- Home
- Volume 18 (2014)
- Numéro 1
- Study of the impact of growth substance treatment and maize (Zea mays L.) variety in spelt (Triticum spelta L.) haplodiploidization
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Study of the impact of growth substance treatment and maize (Zea mays L.) variety in spelt (Triticum spelta L.) haplodiploidization

Editor's Notes
Received on January 21, 2013; accepted on January 13, 2014
Résumé
Étude de l’influence du traitement hormonal et de la variété de maïs (Zea mays L.) dans l’haplodiploïdisation de l’épeautre (Triticum spelta L.). D’après nos connaissances, il s’agit de la première étude sur l’haplodiploïdisation de l’épeautre (Triticum spelta L.). La technique utilisée implique un croisement intergénérique avec le maïs (Zea mays L.). Le taux d’embryons/100 fleurs pollinisées était de 16,1. La lignée d’épeautre n’avait pas d’effet significatif sur la production d’embryon, au contraire de la variété de maïs et une interaction entre la lignée d’épeautre et la variété de maïs a été observée. Le meilleur taux a été obtenu avec une variété de maïs de type popcorn. Le taux de formation de nouaison était faible (66,2 nouaisons/100 fleurs pollinisées). Le taux de régénération de plantules était très faible (38 plantules/100 embryons), avec un impact de la variété de maïs, mais pas de la lignée d’épeautre.
Abstract
So far as we know, this is the first study on spelt (Triticum spelta L.) haplodiploidization. The technique used involved an inter-generic cross with maize (Zea mays L.). The rate of embryos/100 pollinated florets was 16.1. The spelt breeding line had no significant effect on embryo production, but the maize variety did, and an interaction between spelt breeding line and maize variety was found. The best rate was obtained with a maize variety of the popcorn type. The rate of caryopsis formation was low (66.2 caryopses/100 pollinated florets). The rate of plantlet regeneration was very low (38 plantlets/100 embryos), with the maize variety having an impact, but not the spelt line.
1The works of Zenkteler et al. (1984), Laurie et al. (1986, 1987, 1988) and Suenaga et al. (1989) enabled to set the techniques of haplodiploidization on wheat through crossing with maize. Since then, haplodiploidization has become an important tool in plant breeding and is increasingly used in pure-line programs. Spelt (Triticum spelta L.) is an ancient subspecies of common wheat (Triticum aestivum L.) mainly cultivated in Europe. So far as we know, no work has been done on spelt haploidiploidization. The objective of the study was to evaluate the impact of the maize genotype on the hybridization with spelt; and of growth substance treatment on the production of caryopses (C).
2Ten F1 breeding lines from CRA-W were selected on the diversity of their genitors (22). Two sweet maize varieties (GB and TS) and one popcorn variety (MPC) were chosen.
3Twenty-seven grains of the spelt line were sown in 4 L pots. The substrate was half blonde and half brown peat. One month after sowing, the plants were vernalized at 4 °C over 8 weeks. They were transplanted and placed in a greenhouse at 16-18 °C, with a photoperiod of 16 h of light and 8 h of dark. The sodium-vapor lamp provided 107 µE·m-2·s-1. Fifteen grains of maize were sown in 10 L pots with the same substrate as for the spelt. About 19-20 weeks later, some mature pollen was available. The plants were transplanted and placed in a greenhouse at 20-35 °C; photoperiod and lighting were the same as for spelt.
4When spelt heading occurred and before the anthers dehisced, only the primary and secondary florets on the central portion of the spike were retained. Emasculation was done manually 1-4 days before anthesis (Sarrafi et al., 1994; Inagaki et al., 1998). When spelt stigma was feathery and fluffy (Campbell et al., 1998), 1-4 days after emasculation, fresh maize pollen was placed on the florets. Two solutions of 2,4-dicholorphenoxy acetic acid (2,4-D) and gibberellic acid (GA3) both at 100 mg·l-1 were stored at 4 °C. The 2,4-D acts as a growth substance and GA3 plays a role in the growth of the caryopsis (C). Twenty-four hours after pollination, spike was pulverised.
5Fourteen days after pollination, Cs were removed from the spikes and disinfected under laminar flow. The Cs were rinsed three times over 5 min with distilled water and the Cs were then dissected to check for the presence of an embryo (E). The Es were controlled to be haploids (H) with the aspect of the C: a C from an interspecific crossing has an aqueous endosperm while a C from self-pollination has a milky endosperm. H Es were removed under a microscope Leica M26 (4 x 10) and set in culture in a Petri dish with culture media B5 (Gamborg et al., 1968). The Petri dish was closed and placed in darkness in an incubator at 28 °C until germination and the appearance of the first roots. The Petri dish was moved to a culture room at 20 °C, with a photoperiod of 16 h light and 8 h dark under 28.5 µE·m-2·s-1. After 14 days, plantlets (P) were transplanted into a 55 mm diameter container with culture media B5.
6In the first experiment, the spelt breeding lines GE 10, 12, 21, 28, 33 and 43 were chosen. Ten non-emasculated spikes per breeding line were pollinated with each maize variety. This represented 180 spikes for the experiment: 5,404 pollinated florets (PFs). Only the 2,4-D solution was pulverised.
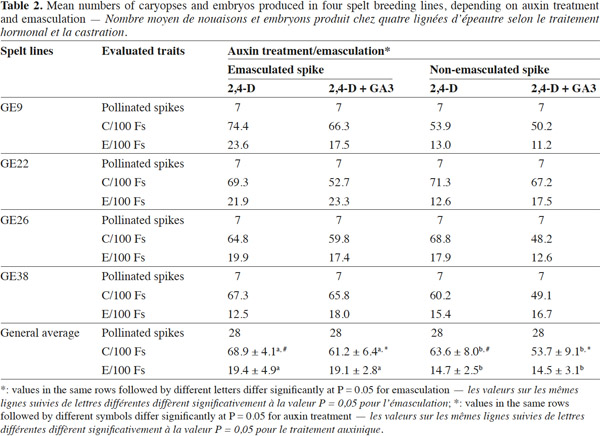
7In the second experiment, the spelt breeding lines GE 9, 22, 26 and 38 were selected. Fourteen spikes per breeding line were pollinated (seven emasculated spikes and seven non-emasculated spikes) and two treatments were applied to both sets of spikes: (a) 2,4-D alone 24 h after pollination and (b) 2,4-D 24 h after pollination plus GA3 48 h after pollination. In total, 28 spikes were worked per breeding line: 112 spikes or 3,374 PFs. Number of Cs, Es and Ps was collected. The results were reported on a per-PF or per-E basis. The proportions of Es per 100 PFs (E/100 PF), P per E (P/E), Cs per PF (C/PF) and P per PF (P/PF) were calculated. An analysis of variance (ANOVA) evaluated the impact of the treatments, based on the general linear model, using the software Statbox 7.1 (Grimmersoft, Issy-les-Moulineaux, France).
8In the first experiment, 3,484 Cs, 872 Es and 280 Ps were obtained.
9Production of embryos. For the six breeding lines and the three maize varieties, 16.1 E/100 PF were obtained on average, with the highest being 18.5 ± 6.8 E/100 PF for MPC (Table 1). The rates of embryo formation reported in literature were: 14.4-28.0% (Laurie et al., 1987), 20.6% (Laurie et al., 1988); 8.3-21.1% (Inagaki et al., 1992); 15.1% (Verma et al., 1999); 22.5% (Martins-Lopes et al., 2001); 13.9% (Sharma et al., 2002); and 3.8% E/PF (Torres et al., 2010).
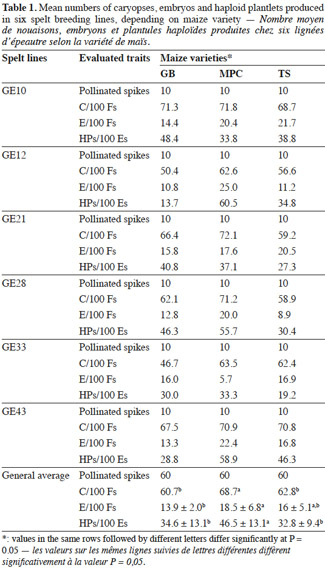
10In the present study, the ANOVA showed that the maize variety had a significant effect on the success of embryo production (p = 0.049) which is consistent with literature (Suenaga et al., 1989; Matzk et al., 1994; Zhang et al., 1996) and the best maize variety was the popcorn type, which is consistent with the work of Verma et al. (1999).
11Here however, the ANOVA showed that the breeding line had no effect on the success of embryo production. In the literature, on the one hand, a considerable varietal difference in efficiency among wheat varieties has been observed in embryo production (Bitsch et al., 1998; Martins-Lopes et al., 2001; Torres et al., 2010) or on C/PF and E/C rates (Sharma et al., 2002). While on the other hand, several authors have reported that wheat genotypes do not affect embryo production (Suenaga et al., 1989; Matzk et al., 1994; Zhang et al., 1996). In the present study, a significant interaction between spelt breeding line and maize variety was observed (p = 0.004). Bitsch et al. (1998) and Lefebvre et al. (1996) reported that interaction was significant for HP formation and for the number of P/100 PF, respectively.
12Regeneration of embryos to plantlets. In the present study, the number of P obtained from the number of Es placed in culture was 38.0% (Table 2). The literature reports rates of 68.1% (Laurie et al., 1988), 85% (Laurie et al., 1991), 43.1% (Inagaki et al., 1992), 43% (Sharma et al., 2002) and 26.1% (Torres et al., 2010). Lefebvre et al. (1996) reported a rate of HP production of 9.1 (4.4-14.7) per 100 florets, compared with 4.8-8.6 P/100 PF in the present study, which is lower.
13The ANOVA showed an effect of the maize variety (p = 0.02), but no effect of the spelt breeding line, nor an interaction between the two factors on the rate of regeneration. The highest rate for MPC was 46.5%; it was 34.6% for GB and 32.8 % for TS. Even the best rate obtained in the present study was not as good as those obtained by Laurie, but it was close to others from literature.
14In this second experiment, 2,099 Cs and 574 Es were obtained.
15Formation of caryopses. The number of Cs obtained is attributed to the application of growth substances. The ANOVA showed that pulverisation had a significant effect (p = 0.002). The number of C/100 PF was: 66.2 with one pulverisation of 2,4-D 24 h after pollination; and 57.4 C/100 PF with one pulverisation of 2,4-D after 24 h and one of GA3 48 h after pollination. The simple use of 2,4-D 24 h after pollination therefore gave the highest number of Cs. These rates were low compared with those reported in the literature for wheat, 87% (Sharma et al., 2002) and 69.4% (Torres et al., 2010). The results obtained from other studies (Laurie et al., 1991; Inagaki et al., 1995) agreed with the finding of the present study that 2,4-D alone performs better than 2,4-D plus GA3. The C/PF increased significantly (p = 0.019) with emasculation, from 58.6 to 65.1%. Emasculation probably enabled the glumes to be opened so that hormonal treatments would have better access to the stigma and better penetration.
16Production of embryos. The ANOVA showed that neither the spelt breeding line nor the nature of growth substance influenced the rate of production of Es. The emasculation factor, however, played a significant role (p = 0.004). When spikes were emasculated 1-4 days prior to anthesis, 19.3 E were obtained for 100 PF whereas only 14.6 were obtained when they were not. Emasculation has been done as early as 4 (Sarrafi et al., 1994) to 5 days before anthesis (Suenaga et al., 1989), 2-3 days before anthesis (Zhang et al., 1996; Suenaga et al., 1997), 1-2 days before anthesis (Matzk et al., 1994; Campbell et al., 1998) and 1 day before anthesis (Inagaki et al., 1998). According to Laurie (1989) however, intact glumes result in higher embryo production than when glumes are cut back for emasculation, but Suenaga et al. (1997) found that there was no difference. The results obtained in the present study contradict those reported in the literature but emasculation in the case of spelt seemed promising.
17For the first experiment on spelt haplodiploidization, the rate of E production was satisfying. The rate of P regeneration was weak overall except for one variety of maize; this suggests that a better rate could be obtained and integrated into a breeding program. The rate of C formation was weak, although in the present study it did not have an impact on the rate of E production. Deeper investigations and new procedures need to be further experimented in order to propose new protocols for spelt haplodiploidization to the scientific community.
18Abbreviations
19C: caryopse
202,4-D: 2,4-dicholorphenoxy acetic acid
21E: embryo
22GA3: gibberellic acid
23HP: haploid plant
24P: plantlet
25PF: pollinated floret
26Acknowledgements
27We wish to thank the Institut de Genech team – Christophe Brame, Sophie Fouchet, Marion Levaux, and Mélanie Wostyn – and the Centre wallon de Recherches agronomiques team – Luc Watelet – for their technical assistance.
28The authors declare that they have no conflict of interest.
Bibliographie
Bitsch C., Groeger S. & Lelley T., 1998. Effect of parental genotypes on haploid embryo and plantlet formation in wheat x maize crosses. Euphytica, 103, 319-323.
Campbell A.W. et al., 1998. The effects of temperature and light intensity on embryo numbers in wheat doubled haploid production through wheat x maize crosses. Ann. Bot., 82, 29-33.
Gamborg O.L., Miller R.A. & Ojima K., 1968. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell. Res., 50, 151-158.
Inagaki M.N. & Tahir M., 1992. Production of haploid through intergeneric crosses. Hereditas, 116, 117-120.
Inagaki M.N & Tahir M., 1995. Comparison of crossabilities of tetraploid wheat with Hordeum bulbosum and maize. Cereal Res. Commun., 23, 339-343.
Inagaki M.N., Pfeiffer W.H., Mergoum M. & Mujeeb-Kazi A., 1998. Variation of the crossability of durum wheat with maize. Euphytica, 104, 17-23.
Laurie D.A., 1989. Factors affecting fertilization frequency in crosses of Triticum aestivum cv. ‘Highbury’ x Zea mays cv. ‘Seneca 60’. Plant Breeding, 103, 133-140.
Laurie D.A. & Bennett M.D., 1986. Wheat x maize hybridization. Can. J. Genet. Cytol., 28, 313-316.
Laurie D.A. & Bennett M.D., 1987. The effect of crossability loci Kr1 and Kr2 on fertilization frequency in hexaploid wheat x maize crosses. Theor. Appl. Genet., 73, 403-409.
Laurie D.A. & Bennett M.D., 1988. The production of haploid wheat plants from wheat x maize crosses. Theor. Appl. Genet., 76, 393-397.
Laurie D.A. & Reymondie S., 1991. High frequencies of fertilisation and haploid seedling production in crosses between commercial hexaploid wheat varieties and maize. Plant Breeding, 106, 182-189.
Lefebvre D. & Devaux P., 1996. Doubled haploids of wheat from wheat x maize crosses: genotypic influence, fertility and inheritance of the 1BL-1RS chromosome. Theor. Appl. Genet., 93, 1267-1273.
Martins-Lopes P.F., Guedes-Pinto H., Pinto-Carnide O. & Snape J., 2001. The effect of spikelet position on the success frequencies of wheat haploid production using the maize cross system. Euphytica, 121, 265-271.
Matzk F. & Mahn A., 1994. Improved techniques for haploid production in wheat using chromosome elimination. Plant Breeding, 113, 125-129.
Sarrafi A., Amrani N. & Alibert G., 1994. Haploid regeneration from tetraploid wheat using maize pollen. Genome, 37, 176-178.
Sharma H., Yang Y. & Ohm H., 2002. An assessment of doubled haploid production in soft red winter wheat by wheat x corn wide crosses. Cereal Res. Commun., 30, 269-275.
Suenaga K. & Nakajima K., 1989. Efficient production of haploid wheat (Triticum aestivum) through crosses between Japanese wheat and maize (Zea mays). Plant Cell Rep., 8, 263-266.
Suenaga K., Morshedi A.R. & Darvey N.L., 1997. Haploid production of Australian wheat (Triticum aestivum L.) cultivars through wheat x maize (Zea mays L.) crosses. Aust. J. Agric. Res., 48, 1207-1211.
Torres L.E. et al., 2010. Production of haploid plants from ten hybrids of bread wheat (Triticum aestivum L.) through wide hybridization with maize (Zea mays L.). Agriscientia, XXVII, 79-85.
Verma V. et al., 1999. Maize genotypes show striking differences for induction and regeneration of haploid wheat embryos in the wheat x maize system. Crop Sci., 39, 1722-1727.
Zenkteler M. & Nitzsche W., 1984. Wide hybridization experiments in cereals. Theor. Appl. Genet., 68, 311-315.
Zhang J. et al., 1996. Wheat embryogenesis and haploid production in wheat x maize hybrids. Euphytica, 90, 315-324.






