Refinement of the production of antigen-specific hen egg yolk antibodies (IgY) intended for passive dietary immunization in animals. A review
Univ. Liege - Gembloux Agro-Bio Tech. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Animal and Microbial Biology Unit. Avenue Maréchal Juin, 27. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: yves.beckers@ulg.ac.be
Received on September 27, 2012; accepted on March 7, 2013
Résumé
Amélioration dans l’obtention d’anticorps du jaune d’œuf (IgY) spécifiques d’un antigène et destinés à l’immunisation passive par voie alimentaire chez l’animal (synthèse bibliographique). Les anticorps sont devenus au fil des années des outils essentiels avec des applications variées tant au laboratoire qu’en médecine humaine ou vétérinaire. L’utilisation de poules pondeuses plutôt que de mammifères pour leur obtention représente déjà en soi une avancée majeure en termes de bien-être animal puisque cette option permettant une collecte des anticorps dans les œufs rend tout simplement obsolète la saignée de l’animal producteur. Les avantages de cette technologie sont cependant multiples et vont bien au-delà de l’aspect de protection de l’animal. En optimisant le protocole d’immunisation, il est possible d’améliorer à la fois la réponse immunitaire de la poule et son bien-être en période de production. Cette synthèse bibliographique propose des recommandations à cette fin. Les approches les plus récentes pour améliorer la technologie sont également discutées.
Abstract
Antibodies have become essential tools in recent decades, with a wide range of applications in the laboratory and in human and veterinary medicine. The use of laying hens, instead of mammals, to obtain the necessary antibodies from the eggs is a major advance in terms of animal welfare because it makes blood sampling obsolete. However, the advantages of this technology are numerous, in addition to the animal welfare aspect. With a carefully designed immunization protocol, it is possible to enhance both the hen’s immune response and its welfare during the process. This review puts forward recommendations how to do this and discusses recent approaches on improving the technology.
1. Introduction
1Hens’ eggs have long been known as an excellent source of nutrients for humans. They are also an important source of antibodies, the most abundant being immunoglobulin (Ig) Y. This characteristic has attracted increasing interest in recent decades (Yegani et al., 2010). The natural transfer of antibodies that occurs from hen to chick via the egg yolk can be exploited to produce antibodies specific to a given pathogen, simply by immunizing the laying hens with an antigen from this targeted pathogen (Kovacs-Nolan et al., 2012). Feeding these specific antibodies to other animals is therefore an extension of the passive maternal protection. Although it has had a reputation for being a source of human foodborne infections, such as salmonellosis and campylobacteriosis, the hen could thus become a serious ally in fighting these pathogens and others, thanks to its ability to produce massive amounts of antibodies specific to targeted bacteria. These antibodies could help address the worldwide emergence of drug-resistant microorganisms and the resultant reduction in antibiotic use in the livestock industry. They also offer a solution to the inability to treat or prevent some diseases with conventional vaccines in some production sectors, such as in industrial broiler chickens whose lifespan is limited (about 42 days) (Namata et al., 2009). Apart from the control of pathogens, hen egg yolk antibodies could also be used to modulate normal gut microflora, as described in recent ruminant studies in order to control ruminal fermentations (Marino et al., 2011).
2Currently, these antibodies remain underused in both veterinary and human medicine. This review focuses on the development of hen egg yolk antibodies for the therapy and prophylaxis of animal diseases. After describing passive immunization and its potential, the paper puts forward recommendations on producing antigen-specific IgY in laying hens. It then explores recent progress in optimizing this technology, with particular emphasis on animal welfare. Other aspects, such as the mode of action of IgY, its molecular properties and its application in human and veterinary medicine, have been described elsewhere (Chalghoumi et al., 2009; Xu et al., 2011; Kovacs-Nolan et al., 2012).
2. The passive immunization concept
3Passive immunization involves transferring preformed antibodies from one individual to another, unlike active immunization where an animal has to produce its own antibodies. The best-known form of passive immunization is the transfer of maternal antibodies from a mother to her descendants. In mammals, it occurs through colostrum ingestion and/or placental transfer; in birds, all the antibodies needed to protect the offspring are transmitted via the egg (Brambell, 1970).
4Three immunoglobulin classes are deposited into the egg: IgA, IgM, and IgY. Maternal IgA and IgM are present at low concentrations (0.7 and 0.15 mg·ml-1, respectively), predominantly in the egg white, whereas IgY, which is by far the most abundant egg Ig, is present in the egg yolk at concentrations up to 25 mg·ml-1 (Rose et al., 1974).
5As the adaptive immune system develops during the first 2 weeks post-hatch, early humoral protection in the chick depends heavily upon this maternal transfer (Smith et al., 2008). The given protection is efficient, but it is short-term and is limited to infections present in the hen’s environment at the time of lay (Smith et al., 2008). Nevertheless, it is possible to take advantage of this natural transfer of antibodies from hen to chick. The concentration of IgY deposited in the egg is closely linked to that in the maternal serum (Hamal et al., 2006). Therefore, by immunizing laying hens with a specific target antigen, we can manage their immune system and the composition of the pool of antibodies, first in the serum, then in the eggs. The specific antibodies obtained can then be exploited to immunize other individuals via a feed additive (Xu et al., 2011). Commercial vaccines have also been developed (e.g., CoxAbic® against coccidiosis), based on the maternal transfer of immunity (Sharman et al., 2010).
3. Advantages of IgY technology
6The growing interest in IgY technology stems from the numerous advantages it offers compared with using its mammalian equivalent, IgG.
7The primary advantage of obtaining Ig via laying hens instead of mammals is improved animal welfare. This is in complete accordance with the principle of the 3 R’s – reduction, refinement and replacement – as defined by Russel et al. (1959) and this method has therefore been strongly recommended for some time by the European Centre for the Validation of Alternative Methods (Schade et al., 1996). It is a refinement of the antibody production protocol because it does not involve bleeding the antibody producer animals, unlike the mammals models. The long-lasting titers obtained from laying hens also reduce the need for frequent booster injections (Schade et al., 2005). Another advantage is that laying hens are able to produce Ig in higher amounts (e.g., 5-6 times more than a rabbit; Narat, 2003), which drastically reduces the number of animals needed to obtain the antibodies.
8This high yield is also associated with an obvious advantage from an economic point of view, the more so because the cost of feeding and housing laying hens tends to be lower than for mammals. The numerous IgY extraction processes described in the literature (De Meulenaer et al., 2001) are usually both efficient and cheap. The hyper-immune yolk can also be used just as it is, as discussed later. The exploitation of antibodies obtained from the egg is therefore less labor-intensive and more cost-effective than traditional Ig production using mammals.
9Oral immunotherapy through the use of IgY is also attractive because of its high specificity compared with other alternatives to antibiotics, such as organic and inorganic acids, oligosaccharides, probiotics and herbal extracts. Nevertheless, even at the risk of developing tools that are too specific, as noted by Sirsat et al. (2009), we consider that this risk is minimal in the case of polyclonal egg yolk antibodies. When Chalghoumi et al. (2008) developed IgY specific to two Salmonella serovars, they demonstrated a high level of cross-reactivity of IgY developed against a particular serovar with antigens of the other one, and vice versa. Thus, using vaccine antigens shared among several serovars addresses the risk of the developed IgY being too highly specific. In addition, the fact that Chalghoumi et al. (2008) were able to raise IgY against two Salmonella serovars in a single egg yolk indicates that it could soon be possible to develop real “cocktail eggs” targeting a diverse set of organisms. Finally, the use of IgY does not lead to undesirable side effects, disease resistance or toxic residues (Xu et al., 2011), unlike other drug strategies (e.g., antibiotics).
4. Standard protocols for IgY production
10The standard protocol for producing antigen-specific IgY intended for passive dietary immunization in animals is illustrated in figure 1.
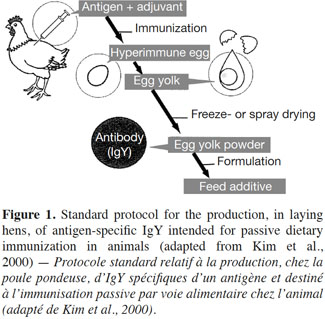
11Hens are usually exposed to the targeted antigen through an injection. This triggers a humoral immune response that manifests itself initially by the production of specific IgY in the blood serum of the immunized hen, followed by its export in the yolk of laid eggs. Once the immune response has been induced, the transovarial passage of IgY takes about 6-7 days (Bollen et al., 1997). The composition of the pool of IgY in the yolk is clearly related to that in the hens’ circulating blood (Hamal et al., 2006). Nevertheless, discrepant results have been published on yolk IgY and serum IgY levels, some authors reporting yolk titers higher than serum titers, and vice versa (Woolley et al., 1995; Malik et al., 2006). These inconsistent data could be explained, at least partly, by the biological oscillations in egg IgY concentrations (Pauly et al., 2009). It has also been shown that 10-15% of immunized hens might be low responders to certain antigens (Schade et al., 1996).
12Basically, obtaining specific IgY involves injecting an antigen-adjuvant combination at certain intervals. Numerous protocols, using different antigens, adjuvants, injection routes and intervals between injections, have been described over the years. All these factors are critical because they influence both the outcome of the immunization procedure (amount and specificity of the obtained IgY) and the welfare of the hens. This section, however, provides general advice about IgY production (Table 1), rather than an exhaustive description of all the variations that can be used.
4.1. Antigen
13The first step in specific IgY production is to choose the target antigen. This can be a single antigen (protein, peptides or polysaccharides) or a complex multi-antigen (bacteria, mold, viruses or parts of these). The molecules exhibiting the best immunogenicity are proteins (Schwarzkopf et al., 2001). In the case of small antigens with a molecular weight below 12,000 da (known as “haptens”), conjugation to a carrier protein (e.g., bovine gamma globulin) is often required (Cook et al., 2010). Carbohydrates and nucleic acids could also be coupled advantageously with carriers because of their reduced immunogenicity (Schwarzkopf et al., 2001). Apart from the intrinsic immunogenicity of the target antigen, its quality and quantity should also be taken into account. The purity of the antigen is a crucial parameter because impurities could lead to IgY with more activity against the impurities themselves than against the antigen of interest (Leenaars et al., 2005). In addition, contaminations of the antigen with microbes, endotoxins or chemical residues from the inactivation/extraction process could have a negative effect on animal welfare as well as on immune response (Leenaars et al., 2005). The antigen dose is also critical because too much or too little antigen can lead to suppression, sensitization, tolerance or other undesirable immunomodulatory effects (Schwarzkopf et al., 2001). The recommended amount of a soluble protein to be administered in a given vaccine dose is usually in the range of 0.01 mg to 1 mg (Schwarzkopf et al., 2001; Cook et al., 2010).
4.2. Adjuvant
14The aqueous portion of the vaccine dose is diluted in a physiological saline solution and the antigen solution thus obtained is commonly combined with an adjuvant to ensure effective immune response. The induced response can be more cellular than humoral, or vice versa, depending on the chosen adjuvant. In the case of antibody production, the humoral response should be favored. There are dozens of commercially available adjuvants that have been described in reviews (e.g. Stills, 2005; Wilson-Welder et al., 2009). Among these multiple adjuvants, Freund’s adjuvants (FA) remain the “gold standard” and are widely used for experimental antibody production. Freund’s complete adjuvant (FCA) is the most effective in terms of productivity; it has not been surpassed by any adjuvant (Stills, 2005). FCA has been associated, however, with a variety of undesirable side effects, particularly in mammals. These findings have led to numerous regulatory guidelines controlling the use of FCA in experimental animals. Nevertheless, it is worth noting that FCA is less problematic in birds (Bollen et al., 1996; Chalghoumi et al., 2008), although this observation has not always been consistent (Olbrich et al., 2002). From our experience, this discrepancy in reports on FCA consequences in birds can be explained by two factors. First, it is possible that the injection route used for laying hens (mainly intramuscular, see 4.3.) might hide the resulting local inflammation, whereas other injection routes (subcutaneous or intradermal) used more frequently in mammals might facilitate the observation of the tissue reaction. The most recent findings in our laboratory (data to be published) corroborate this argument. The second factor is the quality of emulsion. Even in mammals, it seems that FCA is not as damaging as previously reported, at least when a limited volume of high-quality emulsion is injected (Leenaars et al., 2005). At the laboratory level, we advise following the “T”-connector emulsifying protocol proposed by Moncada et al. (1993), where the final vaccine emulsion is obtained by repeated passages of the adjuvant and antigen mixture through a three-way “T”-connector to which two Luer-lock syringes are attached. To limit the risk of local tissue reaction, the use of FCA is often restricted to the first immunization, whereas Freund’s incomplete adjuvant (FIA), which does not contain mycobacteria extracts, is preferred for booster immunization (Chalghoumi et al., 2008). This seems to prevent the adverse side effects while still allowing high IgY levels to be obtained. The use of FIA is sometimes recommended even for the first immunization (Narat, 2003).
4.3. Injection route
15The vaccine is usually injected through the intramuscular route, most often in the pectoralis major muscle (Schade et al., 2005). The subcutaneous route has also been used (Mayo et al., 2009; Lakeh et al., 2011), but it is not recommended in terms of welfare considerations (Schade et al., 1996). In addition, the intramuscular route results in levels of specific IgY nearly 10 times higher than in the case of the subcutaneous route (Chang et al., 1999). In terms of animal welfare, it is imperative to limit the quantity injected to that which is sufficient to induce the antibody response, without exceeding the maximal volume of 1 ml and a maximum of four injection sites (Leenaars et al., 2005).
4.4. Immunization schedule
16Immunization should be performed when the animals are of egg-laying age (Leenaars et al., 2005). The goal is often to make the peak of lay and the peak of antibody production coincide. This peak is reached at about 28-30 weeks old, and the first injection should therefore take place at about 20 weeks old.
17Booster injections are needed in order to take advantage of the memory of the adaptative immune system. The interval between injections ranges from 1 (Cook et al., 2010) to 8 weeks (Pauly et al., 2009), the usual interval being 3-4 weeks. Frequency and interval depend on the immunogenicity of the antigen and on the adjuvant used. The general rule is to administer a booster immunization when the IgY titer reaches a plateau or begins to decrease (Leenaars et al., 2005). Injecting boosters too quickly can lead to a delayed selection of high-affinity B-cells and is therefore less effective (Stills, 2012). Persistent IgY production can be obtained via booster injections repeated throughout the laying period and even beyond it, as discussed by Pauly et al. (2009). The harvest of hyper-immune eggs can begin as early as 1 week after the first injection (Bollen et al., 1997), but the IgY titer peak has been reported from 3 weeks after the first immunization (Trott et al., 2008) to 2 weeks after the second booster injection (Schade et al., 2005).
4.5. Extraction and processing of IgY
18The extraction of IgY from the egg can be achieved using several methods, resulting in variations in the recovery and purity of the extract. Usually, the yolk is separated from the white, but sometimes the whole egg is used as a feed additive (Gürtler et al., 2004). The antibodies can then be purified, from completely purified IgY to unpurified whole yolk options. The choice of IgY extraction method is influenced mainly by the required purity of antibodies versus the cost effectiveness of the method. A number of methods of extracting IgY involving various chemicals have been described (for a review of current protocols, see De Meulenaer et al., 2001). Each method has specific purposes and it is almost impossible to provide a recommendation for each of the many possible applications of IgY. Some general recommendations are provided, however, in table 2 as a first line of approach. Usually, eggs used for laboratory reagent production are purified, whereas eggs used in animal experiments are used as whole yolk (Cook et al., 2010), which has economic advantages. Indeed, the commercial IgY purification kits available on the market are still expensive (Tan et al., 2012). The whole yolk option allows one to take advantage of other egg yolk components that have also been suggested as protective, such as high-density lipoproteins (Kassaify et al., 2005) or sialyloligosaccharides and their derivatives (Sugita-Konishi et al., 2002). The obtained hyper-immune preparation, whether purified or not, needs to be processed before being orally administered to animals. It is usually supplied as freeze- or spray-dried powder (Yegani et al., 2010; Xu et al., 2011), but some have used a liquid form (Rahimi et al., 2007). IgY could possibly be provided in ovo (Yegani et al., 2010), but higher mortality, reduced hatchability and reduced growth of chicks have been reported using this approach (Eterradossi et al., 1997).

5. Optimizing the IgY production
19The annual yield of IgY per laying hen has been reported to be as low as 20 g (Xu et al., 2011) and as high as 100 g by more optimistic authors (Yegani et al., 2010). It is reasonable to think that the truth lies somewhere in between (Cook et al., 2010) and that the quantitative method used alongside the extraction process has a great influence on the yield recovered (Tan et al., 2012). Within the total amount of IgY obtained, an average of 9% can be expected to be antigen-specific (Li et al., 1998). These yields are certainly impressive, but an improvement in the percentage of antigen-specific IgY in the eggs and a reduction in the time needed to reach maximal production would significantly extend the application of IgY technology at the commercial level. In addition, although this technology is aimed primarily at the economic production of the highest amount of highly specific antibodies, welfare issues cannot be neglected. These issues already play an important role in the way researchers design their immunization protocols, and this is expected to increase.
20There are several variation parameters for optimizing the IgY production protocol in terms of both yield and welfare. On the one hand, the focus can be on the vaccine; on the other, it can be on the producer animal itself.
5.1. The producer animal and its environment
21IgY concentration resulting from a vaccination can vary significantly among genetic lines (Hamal et al., 2006). This indicates that it could be possible to increase IgY production by genetic selection within high-producing lines. Nevertheless, for Cook et al. (2010), there is very little difference in the ability of commercial lines to produce antibodies, the most important parameter governing the production of IgY over a year being egg size and rate of lay. IgY concentration (mg·ml-1) in the yolk is independent of the rate of lay or egg size (Li et al., 1998; Trott et al., 2009; Ulmer-Franco et al., 2012). Hence, a larger egg yolk leads to a higher amount of antibody per egg. The productivity of the line is therefore the key parameter to consider when selecting birds intended for IgY production. As a consequence, every method aimed at improving laying performance would also lead to an improvement in the yield of the immunization process.
22The production period can be extended for a second year because an interruption during the lay or the practice of molting hens before or after initiating antibody production has little or no impact on the collected level of IgY (Pauly et al., 2009; Trott et al., 2009). In contrast, egg yolk weight increases with flock age, thus increasing the amount of IgY recovered (Pauly et al., 2009; Ulmer-Franco et al., 2012). Pauly et al. (2009) determined that the maintenance period should not be prolonged, from an economic point of view, when lay decrease to about 4 eggs per week. In the case of extended production, late booster injections can strongly increase the IgY titer deposited in the egg yolk (Schwarzkopf et al., 2001).
23Environmental conditions (e.g., cage density or temperature) can also affect a hen’s ability to transfer IgY to her eggs (Mashaly et al., 2004; Leandro et al., 2011). Any stress that a hen encounters reduces her immune responsiveness (Leandro et al., 2011). Therefore, optimal housing conditions should be provided. The recent ban on conventional cages for laying hens in the European Union (Council Directive No. 1999/74/EC) could complicate the development of research related to IgY technology because the association of hens and laid eggs is easier with conventional cages than in free-range or coop systems. In mass production, however, where immunization protocols are already well established, housing in groups presents no particular problem and the production could take place in commercial egg production units. Schwarzkopf et al. (2001) studied the influence of hen housing conditions on the development of specific IgY and concluded that the use of SPF-hens will remain an exception because it does not lead to any improvement in IgY deposition in the egg yolk and involves significant additional cost compared with conventional housing.
5.2. The vaccine
24Maximizing IgY deposition seems to be achieved mainly by optimizing vaccination procedures. The most critical point is the composition of the vaccine, particularly the choice of adjuvant added to the antigen to enhance the immune response.
25Vaccine composition. Although FA are still used as standard adjuvants in laying hens, it is likely that alternatives will be used to a greater extent in the future because of animal welfare considerations. FA are judged to be potentially toxic and their use has been discouraged or banned by many institutional animal care and use committees. A balance needs to be found between efficacy and safety, and the best alternative to FA would be one that allows similar levels of highly specific IgY to be obtained from the eggs without leading to undesirable side effects.
26Various alternatives have been evaluated in birds, including aluminum salts (de Paula et al., 2011), carbopol formulations (Kim et al., 2012), the immunostimulating complexes matrix (Chalghoumi et al., 2008), lipohexapeptide Pam3Cys-Ser-(Lys)4-OH (PCSL) (Schwarzkopf et al., 2001), Montanide™ oils (Dungu et al., 2009), poxvirus constructs (Chen et al., 2010) and DNA-based formulations (Loots et al., 2006). Many claim to be less damaging and painful than FCA, but none has been shown, so far as we know, to surpass FCA in terms of antibody response.
27Apart from the choice of an alternate adjuvant, the addition of immunostimulating components to a vaccine can markedly increase IgY deposition in the egg yolk. Table 3 gives an overview of some promising products that appear to achieve this.
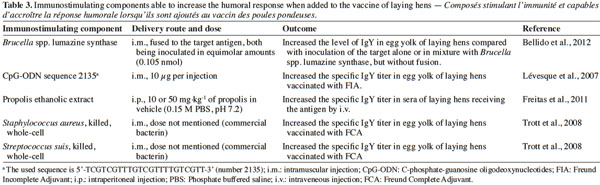
28The resulting enhancement of these additions is variable. Lévesque et al. (2007) demonstrated that the supplementation of FIA with oligodeoxynucleotides (ODN) containing C-phosphate-guanosine motifs (CpG) resulted in a yield increase of up to 480% while they did not observe any improvement with the 1α,25-dihydroxyvitamin D3 supplementation. Adding CpG-ODN is a very promising way of improving the immune response of immunized animals. The activity of CpG-ODN is motif-dependent and the CpG sequences that stimulate optimal responses differ among species (Rankin et al., 2001). The GTCGTT sequence is recognized as most active in avian species (Lévesque et al., 2007; Linghua et al., 2007). A phosphorothioate backbone renders CpG-ODN stable against nucleases and allows the use of smaller amounts (Stacey et al., 2002). This supplementation not only increases the IgY level, but also induces longer-lasting production (Vleugels et al., 2002) without any adverse side effects (Weeratna et al., 2000). The synthetic CpG motifs mimic the immunostimulatory effect of the bacterial DNA (Klinman et al., 2004). The addition of killed whole cell has also been shown to improve humoral immune response in chickens (Trott et al., 2008). Trott et al. (2008) investigated the effect of adding various commercial bacterins to FCA on the antibody response of laying hens to a protein antigen. The addition of Gram+ Staphylococcus aureus or Streptococcus suis to FCA increased the IgY response. In contrast, Gram- Escherichia coli killed whole cells reduced the resulting antibody titer compared with that observed for FCA alone. Producers of IgY should therefore be aware that components added to vaccines do not always improve the response, but can sometimes reduce it (Trott et al., 2008).
29Recently, the enzyme lumazine synthase from Brucella spp. has been reported to increase IgY production in laying hens when fused to the target antigen (Bellido et al., 2012). Another recent study (Freitas et al., 2011) dealt with the potential immunostimulatory effect of propolis, and reported a dose-dependent response of IgY level in blood serum following the intra-abdominal administration of an ethanolic extract of propolis prior to intravenous vaccination.
30Such supplementation allows a reduction in the amount of antigen and/or the necessary amount of adjuvant to be injected, thereby enhancing the overall cost-efficiency of the vaccination. In addition, these supplementations can also improve the efficiency of an adjuvant that would be intrinsically less efficient than the FA but could compete, thanks to the supplementation, with a reduced risk of undesirable side effects.
31The suppression of the adjuvant could even be envisaged when injecting potent immunostimulatory molecules as vaccine antigens (e.g., Salmonella porins, Gomez-Verduzco et al., 2010). These authors did not, however, compare the level of IgY obtained in this case with that obtained with FA. The prudent course is to reduce only the adjuvant/antigen ratio, moving from a conventional 50/50 (v/v) ratio to 30/70, for example.
32Alternative immunization routes. Classic immunization protocols involve injection, but oral routes have been proposed (voluntary intake or gavage, or via oral-nasal administration through exposure of the bird to an aerosol). These routes are considered less stressful and are therefore in line with the 3 R’s principle (Hau et al., 2005). In addition, they potentially allow the easier administration of frequent boosters. The development of these oral immunization protocols is still in its infancy and they need further refinement if they are to compete with parenteral immunization protocols (Mayo et al., 2009). As for the classic protocol, the outcome of immunization through the oral route could be enhanced via immunostimulating components. For example, the oral administration of CpG-ODN has been tested, but exhibited only a slight and temporary increase of serum IgY titer in broilers (Ameiss et al., 2006). Such oral supplementation for enhancing IgY production needs further research.
33In the particular case of DNA vaccines, various methods have been used to improve their delivery and immunogenicity. Among these is the “gene gun” method, which has recently gained more attention for birds’ immunization (Niederstadt et al., 2012). Developed in the early 1980s, it involves delivering DNA or RNA coated in microscopic gold or titanium particles into living tissues. This immunization route might lead to enhanced antibody titers, allowing a wider use of DNA vaccination in birds in the future. DNA vaccines still suffer from poor cost efficiency, partly because of their poor immunogenicity (Singh et al., 2003). The studies to date, so far as we know, have investigated the effects of gene gun immunization on IgY production and laying capacity, but none has provided any evaluation of this approach in terms of animal welfare. In case of proven enhancement, it is worth noting that recent work on mice suggests that gene guns might also successfully deliver protein antigens (Scheiblhofer et al., 2013).
34Nutrition and immunomodulation. If nutrition affects antibody production and the transfer of immunity to chicks (Leandro et al., 2011), supplementing the diet could also be considered as a way to promote IgY production. For example, the hydroxylated form of vitamin D3, 25-hydroxycholecalciferol increased the level of IgY in the serum of Salmonella typhimurium-challenged chickens (Chou et al., 2009). Dietary L-carnitine (β-OH-(γ-N-trimethylamino)-butyrate) supplementation (100 mg·kg-1) has been shown to enhance antigen-specific IgY in vaccinated broilers (Mast et al., 2000). The level of supplementation, however, can have a strong influence on the outcome of these immunomodulation trials; de Beer et al. (2009) did not measure any increase in total IgY level in egg yolks following the addition of L-carnitine at 50 mg·kg-1 to the diet of broiler breeder hens. A “more is better” approach cannot be viewed as a panacea when using nutrition to modulate immunity, as recently discussed by Korver (2012) using the example of vitamin E, which could improve immune response but could also become immunosuppressive if there is excessive supplementation. Diet supplementation via immunomodulating ingredients, however, is an approach that deserves greater attention because it also represents a form of refinement of IgY technology.
6. Conclusion
35IgY will undoubtedly be used more extensively in the future in a wide range of applications, from human and veterinary medicine to diagnostics and research. The generation of these antibodies via laying hens represents a reduction and refinement in animal use compared with the conventional methods for obtaining Ig via mammals. This technology could be further refined thanks to recent progress made in adjuvantal methods as well as other approaches, such as oral immunization and nutritional immunomodulation. Future developments in this technology will also be driven by the economics of immunization.
36List of abbreviations
37CpG: C-phosphate-guanosine motifs
38FA: Freund Adjuvant
39FCA: Freund Complete Adjuvant
40FIA: Freund Incomplete Adjuvant
41Ig: Immunoglobulin
42i.m.: Intramuscular injection
43i.p.: Intraperitoneal injection
44i.v.: Intraveneous injection
45ODN: oligodeoxynucleotides
46PCSL: Pam3Cys-Ser-(Lys)4-OH
47PBS: Phosphate buffered saline.
48Acknowledgements
49This work was supported by the Operational Directorate-General for Agriculture, Natural Resources and Environment of Wallonia (DGARNE, Namur, Belgium).
Bibliographie
Ameiss K.A. et al., 2006. Influence of orally administered CpG-ODNs on the humoral response to bovine serum albumin (BSA) in chickens. Vet. Immunol. Immunopathol., 110, 257-267.
Bellido D. et al., 2012. Brucella spp. Lumazine synthase as a novel immunomodulator to produce egg yolk antibodies. Adv. Biosci. Biotechnol., 3, 80-86.
Bollen L.S., Crowley A., Stodulski G. & Hau J., 1996. Antibody production in rabbits and chickens immunized with human IgG – A comparison of titre and avidity development in rabbit serum, chicken serum and egg yolk using three different adjuvants. J. Immunol. Methods, 191, 113-120.
Bollen L.S. & Hau J., 1997. Immunoglobulin G in the developing oocytes of the domestic hen and immunospecific antibody response in serum and corresponding egg yolk. In Vivo, 11, 395-398.
Brambell F.W.R., 1970. Transmission of immunity in birds. In: Neuberger A. & Tatum E.L., eds. Transmission of immunity from mother to young. New York, NY, USA: Elsevier, 20-41.
Chalghoumi R., Thewis A., Portetelle D. & Beckers Y., 2008. Production of hen egg yolk immunoglobulins simultaneously directed against Salmonella enteritidis and Salmonella typhimurium in the same egg yolk. Poult. Sci., 87, 32-40.
Chalghoumi R., Beckers Y., Portetelle D. & Thewis A., 2009. Hen egg yolk antibodies (IgY), production and use for passive immunization against bacterial enteric infections in chicken: a review. Biotechnol. Agron. Soc. Environ., 13, 295-308.
Chang H.M., Ou-Yang R.F., Chen Y.T. & Chen C.C., 1999. Productivity and some properties of immunoglobulin specific against Streptococcus mutans serotype c in chicken egg yolk (IgY). J. Agric. Food Chem., 47, 61-66.
Chen H.-Y. et al., 2010. Construction and immunogenicity of a recombinant fowlpox vaccine coexpressing S1 glycoprotein of infectious bronchitis virus and chicken IL-18. Vaccine, 28, 8112-8119.
Chou S.H., Chung T.K. & Yu B., 2009. Effects of supplemental 25-hydroxycholecalciferol on growth performance, small intestinal morphology, and immune response of broiler chickens. Poult. Sci., 88, 2333-2341.
Cook M.E. & Trott D.L., 2010. IgY – immune component of eggs as a source of passive immunity for animals and humans. World’s Poult. Sci. J., 66, 215-225.
de Beer M. & Coon C.N., 2009. The effect of different feed restriction programs and dietary L-carnitine supplementation on hepatic lipogenesis, plasma heterophil to lymphocyte ratio and yolk IgY content of broiler breeder hens. Int. J. Poult. Sci., 8, 328-341.
De Meulenaer B. & Huyghebaert A., 2001. Isolation and purification of chicken egg yolk immunoglobulins: a review. Food Agric. Immunol., 13, 275-288.
de Paula V.S. et al., 2011. Applied biotechnology for production of immunoglobulin Y specific to hepatitis A virus. J. Virol. Methods, 171, 102-106.
Dungu B. et al., 2009. Study on the efficacy and safety of different antigens and oil formulations of infectious coryza vaccines containing an NAD-independent strain of Avibacterium paragallinarum. Onderstepoort J. Vet. Res., 76, 299-309.
Eterradossi N. et al., 1997. Passive protection of specific pathogen free chicks against infectious bursal disease by in ovo injection of semi-purified egg-yolk antiviral immunoglobulins. J. Vet. Med. B Infect. Dis. Vet. Public Health, 44, 371-383.
Freitas J.A. et al., 2011. The effects of propolis on antibody production by laying hens. Poult. Sci., 90, 1227-1233.
Gomez-Verdusco G. et al., 2010. Humoral immune response in breeding hens and protective immunity provided by administration of purified Salmonella gallinarum porins. Poult. Sci., 89, 495-500.
Gürtler M., Methner U., Kobilke H. & Fehlhaber K., 2004. Effect of orally administered egg yolk antibodies on Salmonella enteritidis contamination of hen's eggs. J. Vet. Med. B Infect. Dis. Vet. Public Health, 51, 129-134.
Hamal K.R., Burgess S.C., Pevzner I.Y. & Erf G.F., 2006. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult. Sci., 85, 1364-1372.
Hau J. & Hendriksen C.F., 2005. Refinement of polyclonal antibody production by combining oral immunization of chickens with harvest of antibodies from the egg yolk. ILAR J., 46, 294-299.
Kassaify Z.G., Li E.W.Y. & Mine Y., 2005. Identification of antiadhesive fraction(s) in nonimmunized egg yolk powder: in vitro study. J. Agric. Food Chem., 53, 4607-4614.
Kim D.K. et al., 2012. Effects of novel vaccine/adjuvant complexes on the protective immunity against Eimeria acervulina and transcriptome profiles. Avian Dis., 56, 97-109.
Kim M. et al., 2000. Egg yolk antibody and its application. Biotechnol. Bioprocess Eng., 5, 79-83.
Klinman D.M., Currie D., Gursel I. & Verthelyi D., 2004. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol. Rev., 199, 201-216.
Korver D.R., 2012. Implications of changing immune function through nutrition in poultry. Anim. Feed Sci. Technol., 173, 54-64.
Kovacs-Nolan J. & Mine Y., 2012. Egg yolk antibodies for passive immunity. Annu. Rev. Food Sci. Technol., 3, 163-182.
Lakeh A.A.B. et al., 2011. GH and IGF-I induction by passive immunisation of rainbow trout Oncorhynchus mykiss (Walbaum) using a somatostatin-14 antibody. Aquaculture, 316, 99-103.
Leandro N.M. et al., 2011. Maternal antibody transfer to broiler progeny varies among strains and is affected by grain source and cage density. Poult. Sci., 90, 2730-2739.
Leenaars M. & Hendriksen C.F., 2005. Critical steps in the production of polyclonal and monoclonal antibodies: evaluation and recommendations. ILAR J., 46, 269-279.
Lévesque S., Martinez G. & Fairbrother J.M., 2007. Improvement of adjuvant systems to obtain a cost-effective production of high levels of specific IgY. Poult. Sci., 86, 630-635.
Li X. et al., 1998. Effect of egg and yolk weights on yolk antibody (IgY) production in laying hens. Poult. Sci., 77, 266-270.
Linghua Z., Xingshan T. & Fengzhen Z., 2007. Vaccination with Newcastle disease vaccine and CpG oligodeoxynucleotides induces specific immunity and protection against Newcastle disease virus in SPF chicken. Vet. Immunol. Immunopathol., 115, 216-222.
Loots K. et al., 2006. Evaluation of the persistence and gene expression of an anti-Chlamydophila psittaci DNA vaccine in turkey muscle. BMC Vet. Res., 2, 18.
Malik M.W., Ayub N. & Qureshi I.Z., 2006. Passive immunization using purified IgYs against infectious bursal disease of chickens in Pakistan. J. Vet. Sci., 7, 43-46.
Marino C.T. et al., 2011. Effects of adding polyclonal antibody preparations on ruminal fermentation patterns and digestibility of cows fed different energy sources. J. Anim. Sci., 89, 3228-3235.
Mashaly M. et al., 2004. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci., 83, 889-894.
Mast J., Buyse J. & Godderis B.M., 2000. Dietary L-carnitine supplementation increases antigen-specific immunoglobulin G production in broiler chickens. Brit. J. Nutr., 83, 161-166.
Mayo S. et al., 2009. Enhancement of anamnestic immunospecific antibody response in orally immunized chickens. J. Immunol. Methods, 342, 58-63.
Moncada C., Torres V. & Israel Y., 1993. Simple method for the preparation of antigen emulsions for immunization. J. Immunol. Methods, 162, 133-140.
Namata H. et al., 2009. Identification of risk factors for the prevalence and persistence of Salmonella in Belgian broiler chicken flocks. Prev. Vet. Med., 90, 211-222.
Narat M., 2003. Production of antibodies in chickens. Food Technol. Biotechnol., 41, 259-267.
Niederstadt L. et al., 2012. Stimulation of IgY responses in gene gun immunized laying hens by combined administration of vector DNA coding for the target antigen Botulinum toxin A1 and for avian cytokine adjuvants. J. Immunol. Methods, 382, 58-67.
Olbrich C. et al., 2002. Stable biocompatible adjuvants – a new type of adjuvant based on solid lipid nanoparticles: a study on cytotoxicity, compatibility and efficacy in chicken. Altern. Lab. Anim., 30, 443-458.
Pauly D. et al., 2009. Monitoring of laying capacity, immunoglobulin Y concentration, and antibody titer development in chickens immunized with ricin and botulinum toxins over a two-year period. Poult. Sci., 88, 281-290.
Rahimi S. et al., 2007. Prevention of Salmonella infection in poultry by specific egg-derived antibody. Int. J. Poult. Sci., 6, 230-235.
Rankin R. et al., 2001. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev., 11, 333-340.
Rose M.E., Orlans E. & Buttress N., 1974. Immunoglobulin classes in the hen's egg: their segregation in yolk and white. Eur. J. Immunol., 4, 521-523.
Russell W.M.S. & Burch L., 1959. The principles of humane experimental technique. London: Methuen & Co.
Schade R. et al., 1996. The production of avian (egg yolk) antibodies: IgY – the report and recommendations of ECVAM Workshop 21. Altern. Lab. Anim., 24, 925-934.
Schade R. et al., 2005. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Altern. Lab. Anim., 33, 129-154.
Scheiblhofer S., Ritter U., Thalhamer J. & Weiss R., 2013. Protein antigen delivery by gene gun-mediated epidermal antigen incorporation (EAI). In: Sudowe S. & Reske-Kunz A.B., eds. Biolistic DNA delivery. New York, NY, USA: Humana Press, 401-411.
Schwarzkopf C., Staak C., Behn I. & Erhard M., 2001. Immunisation. In: Schade R. et al., eds. Chicken egg yolk antibodies, production and application: IgY Technology. Berlin; Heidelberg, Germany: Springer Verlag, 25-64.
Sharman P.A., Smith N.C., Wallach M.G. & Katrib M., 2010. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol., 32, 590-598.
Singh M. & O’Hagan D., 2003. Recent advances in veterinary vaccine adjuvants. Int. J. Parasitol., 33, 469-478.
Sirsat S.A., Muthaiyan A. & Ricke S.C., 2009. Antimicrobials for foodborne pathogen reduction in organic and natural poultry production. J. Appl. Poult. Res., 18, 379-388.
Smith A.L. & Beal R., 2008. The avian enteric immune system in health and disease. In: Davison F., Kaspers B. & Schat K.A., eds. Avian immunology. Oxford, UK: Elsevier Ltd, 243-271.
Stacey K.J. et al., 2002. Phosphorothioate backbone modification changes the pattern of responses to CpG. In: Raz E., ed. Microbial DNA and host immunity. Totowa, NJ, USA: Humana Press Inc., 63-75.
Stills H.F., 2005. Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR J., 46, 280-293.
Stills H.F., 2012. Polyclonal antibody production. In: Suckhow M.A., Stevens K.A. & Wilson R.P., eds. The laboratory rabbit, guinea pig, hamster and other rodents. Oxford, UK: Elsevier Inc., 259-274.
Sugita-Konishi Y. et al., 2002. Inhibition of bacterial adhesion and Salmonella infection in BALB/c mice by sialyloligosaccharides and their derivatives from chicken egg yolk. J. Agric. Food Chem., 50, 3607-3613.
Tan S.H. et al., 2012. A novel, cost-effective and efficient chicken egg IgY purification procedure. J. Immunol. Methods, 380, 73-76.
Trott D.L., Hellestad E.M., Yang M. & Cook M.E., 2008. Additions of killed whole cell bacteria preparations to Freund complete adjuvant alter laying hen antibody response to soluble protein antigen. Poult. Sci., 87, 912-917.
Trott D.L. et al., 2009. Utility of spent Single Comb White Leghorn hens for production of polyclonal egg yolk antibody. J. Appl. Poult. Res., 18, 679-689.
Ulmer-Franco A.M. et al., 2012. Hatching egg and newly hatched chick yolk sac total IgY content at 3 broiler breeder flock ages. Poult. Sci., 91, 758-764.
Vleugels B., Ververken C. & Godderis B., 2002. Stimulatory effect of CpG sequences on humoral response in chickens. Poult. Sci., 81, 1317-1321.
Weeratna R.D., McCluskie M.J., Xu Y. & Davis H.L., 2000. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine, 18, 1755-1762.
Wilson-Welder J.H. et al., 2009. Vaccine adjuvants: current challenges and future approaches. J. Pharm. Sci., 98, 1278-1316.
Woolley J.A. & Landon J., 1995. Comparison of antibody production to human interleukin-6 (IL-6) by sheep and chickens. J. Immunol. Methods, 178, 253-265.
Xu Y. et al., 2011. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review. Biotechnol. Adv., 29, 860-868.
Yegani M. & Korver D.R., 2010. Application of egg yolk antibodies as replacement for antibiotics in poultry. World’s Poult. Sci. J., 66, 27-37.

