- Home
- Volume 17 (2013)
- numéro 4
- Description of Phaseolus vulgaris L. aborting embryos from ethyl methanesulfonate (EMS) mutagenized plants
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Description of Phaseolus vulgaris L. aborting embryos from ethyl methanesulfonate (EMS) mutagenized plants

Editor's Notes
Received on December 10, 2012; accepted on October 8, 2013
Résumé
Description des embryons de Phaseolus vulgaris en cours d’avortement issus de plantes mutagénisées au méthanesulfonate d’éthyle (EMS). Le but de cette étude était de décrire le processus d'avortement des embryons et d’étudier la transmission du caractère avortement des embryons chez les plantes de Phaseolus vulgaris déficientes dans le développement normal de la graine. Ces plantes ont été identifiées au sein de la deuxième génération d'une population TILLING de P. vulgaris cv. ‘BAT93’ obtenue par mutagénèse au méthanesulfonate d’éthyle (EMS). Les embryons mutants présentent des anomalies au niveau du suspenseur, du méristème apical caulinaire et des cotylédons, du stade globulaire jusqu’au stade cotylédonaire et avortent avant la maturité, comparé à ce qui se passe chez les embryons normaux. Les embryons mutants montrent également une hyperhydricité et contiennent une quantité faible de chlorophylle. Les analyses génétiques des populations F1, F2 et F3 issues des croisements effectués entre les plantes mutantes avec des embryons qui avortent et les plantes de type sauvage ont indiqué que le phénotype avortement des embryons est à hérédité maternelle et contrôlé par un couple d’allèles récessifs. Ces plantes mutantes de Phaseolus constituent un matériel précieux pour les études sur l’embryogenèse des plantes.
Abstract
The aim of this study was to describe the embryos abortion process and the inheritance of the embryos abortion trait in Phaseolus vulgaris plants deficient in seed development. These plants were isolated within the second generation of an ethyl methanesulfonate (EMS) TILLING population of P. vulgaris cv. ‘BAT93’. Mutant embryos show abnormalities mainly in suspensors, shoot apical meristem (SAM) and cotyledons from the globular to the cotyledon stages and abort before maturity compared to those observed in wild-type samples. Mutant embryos show also hyperhydricity and contain low amount of chlorophyll. Genetic analyses of F1, F2 and F3 populations from the crosses carried out between the mutagenized plants with aborting embryos and the wild-type plants indicated that the embryo abortion phenotype is maternally inherited and controlled by a single recessive gene. These Phaseolus mutant plants with aborting embryos constitute a valuable material for plant embryogenesis studies.
Table of content
1. Introduction
1Fertilization and embryogenesis are the first stages in the development of new life in both animals and plants. In early dicot plant embryogenesis, cell division occurs regularly and gives rise to the suspensor and the embryo proper. The suspensor anchors the embryo in the ovular tissues and plays a direct role in its nutrition by absorbing and transmitting nutrient materials from the surrounding tissues to the embryo (Raghavan, 2006). It is also a site for the biosynthesis of several phytohormones during plant early embryogenesis (Kawashima et al., 2010); and a site of high metabolic activity and polytene chromosomes, certainly in beans (Nagl, 1974; Nagl, 1981; Nenno et al., 1998). Embryo proper goes through four major stages: globular, heart, torpedo and cotyledonary. Cotyledons are the first aerial organs to differentiate during embryogenesis and function as lipid and protein storage organs. During embryo organogenesis, the radial symmetry in the apical embryo domain is converted towards bilateral symmetry, at the time of the transition from the globular to the heart-shaped stage (Madishetty et al., 2006; Chandler, 2008). This creates an apical central domain and an apical peripheral domain. The evolution of these two domains gives rise to the establishment of the shoot apical meristem (SAM) in the central domain and the mature cotyledons in the peripheral domain (Chandler et al., 2008).
2Embryogenesis studies in higher plants have been approached in some cases through the isolation of embryo-lethal mutants (Meinke, 1985; Devic, 1995; McElver et al., 2001; Tzafrir et al., 2004; Vernoud et al., 2005) and these mutagenized lines allowed a better understanding of embryo abortion mechanism in plants. Several embryo-lethal mutants were described in monocots and dicots (Neuffer et al., 1980; Schwartz et al., 1994; Yadegari et al., 1994; Liu et al., 1999; Apuya et al., 2002; Madishetty et al., 2006). Two types of embryo-defective mutants were revealed: the defective-kernel (dek) mutants in maize where both, the embryo and the endosperm were affected (Neuffer et al., 1980) and in the embryonic defective mutants (emb) found in maize and Arabidopsis only the embryo was affected (Meinke et al., 1979; Clark et al., 1991). In common bean, chemical mutagenesis is an efficient, reliable, and well understood process (Davis et al., 1988; Park et al., 1992; Pankhurst et al., 2004; Porch et al., 2009). Although different agents have been used, mutagenesis with ethyl methanesulfonate (EMS) was the most successful in many laboratory experiments and several mutants in various biological processes such as nodulation, flowering, fatty acid synthesis and embryogenesis were identified (Park et al., 2006; Silué et al., 2006; Borkar et al., 2010; Abid et al., 2011). EMS alkylates primarily guanine bases and leads to mispairing: alkylated G pairs with T instead of C, resulting in primarily G/C to A/T. Typically, seeds are soaked in EMS solution, rinsed and sown. Because each cell of the embryo is mutagenized independently of the other cells, plants of the first generation (M1) are chimeras. The identification of specific phenotypes starts by screening of the second generation (M2) where mutant genes are homozygous (Henikoff et al., 2003).
3This paper aims at describing the development of P. vulgaris embryos produced by mutagenized plants before they abort, using histological sections and direct visualization, and to present the inheritance of the embryo abortion trait induced by EMS mutagenesis.
2. Materials and methods
2.1. EMS mutagenesis, plant material and growth conditions
4The mutagenesis protocol used was established by Pankhurst et al. (2004) in the University of Geneva (Switzerland). Common bean line BAT93 obtained from the Centro Internacional de Agricultura Tropical (CIAT) in Colombia was used as starting plant material. BAT93 is a breeding line, coming from the cross (Veranic x Tlalnepantla 64) x (Jamapa x Tara) and combining resistance to Bean Common Mosaic Virus, common bacterial blight, rust and anthracnose (Kami et al., 2000). Mutagenesis was done in order to select plants that do not produce mature embryos, i.e. plants with embryos aborting before maturity. To perform mutagenesis, 40 g (~ 200 seeds) of BAT93 seeds were treated with 200 ml of 30 mM EMS overnight (approximately 16 h) at the room temperature with slow shaking then rinsed with sterile water (twenty times for twenty minutes each) and sown to generate M1 plants. Seeds were harvested from the M1 plants and sown to generate M2 plants. For our study, screening was done within the M2 population in order to select plants deficient in seed development. Plants were grown in growth chambers under controlled environmental conditions including 12 h photoperiod, 75% relative humidity and 27 °C/23 °C (day/night) temperature.
2.2. Histological studies
5The histological observations were carried out to determine the abnormalities occurring during embryo abortion in the mutagenized samples compared to those observed in wild-type. The protocol was adapted from Bonhomme et al. (2000) and Geerts et al. (2002). Developing seeds (n = 4) from wild-type and one from the selected mutagenized plants, deficient in seed development of P. vulgaris were harvested at 0, 3, 5, 7, 10 and 12 days after anthesis (DAA) and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7) containing 1.25% glutaraldehyde for at least 24 h at 4 °C. Fixed tissues were dehydrated in a graded ethanol series, infiltrated and embedded in methacrylate resin (Technovik 7100) according to standard procedures. Embedded samples were sectioned with a microtome at 5-7 μm. The sections were stained with toluidine blue and examined by a Nikon microscope (Model Eclipse E800). Observations were focused on suspensor, cotyledons and shoot apical meristem of the two types of embryos.
2.3. Genetic analysis
6One of the selected EMS-mutagenized plants, defective in embryo development, was reciprocally crossed (as male and female) with wild-type plants to study the inheritance of the embryo abortion trait. F2 plants derived from seven F1 plants using wild-type plants as female parents were screened for seed setting to determine the proportions of plants defective in seed development and plants with normal seeds. Seeds from sixteen F2 plants (F3 progenies, F2:3) derived from one of the seven F1 plants were sown to evaluate the rate of homozygous and heterozygous among these sixteen F2 plants. The presence of at least one mutagenized plant with aborting embryos in an offspring demonstrates the heterozygosity of the F2 plant. The observed ratios were tested against the expected ratio according to Mendel’s laws using a chi-square test.
3. Results
3.1. Embryo patterns in Phaseolus vulgaris wild-type plants and EMS mutagenized plants deficient in seed development
7For our study, 365 plants from the second generation (M2) of the TILLING population were screened in order to isolate plants deficient in embryo development. These 365 M2 plants were generated from 60 independent plants from the first generation (M1) obtained after seed treatment. In the M2 progenies, only two plants from one individual M1 plant (numbered 522) showed the desired trait, i.e. embryo abortion before maturity. Future observations and studies have been conducted on one of these two desired plants.
8During their development in the wild-type plants, embryos pass through the four major stages: globular, heart, torpedo and cotyledonary as all dicot embryos. BAT93 embryos reach the globular stage at 2-3 DAA, the heart stage at 4-5 DAA, the torpedo stage at 6 DAA, the early cotyledonary stage at 7-8 DAA and the late cotyledonary stage at 12 DAA. Figures 1a, 1b and 1c show the wild-type embryos at 5, 8 and 12 DAA, respectively. Their development is normal and the different parts of the embryos are well formed. Embryos development from the M2 selected plant 522 is delayed compared to normal embryos and they abort before maturity. Figures 1d, 1e and 1f show mutant embryos extracted from single pods at 8, 11 and 18 DAA, respectively. Many different stages of developmental arrest are included among the embryo mutants. In figures 1d, 1e and 1f, embryos stop growing at cotyledonary, torpedo, heart and globular stages, respectively. Mutant phenotypes included the presence of unusually large suspensors, distorted, asymmetric, single (g), fused (h) and triple (i) cotyledons (Figure 1). One of the main features of these mutant embryos is the lack of a SAM at the late cotyledonary stage, compared to the wild-type embryos (Figures 1k and 1j, respectively). Mutant embryos were also characterized by translucent and turgid aspects. They are pale green, pale yellow, almost white, as opposed to wild-type embryos, which are green at the cotyledonary late stage.
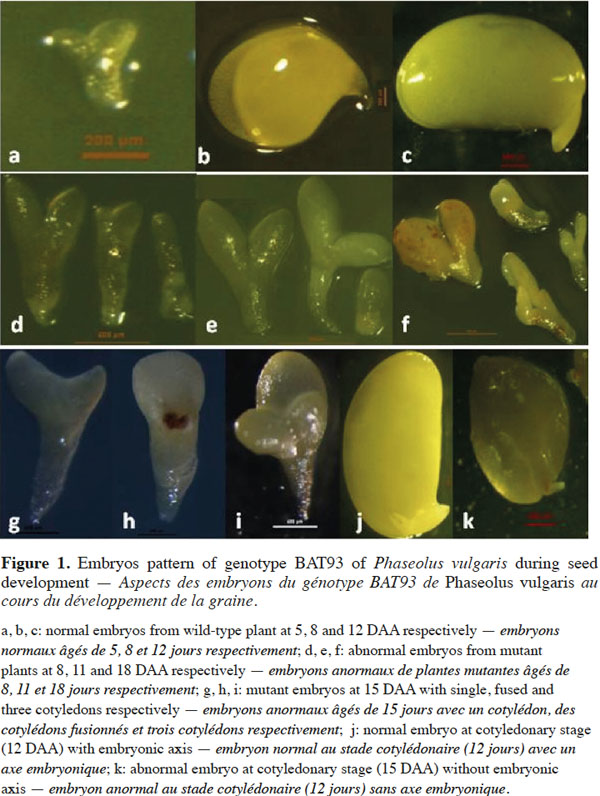
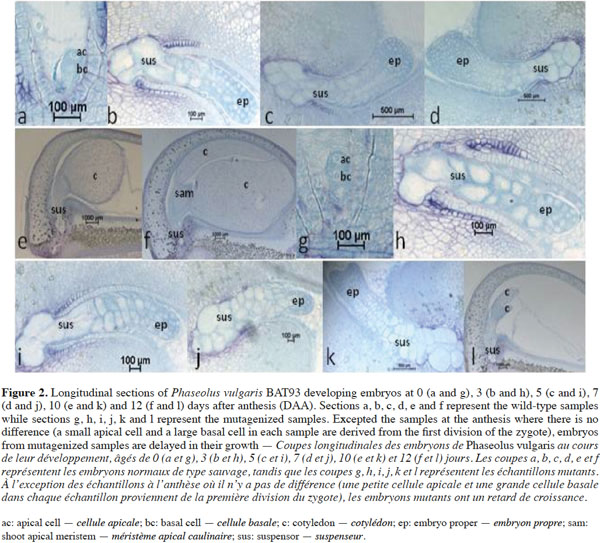
9Histological sections in developing seeds of the wild-type and selected mutagenized samples confirm data observed here above, i.e. delayed growth of the embryos and abnormalities in the suspensor size and structure of the mutagenized samples, as shown by Silué (2009) and Abid et al. (2011) and the lack of the shoot apical meristem in abnormal embryos. These abnormalities occurred during seed development and led to embryo abortion before maturity. Figure 2 shows the histological patterns of normal and abnormal embryos from wild-type and mutagenized plants, respectively, from anthesis to 12 DAA. As shown in figures 2a and 2g, no difference can be observed between the two samples at the anthesis. There are small apical and large basal cells in each sample derived from the transversal and asymmetric divisions of the zygote. From 3 to 12 DAA, the wild-type embryos (Figure 2b, 2c, 2d, 2e and 2f) grow normally from the globular stage to the late cotyledonary one. From 3 to 5 DAA, the wild-type embryo has regular cells and is composed by three cytological zones: the embryo proper containing small isodiametric cells; the basal part of the suspensor consisting of hypertrophic cells, which elongate, invade the micropylar integument tissue and enlarge during this period; and the suspensor “neck zone” (i.e. the zone between the basal part of the suspensor and the embryo proper) consisting of enlarged isodiametric cells. At 5 DAA, the wild-type embryo reaches the heart-shaped stage and primordia of the cotyledons are initiated at the chalazal side of the embryo. Cotyledons continue their growth and the embryo reaches the late cotyledonary stage about two weeks after anthesis, while the size and the number of suspensor cells decrease (Figure 2f). During the same period of development in mutant samples, embryo growth is disturbed and the embryo proper is still globular at 7 DAA (Figure 2j). It reaches heart-shaped stage at 10 DAA (Figure 2k). At 12 DAA (Figure 2l), it forms small cotyledons occupying about ¼ of the seed volume; the suspensor contains large cells, compared to that observed in the wild-type sample where the embryo occupies the whole seed with the presence of a shoot apical meristem between the two cotyledons. Mutant embryos are delayed in their growth, reduced in their size, have a large suspensor and abort before seed maturity.
3.2. Inheritance of the “embryo abortion” trait
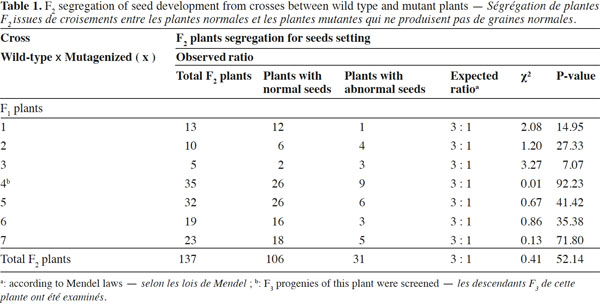
10Genetic analysis of F1 plants reveals that this trait is maternally inherited: if a plant defective in seed development is pollinated with wild-type pollen, the F1 seeds abort before maturity; if a wild-type plant is pollinated with pollen from mutant plant, the F1 seeds have the normal phenotype (embryo without abnormalities) and germinated to produce normal F1 plants. The F1 plants, from the cross wild-type (♀) x mutagenized (♂), were self-pollinated to produce F2 seeds. These F2 seeds were sown to generate F2 plants and a total of 137 plants were scored for seed development: 106 plants have wild-type phenotype and produce normal seeds while 31 plants produce abnormal seeds with embryos that all abort before maturity. These data fit the Mendelian 3:1 (wild-type phenotype:mutant phenotype) segregation ratio as shown in table 1, indicating that the “embryo abortion” might be controlled by a single recessive gene (χ² = 0.41, df = 1, P > 0.05). The different mutation phenotypes shown in figures 1 and 2 are found in the progenies of all seven F1 plants. To support this hypothesis, the progenies of sixteen F2 plants from one of the seven F1 plants were analyzed to determine the ratio of homozygotes and heterozygotes among the F2 plants with normal phenotype. A 2:1 ratio of heterozygotes and homozygotes was observed within these 16 F2 plants as presented in table 2, suggesting that the mutation trait is monogenic.

4. Discussion
11In the present paper we describe the phenotypic patterns of embryo abortion and the inheritance of this abortion trait in P. vulgaris plants deficient in seed development. After seeds treatment by EMS, different phenotypes in embryo pattern were observed. Four major traits can be used to describe these aborting embryos: uncolored embryos with translucent and turgid aspects which are symptoms of hyperhydricity; a large suspensor during embryo development; an abnormal number of cotyledons associated with malformations; and the absence of a SAM between the cotyledons.
12The SAM is the fundamental structure that is located at the growing tip and gives rise to all aerial parts, i.e. stems, leaves and flowers (Kinoshita et al., 2010). There is a link between a good establishment of the SAM and cotyledon separation during embryogenesis (Aida et al., 1999). These two events are correlated with changes in auxin distribution mediated by the polar auxin transport (PAT) (Friml et al., 2003; Blilou et al., 2005; Vieten et al., 2005; Chandler, 2008). The PAT is also involved in the good establishment of the basal part of the embryo, i.e. the suspensor and the root apical meristem as showed during Norway spruce somatic embryo development (Larsson et al., 2008). The distribution of auxin within plants requires directional transport involving auxin transporters of the PINFORMED (PIN) family (Tanaka et al., 2006). Misregulation of the PAT leads to distinct phenotypes of embryo defects in both apical and basal parts. These phenotypes reveal specific cell divisions and cell specification events that are controlled by auxin during embryogenesis. The auxin-related phenotypes occur at three developmental stages: during division of the apical daughter cell just after zygote division; during hypophysis division at the 32-cell stage and during the transition from the globular stage to heart-shaped stage. At the transition stage, the initiation, outgrowth and correct separation of cotyledons, SAM initiation and maintenance are affected in many mutants defective in auxin signaling. These defects result in seedlings with lack or dysfunction of the SAM, fused cotyledons, aberrant number (one or more) of cotyledons, asymmetric cotyledons, or complete absence of cotyledons, and abnormal differentiation of the suspensor (Long et al., 1998; Breuninger et al., 2008; Larsson et al., 2008; Möller et al., 2009).
13In our case, lack of the SAM, the irregular number of cotyledons associated with malformation and abnormalities in suspensor could be partially attributed to abnormalities in auxin biosynthesis and distribution. The poc mutants of tomato, resulting from induced mutation (Al-Hammadi et al., 2003; Madishetty et al., 2006), showed pleiotropic phenotype and included the formation of extra cotyledons. In this species the earliest defects were observed at the transition from the globular to the heart stage of embryogenesis with the formation of multiple cotyledons. The POC gene in tomato is involved in the negative regulation of polar auxin transport, which is likely the reason for the pleiotropic phenotype in the mutant.
14The uncolored phenotype observed in the mutant embryos could be linked with their translucent and turgid aspects, indicating that these embryos did not accumulate appreciable amounts of chlorophyll. These observations suggest that the mutation that occurred affects chloroplast formation during embryogenesis, as observed in Arabidopsis cristal (cri) mutants (Delarue et al., 1997). On the other hand, turgid aspect could be the result of the high content of water (hyperhydricity) in mutant embryo tissues. Mutation could disturb the distribution of the water between the embryo and the maternal tissues.
15Disrupted embryos with abnormal suspensor and malformed cotyledons are common features observed in mutants obtained following induced mutations in some species. In Arabidopsis sus mutants, the embryo proper possesses an enlarged suspensor and exhibits aberrant development. In sus mutants, aberrant morphogenesis in the embryo proper consistently results in the formation of a large suspensor. Defects in the embryo proper appear at the globular stage of development while abnormalities in the suspensor are detected soon after at the heart stage. All these sus mutants produce defective embryos, which aborted before maturity (Schwartz et al., 1994). The raspberry (rsy) mutant embryos, obtained by T-DNA mutagenesis in Arabidopsis, fail to undergo the transition to heart stage, remain globular shaped, and produce an enlarged suspensor region. The suspensor enlargement observed in rsy3 embryos is not as severe as that observed in rsy1 or rsy2 mutants (Yadegari et al., 1994; Apuya et al., 2002).
16In pea (Liu et al., 1999), three single cotyledon (sic) mutants were identified following induced mutation. All of which show some degree of fusion between the cotyledons. The fusion in sic1 is along the margin of one cotyledon and is less complete than in sic2 embryos, but the effects of the mutations are additive in the double mutant. Occasionally sic2 mutants show fusion of the two cotyledons into one cylindrical embryo in which the shoot apex becomes surrounded by the cotyledons. Both sic1 and sic2 mutants produce fertile plants. In the sic3 embryo, a single cotyledon is generated under the shoot apex that breaks the vascular connection between root and shoot, causing embryo lethality.
17On the other hand, in Phaseolus interspecific crosses, embryo abortion is attributed in parts to the disruption of suspensor morphogenesis (Lecomte et al., 1998). Indeed, in the crosses between Phaseolus coccineus or Phaseolus dumosus Macfad. (as female parents) and P. vulgaris, the hybrid embryo showed an enlarged suspensor, resulting in smaller and delayed embryo proper, compared to embryo from the parental lines. At the micropylar end, basal cells of the suspensor of hybrids were bigger and contained larger vacuoles compared to the self-pollinated embryos (Lecomte et al., 1998; Nguema et al., 2007). These abnormalities in the suspensor morphology in Phaseolus interspecific and mutagenized aborting embryos could limit the nutrient transfer to the embryo and lead to its abortion.
18The genetic analysis of the embryo abortion trait in F1 and F2 populations revealed the maternal inheritance of this trait and the possible control by a single recessive mutant allele. The hypothesis that the abortion trait is controlled by a single recessive locus was also supported by data from F3 population studies. Identical results, i.e. maternal inheritance of embryo abortion trait and its monogenic recessive control, were obtained with several traits following induced mutations. In Arabidopsis, Léon-Kloosterziel et al. (1994) isolated from an EMS treated population a testa mutant in which the heart-shaped phenotype was maternally inherited and controlled by a single recessive gene. The other induced mutants cited above: sus and rsy mutants obtained by T-DNA mutagenesis and EMS mutagenesis in Arabidopsis (Schwartz et al., 1994; Apuya et al., 2002), poc mutants obtained by EMS mutagenesis in tomato (Al-Hammadi et al., 2003) and sic mutants obtained by EMS mutagenesis in pea (Liu et al., 1999) are also under the control of single recessive genes.
19The female inheritance of the abortion trait suggests an interaction between the mother tissues and the embryo itself (including the suspensor) occuring just after fecundation. This negative interaction does not exist for the reciprocal crosses (use of non-mutant plant as female parent). The very early abortion of the embryo makes their rescue very complicated because the negative interaction may already occur directly after fecundation. We also attempted to rescue the aborting embryos using different growing media (Baus et al., 1986; Franzmann et al., 1989; Mergeai et al., 1997), but no plantlet has been regenerated from mutant embryos.
5. Conclusion and prospects
20EMS mutagenesis led to P. vulgaris plants with aborting embryos. The pleiotropic action of the mutation is reflected by the different traits that are affected including uncolored and hyperhydric embryos, large suspensor present during embryo development, aberrant number of cotyledons associated with malformation and the lack of SAM. Genetic analyses indicated that the mutation is maternally inherited and could be controlled by a single recessive locus. The mutant plants obtained in Phaseolus constitute a valuable material to unravel the mechanisms necessary for plant embryogenesis. Such mutants could be helpful to isolate and characterize different genes interacting during embryo formation, development and abortion. From our results, we cannot identify precisely which gene(s) is/are affected in these mutants.
21In future studies for better understanding the genetics of embryo defect and abortion, several methods can be applied to characterize such genes. For example, the combination of Suppression Subtractive Hybridization (SSH), dot blot hybridization and quantitative RT-PCR could help to identify cDNAs differentially expressed in the aborted embryos. Due to the existing synteny between species, primers could be designed from genes such as PIN, POC, SUS, SIC, RSY, CRI and their expression pattern could be followed in P. vulgaris wild-type and mutant embryos in order to reveal their putative function in embryogenesis and embyo abortion. In addition, genes involved in the process of embryo abortion can be mapped using the map-based cloning with SSRs and/or RAPD markers (Galeano et al., 2011; Blair et al., 2012). For the last two methods, PhaseolusGenes database (http://phaseolusgenes.bioinformatics.ucdavis.edu/) is an useful tool for searching such genes and markers.
22Acknowledgements
23We would like to thank Prof. W.J. Broughton, Dr C. Pankhurst and Dr P. Lariguet for allowing us to screen the EMS mutants of Phaseolus vulgaris when they were at the University of Geneva in 2005. This work is supported by federal grant for research (doc.CA 15.731 / 16-02-2011) of University of Liege in Belgium.
Bibliographie
Abid G. et al., 2011. In silico identification and characterization of putative differentially expressed genes involved in common bean (Phaseolus vulgaris L.) seed development. Plant Cell Tissue Organ Culture, 107(2), 341-353.
Aida M., Ishida T. & Tasaka M., 1999. Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development, 126(8), 1563-1570.
Al-Hammadi A.S.A. et al., 2003. The polycotyledon mutant of tomato shows enhanced polar auxin transport. Plant Physiol., 133(1), 113-125.
Apuya N.R. et al., 2002. RASPBERRY3 gene encodes a novel protein important for embryo development. Plant Physiol., 129(2), 691-705.
Baus A.D., Franzmann L. & Meinke D., 1986. Growth in vitro of arrested embryos from lethal mutants of Arabidopsis thaliana. Theor. Appl. Genet., 72(5), 577-586.
Blair M.W. et al., 2012. Development of a Mesoamerican intra-genepool genetic map for quantitative trait loci detection in a drought tolerant x susceptible common bean (Phaseolus vulgaris L.) cross. Mol. Breeding, 29, 71-88.
Blilou I. et al., 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature, 433(7021), 39-44.
Bonhomme F. et al., 2000. Cytokinin and gibberellin activate SaMADS A, a gene apparently involved in regulation of the floral transition in Sinapis alba. Plant J., 24(1), 103-111.
Borkar A.T. & More A.D., 2010. Induced flower colour mutations in Phaseolus vulgaris L. through physical and chemical mutagens. Adv. Biores., 1(1), 22-28.
Breuninger H. et al., 2008. Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev. Cell, 14(6), 867-876.
Chandler J.W., 2008. Cotyledon organogenesis. J. Exp. Bot., 59(11), 2917-2931.
Chandler J.W., Cole M. & Werr W., 2008. Plant development revolves around axes. Trends Plant Sci., 13(2), 78-84.
Clark J.K. & Sheridan W.F., 1991. Isolation and characterization of 51 embryo-specific mutations of maize. Plant Cell, 3(9), 935-951.
Davis J.H.C., Giller K.E., Kipe-Nolt J. & Awah M., 1988. Non-nodulating mutants in common bean. Crop Sci., 28(5), 859-860.
Delarue M., Santoni V., Caboche M. & Bellini C., 1997. Cristal mutations in Arabidopsis confer a genetically heritable, recessive, hyperhydric phenotype. Planta, 202(1), 51-61.
Devic M., 1995. L’embryogenèse d’Arabidopsis. Biofutur, 151, 32-37.
Franzmann L., Patton D.A. & Meinke D.W., 1989. In vitro morphogenesis of arrested embryos from lethal mutants of Arabidopsis thaliana. Theor. Appl. Genet., 77(5), 609-616.
Friml J. et al., 2003. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature, 426(6963), 147-153.
Galeano C.H. et al., 2011. Saturation of an intra-gene pool linkage map: towards a unified consensus linkage map for fine mapping and synteny analysis in common bean. PLoS ONE, 6(12), e28135, doi:10.1371/journal.pone.0028135.
Geerts P., Toussaint A., Mergeai G. & Baudoin J.P., 2002. Study of the early abortion in reciprocal crosses between Phaseolus vulgaris L. and Phaseolus polyanthus Greenm. Biotechnol. Agron. Soc. Environ., 6(2), 109-119.
Henikoff S. & Comai L., 2003. Single-nucleotide mutations for plant functional genomics. Annu. Rev. Plant Biol., 54, 375-401.
Kami J. & Gepts P., 2000. Development of a BAC library in common bean genotype BAT93. Annu. Rep. Bean Improv. Cooperative, 43, 208-209.
Kawashima T. & Goldberg R.B., 2010. The suspensor: not just suspending the embryo. Trends Plant Sci., 15(1), 23-30.
Kinoshita A. et al., 2010. RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development, 137(22), 3911-3920.
Larsson E., Sitbon F., Ljung K. & von Arnold S., 2008. Inhibited polar auxin transport results in aberrant embryo development in Norway spruce. New Phytologist, 177, 356-366.
Lecomte B., Longly B., Crabbe J. & Baudoin J.P., 1998. Étude comparative du développement de l’ovule chez deux espèces de Phaseolus: P. polyanthus et P. vulgaris. Biotechnol. Agron. Soc. Environ., 2(1), 77-84.
Léon-Kloosterziel K.M., Keijzer C.J. & Koornneef M., 1994. A seed shape mutant of Arabidopsis that is affected in integument development. Plant Cell, 6(3), 385-392.
Liu C.M., Johnson S., Di Gregorio S. & Wang T.L., 1999. Single cotyledon (sic) mutants of pea and their significance in understanding plant embryo development. Dev. Genet., 25(1), 11-22.
Long J.A. & Barton M.K., 1998. The development of apical embryonic pattern in Arabidopsis. Development, 125(15), 3027-3035.
Madishetty K. et al., 2006. Genetic characterization of the polycotyledon locus in tomato. Theor. Appl. Genet., 113(4), 673-683.
McElver J. et al., 2001. Insertional mutagenesis of genes required for seed development in Arabidopsis thaliana. Genetics, 159(4), 1751-1763.
Meinke D.W., 1985. Embryo-lethal mutants of Arabidopsis thaliana: analysis of mutants with a wide range of lethal phases. Theor. Appl. Genet., 69(5-6), 543-552.
Meinke D.W. & Sussex I.M., 1979. Embryo-lethal mutants of Arabidopsis thaliana: a model system for genetic analysis of plant embryo development. Dev. Biol., 72(1), 50-61.
Mergeai G., Schmit V., Lecomte B. & Baudoin J.P., 1997. Mise au point d'une technique de culture in vitro d'embryons immatures de Phaseolus. Biotechnol. Agron. Soc. Environ., 1(1), 49-58.
Möller B. & Weijers D., 2009. Auxin control of embryo patterning. Cold Spring Harbor Perspect. Biol., 1(5):a001545.
Nagl W., 1974. The Phaseolus suspensor and its polytene chromosomes. Z. Pflanzenphysiol., 73, 1‐44.
Nagl W., 1981. Polytene chromosomes of plants. Int. Rev. Cytol., 73, 21‐53.
Nenno M. & Nagl W., 1998. Identification of polytene chromosomes of Phaseolus coccineus on the basis of centromeric heterochromatin morphology. Annu. Rep. Bean Improv. Cooperative, 41, 105‐106.
Neuffer M.G. & Sheridan W.E., 1980. Defective kernel mutants of maize. I. Genetic and lethality studies. Genetics, 95(4), 929-944.
Nguema N.P., Toussaint A. & Baudoin J.P., 2007. Embryo abortion and histological features in the interspecific cross between Phaseolus vulgaris L. and P. coccineus L. Plant Cell Tissue Organ Culture, 88(3), 329-332.
Pankhurst C., Blair M.W. & Broughton W.J., 2004. Tilling the beans. In: Hernandez G., Gerber D., Pankhurst C. & Broughton W., eds. Proceedings of the 3rd International Scientific Meeting, Phaseomics III, June 13-15, Geneva, Switzerland, 28.
Park S.J. & Buttery B.R., 1992. Ethyl-methane sulphonate (EMS) induced nodulation mutants of common bean (Phaseolus vulgaris L.) lacking effective nodules. Plant Soil, 139(2), 295-298.
Park S.J. & Buttery B.R., 2006. Registration of ineffective nodulation mutant R69 and non nodulation mutant R99 common bean genetic stocks. Crop Sci., 46(3), 1415-1416.
Porch T.G. et al., 2009. Generation of a mutant population for TILLING common bean genotype BAT 93. J. Am. Soc. Hortic. Sci., 134(3), 348-355.
Raghavan V., 2006. Life and times of the suspensor - cell signaling between the embryo and suspensor. In: Double fertilization: embryo and endosperm development in flowering plants. Berlin: Springer, 81-100.
Schwartz B., Yeung E. & Meinke D., 1994. Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development, 120(11), 3235-3245.
Silue S. et al., 2006. Screening Phaseolus vulgaris L. EMS mutants to isolate plants which failed in seed development. Annu. Rep Bean Improv. Cooperative, 49, 149-150.
Silué S., 2009. Genetical mechanisms of Phaseolus embryogenesis and application in interspecific hybridization. PhD thesis: Gembloux Agricultural University (Belgium).
Tanaka H., Dhonukshe P., Brewer P.B. & Friml J., 2006. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell. Mol. Life Sci., 6, 2738-2754.
Tzafrir I. et al., 2004. Identification of genes required for embryo development in Arabidopsis. Plant Physiol., 135(3), 1206-1220.
Vernoud V. et al., 2005. Maize embryogenesis. Maydica, 50(3-4), 469-483.
Vieten A. et al., 2005. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development, 132(20), 4521-4531.
Yadegari R. et al., 1994. Cell differentiation and morphogenesis are uncoupled in Arabidopsis raspberry embryos. Plant Cell, 6(12), 1713-1729.
To cite this article
About: Souleymane Silué
Univ. Liege - Gembloux Agro-Bio Tech. Unit of Tropical Crop Husbandry and Horticulture. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: silsouley@hotmail.com – Peleforo Gon Coulibaly University of Korhogo. Biological Sciences Department. BP 1328. CI-Korhogo (Côte d’Ivoire) – Walloon Agricultural Research Centre. Department of Life Sciences. Unit of Breeding and Biodiversity. Chaussée de Charleroi, 234. B-5030 Gembloux (Belgium).
About: Nafan Diarrassouba
Peleforo Gon Coulibaly University of Korhogo. Biological Sciences Department. BP 1328. CI-Korhogo (Côte d’Ivoire).
About: Inza Jesus Fofana
Peleforo Gon Coulibaly University of Korhogo. Biological Sciences Department. BP 1328. CI-Korhogo (Côte d’Ivoire).
About: Yordan Muhovski
Walloon Agricultural Research Centre. Department of Life Sciences. Unit of Breeding and Biodiversity. Chaussée de Charleroi, 234. B-5030 Gembloux (Belgium).
About: André Toussaint
Univ. Liege - Gembloux Agro-Bio Tech. Unit of Tropical Crop Husbandry and Horticulture. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
About: Guy Mergeai
Univ. Liege - Gembloux Agro-Bio Tech. Unit of Tropical Crop Husbandry and Horticulture. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
About: Jean-Marie Jacquemin
Walloon Agricultural Research Centre. Department of Life Sciences. Unit of Breeding and Biodiversity. Chaussée de Charleroi, 234. B-5030 Gembloux (Belgium).
About: Jean-Pierre Baudoin
Univ. Liege - Gembloux Agro-Bio Tech. Unit of Tropical Crop Husbandry and Horticulture. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






