- Accueil
- Volume 28 (2025)
- number 3-4
- Two cases of sclerobionts including foraminifers on Lower Devonian asteropygine trilobites from Germany and Luxembourg
Visualisation(s): 1264 (19 ULiège)
Téléchargement(s): 462 (6 ULiège)
Two cases of sclerobionts including foraminifers on Lower Devonian asteropygine trilobites from Germany and Luxembourg

Abstract
Two Devonian asteropygine trilobite specimens exhibiting rare cases of encrustation are recorded. An almost fully articulated exoskeleton of Rhenops australocustos from the lower Emsian in Luxembourg carries hederelloid colonies on its cephalon and thorax. The colonies are interpreted to have attached syn vivo (i.e. to the living trilobite) and the relation with their host was likely commensal. A cephalon of Philonyx philonyx from the upper Emsian in Germany exhibits five putative morphotype encrusters identified as hederelloids, auloporid corals, cornulitids, bryozoans and foraminifers. This is the first documented evidence of a foraminifer on a trilobite host. This cephalon is either a moult or a carcass.
Table des matières
1. Introduction
1Associations of fossil sclerobionts and biotic hosts present unique windows into the past, offering valuable clues about ecology, community dynamics, environmental conditions and life habits. Sclerobiont-host relationships have been studied extensively in miscellaneous host groups including trilobites, brachiopods, cephalopods, crinoids, decapods, gastropods and bivalves. In many cases it is difficult to assess whether the host was still alive at the time of encrustation. Trilobites were almost ubiquitous in marine environments of the Palaeozoic, having adopted a wide variety of life habits, ranging from pelagic to endobenthic. The mineralised trilobite exoskeleton encompassed an astonishing range of morphologic forms some of which may have been suitable, if not adapted at times, for epizoans to grow on. This notion seems to stand in sheer contrast with the comparatively few published examples of encrusted trilobite specimens (e.g. Solle, 1968; Tetreault, 1992; Kloc, 1992, 1993, 1997; Kácha & Šarič, 1995, 2009; Brandt, 1996; Müller, 1997; Basse, 1998; Key et al., 2000, 2010; Vinn et al., 2017; Basse & Müller, 2004, 2016; Alberti, 2014, 2018; Zapalski & Klug, 2018; Vinn et al., 2024a). Since trilobites are extinct there are no close living analogies to assess their frequency and behaviour as hosts, although certain comparisons might be made with other marine arthropod groups. For instance, Waugh et al. (2004) noticed that epizoans are more common on living marine decapod crustaceans than on fossils, which they attributed to a preservation bias. Other possible causes for the loss of epizoic growth on trilobites include the opportunistic nature of the epizoan’s settlement and the life habit of the host (Brandt, 1996). Indeed, antifouling behaviours such as burrowing, grooming, frequent ecdysis and a nocturnal mode of life have been shown to be effective in lowering epibiont occurrences in extant brachyurans (e.g. Becker & Wahl, 1996; Bauer, 2013; Key et al., 2024).
2Here we present two examples of encrustation in asteropygine trilobites from the Lower Devonian (Emsian) of Germany and Luxembourg.
2. Material and methods
3The first specimen is an exoskeleton of Rhenops australocustos Basse et al., 2006, which was collected from a pelitic sequence of earliest(?) Emsian (Early Devonian) age in the Réideschbaach locality near Heiderscheid, Éislek, northern Luxembourg (van Viersen & Müller, 2024). A silicone cast was made from the external mould. The specimen is kept by the Musée national d’histoire naturelle, Luxembourg, under registration number EIA 750.
4The second specimen is the holotype (silicone cast of the external mould) of Philonyx philonyx (Richter & Richter, 1952), Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt am Main; old number SMF X 1282a, new number SMF 58393a (Basse, 2006, p. 276, fig. 495). The specimen is from the Emsian Kahleberg-Group of Festenburg near Oberschulenberg, TK 25 sheet 4128 Clausthal-Zellerfeld, Ober-Harz, Germany.
5Both silicone casts were whitened with magnesium oxide prior to photography.
3. Encrustation along the exoskeletal fringe
6Previously, epizoic growth on asteropygine trilobites had been reported on the long pleural spines of Psychopyge from the Emsian of the Lahn Syncline in Germany (e.g. Basse & Müller, 2016), similar to cases in spiny odontopleurids (e.g. Kloc, 1997; Basse & Müller, 2004). Alberti (2018) recorded a Psychopyge cephalon from the same area with the long anterior ledge covered by various encrusters. Spines on marine organisms have been attributed various functions ranging from sensory devices, structural deterrents against predators, aids for floating or stabilisation on the substrate, attachment points for epibionts as a means of camouflage, and as an energetically inexpensive way of entering size refuge at an early development stage (e.g. Johnsen et al., 2013). Van Viersen & Kloc (2022) considered exceedingly spiny asteropygines such as Psychopyge to be obligate bottom dwellers with limited capabilities of coaptation and thus, not heavily reliant on pleural spines for their defence against predators. According to Wahl (1997), epibiotic cover inducing a change of contour, shape or colour of its host can prevent detection from optically searching predators. Feifarek (1987) studied contemporary spiny bivalves and concluded that rather than having a defensive function, their spines evolved to attract fouling organisms that aid in concealing the host. Perhaps epizoic growth on the pleural spines along the exoskeletal fringe and the anterior cephalic ledge was beneficial to members of Psychopyge in effectively masking their characteristic appearances as to avoid recognition as potential prey. Although the relatively scanty encrusters in the aforementioned examples may not have sufficed to achieve this, other (soft bodied) epizoans may have been attached to the trilobite yet not preserved. For instance, the cephalon of Philonyx discussed below shows various traces of epizoic growth, some of which have deteriorated almost beyond recognition.
4. The Rhenops case
4.1. Description of the host specimen
7A single fouled exoskeleton among dozens of Rhenops australocustos specimens collected at the Réideschbaach locality is available for study (Fig. 1A, B). This individual shows damage to the left pleural lobe and the pygidium is slightly dislocated. The hypostome was preserved in situ in the counterpart specimen but unfortunately it was lost during preparation. The silicone cast reveals many fine details of the dorsal cuticle, including a range of fine to coarser sculpture, pitted areas on the axial and pleural lobes of thorax and pygidium, and the presence of a narrow band enclosed by a dorsally and ventrally disposed granule rows along the horizontal fringe of the exoskeleton. This band was previously documented by van Viersen & Kloc (2022) in an exceptionally well-preserved specimen of the Devonian asteropygine Hollardops and shown to be densely pitted. The pits were construed by van Viersen & Kloc as inset points for setae as part of the sensory apparatus. The cephalon and anterior thoracic segments of the Rhenops specimen are slightly tectonically deformed (i.e. skewed along the sagittal line) and the thorny tips on the extremities of the genal spines shown by other specimens of this species (see, e.g., van Viersen & Müller, 2024, pl. 1, fig. 4) are lost. The thoracic pleural spines are also incompletely preserved. The anterior three to four pleural extremities reveal a truncated morphology giving way to the large genal spines; the remaining pleurae are damaged. Species of Rhenops from the Rhenish Massif (see, e.g., Basse, 2003) generally adhere to the fan-like pleural spine design discussed by van Viersen & Kloc (2022) and it is conservative to assume that this also applies to R. australocustos. In that case the posterior thoracic pleurae almost certainly carried consecutively longer spines posteriorly, with the last thoracic pleural spines being about as long as the anteriormost pygidial pleural spines. Metamerically repeated median thorns are located on the occipital ring, all thoracic axial rings and the first seven or eight pygidial axial rings. Pits are scarce on the posterior half of the pygidium which is probably a preservation artefact since sculpture is equally missing here.
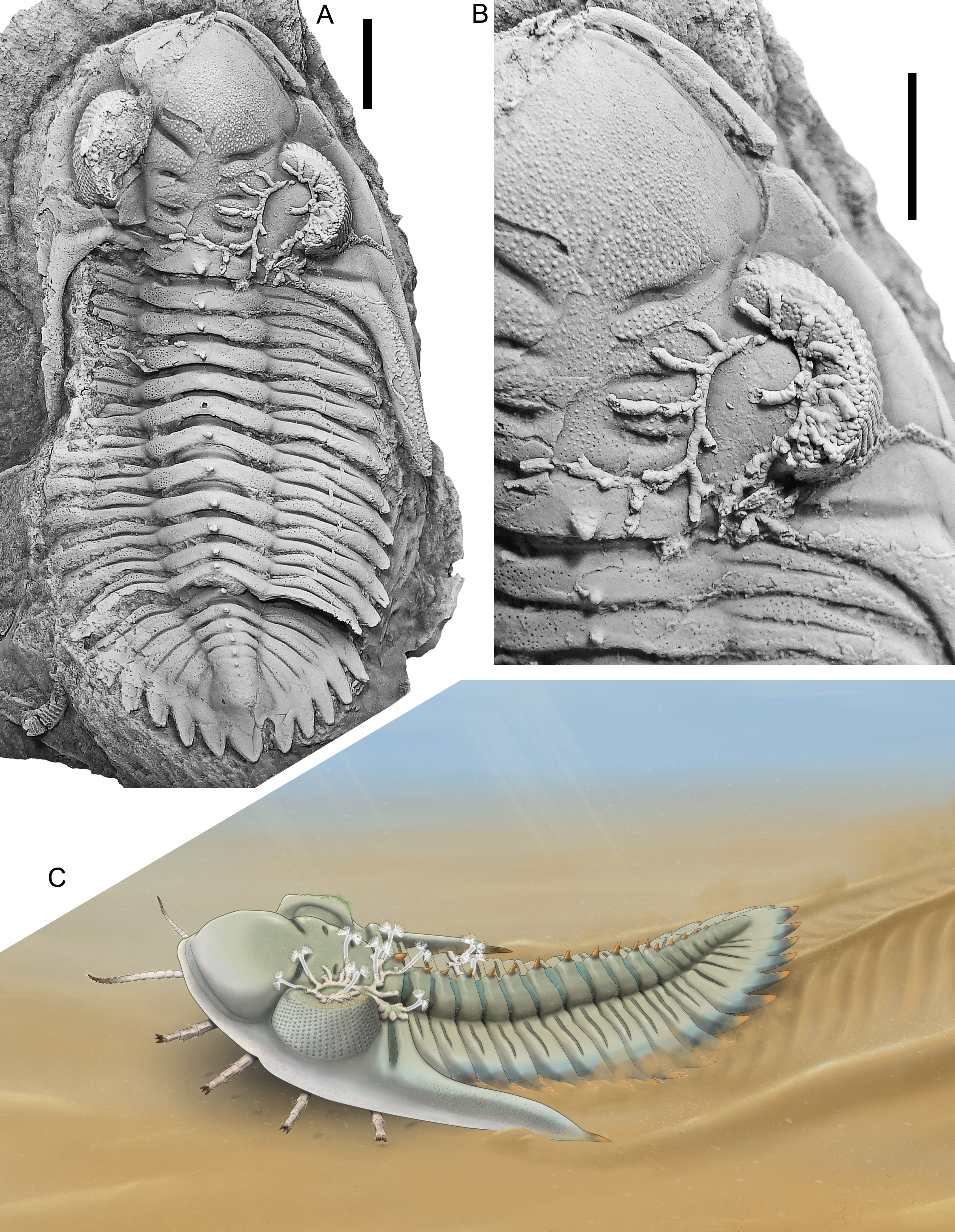
Figure 1. Rhenops australocustos Basse et al., 2006, silicone cast of EIA 750; Lower Devonian, lower Emsian of Réideschbaach, Éislek, Luxembourg; cephalon and thorax carrying hederelloids. A. Dorsal view of exoskeleton. B. Close-up of cephalon and anterior two thoracic segments. C. Reconstruction of studied individual carrying hederelloids. All scale bars equal 10 mm.
4.2. Taxonomic placement of the sclerobiont
8The sclerobiont gross morphology is similar to auloporid tabulate corals, cyclostome bryozoans, and hederelloids. The latter is a predominantly Devonian group of runner-like colonial encrusters that were previously regarded as cyclostome bryozoans (Bassler, 1939), but this view is no longer supported by more recent analyses of morphological details (Alvarez & Taylor, 1987; Taylor & Wilson, 2003). Instead, Taylor & Wilson (2008) provided arguments to associate the group with phoronid worms. According to Alvarez & Taylor (1987), hederelloid colonies have long, tubular zooecia, usually somewhat vermiform, ornamented by faint transverse annulations. The zooecia bud from the sides of a stolon ranging from 0.35 mm in width in the smallest colonies to 0.59 mm in the largest.
4.3. Palaeoecology of the sclerobiont-host association
9Hederelloids are known from the Lower Devonian of the Rhenish Massif, but are not abundant here (e.g. Solle, 1952, 1968; Brassel, 1977). They have been found to preferentially colonise the inner shells of dead brachiopods, bivalves, orthoconic nautiloids, and were also reported from the rostral plate and pygidium of homalonotid trilobites (Solle, 1968; Müller, 1997). In contrast, Stilkerich et al. (2017) reported in vivo encrustation by hederelloids of the ammonoid Ivoites from the Hunsrück Slate that evidently influenced the growth pattern of the host.
10Generally, hederelloids are found less commonly on living mobile substrata than on dead biological substrata. In analogy to palaeoecologically comparable bryozoans, this is particularly so when it comes to ephemeral substrata where the host regularly sheds its integument (Key et al., 1996a). Establishing breeding colonies on those moulting hosts indicates short life cycles, high growth rates, and early reproduction for sessile epizoan organisms (Abelló et al., 1990). However, if these requirements are successfully met, there are many benefits for epizoans on mobile substrata, such as reduced competition for a suitable substrate, reduced risk of predation, enhanced gene dispersal, and ample food supply (Key et al., 1996a). Therefore, extant arthropods, such as brachyuran crabs, horseshoe crabs, and pycnogonids, are regular hosts of epizoic bryozoans and similar organisms (e.g. Key et al., 1996b, 2000, 2010, 2024).
11Alvarez & Taylor (1987) observed auloporid corals growing over the commissure from the dorsal to the ventral valve of a brachiopod and interpreted this as evidence for colony growth continuing after the death of the host. This is also a possibility with respect to the Rhenops specimen. Van Viersen & Müller (2024) gave reasons to suggest that the hederelloid colony grew here in the course of the trilobite’s life. The facial sutures of asteropygines were functional and played a key role in ecdysis. The observations that the hypostome and librigenae are in place indicate that the studied specimen represents a deceased animal (e.g. Whittington, 1997). In that case, the colony may have settled on the living trilobite or, less likely, it settled postmortem, on the carcass. On the other hand, the slightly dislocated and rotated pygidium, might be taken as evidence to suggest that the specimen is, in fact, an exuvia. In that case, the colony may have grown on the living trilobite and was disposed of along with the moult, or settlement and growth fully took place on the moult (i.e. after ecdysis). This last scenario is not credible because the moult would have been prone to disarticulation faster than the colony could grow to its current extent. This might have taken up to several weeks, although it is a rough estimate based on growth rates in bryozoans of overall similarity (van Viersen & Müller, 2024).
12Considering the scenario that the colony attached to the living host, we do not believe that there was a trophic exchange between the two because the central area of the dorsal exoskeleton is too far away from the mouth and appendages to be able to interact with the hederelloids. Rather the relationship was commensal. The location of the large hederelloid on the cephalon was haphazard yet favourable, as it encompasses a large, stable, dorsally high surface on the trilobite, affording optimal prospect of gathering nutrients. The epizoic presence was not lethal and questionably beneficial to its host other than that it may have afforded some sort of camouflage. Van Viersen & Kloc (2022) elaborated on the feeding habits of Hollardops, suggesting that it used its shovel-like cephalon to plough through the top layer of the substrate which was guided and disposed of laterally along the cephalic border and the steep front of the genal spine, and so exposing the appendages to fresh sediment. Taking into account the morphologically very similar cephala of both taxa, it is likely that Rhenops had the same feeding habits. The epizoic growth was sustainable in that a firm base was established in the deep axial, palpebral, occipital and posterior border furrows, while remaining at a distance from the regions of the exoskeleton that would have been involved in ploughing activities (van Viersen & Müller, 2024). A reconstruction of the R. australocustos specimen is proposed here, assuming close analogies of hederelloids with extant phoronid worms (Fig. 1C).
5. The Philonyx case
5.1. Description of the host specimen
13The asteropygine trilobite Philonyx was erected by Richter & Richter (1952) as a monotypic subgenus tentatively placed in Asteropyge. To date, the type species, Philonyx philonyx from the upper Emsian in the Harz Mountains, Germany, is only known from the holotype incomplete cephalon (Figs 2, 3). Philonyx as a taxon of independent generic rank has been questioned recurrently in view of its inadequately documented morphology. Van Viersen (2025) argued that Philonyx be provisionally retained as an uncertain subgenus of Comura. Well-preserved specimens of morphologically similar species placed in Quadrops and Comura have been recorded from coeval strata in southern Morocco (e.g. Lebrun, 2018). Those specimens afford insights into the potential thoracic and pygidial morphologic ranges of Philonyx, which may have included both long pleural and dorsal spines.
14The cephalon is crushed resulting in the opening of the preocular facial sutures, fractures in the cephalic border and exaggerated overhang of the librigenal fields. There are a small convex anterior ledge and a possible pair of exsagittally positioned broad spines. The anterior glabellar and lateral L2 lobes bear large pustules of varying sizes and shapes. The librigenae are in place, suggesting that theoretically, the specimen is a carcass, but the genal angles and posterior borders are almost fully lacking. Perhaps this damage was caused by natural mechanical wearing (transportation?) or durophagous predation activity. The symmetry of the damage on both ends of the cephalon, however, is conspicuous and could nevertheless indicate a moult. Only the bases of the palpebral spines are preserved; these spines were probably moderately long when comparing the Moroccan taxa. The stout occipital spine is broken off but almost fully present in the internal mould (see Basse, 2003, figs 496, 497).
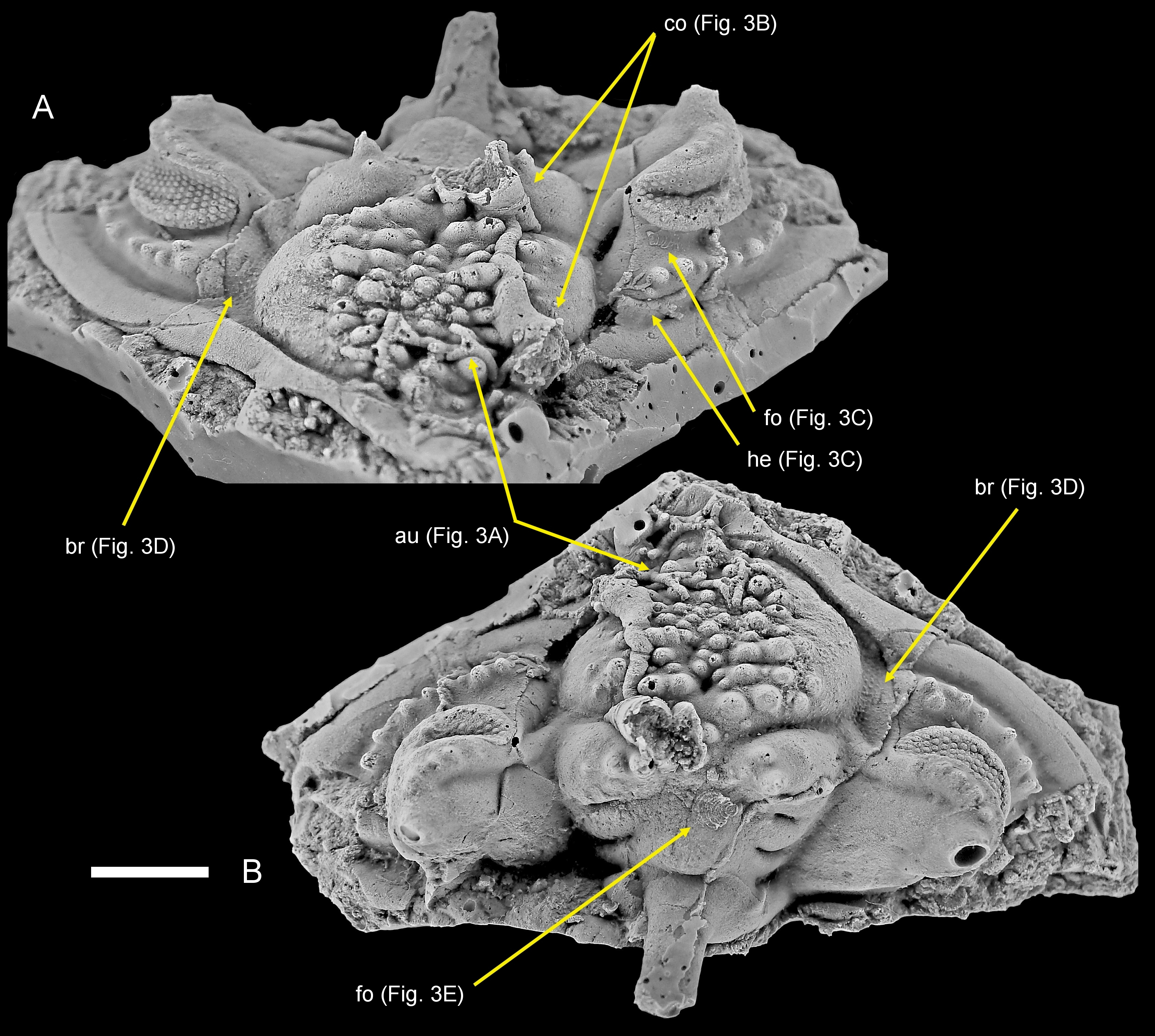
Figure 2. Philonyx philonyx (Richter & Richter, 1952), silicone cast of SMF 58393a; Lower Devonian, upper Emsian of Festenburg near Oberschulenberg, Harz Mountains, Germany; cephalon with various sclerobionts. A. Oblique anterior view. B. Dorsal view. Abbreviations: au, auloporid; br, bryozoan; co, cornulitid; fo, foraminifer; he, hederelloids. Photographs courtesy of M. Basse. Scale bar equals 10 mm.
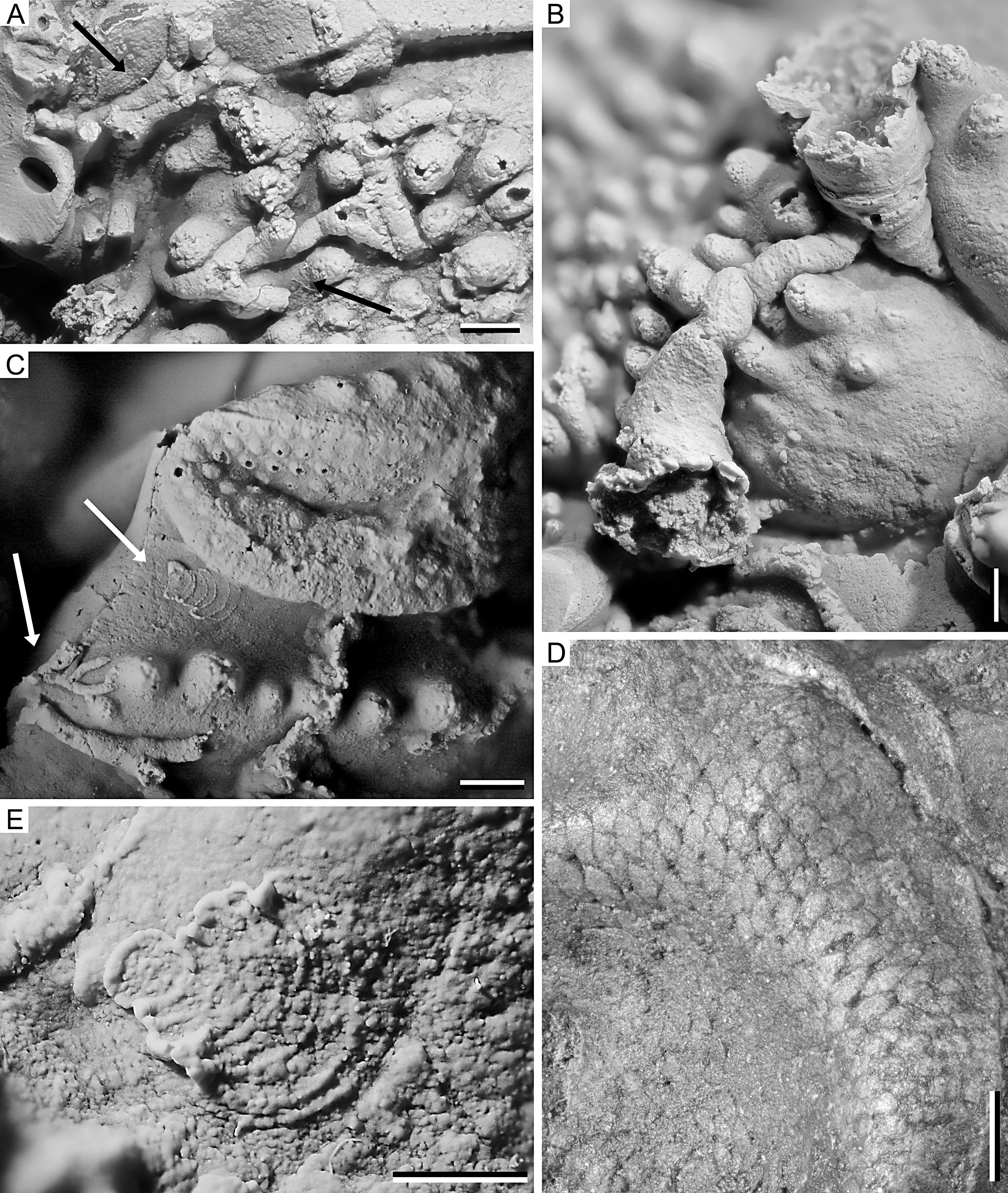
Figure 3. Philonyx philonyx (Richter & Richter, 1952), silicone cast of SMF 58393a; Lower Devonian, upper Emsian of Festenburg near Oberschulenberg, Harz Mountains, Germany; details of sclerobionts. A. Morphotype attributable to auloporid corals (indicated by black arrows). B. Two cornulitid tubeworms. C. Encrusting foraminifer (upper white arrow) and putative hederelloid (lower white arrow). D. Encrusting (trepostome) bryozoan. E. Encrusting foraminifer with proloculus to the left. All scale bars equal 1 mm.
5.2. Taxonomic placements of the sclerobionts
15The cephalon is covered with various epizoans, many of which are too fragmentary to be discussed in detail. At least five different morphotypes of epizoans are identified. The first morphotype shows long, tubular, slender stolons with an approximately uniform width and regular branching pattern (Fig. 3C). This morphotype is strongly reminiscent of the hederelloids on the Réideschbaach specimen.
16In the second morphotype, the stolons are larger and, again, zooecia are not clearly preserved, but were apparently only slightly wider than the stolons, if wider at all, and situated on slightly bulbous sections along the stolons (Fig. 3A). We suppose that the second morphotype represents auloporid corals rather than hederelloids, but we cannot exclude the possibility that both morphotypes represent different ontogenetic stages of one and the same organism.
17The third morphotype differs from the first by its greater size and especially in having a distinctly bag-shaped morphology, i.e. strongly widening towards the aperture. The latter is markedly raised from the otherwise creeping exoskeleton. The presence of outer growth rings on the former exoskeleton is indicated in the upper specimen in Figure 3B; otherwise, an outer ornament is lacking or not preserved. The morphology of this morphotype is consistent with its interpretation as cornulitid tubeworms (e.g. Morris & Rollins, 1971; Sparks et al., 1980; Vinn et al., 2024b). It is apparent that at one point the slender tubeworm overgrew the previously established coral or hederelloid specimen. The conical shells of possible cornulitids clearly represent two species. The broadly conical form is similar to Cornulites devonicus (Pacht, 1858) while the slender form resembles somewhat C. sokiranae Vinn et al., 2019. Both cornulitid species occur as epibionts on living brachiopod shells in the Upper Devonian of central Russia, and likely exploited feeding currents of their brachiopod hosts (Vinn et al., 2019).
18The fourth morphotype is represented by only two examples, one of which is comparatively well-preserved (Fig. 3E). It consists of nine chambers, starting with a small subcircular proloculus with a diameter of about 0.6 mm followed by uniserially arranged, closely appressed, arch-shaped adult chambers. Adult chambers expand laterally and become flared and may afford the test a leaf or fan-like appearance. The aperture consists of multiple openings at the periphery of the final chamber. The inner septa are subdivided and resemble irregular dashed lines. They represent the openings of the previous chambers (foramina), allowing efficient protoplasmic communication in both longitudinal and transverse directions. The test is attached to the substrate and reaches a size that is about 2.2 mm long and 1.9 mm wide. The second, incompletely preserved specimen is attached to the left eye socket of the trilobite (Fig. 3C, upper arrow). The morphology of these sclerobionts is consistent with an interpretation as encrusting, multichambered foraminifers.
19The fifth morphotype is indicated by a regular pattern of subrectangular cells, each typically about 0.3 mm wide, consistent with an interpretation as a thin sheet-like colony of encrusting bryozoans (Fig. 3D). A closer determination seems impossible due to the lack of skeletal material.
5.3. Palaeoecology of the sclerobiont-host association
20Tentaculitoid tubeworms are common components of Palaeozoic encrusting communities and lived as active suspension feeders using a lophophore to gain their food (Richards, 1974; Taylor & Vinn, 2006). Such tubeworms were found previously encrusting the inner surface of an Ordovician trilobite pygidium (Vinn et al., 2017). However, the encrustation of trilobite pygidia in the Ordovician of Estonia most certainly took place post mortem. On the other hand, if the studied trilobite cephalon was encrusted while the host was alive, it is possible that cornulitids benefitted from water currents created by the movement of the trilobite host. The trilobite provided a hard substrate to the cornulitids on the otherwise soft seafloor. Moreover, selecting the appropriate living substrate can equip an epibiont with many advantages similar to those of a mobile lifestyle, such as the ability to evade adverse conditions, elude predators, and endure burial (Coletti et al., 2023). The trilobite host may have protected the cornulitids from burial in the conditions of fast sedimentation or sudden sediment flows.
21Epizoic and epiphytic foraminifers were recorded, e.g., from Carboniferous algae (Cossey & Mundy, 1990) and hardgrounds (Vinn & Mironenko, 2025), extant seagrasses (Langer, 1988, 1993), as extant epizoan commensals on brachiopods (Zumwalt & DeLaca, 1980), on agglutinated tubes of gammarid amphipods (Langer & Long, 1994), and on arthropods such as the Norwegian lobster Nephrops (Farmer, 1977). In contrast, Devonian foraminifers are very scarce before the Givetian and are thought to have been endobenthic (Vachard et al., 2010). Kloc (1997) mentioned encrusting foraminifers on the trilobite Dicranurus from the Lower Devonian of Oklahoma, but these were neither described nor figured. The case reported herein is, to our knowledge, the second report of encrusting foraminifers on trilobites and the first case where these are figured and described. Morphology and preservation indicate that we are dealing with multichambered, calcareous foraminifers with a leaf-like test that resemble semitextulariids such as Semitextularia Miller & Carmer, 1933. Semitextulariid foraminifers, however, are characterised by a short early biserial portion with up to four pairs of biserially arranged chambers and an interior that is subdivided by vertical chamber partitions. Both features are absent in the encrusting foraminifers recorded here. They may therefore represent a new genus yet to be described. Semitextulariids are regarded as the oldest known plurilocular foraminifers previously recorded from the Eifelian to Frasnian with a former global distribution in shallow marine, well-illuminated habitats of the inner shelves, such as tropical reefs and lagoons (Vachard & Massa, 1989; Dubicka et al., 2021). It also has been hypothesised that these taxa represent the earliest photosynthetically active symbiont-bearing benthic foraminifers (Dubicka et al., 2021). If this is also true for the epizoic foraminifers found here, this would suggest a habitat within the euphotic zone, probably in a warm, clear, nutrient poor environment most favourable to photosymbiosis (e.g. Hallock, 1981, 1987; Lee et al., 2010; Schmidt et al., 2004).
Acknowledgements
22We are greatly indebted to Mónica M. Solórzano Kraemer and especially Robin Kunz (both Senckenberg Forschungsinstitut und Naturmuseum) for photographs of the original specimen SMF58393 and to Martin Basse for photographs and for providing the silicone cast of this specimen. We thank the reviewers, Martin Basse (Senckenberg Forschungsinstitut und Naturmuseum), Danita Brandt (Michigan State University) and Andrej Ernst (Universität Hamburg), for their constructive comments which improved the manuscript.
Author contribution
23AV, MP, BT and PM conceptualised the initial draft of the paper, ML and OV contributed their long-standing expertise, FL drew the reconstruction of the encrusted Rhenops specimen (Fig. 1C).
Data availability
24The studied specimens are all housed in official repositories guaranteeing their long-term safekeeping and availability to other researchers for future studies.
References
25Abelló, P., Villanueva, R. & Gili, J.M., 1990. Epibiosis in deep-sea crab populations as indicator of biological and behavioural characteristics of the host. Journal of the Marine Biological Association of the United Kingdom, 70, 687–695. https://doi.org/10.1017/S0025315400058975
26Alberti, M., 2014. Im Leben und im Tod vereint – Trilobit mit Koralle. Fossilien, 31/3, 23–25.
27Alberti, M., 2018. Jeder Hartgrund zählte – Die Epizoenfauna im frühen Rupbach-Schiefer. Fossilien, 35/5, 10–20.
28Alvarez, F. & Taylor, P.D., 1987. Epizoan ecology and interactions in the Devonian of Spain. Palaeogeography, Palaeoclimatology, Palaeoecology, 61, 17–31. https://doi.org/10.1016/0031-0182(87)90039-3
29Basse, M., 1998. Trilobiten aus mittlerem Devon des Rhenohercynikums: III. Proetida (3), Phacopida (2), Lichida (Lichoidea, Odontopleuroidea) und ergänzende Daten. Palaeontographica (A), 249, 1–162. https://doi.org/10.1127/pala/249/1998/1
30Basse, M., 2003. Eifel-Trilobiten. 2. Phacopida 1. Goldschneck Verlag, Korb, 198 p.
31Basse, M., 2006. Eifel-Trilobiten IV. Proetida (3), Phacopida (3). Quelle & Meyer-Verlag, Wiebelsheim, 304 p.
32Basse, M. & Müller, P., 2004. Eifel-Trilobiten III. Corynexochida, Proetida (2), Harpetida, Phacopida (2), Lichida. Quelle & Meyer-Verlag, Wiebelsheim, 261 p.
33Basse, M. & Müller, P., 2016. Trilobiten aus dem Ober-Emsium und frühen Eifelium der südlichen Lahnmulde (Rupach-Schiefer, Leun-Schiefer und Ballersbach-Kalk). Abhandlungen der Senckenberg Gesellschaft für Naturforschung, 572, 1–329.
34Basse, M., Müller, P. & Franke, C., 2006. Neue Daten zu den Trilobiten aus dem frühen Unteremsium (Ulmen-Unterstufe; Unterdevon) vom Reideschbaach (Luxemburg; Givonne-Oesling-Antiklinorium; Rhenohercynikum). Senckenbergiana lethaea, 86, 243–259. https://doi.org/10.1007/BF03043491
35Bassler, R.S., 1939. The Hederelloidea, a suborder of Paleozoic cyclostomatous Bryozoa. Proceedings of the United States National Museum, 87, 25–91. https://doi.org/10.5479/si.00963801.87-3068.25
36Bauer, R.T., 2013. Adaptive modification of appendages for grooming (cleaning, antifouling) and reproduction in the Crustacea. In Watling, L. & Thiel, M. (eds), The natural History of the Crustacea, Volume 1: Functional Morphology and Diversity. Oxford University Press, New York, 337–375. https://doi.org/10.1093/acprof:osobl/9780195398038.003.0013
37Becker, K. & Wahl, M., 1996. Behaviour patterns as natural antifouling mechanisms of tropical marine crabs. Journal of Experimental Marine Biology and Ecology, 203, 245–258. https://doi.org/10.1016/0022-0981(96)02575-0
38Brandt, D.S., 1996. Epizoans on Flexicalymene (Trilobita) and implications for trilobite paleoecology. Journal of Paleontology, 70, 442–449. https://doi.org/10.1017/S0022336000038373
39Brassel, G., 1977. Der erste Fund von Hederelloideen (Bryozoa) im Hunsrückschiefer (Unterdevon, Rheinisches Schiefergebirge). Geologisches Jahrbuch Hessen, 105, 41–45.
40Coletti, G., Collareta, A., Di Cencio, A., Bosio, G. & Casati, S., 2023. Symbiont-bearing colonial corals and gastropods: An odd couple of the shallow seas. Journal of Marine Science and Engineering, 11, 260. https://doi.org/10.3390/jmse11020260
41Cossey, P.J. & Mundy, D.J.C., 1990. Tetrataxis: a loosely attached limpet-like foraminifer from the Upper Palaeozoic. Lethaia, 23, 311–322. https://doi.org/10.1111/j.1502-3931.1990.tb01457.x
42Dubicka, Z., Gajewska, M., Kozłowski, W., Hallock, P. & Hohenegger, J., 2021. Photosynthetic activity in Devonian Foraminifera. Biogeosciences, 18, 5719–5728, https://doi.org/10.5194/bg-18-5719-2021
43Farmer, A.S.D., 1977. Epizoic Foraminifera on Nephrops norvegicus. Journal of the Marine Biological Association of the United Kingdom, 57, 877–878. https://doi.org/10.1017/S0025315400025236
44Feifarek, B.P., 1987. Spines and epibionts as antipredator defenses in the thorny oyster Spondylus americanus Hermann. Journal of Experimental Marine Biology and Ecology, 105, 39–56. https://doi.org/10.1016/S0022-0981(87)80028-X
45Hallock, P., 1981. Light dependence in Amphistegina. Journal of Foraminiferal Research, 11, 40–46. https://doi.org/10.2113/gsjfr.11.1.40
46Hallock, P., 1987. Fluctuations in the trophic resource continuum: a factor in global diversity cycles? Paleoceanography, 2, 457–471. https://doi.org/10.1029/pa002i005p00457
47Johnsen, S.A.L., Ahmed, M. & Leighton, L.R., 2013. The effect of spines of a Devonian productide brachiopod on durophagous predation. Palaeogeography, Palaeoclimatology, Palaeoecology, 375, 30–37. https://doi.org/10.1016/j.palaeo.2013.02.009
48Kácha, P. & Šarič, R., 1995. Bryozoans attached to exuvia of the Ordovician trilobite Dalmanitina (D.) proeva. Věstník Českého geologického ústavu, 70/4, 43–46.
49Kácha, P. & Šarič, R., 2009. Host preferences in Late Ordovician (Sandbian) epibenthic bryozoans: example from the Zahořany Formation of Prague Basin. Bulletin of Geosciences, 84, 169–178. https://doi.org/10.3140/bull.geosci.1048
50Key, M.M., Jeffries, W.B., Voris, H.K. & Yang, C.M., 1996a. Epizoic bryozoans and mobile ephemeral host substrata. In Gordon, D.P., Smith, A.M. & Grant-Mackie, J.A. (eds), Bryozoans in Space and Time. Proceedings of the 10th International Bryozoology Conference, Wellington, New Zealand, 1995, 157–165.
51Key, M.M., Jeffries, W.B., Voris, H.K. & Yang, C.M., 1996b. Epizoic bryozoans, horseshoe crabs, and other mobile benthic substrates. Bulletin of Marine Science, 58, 368–384.
52Key, M.M., Jeffries, W.B., Voris, H.K. & Yang, C.M., 2000. Bryozoan fouling pattern on the horseshoe crab Tachypleus gigas (Müller) from Singapore. In Herrera, C.A. & Jackson, J.B.C. (eds), Proceedings of the 11th International Bryozoology Association Conference. Smithsonian Tropical Research Institute, Balboa, Panama, 265–271.
53Key, M.M., Schumacher, G.A., Babcock, L.E., Frey, R.C., Heimbrock, W.P., Felton, S.H., Cooper, D.L., Gibson, W.B., Scheid, D.G. & Schumacher, S.A., 2010. Paleoecology of commensal epizoans fouling Flexicalymene (Trilobita) from the upper Ordovician, Cincinnati Arch region, USA. Journal of Paleontology, 84, 1121–1134. https://doi.org/10.1666/10-018.1
54Key, M.M., Hyžný, M., Zágoršek, K. & Dulai, A., 2024. Cheilostome bryozoan epibiosis on brachyuran crabs in the Paratethys Sea during the late Badenian (middle Miocene). PalZ, 98, 563–578. https://doi.org/10.1007/s12542-024-00707-8
55Kloc, G.J., 1992. Spine function in the odontopleurid trilobites Leonaspis and Dicranurus from the Devonian of Oklahoma. In Lidgard, S. & Crane, P.R. (eds), Fifth North American Paleontological Convention - Abstracts and Program. The Paleontological Society Special Publications, 6, 167. https://doi.org/10.1017/S2475262200007279
56Kloc, G.J., 1993. Epibionts on Selenopeltinae (Odontopleurida) trilobites. Geological Society of America Abstracts with Programs, 25/6, 103.
57Kloc, G.J., 1997. Epibionts on Dicranurus and some related genera. 2nd International Trilobite Conference. Brock University, St. Catharines, Ontario, August 22-24, 1997. Abstracts.
58Langer, M.R., 1988. Recent epiphytic Foraminifera from Vulcano (Mediterranean Sea). Revue de Paléobiologie, 2, 827–832.
59Langer, M.R., 1993. Epiphytic foraminifera. Marine Micropaleontology, 20, 235–265. https://doi.org/10.1016/0377-8398(93)90035-V
60Langer, M.R. & Long, D.J., 1994. Associations of benthic foraminifera with a gammarid amphipod on tidal flats of San Francisco Bay, California. Journal of Coastal Research, 10, 877–883.
61Lebrun, P., 2018. Fossiles du Maroc. Tome I. Gisements emblématiques du Paléozoïque de l’Anti-Atlas. Les Editions du Piat, Saint-Julien-du-Pinet, 298 p.
62Lee, J.J., Cervasco, M., Morales, J., Billik, M. & Fine, M., 2010. Symbiosis drove cellular evolution: Symbiosis fuelled evolution of lineages of Foraminifera (eukaryotic cells) into exceptionally complex giant protists. Symbiosis, 51, 13–25. https://doi.org/10.1007/s13199-010-0056-4
63Miller, A.K. & Carmer, A.M., 1933. Devonian Foraminifera from Iowa. Journal of Paleontology, 7, 423–431.
64Morris, R.W. & Rollins, H.B., 1971. The distribution and paleoecological interpretation of Cornulites in the Waynesville Formation (Upper Ordovician) of southwestern Ohio. Ohio Journal of Science, 71, 159–179.
65Müller, P., 1997. Fossillagerstätten im Westerwald. Westerburger Hefte, 25, 1–52.
66Pacht, R., 1858. Geognostische Untersuchungen zwischen Orel, Woronesh und Simbirsk im Jahr 1853. In von Helmersen, G. (ed.), Beiträge zur Kenntniss des Russischen Reiches und der angränzenden Länder Asiens, 21, 61–108.
67Richards, P.R., 1974. Ecology of the Cornulitidae. Journal of Paleontology, 48, 514–523.
68Richter, R. & Richter, E., 1952. Phacopacea von der Grenze Emsium/Eiflium. (Tril.). Senckenbergiana, 33, 79–107.
69Schmidt, D.N., Renaud, S., Bollmann, J., Schiebel, R. & Thierstein, H.R., 2004. Size distribution of Holocene planktic foraminifer assemblages: Biogeography, ecology and adaption. Marine Micropaleontology, 50, 319–338. https://doi.org/10.1016/S0377-8398(03)00098-7
70Solle, G., 1952. Neue Untergattungen und Arten der Bryozoen-Gattung Hederella und eine Hernodia im rheinischen Unterdevon. Notizblatt des Hessischen Landesamtes für Bodenforschung, 6, 35–55.
71Solle, G., 1968. Hederelloidea (Cyclostomata) und einige ctenostome Bryozoen aus dem Rheinischen Devon. Abhandlungen des Hessischen Landesamtes für Bodenforschung, 54, 1–40.
72Sparks, D.K., Hoare, R.D. & Kesling, R.V., 1980. Epizoans on the brachiopod Paraspirifer bownockeri (Stewart) from the Middle Devonian of Ohio. Papers on Paleontology, 23, 1–105.
73Stilkerich, J., Smrecak, T.A. & De Baets, K., 2017. 3D-Analysis of a non-planispiral ammonoid from the Hunsrück Slate: natural or pathological variation? PeerJ, 5, e3526. https://doi.org/10.7717/peerj.3526
74Taylor, P.D. & Vinn, O., 2006. Convergent morphology in small spiral worm tubes (‘Spirorbis’) and its palaeoenvironmental implications. Journal of the Geological Society, London, 163, 225–228. https://doi.org/10.1144/0016-764905-145
75Taylor, P.D. & Wilson, M.A., 2003. Palaeoecology and evolution of marine hard substrate communities. Earth-Science Reviews, 62, 1–103. https://doi.org/10.1016/S0012-8252(02)00131-9
76Taylor, P.D. & Wilson, M.A., 2008. Morphology and affinities of hederelloid “Bryozoans”. In Hageman, S.J., Key, M.M. & Winston, J.E. (eds), Bryozoan studies 2007. Proceedings of the 14th International Bryozoology Association Conference, Boone, North Carolina, July 1-8, 2007. Virginia Museum of Natural History, Publications, Special Publication, 15, 301–309.
77Tetreault, D.K., 1992. Paleoecologic implications of epibionts on the Silurian lichid trilobite Arctinurus. In Lidgard, S. & Crane, P.R. (eds), Fifth North American Paleontological Convention - Abstracts and Program. The Paleontological Society Special Publications, 6, 289. https://doi.org/10.1017/S2475262200008492
78Vachard, D. & Massa, D., 1989. Apparition précoce du genre Nanicella (Foraminifère) dans le Dévonien inférieur du Sud-Tunisien. Bulletin de la Société belge de Géologie, 98, 287–293.
79Vachard, D., Pille, L. & Gaillot, J., 2010. Palaeozoic Foraminifera: Systematics, palaeoecology and responses to global changes. Revue de Micropaléontologie, 53, 209–254. https://doi.org/10.1016/j.revmic.2010.10.001
80van Viersen, A.P., 2025. The curious case of Philonyx Richter & Richter, 1952 (Trilobita, Acastidae) and its allies from the Lower Devonian. Mainzer geowissenschaftliche Mitteilungen, 53, 111–122. https://doi.org/10.23689/fidgeo-9315
81van Viersen, A.P. & Kloc, G.J., 2022. Functional morphology, coaptation and palaeoecology of Hollardops (Trilobita, Acastidae), with descriptions of new species and two new genera from the Devonian of Morocco. Geologica Belgica, 25, 99–144. https://doi.org/10.20341/gb.2022.005
82van Viersen, A.P. & Müller, P., 2024. Taxonomy and palaeogeographic affinities of early Emsian (Lower Devonian) trilobites from near Heiderscheid (Éislek, Luxembourg). In Thuy, B. & Franke, C. (eds), The Réideschbaach Fossil Fauna. Ferrantia, 91, 89–128.
83Vinn, O. & Mironenko, A.A., 2025. New species of encrusting foraminifera from the Carboniferous (Upper Mississippian) of Central Russia. Revue de Micropaléontologie, 86, 100819. https://doi.org/10.1016/j.revmic.2024.100819
84Vinn, O., Toom, U. & Isakar, M., 2017. Earliest cornulitid on the internal surface of illaenid pygidium from the Middle Ordovician of Estonia. Estonian Journal of Earth Sciences, 66, 193–197. https://doi.org/10.3176/earth.2017.19
85Vinn, O., Musabelliu, S. & Zatoń, M., 2019. Cornulitids from the Upper Devonian of the Central Devonian Field, Russia. GFF, 141, 68–76. https://doi.org/10.1080/11035897.2018.1505777
86Vinn, O., Almansour, M.I., Al Farraj, S. & El Hedeny, M., 2024a. The abundance of Arachnostega in trilobite moulds remained unaffected by the climatic warming during the Ordovician of Baltica. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 312, 109–116. https://doi.org/10.1127/njgpa/2024/1204
87Vinn, O., Jäger, M., Słowiński, J. & Zatoń, M., 2024b. Convergent evolution of encrusting calcareous tubeworms. Palaeoworld, 33, 267–283. https://doi.org/10.1016/j.palwor.2023.04.001
88Wahl, M., 1997. Living attached: Aufwuchs, fouling, epibiosis. In Nagabhushanam, R. (ed.), Fouling Organisms of the Indian Ocean. CRC Press, London, 31–83. https://doi.org/10.1201/9781003077992-2
89Waugh, D.A., Feldmann, R.M., Crawford, R.S., Jakobsen, S.L. & Thomas, K.B., 2004. Epibiont preservational and observational bias in fossil marine decapods. Journal of Paleontology, 78, 961–972. https://doi.org/10.1666/0022-3360(2004)078%3C0961:EPAOBI%3E2.0.CO;2
90Whittington, H.B., 1997. Mode of life, habits, and occurrence. In Kaesler, R.L. (ed.), Treatise on Invertebrate Paleontology, Part O, Arthropoda 1. Trilobita, revised. Volume 1: Introduction, Order Agnostida, Order Redlichiida. University of Kansas Press, Lawrence, KS and Geological Society of America, Boulder, CO, 137–169.
91Zapalski, M. & Klug, C., 2018. Trilobite sclerites as attachment surface for Emsian tabulate corals of Hamar Laghdad (Anti-Atlas, Morocco). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 290, 111–115. https://doi.org/10.1127/njgpa/2018/0769
92Zumwalt, G.S. & DeLaca, T.E., 1980. Utilization of brachiopod feeding currents by epizoic foraminifera. Journal of Paleontology, 54, 477–484.
93Manuscript received 25.04.2025, accepted in revised form 25.07.2025, available online 28.11.2025.






