Agro-physiological and biochemical responses of faba bean (Vicia faba L. var. ‘minor’) genotypes to water deficit stress
Received on July 28, 2016; accepted on March 31, 2017
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
Résumé
Comportement agro-physiologique et biochimique de différents génotypes de féverole (Viciafaba L. var. ‘minor’) soumis au déficit hydrique
Description du sujet. La sècheresse est l'un des facteurs abiotiques majeurs affectant la croissance et la productivité des espèces cultivées. Cependant, le déficit hydrique provoque chez les plantes un ensemble de modifications d'ordre morphologique, physiologique et biochimique.
Objectifs. L’objectif de ce travail est d’étudier quelques réponses morphologiques, physiologiques et biochimiques à la sècheresse chez la féverole et d’évaluer la contribution du facteur génétique à l’amélioration de la tolérance de cette espèce à la sècheresse.
Méthode. Des plantes de 11 cultivars de fèverole ont été cultivées dans des conditions contrôlées et ont été soumises à trois régimes de déficit hydrique (90, 50 et 30 % de la capacité au champ) durant 20 jours selon un dispositif en bloc aléatoire. L’effet de déficit hydrique sur la croissance de la plante, la teneur relative en eau (TRE), les échanges gazeux, la teneur en chlorophylle b (Chlb), l’accumulation des osmoprotectants (proline et sucres totaux), l’activité des enzymes antioxydantes et le rendement en grain ont été déterminés.
Résultats. Le déficit hydrique a réduit la croissance des plantes et a provoqué un déséquilibre physiologique. Il a également diminué l’activité des enzymes antioxydantes. Ce déficit hydrique a augmenté la teneur de la proline, des sucres solubles et des protéines. Les cultivars étudiés différaient significativement dans leurs réponses au déficit hydrique. Les paramètres photosynthétiques ont été moins touchés chez le cultivar ‘Hara’. Ce cultivar ayant le rendement le plus élevé en grain à 30 % de capacité au champ (CC) a montré une activité des enzymes antioxydantes (CAT, GPX et APX), une teneur en Chlb et une TRE relativement plus élevées que les autres génotypes étudiés. Ceci permet de suggérer que le cultivar Hara est relativement tolérant au déficit hydrique par comparaison aux autres génotypes étudiés.
Conclusions. Nos résultats peuvent être utilisés pour évaluer la réponse des ressources génétiques à la sècheresse et développer des programmes d’amélioration génétique pour améliorer la tolérance de la fève vis-à-vis du stress hydrique.
Abstract
Description of the subject. Drought is one of the major abiotic factors affecting growth and productivity of plants by imposing certain morphological, physiological and biochemical changes at different growth stages.
Objectives. The objective of this work is to study key morphological, physiological and biochemical responses of faba bean (Vicia faba L. var. ‘minor’) to soil water deficit stress and to assess the contribution of genetic factors in improving faba bean tolerance to water deficit.
Method. Plants of 11 faba bean cultivars were grown in the greenhouse and subjected to three levels of water deficit (90, 50 and 30% of field capacity [FC]) in a simple randomized design for 20 days. Water deficit effects on plant growth, relative water content (RWC), gas exchange, chlorophyll a (Chla) and chlorophyll b (Chlb) content, osmoprotectant accumulations (such as proline and soluble sugars), antioxidant enzyme activities and grain yield were determined.
Results. Soil water deficit stress reduced growth and affected physiological parameters, especially antioxidant enzyme activities. Water deficit also increased proline, soluble sugars and protein contents. The studied cultivars significantly differed in their responses to water deficit stress. Photosynthetic parameters were less affected in the ‘Hara’ cultivar. Furthermore, this cultivar produced the highest value of grain yield at 30% FC, and showed higher antioxidant enzyme activities (CAT, GPX and APX), osmoprotectant accumulations, Chlb and RWC. The ‘Hara’ cultivar was found to be more tolerant to water deficit stress than the other cultivars.
Conclusions. Our methodology can be used for assessing the response of faba bean genetic resources to soil water deficit. The identified tolerant cultivar can be utilized as a source for water stress tolerance in faba bean breeding programs aimed at improving drought tolerance.
1. Introduction
1Faba bean (Vicia faba L.) is an important legume crop worldwide, ranking as the fourth most important grain legume after dry beans, dry peas and chickpeas (Lopez-Bellido et al., 2005). It is one of the oldest and most important grain legumes grown in the Mediterranean region, where it is used for human consumption and animal feed. Moreover it is still considered an important improving crop in wheat-legume rotations (Kharrat & Ouchari, 2011).
2In Tunisia, two botanicals of faba bean, namely Vicia faba var. ‘major’ and Vicia faba var. ‘minor’, are cultivated. The first is produced for human consumption (fresh or dry seeds), whereas the second type is used mainly for animal feed. During the past 20 years, faba bean has been the major food legume crop grown in Tunisia: the average area allocated to this crop is about 68% of the total grain legume area (Kharrat & Ouchari, 2011). The average dry grain yield is 0.99 t·ha-1, which is lower than the worldwide average yield (1.7 t·ha-1). Grain production is also very variable. There has however been great inconsistency in the grain harvest (Maalouf, 2011), which can be explained by the effect of various biotic and abiotic (mainly drought) stresses (Kharrat & Ouchari, 2011).
3Water deficit is a major environmental and multidimensional stress leading to reduced crop yield worldwide. Drought severely affects plant growth, grain yield and quality, and causes morphological, physiological, biochemical, and molecular changes in plants (Zarafshar et al., 2014). However, different plant species can vary in their sensitivity and response to water shortage. According to Amede & Schubert (2003), faba bean is more sensitive to water deficits than common bean, pea and chickpea.
4Drought severely affects plant biomass production (Shao et al., 2008) and modifies their morphological components through a decrease in height, leaf area, number of leaves and consequently plant biomass production. Furthermore, yield constituents such as grain number and size are decreased (Jaleel et al., 2009). Water deficit in faba bean causes a significant reduction in internode length, number and size of leaves, shoot dry matter, number of pods per plant and seed production (Mwanamwenge et al., 1999; Mohamad Zabawi & Dennett, 2010). Reduction in fresh and dry weight of plant organs, and in leaf area and early maturity, are key responses to mitigate the effect of drought on plants (Farooq et al., 2009).
5Drought affects many aspects of plant physiology, including net photosynthesis, relative water content, chlorophyll content and photosystem II (PSII) activity (Pandey & Shukla, 2015). Values of all these parameters are reduced under water stress in faba bean (Ammar et al., 2014; Siddiqui et al., 2015). The maintenance of high net photosynthesis and the maintenance of relative water content constitute the mechanisms by which drought-tolerant soybean genotypes cope with water deficit (Hossaina et al., 2014). Various physiological traits such as stomatal conductance and leaf temperature, which were a marker for both low stomatal conductance and high transpiration efficiency, were used to screen breeding materials in order to identify drought stress tolerance (Benešová et al., 2012). Genetic variation in stomatal conductance among nine faba bean genotypes previously screened for drought response in the field was reported by Khan et al. (2007) in growth chamber conditions: in these drought tolerant faba bean genotypes, lower stomatal conductance was associated with warmer leaves. Among various mechanisms, osmotic adjustment provided by the synthesis of osmoprotectants like proline may confer tolerance to drought injuries by maintaining high tissue water potential (Mohammadkhani & Heidari, 2008). In several plant species, such as chick pea and tea, the accumulation of proline is significantly higher in drought tolerant cultivars and is used as a marker for drought tolerance selection (Rozrokh et al., 2012; Maritimi et al., 2015). Khalafallah et al. (2008) reported that drought stress resulted in a significant accumulation of free proline in shoots of faba bean varieties, and the magnitude of increase in free proline accumulation was higher in the tolerant cultivars than in the sensitive ones.
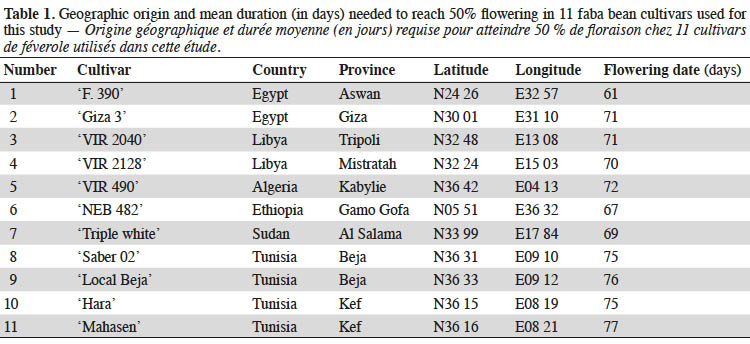
6Various environmental stresses, including drought, often lead to reactive oxygen species (ROS) in plant cells. Enhanced level of ROS causes oxidative damage to membrane lipids, inhibition of protein synthesis, damage to nucleic acids, and loss of enzyme activity, ultimately resulting in cell death (Sharma et al., 2012). Scavenging of excess ROS is achieved by an efficient antioxidative system including nonenzymatic mechanisms (such as ascorbate, glutathione, carotenoids, tocopherols, and phenolics) as well as enzymatic mechanisms (involvement of superoxide dismutase, catalase, guaiacol peroxidase and ascorbate peroxidase) (Sharma et al., 2012). Wu et al. (2012) reported that increased activities of enzymes implicated in the plant’s antioxidant defense system controlled optimum ROS levels and therefore improved drought stress tolerance. Antioxidant enzyme activities are regarded as an indicator of genotype tolerance to stress conditions (Harb et al., 2015.). Recently, antioxidant enzyme profiles provided a meaningful tool for depicting drought tolerance in faba bean genotypes (Siddiqui et al., 2015).
7Selecting adapted genotypes under environmental stress conditions helps to improve adaptation and stress tolerance in cultivars (Lopes et al., 2012). Although the growth and development of faba bean in relation to water deficit have been extensively studied, the physiological and biochemical processes involved in tolerance to water deficit are less well described in this species. Furthermore, marker traits that could be used as indicators of plant tolerance to drought stress in faba bean have not yet been elucidated.
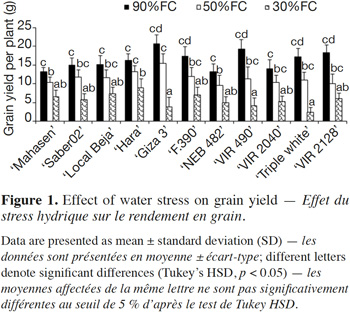
8Therefore, the objectives of this study are to analyze the morphological, physiological and biochemical responses of faba bean genotypes to drought stress, to determine potential traits related to the tolerance of water deficits, and to identify sensitive and tolerant genotypes.
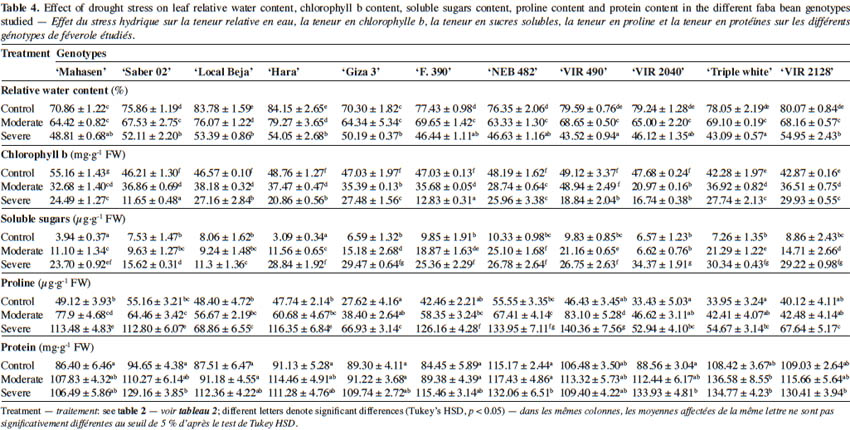
2. Materials and methods
2.1. Plant materials and culture conditions
9The trials were carried out at the Experimental Station of the Biotechnology Center of Borj Cedria, Tunisia, during the period 2013-2014. All experiments were conducted in a greenhouse under controlled conditions (temperature of 23 ± 2° C, relative humidity 55%-65%, light 270 μmol of photons·m-2·s-1 photosynthetic active radiations and a 16/8 h day/night photoperiod). Eleven cultivars showing morphological variability and a high level of genetic polymorphism were chosen (Abid et al., 2015). Seven cultivars (‘Giza 3’, ‘F. 390’, ‘NEB 482’, ‘VIR 490’, ‘VIR 2040’, ‘VIR 2128’ and ‘Triple white’) were received from the International Center for Agricultural Research in the Dry Areas (ICARDA, Aleppo, Syria), while the remaining four genotypes are cultivated in Tunisia (Table 1; ‘Mahasen’, ‘Saber 02’, ‘Local Beja’ and ‘Hara’). ‘NEB 482’ and ‘Triple white’ cultivars originated from Ethiopia and Sudan respectively, and are adapted to a tropical climate. ‘Giza 3’ and ‘F. 390’ originated from Egypt; ‘VIR 2040’ and ‘VIR 2128’ from Libya; ‘VIR 490’ from Algeria; ‘Mahasen’, ‘Saber 02’, ‘Local Beja’ and ‘Hara’ from Tunisia. All these nine cultivars are adapted to Mediterranean climate. Moreover, ‘Giza 3’ is known as the most sensitive genotype to water stress, and is used as a standard for the drought tolerance test of faba bean worldwide (Abdellatif et al., 2012). Seeds of all genotypes were surface disinfected by soaking them in a 5% sodium hypochlorite solution for 5 min and rinsed 3 times with sterile distilled water. The seeds were then sown into plastic pots (20 cm diameter and 30 cm depth) filled with 5 kg of air-dried soil. The experimental soil was loamy sand in texture (86% sand, 3% slit, 11% clay) and the chemical attributes of the soil were as follows: pH: 8.15; organic matter content: 0.4%; N: 0.28%; P: 16 ppm; K: 120 ppm and EC: 41.35 mS·cm-1. The pots were arranged in a completely randomized design and each pot was irrigated with half-strength Hoagland solution (Hoagland & Arnon, 1950) before exposure to drought stress, which was imposed 25 days after sowing when seedlings reached the four leaf stage. All plants were grown in individual pots. Deficit irrigation treatments were applied as follows: well-watered control (90% FC); moderate drought stress (50% FC) and severe drought stress (30% FC). Cultivars and stress treatment consisted of three replications, with five pots per replication and a single plant in each pot (n = 15 plants for each cultivar per drought stress level) (Alghamdi et al., 2015; Ziadi Backouchi et al., 2015). Every day, the pots were weighed at 10.00 a.m. to compensate the water loss by evapotranspiration and therefore, the soil moisture was kept at 90%, 50% and 30% FC according to the treatments. All morphological parameters were evaluated at the flowering stage; however, physiological and biochemical parameters were determined after 30 days of water stress induction. Grain yield was measured at grain maturity stage.
2.2. Measurements of photosynthetic gas exchange
10The third totally expanded leaf was used to evaluate physiological parameters. Stomatal conductance (gs), net photosynthesis (Pn), transpiration rate (E) and sub-stomatal CO2 concentration (Ci) were determined at 10.00 a.m. under atmospheric CO2 and full sunlight in five plants per treatment using a Portable Photosynthesis System (LCpro+, Inc., UK).
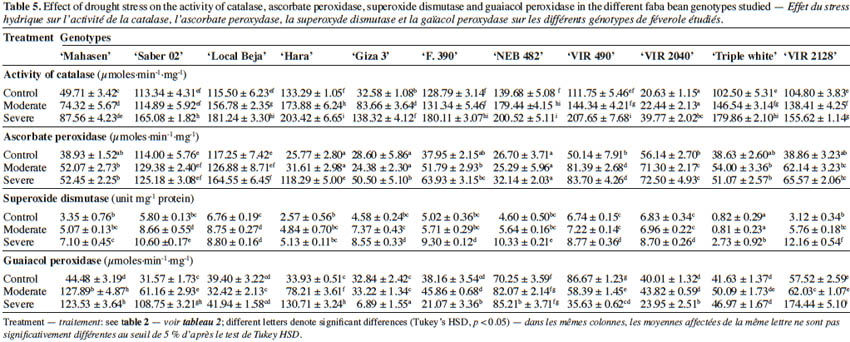
2.3. Relative water content (RWC)
11Relative water content was determined as described by Galmes et al. (2007). The uppermost fully expanded leaf of the main stem was weighed (fresh weight, FW) and then turgid leaf weight (TW) was obtained after submerging samples in distilled water for 24 h. The samples were then directly dried at 70 °C for 72 h and weighed (DW). The RWC is determined according to the equation:
12RWC (%) = [(FW-DW)/(TW-DW)] X 100
2.4. Chlorophyll content
13The youngest fully expanded leaves (1 g) were homogenized in 5 ml of 80% acetone. The homogenate was centrifuged at 3000 x g for 5 min and the supernatant was collected. The absorbance of the extract was read at 663 and 645 nm. Chlorophyll a (Chla) and b (Chlb) and total chlorophyll (Chlt) content were calculated according to Arnon (1949) using the following formulae:
14Chla = 12.7(A663) - 2.69(A645)
15Chlb = 22.9(A645) - 4.68(A663)
16Chlt = 20.2(A645) + 8.02(A663)
2.5. Proline content
17The proline content was determined according to Yooyongwech et al. (2012). One hundred milligrams of fresh material was homogenized with 10 ml of a 3% (w/v) sulfosalicylic acid solution and the extract was filtered through filter paper. One milliliter of acid-ninhydrin, 1 ml of glacial acetic acid, and 1 ml of the filtrate were mixed and incubated at 100 °C for 1 h. The reaction was terminated at room temperature (25 °C) for 5 min. The absorbance at 520 nm was measured immediately by spectrophotometer (Spectro UV-Vis Dual Beam PC, UV-S-2007; LABOMED, INC.). L-proline was used for standard curve construction.
2.6. Soluble sugar content
18Soluble sugar content was defined using the method described by Nazarli & Faraji (2011). Briefly, 0.5 g of fresh weight of leaves was homogenized with 5 ml of 95% ethanol for 48 h and subsequently dried under a hot air stream. The residue was homogenized with 20 ml of water. One milliliter of alcoholic extract was treated with 5 ml of 5% phenol (v/v) and 5 ml of sulphuric acid. The mixture was kept for 20 min at 30 °C. The soluble sugar content was revealed through absorbance at 625 nm. The standard curve was constructed using glucose.
2.7. Protein content
19The total protein content was determined according to the Bradford (1976) method using bovine serum albumin (BSA) as a standard.
2.8. Assays of antioxidant enzymes
20Fresh and fully expanded leaves (1 g) were collected after 30 days of water stress at different water deficit treatments for enzymatic assays. Total superoxide dismutase (SOD) activity was assayed using the nitroblue tetrazolium (NBT) method of Beauchamp & Fridovich (1971). The 2 ml assay reaction mixture consisted of 50 mM of phosphate buffer (pH 7.8), 2 mM of EDTA, 9.9 mM of methionine, 55 µM of NBT, 2 µM of riboflavin and 20 µl of the supernatant. The reaction was initiated by illuminating the samples under a light source for 15 min. Absorbance of the samples was measured immediately after stopping the reaction at 560 nm (Hasheminasab et al., 2012). Catalase (CAT) activity was determined according to the method described by Dhindsa et al. (1981). The H2O2 decomposition was followed as a decrease in absorbance at 240 nm. The assay mixture contained 50 mM of potassium phosphate buffer (pH 7.0) and 15 mM of H2O2. The extinction coefficient of H2O2 (ε = 39.4 mM·cm-1 at 240 nm) was used to calculate the enzyme activity, which was expressed in terms of millimoles of H2O2 per minute per gram fresh weight (Hasheminasab et al., 2012). Guaiacol peroxidase (GPX) activity was determined using the method of Plewa et al. (1991). The extinction coefficient (ε = 25.5 mM·cm-1 at 470 nm) was used to calculate the enzyme activity, which was expressed in terms of micromoles of H2O2 per minute. The reaction mixture contained guaiacol, H2O2 and phosphate buffer (pH 7.0) in the concentrations of 1%, 40 mM and 100 mM respectively. The use of H2O2 and guaiacol as substrates caused the enzyme to produce a colorful product. Ascorbate peroxidase (APX) activity was measured by the decrease in absorbance at 290 nm (Hasheminasab et al., 2012) as the ascorbate was oxidized using the method of Nakano & Asada (1981) with minor modification. The reaction mixture (1 ml) contained ascorbic acid, H2O2 and phosphate buffer (pH 7.0) in the concentrations of 0.5 mM, 1.5 mM and 50 mM respectively.
2.9. Statistical analysis
21Data were subjected to an analysis of variance (ANOVA), and means and standard errors were calculated. All measurements were performed at the three levels of water deficit and in the three replicates. All morphological, physiological, and biochemical parameters were subjected to a one-way analysis (p < 0.001) and compared using Tukey’s test at 5% of probability. The statistical analysis was performed using SYSTAT 8.0 software (https://systatsoftware.com/).
3. Results
3.1. Effects of water deficit stress on growth of faba bean plants
22The effects of water deficit stress on the growth parameters of faba bean plants are presented in table 2. The data revealed significant differences (p < 0.05) among the treatments and cultivars for all the studied traits except for days to flowering. Water deficit significantly (p < 0.05) reduced the plant height of all cultivars, and the shortest plants were observed at 30% FC. Furthermore, drought stress conditions caused a decrease in the leaf area compared to non-stress conditions. In general, plant leaf area differed significantly between studied cultivars. Under control conditions, ‘VIR 490’ had the largest leaf area (703.85 ± 19.59 cm2·plant-1), while ‘Mahasen’ and ‘F. 390’ showed the smallest (201.25 ± 12.86 and 204.55 ± 9.55 cm2·plant-1 respectively). The leaf area decreased more at 30% FC than at 50% FC. Exposed to drought stress, ‘Giza 3’ and ‘NEB 482’ were able to maintain leaf surface similar to control plants at 50% FC. However, leaf area reduction in other cultivars ranged from 12.30% to 50.21% in ‘Hara’ and ‘VIR 490’ respectively. At 30% FC, leaf area decreased from 16.90% to 75.00% in ‘Mahasen’ and ‘Saber 02’ respectively. Differences in shoot and root fresh weight as responses to drought were found among the studied genotypes: water deficit conditions significantly decreased shoot and root fresh weights. Water-stressed plants had significantly lower root, leaf and whole plant fresh weight values than the control, although absolute values varied among cultivars. The largest decrease of shoot fresh weight was found in ‘Giza 3” (68.50%) at 30% FC, while the lowest decrease occurred in ‘F.390’ and ‘Mahasen’ (37.40% and 35.04% respectively) under the same stress level. At 50% FC level, shoot fresh weight was not affected by water shortage stress. There were no significant differences in root fresh weight under drought stress and the reduction was found to be more marked in ‘Triple white’ (71.54%) than in other cultivars.
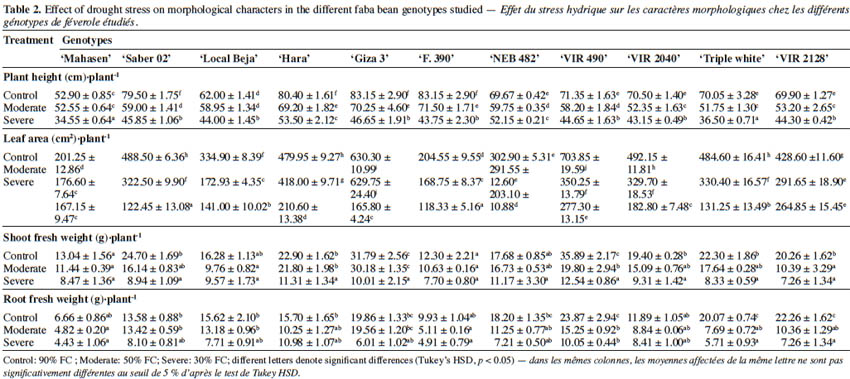
23Water deficit reduced mean grain yield as shown in figure 1. The level of grain yield reduction varied according to the cultivar. ‘Giza 3’, ‘Hara’, and ‘NEB 482’ showed a reduction in grain yield at 50% FC of 18.9%, 25.7% and 28.1% respectively, while other cultivars such as ‘Triple white’, ‘VIR 490’, and ‘VIR 2128’ exhibited higher reductions (35.7%, 40.9%, and 44.7% respectively) at this FC. Interestingly, the obtained data revealed that some genotypes displayed differential response to drought stress. Thus, ‘Giza 3’ had the highest grain yield (20.7 ± 2.47 g·plant-1) under control conditions, but this yield decreased dramatically at 30% FC (4.81 ± 2.53 g.plant-1). In contrast, ‘Hara’, which had a medium yield at 50% FC (16.34 ± 1.87 g·plant-1), showed the best yield at 30% FC (8.95 ± 2.36 g·plant-1).
3.2. Effects of water deficit stress on leaf gas exchange
24Table 3 shows the effects of water deficit stress on the leaf gas exchange parameters in the studied cultivars. The net CO2 assimilation rate (A) decreased in all genotypes under drought stress as compared to control. This rate was slightly reduced at 50% FC in ‘Hara’, ‘Saber 02’ and ‘Local Beja’, whereas a significant reduction was observed in all studied cultivars at 30% FC as compared to control conditions (Table 3). In relation to the control treatment, stomatal conductance (gs) was slightly lower at 50% FC in the studied plant material, with the exception of ‘VIR 490’ and ‘Triple white’; on the other hand, a large decrease in gs occurred in all at 30% FC, with the exception of ‘Giza 3’. In well-watered treatment, no significant difference in transpiration rate (E) was found among studied cultivars, while in drought-stressed plants the transpiration rate (E) decreased gradually throughout the assay, without a significant difference between cultivars. A similar response was observed for the internal concentration of CO2 (Ci). However, an increase in water use efficiency (A/gs) is a means to offset the decrease in A, as stomata closure was found in all water-stressed cultivars. With regard to mesophyl conductance (A/Ci) for all cultivars, drought-stressed plants showed a significant decrease in the A/Ci compared with well-watered plants.
3.3. Effects of water deficit stress on relative water content
25The effect of water deficit on leaf relative water content (RWC) is shown in table 4. The RWC showed differences between cultivars and was significantly (p < 0.05) reduced under water deficit stress. The decrease was dependent on genotype and drought stress level. Under severe drought stress (30% FC), the RWC of leaves was significantly reduced in all faba beans, causing a marked turgor loss. The lowest value of RWC was recorded in ‘VIR 490’ and ‘Triple white’ cultivar. Conversely, higher values were noted in ‘VIR 2128’, followed by ‘Hara’, ‘Local Beja’ and ‘Saber 02’.
3.4. Effects of water deficit stress on chlorophyll, soluble sugars, proline and protein content
26Induced drought stress significantly influenced chlorophyll, soluble sugars, proline and protein content (Table 4). In all studied genotypes, chlorophyll b (Chlb) content was noticeably higher than chlorophyll a (Chla) under well-watered conditions (data not shown for Chla). Water deficit stress did not affect the Chla rate, but significantly reduced the Chlb content with respect to the controls. At 30% FC, the Chlb amount decreased in all cultivars from 30.18% in ‘VIR 2128’ to 74.78% in ‘Saber 02’, while protein content increased in plants under water deficit stress conditions.
27Under non stress conditions, the tested genotypes did not differ significantly for protein quantity. However, water deficit stress increased protein content in all studied cultivars except for ‘VIR 490’ (Table 4). This increase was more pronounced in ‘VIR 2040’ (151.23%), ‘Saber 02’ (136.46%) and ‘F. 390’ (136.17%).
28Proline amount also increased as a result of water deficit stress for all genotypes. However, the highest increases were observed in leaves of ‘VIR 490’ (202.30%) and ‘F. 390’ (197.12%), and the lowest in ‘Local Beja’ (42.27%). It is noteworthy that this increase was more evident at 30% FC than at 50% FC.
29As with the protein and proline rate, the increases in leaf soluble sugars (Table 4) were more conspicuous in all studied cultivars. The highest increases were observed in ‘Hara’ (833%) and the lowest in ‘Local Beja’ (52%) at 30% FC.
3.5. Effect of water deficit stress on antioxidant enzyme activities
30The activities of antioxidant enzymes are shown in table 5. Total CAT, SOD, APX and GPX activities in leaves changed among cultivars, when compared to the controls and water deficit treatments. Indeed, a significant increase was observed in CAT, SOD, APX and GPX activities under water deficit when compared to the control for all the tested cultivars. The activity of catalase in all studied cultivars gradually increased as drought increased. However, CAT activity in ‘Saber 02’, ‘F. 390’, and ‘VIR 2040’ augmented only under severe stress and in the other eight cultivars under moderate and severe stress. The highest activity of CAT was noted in ‘VIR 490’, ‘Hara’ and ‘NEB 482’ at 30% FC, while the lowest was shown in ‘VIR 2040’ at 30% FC. The cultivar ‘Hara’ showed the maximum increase in APX activity (3.59 times) under 30% FC, whereas APX activity either slightly increased or remained unchanged for other cultivars under drought stress. The maximum increase in the GPX activities was observed in ‘Hara’ and ‘Saber 02’ (2.85 times and 2.44 times respectively), while the minimum increase was found in the cultivar ‘Local Beja’ (0.6 times) compared to the control. Among the cultivars, ‘Giza 3’, ‘F. 390’, ‘VIR 490’ and ‘VIR 2040’ exhibited a decrease in GPX activity at 30% FC. The activity of SOD was lower compared to other antioxidant enzymes in all studied genotypes and under both moderate and severe water deficit stress. Water deficit stress treatment enhanced the SOD activity by 289.74% in the leaves of ‘VIR 2128’ at 30% FC, while the minimum increase was found in ‘Local Beja’, ‘VIR 490’ and ‘VIR 2040’ (30.17%, 30.11 % and 27.37% respectively) compared to control plants.
4. Discussion
31Drought is one of the major abiotic stress factors that affect almost all plant functions (Anjum et al., 2011). Effects of water deficits on physiological, biochemical, growth and yield processes have been discussed and reviewed extensively in various crop plants. Among the crops, faba bean is reputed to be more susceptible to drought than other grain legumes (Amede & Schubert, 2003). Drought stress induces variability of grain harvest in faba bean and can drastically reduce grain yield (Link et al., 1999). Cultivating tolerant genotypes is the most attractive approach to attenuate the negative effects of drought on faba bean production. Numerous agro-morphological, physiological and biochemical indicators for drought responses have been used in rapid screening of genotypes for drought stress tolerance (Zarafshar et al., 2014). However, the development of drought tolerant faba bean cultivars has been a slow process, mainly due to a lack of efficient screening techniques.
32The drought stress negatively affected all studied faba bean genotypes. Growth parameters (plant height, leaf area, shoot and root fresh weight) of the cultivars were reduced under water deficit stress compared to the control, which may have been due to the loss of turgidity and reduction of relative water content (Table 4). Drought challenge may decrease cell division and elongation, which can lead to reduction in plant height and leaf area. Similar results have been reported in several crop species, such as common bean (Emam et al., 2010). These data are in agreement with those obtained by Siddiqui et al. (2015), revealing that faba bean growth performance was affected significantly and depends on the level of water deficit stress. Among the studied cultivars, drought drastically decreased leaf area, shoot and root fresh weight in ‘VIR 490’. This trend may be indicative of sensitivity to drought stress. Drought stress decreases net CO2 assimilation rate (A), stomatal conductance (gs) and transpiration rate (E) and reduces growth of crop plants (Saeidi & Abdoli, 2015). In the present study, drought stress significantly affected these parameters in all studied faba beans, which is in line with previous results (Mwanamwenge et al., 1999). Significant differences were observed between studied genotypes for the photosynthetic parameters. ‘Hara’ and ‘VIR 2128’ exhibited less reduction in photosynthesis activity than other cultivars under moderate and severe water deficit stress. Moreover, a genotypic variability in stomatal characteristics was found. ‘Hara’ cultivar displayed higher water use efficiency (A/gs) than other cultivars, which may be related to lower gs in ‘Hara’ under severe drought stress conditions. The high A/gs observed in ‘Hara’ cultivar indicates increased capacity for water saving in comparison to others. According to Ghaderi & Siosemardeh (2011), a high A/gs ratio reflects an ability to maintain photosynthetic capacity under drought stress conditions and a better drought tolerance. These findings could indicate that in faba bean, sensitive cultivars react to water availability by substantially decreasing their photosynthesis rate, while ‘Hara’, as a tolerant cultivar, has better photosynthesis performance under water shortage conditions. These facts are consistent with previously reported data in other plants, such as Glycine max (Zhou et al., 2013).
33Water deficit significantly decreased leaf stomatal conductance (gs) but slightly increased internal concentration of CO2 (Ci), which could be explained by a decrease in CO2 assimilation (Lu & Zhang, 1998). According to Fischer et al. (1998), mesophyl conductance (A/Ci) is an indicator of non-stomatal factor involved in CO2 assimilation. A/Ci was greater in ‘Hara’ than in other cultivars. These results suggest that ‘Hara’ may have higher CO2 assimilatory capacity of mesophyll cells than other genotypes under drought conditions. Ahmadi & Siosemardeh (2005) found that under drought stress A/Ci is greater in tolerant than in susceptible cultivars. Moreover, Ratnayaka & Kincaid (2005) found that the ability to maintain a high carbon gain appears to confer stress tolerance in crops. The results of this study suggest that under drought stress, photosynthesis in faba bean species could be limited by stomatal and non-stomatal factors. The results obtained for photosynthesis parameters are in agreement with earlier reports concerning chickpea (Mafakheri et al., 2010) and triticale, bread and barley (Roohi et al., 2013). Among the cultivars, ‘Hara’, with a higher stomatal control, was found to be more tolerant to drought stress than other faba beans.
34Leaf relative water content (RWC) is the appropriate measure of plant water status in terms of the physiological consequence of cellular water deficit (Yamasaki & Dillenburg, 1999). In the present experiment, leaf RWC of all cultivars decreased significantly depending on water deficit level, which suggested differences in leaf hydration, leaf water deficit and physiological water status in the different studied cultivars. This result strongly supports the findings of Siddiqui et al. (2015) in faba bean genotypes. The authors suggest that the differences in RWC in all genotypes could be associated with their capacity for water absorption from the soil. Among the studied cultivars, ‘Hara’ exhibited the highest RWC, while ‘Triple white’ had the lowest RWC under moderate and severe water deficit stress. Leaf RWC remained similar under well-watered conditions in both cultivars (Table 4). These results indicate that ‘Triple white’ leaves lost water more than ‘Hara’ during drought stress. The physiological analysis of RWC demonstrates that ‘Hara’ was more tolerant to drought stress than the other studied plant material. In common bean, Türkan et al. (2005) reported that drought-resistant cultivars have higher leaf RWC than drought-sensitive cultivars.
35In the present research, there was a significant decrease in the total chlorophyll amount due to the decrease in the Chlb rate of all studied faba beans. Chlorophyll content loss in plants, including faba bean, is a negative consequence of water deficit stress (Siddiqui et al., 2015). Our data are in agreement with previous studies indicating that Chla is less affected than Chlb in water deficiency (Mafakheri et al., 2010). This decrease in chlorophyll in faba bean under drought stress is mainly the result of inhibition of photosynthetic electron transport chain and the enzymes of chlorophyll biosynthesis (Tavakkoli et al., 2010).
36Drought stress induces changes in metabolic parameters that are associated with the tolerance of plants to water shortage. Accumulation of free amino acids (especially proline) and soluble carbohydrates by plant tissue under water deficit conditions is an adaptive response (Sheela Devi & Sujatha, 2014). Hayat et al. (2012) reported that overproduction of proline in plants exposed to various environmental stress imparts stress tolerance by maintaining cell turgor, stabilizing membranes and bringing concentrations of reactive oxygen species (ROS) to normal ranges. In this study, proline accumulation was significant in stressed plants compared to the control. We infer that accumulation of proline may have a role in tolerance of faba bean to drought stress. These facts are consistent with previously reported data for faba bean (Siddiqui et al., 2015), where drought tolerant genotypes accumulate more proline than sensitive genotypes.
37Soluble sugars are involved in various metabolic events (Rosa et al., 2009). However, they also act as molecules regulating different genes involved in osmolyte synthesis in response to environmental stresses (Rosa et al., 2009). Our results showed that soluble sugar content was positively influenced by water deficit stress (table 4): in all tested cultivars, soluble sugars increased with increasing water deficit levels. The current results support the hypothesis that soluble sugars play a main role for osmotic adjustment in plants. This finding is consistent with the data obtained by Ali et al. (2016), who reported an increase in soluble sugar content in the leaves of water stressed plants. Thus we suggest that accumulation of soluble sugars is important in response to water deficit stress in faba bean, contributing to osmotic adjustment in this species. Our results are in disagreement with previous research by Khalafallah et al. (2008), which revealed that drought stress did not affect soluble sugar content in faba bean.
38It is well established that various environmental stresses, including drought, lead to excessive production of reactive oxygen species (ROS), which can cause severe damage to protein, DNA, RNA and lipids, and consequently affects the whole cellular life (Richardson et al., 2015).
39Interestingly, ROS accumulation is counteracted by enzymatic antioxidant systems involving a variety of scavengers like catalase (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD) and guaiacol peroxidase (GPX) and non-enzymatic low molecular metabolites such as α-tocopherol, carotenoids and flavonoids (Uzilday et al., 2012).
40In the current study, in general, CAT, APX, SOD and GPX activities augmented with increasing levels of water deficit in all studied genotypes. At 30% FC the highest enzyme activities of CAT were noted in ‘Hara’ and ‘VIR 490’. On the other hand, ‘VIR 2128’ followed by ‘Hara’ had the highest GPX activity. The highest APX was found in ‘Local Beja’, followed by ‘Saber 02’ and ‘Hara’ at 30% FC. Superoxide dismutase activity was also influenced by water deficit stress and significantly increased under drought stress, but its activity showed lower levels than in other antioxidant enzymes activities under control and drought stress treatments (Table 5). Overall, the obtained results could suggest that the higher CAT, GPX and SOD activities may be important in giving ‘Hara’ tolerance to drought stress. The very high level of activity of these enzymes may help ‘Hara’ to be more tolerant than the other cultivars. These data are in agreement with other studies reporting the increased activity of antioxidant enzymes (POD, CAT and SOD) in faba bean in response to drought stress (Siddiqui et al., 2015). These authors found higher values for these enzymatic activities in tolerant genotypes ‘C5’ and ‘Zafar 1’ than in sensitive genotypes.
5. Conclusions
41The studied faba bean genotypes significantly differed in their tolerance to water deficit stress. All agro-physiological and biochemical traits of faba bean cultivars are strongly influenced under water deficit stress. However, the degree of water shortage tolerance depends on the interactions between the faba bean cultivars and the levels of water deficit stress. We suggest that ‘Hara’ cultivar could manage the water deficit condition better than other studied cultivars by adjusting its gas exchange and stomatal control, and through its ability to accumulate proline and soluble sugars. In addition, ‘Hara’ developed a more efficient antioxidant system to scavenge ROS than other cultivars by increasing CAT, APX and GPX activities. Even though under well-watered conditions, ‘Hara’ did not show the best grain yield potential, under water deficit stress it had the best grain harvest. From the outcome of the obtained results, ‘Hara’ cultivar could be considered as tolerant. For further study, this genotype can be used as a basis for studying the molecular mechanisms underlying faba bean tolerance to water deficit stress and could be utilized in faba bean breeding programs to combine its water deficit tolerance with other higher yield potential cultivars. Further studies are needed to verify the response of the studied genotypes to water deficit and to investigate the water deficit tolerance of faba bean at other growth stages and under field conditions.
Bibliographie
Abdellatif K.F., El Absawy S.A. & Zakaria A.M., 2012. Drought stress tolerance of faba bean as studied by morphological traits and seed storage protein pattern. J. Plant Stud., 1, 47-54.
Abid G. et al., 2015. Genetic relationship and diversity analysis of faba bean (Vicia faba L. var. minor) genetic resources using morphological and microsatellite molecular markers. Plant Mol. Biol. Rep., 33, 1755-1767.
Ahmadi A. & Siosemardeh A., 2005. Investigation on the physiological basis of grain yield and drought resistance in wheat: leaf photosynthetic rate, stomatal conductance, and non-stomatal limitations. Int. J. Agric. Biol., 7, 807-811.
Alghamdi S.S. et al., 2015. Physiological and molecular characterization of faba bean (Vicia faba L.) genotypes for adaptation to drought stress. J. Agron. Crop Sci., 201, 401-409.
Ali M.B.M. et al., 2016. Association analyses to genetically improve drought and freezing tolerance of faba bean (Vicia faba L.). Crop Sci., 56, 1036-1048.
Amede T. & Schubert S., 2003. Mechanisms of drought resistance in seed legumes. I. – Osmotic adjustment. Ethiop. J. Sci., 26, 37-46.
Ammar M.H. et al., 2014. Physiological and yield responses of faba bean (Vicia faba L.) to drought stress in managed and open field environments. J. Agron. Crop Sci., 201, 280-287.
Anjum S.A. et al., 2011. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res., 6, 2026-2032.
Arnon D.I., 1949. Copper enzymes in isolated chloroplasts, polyphenol oxidase in Beta vulgaris. Plant Physiol., 24, 1-15.
Beauchamp C. & Fridovich I., 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem., 44, 276-287.
Benešová M. et al., 2012. The physiology and proteomics of drought tolerance in maize: early stomatal closure as a cause of lower tolerance to short-term dehydration? PLoS One, 7, 1-17.
Bradford M.M., 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 722, 248-254.
Dhindsa R.S., Plumb-Dhindsa P. & Thorpe T.A., 1981. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot., 32, 93-101.
Emam Y., Shekoofa A., Salehi F. & Jalali A.H., 2010. Water stress effects on two common bean cultivars with contrasting growth habits. Am.-Eurasian J. Agric. Environ. Sci., 9, 495-499.
Farooq M. et al., 2009. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev., 29, 185-212.
Fischer R.A. et al., 1998. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci., 38, 1467-1475.
Galmes J., Flexas J., Save R. & Medrano H., 2007. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant Soil, 290, 139-155.
Ghaderi N. & Siosemardeh A., 2011. Response to drought stress of two strawberry cultivars (cv. Kurdistan and Selva). Hortic. Environ. Biotechnol., 52, 6-12.
Harb A., Awada D. & Samarah N., 2015. Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. J. Plant Interact., 10, 109-116.
Hasheminasab H., Assad M.T., Aliakbari A. & Sahhafi S.R., 2012. Influence of drought stress on oxidative damage and antioxidant defense system in tolerant and susceptible wheat genotypes. J. Agric. Sci., 4, 8-30.
Hayat S. et al., 2012. Role of proline under changing environments. Plant Signaling Behav., 7, 1456-1466.
Hoagland D.R. & Arnon D.I., 1950. The water culture method for growing plant without soil. Circular 347. Berkeley, CA, USA: University of California, The College of Agriculture, California Agricultural Experiment Station.
Hossaina M.M. et al., 2014. Differences between soybean genotypes in physiological response to sequential soil drying and rewetting. Crop J., 2, 366-380.
Jaleel C.A. et al., 2009. Drought stress in plants: a review on morphological characteristics and pigments composition. Int. J. Agric. Biol., 11, 100-105.
Khalafallah A.A., Tawfik K.M. & Abd El-Gawad Z.A., 2008. Tolerance of seven faba bean varieties to drought and salt stresses. Res. J. Agric. Biol. Sci., 4, 175-186.
Khan H.R., Link W., Hocking T.J. & Stoddard F.L., 2007. Evaluation of physiological traits for improving drought tolerance in faba bean (Vicia faba L.). Plant Soil, 292, 205-217.
Kharrat M. & Ouchari H., 2011. Faba bean status and prospects in Tunisia. Grain Legumes, 56, 11-12.
Link W. et al., 1999. Genotypic variation for drought tolerance in Vicia faba. Plant Breed., 118, 477-483.
Lopes M.S. et al., 2012. The yield correlations of selectable physiological traits in a population of advanced spring wheat lines grown in warm and drought environments. Field Crops Res., 128, 129-136.
Lopez-Bellido F.J., Lopez-Bellido L. & Lopez-Bellido R.J., 2005. Competition, growth and yield of faba bean (Vicia faba L.). Eur. J. Agron., 23, 359-378.
Lu C. & Zhang J., 1998. Change in photosynthesis II function during senescence of wheat leaves. Physiol. Plant., 104, 239-247.
Maalouf F., 2011. Faba bean improvement at ICARDA: constraints and challenges. Grain Legumes, 56, 13-14.
Mafakheri A. et al., 2010. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci., 4, 580-585.
Maritimi T.K. et al., 2015. Physiological and biochemical response of tea [Camellia sinensis (L.) O. Kuntze] to water-deficit stress. J. Hortic. Sci. Biotechnol., 90, 395-400.
Mohamad Zabawi A.G. & Dennett M.D.D., 2010. Responses of faba bean (Vicia faba) to different levels of plant available water: I. Phenology, growth and biomass partitioning. J. Trop. Agric. Food Sci., 38, 11-19.
Mohammadkhani N. & Heidari R., 2008. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J., 3, 448-453.
Mwanamwenge J., Loss S.P., Siddique K.H.M. & Cocks P.S., 1999. Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). Eur. J. Agron., 11, 1-11.
Nagahatenna D.S.K., Langridge P. & Whitford R., 2015. Tetrapyrrole-based drought stress signaling. Plant Biotechnol. J., 13, 447-459.
Nakano Y. & Asada K., 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol., 22, 867-880.
Nazarli H. & Faraji F., 2011. Response of proline, soluble sugars and antioxidant enzymes in wheat (Triticum aestivum L.) to different irrigation regimes in green house condition. Cercetări Agronomice Moldova, 148, 27-33.
Plewa M.J., Smith S.R. & Wanger E.D., 1991. Diethyldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutat. Res., 247, 57-64.
Ratnayaka H.H. & Kincaid D., 2005. Gas exchange and leaf ultrastructure of tinnevelly senna, Cassia angustifolia, under drought and nitrogen stress. Crop Sci., 45, 840-847.
Richardson C., Yan S. & Vestal C.G., 2015. Oxidative stress, bone marrow failure, and genome instability in hematopoietic stem cells. Int. J. Mol. Sci., 16, 2366-2385.
Roohi E., Tahmasebi-Sarvestani Z., Modarres-Sanavy S.A.M. & Siosemardeh A., 2013. Comparative study on the effet of soil water stress on photosynthesis function of triticale, bread wheat, and barley. J. Agric. Sci. Technol., 15, 215-228.
Rosa M. et al., 2009. Soluble sugars-metabolism, sensing and abiotic stress. Plant Signal. Behav., 4, 388-393.
Rozrokh M., Sabaghpour S.H., Armin M. & Asgharipour M., 2012. The effects of drought stress on some biochemical traits in twenty genotypes of chickpea. Eur. J. Exp. Biol., 2, 1980-1987.
Saeidi M. & Abdoli M., 2015. Effect of drought stress during grain filling on yield and its components, gas exchange variables, and some physiological traits of wheat cultivars. J. Agric. Sci. Technol., 17, 885-898.
Shao H.B. et al., 2008. Higher plant antioxidants and redox signaling under environmental stresses. C.R. Biol., 331, 433-441.
Sharma P., Jha A.B., Dubey R.S. & Pessarakli M., 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot., 2012, ID 217037.
Sheela Devi S.P. & Sujatha B., 2014. Drought-induced accumulation of soluble sugars and proline in two pigeon pea (Cajanus Cajan L. Millsp.) cultivars. Int. J. Innovative Res. Dev., 3, 302-306.
Siddiqui M.H. et al., 2015. Response of different genotypes of faba bean plant to drought stress. Int. J. Mol. Sci., 16, 10214-10227.
Tavakkoli E., Rengasamy P. & McDonald G.K., 2010. High concentrations of Na+ and Cl- ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot., 61, 4449-4459.
Türkan I., Bor M., Ozdemir F. & Koca H., 2005. Differential response of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris subjected to polyethylene glycol mediated water stress. Plant Sci., 168, 223-231.
Uzilday B. et al., 2012. Comparaison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci., 182, 59-70.
Wu G.Q., Zhang L.N. & Wang Y.Y., 2012. Response of growth and antioxidant enzymes to osmotic stress in two different wheat (Triticum aestivum L.) cultivars seedlings. Plant Soil Environ., 58, 534-539.
Yamasaki S. & Dillenburg L.R., 1999. Measurement of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg., 11, 69-75.
Yooyongwech S., Cha-um S. & Supaibulwatana K., 2012. Proline related genes expression and physiological changes in Indica rice response to water-deficit stress. Plant Omics, 5(6), 597-603.
Zarafshar M. et al., 2014. Morphological, physiological and biochemical responses to soil water deficit in seedlings of three populations of wild pear tree (Pyrus boisseriana). Biotechnol. Agron. Soc. Environ., 18, 353-366.
Zhou S. et al., 2013. How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress. Agric. For. Meteorol., 182, 204-214.
Ziadi Backouchi I., Aouida M. & Jebara M., 2015. Drought stress response in Tunisian populations of faba bean (Vicia faba L.). J. Plant Biol. Res., 4, 55-72.

