- Home
- Volume 17 (2013)
- numéro 3
- Pheromone-based management strategies to control the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). A review
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Pheromone-based management strategies to control the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). A review

Editor's Notes
Received on October 5, 2012; accepted on May 8, 2013
Résumé
Synthèse bibliographique : les stratégies de lutte phéromonale utilisées pour contrôler la mineuse de la tomate, Tuta absoluta (Lepidoptera : Gelechiidae). Cette synthèse bibliographique fait le point sur les stratégies de lutte phéromonale développées spécifiquement pour lutter contre la mineuse de la tomate, Tuta absoluta (Lepidoptera: Gelechiidae). Ce ravageur provient d’Amérique du Sud et est actuellement considéré comme un des plus importants ravageurs de tomates dans les pays européens et nord-africains du bassin méditerranéen. Après avoir présenté les principes généraux des stratégies de lutte phéromonale, les stratégies utilisées pour contrôler T. absoluta, comme les techniques de détection de ravageurs, de suivi des populations, de piégeage de masse et de confusion sexuelle, sont décrites successivement dans cette synthèse.
Abstract
We here review pheromone control strategies for species-specific and environmentally safe management of the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). This insect pest originates from South America and is now considered to be one of the most damaging invasive pests of tomatoes in the Mediterranean Basin countries of Europe and North Africa. After presenting the general principles of sex pheromone-based control strategies, we describe strategies used to control T. absoluta including pest detection, population monitoring, mass annihilation and mating disruption techniques.
Table of content
1. Introduction
1The tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is a major pest of processed and fresh tomatoes, both in greenhouses and open field crops (Desneux et al., 2010). This pest has spread rapidly since its introduction to Europe in 2006, and within just a few years its global status has changed completely, from a South American tomato pest into a major threat to world tomato production (Roditakis et al., 2010; Desneux et al., 2011). Presently, the pest threatens other cultivated solanaceous plants such as eggplant and potato (Desneux et al., 2010; Unlu, 2012; Caparros Megido et al., 2013). Since its introduction in many countries, chemical sprays have been the main method of control used against T. absoluta (Galarza et al., 1984). However, the efficiency of chemical control of tomato leafminer infestations has been poor because of (1) the endophytic habit of its larvae, which are protected in the leaf mesophyll or inside fruits (Cocco et al., 2013), and (2) pest resistance against a number of applied insecticides (Siqueira et al., 2000a; Siqueira et al., 2000b; Siqueira et al., 2001; Lietti et al., 2005; Silva et al., 2011; Reyes et al., 2012). In order to reduce the excessive use of insecticides in tomato fields, environmentally sound control strategies have been developed, including cultural control measures (e.g. crop rotation, selective removal and destruction of infested plant material) (Korycinska et al., 2009), the use of natural enemies (parasitoids, predators, entomopathogens and nematodes) (Desneux et al., 2010; Urbaneja et al., 2012) and resistant varieties of tomato (de Oliveira et al., 2012). Additional alternative control methods, based on the use of the insect’s sex pheromones, have also been developed to control T. absoluta. After presenting the principles of sexual pheromone-based control strategies, this review will focus on pheromone control strategies developed to specifically control the tomato leafminer.
2. General principles of sex pheromone-based control strategies
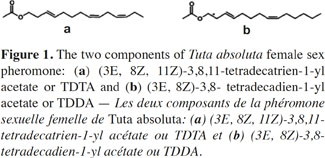
2Sex pheromones are chemical signals released by an organism to attract an individual of the same species of the opposite sex for mating. Among the 120,000 or so known moth species, the preponderant system for finding a mate is upwind flight by the male toward an attractant sex pheromone released by the female (Cardé et al., 1995). The majority of female sex pheromones identified in Lepidoptera consist of a mixture of two or more compounds, which not only evoke long-range male attraction but also elicit courtship behavior (Linn et al., 1987). As an example, the female sex pheromone of T. absoluta consists of two components. The major component, which represents about 90% of the volatile material found in the sex gland of calling females, is (3E, 8Z, 11Z)-3,8,11-tetradecatrien-l-yl acetate or TDTA (Figure 1) (Attygalle et al., 1996; Griepink et al., 1996; Svatoš et al., 1996). The minor constituent (10%) was identified as (3E, 8Z)-3,8- tetradecadien-l-yl acetate or TDDA (Figure 1) (Svatoš et al., 1996).
3Sex pheromones have been widely used to monitor, forecast or control populations of moth pests (Prasad et al., 2012). The most widespread and successful applications of sex pheromones concern their use in detection and population monitoring (Witzgall et al., 2010). They are also used to control insect populations, which is achieved by two main techniques: mass annihilation and mating disruption (Witzgall et al., 2010). Sex pheromone management strategies are based on the sexual reproduction of the targeted pest. A study by Caparros Megido et al. (2012) recently confirmed that T. absoluta females were also able to reproduce without mating (i.e. parthenogenetically). Asexual reproduction as well as the polygynic nature of T. absoluta males could have strong implications for the efficiency of sex pheromone management strategies and must be considered in further studies on these control strategies (Silva, 2008; Caparros Megido et al., 2012).
4The sex pheromone management techniques actually used to control T. absoluta are discussed below in separate paragraphs.
3. Pest detection and population monitoring
5Captures in traps baited with synthetic pheromone lures accurately show whether a specific insect species is present, and when its seasonal flight period starts (Witzgall et al., 2010). Detection of presence or absence is all that is required for early warning of emergence, for warning of arrival or departure of a pest within a crop, and for survey and quarantine work (Howse, 1998). After pest detection, synthetic sex pheromones are principally used to monitor population levels and trigger applications of chemicals or other control methods (Salas, 2004). The basic components of a monitoring system are the attractant source, the trap design and where to place them (Howse, 1998).
3.1. The attractant source
6Although the female sex pheromone of T. absoluta was identified in 1995, virgin females were already used to capture more than 100 males per trap per day (Quiroz, 1978). Uchôa-Fernandes et al. (1994) also used virgin females to compare different trap designs, heights, and displacement in tomato fields for capturing T. absoluta males. A high specificity and sensitivity of traps baited with virgin females was recorded and they seemed to be more economical and convenient than light traps. Moreover, wind tunnel experiments under laboratory conditions indicated that the mixture of the two pheromone components was more effective in evoking long-range female location and short-range courtship behavior in males than TDTA alone (Griepink et al., 1996; Svatoš et al., 1996). For more details about the mating behavior of T. absoluta, see Hickel et al. (1991). However, it has been observed in laboratory experiments that T. absoluta males were far less sensitive to the absence of the minor component than most other lepidopterans, which are characteristically highly sensitive to small qualitative or even quantitative changes in the composition of pheromone blends (Svatoš et al., 1996). Field experiments corroborate these laboratory results, although T. absoluta males were not particularly sensitive to the presence of TDDA in pheromone traps. Finally, it appears that monitoring pheromone traps could be loaded with just 100 μg of the synthetic major pheromone component (TDTA), enabling a significant reduction in production costs without reducing trap efficacy (Michereff Filho et al., 2000a; Ferrara et al., 2001). Actually, different loadings of pheromone are suggested for monitoring T. absoluta populations: 0.5 mg in greenhouses for 4–6 weeks of longevity, 0.8 mg in open fields for 4–6 weeks of longevity and 3.0 mg in open fields in hot desert climates for a long lasting lure (Hassan et al., 2010a). Russell IPM Ltd. (United Kingdom) provides a new trap formulation in which 0.8 mg of both pheromone constituents are loaded on a pheromone lure. According to the manufacturer, this new formulation showed better results and this loading delivers the additional benefits of a constant release of pheromone for a longer period in hotter climates and a higher trap catch over the standard 0.5 mg lure (Russell IPM, 2012).
3.2. Traps
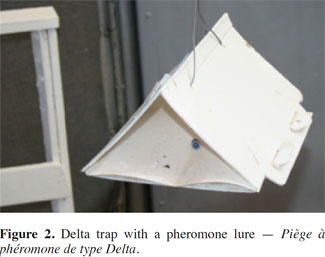
7The variety of trap designs used for insect pest monitoring is extensive (Howse, 1998). The most common types of traps involve a sticky surface to retain the attracted insect (Cardé, 1984). To monitor T. absoluta, pheromone lures are principally coupled with Delta traps (Hassan et al., 2010a; Russell IPM, 2012). Various companies such as ISCA Technologies (United States), Russell IPM Ltd. (United Kingdom), Koppert Biological Systems (The Netherlands), and PRI Pherobank (The Netherlands) manufacture these traps (USDA APHIS, 2011). Delta traps consist of a triangular-shaped body (Figure 2) (manufactured of paper or plastic) open at ends, a removable sticky insert placed inside on the floor of the triangle, and a pheromone lure suspended above the sticky insert. In heavy infestations, the sticky inserts can become saturated with trapped males, losing their effectiveness at capturing and retaining additional moths (USDA APHIS, 2011). The color of the trap also seems to affect its effectiveness, with dark colors (black, red, green and blue) giving higher catches of males than lighter colors (yellow and white) (Uchôa-Fernandes et al., 1994). The use of completely open traps could also increase the number of catches (Ferrara et al., 2001).
3.3. Trap position
8Three important elements related to trap placement are trap height, position with respect to vegetation and trap density (Howse, 1998).
9Trap height and position with respect to vegetation. The height of the trap in the crop influences male capture and is related to the height of the vegetation (Uchôa-Fernandes et al., 1994; Ferrara et al., 2001). The trap height must be adapted according to the growth stage of the plant, knowing that a higher proportion of moths are found in the upper parts of the canopy but never beyond 1 m high (Coelho et al., 1987; Uchôa-Fernandes et al., 1994; Laore, 2010). Traps placed up to 60 cm high captured significantly more males than traps at higher heights, irrespective of the stage of plant growth (before planting, 20 cm high plants, or blooming plants). Therefore, it was suggested to deploy monitoring traps in a field at a height of 20 cm before planting and then move them up to 60 cm high as the plants grow (Ferrara et al., 2001).
10Trap density. For monitoring T. absoluta populations in greenhouses, different trap densities have been proposed and different sources are not always in agreement. It is usually suggested to use 1 trap·ha-1 in greenhouses smaller than 2,500 m2 and 2–4 traps·ha-1 in greenhouses wider than 2,500 m2 (Bolckmans, 2009; Fredon, 2009; Laore, 2010). Russell IPM advised that a total of 4–5 traps·ha-1 should be deployed, with one trap near the entrance and 1–2 traps in the warmest part of the greenhouse (Al-Zaidi, 2009). Laore (2010) suggested placing traps only in a central position in the greenhouse.
11In open-field crops, Russell IPM suggested a trap density of 2–3 traps·ha-1; in order to determine the direction of the infestation, two more traps can be added along all four edges of the field (Al-Zaidi, 2009). In plant propagation greenhouses, Bolckmans (2009) suggested using 10–20 traps·ha-1, which seems to be a mixed monitoring and mass trapping system. Finally, it is recommended to count the trap catches every week to follow the evolution of the insect population and change the traps every 4–6 weeks (Bolckmans, 2009; Fredon, 2009; Laore, 2010).
3.4. Level of infestation
12Several ways have been proposed to evaluate the infestation level of the tomato leafminer in a tomato crop, such as counting eggs, larvae, leaf mines or adult males (with the help of pheromone traps) (Gomide et al., 2001). Although counting larvae or leaf mines provides a reliable assessment of the infestation level, it seems better, for preventive purposes, to focus on egg and adult counts to predict the infestation level (Benvenga et al., 2007). Indeed, Integrated Pest Management (IPM) programs are principally based on environmentally sound strategies such as the use of predators, parasitoids or Bacillus thuringiensis to control eggs and first instar larvae of T. absoluta (Desneux et al., 2010; Urbaneja et al., 2012). If the evaluation of the infestation level is based on adult and egg populations, the pest management program can be set up before oviposition or before emergence of the first instar larvae, respectively (Benvenga et al., 2007). However, counting eggs is time demanding and difficult to carry out because of the small size of the egg (0.35 mm long and 0.22 mm wide) and the number of leaves that must be examined (the last three expanded leaves of the tomato plant) (Gomide et al., 2001; EPPO/OEPP, 2005). Since the adult male population caught in pheromone traps should also reflect any increase in the larval population, it seems easier to use this method to evaluate the infestation level. Based on the fact that the number of males caught in pheromone traps is linearly and negatively correlated with tomato production in the crop, a table with indicative values of risk was created (Monserrat Delgado, 2008). Based on the numbers of adult males of T. absoluta caught in pheromone traps, the risk of infestation is considered to be low for 1 to 3 captured moths per week, moderate for 4 to 30 captured moths per week and high for more than 30 moths per week (Stol et al., 2009).
4. Mass annihilation
13Mass annihilation, conducted either by mass trapping or lure and kill techniques, relies on the attraction of one or both sexes to a lure in combination with a large-capacity trap or an insecticide-impregnated target (Witzgall et al., 2010). Mass trapping consists of the use of a lure (semiochemicals or a light source) combined with a physical device to “entrap” insects, like an adhesive surface or water bath, while lure and kill consist of the use of semiochemical lures combined with a killing or sterilizing agent (Jones, 1998). The mate-finding communication system of T. absoluta is guided by a female-produced sex pheromone, so only males are caught in traps, which decreases their efficiency (Jones, 1998; Witzgall et al., 2010). Since tomato leafminer males are polygynic and mate on average 6.5 times (Silva, 2008), a very high proportion of males must be removed before the number of eggs oviposited in a population starts to be affected (Jones, 1998; Witzgall et al., 2010). Moreover, Caparros Megido et al. (2012) have demonstrated that females are able to lay eggs without male fertilization, which could increase the difficulty in affecting the pest population density.
4.1. Mass trapping
14For mass trapping, a high density of pheromone-baited traps are placed in strategic positions within a crop (Jones, 1998). Large numbers of adult males are trapped, resulting in an imbalance in the sex ratio, which impacts the mating pattern of the pest (USDA APHIS, 2011). The pheromone trap density should be 20 to 25 traps·ha-1 inside greenhouses (30 traps·ha-1 in greenhouses destined for plant propagation) and 40 to 50 traps·ha-1 in open fields (Bolckmans, 2009). The most common pheromone traps used for mass trapping of T. absoluta are water traps, which are easier to maintain and less sensitive to dust than Delta or light traps and also have a larger trapping capacity than Delta traps (Salas, 2004; USDA APHIS, 2011). Unfortunately, when mass trapping was used alone for controlling male T. absoluta populations, it was not effective in reducing leaf and fruit damage (Cocco et al., 2012). This could be explained by the traps’ inability to catch all of the male population and by the ability of the females to reproduce parthenogenetically (Silva, 2008; Caparros Megido et al., 2012). In addition to mass trapping techniques, the use of isolation measures such as insect-proof nets or a double door system at the greenhouse entrance was shown to decrease leaf and fruit damage considerably by preventing the entry of adults from outside and reducing mating probabilities (Harbi et al., 2012). In addition to the use of isolation measures, the efficiency of mass-trapping is dependent on the choice of pheromone capsule (a sufficient longevity is needed) and regular renewal of the capsules according to the manufacturer’s recommendations (Abbes et al., 2011).
15Water traps. Water traps consist of a plastic container holding water and a pheromone lure (Figure 3) (USDA APHIS, 2011). The lure is secured above the water with a wire attached at both ends of the container. A small amount of vegetable oil or soap should be added to the water to reduce surface tension (and consequently reduce the insect’s capacity to escape from the trap) and limit water evaporation (consequently reducing the frequency of water refills) (USDA APHIS, 2011; Chermiti et al., 2012). This type of trap can capture large numbers of adult males without becoming saturated with insects (USDA APHIS, 2011). Chermiti et al. (2012) compared the effectiveness of three commonly used commercial pheromone dispensers for mass trapping of T. absoluta in open-field tomato crops: Pherodis® produced by Koppert Biological System (capsule loaded with 0.5 mg of TDTA), TUA-500® produced by Russell IPM (capsule loaded with 0.5 mg of TDTA) and TUA-Optima®, also manufactured by Russell IPM (capsule loaded with 0.8 mg of TDTA). An efficient reduction in the male population was achieved with a trap density of 32 traps·ha-1 (or 1 trap·312 m-2) and was correlated with reductions in the leaf infestation rate and in the number of larvae found on leaves. The TUA-Optima® dispenser (0.8 mg of TDTA) was more attractive than the standard emitters (0.5 mg of TDTA) but all three tested pheromone plugs suffered at high temperatures and water had to be continuously supplied. To conclude, Chermiti et al. (2012) suggested using TUA-Optima® dispensers for high level infestations (more than 30 males per trap per week) and the two other types of plugs for low level infestations (under 30 males per trap per week). On the other hand, Cocco et al. (2012) compared the effectiveness of mass trapping using light and pheromone water traps in greenhouses equipped with insect-proof nets and did not find any reduction in leaf damage even with a high trap density (up to 1 trap·350 m-2 for light traps and 1 trap·100 m-2 for pheromone water traps). Some possible hypotheses explaining the differences between the two experiments could be the greater activity of biocontrol agents in open fields than in high-containment greenhouses or differences in the initial pest population.

16Recently, Russell IPM Ltd (United Kingdom) designed new traps based on a combination of a water trap, a sex pheromone and a specific light frequency that is highly attractive to T. absoluta adults (Hassan et al., 2010a; USDA APHIS, 2011; Russell IPM, 2012). These traps attract males with the pheromone lure but also females with the specific light frequency, entrapping both sexes in a water pan (Hassan et al., 2010a; Russell IPM, 2012). These new traps are considered, by the manufacturer, to be 200–300% more effective than standard pheromone traps (Hassan et al., 2010a; Russell IPM, 2012).
17Sticky rolls. Sticky rolls are rolls with T. absoluta pheromone incorporated into the sticky glue, with the pheromone gradually released from the adhesive layer (Hassan et al., 2010b; Russell IPM, 2012). Two types of sticky rolls are provided: clear and yellow sticky film. The clear film is used in greenhouses containing beneficial insects as biocontrol agents (Russell IPM, 2012). Yellow sticky rolls are used to catch T. absoluta and, additionally, whiteflies and aphids. It is recommended not to use the yellow sticky rolls in greenhouses with beneficial insects because the yellow color, which attracts different kinds of pests, could also attract and glue beneficial insects (Hassan et al., 2010b). It is recommended to apply the sticky rolls 1.5 m high and 60–70 cm away from the plant canopy (Hassan et al., 2010b). In greenhouses, placing sticky rolls vertically at a higher plant level can also give an indication of the preferred height of insect oviposition and consequently help to decide where to focus management efforts (Hassan et al., 2010b).
4.2. Lure and kill technique
18The lure and kill technique involves two components: the lure, which may consist of odors, visual cues or a combination of both, and an affector, which eliminates the attracted insect from the population (Jones, 1998). This control method can be applied through two different strategies:
19– the semiochemical attractant and the insecticide are applied separately or mixed to obtain a lure and kill formulation which is applied in the field;
20– the attractant and the insecticide are combined in a single matrix and deployed as a stand alone application (Witzgall et al., 2010).
21Lure and kill formulations are targeted to a specific pest, are applied separately or to a limited part of the plant, reducing the development of insecticide resistance, and can be integrated with other beneficials. For the control of T. absoluta, a matrix formulated with 0.3% sex pheromone and 3% cypermethrin is available (Al-Zaidi, 2010; Hassan et al., 2010a).
5. Mating disruption
22The mating disruption technique aims at creating sexual confusion in males by saturating the atmosphere with a synthetic female pheromone in order to prevent the pest mating and, consequently, reduce the pest population (Cardé, 2007; Cocco et al., 2013). Studies on the application of the mating disruption technique against T. absoluta in open fields and protected tomato crops showed mixed results (Michereff Filho et al., 2000b; Vacas et al., 2011; Cocco et al., 2013). Michereff Filho et al. (2000b) examined the use of mating disruption for T. absoluta in small plots of fresh market tomatoes in Brazil, finding high levels of interruption in male orientation (60–90%) in plots treated with 35 to 50 g·ha-1 of sex pheromone. However, the pheromone treatment did not significantly reduce the damage to leaflets and fruits, probably due to the synthetic pheromone’s composition and dose, the high pest population density, or the migration of mated females to the treated area. Vacas et al. (2011) reported satisfactory control of T. absoluta by mating disruption in high-containment greenhouses with a dose of 30 g·ha-1 (dispensers loaded with 60 mg of sex pheromone applied at a density of 500 dispensers·ha-1). Conversely, dispensers at the same loading and density were ineffective in reducing crop damage in low-containment greenhouses (Vacas et al., 2011). This suggests the importance of the degree of containment to the success of pheromone treatment on T. absoluta, as it prevents migration of the pest from outside the greenhouses (Vacas et al., 2011). Cocco et al. (2013) confirmed this hypothesis by showing disruption of the sexual communication of T. absoluta and reductions in leaf and fruit damages in high-containment greenhouses with a dose of 60 g·ha-1 (dispensers loaded with 60 mg of sex pheromone at a density of 1,000 dispensers·ha-1), but not in low-containment greenhouses. These results show that T. absoluta can be controlled by means of sexual confusion if the treatments are carried out in greenhouses with good isolation that prevents new moths from entering. Although the effectiveness of this method seems demonstrated, the final viability of this control technique will depend on the pheromone’s end price (Vacas et al., 2011).
6. Conclusion
23Tuta absoluta is a very challenging pest to control (Korycinska et al., 2009). Sex pheromone-based strategies (i.e. mass trapping and mating disruption) are promising techniques to control this invading pest. However, even if large numbers of individual males can be caught by coupling pheromone releasers and insect trapping devices, the success of mass trapping strategies is usually low (Hassan et al., 2010a). This failure could be explained by the parthenogenetic reproduction of the pest or by the polygynic nature of T. absoluta males. If asexual reproduction occurs in natural populations of T. absoluta, a reduction in the efficiency of mass trapping strategies is clearly expected (Caparros Megido et al., 2012). The mating disruption technique used against T. absoluta was effective in high-containment greenhouses, while poor results were observed in low-containment greenhouses and open-field crops (Michereff Filho et al., 2000b; Vacas et al., 2011; Cocco et al., 2013). The degree of containment seems to be the major component contributing to the success of T. absoluta control with mating disruption, as it prevents the migration of mated females into the greenhouses (Cocco et al., 2013). Moreover, some enhancements to the dispensers are also needed (e.g. optimization of release rate, maximization of the saturation rate of the trap, modifications in trap design, etc.). For these reasons, mating disruption cannot yet be effectively applied in open field crops. Finally, sex pheromone strategies are sometimes more expensive than conventional chemical control of pests. It is therefore important to reduce the production cost of T. absoluta pheromone in order to promote their use by farmers.
24Acknowledgments
25Rudy Caparros Megido is financially supported by a PhD grant from the Service Public de Wallonie (SPW–DGO3, project D31-1263), Belgium.
Bibliographie
Abbes K. & Chermiti B., 2011. Comparison of two marks of sex pheromone dispensers commercialized in Tunisia for their efficiency to monitor and to control by mass-trapping Tuta absoluta under greenhouses. Tunisian J. Plant Prot., 6(2), 133-148.
Al-Zaidi S., 2009. Recommendations for the detection and monitoring of Tuta absoluta, http://www.russellipm-agriculture.com/uploads/files/recommendationdetectionmonitoring.pdf, (16/08/12).
Al-Zaidi S., 2010. Manejo de Tuta absoluta mediante feromonas. Phytoma Espana, 217, 41.
Attygalle A.B. et al., 1996. (3E,8Z,11Z)-3,8,11-tetradecatrienyl acetate, major sex pheromone component of the tomato pest Scrobipalpuloides absoluta (Lep., Gelechiidae). Bioorg. Med. Chem., 4(3), 305-314.
Benvenga S.R., Fernandes O.A. & Gravena S., 2007. Decision making for integrated pest management of the South American tomato pinworm based on sexual pheromone traps. Hortic. Bras., 25(2), 164-169.
Bolckmans K., 2009. Integrated pest management of the exotic invasive pest Tuta absoluta. In: International Biocontrol Manufacturers Association and Research Institute of Organic Agriculture, eds. Proceedings of the 4th Annual Biocontrol Industry Meeting Internationals, Lucerne, Switzerland.
Caparros Megido R., Haubruge E. & Verheggen F.J., 2012. First evidence of deuterotokous parthenogenesis in the tomato leafminer, Tuta absoluta (Meyrick) (Lep., Gelechiidae). J. Pest Sci., 85(4), 409-412.
Caparros Megido R., Brostaux Y., Haubruge E. & Verheggen F.J., 2013. Propensity of the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae), to develop on four potato plant varieties. Am. J. Potato Res., 90, 255-260.
Cardé R.T., 1984. Field trapping with attractants: methods and interpretation. In: Cardé R.T. & Elkinton J.S., eds. Techniques in pheromone research. New York, USA: Springer Verlag, 111-129.
Cardé R.T., 2007. Using pheromones to disrupt mating of moth pests. In: Kogan M. & Jepson P., eds. Perspectives in ecological theory and integrated pest management. Cambridge, UK: Cambridge University Press, 122-169.
Cardé R.T. & Minks A.K., 1995. Control of moth pests by mating disruption: successes and constraints. Annu. Rev. Entomol., 40, 559-585.
Chermiti B. & Abbes K., 2012. Comparison of pheromone lures used in mass trapping to control the tomato leafminer Tuta absoluta (Meyrick, 1917) in industrial tomato crops in Kairouan (Tunisia). EPPO Bull., 42(2), 241-248.
Cocco A., Deliperi S. & Delrio G., 2012. Potential of mass trapping for Tuta absoluta management in greenhouse tomato crops using light and pheromone traps. IOBC-WPRS Bull., 80, 319-324.
Cocco A., Deliperi S. & Delrio G., 2013. Control of Tuta absoluta (Meyrick) (Lep., Gelechiidae) in greenhouse tomato crops using the mating disruption technique. J. Appl. Entomol., 137(1-2), 16-28.
Coelho M.C.F. & França F.H., 1987. Biologia e quetotaxia da larva e descrição da pupa e adulto da traça do tomateiro. Pesqui. Agropecu. Bras., 22, 129-135.
de Oliveira C.M. et al., 2012. Resistance of tomato strains to the moth Tuta absoluta imparted by allelochemicals and trichome density. Ciênci. Agrotecnol., 36(1), 45-52.
Desneux N. et al., 2010. Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J. Pest Sci., 83(3), 197-215.
Desneux N., Luna M.G., Guillemaud T. & Urbaneja A., 2011. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J. Pest Sci., 84(4), 403-408.
EPPO/OEPP, 2005. Tuta absoluta - Data sheets on quarantine pests, http://www.eppo.int/QUARANTINE/insects/Tuta_absoluta/DS_Tuta_absoluta.pdf, (18/08/12).
Ferrara F.A.A. et al., 2001. Evaluation of the synthetic major component of the sex pheromone of Tuta absoluta (Meyrick) (Lep., Gelechiidae). J. Chem. Ecol., 27(5), 907-917.
Fredon, 2009. Mesures de lutte contre Tuta absoluta, http://www.fredon-corse.com/standalone/1/CE5Bk98q7hNOOAd4qo4sD67a.pdf, (10/12/12).
Galarza J. & Larroque O., 1984. Control de Scrobipalpula absoluta (Meyrick) (Lep., Gelechidae) en tomate. Idia, 421-424, 15-18.
Gomide E.V.A., Vilela E.F. & Picanço M., 2001. Comparacao de procedimentos de amostragem de Tuta absoluta (Meyrick) (Lep., Gelechiidae) em tomateiro estaqueado. Neotrop. Entomol., 30(4), 697-705.
Griepink F.C. et al., 1996. Identification of the sex pheromone of Scrobipalpula absoluta; determination of double bond positions in triple unsaturated straight chain molecules by means of dimethyl disulphide derivatization. Tetrahedron Lett., 37(3), 411-414.
Harbi A., Abbes K. & Chermiti B., 2012. Evaluation of two methods for the protection of tomato crops against the tomato leafminer Tuta absoluta (Meyrick) under greenhouses in Tunisia. EPPO Bull., 42(2), 317-321.
Hassan N. & Al-Zaidi S., 2010a. Tuta absoluta - pheromone mediated management strategy. Int. Pest Control, 52(3), 158-160.
Hassan N. & Al-Zaidi S., 2010b. Tutaroll – an innovative solution for Tuta absoluta. Int. Pest Control, 52(5), 262-264.
Hickel E.R. & Vilela E.F., 1991. Comportamento de chamamento e aspectos do comportamento de acasalamento de Scrobipalpula absoluta (Lep., Gelechiidae), sob condicoes de campo. Ann. Soc. Entomol. Bras., 20, 173-182.
Howse P., 1998. Pheromones and behaviour. In: Howse P., Stevens I. & Jones O., eds. Insect pheromones and their use in pest management. London, UK: Chapman & Hall, 1-130.
Jones O., 1998. Practical applications of pheromones and other semiochemicals. In: Howse P., Stevens I. & Jones O., eds. Insect pheromones and their use in pest management. London, UK: Chapman & Hall, 263-355.
Korycinska A. & Moran H., 2009. South American tomato moth (Tuta absoluta): plant pest factsheet. Sand Hutton, York, UK: FERA.
Laore, 2010. Tignola o falena del pomodoro, Tuta absoluta (Meyrick, 1917), Povolny (1994): riconoscimento e lotta. Cagliari, Italia: Laore, agenzia regionale per lo sviluppo in agricoltura, http://www.sardegnaagricoltura.it/documenti/14_43_20100420134239.pdf, (10/12/12).
Lietti M.M.M., Botto E. & Alzogaray R.A., 2005. Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol., 34(1), 113-119.
Linn J.C.E., Campbell M.G. & Roelofs W.L., 1987. Pheromone components and active spaces: what do moths smell and where do they smell it? Science, 237(4815), 650-652.
Michereff Filho M. et al., 2000a. Field trapping of tomato moth, Tuta absoluta with pheromone traps. J. Chem. Ecol., 26(4), 875-881.
Michereff Filho M. et al., 2000b. Initial studies of mating disruption of the tomato moth, Tuta absoluta (Lep., Gelechiidae) using synthetic sex pheromone. J. Braz. Chem. Soc., 11(6), 621-628.
Monserrat Delgado A., 2008. La polilla del tomate Tuta absoluta en la region de Murcia : bases para su control. Murcia, Spain: Ministry of Agriculture and Water.
Prasad Y. & Prabhakar M., 2012. Pest monitoring and forecasting. In: Shankar U. & Abrol D.P., eds. Integrated pest management: principles and practice. Oxfordshire, UK: Cabi, 41-57.
Quiroz C.E., 1978. Utilizacion de trampas con hembras virgenes de Scrobipalpula absoluta (Meyrick) (Lep., Gelechiidae) en estudios de dinamica de poblacion. Agric. Tec. (Chile), 38, 94-97.
Reyes M. et al., 2012. Metabolic mechanisms involved in the resistance of field populations of Tuta absoluta (Meyrick) (Lep., Gelechiidae) to spinosad. Pestic. Biochem. Physiol., 102(1), 45-50.
Roditakis E., Papachristos D. & Roditakis N.E., 2010. Current status of the tomato leafminer Tuta absoluta in Greece. EPPO Bull., 40(1), 163-166.
Russell IPM, 2012. Tuta absoluta products, http://russellipm-agriculture.com/solutions.php?id_ctg=1&lang=en, (10/08/12).
Salas J., 2004. Capture of Tuta absoluta (Lepidoptera: Gelechiidae) in traps baited with its sex pheromone. Rev. Colomb. Entomol., 30(1), 75-78.
Silva G.A. et al., 2011. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manage. Sci., 67(8), 913-920.
Silva S.S., 2008. Fatores da biologia reprodutiva que influenciam o manejo comportamental de Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). MS thesis: Universidade Federal Rural de Pernambuco (Brasil).
Siqueira H.A., Guedes R.N. & Picanco M.C., 2000a. Cartap resistance and synergism in populations of Tuta absoluta (Lep., Gelechiidae). J. Appl. Entomol., 124(5-6), 233-238.
Siqueira H.A., Guedes R.N. & Picanço M.C., 2000b. Insecticide resistance in populations of Tuta absoluta (Lep., Gelechiidae). Agric. For. Entomol., 2(2), 147-153.
Siqueira H.A., Guedes R.N., Fragoso D.B. & Magalhaes L.C., 2001. Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lep., Gelechiidae). Int. J. Pest Manage., 47(4), 247-251.
Stol W., Griepink F.C. & Van Deventer F., 2009. Tuta absoluta: a new pest for tomato production in Europe. In: Segundo Jornadas Feromonas Murcia, 18-19 November 2009, Spain, http://www.feromonasmurcia.es/espanol/files/presentacion/hortalizas/H-1%20W%20STOL%20TUTA.pdf, (20/08/13).
Svatoš A. et al., 1996. Sex pheromone of tomato pest Scrobipalpuloides absoluta (Lepidoptera: Gelechiidae). J. Chem. Ecol., 22(4), 787-800.
Uchôa-Fernandes M.A. & Vilela E.F., 1994. Field trapping of the tomato worm Scrobipalpula absoluta (Meyrick) (Lep., Gelechiidae). Ann. Soc. Entomol. Bras., 23, 271-277.
Unlu L., 2012. Potato: a new host plant of Tuta absoluta Povolny (Lepidoptera: Gelechiidae) in Turkey. Pak. J. Zool., 44(4), 1183-1184.
Urbaneja A., González-Cabrera J., Arnó J. & Gabarra R., 2012. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manage. Sci., 68(9), 1215-1222.
USDA APHIS, 2011. New pest response guidelines: tomato leafminer (Tuta absoluta). Washington, DC: United States Department of Agriculture.
Vacas S., Alfaro C., Primo J. & Navarro-Llopis V., 2011. Studies on the development of a mating disruption system to control the tomato leafminer, Tuta absoluta Povolny (Lepidoptera: Gelechiidae). Pest Manage. Sci., 67(11), 1473-1480.
Witzgall P., Kirsch P. & Cork A., 2010. Sex pheromones and their impact on pest management. J. Chem. Ecol., 36(1), 80-100.
To cite this article
About: Rudy Caparros Megido
Univ. Liege - Gembloux Agro-Bio Tech. Department of Functional and Evolutionary Entomology. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: entomologie.gembloux@ulg.ac.be
About: Éric Haubruge
Univ. Liege - Gembloux Agro-Bio Tech. Department of Functional and Evolutionary Entomology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
About: François Jean Verheggen
Univ. Liege - Gembloux Agro-Bio Tech. Department of Functional and Evolutionary Entomology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






