Evaluation of biocide effects of Aloysia gratissima (Gillies & Hook.) Tronc. essential oils
Résumé
Évaluation des effets biocides des huiles essentielles d'Aloysia gratissima (Gillies & Hook.) Tronc.
Description du sujet. Les huiles essentielles sont des fractions volatiles produites par le métabolisme particulier des plantes. Une application biologique importante des huiles essentielles ces dernières années a été leur utilisation comme bioherbicide, en raison de leur effet biocide important.
Objectifs. Cette étude vise à identifier les constituants chimiques de l’huile essentielle d’Aloysia gratissima (Gillies & Hook.) Tronc. et à évaluer son potentiel biocide.
Méthode. Cette étude décrit, pour la première fois, la constitution chimique des huiles essentielles des graines d'A. gratissima. Pour vérifier un éventuel effet biocide, les graines de laitue ont été soumises à différentes concentrations d'huiles essentielles provenant des feuilles (AG-LE-EO), des fleurs (AG-FL-EO) et des graines (AG-SE-EO) d'A. gratissima afin d'évaluer le pourcentage de germination, le temps moyen de germination, la synchronie de la germination et la longueur des plantules.
Résultats. À des concentrations de 800 μl·l-1, la synchronie a été positivement influencée par (AG-LE-EO), passant de 0,38 à 0,54. D’autre part, l’AG-SE-EO à des concentrations de 800 μl·l-1 a réduit la germination de la laitue de 90,8 % à 64,4 % et la longueur des semis de 1,35 à 0,8 cm, en plus d’augmenter le temps moyen de germination, ce qui indique des effets biocides.
Conclusions. Ces résultats devraient conduire à d’autres essais visant à comprendre comment les constituants des huiles essentielles d’A. gratissima inhibent la germination, ce qui en fait un bioherbicide potentiel.
Abstract
Description of the subject. Essential oils are volatile fractions produced through the special metabolism of plants. One significant biological application of essential oils in recent years has been their use as bioherbicides, due to their important biocide effect.
Objectives. This study aims to identify the chemical constituents of Aloysia gratissima (Gillies & Hook.) Tronc. essential oil and assess its biocide potential.
Method. This study describes, for the first time, the chemical constitution of the essential oils from seeds of A. gratissima. To verify possible biocide effect, lettuce seeds were submitted to different concentrations of essential oils from A. gratissima leaves (AG-LE-EO), flowers (AG-FL-EO), and seeds (AG-SE-EO) to evaluate germination percentage, mean germination time, synchrony of germination, and seedling length.
Results. At concentrations of 800 μl·l-1, synchrony was positively influenced by (AG-LE-EO), increasing from 0.38 to 0.54. On the other hand, AG-SE-EO at concentrations of 800 μl·l-1 reduced lettuce germination from 90.8% to 64.4% and seedling length from 1.35 to 0.8 cm, besides increasing mean germination time, indicating biocide effects.
Conclusions. These results are expected to lead to further trials to understand how the constituents of A. gratissima essential oils inhibit germination, making them a potential bioherbicide.
* These authors equally contributed to this work.
Received 17 March 2024, accepted 17 December 2024, available online 20 January 2025.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1Essential oils (EO) are a class of volatile, lipophilic, and low molecular weight compounds that are produced through the special metabolism of plants. Typically, they are liquid at room temperature and possess an attractive smell (Bakkali et al., 2008). This class of compounds is used by plant organisms to attract pollinators, deter predators, and perform other secondary plant functions. The utilization of volatile fractions for various biochemical purposes has shown promising potential in the development of new technologies, including the use of these metabolites for agrochemical purposes (El-Remaly et al., 2022; Morais, 2009).
2Weed growth negatively impacts the functionality of numerous ecosystems and crop yield (Jabran et al., 2015). Currently, biochemical methods of pest control, like the use of bioherbicides based on chemicals that promote allelopathy, are preferred, as this practice may create a form of defensive chemical adaptation between plants, directly and indirectly affecting plant properties (Bezerra et al., 2021; El-Remaly et al., 2022). Allelochemicals in biological systems play a crucial role in this process. Once these chemicals are released into the environment, they can influence the growth and development of the surrounding plant systems. This influence can be seen in terms of plant dominance and succession, formation of plant communities, yield, and management of the cultivated area (Ribeiro et al., 2019). Allelochemicals occurring naturally on plants, and can be act as an interesting source of ecological biocidal compounds (Gatger & Dar, 2021).
3Therefore, metabolites from cultivable plants are an important alternative in the search for new bioactive compounds that can be used as bioherbicides (Macias et al., 2007). Bioherbicides based on allelochemicals can be biodegradable and have little or no autotoxicity and heterotoxicity. They also represent a gain in terms of green chemistry, allowing for the flexible adoption of allelopathy for weed management and making agronomic practices sustainable and organic (Scavo & Mauromicale, 2021). One of the species that stands out due to its chemical and cultivation characteristics is Aloysia gratissima (Gillies & Hook.) Tronc., popularly known as Brazilian lavender. Aloysia gratissima is a plant native to America and is easily adapted to tropical climates. It has a variety of very interesting biological effects described in the literature, such as anesthetic, leishmanicidal, antimicrobial, antiproliferative, antioxidant, and antiviral activity. Hence, it can be easily explored for other biochemical purposes (Souza & Wiest, 2007; Hister et al., 2009; Vandresen et al., 2010; Santos et al., 2013; Zeni et al., 2013; Benovit et al., 2015; Santos et al., 2015; Garcia et al., 2018).
4Aloysia gratissima is a species with remarkable biological potential, as explored in several works published in the literature. Benovit et al. (2015) conducted studies on the anesthetic biological activity of A. gratissima EO and examined the effects of five of its major compounds tested alone. These studies have demonstrated the potential use of the plant's EO for anesthesia purposes. Garcia et al. (2018) investigated the in vitro effects of A. gratissima EO and its major sesquiterpenes against Leishmania amazonensis. The study revealed that the EO was effective in the mortality of promastigote and amastigote species at concentrations of 25 and 0.16 μg·ml-1, respectively. Additionally, the authors described that the EO of A. gratissima directly affects the mitochondrial matrix and plasma membrane of the parasites, making it a promising drug candidate for the development of drugs against leishmaniasis (Garcia et al., 2018). Apart from these findings, several other interesting biological activities have been described in the literature, as mentioned above. However, the biocide activity of A. gratissima has not been explored yet.
5Despite being an easy-to-grow plant species, studies on the potential of A. gratissima EO as a bioproduct are scarce (Bettega & Trevisan, 2022; Souza et al., 2022). Considering that several EOs have been employed as botanical pesticides, and often used as antimicrobial agents (Ordóñez et al., 2023), the objective of this study is to access the chemical constituents of the EO present in the A. gratissima leaves, flowers, and seeds and investigate their potential biocide effects.
2. Materials and methods
2.1. Extraction of EO of A. gratissima
6Parts of the plant absent from herbivory were collected in the city of Ribeirão Preto (São Paulo, Brazil), at the geographic coordinates 21°12'38''S 47°46'60''W in September 2021. The collected material, which contained reproductive parts of the plant was used to prepare voucher specimens. The taxonomic identification of the plant species was conducted by Prof. Milton Groppo and the specimens were deposited in the herbarium of Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto (São Paulo, Brazil). The fresh plant (115 g) of each plant part (leaves, flowers and seeds) of A. gratissima was equally divided and placed into three round bottom flasks coupled to a Clevenger-type apparatus containing 0.5 l of distilled water and submitted to hydrodistillation for 3 h (Martins et al., 2017; Alcoba et al., 2018). After the extraction of the EO, the organic layer obtained was treated with anhydrous magnesium sulfate (Synth, Diadema, Brazil) and filtered off. The EO samples were stored in a hermetically closed amber flask, which was kept in a refrigerator at -10 °C until chemical and biological analyses were carried out. The yield was calculated by the ratio between the mass of EO collected and the mass of leaves, flowers or seeds used in each extraction.
2.2. Identification of A. gratissima EO constituents
7Gas chromatography mass spectrometry (GC-MS) analyses were conducted on a Shimadzu QP2010 Plus equipped with an AOC-20i autosampler (Shimadzu Corporation, Kyoto, Japan). An Rtx-5MS (Restek Co., Bellefonte, USA) fused silica capillary chromatograph column (30 m x 0.25 mm i.d. x 0.25 m film thickness) was used. The electron ionization mode was used at 70 eV, with helium (99.999%) as the carrier gas at a constant flow rate of 1.0 ml·min-1. Mass spectra were taken at a scan interval of 0.5 s for mass ranging from 40 to 600 Da. Gas chromatography – flame ionization detection (GC-FID) analyses were performed on a Shimadzu GC2010 Plus gas chromatograph equipped with an AOC-20s autosampler fitted with FID. An Rtx-5 (Restek Co., Bellefonte, USA) fused silica capillary column (30 m x 0.25 mm i.d. x 0.25 m film thickness) was employed. Helium (99.999%) was used as the carrier gas at a constant flow of 1.0 ml·min-1.
8The EOs were dissolved in n-hexane (Neon Química, Suzano, Brazil) and analyzed by GC-MS and GC-FID. The injection volume was 0.1 l at split ratio of 1:10. The injector source and detector temperature were set at 240 °C and 280 °C, respectively. The column temperature was programmed to rise from 60 to 240 °C at a rate of 3 °C·min-1, then held at 240 °C for 5 min; the injection volume was 0.1 l at a split ratio of 1:10; the injector and ion source were set at 240 °C and 280 °C, respectively.
9The relative concentrations of the constituents of the essential oils of A. gratissima leaves (AG-LE-EO), flowers (AG-FL-EO), and seeds (AG-SE-EO) were estimated by peak area normalization (%) and expressed as the average of three GC-FID analyses. The AG-EO components were accessed based on the retention index on Rtx-5 and Rtx-5MS capillary columns under the same operating conditions relative to a homologous series of n-alkanes (C8-C20, Sigma-Aldrich, Saint Louis, USA). The structures were computer-matched with the Wiley 7, NIST 8, and FFNSC 1.2 spectra libraries and compared with literature data (Adams, 2017; Liu et al., 2023).
2.3. Evaluation of biocide response of A. gratissima EO
10The experiments on biocide activity were conducted at the Laboratory of Biotechnology located in the Instituto Federal de Educação, Ciência e Tecnologia Goiano - Campus Urutaí (Urutaí, Goiás, Brazil) in September 2022. The trials aim to verify the biocide effect of A. gratissima EO on lettuce (Lactuca sativa) seed germination. Lettuce is commonly used as a model plant in biocide bioassays due to its sensibility to phytochemicals and fast germination rate (Chon et al., 2005; Mirmostafaee et al., 2020). Essential oils from A. gratissima leaves (AG-LE-EO), flowers (AG-FL-EO), and seeds (AG-SE-EO) were diluted in distilled water + 0.01% of MeOH (Sigma-Aldrich, St. Louis, USA) at concentrations of 800, 600, 400 and 200 l·l-1. Distilled water + 0.01% MeOH was used as a negative control, resulting in a total of five treatments. The experiments followed a completely randomized design with five replications of 50 lettuce seeds per plot. Each plot consisted of a germination box with two sheets of germination paper. The paper was weighed, and it was moistened with 2.5-fold its volume (12.5 ml) of distilled water or the solution in the respective concentration. Then, 50 lettuce seeds were evenly spaced on the already moistened germination paper.
11The boxes were kept in a germination chamber for a photoperiod of 12 h and a temperature of 25 °C ± 3 °C. The evaluations were conducted daily, and seeds with a radicle measuring at least 2 mm were considered germinated. Based on the daily readings, the germination percentage (G, %), mean germination time (MGT, days), synchrony of the germination process (Z), and seedling length (SL, cm) were determined. The traits G, MGT, and Z were calculated following the method proposed by Ranal et al. (2009):

12where ti: time from the start of the experiment to the ith observation (days); ni: number of seeds germinated in the ith time correspondent to the ith observation, and k: last time of germination.
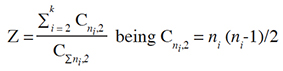
13where Cni,2: combination of the seeds germinated in the ith time, two by two, and ni: number of seeds germinated in the ith time.
14Seven days after the test was set up, the final germination count was performed, and the length of the seedling was measured using a ruler graduated in millimeters. The evaluations were conducted following the specifications outlined in the Brazilian Seed Analysis Rules (MAPA/ACS, 2009). After confirming that, the assumptions for the analysis of variance were met and the data were subjected to Anova. In cases where differences were detected, regression analysis was performed at a significance level of α = 5%.
3. Results
3.1. Identification of A. gratissima EO constituents
15The EO obtained from A. gratissima leaves (AG-LE-EO), flowers (AG-FL-EO), and seeds (AG-SE-EO) showed a yellowish color. These EOs were obtained in 0.75 ± 0.12%, 0.85 ± 0.24%, and 1.44 ± 0.24% yield (w/w), respectively. The chemical composition of AG-LE-EO, AG-FL-EO, and AG-SE-EO is shown in table 1. GC-MS and GC-FID analyses revealed that AG-LE-EO is composed of monoterpene hydrocarbons (14.2%), oxygenated monoterpenes (31.3%), sesquiterpene hydrocarbons (26.0%), oxygenated sesquiterpenes (22.3%). A total of 25 compounds were identified in AG-LE-EO, with trans-pinocamphone (11.7%), guaiol (10.1%), germacrene B (8.3%), β-pinene (7.4%) and caryophyllene oxide (8.5%) as the major compounds.
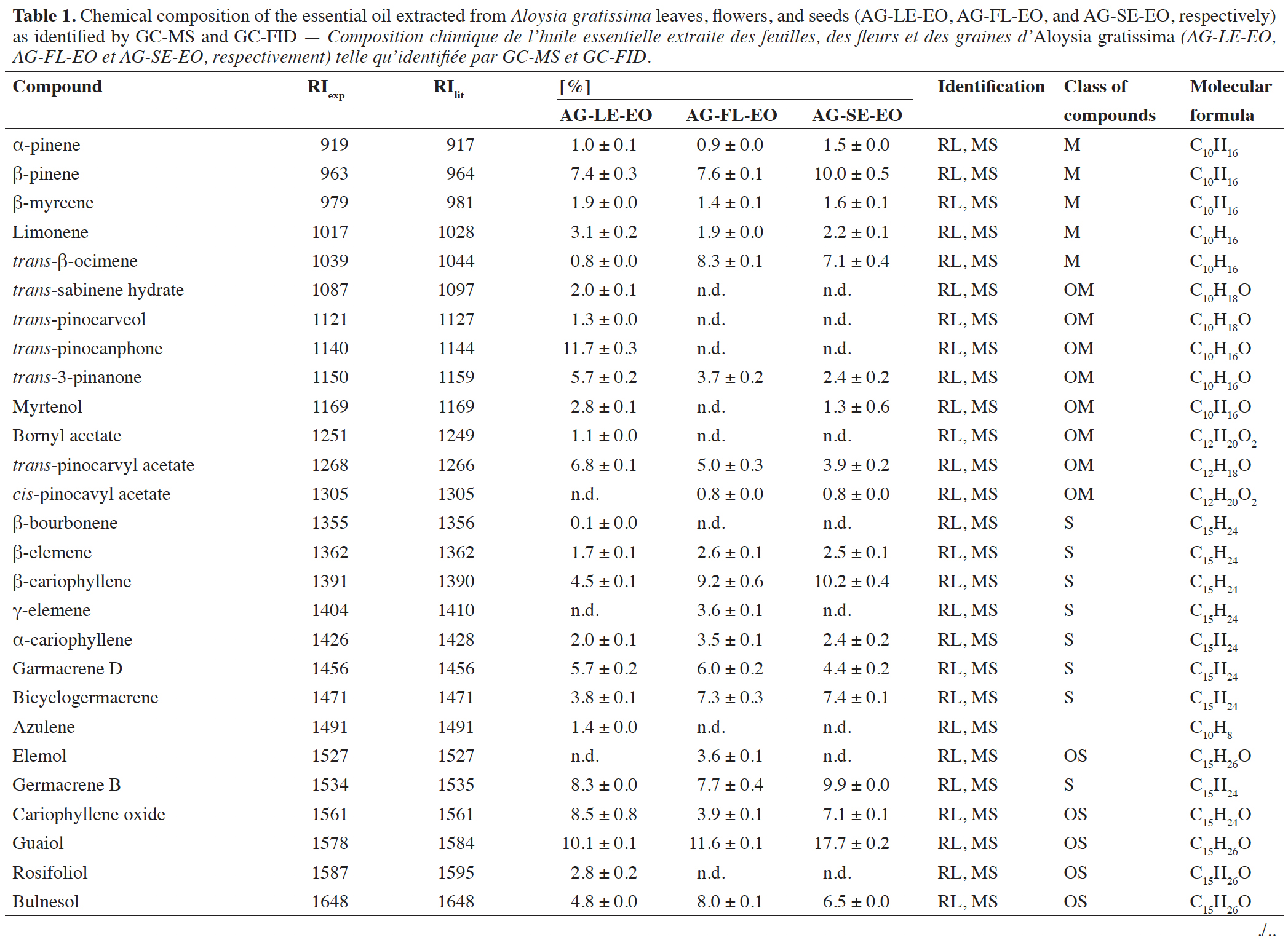
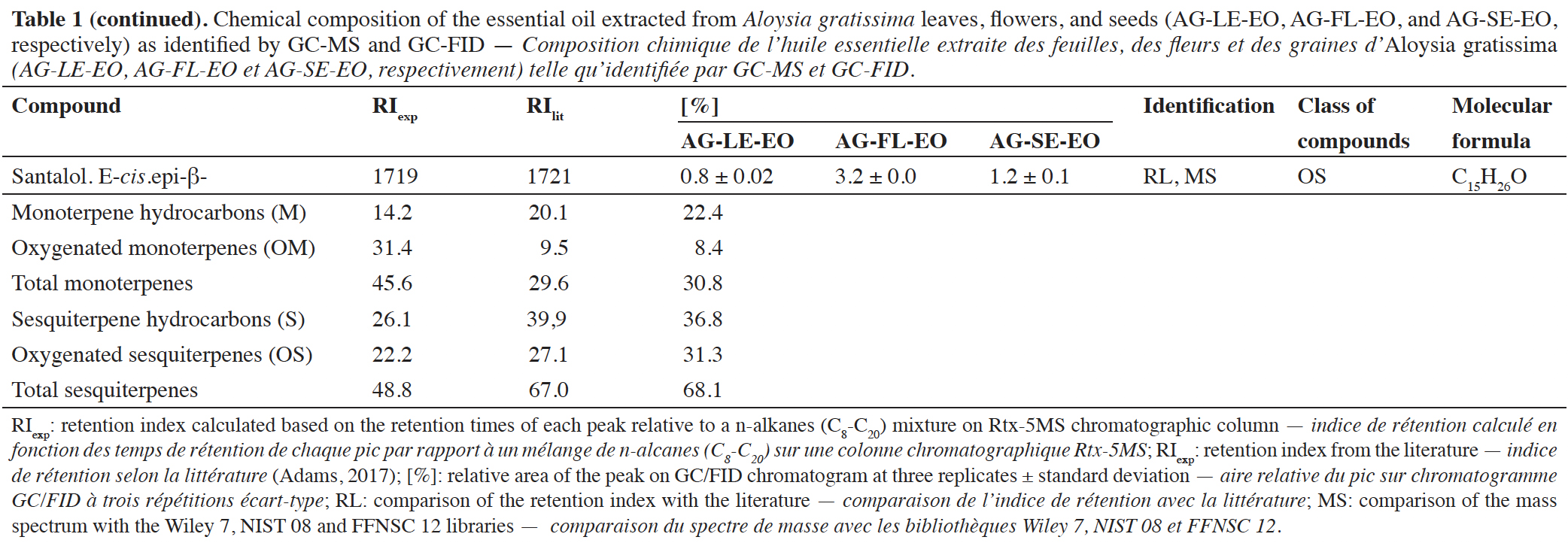
16In the EO of A. gratissima flowers (AG-FL-EO), 20 compounds were observed. The chemical composition of this EO includes monoterpenes (20.2%), oxygenated monoterpenes (9.5%), sesquiterpene hydrocarbons (40.0%), and oxygenated sesquiterpenes (22.2%). The main constituents were guaiol (11.7%), caryophyllene (9.2%), trans-β-ocimene (8.3%), bulnesol (8.0%) and germacrene B (7.7%) (Table 1). In the case of EO of A. gratissima seeds (AG-SE-EO), GC-MS analysis revealed 19 compounds. These compounds included monoterpenes (23.7%), oxygenated monoterpenes (7.0%), sesquiterpenes (36.8%), and oxygenated sesquiterpenes (26.0%). The majority constituents identified in AG-SE-EO were guaiol (17.7%), β-caryophyllene (10.2%), β-pinene (10.0%), germacrene B (9.9%) and bicyclogermacrene (7.4%) (Table 1).
3.2. Evaluation of biocide response of A. gratissima EO
17Allelopathy refers to the influence of plants on neighboring plants through the release of allelochemicals, which can affect their growth. This influence on the development of the surrounding plants can be either positive or negative (Khamare et al., 2022). Allelochemicals arise as an alternative source of natural biocides and can be act in reducing the use of synthetic biocides or herbicides (Gatger & Dar, 2021). The effects of the concentration of the EOs extracted from A. gratissima leaves, flowers, and seeds on the initial development of lettuce seedlings are presented in table 2. Significant differences were found in the germination (Z) and seedling lengh (SL) for treatments with AG-LE-EO, whereas Z was affected by treatments with AG-FL-EO, and for all traits when evaluating the influence of AG-SE-EO. Exploring the results, the trait Z was positively influenced by an increase in the concentration of the AG-LE-EO. Synchrony increased from 0.38 for the control to 0.54 at 800 μl·l-1 (Figure 1).
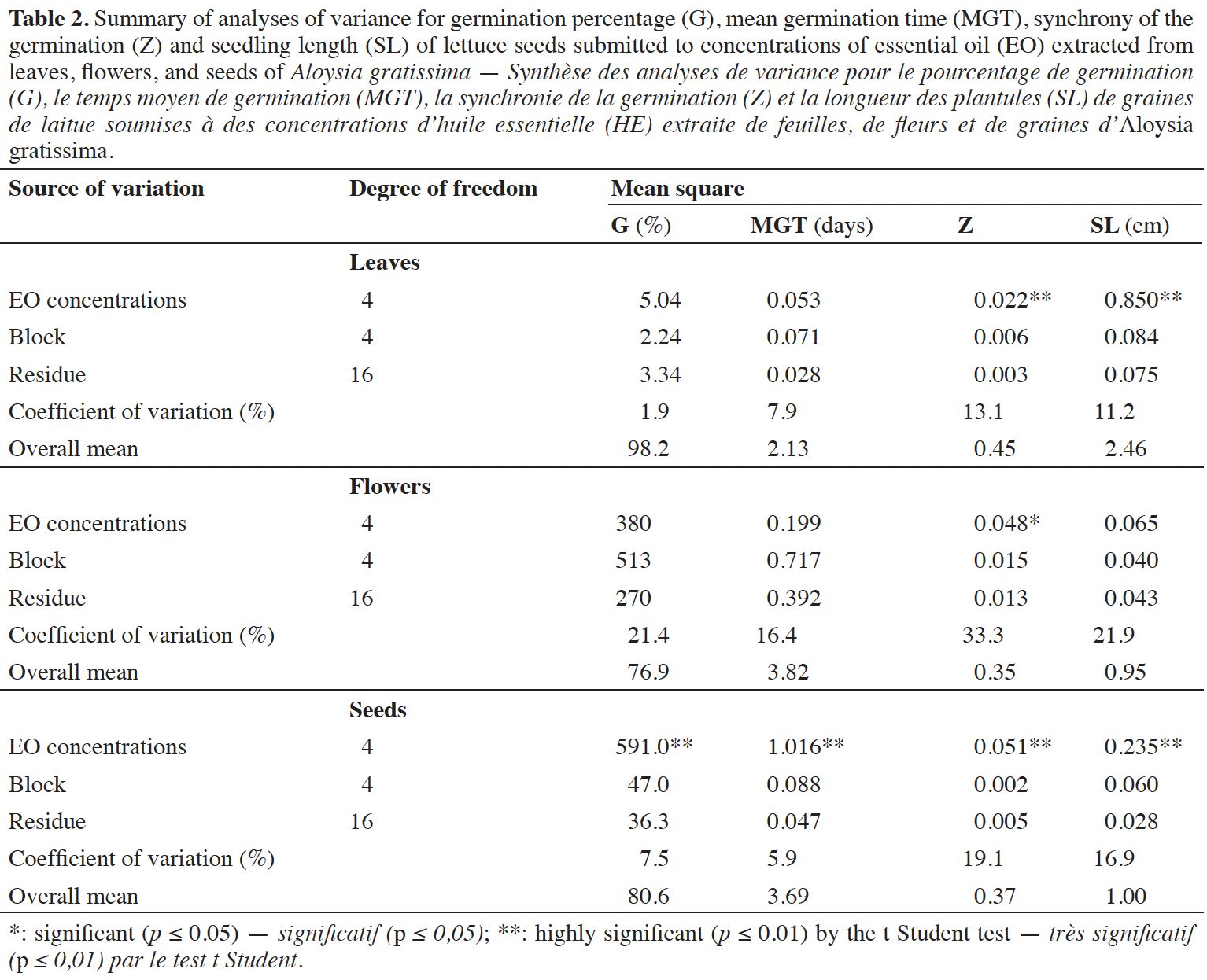
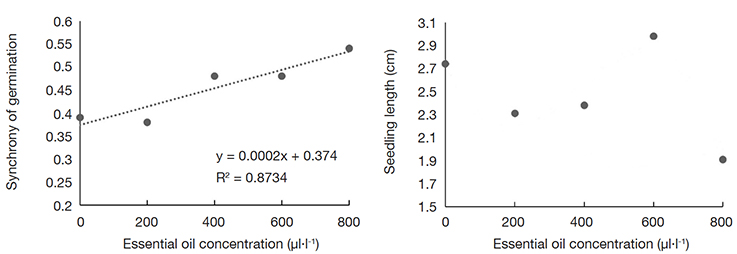
Figure 1. Synchrony of germination and seedling length (cm) of lettuce seeds submitted to concentrations of essential oil extracted from Aloysia gratissima leaves (AG-LE-EO) — Synchronisation de la germination et de la longueur des plantules (cm) des graines de laitue soumises à des concentrations d’huile essentielle extraite des feuilles d’Aloysia gratissima (AG-LE-EO).
18Although there were significant differences between the doses of OE, it was not possible to find a clear trend or fit any linear or non-linear model that could coherently explain the results based on biological aspects for the trait SL influenced by AG-LE-OE (Figure 1). The same dynamic was observed for the trait Z when the lettuce seeds were exposed to AG-FL-EO (Figure 2) and AG-SE-EO (Figure 3). The increase in the concentration of AG-SE-EO reduced germination from 90.8% (control) to 64.4% (800 μl·l-1) and decreased the SL from 1.35 cm to 0.8 cm (Figure 3). On the other hand, the longest MGT (4.23 days) was observed when the highest concentration of AG-SE-EO was applied, whereas the seeds of the control treatment showed a MGT of 2.99 days.
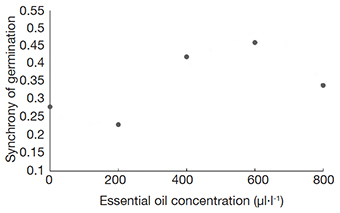
Figure 2. Synchrony of germination of lettuce seeds submitted to concentrations of essential oil extracted from Aloysia gratissima flowers (AG-FL-EO) — Synchronie de germination des graines de laitue soumises à des concentrations d’huile essentielle extraite des fleurs d’Aloysia gratissima (AG-FL-EO).
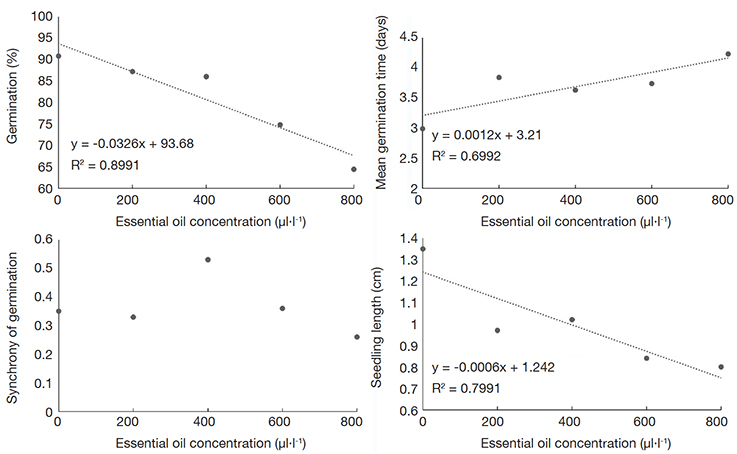
Figure 3. Germination percentage (%), mean germination time (days), synchrony of the germination and seedling length (cm) of lettuce seeds submitted to concentrations of essential oil extracted from Aloysia gratissima seeds (AG-SE-EO) — Pourcentage de germination (%), temps de germination moyen (jours), synchronie de la germination et longueur des plantules (cm) des graines de laitue soumises à des concentrations d’huile essentielle extraite de graines d’Aloysia gratissima (AG-SE-EO).
4. Discussion
19Considering the extraction of EOs, the results obtained for AG-LE-EO and AG-FL-EO are consistent with data previously reported in the literature. For exemple, Garcia et al. (2018) reported an extraction yield of 0.52% (w/w) for the EO from A. gratissima leaves, whereas Santos et al. (2015) described an extraction yield of 0.56% (w/w) for an EO obtained from A. gratissima flowers. It is worth noting that this is the first study to report data on the extraction and chemical composition of the EO from A. gratissima seeds. By means of the chemical composition of AG-LE-EO, AG-FL-EO, and AG-SE-EO, the EOs are very similar. However, some compounds are only present in AG-FL-EO and AG-SE-EO, and the relative percentages of constituents differ significantly. When comparing the data with Santos et al. (2015), we observe a certain similarity with the data obtained through GC-MS analyses. The main constituents were found in the study to be trans-pinocampone (11.7%), guaiol (10.1%), and germacrene B (8.3%) (Santos et al., 2015). It is important to note that the comparison is more similar with Santos et al. (2015) than with Garcia et al. (2018).
20The literature still highlights other major constituents such as β-pinene (7.4%) and caryophyllene oxide (8.5%), although they are reported in smaller proportions (Garcia et al., 2018; Santos et al., 2015). These differences in determining the chemical composition of EO are common, as variations in locations and cultivation conditions can lead to slight differences in the chemical constitution of the analyzed EO (Woolf, 1999). Although the major constituents of AG-LE-EO, AG-FL-EO, and AG-SE-EO do not change drastically, their relative percentages demonstrate marked differences. Several interesting biological activities have been described for A. gratissima EOs in the literature, as mentioned above. However, the biocidal activity has not yet been explored. This prompts us to investigate these differences in biological trials, as described in this section.
21Table 2 presents an interesting correlation between germination percentage (G), mean germination time (MGT), synchrony of the germination (Z) and seedling length (SL) of lettuce seeds in different concentrations of A. gratissima EOs. In the case of AG-LE-EO, the concentration of the EO influenced both the synchrony of germination (Z) and seedling length (SL). The effect of the AG-FL-EO concentration was only observed in the trait Z. However, the concentration of AG-SE-EO affected all traits, including germination (G), mean germination time (MGT), Z and SL.
22We observed for AG-LE-EO a positive influence of trait Z in increasing concentrations in EO (Figure 1). Thiesen et al. (2019) analyzed the effect of Lippia alba EO on the germination, vigor, and emergence of lettuce, and observed a positive effect on the dry mass of seedlings with an increase in the EO concentration for one batch of lettuce seeds. These results could be explored to utilize AG-LE-EO in increasing the germination synchrony of species that exhibit high variability (Thiesen et al., 2019). The index Z ranges from 0 to 1, with 0 indicating that at least two seeds germinated at different times, and 1 indicating that all seeds germinated simultaneously (Ranal & Santana, 2006; Ranal et al., 2009). The overall mean for Z in AG-FL-EO treatment was 0.35, and it ranged from 0.23 (at 200 μl·l-1) to 0.46 (600 μl·l-1), low to medium value, indicating high to medium variability.
23Variations in the responses of plants to different doses and biochemicals present in EOs are common. To mention, Thiesen et al. (2019) verified cubic trends for many traits, such as germination, percentage of normal and abnormal seedlings, radicle and shoot length, showing that lettuce is affected by the application of L. alba EO (Thiesen et al., 2019). In contrast, Tigre et al. (2012) observed no influence of extracts of Cladonia verticillaris on the germination of lettuce seeds, but they did observe modifications in leaf area and seedling hypocotyl and root development. Essential oils and extracts can induce different effects, either stimulating or inhibiting physiological processes. The responses depend on diverse factors, such as the plant species, concentration of the compound, and interaction with biotic and abiotic factors. Physiological processes have different responses depending on the doses of each specific allelochemical, and this determines varied responses in plants (Reigosa et al., 1999; Tigre et al., 2012).
24For AG-SE-EO consistent results were found indicating a negative influence of the mixture of phytochemical compounds, which suggests biocide effects on lettuce seeds. The increase in AG-SE-EO led to a reduction in lettuce G and SL, while increasing MGT (Figure 3). Although differences were detected (Table 2), it was not possible to explain the influence of the increase in AG-SE-EO concentration on Z. Crucial elements that can be assessed to provide insights into the dynamics of the germination process are time, rate, homogeneity, and synchrony (Ranal & Santana, 2006; Ranal et al., 2009). At the highest concentrations, AG-SE-EO influenced the development of lettuce seedlings, suggesting its potential as a bioherbicide.
25There is a growing interest in EOs as a means to reduce reliance on synthetic herbicides, and promising results have been observed for various species (Tigre et al., 2012; Thiesen et al., 2019; Verdeguer et al., 2020). Generally, EOs are associated with negative effects on germination, especially at higher concentrations. However, many biocide effects of EO on the germination, vigor, and emergence of some lettuce batches were reported in the literature. Mirmostafaee et al. (2020) investigated the inhibitory effects of 112 EOs on lettuce seeds and seedlings, and found biocide interaction effects with an increase in the dose.
26Although A. gratissima is widely used in folk medicine, studies of A. gratissima EO as a bioproduct in the agronomic field as a potential bioherbicide are scarce (Souza et al., 2022), and that includes the use of A. gratissima EO as a potential bioherbicide. As a botanical bioproduct, recently, the biocide effect of EO and hydrolate obtained from A. gratissima leaves was evaluated in the control of the exotic grass Eragrostis plana germination. The reports suggested promising results in the control of this weed (Bettega & Trevisan, 2022).
27Analyzing the compounds (Table 1), it was observed that for AG-SE-EO the major compound was guaiol, and it is present in higher concentrations in seeds (17.7%) compared to leaves (10.1%) and flowers (11.6%). In recent studies, guaiol has been reported as an allelochemical that might exhibit negative allelopathic effects (Ojija, 2023). This could explain, in principle, the prominent influence of AG-SE-EO on lettuce seeds germination and initial development. In addition, guaiol is reported to affect the plasma membranes of cells and organelles. According to Garcia et al. (2018), the antileishmanial activity of A. gratissima EO is due to the presence of this sesquiterpene (Garcia et al., 2018). These researchers proposed that A. gratissima EO and guaiol act on the mitochondrial matrix, plasma membrane, and other structures resulting in an antileishmanial effect. It is worth investigating if this action on plants could be similar.
28The analysis of AG-SE-EO composition leads us to discuss the role of compound caryophyllene (10.2%) which plays a key role in the biocide effect. Reports in the literature describe this substance as an indirect defense mechanism plant (Wang et al., 2010). Another major constituent of AG-SE-EO is β-pinene, an oxygenated monoterpene found in some flavor plants of Myrtaceae and Rutaceae, which is known to have important roles in mediating intra- and inter- plant interactions, favoring biocide actions (Singh et al., 2006; Batish et al., 2008; Chowhan et al., 2011). Studies show that β-pinene acts as a growth inhibitor by inducing a stress in plant organisms, which alters the biochemical profile of enzymes involved in plant defense (Chowhan et al., 2011). Both chemicals are major compounds in AG-SE-EO and are present in larger quantities compared to other EOs analyzed. This suggests, in principle, the possibility of synergy in the action of these compounds as allelochemicals. In addition, investigating the environmental influence on the efficacy of these compounds, such as temperature, soil water stress, humidity, and others, as suggested in recent report (Roberts et al., 2022), can clarify the action mode of A. gratissima EOs as potential bioherbicides.
5. Conclusions
29In this work, we identified the chemical constituents of A. gratissima EO obtained from leaves, flowers, and seeds, being the last one described for the first time. The chemical constituents of the EO from A. gratissima seeds were found to be like that of the EO obtained from leaves and flowers, which are rich in sesquiterpenes. We highlight important differences in relative percentages of the major compounds in the complex mixtures of EOs, being trans-pinocamphone, guaiol, germacrene B, β-pinene and caryophyllene oxide for AG-LE-EO, guaiol, caryophyllene, trans-β-ocimene, bulnesol and germacrene B for AG-FL-EO, and guaiol, caryophyllene, β-pinene, germacrene B and bicyclogermacrene for AG-SE-EO In addition, the EO of A. gratissima seeds played biocidal effects mainly at a concentration of 800 μl·l-1, inhibiting a decrease in germination percentage from 90.8% to 64.4%, increasing mean germination time, and decreasing seedling length 1.35 cm to 0.8 cm. The results conducted us to infer the presence of a higher percentage of substances such as guaiol, caryophyllene, and β-pinene in A. gratissima seeds EO can, in principle, explain the better action of EO as a bioherbicide. Although A. gratissima seeds EO represents an important step in developing new eco-friendly bioherbicides, understanding the relationship between the phytochemical compounds in plants and their influence on germination processes is still complex and challenging. For instance, new studies with AG-SE-EO, exploring the influence of the EO on weed seeds, experiments involving the combination of major constituents of AG-SE-EO, and investigating the environmental influence on the efficacy of the agrochemical candidates here tested still need to be assessed.
Acknowledgements
30The authors would like to thank Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação – PROPPI-IFGoiano (grant number 23216.000929.2022-22), Centro de Excelência em Bioinsumos – Urutaí (CEBIO-Urutaí), CNPq (grant # 311969/2019-4), Fapesp (grants #2016/06260-2; #2022/12597-0; #2022/11451-2), IAPT, INCT-Herbário Virtual da Flora e dos Fungos (grant #465420/2014-1), and IF Goiano for the financial support. We also would like to thank Dr Anderson Rodrigo da Silva, Dr Luiz Fernando de Camargos and Dra Erica Fernandes Leão Araújo for statistical and experimental support.
Bibliographie
Adams R.P., 2017. Identification of essential oil components by gas-chromatography/mass spectroscopy (5 online ed.). Gruver, TX, USA: Texensis Publishing.
Alcoba A.E. et al., 2018. Chemical composition and in vitro antileishmanial and cytotoxic activities of the essential oils of Ocotea dispersa (Nees) Mez and Ocotea odorifera (Vell) Rohwer (Lauraceae). Nat. Prod. Res., 32, 2865-2868, doi:10.1080/14786419.2017.1385007
Bakkali F., Averbeck S., Averbeck D. & Idaomar M., 2008. Biological effects of essential oils-a review. Food Chem. Toxicol., 46, 446-475, doi:10.1016/j.fct.2007.09.106
Batish D.R., Singh H.P., Kohli R.K. & Kaur S., 2008. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manage., 256, 2166-2174, doi:10.1016/j.foreco.2008.08.008
Benovit S.C. et al., 2015. Anesthetic activity and bio-guided fractionation of the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. in silver catfish Rhamdia quelen. Anais Acad. Bras. Cienc., 87, 1675-1689, doi:10.1590/0001-3765201520140223
Bettega R.d.P. & Trevisan A.C.D., 2022. Control of Eragrostis plana Nees (capin-annoni) germination: innovation with botanical bioproducts. Res. Soc. Dev., 11, e267111739142, doi:10.33448/rsd-v11i17.39142
Bezerra R.H.S., Sousa-Souto L., Santana A.E.G. & Ambrogi B.G., 2021. Indirect plant defenses: volatile organic compounds and extrafloral nectar. Arthropod-Plant Interact., 15, 467-489, doi:10.1007/s11829-021-09837-1
Chon S.-U. et al., 2005. Allelopathic potential in lettuce (Lactuca sativa L.) plants. Sci. Hortic., 106, 309-317, doi.org/10.1016/j.scienta.2005.04.005
Chowhan N., Singh H.P., Batish D.R. & Kohli R.K., 2011. Phytotoxic effects of β-pinene on early growth and associated biochemical changes in rice. Acta Physiol. Plant, 33, 2369-2376, doi:10.1007/s11738-011-0777-x
El-Remaly E. et al., 2022. Bio-management of root-knot nematodes on cucumber using biocidal effects of some Brassicaceae crops. Horticulturae, 8, 699, doi:10.3390/horticulturae8080699
Garcia M.C.F. et al., 2018. The in vitro antileishmanial activity of essential oil from Aloysia gratissima and guaiol, its major sesquiterpene against Leishmania amazonensis. Parasitology, 145, 1219-1227, doi:10.1017/S0031182017002335
Gatger T.I. & Dar S.A., 2021. Plant allelochemicals as sources of insecticides. Insects, 12, 189, doi:10.3390/insects12030189
Hister C. et al., 2009. Evaluation of the antiproliferative effects of infusions and essential oil of Aloysia gratissima. Pak. J. Biol. Sci., 12, 1581-1584.
Jabran K., Mahajan G., Sardana V. & Chauhan B.S., 2015. Allelopathy for weed control in agricultural systems. Crop Prot., 72, 57-65, doi:10.1016/j.cropro.2015.03.004
Khamare Y., Chen J. & Marble S.C., 2022. Allelopathy and its application as a weed management tool: a review. Front Plant Sci., 13, 1034649, doi:10.3389/fpls.2022.1034649
Liu X. et al., 2023. Chemical composition, antioxidant activity, and anti-bacterial activity of essential oils from different organs of Cinnamomum burmanni. J. Essent. Oil Bear. Plants, 26, 787-801, doi:10.1080/0972060X.2023.2239847
Macias F.A., Molinillo J.M., Varela R.M. & Galindo J.C., 2007. Allelopathy—a natural alternative for weed control. Pest Manage. Sci., 63, 327-348, doi:10.1002/ps.1342
MAPA/ACS, 2009. RAS: Regras para análise de sementes. Brasilia: Ministério da Agricultura, Pecuária e Abastecimento, Secretaria de Defesa Agropecuária.
Martins M.H. et al., 2017. Schistosomicidal effects of the essential oils of Citrus limonia and Citrus reticulata against Schistosoma mansoni. Chem. Biodivers., 14, e1600194, doi:10.1002/cbdv.201600194
Mirmostafaee S., Azizi M. & Fujii Y., 2020. Study of allelopathic interaction of essential oils from medicinal and aromatic plants on seed germination and seedling growth of lettuce. Agronomy, 10, 163, doi:10.3390/agronomy10020163
Morais L.A.S., 2009. Óleos essenciais no controle fitossanitário. In : Biocontrole de doenças de plantas: uso e perspectivas. São Paulo, Brazil: Embrapa Meio Ambiente, 139-152.
Ojija F., 2023. Allelopathic effects of Sphaeranthus suaveolens (Forssk.) DC. and Argemone mexicana L. leaf crude extract on Zea mays L. germination and growth. Agric. Biol. Res., 39, 651-656, doi:10.35248/0970-1907.23.39.651-656
Ordóñez Y.F. et al., 2023. In vitro antimicrobial activity of plant species against the phytopathogens Ralstonia solanacearum, Phytophthora infestans, and Neopestalotiopsis javaensis. Agriculture, 13, 2029, doi:10.3390/agriculture13102029
Ranal M.A. & Santana D.G.d., 2006. How and why to measure the germination process? Braz. J. Bot., 29, 1-11, doi:10.1590/S0100-84042006000100002
Ranal M.A., Santana D.G.d., Ferreira W.R. & Mendes-Rodrigues C., 2009. Calculating germination measurements and organizing spreadsheets. Braz. J. Bot., 32, 849-855, doi:10.1590/S0100-84042006000100002
Reigosa M.J., Sánchez-Moreiras A. & González L., 1999. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci., 18, 577-608, doi:10.1080/07352689991309405
Ribeiro V.E. et al., 2019. Enhanced essential oil and leaf anatomy of Schinus molle plants under lead contamination. Ind. Crop Prod., 132, 92-98, doi:10.1016/j.indcrop.2019.02.014
Roberts J., Florentine S., Fernando W.G.D. & Tennakoon K.U., 2022. Achievements, developments and future challenges in the field of bioherbicides for weed control: a global review. Plants, 11, 2242, doi:10.3390/plants11172242
Santos F. et al., 2013. Chemical composition and antimicrobial activity of the essential oil from the leaves and flowers of Aloysia gratissima. Braz. J. Med. Plant, 15, 583-588, doi:10.1590/S1516-05722013000400015
Santos T.G. et al., 2015. Chemical composition and antimicrobial activity of Aloysia gratissima (Verbenaceae) leaf essential oil. J. Essent. Oil Res., 27, 125-130, doi:10.1080/10412905.2015.1006737
Scavo A. & Mauromicale G., 2021. Crop allelopathy for sustainable weed management in agroecosystems: knowing the present with a view to the future. Agronomy, 11, 2104, doi:10.3390/agronomy11112104
Singh H.P. et al., 2006. α-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot., 98, 1261-1269, doi:10.1093/aob/mcl213
Souza A. & Wiest J.M., 2007. Atividade antibacteriana de Aloysia gratissima (Gill et Hook) Tronc. (garupá, erva-santa) usada na medicina tradicional no Rio Grande do Sul-Brasil. Rev. Bras. Plant. Med., 9, 23-29.
Souza M.A. et al., 2022. Biological properties of Aloysia gratissima (Gillies & Hook.) Tronc. (Verbenaceae). Evidence-Based Complementary Altern. Med., 2022, 1119435, doi:10.1155/2022/1119435
Thiesen L.A. et al., 2019. Essential oil of Lippia alba (Mill.) N.E.Br. influences the germination, vigor and emergence of lettuce seeds. Rev. Colomb. Cienc. Horticolas, 13, 416-425, doi:10.17584/rcch.2019v13i3.8033
Tigre R.C. et al., 2012. Allelopathic and bioherbicidal potential of Cladonia verticillaris on the germination and growth of Lactuca sativa. Ecotoxicol. Environ. Saf., 84, 125-132, doi.org/10.1016/j.ecoenv.2012.06.026
Vandresen F. et al., 2010. Constituintes químicos e avaliação das atividades antibacteriana e antiedematogênica de Aloysia gratissima (Gillies & Hook.) Tronc. e Aloysia virgata (Ruiz & Pav.) Pers., Verbenaceae. Braz. J. Pharmacogn., 20, 317-321, doi:10.1590/S0102-695X2010000300005
Verdeguer M. et al., 2020. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus essential oils. Molecules, 25, 562, doi:10.3390/molecules25030562
Wang R.-L. et al., 2010. Responses of Mikania micrantha, an invasive weed to elevated CO2: induction of β-caryophyllene synthase, changes in emission capability and allelopathic potential of β-caryophyllene. J. Chem. Ecol., 36, 1076-1082, doi.org/10.1007/s10886-010-9843-x
Woolf A., 1999. Essential oil poisoning. J. Toxicol. Clin. Toxicol., 37, 721-727, doi:10.1081/CLT-100102450
Zeni A.L.B. et al., 2013. Phytochemical profile, toxicity and antioxidant activity of Aloysia gratissima (Verbenaceae). Quim. Nova, 36, 69-73, doi:10.1590/S0100-40422013000100013

