- Accueil
- Volume 78 (2025)
- The entomofauna of olive groves in Northern Tunisia: Identification, distribution and abundance
Visualisation(s): 579 (0 ULiège)
Téléchargement(s): 142 (0 ULiège)
The entomofauna of olive groves in Northern Tunisia: Identification, distribution and abundance

Document(s) associé(s)
Version PDF originaleRésumé
Un inventaire d’insectes a été réalisé dans 40 oliveraies tunisiennes situées dans six gouvernorats (Béja, Seliana, Jendouba, Nabeul, Bizerte et Zaghouan) au cours de deux années d’étude (2017 et 2019). L’inventaire a révélé la présence de dix-sept espèces d’insectes regroupées en cinq ordres et onze familles. L'étude a montré la présence de trois espèces de coléoptères (Otiorhynchus cribricollis, Phloeotribus scarabaeoides et Hylesinus Oleiperda), de huit hémiptères (Parlatoria oleae, Lepidosaphes ulmi, Aspidiotus nerii, Saissetia oleae, Lichtensia viburni, Pollinia pollini, Macrosiphum sp., Euphyllura olivina), trois espèces de thrips (Liothrips oleae, Frankliniella occidentalis, Thrips tabaci), deux insectes lépidoptères (Palpita vitrealis, Prays oleae) et un insecte diptère (Bactrocera oleae). Au cours de la première année d'étude, nous avons identifié quatre agents bénéfiques appartenant à trois ordres et quatre familles. Par ailleurs, nous avons étudié l'impact du mode d'irrigation (irrigation vs pluie) sur la distribution des insectes ravageurs dans les oliveraies situées dans les gouvernorats de Bizerte, Zaghouan, Beja et Seliana. Nos résultats ont montré qu'Anthocoris nemoralis était l'espèce la plus abondante représentant 67,44% du total des ennemis naturels suivi de Psyllaephagus euphyllurae (18,60%), Chrysoperla carnea (9,30%) et Alloxysta eleaphila (4,65%). Nos résultats ont indiqué que le mode d'irrigation n'avait pas d'impact significatif sur le nombre d'insectes ravageurs identifiés, sauf à Beja, uniquement sur les feuilles des oliviers. Les implications des données obtenues pour le développement et la mise en œuvre d’outils de gestion alternatifs en réponse au changement climatique et à la répartition des ravageurs sont discutées.
Abstract
An inventory of insect species was performed in 40 Tunisian olive groves in six governorates (Beja, Seliana, Jendouba, Nabeul, Bizerte and Zaghouan) during two study years (2017 and 2019). The survey revealed seventeen insect species grouped in five orders and eleven families. The study showed the presence of three coleopteran species (Otiorhynchus cribricollis, Phloeotribus scarabaeoides and Hylesinus Oleiperda), eight hemiptera individuals (Parlatoria oleae, Lepidosaphes ulmi, Aspidiotus nerii, Saissetia oleae, Lichtensia viburni, Pollinia pollini, Macrosiphum sp., Euphyllura olivina), three thrips species (Liothrips oleae, Frankliniella occidentalis, Thrips tabaci), two lepidopteran insects (Palpita vitrealis, Prays oleae), and one dipteran insect (Bactrocera oleae). We identified four beneficial agents from three orders and four families during the first study year. Furthermore, we investigated the impact of the irrigation mode (irrigation vs rainfall) on the distribution of insect pests in olive groves in the governorates of Bizerte, Zaghouan, Beja and Seliana. Our results showed that Anthocoris nemoralis was the most abundant species representing 67.44% of the total natural enemies followed by Psyllaephagus euphyllurae (18.60%), Chrysoperla carnea (9.30%) and Alloxysta eleaphila (4.65%). Our findings indicated that the irrigation mode had no significant impact on the number of identified insect pests, except in Beja, only on the leaves of olive trees. The implications of the obtained data for developing and implementing alternative management tools in response to climate change and pest distribution are discussed.
Reçu le 23 août 2024, accepté le 5 mars 2025.
Cet article est distribué suivant les termes et conditions de la licence CC-BY (http://creativecomons.org/licenses/by/4.0/deed.fr)
INTRODUCTION
1The olive (Olea europaea L.) is a worldwide horticultural crop of great socio-economic importance. It is cultivated over 10 million hectares (Faostat, 2017), more than 98% of which are in the Mediterranean basin (Civantos, 2001; Gonçalves & Andrade, 2012). In Tunisia, olive covered an area estimated to 1.7 million hectares and presented a total plantation of about 66 million trees (Khlif, 2002; Gharbi & Ben Abdallah, 2016; Gharbi, 2021). This crop may be threatened by many insect pests belonging to different orders (e.g., Diptera, Hemiptera, Lepidoptera, Coleoptera…) and families (e.g., Curculionidae, Diaspididae, Psyllidae, Tephritidae, Hyponeumotidae…) that may cause significant yield losses (Herz et al., 2005). Various management strategies including agronomic practices, pesticides sprays and releases of beneficial insects have been deployed to manage olive pests in many countries around the world (Haniotakis, 2005; Petacchi et al. 2024). Many natural enemies are known to be effective biological control agents of several olive pests (Jerraya, 2003). Outbreaks of olive pest populations, such as the case of psyllid species, may be associated with different factors, including weather conditions (Chermiti, 1989). Climate changes may directly impact insect species by affecting their biological and physiological characteristics (Thomson et al., 2010; Eigenbrode et al., 2022). In response to weather anomalies (e.g., changes in temperature values, atmospheric CO2 levels and precipitation patterns), insects may expand their geographic distribution and change their behaviour (Skendžić et al., 2021; Subedi et al., 2023). In fact, some insect species will be able to develop additional generations which may increase the number of newly damaged areas (Skendžić et al., 2021).
2The interaction between the insect pests and natural enemies may also be altered, reducing the success of biological control methods (Skendžić et al., 2021; Subedi et al., 2023). Consequently, severe crop damages may be registered, which require integrating new control methods considering climate changes and insect populations (Masood et al., 2022). Thus, knowledge of the entomofauna associated with olive groves that may differ due to many factors (e.g., climate changes, biological factors…), is crucial to develop effective control methods.From this perspective, the objective of the present study was to update the status of insect pests and their natural enemies in the northern region of Tunisia, which we lack information on. We also studied the impact of the irrigation mode (irrigation vs rainfall) on the abundance of pests in the prospected olive groves.
MATERIALS & METHODS
3Study sites
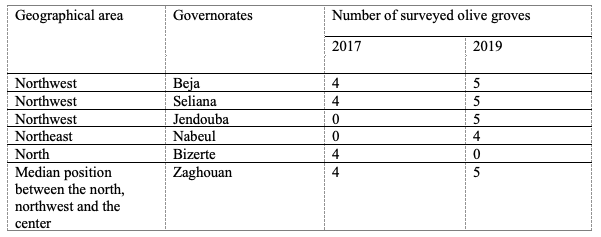
4The study was conducted in six Tunisian governorates from March to May in 2017 and 2019 (Table 1). A total of 40 olive groves were surveyed and cultivated with various varieties (Table 1). The spaces between olive trees were 10×10 m or 4 ×1.5m, respectively, for groves conducted in rainfall or irrigation.
Table 1: Geographic characteristics of the prospected olive groves in the north of Tunisia
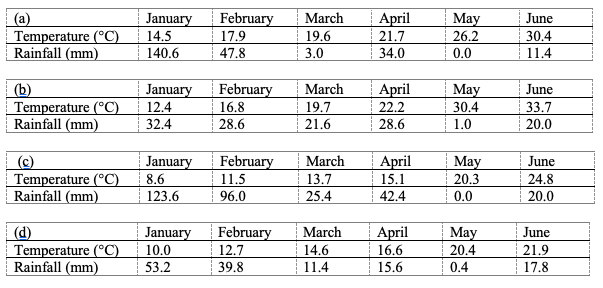
5The climatic conditions in the surveyed olive groves during the first study period are indicated in Table 2 (http://www.accuweather.com/fr/tn/tunisia-weather).
Table 2: Average monthly temperature and rainfall in the prospected governorates (Bizerte (a), Seliana (b), Beja (c) and Zaghouan (d)) during 2017
6Sampling and morphological identification of the specimens collected
7Specimens were collected by randomly harvesting 100 leaves and 10 branches from each olive grove or hitting branches onto a funnel (Fauvel et al., 1981). Parasitized specimens were kept in vials until the parasitoids emerged. Thrips and beneficial insects were preserved in small plastic vials containing 70% alcohol. Thrips were identified using several keys, such as those of Mound & Walker (1982), Mound & Kibby (1998), and Zur Strassen (1996; 2003).
8The keys of Herring (1976), Trjapitzin (1982), and Mazel et al. (2006) were used to identify the collected natural enemies. The abundance of the insect species collected in the first year of the study (2017) was compared in the groves conducted in the rainfall or irrigation mode.
9Statistical analysis
10All data were checked for their homogeneity and normality using Levene and Shapiro–wilk tests, respectively. A generalized linear model (GLM) followed by one-way ANOVA was used to determine the effect of “the irrigation mode and the insect species” or “the region and the insect species” and their interactions on the number of recorded insects on leaves and branches of olive trees. Duncan’s post-hoc test was used for mean separation at P<0.05 (SPSS 2012).
RESULTS
Morphological identification of the specimens collected
11Insect species
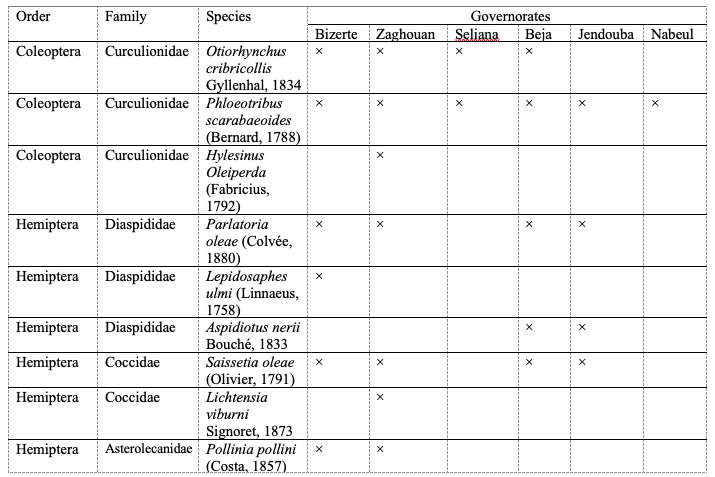
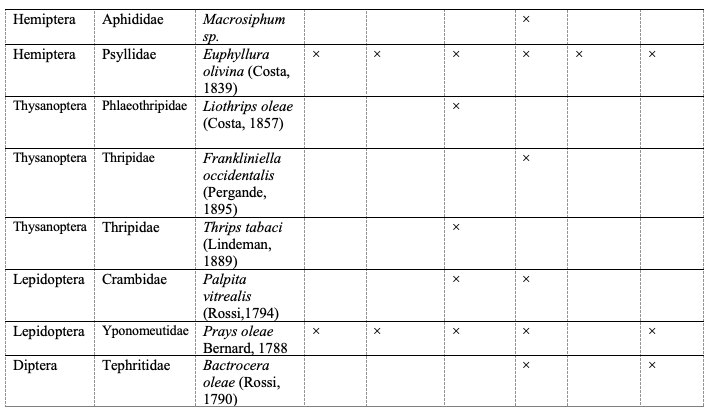
12Seventeen 1insect species belonging to five orders and eleven families were collected and identified from olive groves during 2017 and 2019 in the north of Tunisia (Table 3). The distribution of these species differed from one governorate to another (Table 3). Only the olive dark beetle Phloeotribus scarabaeoides (Bernard, 1788) and the olive psyllid Euphyllura olivina (Costa, 1839) were found in all prospected governorates (Table 3).
Table 3: List of insect species collected from olive
13Natural enemies
14An important number of beneficial insects were recorded in the olive groves surveyed in 2017 (Figure 1). Four species were identified: Chrysoperla carnea (Stephens, 1836) (Neuroptera, Chrysoperla), Anthocoris nemoralis (Fabricius, 1794) (Hemiptera, Anthocoridae), Psyllaephagus euphyllurae (Masi, 1911) (Hymenoptera, Encyrtidae) and Alloxysta eleaphila (Silvestri, 1915) (Hymenoptera, Figitidae) (Figure 1). The highest abundance of beneficial agents was recorded in the governorate of Zaghouan with a total number of parasitoids (Figure 1). Most of them are recognized as natural enemies of E. olivina.
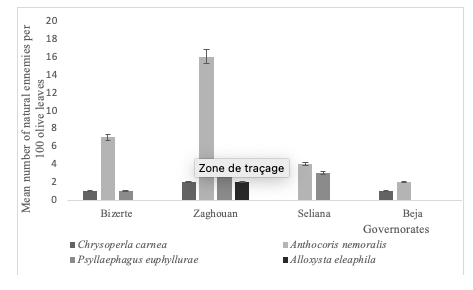
Figure 1: Natural enemies collected from olive groves (Northern Tunisia, 2017)
15Abundance of insect pests in olive groves in 2017
16The abundance of insect pests on the leaves and branches of olive trees differed among the olive groves conducted in irrigation (mode 1) or rainfall (mode 2) during 2017 (Figure 2, 3). On leaves (Figure 2), GLM analysis indicated that the insect species had a significant impact on the number of recorded insects in all governorates ((F1,3= 11.82, P<0.01); (F1,4=31.36, P<0.001); (F1,5=105.73, P<0.001) and (F1,5=13.65, P<0.01) respectively for the governorates of Bizerte, Zaghouan, Beja and Seliana). However, the irrigation mode significantly affected the number of insects only in the governorate of Beja (F1,5= 24.09, P<0.001). The interaction between the two factors “insect species” and “irrigation mode” was significant only in the governorates of Zaghouan (F1,5=9.76; P=0.04) and Beja (F1,5=90.66, P<0.001).
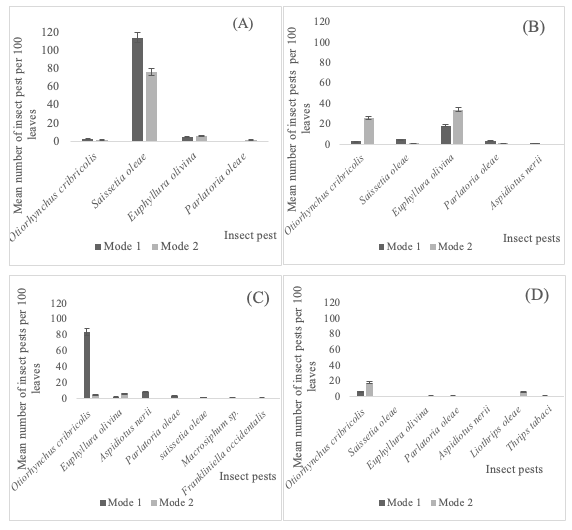
Figure 2: Distribution of insect pest on the leaves of olive trees depending on the irrigation application mode (mode 1= irrigation; mode 2=rainfall) in the governorate of Bizerte (A), Zaghouan (B), Beja (C) and Seliana (D) during 2017
17On branches (Figure 3), GLM analysis indicated that only the insect species (P<0.05), neither the irrigation mode (P>0.05) nor their interactions (P>0.05), had a significant impact on the number of recorded insect pests in all surveyed governorates.
18There is no significant difference between the two irrigation systems concerning the number of found insect species in the surveyed olive groves ((F1,7=0.38, P=0.55); (F1,9=0.07, P=0.79); (F1,9=0.37, P=0.55) and (F1,7=0.13, P=0.72) respectively for the governorates of Bizerte, Zaghouan, Beja and Seliana).
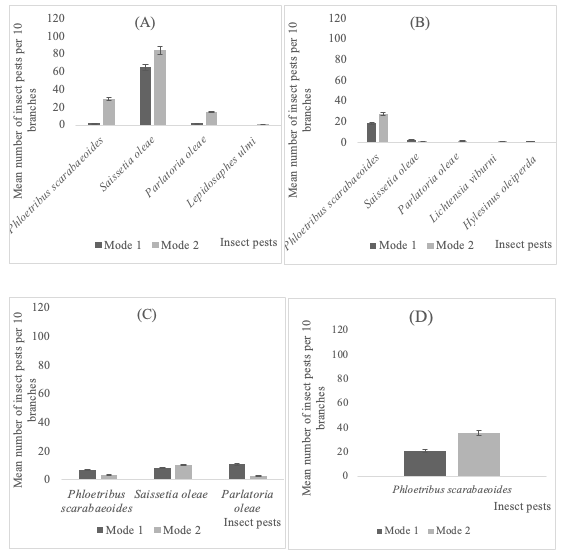
Figure 3: Distribution of insect pest on the branches of olive trees depending on the irrigation application mode (mode 1=irrigation; mode 2=rainfall) in the governorate of Bizerte (A), Zaghouan (B), Beja (C) and Seliana during 2017
19Abundance of insect pests on leaves and branches of olive trees in 2017
20Leaves
21Many Otiorhynchus cribricollis Gyllenhal, 1834, Saissetia oleae (Olivier, 1791) and Euphyllura olivina (Costa, 1839) insects exceeding 50 adults per 100 olive leaves, was recorded respectively in the governorate of Beja, Bizerte and Zaghouan compared to the other insect species (Figure 4).
22Liothrips oleae (Costa, 1857) specimens were found in high numbers only in the governorate of Seliana compared to F. occidentalis (Pergande, 1895) and T. tabaci (Lindeman, 1889) reported in the governorate of Beja and Seliana respectively (Figure 4). GLM analysis indicated that the governorate (F1,3=8.49; P<0.001) had a significant impact on the abundance of the specimens collected compared to the insect species (F1,6=11.18; P=0.08). The interaction between the two tested factors (governorates and insect species) significantly impacted the distribution of the recorded insects.
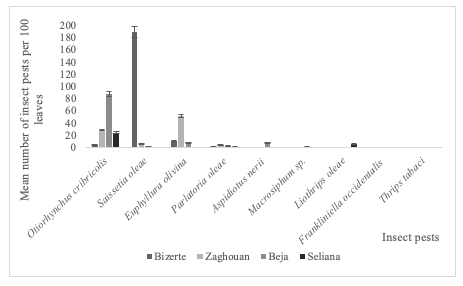
Figure 4: Distribution of insect pest on the leaves of olive trees in four Tunisian governorates during 2017
23Branches
24Phloeotribus scarabaeoides (Bernard, 1788) appeared in all surveyed olive groves compared to the other insect species (Figure 5). Saissetia oleae (Olivier, 1791) was the predominant species in the governorate of Bizerte, representing 87% of the total specimens collected (Figure 5). GLM analysis showed that the governorates (F1,3=29.96; P<0.001), insect species (F1,5= 62.03; P<0.001) and their interaction (F1,4=120.84; P<0.001) had a significant effect on the abundance of the recorded insects in the olive groves of all surveyed governorates.
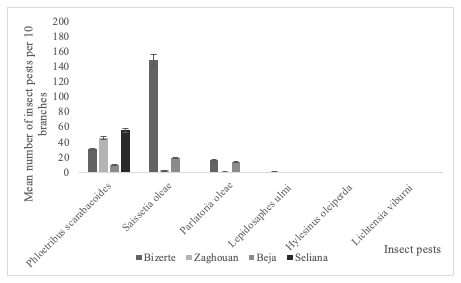
Figure 5: Distribution of insect pest on the branches of olive trees in four Tunisian governorates during 2017
DISCUSSION
25Our results highlighted the occurrence of seventeen insect species collected from olive groves of North Tunisia. Hemiptera was the most presented order with five families and eight species. Three coleopteran and two lepidopteran species were reported herein. Only Liothrips oleae (Costa, 1857) among the three identified thrips species, was known as an olive pest. The olive fly Bactrocera oleae (Rossi, 1790) was the only diptera species in this survey. Our results are almost similar to those obtained in the Mediterranean area. Indeed, in northeastern Algeria, Chafaa et al. (2019) indicated that the entomofauna associated with olives included several species as B. oleae, Parlatoria oleae (Colvée, 1880), Euphyllura olivina (Costa, 1839) and L. oleae.
26Furthermore, in southern Portugal, it has been shown that B. oleae and Prays oleae Bernard, 1788 are the primary insect species of olive groves (Gonçalves & Andrade 2012). These authors highlighted also the occurrence of other insect species including E. olivina, Phloeotribus scarabaeoides (Bernard, 1788), Saissetia oleae (Olivier, 1791) and L. oleae (Gonçalves & Andrade 2012). Our results indicated that the irrigation mode had a significant effect on the number of recorded insect species only in the governorate of Beja compared to the other governorates (Bizerte, Zaghouan and Seliana) on the leaves of olive trees during 2017. We demonstrated also that the distribution of insect species significantly differed among the surveyed governorates. In Tunisia, it has been shown previously that six scale insects including Aspidiotus nerii Bouché, 1833, Lepidosaphes ulmi (Linnaeus,1758), P. oleae and S. oleae, occurred in olive groves (Mansour et al., 2011). According to these authors, S. oleae was the predominant scale insect and P. oleae was the least abundant species in olive groves of northeastern Tunisia.
27Contrary to our results, Mansour et al. (2011) stated that S. oleae was absent in olives in the Northwest region. Concerning E. olivina, Jerraya (2003) indicated that, in Tunisia, this pest occurred mainly in the coastal zone, from the Sahel (governorate of Sousse) to the South (Zarzis region) which is in total contradiction with our results. The occurrence of S. oleae and E. olivina in the northwest of Tunisia may be linked to many factors, including the abiotic factors (temperature, relative humidity, and rainfall), and the widespread distribution and cultivation of olive trees under different climatic areas. According to Mesbah et al. (2020), only temperature and wind speed, contrary to the relative humidity, positively impacted the population density of S. oleae in olive groves in Egypt. Here, L. oleae was reported in high numbers on the leaves of olive trees in the governorate of Seliana. In Italy, L. oleae was considered one of the most redoubtable pests damaging leaves and fruits of wild and cultivated olive (Vono et al., 2020). Our data showed that Frankliniella occidentalis (Pergande, 1895) and Thrips tabaci (Lindeman, 1889) occurred in olive groves confirming results of Agamy et al. (2017) in Egypt. In our study, we demonstrated that Otiorhynchus cribricollis Gyllenhal, 1834 and P. scarabaeoides were found respectively on the leaves and branches of olive trees in all prospected governorates contrary to Hylesinus Oleiperda (Fabricius, 1792) detected only in Zaghouan. These pests were already reported as olive pests in Tunisia (Jerraya, 2003) and widely distributed in the Mediterranean coast and other countries as the case of Algeria (Arambourg, 1984; Menzer, 2016; Deghiche-Diab et al., 2021).
28Regarding the abundance of natural enemies, our findings indicate that two predators (Chrysoperla carnea (Stephens, 1836) and Anthocoris nemoralis (Fabricius, 1794)) and two parasitoids (Psyllaephagus euphyllurae (Masi, 1911) and Alloxysta eleaphila (Silvestri, 1915)) were observed in the prospected olive groves during 2017. In Tunisia, the species A. nemoralis, P. euphyllurae and A. eleaphila are well known as effective biological control agents of the olive psyllid E. olivina (Chermiti et al., 1986; Jerraya, 2003; Ksantini, 2003; Gharbi, 2021). According to Gharbi et al. (2012), A. nemoralis was the most abundant predator of E. olivina in Tunisian olive groves presenting more than 49% of the total number of beneficial insects. This predator showed great potential in decreasing E. olivina population in the spring when releasing 40 nymphs/olive tree in Tunisian olive orchards (Gharbi 2021). Chrysoperla carnea was already found in olive groves of different countries as the case of Spain (Alvareza et al., 2019) and was considered a voracious predator of soft-bodied insects including juveniles of S. oleae (Mahzoum et al., 2020). In southern Spain, Corrales & Campos (2004) demonstrated that C. carnea was the most abundant beneficial with 95% of the total captured natural enemies in the conventional olive grove.
29In conclusion, our research provided knowledge about the entomofauna of olive groves in the north of Tunisia. Our study demonstrated an unequal abundance and variable geographical distribution of insect pests and natural enemies in Tunisian olive groves. This finding may be linked to climate changes that can significantly impact crops and agricultural insects (Skendžić et al., 2021). So, management tools must be adjusted to adapt to climate change for better pest control and less crop losses. Future studies on the entomofauna of olive groves in other important areas in Tunisia should be performed to update the new distribution of insect pests and their natural enemies in response to climate changes.
Acknowledgements
We thank the farmers for their valuable contribution to this work.
Disclosure Statement
The authors declare that there is no conflict of interest.
Bibliographie
Alvareza H.A., Morente M., Oia F.S., Estefania Rodriguez E., Campos M. & Ruano F. 2019. Semi-natural habitat complexity affects abundance and movement of natural enemies in organic olive orchards. Agriculture, Ecosystems and Environment, 285, 106618. DOI: https://doi.org/10.1016/j.agee.2019.106618.
Agamy E.A., El-Husseini M.M., El- Sebaey I.I. & Wafy M.E. 2017. The Egyptian Thripid Species in Olive Groves at Ismaialia, Egypt. Egyptian Academic Journal of Biological Sciences, 10(4), 1- 17.
Arambourg Y. 1984. La fauna entomológica del olivo. Olivae, 4, 14–21.
Chafaa S., Mimeche F. & Haroun Chenchouni H. 2019. Diversity of insects associated with olive (Oleaceae) groves across a dryland climate gradient in Algeria. Canadian Entomologist, 00, 1–19. DOI: https://doi.org/10.4039/tce.2019.35.
Chermiti B., Hawlitzky N., Boulay C. & Onillon J.C. 1986. Quelques caractéristiques du développement de l’endoparasite Psyllaephagus euphyllurae [Hymenoptère : Encrytidae] et exploitation de son hôte Euphyllura olivina [Homoptère : Psylliade]. Entomophaga, 31(4), 351-361.
Chermiti B. 1989. Dynamique des populations du psylle de l’olivier Euphyllura olivina, en conditions méditerranéennes. PhD thesis, Université Aix Marseille, France, 165 pp.
Civantos L. 2001. La olivicultura en el mundo y en Espaňa. In Barranco D. & Fernández-Escobary L.R. (eds.), Revisada y ampliada. 17-36. Junta de Andalucía y Ediciones Mundiprensa, Madrid.
Corrales N. & Campos M., 2004. Populations, longevity, mortality and fecundity of Chrysoperla carnea (Neuroptera, Chrysopidae) from olive-orchards with different agricultural management systems. Chemosphere, 57 (11), 1613-1619.
DOI : https://doi.org/10.1016/j.chemosphere.2004.09.019
Deghiche-Diab N., Deghiche L. & Belhamra Y.I., 2021. New record of Phloeotribus scarabaeoides (Bernard, 1788) on introduced olive trees in Biskra region–Algeria. Munis Entomology & Zoology, 16 (2), 1093-1102.
Eigenbrode S.D., Adhikari S., Kistner-Thomas E. & Neven L. 2022. Introduction to the Collection: Climate Change, Insect Pests, and Beneficial Arthropods in Production Systems. Journal of Economic Entomology, 115(5), 1–5. DOI: https://doi.org/10.1093/jee/toac107.
Faostat. 2017. Statistical Data. Food and Agriculture Organization of the United Nations. Rome, Italy, http://www.fao.org/faostat/en/#data, (19/08/2024).
Fauvel G., Rambier A. & Balduque-Martin R. 1981. La technique du battage pour la surveillance des ravageurs en cultures fruitière et florale. I. – Comparaison des résultats obtenus en verger de pommiers avec des entonnoirs rigides de taille moyenne et avec des entonnoirs en toile. Étude de l’influence de quelques facteurs sur l’efficacité du battage. Agronomie, 1(2), 105-111.
Gharbi N., Debo A. & Ksantini, M. 2012. Observation of arthropod populations during outbreak of olive psyllid Euphyllura olivina in tunisian olive groves. Tunisian Journal of Plant Protection, 7, 35–42.
Gharbi N. & Ben Abdallah S. 2016. Effectiveness of kaolin treatment for the control of the olive fruit fly Bactrocera oleae in Tunisian olive groves. Tunisian Journal of Plant Protection, 11, 73-81.
Gharbi N. 2021. Effectiveness of inundative releases of Anthocoris nemoralis (Hemiptera: Anthocoridae) in controlling the olive psyllid Euphyllura olivina (Hemiptera: Psyllidae). European Journal of Entomology, 118, 135–141. DOI: https://doi.org/10.14411/eje.2021.014.
Gonçalves M.A. & Andrade L. 2012. Entomofauna associated with the olive tree in southern Portugal. IOBC-WPRS Bulletin, 79, 91-99.
Haniotakis, G.E., 2005. Olive Pest Control: Present Status and Prospects. In Proceedings of the IOBC/WPRS Conference on Integrated Protection of Olive Crops, 29-31 May 2003, Chania, Greece, 178pp.
Herring J.L. 1976. Keys to the genera of Anthocoridae of America north of Mexico, with description of a new genus (Hemiptera: Heteroptera). Florida Entomologist, 59, 143–150
Herz A., Hassan S.A., Hegazi E., Nasr F.N., Youssef A.A., Khafagi W.E., Agamy E., Ksantini M., Jardak T., Mazomenos B.E., Konstantopoulou M.A., Torres L., Gonçalves F., Bento A. & Pereira J.A. 2005. Towards sustainable control of Lepidopterous pests in olive cultivation. Gesunde Pflanzen, 57, 117–128. DOI: https://doi.org/10.1007/s10343-005-0076-9.
Jerraya A. 2003. Principaux Nuisibles des Plantes Cultivées et des Denrées Stockées en Afrique du Nord : Leur Biologie, leurs Ennemis Naturels, leurs Dégâts, leur Contrôle. Climat Publications, Tunis, 415 pp.
Khlif M., Ayadi M., Grati-Kammoun N., Arous M.N., Rekik H., Hamdi M.T. & Rekik-Fakhfakh, B. 2002. Identifying chemlali olive variety in its traditional area. Acta Horticulturae, 586, 117-120.
Ksantini M. 2003. Contribution à l’Etude de la Dynamique des Populations du Psylle de l’Olivier Euphyllura olivina (Costa) (Homoptera - Sternorhyncha - Aphalaridae) et de sa Nuisibilité dans la Région de Sfax. PhD thesis, Faculté des Sciences, Tunisie, 344 pp.
Mahzoum A.M., Villa M., Benhadi-Marín J. & Pereira. J.A. 2020. Functional Response of Chrysoperla carnea (Neuroptera: Chrysopidae) Larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for Biological Control. Agronomy, 10(10), 1511. https://doi.org/10.3390/agronomy10101511.
Masood N., Akram R., Fatima M., Mubeen M., Hussain S., Shakeel M., Khan N., Adnan M., Wahid A., Shah A.N., Ihsan M.Z., Rasool A., Ullah K., Awais M., Abbas M., Hussain D., Shahzad K., Bibi F., Ahmad I., Khan I., Hussain K. & Nasim W. 2022. Insect Pest Management Under Climate Change. In Jatoi W.N., Mubeen M., Ahmad A., Cheema M.A., Lin Z., Hashmi M.Z. (eds.), Building Climate Resilience in Agriculture, 225-237. Springer, Cham, Switzerland. DOI: https://doi.org/10.1007/978-3-030-79408-8_15.
Mansour R., Mkaour R., Grissa Lebdi K., Suma P. & Russo A. 2011. A survey of scale insects (Hemiptera: Coccoidea) occurring on olives in Tunisia. Journal of Entomological and Acarological Research, Ser. II 43(3), 315-322.
Mazel R., Canard M. & Thierry D. 2006. Clé synoptique des Chrysopidae de France (Neuroptera). R.A.R.E. T., XV (1), 29-45.
Menzer N. 2016. Entomofaune de l’olivier dans quelques régions d’Algérie : Etude des principaux ravageurs. Ph.D. thesis, National Higher Agronomic School El–Harrach, Algeria, 79pp.
Mesbah H.A., Moursi K.S., EL-Kady M.B., Abdel-Megeed A.A.M. & Ghaith A. 2020. Population Dynamic of Olive Black Scale, Saissetia oleae (Olivier) (Hemiptera: Coccidae) Infesting Olive Trees in Irrigated Farms in Matrouh Governorate, Egypt. Journal of the Advances in Agricultural Researches, 25(1), 38-47.
Mound L.A. & Walker, A.K. 1982. Fauna of New Zealand, No. 1. Terebrantia (Insecta: Thysanoptera). Science Information Division, DSIR Wellington, New Zealand, 113 pp.
Mound L.A. & Kibby G. 1998. Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp.
Petacchi, R., Picchi, M., Canale, A., Benelli, G., Zeni, V., Sacchetti, P., Belcari, A. 2024. Pest Management in Olive Orchards. In Fabbri A., Baldoni L., Caruso T. (eds.), The Olive: Botany and Production, 529-564. London, CAB International. DOI: https://doi.org/10.1079/9781789247350.0007.22024
Skendžić S., Zovko M., Živković I.P., Lešić V. & Lemić D. 2021. The Impact of Climate Change on Agricultural Insect Pests. Insects, 12, 440. DOI: https://doi.org/10.3390/insects12050440.
SPSS. 2012. SPSS 21.0 brief guide. SPSS Incorporation.
Subedi B., Poudel A. & Aryal S. 2023. The impact of climate change on insect pest biology and ecology: Implications for pest management strategies, crop production, and food security. Journal of Agriculture and Food Research, 14, 100733. DOI: https://doi.org/10.1016/j.jafr.2023.100733.
Thomson L.J., Macfadyen S. & Hoffmann A.A. 2010. Predicting the effects of climate change on natural enemies of agricultural pests. Biological Control, 52(3), 296–306. DOI: https://doi.org/10.1016/j.biocontrol.2009.01.022.
Trjapitzin, V.A., 1982. A key for identification of superfamilies, families and some subfamilies of parasitic Hymenoptera. In Trjapitzin V.A., Shapiro V.A., Scheptilnikova V.A. (eds.), Parasites and Predators of Agricultural Crop Pests, 237-254. Lemingrad, Kolos.
Vono G., Bonsignore C.P., Gullo G. & Marullo R., 2020. Olive Production Threatened by a Resurgent Pest Liothrips oleae (Costa, 1857) (Thysanoptera: Phlaeothripidae) in Southern Italy. Insects, 11(12), 887. DOI: https://doi.org/10.3390/insects11120887
Zur Strassen, R., 1996. Neue daten zur Systematik und Verbreitung einiger west-paläarktischer Terebrantia-Arten (Thysanoptera). Entomologische Nachrichten und Berichte, 40, 111-118.
Zur Strassen R. 2003. Die Terebranten Thysanoptera Europas und des Mittelmeer-Gebeties. Die Tierwelt Deutschlands, 74, 1-277.
(39 Réf.)
Notes
1 The full scientific names of all studied species are shown by Table 3