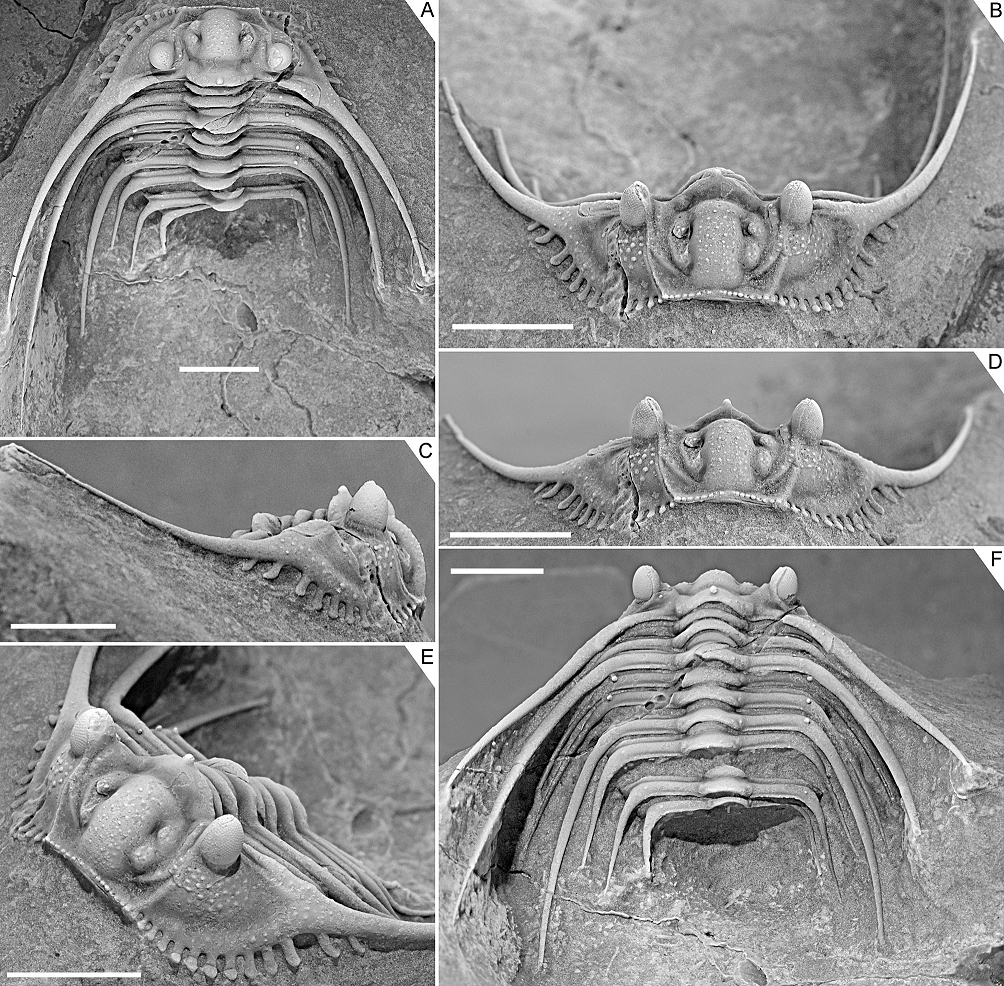
Figure 4. Kettneraspis freitagi sp. nov., Emsian–Eifelian transition, Hamar Laghdad, Tafilalt, Morocco. Paratype, IRSNB a13910, in dorsal (A), oblique anterior (B), lateral (C), anterior (D), anterolateral (E) and oblique posterior (F) views. Scale bars are 5 mm.
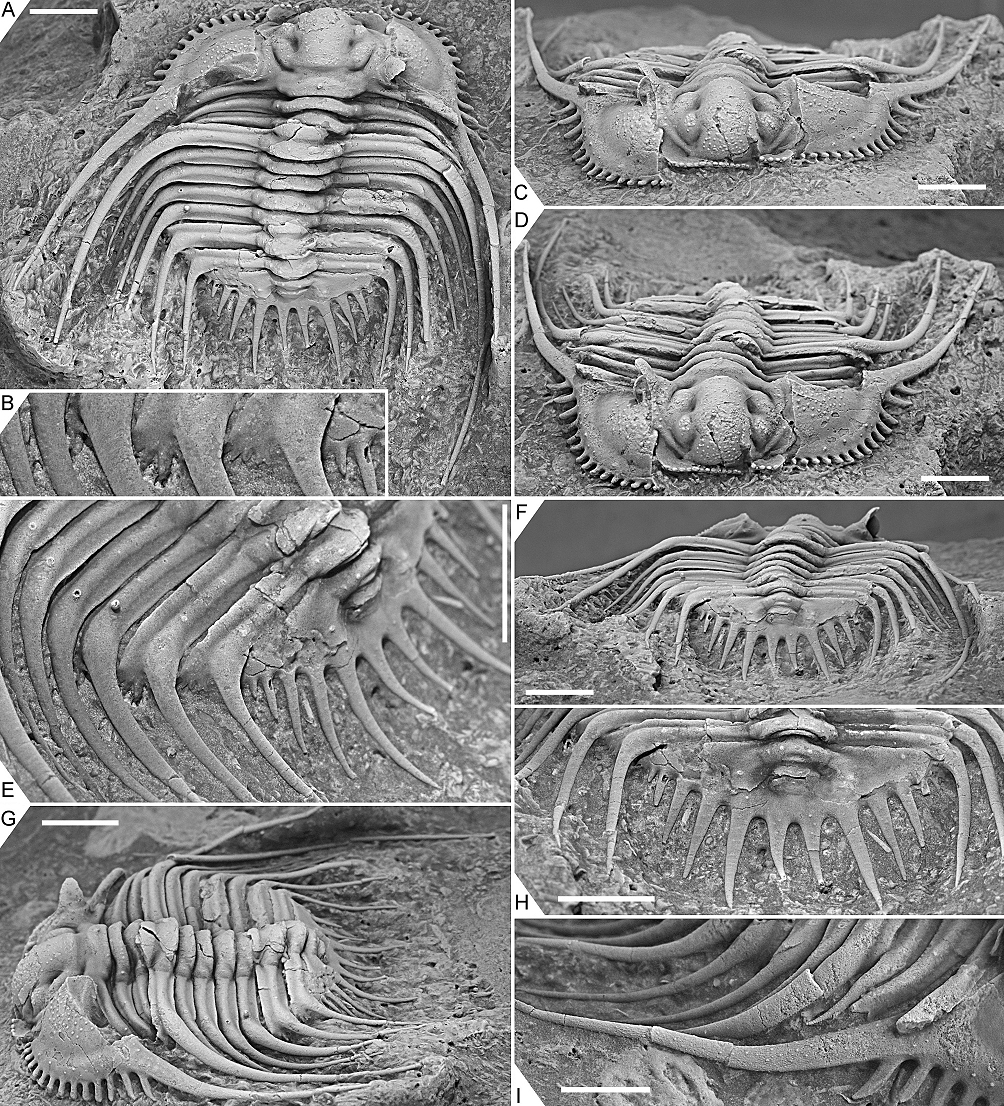
Figure 5. Kettneraspis freitagi sp. nov., Emsian–Eifelian transition, Hamar Laghdad, Tafilalt, Morocco. Paratype, IRSNB a13911, in dorsal view (A), close-up of left posterolateral part of thorax and extremity of pygidium showing the anterior pleural spines (B), anterior view (C), oblique anterior view (D), close-up of left posterior part of exoskeleton (E), posterior view (F), oblique lateral view (G), close-up of pygidium (H) and close-up of right genal spine (I). Scale bars are 5 mm except I which is 2 mm.
36Kettneraspis propinqua (Barrande, 1852)
37(Fig. 6A–E, M, Q)
38* 1852 Acidaspis propinqua Barrande, p. 733, pl. 39, figs 25–27.
391949 Acanthaloma (Kettneraspis) propinqua; Prantl & Přibyl, p. 169, pl. 1, fig. 17, pl. 3, fig. 15.
40e.p. 1968 Leonaspis dormitzeri; Bruton, pp. 22, 23.
411987 Leonaspis propinqua; Chlupáč, p. 173.
421991 propinqua; Ramsköld & Chatterton, p. 367 [Kettneraspis species inquirenda].
43Material. Lectotype incomplete specimen, NM L 15697, from the Motol Formation (Wenlock) in Lištice near Beroun, Barrandian.
44Discussion. Synonymy of Acidaspis propinqua with Odontopleura dormitzeri as a species of Leonaspis was proposed by Bruton (1968) and tentatively accepted by Ramsköld & Chatterton (1991), who transferred them to Kettneraspis. The pygidial configuration of the lectotype of K. propinqua is consistent with its contemporary generic assignment. Other characters are the anteriorly steeply inclined median glabellar lobe, moderately broad (tr.) fixigena carrying a row of pustules, stunted anterior two thoracic segments, remaining thoracic segments carrying P2 tubercles (with occasionally a P1), with distinctive fulcral swellings and distally extended into robust spines, pygidium with well-developed pseudo-articulating half ring, swollen posterior border, markedly tapered border spines with thick bases, and two pairs of lateral border spines. Many of these features not only serve to discriminate Kettneraspis propinqua and Bruthansovaspis dormitzeri comb. nov., but also these genera as outlined below. According to Chlupáč (1987), K. propinqua occurs in the volcano-carbonate facies of the Motol Formation, slightly above the Bumastus-Sphaerexochus-Cheirurus assemblage that includes B. dormitzeri. No co-occurrences of these species have been reported.

Figure 6. A–E, M, Q. Kettneraspis propinqua (Barrande, 1852), Motol Formation, Lištice near Beroun, Barrandian. Lectotype incomplete specimen, NM L 15697, in dorsal view (A, M), left lateral view of cephalon (B), oblique lateral view (C), oblique anterolateral view (D), close-up of cranidium (E), and dorsal view of cephalon (Q).
F, P. Kettneraspis lindackeri (Hawle & Corda, 1847), Kopanina Formation, Koledník, Barrandian. Proposed neotype pygidium, NM L 1867, in dorsal view.
G, J, L. Bruthansovaspis dormitzeri (Hawle & Corda, 1847), Motol Formation, Svatý Jan, Barrandian. Incomplete specimen, NM L 5359 (= holotype of Odontopleura zenkeri), in dorsal (G, L) and left lateral (J) views.
H, I, N. Bruthansovaspis dumortieri (Hawle & Corda, 1847), Kopanina Formation, Koledník near Beroun, Barrandian. Holotype pygidium, NM L 5371, in dorsal (H, N) and oblique lateral (I) views.
K, O. Bruthansovaspis dormitzeri (Hawle & Corda, 1847), Motol Formation, Svatý Jan, Barrandian. Paralectotype pygidium, NM L 15678, in dorsal view.
Scale bars are 2 mm. Fig. L–Q untreated before photography. All photos courtesy of J. Bruthansová.
45Kettneraspis lindackeri (Hawle & Corda, 1847)
46(Fig. 6F, P)
47* 1847 Odontopleura Lindackeri Hawle & Corda, p. 153.
48e.p. 1852 Acid. Dormitzeri; Barrande, pp. 728, 729.
49e.p. 1949 Acanthaloma (Acanthaloma) dormitzeri; Prantl & Přibyl, pp. 159, 160.
50e.p. 1968 Leonaspis dormitzeri; Bruton, pp. 22, 23.
511970 Leonaspis (Leonaspis) dormitzeri; Horný & Bastl, p. 193.
521984b Odontopleura Lindackeri; Šnajdr, p. 169, pl. 13, fig. 8 [= lectotype].
531991 lindackeri; Ramsköld & Chatterton, p. 366 [as a synonym of Kettneraspis dormitzeri].
54Material. Pygidium, NM L 1867 (proposed neotype), from Koledník, Barrandian; Kopanina Formation (Ludlow).
55Discussion. Šnajdr (1984b) selected syntype NM L 17635 as the lectotype while mentioning that a second syntype, NM L 1867, had also been found. I was informed by Dr J. Bruthansová (pers. comm. in 2023) that the lectotype could not be traced in the National Museum, Prague, and that it has to be presumed lost. Therefore, I propose as the neotype, paralectotype pygidium NM L 1867. According to its label, the neotype was collected from the Kopanina Formation in Koledník. Kettneraspis lindackeri comb. nov. occurs in boundary beds in the horizon with Ananaspis fecunda and Prionopeltis archiaci (Šnajdr, 1984b). This is a shallow-water interval near the top of the Kopanina Formation that contains the Ananaspis-Coniproetus assemblage graded upward into the Prionopeltis archiaci assemblage (Chlupáč, 1987).
56Many workers (e.g., Barrande, 1852; Prantl & Přibyl, 1949; Bruton, 1968; Horný & Bastl, 1970; Ramsköld & Chatterton, 1991) have regarded K. lindackeri as a synonym of B. dormitzeri. Šnajdr (1984b) and Vaněk & Valíček (2002) placed K. lindackeri in synonymy of Leonaspis leonhardi without an explanation. The identity of the lectotype is difficult to assess based on the single published photograph by Šnajdr (1984b, pl. 13, fig. 8), except that it is not a Leonaspis in having a single pair of interior border spines and in lacking an inner pleural ridge or wide space here. The neotype is a typical, but slightly effaced (weathered?) Kettneraspis pygidium preserved in a bioclastic limestone. It is principally different from B. dormitzeri in having a pseudo-articulating half ring, (weakly) isolated terminal axial piece, inflated pleural ridges running subtransversely before flexing posteriorly, and robust, markedly tapered border spines.
57Genus Leonaspis Richter & Richter, 1917
582021 Rupbachella Alberti, pp. 58–63.
59Type species. Odontopleura leonhardi Barrande, 1846, from the Kopanina Formation (Ludlow) in the Barrandian.
60Discussion. Holloway (2021) reviewed features that had previously been deemed characteristic of Leonaspis and which he found to be unreliable. I add to that, the fact that well-preserved specimens of L. leonhardi lack the irregular tuberculation/granulation on the anterior border that was considered by Ramsköld & Chatterton (1991) to be typical of Leonaspis (their character state 1:2). Although this condition is inconclusive in the lectotype due to inadequate preservation (Basse & Müller, 2016, pl. 31, fig. 326), other specimens (e.g., Flick & Flick, 2021, p. 114, fig. 3, and photos shown to me by F. Hartl, pers. comm. in 2022) clearly show a single row of 20 tubercles (character state 1:1) which, according to Ramsköld & Chatterton (1991), is characteristic of Kettneraspis. This information is pertinent because L. leonhardi is not only the type species but it is also one of the stratigraphically earliest known Leonaspis species.
61The junior synonymy of the monotypic Rupbachella was explained above. Its type species, R. paedomorpha Alberti, 2021, is regarded here as a degree 8 meraspis of Leonaspis kleini.
62Leonaspis sp.
63(Fig. 7)
641933 Acidaspis elliptica; Maillieux, p. 62.
652013 Leonaspis? sp.; van Viersen, p. 2, fig. 2A.
662016 Leonaspis; Basse & Müller, p. 180.
67Material. External mould of an articulated exoskeleton with very fragmentary cephalon, IRSNB a12829, from the Hierges Formation (Emsian) in Olloy-sur-Viroin, southern Belgium.
68Discussion. Ramsköld & Chatterton (1991) believed Leonaspis to be exclusively Gondwanan and never to have crossed the Rheic Ocean. The occurrence of a potential Leonaspis species in the Hierges Formation of Belgium (Avalonia) was briefly mentioned by van Viersen (2013) based on a single external mould of a complete specimen from the old Maillieux collections in the IRSNB. The thorax carries P2 and Pa tubercles, and there is a very wide space between the pygidial pleural ridge and the second axial ring, combined with a comparatively narrow (tr.) pleural region. There can thus be little doubt that the specimen has four interior border spines, and that it should be assigned to Leonaspis. Franke (2010) recorded Leonaspis from the coeval Wiltz Formation, a lateral facies variant of the Hierges Formation in the Oesling and the Eifel.

Figure 7. Leonaspis sp., Hierges Formation (Emsian), Olloy-sur-Viroin, southern Belgium. External mould of an articulated exoskeleton with very fragmentary cephalon, IRSNB a12829, in dorsal view (digitally inverted image). Scale bar is 5 mm.
69Leonaspis? strix Lütke, 1965
70(Fig. 2T)
71* 1965 Leonaspis (Leonaspis) strix Lütke, pp. 219–221, pl. 22, fig. 8, p. 220, fig. 33.
721991 strix; Ramsköld & Chatterton, p. 367 [Leonaspis? species inquirenda].
73? 2009 Leonaspis strix (?); Basse, pl. 6, figs 177, 178.
74Discussion. Leonaspis strix has long been known exclusively on the basis of a single damaged cranidium from the Emsian in the Harz Mountains. Lütke (1965) noticed the unusually large palpebral lobes of this species and drew attention to similarities with the large-eyed Leonaspis truncata (Hawle & Corda, 1847) (Bruton, 1968, pl. 4, figs 1–7) from the Pragian of the Barrandian, which is known only from cephalic remains. Leonaspis glaux Flick & Flick, 2021 from the Emsian–Eifelian transition in the Rhenish Mountains, too, shares large palpebral lobes but like the other two species it remains inadequately documented.
75Basse (2009) recorded a large-eyed librigena and a pygidium with four interior border spines that he tentatively identified as L. strix. The pygidium is peculiar in lacking the inner pleural ridge or space characteristically associated with Leonaspis as alluded to above, and in having an exceedingly wide (tr.) pleural region. In all the previously listed examples of abnormal odontopleurine pygidia, the distance between the pleural ridge and the second axial ring is barely affected, regardless of how many interior border spines are developed. In normal, four-spined pygidia of Sinespinaspis nehedensis, the inner pleural space is marked by P2 and Pa tubercles (Fig. 2S1). In pygidia of this species with two interior border spines, both tubercles have merely migrated anteriorly, standing slightly away from the posterior margin (Fig. 2S2). Teratological pygidia of L. leonhardi (Fig. 2A3–6) show many variations of the inner pleural ridge but similarly to the abnormal S. nehedensis pygidia, the amount of inner pleural space remains fundamentally the same. The inner pleural areas of two-spined and four-spined pygidia of K. jaanussoni, and two-spined and single-spined pygidia of Edgecombeaspis longstaffei, are also not noticeably different. This leads to consideration of another option, of L.? strix being an abnormal Kettneraspis, but that assignment is equally doubtful because the major border spines are barely tapered and rather poorly differentiated from the long(?) secondary border spines. Instead, these characters are suggestive of other affinities, e.g., with some odontopleurines previously included in Diacanthaspis. The pygidium of D. (Diacanthaspis)? tricosa Holloway, 2021 (e.g., Holloway, 2021, pl. 8, fig. 4) from the Silurian of North America, for instance, is very similar to that of L.? strix?. However, Diacanthaspis is Ordovician (see Adrain & Pérez-Peris, 2023, for a revised concept), the generic affinity of Holloway’s species is in doubt, and there are no transitional forms known from the Lower Devonian. The case of L.? strix hinges on the uncertain relationship between Basse’s (2009) pygidium, which resides in a private collection, and the holotype cranidium described by Lütke (1965). If these are shown to be conspecific, then it is certain that the current assignment to Leonaspis needs to be reconsidered.
76Genus Bruthansovaspis nov.
77Etymology. Named after Dr Jana Bruthansová, curator at the National Museum, Prague, and noted trilobite researcher, whose help enabled the recognition of the new genus, in combination with -aspis (Greek: ἀσπίς, shield), a common suffix for odontopleurine trilobites. Gender femininum.
78Type species. Acidaspis roemeri Barrande, 1852, from the Motol Formation (Wenlock) in the Barrandian.
79Diagnosis. Long cephalon (sag.), measuring about half the length of thoracopygidium. Median glabellar lobe markedly expanded (tr.) anteriorly. Fixigena very rudimentary (tr.), especially near ocular ridge. Posterior border of cranidium narrow (exsag.), lacking a transverse furrow. Posterior margin of librigena markedly recurved near ω. Slender, straight, consecutively longer thoracic pleural spines, arising from narrow bases on the posterior extremity of the posterior pleural band alone. Dorsally weakly convex, proximally smoothly posteriorly flexed pleural ridge. P2 tubercles opposite terminal axial piece.
80Species assigned. Odontopleura dormitzeri Hawle & Corda, 1847 from the Motol Formation (Wenlock) in the Barrandian; Odontopleura dumortieri Hawle & Corda, 1847 from the Kopanina Formation (Ludlow) in the Barrandian.
81Discussion. Bruthansovaspis roemeri comb. nov. had been considered a species of Leonaspis (e.g., Přibyl & Vaněk, 1966; Bruton, 1968) and was subsequently placed in Kettneraspis by Ramsköld & Chatterton (1991) where it has remained since. But the similarities to either genus are superficial, and its current generic assignment was probably chiefly inferred from the single pair of interior border spines. Prantl & Přibyl (1949) already noticed marked differences between B. roemeri and species assigned by them to their new subgenus Acanthaloma (Kettneraspis), with in particular the rudimentary fixigena between the glabella and the ocular ridge. This feature may serve to discriminate B. roemeri from the far majority of Kettneraspis and Leonaspis species and even Edgecombeaspis, which have well-developed fixigenae commonly accentuated by a row of large pustules. In some derived species from the Devonian, the fixigenae can be narrow and almost fully deflated (e.g., K. clavata (Chatterton, 1971), L. kleini). This secondary loss, however, has no bearing on the significance of this character as a synapomorphy of Kettneraspis and Leonaspis.
82Bruthansovaspis predates the earliest Leonaspis from the Ludlow and postdates the earliest Kettneraspis species from the Llandovery, and so it might be conceived as having been derived from the Kettneraspis rootstock. This is unlikely to be the case, however, since in certain aspects, Bruthansovaspis (Fig. 8B) is more primitive than Kettneraspis (Fig. 8A) in bearing a number of symplesiomorphies. These include the weakly vaulted cranidium of Bruthansovaspis (this makes a quarter circle in lateral view of Kettneraspis species), the narrow cephalic border and weakly impressed lateral border furrow, the exceedingly slender genal spines with thin bases (Kettneraspis has very robust spines that appear as fluent continuations of the posterior and lateral cephalic borders where these merge at the genal angle), a pair of subvertical spines on the occipital ring (reduced to tubercles or absent in Kettneraspis), the markedly and very steadily convergent axial furrows representing a straight line between occipital ring and terminal axial piece, the slender, thin-based, uniformly shaped thoracic posterior pleural spines (instead of the stunted anterior, and subsequent robust macropleural spine pairs with marked fulcral swellings of Kettneraspis) the last pair of which steadily converges behind the pygidium for its entire length, the fully developed first pygidial axial ring (lacking the distinctive posterior indentation of the pseudo-articulating half ring of all Kettneraspis species), the fused terminal axial piece, the pleural regions and postaxial area which form a uniform plain reaching the posterior margin without the development of a posterior border (typically developed in Kettneraspis along with a depressed area between the pleural ridges), and the weakly tapered and slender pygidial border spines. Šnajdr (1990) characterised the dorsal sculpture of B. roemeri as exceedingly spiny, comprising sparsely distributed tiny spines or thorn-like tubercles on the glabella, eye ridges, cephalic borders and on the librigenal field (these have never been reported in large holaspides of Kettneraspis). Šnajdr (1990) also reported large subvertical spines on the occipital ring and thoracic and pygidial axial rings (see remarks under B. roemeri below). The comparatively large cephalon of B. roemeri was previously noticed by Basse & Müller (2017). Some of the above-mentioned traits are potentially synapomorphic with the conditions of other odontopleurines such as Ivanopleura Šnajdr, 1984a, Odontopleura Emmrich, 1839, Sinespinaspis Adrain & Chatterton, 1990, Acanthalomina, and species traditionally associated with Diacanthaspis, and it would be illogical to dismiss all of them as mere reversals. Bruthansovaspis is principally different from these genera in exhibiting a very dissimilar course of the facial sutures, narrow fixigenae, proximally posteriorly flexed pygidial pleural ridges, single pair of interior border spines, and in lacking the large, paired occipital spines (perhaps homologous with one of the tubercle or spine pairs of B. roemeri). As a final remark, the morphologically very similar pleural ridge and tubercle of the poorly known Orphanaspis Prantl & Přibyl, 1949 (e.g., Horný & Bastl, 1970, pl. 20, fig. 4) are pointed out, although these are doubtfully homologous.
83The recognition of Bruthansovaspis and revision of its type species have implications for generic diversity of Odontopleurinae in peri-Gondwana during the middle Silurian. Feist & Clarkson (2023) counted three Kettneraspis species here, including one from the Barrandian that represents the now obsolete concept of Kettneraspis dormitzeri. The independent specific ranks of Kettneraspis lindackeri and Kettneraspis propinqua were reinstated above, and these two species are currently the only members of this genus in the Wenlock and Ludlow of the Barrandian. Bruthansovaspis encompasses three species which are only known to occur here. This is consistent with Šnajdr’s (1984a) notion of diversified odontopleurid assemblages in the Silurian of the Barrandian including specialised, probably endemic genera.
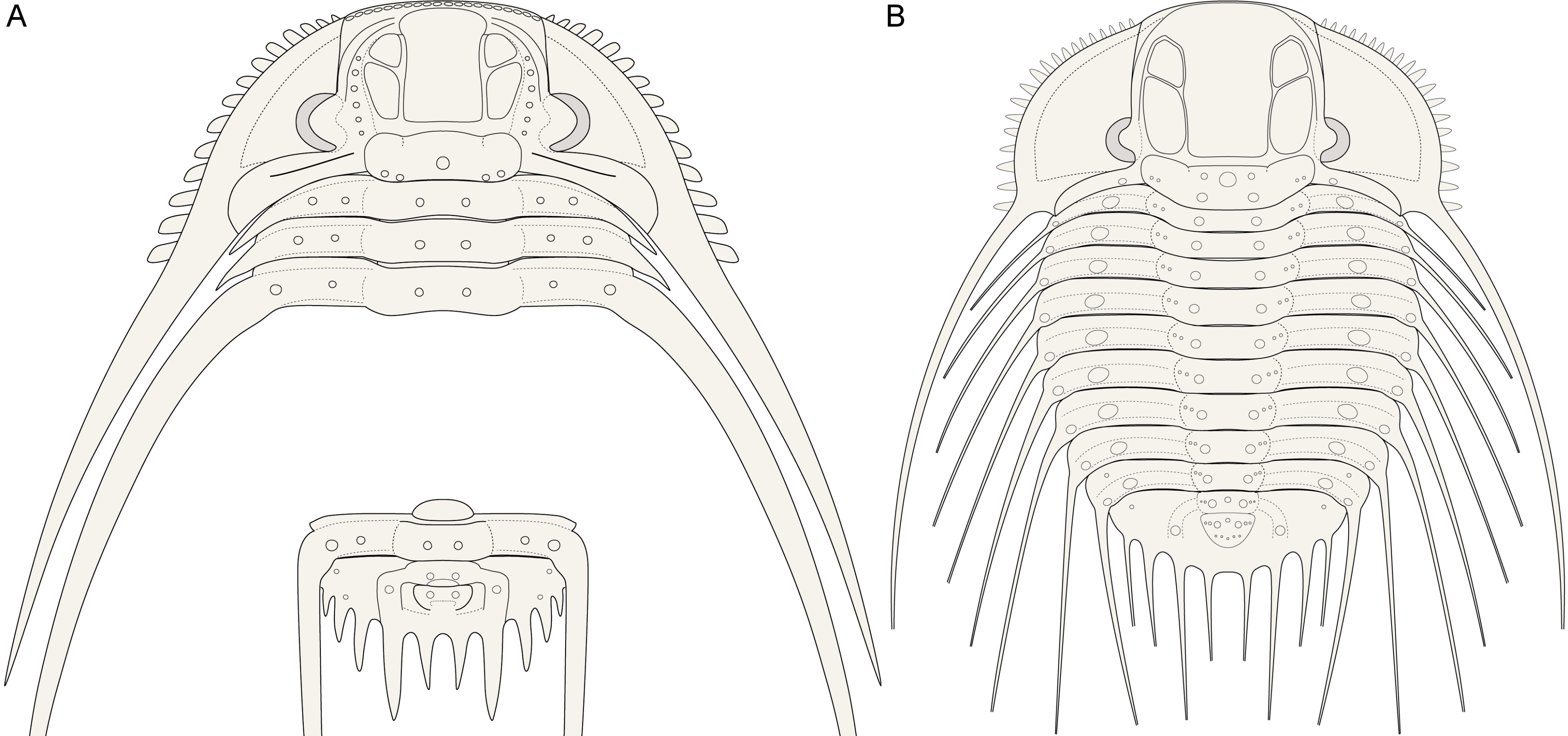
Figure 8. Reconstructions of the cephalon, several thoracic tergites and the pygidium of Kettneraspis (generalised) (A) and the exoskeleton of Bruthansovaspis roemeri (Barrande, 1852) (B).
84Bruthansovaspis roemeri (Barrande, 1852)
85(Figs 8B, 9, 10A, B)
86* 1852 Acidaspis roemeri Barrande, p. 726, pl. 39, figs 29–32.
871949 Acanthaloma (Acanthaloma) roemeri; Prantl & Přibyl, pp. 163, 164, pl. 1, figs 12, 13, pl. 7, figs 7, 8, pl. 10, fig. 10.
881966 Leonaspis (Leonaspis) roemeri; Přibyl & Vaněk, pl. 4, fig. 1 [= paralectotype NM L 36549].
89e.p. 1968 Leonaspis dormitzeri; Bruton, pp. 22, 23 [original syntype ČE 251 in pl. 3, fig. 10].
901987 Leonaspis roemeri; Chlupáč, p. 174.
911990 Leonaspis roemeri; Šnajdr, pp. 248, 249, unnumb. fig.
921991 roemeri; Ramsköld & Chatterton, p. 367 [as a synonym of Kettneraspis dormitzeri].
932017 Kettneraspis? roemeri; Basse & Müller, p. 221, fig. 4f [= plaster cast of lectotype].
94Material. Lectotype complete specimen, NM L 28818 (= ČE 250, IT 742); paralectotype incomplete specimen, NM L 36549 (= IT 744); external mould of an incomplete specimen, CGS JV 504; three specimens on a single rock slab, CGS JV 582. All from the Motol Formation (Wenlock) in Loděnice, Barrandian.
95Discussion. Prantl & Přibyl (1949) selected the specimen in the drawing by Barrande (1852, pl. 39, fig. 29) as the lectotype. Unfortunately, Prantl & Přibyl (1949) did not provide a catalogue number and Barrande’s drawing was idealised and based on multiple specimens. Hence, from their publication it was unclear which of the syntypes Prantl & Přibyl (1949) intended to be the lectotype. Přibyl & Vaněk (1966, pl. 4, fig. 1) illustrated a complete specimen (refigured here as Fig. 9D, E, H, I), declaring that it represents the lectotype carrying catalogue number ČE 250. This number has consistently been used in the literature to identify the lectotype (Horný & Bastl, 1970; Vaněk & Valíček, 2002; Basse & Müller, 2017). However, the trilobite in the photograph of Přibyl & Vaněk (1966, pl. 4, fig. 1) does not bear catalogue number ČE 250. Examination of the original material has revealed that ČE 250 is a different specimen (figured here as Fig. 9A–C, F, G), unambiguously marked on its museum label as being the lectotype; it corresponds to SMF 88196.23 (Senckenberg Museum) figured by Basse & Müller (2017, p. 221, fig. 4f) and identified by them as a plaster cast of the lectotype. Because the information on the specimen label is unambiguous and coherent with the catalogue number used in the literature to identify the lectotype, it has to be assumed that Přibyl & Vaněk (1966) inadvertently illustrated the wrong specimen.
96Bruthansovaspis roemeri was previously regarded as a junior synonym of Bruthansovaspis dormitzeri by Bruton (1968) and Ramsköld & Chatterton (1991). According to Šnajdr (1990), B. roemeri is known only from the Lištice and Loděnice localities, close to the Svatý Jan Volcanic Centre. Chlupáč (1987) reported it as a member of the Aulacopleura konincki assemblage which is characterised by abundant Aulacopleura specimens and markedly diverse odontopleurid component, and restricted to brown-grey tuffaceous shales high in the Motol Formation. The Aulacopleura konincki assemblage occurs lateral to the volcano-carbonate facies that yields the Bumastus-Sphaerexochus-Cheirurus assemblage with B. dormitzeri. The lectotype of B. roemeri is a partially exfoliated exoskeleton with counterpart, showing hallmarks of shale preservation. Some of the differences with B. dormitzeri described on individuals coming from micritic to bioclastic limestones, could be attributed to preservation or intraspecific variation (see, e.g., remarks by Vokáč et al., 2015). Consistent traits of B. dormitzeri that serve to distinguish this species from B. roemeri are the single tubercles abaxially on the thoracic axial rings or lack thereof (as against double tubercles or spines here on all thoracic segments of B. roemeri), more convexly (sag.) rounded pygidium with distally fully deflated pleural ridges, long (sag., exsag.) postaxial area, and exceedingly long border spines. One paralectotype pygidium of B. dormitzeri (Fig. 6K, O) has three lateral spine pairs but it seems that the lectotype has two (Fig. 10F). Bruthansovaspis roemeri may have two or three lateral spine pairs. However, lateral spine pair numbers are generally not very reliable in odontopleurines.
97Prantl & Přibyl (1949) reported large, paired tubercles on the occipital ring and thoracic axial rings of B. roemeri. Most published photos do not show this feature because of extensive damage to the axis of the specimens. The lectotype shows a dark spot centrally on the occipital ring which appears to represent a three-dimensional structure like the paired tubercles on the posterior thoracic axial rings of this same specimen. According to Šnajdr (1990), B. roemeri is characterised by an occipital ring with one pair of large subvertical spines near the posterior margin, a vertical median spine, and several small vertical spines on the posterior part of the ring. The single specimen figured by Šnajdr (1990, p. 249, unnumb. fig.) only shows the broken bases of paired structures posterocentrally on the occipital ring and on some of the thoracic axial rings, and so Šnajdr must have had access to other material revealing their spiny nature. One specimen in the collections of the Czech Geological Survey (Fig. 10B1) clearly shows the bases of a relatively far anteriorly positioned median spine and two pairs of laterally and posterolaterally disposed structures. Judging from the horizontal and vertical sizes of the broken bases, which exceed those of regular tubercles known in many odontopleurines, it seems fair to assume that spines were indeed originally present here. Because I have not observed the subvertical spines and only their damaged bases, this feature was provisionally omitted from the generic diagnosis.

Figure 9. Bruthansovaspis roemeri (Barrande, 1852), Motol Formation, Loděnice, Barrandian. A–C, F, G. Lectotype complete specimen, NM L 28818, in dorsal view (A, C), dorsal view of external mould (B), oblique anterior view (F) and oblique lateral view (G). D, E, H, I. Paralectotype incomplete specimen, NM L 36549, in dorsal view (D, E) and dorsal view of external mould (H, I). Scale bars are 2 mm. Fig. A, B, E, I untreated before photography. All photos courtesy of J. Bruthansová.

Figure 10. Bruthansovaspis roemeri (Barrande, 1852), Motol Formation, Monograptus flexilis Zone, Loděnice (Černidla), Barrandian. A. External mould of an incomplete specimen, CGS JV 504, in dorsal view. B. Three specimens on a single rock slab, CGS JV 582.
C–F. Bruthansovaspis dormitzeri (Hawle & Corda, 1847), Motol Formation, Svatý Jan, Barrandian. Lectotype incomplete specimen, NM L 1567a, in dorsal view (C, D), oblique lateral view (E) and close-up of pygidium and posterior thoracic segments (F).
Scale bars are 2 mm. Fig. D untreated before photography. Fig. 10A, B courtesy of P. Budil; 10C–F courtesy of J. Bruthansová.
98Bruthansovaspis dormitzeri (Hawle & Corda, 1847)
99(Figs 2M, 6G, J, K, L, O, 10C–F)
100* 1847 Odontopleura Dormitzeri Hawle & Corda, p. 150.
1011847 Odontopleura Zenkeri Hawle & Corda, p. 153.
102e.p. 1852 Acidaspis dormitzeri; Barrande, p. 728, pl. 38, figs 22–24.
1031949 Acanthaloma (Acanthaloma) dormitzeri; Prantl & Přibyl, pp. 159, 160, pl. 1, figs 10, 11.
104e.p. 1966 Leonaspis (Leonaspis) dormitzeri; Přibyl & Vaněk, p. 295, pl. 3, fig. 2.
105e.p. 1968 Leonaspis dormitzeri; Bruton, pp. 22, 23 [lectotype in pl. 3, fig. 5].
1061984b Odontopleura Dormitzeri; Šnajdr, p. 152, pl. 13, fig. 5.
1071987 Leonaspis dormitzeri; Chlupáč, pp. 172, 173.
1081991 dormitzeri; Ramsköld & Chatterton, p. 365 [as a species of Kettneraspis].
109Material. Lectotype incomplete specimen NM L 1567a (= ČE 1263); paralectotype pygidium NM L 15678; incomplete specimen NM L 5359 (holotype of Odontopleura zenkeri). All from the Motol Formation (Wenlock) in Svatý Jan, Barrandian.
110Discussion. There is considerable confusion involved with the identity and status of the name-bearing type. Barrande (1852, pl. 38, fig. 22) figured a drawing of a nearly complete specimen while stating that his reconstruction was compiled from material in the collections of Hawle and his own. Prantl & Přibyl (1949) repeated Barrande’s drawing and incorrectly took it as the holotype by monotypy. Bruton (1968, p. 23, pl. 3, fig. 5) figured specimen ČE 1263 (refigured here as Fig. 10C–F) which he referred to as the holotype, probably inadvertently having repeated the error of Prantl & Přibyl (1949). Because multiple syntypes were only elsewhere identified, Prantl & Přibyl’s (1949) designation of the holotype by monotypy does not constitute a valid lectotype selection (article 74.5 of the ICZN code). This problem appears to have been noticed by Přibyl & Vaněk (1966, pl. 3, fig. 2) who figured specimen ČE 1263 while explicitly referring to it as the lectotype in the figure caption. Their statement fulfils the requirements of a valid lectotype designation from before 2000 (article 74 of the ICZN code). The subsequent lectotype designation by Přibyl in Horný & Bastl (1970) involving this same specimen was thus unnecessary and invalid. Šnajdr (1984b) asserted that specimen ČE 1263 was not a syntype of Hawle & Corda, and so the specimen was deemed to lose its status of lectotype (ICZN article 74.2). Šnajdr (1984b) selected a different specimen, pygidium NM L 15678 (refigured here as Fig. 6K, O), as the lectotype which, according to its museum label, was an original syntype of Hawle & Corda. Vaněk & Valíček (2002) reiterated specimen ČE 1263 as the lectotype and considered the designation by Šnajdr (1984b) to be invalid. I was informed by Dr J. Bruthansová that Barrande kept the syntypes of Hawle and Corda separate, and this practice has been continued by the curators to the present day, leaving little doubt about the origins of the specimens. The original label of specimen ČE 1263 bears a “Coll. Hawle” stamp. It must be concluded that the specimen was, indeed, an original syntype of Hawle and Corda, and that the lectotype designation by Přibyl & Vaněk (1966) was valid. Šnajdr’s (1984b) pygidium retains its status as a paralectotype.
111I cannot distinguish between the types of Bruthansovaspis zenkeri comb. nov. (Fig. 6G, J, L) and B. dormitzeri based on the material currently available to me, except that the lectotype of the latter bears putative P1 tubercles or spines just adaxial to P2 on the anterior six thoracic segments and their presence in the holotype of B. zenkeri cannot be validated. Differences with B. roemeri are listed under that species.
112Bruthansovaspis dumortieri (Hawle & Corda, 1847)
113(Fig. 6H, I, N)
114* 1847 Odontopleura Dumortieri Hawle & Corda, p. 154.
115e.p. 1852 Acid. Dormitzeri; Barrande, pp. 728, 729.
116e.p. 1949 Acanthaloma (Acanthaloma) dormitzeri; Prantl & Přibyl, pp. 159, 160.
117e.p. 1968 Leonaspis dormitzeri; Bruton, pp. 22, 23.
1181970 Leonaspis (Leonaspis) dormitzeri; Horný & Bastl, p. 125.
1191984b Odontopleura Dumortieri; Šnajdr, p. 153, pl. 4, fig. 8 [= holotype].
1201991 dumortieri; Ramsköld & Chatterton, p. 365 [as a synonym of Kettneraspis dormitzeri].
121Material. Holotype pygidium NM L 5371, from the Kopanina Formation (Ludlow) in Koledník near Beroun, Barrandian.
122Discussion. Hawle & Corda (1847) recorded this species from the “Kalke von Koledník” which correspond to the Kopanina Formation, and this information is consistent with that of Horný & Bastl (1970, p. 125) and the label of the type. Šnajdr (1984b) provided a new photo of the type while stating that it came from the Liteň Formation in an uncertain locality (probably Svatý Jan, and not Koledník). Since Šnajdr did not provide an argumentation for the adjusted provenance nor change the specimen label, I am assuming this was an inadvertent mistake. Ramsköld & Chatterton (1991) also gave the Liteň Formation as the type horizon while referring to the work of Šnajdr (1984b) for the first published figure of this species, which was probably the source of their erroneous information.
123The type bears a weakly convex furrow posteriorly on the first axial ring, but it is asymmetric (sag.) in joining the inter-ring furrow far abaxially on the right side, and in terminating more adaxially on the left side, never to reach the inter-ring furrow. This pygidium is damaged and deformed, and so better-preserved specimens are needed to assess the doubtful presence of a pseudo-articulating ring. Differences with B. dormitzeri based on the single damaged pygidium are difficult to assess but the latter reveals a prompter flexure of the pleural ridge just anterior to the P2 tubercle or spine here. Considering the very unsatisfactory preservation of the type of B. dumortieri and the distinct stratigraphic origin of B. dormitzeri, I prefer to retain both as independent species, at least provisionally.
Acknowledgements
124Paul Freitag (Rostock) skilfully prepared the Moroccan trilobite specimens and kindly donated these for study. Jana Bruthansová (National Museum, Prague) unwearyingly traced and photographed trilobite specimens in her care, at times helped by Vojtěch Turek and colleagues at the museum; their support was indispensable. Petr Budil (Czech Geological Survey) shared photographs of trilobite specimens. František Hartl (Almere) participated in valuable discussions. Scott Morrison (University of Oregon) helped to find some of the literature used. Martin Basse (Senckenberg Forschungsinstitut und Naturmuseum) and Raimund Feist (Université Montpellier II) reviewed the manuscript and made helpful suggestions for its improvement. I am indebted to these colleagues for their support.
Data availability
125The studied specimens are all housed in official repositories guaranteeing their long-term safekeeping and availability to other researchers for future studies.
References
126Adrain, J.M. & Chatterton, B.D.E., 1990. Odontopleura (Trilobita, Silurian), and a method of constrained congruency analysis. Journal of Paleontology, 64, 600–614. https://doi.org/10.1017/S0022336000042645
127Adrain, J.M. & Pérez-Peris, F., 2023. Funeralaspis n. gen.: a new odontopleurine trilobite from the early Middle Ordovician (Dapingian) of Death Valley, eastern California, USA, and the classification of Ordovician odontopleurines. Zootaxa, 5336, 509–529. https://doi.org/10.11646/zootaxa.5336.4.3
128Adrain, J.M. & Ramsköld, L., 1997. Silurian Odontopleurinae (Trilobita) from the Cape Phillips Formation, Arctic Canada. Journal of Paleontology, 71, 237–261. https://doi.org/10.1017/S0022336000039160
129Alberti, G.K.B., 1967. Neue obersilurische sowie unter- und mitteldevonische Trilobiten aus Marokko, Deutschland und einigen anderen europäischen Gebieten. 2. Senckenbergiana lethaea, 48, 481–409.
130Alberti, G.K.B., 1969. Trilobiten des jüngeren Siluriums sowie des Unter- und Mitteldevons. I. Mit Beiträgen zur Silur-Devon Stratigraphie einiger Gebiete Marokkos und Oberfrankens. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 520, 1–692.
131Alberti, M., 2018. Leonaspis bassei n. sp. (Trilobita, Odontopleuridae) aus dem basalen Rupbach-Schiefer (Ober-Emsium; Rhenoherzynikum). Mainzer geowissenschaftliche Mitteilungen, 46, 7–22.
132Alberti, M., 2021. Der Odontopleurid Rupbachella paedomorpha n. gen., n. sp. aus dem tiefen Rupbach-Schiefer (Trilobita; Ober-Emsium; Rheinisches Schiefergebirge). Mainzer naturwissenschaftliches Archiv, 58, 53–67.
133Alberti, M., 2023. Kleine Raritäten. Juvenile Trilobiten aus dem Rupbach-Schiefer. Fossilien, 2023/4, 53–60.
134Barrande, J., 1846. Notice préliminaire sur le systême silurien et les trilobites de Bohême. C. L. Hirschfeld, Leipzic, 97 p. https://doi.org/10.5962/bhl.title.9142
135Barrande, J., 1852. Systême silurien du centre de la Bohême. 1ère partie : Recherches paléontologiques, 1 : Trilobites. J. Barrande, Prague and Paris, 935 p. https://doi.org/10.5962/bhl.title.14776
136Barrande, J., 1872. Systême silurien du centre de la Bohême, 1ère partie : Recherches paléontologiques. Supplément au Vol. 1 : Trilobites, crustacés divers et poissons. J. Barrande, Prague and Paris, 647 p.
137Basse, M., 2009. Proetoidea Hawle & Corda, 1847 und andere Trilobita aus unterdevonischen Herzynkalken (Zlichovium, Dalejum) der westlichen Harzgeröder Faltenzone (südwestlicher Harz, Rhenoherzynikum): Allgemeiner Teil und Proetinae Hawle & Corda, 1847. Freiberger Forschungshefte, C 532, 1–55.
138Basse, M., 2022. Die Typen der Trilobiten mit deutscher Typuslokalität – Fortschritte in der Forschung von Sommer 2018 bis Dezember 2021. Dortmunder Beiträge zur Landeskunde, Naturwissenschaftliche Mitteilungen, 51, 47–92.
139Basse, M. & Müller, P., 2004. Eifel-Trilobiten III. Corynexochida, Proetida (2), Harpetida, Phacopida (2), Lichida. Quelle & Meyer-Verlag, Wiebelsheim, 260 p.
140Basse, M. & Müller, P., 2016. Trilobiten aus dem Ober-Emsium und frühen Eifelium der südlichen Lahnmulde (Rupbach-Schiefer, Leun-Schiefer und Ballersbach-Kalk). Abhandlungen der Senckenberg Gesellschaft für Naturforschung, 572, 1–329.
141Basse, M. & Müller, P., 2017. Revision einiger Trilobiten aus dem Devon des Lahn-Dill-Gebiets (Grenzbereich Emsium/Eifelium, Rhenoherzynikum). Mainzer geowissenschaftliche Mitteilungen, 45, 203–242
142Basse, M. & Müller, P., 2023. Trilobiten aus dem Leun-Schiefer und Leun-Kalk von Löhnberg und Leun in der zentralen Lahn-Mulde in Hessen (Grenzbereich Unter-/Mitteldevon, Rheinisches Massiv, Varisziden). Mainzer Naturwissenschaftliches Archiv, Beiheft, 37, 1–211.
143Bicknell, R.D.C. & Smith, P.M., 2022. Examining abnormal Silurian trilobites from the Llandovery of Australia. PeerJ, 10, e14308. https://doi.org/10.7717/peerj.14308
144Bruton, D.L., 1967. Silurian odontopleurid trilobites from Sweden, Estonia, and Latvia. Palaeontology, 10, 214–244.
145Bruton, D.L., 1968. A revision of the Odontopleuridae (Trilobita) from the Palaeozoic of Bohemia. Skrifter utgitt av Det Norske Videnskaps-Akademi i Oslo. I. Matematisk-naturvidenskapelig Klasse. Ny Serie, 25, 1–73.
146Burmeister, H., 1843. Die Organisation der Trilobiten aus ihren lebenden Verwandten entwickelt; nebst einer systematischen Übersicht aller seither beschriebenen Arten. Reimer, Berlin, 147 p.
147Chatterton, B.D.E., 1971. Taxonomy and ontogeny of Siluro-Devonian trilobites from near Yass, New South Wales. Palaeontographica (A), 137, 1–108.
148Chatterton, B.D.E. & Perry, D.G., 1979. Acanthalomina Prantl & Přibyl, a valid subgenus of the trilobite genus Diacanthaspis. Journal of Paleontology, 53, 1327–1342.
149Chatterton, B.D.E. & Perry, D.G., 1983. Silicified Silurian odontopleurid trilobites from the Mackenzie Mountains. Palaeontographica Canadiana, 1, 1–127.
150Chatterton, B.D.E. & Speyer, S.E., 1997. Ontogeny. In Kaesler, R.L. (ed.), Treatise on Invertebrate Paleontology, Part O, Arthropoda 1. Trilobita, revised. Volume 1: Introduction, Order Agnostida, Order Redlichiida. University of Kansas Press, Lawrence, Kansas and Geological Society of America, Boulder, Colorado, 173–247.
151Chatterton, B.D.E., Fortey, R.A., Brett, K.D., Gibb, S.L. & McKellar, R.C., 2006. Trilobites from the upper Lower to Middle Devonian Timrhanrhart Formation, Jbel Gara el Zguilma, southern Morocco. Palaeontographica Canadiana, 25, 1–177.
152Chlupáč, I., 1987. Ecostratigraphy of Silurian trilobite assemblages of the Barrandian area, Czechoslovakia. Newsletters on Stratigraphy, 17, 169–186. https://doi.org/10.1127/nos/17/1987/169
153Chlupáč, I., 1993. Geology of the Barrandian. A field trip guide. Verlag Waldemar Kramer, Frankfurt am Main, 163 p.
154Dalman, J.W., 1828. Nya Svenska Palaeder. Årsberättelse om Nyare Zoologiska Arbeten och Upptäcker. Kongliga Svenska Vetenskaps-Akademiens Handlingar, 1828, 121–138.
155Edgecombe, G.D. & Sherwin, L., 2001. Early Silurian (Llandovery) trilobites from the Cotton Formation, near Forbes, New South Wales, Australia. Alcheringa, 25, 87–105. https://doi.org/10.1080/03115510108619215
156Emmrich, H.F., 1839. De trilobitis: dissertatio petrefactologica quam consensu et auctoritate amplissimi philosophorum ordinis in alma litterarum universitate Friderica Guilelma pro summis in philosophia honoribus. Nietackian, Berlin, 56 p.
157Emmrich, H.F., 1844. Zur Naturgeschichte der Trilobiten. Meiningen, 28 p.
158Etheridge, R. & Mitchell, J., 1896. The Silurian trilobites of New South Wales, with references to those of other parts of Australia. Part IV. The Odontopleuridae. Proceedings of the Linnean Society of New South Wales, 21, 694–721. https://doi.org/10.5962/bhl.part.8491
159Feist, R. & Clarkson, E.N.K., 2023. Mid-Silurian odontopleurid trilobites from Roquemaillère, Montagne Noire, Southern France. Bulletin of Geosciences, 98, 247–263. https://doi.org/10.3140/bull.geosci.1885
160Flick, U. & Flick, H., 2021. Eine neue Art der Gattung Leonaspis R. & E. Richter, 1917 (Trilobita) aus dem Ballersbach-Kalk (hohes Unter-tiefes Mitteldevon) von Ballersbach/Lahn-Dill-Gebiet, Rheinisches Schiefergebirge. Philippia, 18, 109–122.
161Franke, C., 2010. Marine Fauna der Wiltz-Schichten (Ober-Emsium, Unter-Devon) der Mulde von Wiltz und der Daleider Mulden-Gruppe (Luxemburg, Deutschland): Teil 1. Ferrantia, 58, 5–62.
162Haas, W., 1968. Trilobiten aus dem Silur und Devon von Bithynien (NW-Türkei). Palaeontographica (A), 130, 60–207.
163Haas, W., 1969. Lower Devonian trilobites from central Nevada and northern Mexico. Journal of Paleontology, 43, 641–659.
164Hall, J., 1859. Containing descriptions and figures of the organic remains of the Lower Helderberg Group and the Oriskany Sandstone. Natural History of New York: Palaeontology, vol. III. Albany, 532 p.
165Hawle, I. & Corda, A.J.C., 1847. Prodrom einer Monographie der böhmischen Trilobiten. Calve, Prague, 176 p.
166Holloway, D.J., 2021. Middle Silurian trilobites from Arkansas and Oklahoma, USA. Orders Lichida and Odontopleurida. Palaeontographica (A), 319, 57–131. https://doi.org/10.1127/pala/2021/0102
167Horný, R. & Bastl, F., 1970. Type specimens of fossils in the National Museum, Prague. Volume 1. Trilobita. National Museum, Prague, 354 p.
168Ivanova, O., Owens, R.M., Kim, I. & Popov, L.E., 2009. Late Silurian trilobites from the Nuratau and Turkestan ranges, Uzbekistan and Tajikistan. Geobios, 42, 715–737. https://doi.org/10.1016/j.geobios.2009.09.001
169Klug, C., 2002. Quantitative stratigraphy and taxonomy of late Emsian and Eifelian ammonoids of the eastern Anti-Atlas (Morocco). Courier Forschungsinstitut Senckenberg, 238, 1–109.
170Lebrun, P., 2018. Fossiles du Maroc. Tome I. Gisements emblématiques du Paléozoïque de l’Anti-Atlas. Les Editions du Piat, Saint-Julien-du-Pinet, 298 p.
171Lütke, F., 1965. Zur Kenntnis herzynischer Trilobiten aus dem Unter- und Mitteldevon des Harzes. Palaeontographica (A), 124, 151–236.
172Maillieux, E., 1933. Terrains, roches et fossiles de la Belgique. Musée royal d’Histoire naturelle de Belgique, Bruxelles, 217 p.
173Perry, D.G. & Chatterton, B.D.E., 1979. Wenlock trilobites and brachiopods from the Mackenzie Mountains, north-western Canada. Palaeontology, 22, 569–607.
174Prantl, F. & Přibyl, A., 1949. Studie o trilobitech nadčeledi Odontopleuracea nov. superfam. Rozpravy státního geologického ústavu ČSR, 12, 1–221. [1–110 in Czech, 111–115 in Russian, 117–221 in English]
175Přibyl, A. & Vaněk, J., 1966. Zur Kenntnis der Odontopleuridae-Trilobiten aus dem böhmischen Altpaläozoikum. Acta Universitatis Carolinae – Geologica, 4, 289–304.
176Ramsköld, L., 1984. Silurian odontopleurid trilobites from Gotland. Palaeontology, 27, 239–264.
177Ramsköld, L., 1991. Patterns and process in the evolution of the Odontopleuridae (Trilobita). The Selenopeltinae and Ceratocephalinae. Transactions of the Royal Society of Edinburgh: Earth Sciences, 82, 143–181. https://doi.org/10.1017/S0263593300007616
178Ramsköld, L. & Chatterton, B.D.E., 1991. Revision and subdivision of the polyphyletic ‘Leonaspis’ (Trilobita). Transactions of the Royal Society of Edinburgh: Earth Sciences, 82, 333–371. https://doi.org/10.1017/S026359330000420X
179Richter, R. & Richter, E., 1917. Über die Einteilung der Familie Acidaspidae und über einige ihrer devonischen Vertreter. Centralblatt für Mineralogie, Geologie und Paläontologie, 1917, 462–472.
180Roemer, F.A., 1843. Die Versteinerungen des Harzgebirges. Hahn, Hannover, 40 p.
181Siveter, D.J., 1989. Silurian trilobites from the Annascaul inlier, Dingle Peninsula, Ireland. Palaeontology, 32, 109–161.
182Šnajdr, M., 1983. New Silurian trilobites from Bohemia. Věstník Ústředního ústavu geologického, 58, 175–178.
183Šnajdr, M., 1984a. Ivanopleura and Borkopleura, new odontopleurid genera from the Bohemian Silurian (Trilobita). Věstník Ústředního ústavu geologického, 59, 49–52.
184Šnajdr, M., 1984b. Revision of the trilobite type material of I. Hawle and A. J. C. Corda, 1847. Acta Musei Nationalis Pragae, Series B, 129–212 [for 1983].
185Šnajdr, M., 1990. Bohemian trilobites. Geological Survey, Prague, 265 p.
186Van Viersen, A.P., 2007. Kettneraspis, Radiaspis and Ceratarges (Trilobita) from the Middle Devonian of the Rochefort area (Ardennes, Belgium). Scripta Geologica, 134, 1–18.
187Van Viersen, A.P., 2013. Latest Early to early Middle Devonian acastid trilobites from the eastern part of the Dinant Synclinorium, Belgium (Rhenohercynian Zone). Memoirs of the Association of Australasian Palaeontologists, 44, 1–10.
188Van Viersen, A.P. & Heising, H., 2015. Description of Kettneraspis? prescheri sp. nov. (Trilobita, Odontopleuridae) from the “couche rouge” (Pragian, Lower Devonian) in Morocco. Geologica Belgica, 18, 15–20.
189Van Viersen, A.P., Taghon, P. & Magrean, B., 2019. Early Middle Devonian trilobites and events in the Nismes – Vireux-Molhain area, southern border of the Dinant Synclinorium (Belgium, northern France). Geologica Belgica, 22, 7–33. https://doi.org/10.20341/gb.2019.001
190Vaněk, J. & Valíček, J., 2002. New index of the genera, subgenera, and species of Barrandian trilobites. Part C–D (Silurian and Devonian). Palaeontologia Bohemiae, 8/1, 1–74.
191Vokáč, V., Hartl, F., Pavlovič, M., Šach, R., Hanák, A. & Grigar, L., 2015. Diverzita trilobitového společenstva s Aulacopleura (A.) konincki (motolské souvrství, homer) z lokality Černidla – Barrandovy jámy u Loděnic (pražská pánev, Česká republika). Erica, Plzeň, 22, 101–140.
192Vokáč, V., Hartl, F., Pavlovič, M. & Krýda, P., 2018. Nové údaje o trilobitových společenstvech středních poloh kopaninského souvrství (ludford, silur) v lomu Kosov u Berouna (pražská pánev, Česká republika). Erica, Plzeň, 25, 69–84.
193Vokáč, V., Hartl, F., Pavlovič, M., Mrázek, T., Bureš, J., Krýda, P., Mottl, M. & Hanák, A., 2021. Někteří noví nebo málo známí silurští (wenlock, ludlow) a spodnodevonští (lochkov) trilobiti v pražské pánvi (Barrandien, Česká republika). Erica, Plzeň, 28, 57–108.
194Whiteley, T.E., Kloc, G.J. & Brett, C.E., 2002. Trilobites of New York. Cornell University Press, Ithaca, 203 p.
195Whittington, H.B., 1941. Silicified Trenton trilobites. Journal of Paleontology, 15, 492–522.
196Whittington, H.B., 1956. Silicified Middle Ordovician trilobites: The Odontopleuridae. Bulletin of the Museum of Comparative Zoology, 114, 155–288.
197Whittington, H.B. & Campbell, K.S.W., 1967. Silicified Silurian trilobites from Maine. Bulletin of the Museum of Comparative Zoology, 135, 447–483.
198Article history
199Received 03.11.2023, accepted in revised form 15.01.2024, available online 15.04.2024.