- Portada
- Volume 18 (2014)
- Numéro 1
- Impacts of earthworms on soil components and dynamics. A review
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Impacts of earthworms on soil components and dynamics. A review

Notes de la rédaction
Received on October 9, 2012; accepted on August 20, 2013
Résumé
Impacts des vers de terre sur les composants et la dynamique du sol (synthèse bibliographique). Les vers de terre sont des décomposeurs importants contribuant à la formation d’aggrégats et aux différents cycles d’éléments nutritifs tels que l’azote, le phosphore et le carbone. Ils sont connus pour leur influence sur la fertilité du sol en participant à la régulation de la structure du sol et à la dynamique de la matière organique. En ingérant d’importantes quantités de sol, les vers de terre modifient la communauté microbienne lors du passage à travers leur tube digestif. Par conséquent, les changements dans les activités de la communauté lombricienne, à la suite de pratiques de gestion des sols, peuvent également être utilisés comme indicateurs de la fertilité et de la qualité des sols. Il est important de comprendre comment les communautés lombriciennes affectent la dynamique des sols. Cette synthèse bibliographique porte sur l’état actuel des connaissances sur les impacts de vers de terre sur la structure des sols et la dynamique de la matière organique, en mettant l’accent sur les impacts des pratiques agricoles sur les communautés lombriciennes.
Abstract
Earthworm populations are important decomposers contributing to aggregate formation and nutrient cycling processes involving nitrogen cycles, phosphorus and carbon. They are known to influence soil fertility by participating to important processes in soil such as soil structure regulation and organic matter dynamics. Earthworms also modify the microbial communities through digestion, stimulation and dispersion in casts. Consequently, changes in the activities of earthworm communities, as a result of soil management practices, can also be used as indicators of soil fertility and quality. It is therefore important to understand how earthworm communities affect soil dynamics. This review adresses the current state of knowledge on earthworm’s impacts on soil structure and soil organic matter (carbon, nitrogen, and phosphorus) dynamics, with special emphasis on the effects of land management practices on earthworm communities.
Tabla de contenidos
1. Introduction
1Soil forms a narrow interface between the atmosphere and the lithosphere. The structure of cultivated soil results from climatic, anthropogenic, and biological processes, but the precise roles of each of these processes is difficult to assess. The impact of earthworms activity on soil structure was underlined long ago, and these organisms are now recognized as major biological drivers in temperate agrosystems. Soil characteristics (pH, organic matter, nitrogen, granulometry, etc.) are influenced by earthworms because they participate in the construction and destruction of the soil particles, as well as in organic matter transfer. The soil ingested by earthworms undergoes chemical and microbial changes when it passes throught the gut. Organic matter is digested and both the pH and the microbial activity of the gut contents increase (Edwards et al., 1996; Lukkari et al., 2006). Earthworms accelerate nitrogen mineralization from organic matter, but the effect depends on the species and their interaction with soil characteristics, organic matter location and soil biota (Butenschoen et al., 2009).
2Soil biodiversity has been widely studied since the soil itself is the base for farming (Stockdale et al., 2006). The conservation of biodiversity is necessary to maintain the sustainable functioning of soil. In 1881, Darwin was one of the first scientists who noted that the topsoil consisted mostly of earthworm castings, thus highlighting the importance of earthworms in pedogenesis processes (soil organo-mineral complex). For example, the earthworm population builds galleries and ingests large quantities of organic and mineral matter, thus modifying the porosity and aggregation of the soil. This earthworm bioturbation may subsequently be reflected in soil profiles (Zhang et al., 1995), for example: soil profile disturbance, soil structure modification, and vertical and horizontal redistribution of soil and organic matter (OM). This redistribution of OM depends on the earthworm ecological groups. Endogeic earthworms keep moving inside the soil to feed on soil organic matter (SOM) while anecic ones feed on plant litter and organic residues at the soil surface and tend to stay in the same burrow (Lavelle et al., 1997). Epigeic species, which consume considerable amounts of raw OM have a broad range of enzymatic capacities, probably mainly originating from ingested microflora (Curry et al., 2007). As discussed by Lavelle (1997), the soil biogenic structure (mixture of casts, burrows, OM, etc.) created by earthworms is commonly termed the “drilosphere” (Brown et al., 2000).
3In agrosystems, the intensification of human activities (tillage, use of mineral fertilizers, etc.) has led to deterioration in structural and biological soil characteristics (Edwards, 1984; Lee, 1985). Soil degradation is often associated with decreases in biodiversity and the abundances of earthworms and other invertebrate communities (Lee et al., 1991; Lavelle, 1997). However, there is a perceived lack of information to characterize adequately their functional role in soil ecosystem processes such as soil carbon sequestration and loss, decomposition of organic residues, and the maintenance of soil structure.
4This paper addresses the current state of knowledge on earthworms’ impacts on soil structure and SOM (carbon, nitrogen, and phosphorus) dynamics, with special emphasis on the effects of land management practices on earthworm communities.
2. The role of earthworms in organic matter decomposition and nutrient dynamics
2.1. Decomposition of organic matter
5Organic matter (OM) is mainly present in the top 20 – 30 cm of most soil profiles and is essentially an array of organic macromolecules consisting principally of combinations of carbon (C), oxygen (O) hydrogen (H), nitrogen (N), phosphorus (P) and sulfur (S). Almost all OM in soil is directly or indirectly derived from plants via photosynthesis. Specifically, atmospheric carbon dioxide is transformed by reduction into simple and complex organic carbon (OC) compounds, which in combination with key nutrients enable the plant to function and grow. Soil organic matter (SOM) provides food and substrates for soil organisms, ranging from macroinvertebrates to heterotrophic bacteria (Lavelle et al., 2001). This is of great importance, given that the soil biota is increasingly recognized as playing a major role in soil functions. In cultivated soils, earthworm communities could play an important role in SOM dynamics through regulation of the mineralization and humification processes (Lavelle et al., 1992). On the basis of the results of current literature, it appears that there are some differences among studies regarding the effects of earthworms on soil organic carbon content. Lachnicht et al. (1997) and Desjardins et al. (2003) found a negative effect of earthworms addition on soil carbon content, whereas Gilot (1997) found an opposite effect. In the experiment of Desjardins et al. (2003), the maize crop was grown under no tillage, and this factor can explain the weak loss of carbon in the non-inoculated plots. The decrease by 28% of the total carbon content in the earthworm-inoculated plots indicates that the endogeic tropical earthworm Pontoscolex corethrurus affects the SOM dynamics dramatically. The observed losses of SOM in continuously cropped fields are often attributed to a rapid mineralization of SOM following cultivation. Earthworms caused a decrease in SOM and carbon mineralization by mobilizing recalcitrant forms of OM. Earthworms enhance mineralization by fragmenting SOM and by mixing SOM, mineral particles and microorganisms, thus creating new contact surfaces between the SOM and microorganisms (Parmelee et al., 1998). Since earthworms of different ecological groups prefer different food resources, they likely affect nutrient mineralization. Anecic earthworms incorporate litter material into the mineral soil thereby making it available for the soil food web (Bossuyt et al., 2006). Endogeic earthworm species, in contrasts, primarily consume soil and associated humified OM in the upper layer of the mineral soil.
6Soil microorganisms, mainly fungi and bacteria, are primarily responsible for the transformation of organic molecules in soil, and their activity is thus a key factor in SOM dynamics (Coq et al., 2007). Aira et al. (2008) characterized changes in fungal populations, bacterivore nematodes communities and the biochemical properties of an organic substrate over a short (72 h) exposure to four densities of the epigeic earthworm Eisenia fetida. Calcium and N-mineralization increased with increasing earthworms density, as did microbial metabolic activity. In addition, Coq et al. (2007) showed that casts of endogeic species Pontoscolex corethrurus were slightly enriched in C and showed significantly higher mineralization than the non-ingested soil. The higher mineralization in casts might indicate a higher concentration of labile compounds (soluble carbon, lignin, etc.), and probably a higher microbial activity. Earthworms have indirect effects on soil organic carbon as determinants of microbial activity. In addition, mucus production associated with water excretion in the earthwom gut is known to enhance the microbial activity (Barois, 1986).
7In soil, earthworms control biomass, diversity and activity of soil microorganisms (Doube et al., 1998). However, microorganisms may constitute an important part of the diet of earthworms, which can feed on them selectively (Moody et al., 1995; Edwards, 2004). In most natural and managed ecosystems, up to half of the OC added to soil on an annual basis by plant detritus and root exudates is rapidly consumed by microorganisms, and released as carbon dioxide (Hopkins et al., 2005; Wolf et al., 2005). The remainder of the added OM, together with organic compounds synthesized by soil organisms during decomposition and which is released mainly as detritus, persist in the soil for an extended period. The importance of soil fauna in the decomposition of OM is well known. However, the complex interactions between earthworm and soil microorganisms are less understood. While soil invertebrates yield about 15% of the C and 30% of the N in some ecosystems (Anderson, 1995), their indirect effects through activation of microflora are likely to be much greater.
2.2. Consumption and humification
8Epigeic earthworm species may feed directly on microorganisms or litter material and inhabit the organic layer of soil. They have been shown to strongly affect decomposition processes (Sampedro et al., 2008) and modify the fungal composition of forest soils (McLean et al., 2000). Generally, effects of earthworms on microbial biomass and activity depend on soil conditions (Shaw et al., 1986; Wolters et al., 1992).
9Aira et al. (2006) showed that microbial biomass and activity in pig slurry were significantly decreased by transit through the gut of the epigeic species Eudrilus eugeniae. It appears that E. eugeniae is able to digest microorganisms present in pig slurry (Aira et al., 2006). The effects of earthworms on microorganisms depend on the kind of food source and availability and the species of earthworms involved (Flegel et al., 2000; Tiunov et al., 2000). McLean et al. (2006) found that invasive earthworms decreased microbial biomass in surface soils with a high organic carbon content and increased microbial biomass in the underlying mineral soils. Zhang et al. (2000) found that large numbers of the anecic earthworm Metaphire guillelmi decreased microbial biomass C, N and P after 24 h, thereby concluding that earthworms used microorganisms as a secondary food source.
10An attempt to distinguish between nutrient-enrichment processes associated with the OM incorporation and gut-associated processes associated with the passage of soil and OM through the gut of Lumbricus terrestris was made by Devliegher et al. (1997). They concluded that nutrient-enrichment processes but not gut associated processes were responsible for the increased microbial biomass and activity reported in the presence of L. terrestris. Meanwhile, endogeic earthworms can transport fresh organic detritus from the soil surface into burrows while mixing it with mineral soil. In the case of tropical endogeic species, it has been demonstrated that the addition of water and readily assimilable intestinal mucus to the ingested soil rapidly stimulates microbial activity. In the second half of the earthworm gut, the mucus will have been almost entirely metabolized and the microorganisms start to degrade the SOM into assimilable OM. This form of OM is then used by both the worms and the microorganisms. Furthermore, the interactions between earthworms and microorganisms occur at several spatial scales in the drilosphere (Brown et al., 2004). The drilosphere concept (Figure 1) was developed by Bouché (1972), originally to describe the 2-mm-thick zone around the earthworm burrow walls. Lavelle (1997) completed the meaning of drilosphere by including earthworm communities, the digestive tract content, and all microbial and invertebrate populations. Up to 60% of the C losses from earthworms during their life span can be in the form of mucus secretion, and this soluble organic carbon is an important microbial stimulant in the drilosphere (Brown et al., 2004).
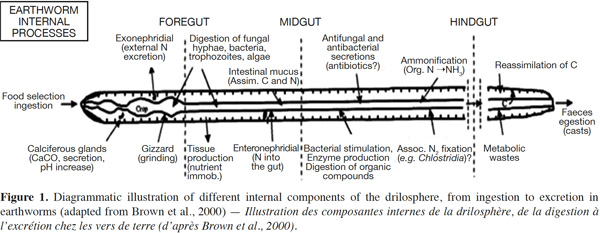
11Different species differ in their ability to digest organic residues and assimilate nutrients (Lattaud et al., 1998). Aporrectodea caliginosa earthworms consume a mixture of soil and OM, often choosing to feed in patches of soil that are relatively rich in OM, or in microsites since they are enriched with bacteria and fungi (Wolter et al., 1999). Lavelle et al. (1994) showed that several temperate earthworm species have a mutualistic digestive system. The mixture of soluble OC, in the form of low-molecular-weight mucus with ingested OM, together with the moist conditions and neutral pH in the foregut, promoted the development of a microbial community that could digest cellulose and other substances that earthworms typically cannot digest. Essentially, the earthworm gut can act like a bioreactor where microbial activity and biomass are increased due to favorable conditions, with readily available C, from mucus, and water. Hence, earthworm casts (EC) may contain large amounts of OM that has not been assimilated, but that has been modified both physically and chemically during passage through the earthworm gut. The EC are usually rich in ammonium-nitrogen and partially digested OM, providing a good substrate for growth of microorganisms. It has been established that there are larger populations of fungi, bacteria, and actinomycetes (Shaw et al., 1986), and higher enzymatic activities in EC than in bulk soil (Figure 1).
12Earthworms produce a huge amount of intestinal mucus composed of gluco-proteins and small glucosidic and proteic molecules (Morris, 1985). The microorganisms entering the worm guts consume these nitrogenous compounds in mucus (Zhang et al., 2000), which largely increases their activity. The biological decomposition of OM is mediated by a variety of biochemical processes in which enzymes play a key role (Garcia et al., 1992). The major constituents of OM, like cellulose, hemicellulose, lignin, and proteins, are degraded by specific enzymes. Earthworms fragment the substrate in the process of feeding and thereby increase the surface area for further microbial colonization. The enhanced microbial activity accelerates the decomposition process leading to humification, thus oxidizing unstable OM into more stable forms. Humification processes are accelerated and enhanced not only by the fragmentation and size reduction of the OM, but also by the greatly increased microbial activities within the intestines of the earthworms and by the aeration and turnover of the OM through earthworm movement and feeding.
2.3. Nutrient inputs, mineralization
13Earthworms are known to be important regulators of major soil processes and functions such as soil structure, OM decomposition, nutrient cycling, microbial decomposition and activity, and plant production. Cortez et al. (2000) reported that the presence of earthworms whatever the ecological category, increased the quantity of inorganic N in the soil. This was caused by enhanced mineralization of N forms, both of a 15N-labelled residue and that of the soil organic matter. Earthworms can impact plant growth by promoting N-availability (Li et al., 2002; Ortiz-Ceballos et al., 2007). Several factors may contribute to the mineral weathering mediated by earthworms, such as low pH and a bacteria-rich microenvironment in the gut of earthworms. However, the presence of earthworms may have an effect on the production of greenhouse gases such as nitrous oxide (N2O). Research by Rizhiya et al. (2007) indicated that earthworms increased N2O fluxes when grass residue was applied to the soil. The formation and production of N2O in soils is determined by microbial processes: nitrification, denitrification, and nitrifier denitrification (Wrage et al., 2001). The earthworm gut provides ideal conditions for N2O producing microorganisms by providing abundant substrate, an anaerobic environment, suitable pH and a high moisture content (Horn et al., 2003; Drake et al., 2007). The powerful mechanical grinding action of the gut is caused by the peristaltic actions used to move food along the gut, and the action of ligands originating from earthworms and their gut microorganisms (Carpenter et al., 2007). Earthworm guts are, consequently, enriched in microorganisms, with concentrations much higher than in the surrounding environment (Carpenter et al., 2007). High numbers of other organisms that are capable of producing N2O (i.e., nitrate-dissimilating and nitrifying bacteria) are also present in the A. caliginosa earthworm gut (Ihssen et al., 2003). Production of N2O by nitrate-dissimilating bacteria is favored in systems that contain high levels of organic carbon, like the rumen or the gastrointestinal tracts of animals. Some nitrifiers are able to use nitrate or nitrite as electron acceptors and, by using this nitrifier denitrification system, can produce N2O and/or N2 under oxygen-limited conditions (Freitag et al., 1987). The in situ conditions of the gut are ideal for activation of dormant bacteria and bacterial spores that might be present in soil. Many endospore-forming bacilli that are abundant in soil (Felske et al., 1998) have been detected in the gut of A. caliginosa species and can reduce nitrate or nitrite to N2O (Ihssen et al., 2003).
14The increased total nitrogen may be due to the release of nitrogenous metabolic products through E. eugeniae earthworm excreta, urine, and mucoproteins (Padmavathiamma et al., 2008). Indeed, Dash et al. (1977; 1979) reported higher levels of N in casts of Lampito mauritii than in the surrounding soil. In the gut of earthworms, it is possible that the mucus secreted from the gut epithelium provides an energy source that stimulates biological N-fixation (Lee, 1985).
15To determine the role of earthworms in agrosystem sustainability, it may be necessary to focus on processes by which earthworms increase or decrease the storage or loss of nutrients, and how they influence productivity and nutrient uptake by crops. As shown in figure 2, the presence of earthworms can change the sizes of various nutrients pools, and the fluxes of C and N, significantly (Bohlen et al., 2004a; figure 2).
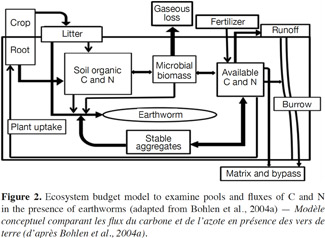
16This model emphasizes the major pathways by which earthworms change the retention and loss of C and N, incorporating the effects of earthworms on soil biological, physical and chemical processes. Through interactions of earthworms with the microbial community and by processing OM, earthworms can increase the system flux of CO2 (gaseous C loss). These same interactions, coupled with earthworm excretion, can also lead to increased availability of N.
2.4. Nutrient dynamics
17Earthworms are important decomposers contributing to nutrient cycling processes involving nitrogen (Lavelle et al., 1992), phosphorus (Chapuis-Lardy et al., 1998) and carbon (Lee, 1985; Lavelle et al., 1992; Zhang et al., 1995; Curry et al., 2007). They ingest organic matter with relatively wide C:N ratios and convert it to earthworm tissues of lower C:N ratios (Syers et al., 1984). This accelerates the cycling of nutrients in soil, particularly N. Some field studies indicate that earthworms feed on organic materials with low C:N ratio, thereby leaving behind a pool of organic materials with a higher C:N ratio (Bohlen et al., 1997; Ketterings et al., 1997).
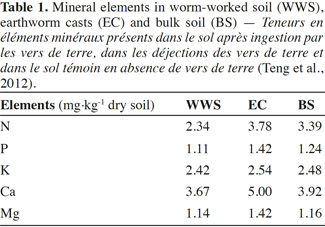
18Field studies have shown variable effects of earthworm invasion on soil N dynamics. Invasion of maple sugar forests in New York by Lumbricus spp. increased leaching of NO3 in a historically plowed site. However, at another site that had never been plowed, the effects have not been observed, which could be attributed to the greater potential for N immobilization in the more C-rich unplowed site (Frelich et al., 2006). Total soil N was not significantly changed by earthworm invasion (Bohlen et al., 2004b). During earthworm feeding, the nutrients, phosphorus (P) and potassium (K), are converted into an available form for plants. Lavelle et al. (1992) highlight the importance of earthworm feeding behaviors, which may contribute to the long-term effects of earthworms on nutrient cycling processes. Suarez et al. (2004) found an increase in P leaching and decrease in P availability on plots in a New York sugar maple forest dominated by Lumbricus rubellus. Sugar maple forests invaded by several species including L. rubellus had lower P availability than control parcels without those earthworm species (Hale et al., 2005). Loss of P with earthworm invasion can be associated with maple decline. The magnitude of earthworm invasion impacts on nutrient cycling depends on the species assemblage of earthworms that invade as well as land-use history. In order to have systems of sustainable agriculture, it is important to maintain a global balance of nutrients to ensure that the outputs and loss of nutrients are offset by nutrient inputs (Giller, 2001). Potassium is one of the major nutrients for plant growth that can significantly affect the growth and production of crops, along with N and P (Amtmann et al., 2007; Sugumaran et al., 2007; Chen et al., 2008). However K, in the form of silicates, can hardly be used by plants (Liu et al., 2006). Earthworms can help in releasing K from silicate minerals. For instance, Basker et al. (1994) reported that exchangeable K-content increased significantly in soils populated by earthworms compared with soils devoid of earthworms (Basker et al., 1992). They concluded that the increase was due to the release of K, from the non-exchangeable K-pool, as soil material passed through the worm gut. Some microorganisms in the earthworm gut can enhance the weathering of minerals by lowering pH or by producing ion-complexing organic ligands (Sanz-Montero et al., 2009). EC are usually found to have greater exchangeable K, calcium (Ca), and magnesium (Mg) contents than bulk soil (Edwards et al., 1996; Mariani et al., 2007). This was also confirmed by Teng et al. (2012) who examined the physical, chemical and biological properties of casts produced by endogeic species Metaphire tschiliensis tschiliensis in clay soil incubated in the dark for two weeks. The findings suggested improved nutrient content in EC as compared to WWS and BS (Table1). This was shown by higher content of macronutrients (N, Ca) in EC than in both WWS and BS. This is probably due to the intimate mixing of OM through the earthworm gut which can further enhance mineralization and humification processes (Lavelle, 1988; Blanchart et al., 1999).
19Improved Ca-content in EC was probably due to the presence of an active calciferous gland in earthworms that actively secretes mucus rich in calcium carbonates into the esophagus (Drake et al., 2007). This leads to the elimination of excess Ca ions via casting activity, and greatly increases Ca availability in soil.
3. Earthworms and microorganisms
20The impact of earthworms on soil OM breakdown has been studied before. However, despite the fact that importance of soil fauna in OM-turnover is well known, the complex interactions between soil fauna and microorganisms, and the indirect effects on microbial communities, are less understood. The biochemical decomposition of OM is primarily accomplished by microorganisms, but earthworms are crucial drivers of the process as they may affect microbial decomposer activity by grazing directly on microorganisms (Monroy et al., 2008; Aira et al., 2009; Gómez-Brandón et al., 2011), and by increasing the surface area available for microbial attack after comminution of OM (Domínguez et al., 2010). Some microorganisms may be a source of food for earthworms, but the amounts consumed and the ability of earthworms to digest and assimilate microbial biomass vary with earthworm species, its ecologogical category, food substrate, and the environmental conditions in which the earthworms are living (Brown et al., 2004). Earthworms affect directly the decomposition of soil through gut-associated processes, via the effects of ingestion, digestion, and stimulation of the OM breakdown and microorganisms (Monroy et al., 2008; Aira et al., 2009). After passage of microorganisms through the earthworm gut (mainly fungal and protozoan spores and some resistant bacteria), they provide inocula for microbial colonization of newly formed EC (Brown, 1995). Some bacteria are activated during passage through the gut, whereas others remain unaffected, and yet others are digested in the intestinal tract and thus decrease in number (Pedersen et al., 1993; Drake et al., 2007). The microbial composition of the earthworm intestine contents has been considered to reflect that of the soil ingested (Brown, 1995). Furthermore, the numbers, biomass, and activity of microbial communities in the earthworm gut have also been shown to be different from that in uningested soil (Schönholzer et al., 1999). Singleton et al. (2003) studied bacteria associated with the intestine and casts of earthworms and found Pseudomonas, Paenibacillus, Azoarcus, Burkholderia, Spiroplasm and Actinobacterium. Some of these bacteria, such as Pseudomonas alcaligenes and Acidobacterium, are known to degrade hydrocarbons (Johnsen et al., 2005). Monroy et al. (2008) observed a reduction in the density of total coliforms by 98%, after the passage of pig slurry through the gut of the epigeic earthworm E. fetida. Accordingly, Pedersen et al. (1993) reported a selective reduction in the coliform Escherichia coli BJ 18 in cattle dung during passage through the gut of several species of earthworms of one genus Lumbricus. The selective effects on ingested microorganisms through the earthworm gut may be caused by competitive interactions between those ingested and the endosymbiotic microrganisms that reside in the gut (Brown et al., 1981). Indeed, Byzov et al. (2007) found that the mid-gut fluid of earthworms possess a selective suppressive activity while stimulating certain soil microorganisms. Meanwhile, Thakuira et al. (2010) found that food resource type can cause shifts in the gut wall-associated bacterial community, but that the magnitude of these shifts did not obscure the delineation between ecological group specificity. For instance, spores of some fungi that survived in the mid-gut environment (Alternaria alternata) started to germinate and grew actively in fresh excrement. The fate of microorganisms passing through the digestive tract of earthworms is an important factor in the formation of the soil microbial community and the degradation of OM. Recently, Rudi et al. (2009) observed a rapid and homogenous change in the microbiota in gut selective effects on the presence and abundance of ingested microorganisms. These selective effects may alter the decomposition pathways, probably by modifying the composition of microbial communities involved in decomposition. Previous studies were mostly aimed to evaluate the effect of gut transit on the microbial population, biomass and enzyme activities of different organic residues (Devliegher et al., 1995; Zhang et al., 2000; Scheu et al., 2002; Aira et al., 2006). But recently Aira et al. (2007a; 2007b) showed that earthworms can modify the microbial community physiology and trigger enzyme activities during vermicomposting of pig slurry. Several enzymes isolated from earthworm guts allowed to digest some bacteria, fungi and microinvertebrates (e.g., protozoa, nematodes) (Brown et al., 2000). Studies using 6 earthworm species and more than 10 soil and litter fungal species (Moody et al., 1995; Bonkowski et al., 2000) have shown that earthworms prefer, and digest, the rapid-growing fungi species typically associated with the early successional stages of decomposition.
4. Effects of earthworms on soil structure
21The beneficial effects of SOM on soil productivity through the supply of plant nutrients, enhancement of cation exchange capacities, and improvements in soil and water retention are well established (Woomer et al., 1994). In addition, SOM supports various soil biological processes by acting as a substrate for decomposer organisms and ecosystem engineers, such as earthworms. They play a role in both acceleration of decomposition and mineralization processes (C loss) and in carbon storage or protection from decomposition (C accumulation) in stable aggregates (Brown et al., 2000). Aggregate stability is a key factor for physical soil fertility and it also affects SOM dynamics (Abiven et al., 2009). Aggregates are formed through the combination of clay, silt, and sand, with organic and inorganic compounds. Their stability is used as an indicator of soil structure (Six et al., 2000). The size, quantity, and stability of soil aggregates reflect a balance between factors such as organic amendments, soil microorganisms, fauna, and disrupting factors as bioturbation and culture (Six et al., 2002) (Figure 3).
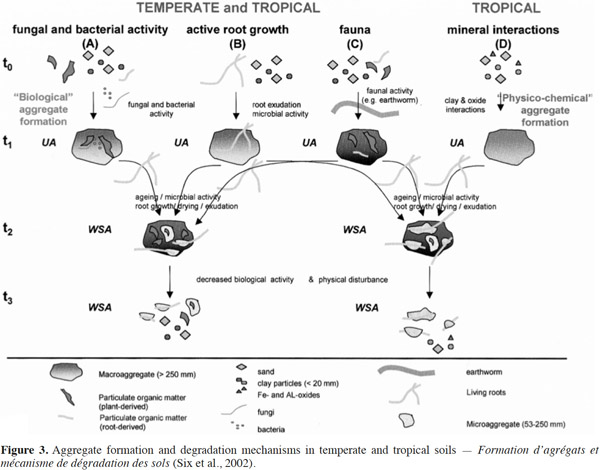
22Aggregation is a complex procedure that includes environmental factors, soil management factors, plant influences, and soil properties such as mineral composition, texture, SOC-concentration, pedogenic processes, microbial activities, exchangeable ions, and moisture availability (Kay, 1998). For several decades, the role of soil organisms in soil structure has been recognized by farmers, but the impact of soil organisms on the formation of aggregates was conceptualized in the hierarchical model of soil aggregates only in the last 25 years (Tisdall et al., 1982). This model shows that the activity of fungi, bacteria, plant roots, and macrofauna (e.g. earthworms) lead to the formation of biological macroaggregates (Six et al., 2002). The breakdown of soil macroaggregates increases over time because the action of binding agents is gradually disrupted. However, despite the disruptive forces, the microaggregates remain stable and become blocks during the formation of new soil macroaggregates. A study conducted by Bossuyt et al. (2006) showed that there was a significant influence of earthworm activity and residue application on stable aggregate formation. Soil aggregates were 4.3 times greater than the control (no earthworms) when A. caliginosa was present in residues-incorporated soils. Further, in the presence of L. rubellus, soil aggregates were three times greater than the control.
23Nunan et al. (2003) reported that bacteria are not randomly distributed throughout the soil; there are variations in biomass and differential colonization among different sizes of aggregates. Using molecular methods, Mummey et al. (2006) examined the bacterial communities associated with different aggregate size-fractions in earthworm-worked soil relative to soil receiving only plant litter. Earthworms altered the bacterial community composition in all soil fractions that were analyzed. When earthworms ingest the soil, the soil particles are broken down and the soil is compacted during passage through the gut prior to excretion. Barré et al. (2009) reported that earthworms were shown to bring initially loose or compacted soil to an intermediate mechanical state that is more favorable for structural stability and root growth. In addition, soil size-distribution is significantly affected by earthworms in the 0 – 2 cm layer of soil (Snyder et al., 2009). Earthworm presence shifted soil aggregate-size to the > 2,000 µm fraction from smaller fractions by reducing the amount of soil in the 200 – 250 and 250 – 253 µm fractions.
5. Effects of agricultural practices on the dynamics of earthworm communities
24Agricultural practices such as tillage, drainage, irrigation, lime application, pesticide use, fertilization and crop rotation, can influence significantly earthworm biomass and activity (Edwards et al., 1996). In a review of several studies exploring the effects of tillage on earthworms, it was concluded that deep ploughing and intensive tilling reduced earthworm populations in clay loam soils. In sandy loams tillage effects were variable and dependent upon several factors including the earthworm species present in the soil (Chan, 2001). No-till management systems promoted earthworm abundance (Edwards et al., 1996; Johnson-Maynard et al., 2007). However, populations tend to recover within one year from less-severe forms of cultivation, provided the disturbance is not repeated. When performed once a year, the effect of tillage on earthworm populations was even found to be less destructive than that of birds feeding on earthworms. Larger, anecic species such as L. terrestris and Aporrectodea longa which require a supply of surface litter and inhabit relatively permanent burrows, are the species most adversely affected by repeated soil disturbance; smaller endogeic species such as Allolobophora chlorotica and A. caliginosa are less affected and can benefit from plowed-in crop residues (Lofs-Holmin, 1983; Edwards, 1984). Eriksen-Hamel et al. (2009) investigated the effects of tillage on the earthworm Aporrectodea turgida and suggested that in cool, humid agrosystems, tillage-induced disturbance probably has a greater impact on earthworm populations and biomass than food availability. Mechanical weeding was found to be responsible for habitat disturbance, physical damage to earthworms, and disturbance in reproduction functions among other factors (Ernst et al., 2009; Peigné et al., 2009).
25Agricultural systems are characterized by high levels of inputs. Biological activity in agricultural soils is driven by organic C inputs. Inputs of organic materials from crop residue, cover crops, manure applications or organic fertilizers have a strong positive effect on the composition, size and activity of the soil biological community (Kirchner et al., 1993). Use of solid materials and organic fertilizers obtained from plants and animal origins were reported to increase earthworm populations (Leroy et al., 2007; Leroy et al., 2008; Reinecke et al., 2008). However, most chemical fertilizers influence earthworms indirectly through an increase in plant yield and consequently an increase in plant residues that remain in the field after harvest. Earthworms play an important role in surface residue decomposition rate, distribution of OM throughout the soil profile, and soil physical property modification. Lowe et al. (2002) found that OM management is important in the development of sustainable earthworm populations and their role in soil amelioration at restored sites. Also the conversion of grassland to arable land can affect the SOM and also decrease earthworm populations. Indeed, Van Eekeren et al. (2008) found a strong decrease in earthworm abundance after conversion of grassland to arable land. On the contrary, conversion of arable land to grassland stimulated the species richness and abundance, even in the second year after conversion (Van Eekeren et al., 2008).
6. Conclusion
26Earthworms are important biological factors in soil ecosystems. They are sensitive to cultivation techniques and consequently may be used as bioindicators of soil health. Earthworms have been suggested as potential indicators of the sustainability of agricultural practices that farmers could use, thereby optimizing different farming systems. Nevertheless, further research regarding the impact of cultivation techniques, crop rotations, and crop residue management on earthworm populations within Europe is required. Also, it will be important to explore the potential role of earthworms in soil fertility and agricultural sustainability.
27List of abbreviations
28Al3+ : Aluminum ions
29BS: Bulk soil
30Ca: Calcium
31Ca2+: Calcium ions
32C: Carbon
33EC: Earthworm casts
34Fe2+: Fer ions
35H: Hydrogen
36K: Potassium
37Mg: Magnesium
38Mg2+: Magnesium ions
39N: Nitrogen
40N2O: Nitrous oxide
41OC: Organic carbon
42OM: Organic matter
43O: Oxygen
44O2-: Oxygen ions
45P: Phosphorus
46SOC: Soil organic carbon
47SOM: Soil organic matter
48WWS: Worm-worked soil
Bibliographie
Abiven S., Menasseri S. & Chenu C., 2009. The effects of organic inputs over time on soil aggregate stability – A literature analysis. Soil Biol. Biochem., 41(1), 1-12.
Aira M., Monroy F. & Dominguez J., 2006. Changes in microbial biomass and microbial activity of pig slurry after the transit through the gut of the earthworm Eudrilus eugeniae (Kinberg, 1897). Biol. Fertil. Soils, 42(4), 371-376.
Aira M., Monroy F. & Dominguez J., 2007a. Earthworms strongly modify microbial biomass and activity triggering enzymatic activities during vermicomposting independently of the application rates of pig slurry. Sci. Total Environ., 385(1-3), 252-261.
Aira M., Monroy F. & Dominguez J., 2007b. Eisenia fetida (Oligochaeta: Lumbricidae) modifies the structure and physiological capabilities of microbial communities improving carbon mineralization during vermicomposting of pig manure. Microb. Ecol., 54(4), 662-671.
Aira M., Sampedro L., Monroy F. & Dominguez J., 2008. Detritivorous earthworms directly modify the structure, thus altering the functioning of a microdecomposer food web. Soil Biol. Biochem., 40(10), 2511-2516.
Aira M., Monroy F. & Dominguez J., 2009. Changes in bacterial numbers and microbial activity of pig slurry during gut transit of epigeic and anecic earthworms. J. Hazard. Mater., 162(2-3), 1404-1407.
Amtmann A. & Armengaud P., 2007. The role of calcium sensor-interacting protein kinases in plant adaptation to potassium-deficiency: new answers to old questions. Cell Res., 17, 483-485.
Anderson J., 1995. Soil organisms as engineers: microsite modulation of macroscale processes. In: Jones C. & Lawton J., eds. Linking species and ecosystems. London: Chapman & Hall, 94-106.
Barois I. & Lavelle P., 1986. Changes in respiration rate and some physico-chemical properties of soil during transit through Pontoscolex corethurus (Glossoscolecidae Oligochaete). Soil Biol. Biochem., 18(5), 539-541.
Barré P., McKenzie B.M. & Hallet P.D., 2009. Earthworms bring compacted and loose soil to a similar mechanical state. Soil Biol. Biochem., 41(3), 656-658.
Basker A., Macgregor A.N. & Kirkman J.H., 1992. Influence of soil ingestion by earthworms on the availability of potassium in soil: an incubation experiment. Biol. Fertil. Soils, 14(4), 300-303.
Basker A., Kirkman J.H. & Macgregor A.N., 1994. Changes in potassium availability and other soil properties due to soil ingestion by earthworms. Biol. Fertil. Soils, 17(2), 154-158.
Blanchart E. et al., 1999. Effects of earthworms on soil structure and physical properties. In: Lavelle et al., eds. Earthworm management in tropical agroecosystems. Wallingford, Oxon, UK; New York, NY, USA: CABI Publisher, 149-172.
Bohlen P.J., Parmelee R.W., McCartney D.A. & Edwards C.A., 1997. Earthworms effects on carbon and nitrogen dynamics of surface litter in corn agroecosystems. Ecol. Appl., 7(4), 1341-1349.
Bohlen P.J., Parmelee R.W. & Blair J.M., 2004a. Integrating the effects of earthworms on nutrient cycling across spatial and temporal scales. In: Edwards C.A., ed. Earthworm ecology. 2nd ed. Boca Raton, FL, USA: CRC Press, 183-200.
Bohlen P.J. et al., 2004b. Influence of earthworm invasion on redistribution and retention of soil carbon and nitrogen in northern temperate forests. Ecosystems, 7(1), 13-27.
Bonkowski M., Griffiths B.S. & Ritz K., 2000. Food preferences of earthworms for soil fungi. Pedobiologia, 44, 666-676.
Bossuyt H., Six J. & Hendrix P.F., 2006. Interactive effects of functionally different earthworm species on aggregation and incorporation and decomposition of newly added residue carbon. Geoderma, 130(1-2), 14-25.
Bouché M.B., 1972. Lombriciens de France. Écologie et systématique. Paris : INRA.
Brown B.A. & Mitchell M.J., 1981. Role of the earthworm, Eisenia foetida, in affecting survival of Salmonella enteriditis ser. typhimurium. Pedobiologia, 21(6), 434-438.
Brown G.G., 1995. How do earthworms affect microfloral and faunal community diversity? Plant Soil, 170(1), 209-231.
Brown G.G., Barois I. & Lavelle P., 2000. Regulation of soil organic matter dynamics and microbial activity in the drilosphere and the role of interactions with other edaphic functional domains. Eur. J. Soil Biol., 36(3-4), 177-198. Copyright © 2013 Elsevier Masson SAS. All rights reserved.
Brown G.G. & Doube B., 2004. Functional interactions between earthworms, microorganisms, organic matter and plants. Earthworm ecology. 2nd ed. London; Boca Raton, FL, USA: CRC Press, 213-240.
Butenschoen O., Marhan S., Langel R. & Scheu S., 2009. Carbon and nitrogen mobilisation by earthworms of different functional groups as affected by soil sand content. Pedobiologia, 52(4), 263-272.
Byzov B.A., Khomyakov N.V., Kharin S.A. & Kurakov A.V., 2007. Fate of soil bacteria and fungi in the gut of earthworms. Eur. J. Soil Biol., 43(1), 149-156.
Carpenter D., Hodson M.E., Eggleton P. & Kirk C., 2007. Earthworm induced mineral weathering: preliminary results. Eur. J. Soil Biol., 43(1), 176-183.
Chan K.Y., 2001. An overview of some tillage impacts on earthworm population abundance and diversity —implications for functioning in soils. Soil Till. Res., 57(4), 179-191.
Chapuis-Lardy L., Brossard M., Lavelle P. & Schouller E., 1998. Phosphorus transformations in a ferralsol through ingestion by Pontoscolex corethrurus, a geophagous earthworm. Eur. J. Soil Biol., 34(2), 61-67.
Chen S., Lian B. & Liu C.Q., 2008. The role of a strain of Bacillus mucilaginosus on weathering of phosphorite rock under experimental conditions. Acta Mineralogica Sinica, 28(1), 77-83.
Coq S. et al., 2007. Earthworm activity affects soil aggregation and organic matter dynamics according to the quality and localization of crop residues – an experimental study (Madagascar). Soil Biol. Biochem., 39(8), 2119-2128.
Cortez J., Billes G. & Bouché M.B., 2000. Effect of climate, soil type and earthworm activity on nitrogen transfer from a nitrogen-15-labelled decomposing material under field conditions. Biol. Fertil. Soils, 30(4), 318-327.
Curry J.P. & Schmidt O., 2007. The feeding ecology of earthworms – a review. Pedobiologia, 50(4), 463-477.
Darwin C., 1881. The formation of vegetable mould through the actions of worms with observations on their habits. London: Murray.
Dash M.C. & Patra U.C., 1977. Density, biomass and energy budget of a tropical earthworm population from a grassland site in Orissa, India. Rev. Ecol. Biol. Sol, 14(3), 461-471.
Dash M.C. & Patra U.C., 1979. Wormcast production and nitrogen contribution to soil by a tropical earthworm population from a grassland site in Orissa, India. Rev. Ecol. Biol. Sol, 16(1), 79-83.
Desjardins T. et al., 2003. Effects of earthworm inoculation on soil organic matter dynamics of a cultivated ultisol. Pedobiologia, 47(5-6), 835-841.
Devliegher W. & Verstraete W., 1995. Lumbricus terrestris in a soil core experiment: nutrient-enrichment processes (NEP) and gut-associated processes (GAP) and their effect on microbial biomass and microbial activity. Soil Biol. Biochem., 27(12), 1573-1580.
Devliegher W. & Verstraete W., 1997. Microorganisms and soil physico-chemical conditions in the drilosphere of Lumbricus terrestris. Soil Biol. Biochem., 29(11-12), 1721-1729.
Doube B.M. & Brown G.G., 1998. Life in a complex community: functional interactions between earthworms, organic matter, microorganisms, and plant growth. In: Edwards C.A., ed. Earthworm ecology. Boca Raton, FL, USA: CRC Press, 179-211.
Drake H.L. & Horn M.A., 2007. As the worm turns: the earthworm gut as a transient habitat for soil microbial biomes. Annu. Rev. Microbiol., 61, 169-189.
Edwards C.A., 2004. Earthworm ecology. 2nd ed. Boca Raton, FL, USA: CRC Press.
Edwards C.A., 1984. Changes in agricultural practice and their impact upon soil organisms. In: Jenkins D., ed. Proceedings of ITE symposium no. 13, The impact of agriculture on wildlife, agriculture and the environment, 28-29 February and 1 March 1984, Monks Wood Experimental Station, Sawtry, Cambridgeshire, UK, 56-65.
Edwards C.A. & Bohlen P.J., 1996. Earthworm ecology and biology. London: Chapman & Hall, 196-212.
Eriksen-Hamel N.S. et al., 2009. Earthworm populations and growth rates related to long-term crop residue and tillage management. Soil Tillage Res., 104(2), 311-316.
Ernst G. & Emmerling C., 2009. Impact of five different tillage systems on soil organic carbon content and the density, biomass and community composition of earthworms after a ten year period. Eur. J. Soil Biol., 45(3), 247-251.
Felske A., Akkermans A.D.L. & De Vos W.M., 1998. In situ detection of an uncultured predominant Bacillus in Dutch grassland soils. Appl. Environ. Microbiol., 64(11), 4588-4590.
Flegel M. & Schrader S., 2000. Importance of food quality on selected enzyme activities in earthworm casts (Dendrobaena octaedra, Lumbricidae). Soil Biol. Biochem., 32(8-9), 1191-1196.
Freitag A., Rudert M. & Bock E., 1987. Growth of Nitrobacter by dissimilatoric nitrate reduction. FEMS Microbiol. Lett., 48(1-2), 105-109.
Frelich L.E. et al., 2006. Earthworm invasion into previously earthworm-free temperate and boreal forests. Biol. Invasions, 8(6), 1235-1245.
Garcia C. et al., 1992. Changes in ATP content, enzyme activity and inorganic nitrogen species during composting of organic wastes. Can. J. Soil Sci., 72(3), 243-253.
Giller K.E., 2001. Nitrogen fixation in tropical cropping systems. 2nd ed. Wallingford, UK: CAB International.
Gilot C., 1997. Effects of a tropical geophageous earthworm, Millsonia anomala (Megascolecidae), on soil characteristics and production of a yam crop in Ivory Coast. Soil Biol. Biochem., 29(3-4), 353-359.
Gómez-Brandón M., Aira M., Lores M. & Domínguez J., 2011. Epigeic earthworms exert a bottleneck effect on microbial communities through gut associated processes. Plos One, 6, e24786.
Hale C.M., Frelich L.E. & Reich P.B., 2005. Effects of European earthworm invasion on soil characteristics in northern hardwood forests of Minnesota. Ecosystems, 8(8), 911-927.
Hopkins D.W. & Gregorich E.G., 2005. Carbon as a substrate for soil organisms. In: Bardgett R.D., Usher M.B. & Hopkins D.W., eds. Biodiversity and function in soils. British Ecological Society Ecological Reviews. Cambridge, UK: Cambridge University Press, 57-79.
Horn M.A., Schramm A. & Drake H.L., 2003. The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms. Appl. Environ. Microbiol., 69(3), 1662-1669.
Ihssen J. et al., 2003. N2O - producing microorganisms in the gut of the earthworm Aporrectodea caliginosa are indicative of ingested soil bacteria. Appl. Environ. Microbiol., 69(3), 1655-1661.
Johnsen A.R., Wick L.Y. & Harms H., 2005. Principles of microbial PAH-degradation in soil. Environ. Pollut., 133, 71-84.
Johnson-Maynard J.L., Umiker K.J. & Guy S.O., 2007. Earthworm dynamics and soil physical properties in the first three years of no-till management. Soil Till. Res., 94(2), 338-345.
Kay B.D., 1998. Soil structure and organic carbon: a review. In: Lal R. et al., eds. Soil processes and the carbon cycle. Boca Raton, FL, USA: CRC Press, 169-197.
Ketterings Q.M., Blair J.M. & Marinissen J.C.Y., 1997. Effects of earthworms on soil aggregate stability and carbon and nitrogen storage in a legume cover crop agroecosystem. Soil Biol. Biochem., 29(3-4), 401-408.
Kiikkilä O., Kitunen V. & Smolander A., 2006. Dissolved soil organic matter from surface horizons under birch and conifers: degradation in relation to chemical characteristics. Soil Biol. Biochem., 38(4), 737-746.
Kirchner M.J., Wollum II A.F. & King L.D., 1993. Soil microbial populations and activities in reduced chemical input agroecosystems. Soil Sci. Soc. Am. J., 57(5), 1289-1295.
Lachnicht S.L., Parmelee R.W., McCartney D. & Allen M., 1997. Characteristics of macroporosity in a reduced tillage agroecosystem with manipulated earthworm populations: implications for infiltration and nutrient transport. Soil Biol. Biochem., 29(3-4), 493-498.
Lattaud C. et al., 1998. The diversity of the digestive systems in tropical geophagous earthworms. Appl. Soil Ecol., 9(1-3), 189-195.
Lavelle P., 1988. Earthworm activities and the soil system. Biol. Fertil. Soils, 6(3), 237-251.
Lavelle P., 1997. Faunal activities and soil processes: adaptive strategies that determine ecosystem function. Adv. Ecol. Res., 27, 93-132.
Lavelle P. & Martin A., 1992. Small-scale and large-scale effects of endogeic earthworms on soil organic matter dynamics in soils of the humid tropics. Soil Biol. Biochem., 24(12), 1491-1498.
Lavelle P. & Gilot C., 1994. Priming effects of macroorganisms on microflora: a key process of soil function? In: Ritz K., Dighton J. & Giller K., eds. Beyond the biomass: compositional and functional analysis of soil microbial communities. Chichester, UK: Wiley, 173-180.
Lavelle P. & Spain A.V., 2001. Soil ecology. Dordrecht, The Netherlands: Kluwer Academic.
Lee K.E., 1985. Earthworms: their ecology and relationships with soil and land use. New York, NY, USA: Academic Press, Inc.
Lee K.E. & Foster R.C., 1991. Soil fauna and soil structure. Austr. J. Soil Res., 29(6), 745-775.
Leroy B.L.M. et al., 2007. The quality of exogenous organic matter: short-term influence on earthworm abundance. Eur. J. Soil Biol., 43(1), 196-200.
Leroy B.L.M. et al., 2008. Earthworm population dynamics as influenced by the quality of exogenous organic matter. Pedobiologia, 52(2), 139-150.
Li X., Fisk M.C., Fahey T.J. & Bohlen P.J., 2002. Influence of earthworm invasion on soil microbial biomass activity in a northern hardwood forest. Soil Biol. Biochem., 34(12), 1929-1937.
Liu W. et al., 2006. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture. Environ. Geochem. Health, 28(1-2), 133-140.
Lofs-Holmin A., 1983. Earthworm population dynamics in different agricultural rotations. In: Satchell J.E., ed. Earthworm ecology – from Darwin to vermiculture. London: Chapman & Hall, 151-160.
Lowe C.N. & Butt K.R., 2002. Influence of organic matter on earthworm production and behaviour: a laboratory-based approach with applications for soil restoration. Eur. J. Soil Biol., 38(2), 173-176.
Lukkari T., Teno S., Vaisanen A. & Haimi J., 2006. Effects of earthworms on decomposition and metal availability in contaminated soil: microcosm studies of populations with different exposure histories. Soil Biol. Biochem., 38(2), 359-370.
Mariani L. et al., 2007. What happens to earthworm casts in the soil? A field study of carbon and nitrogen dynamics in Neotropical savannahs. Soil Biol. Biochem., 39(3), 757-767.
McLean M.A. & Parkinson D., 2000. Field evidence of the effects of the epigeic earthworm Dendrobaena octaedra on the microfungal community in pine forest floor. Soil Biol. Biochem., 32(3), 1671-1681.
McLean M.A., Migge-Kleian S. & Parkinson D., 2006. Earthworm invasions of ecosystems devoid of earthworms: effects on soil microbes. Biol. Invasions, 8(6), 1257-1273.
Monroy F., Aira M. & Dominguez J., 2008. Changes in density of nematodes, protozoa and total coliforms after transit through the gut of four epigeic earthworms (Oligochaeta). Appl. Soil Ecol., 39(2), 127-132.
Moody S.A., Briones M.J.I., Pierce T.G. & Dighton J., 1995. Selective consumption of decomposing wheat straw by earthworms. Soil Biol Biochem., 27(9), 533-537.
Morris G.M., 1985. Secretory cells in the clitellar epithelium of Eisenia fetida (Annelida, Oligochaeta): a histochemical and ultrastructural study. J. Morphol., 185(1), 89-100.
Mummey D.L., Rillig M.C. & Six J., 2006. Endogeic earthworms differentially influence bacterial communities associated with different soil aggregate size fractions. Soil Biol. Biochem., 38(7), 1608-1614.
Nunan N. et al., 2003. Spatial distribution of bacterial communities and their relationships with the micro-architecture of soil. FEMS Microbiol. Ecol., 44(2), 203-215.
Ortiz-Ceballos A.I., Pena-Cabriales J.J., Fragoso C. & Brown G.G., 2007. Mycorrhizal colonization and nitrogen uptake by maize: combined effect of tropical earthworms and velvetbean mulch. Biol. Fertil. Soils, 44(1), 181-186.
Padmavathiamma P.K., Li LY. & Kumari U.R., 2008. An experimental study of vermi-biowaste composting for agricultural soil improvement. Bioresour. Technol., 99(6), 1672-1681.
Parmelee R.W., Bohlen P.J. & Blair J.M., 1998. Earthworms and nutrient cycling processes: integrating across the ecological hierarchy. In: Edwards C., ed. Earthworm ecology. Boca Raton, FL, USA: CRC Press, 179-211.
Pedersen J.C. & Hendriksen N.B., 1993. Effect of passage through the intestinal track of detritivore earthworms (Lumbricus spp.) on the number of selected Gram-negative and total bacterial. Biol. Fertil. Soils, 16(3), 227-232.
Peigné J. et al., 2009. Earthworm populations under different tillage systems in organic farming. Soil Tillage Res., 104(2), 207-214.
Reinecke A.J., Albertus R.M.C., Reinecke S.A. & Larink O., 2008. The effects of organic and conventional management practices on feeding activity of soil organisms in vineyards. Afr. Zool., 43(1), 66-74.
Rizhiya E. et al., 2007. Earthworm activity as a determinant for N2O emission from crop residue. Soil Biol. Biochem., 39(8), 2058-2069.
Rudi K., Ødegard K., Løkken T.T. & Wilson R., 2009. A feeding induced switch from a variable to a homogenous state of the earthworm gut microbiota within a host population. PloS One, 4(10), e7528.
Sampedro L. & Dominguez J., 2008. Stable isotope natural abundance (d13C and d15N) of the earthworm Eisenia fetida and other soil fauna living in two different vermicomposting environments. Appl. Soil Ecol., 38(2), 91-99.
Sanz-Montero M.E. & Rodriguez-Aranda J.P., 2009. Silicate bioweathering and biomineralization in lacustrine microbialites: ancient analogues from the Miocene Duero Basin, Spain. Geol. Mag., 146(4), 527-539.
Scheu S. et al., 2002. Effects of the presence and community composition of earthworms on microbial community functioning. Oecologia, 133(2), 254-260.
Schönholzer F., Hahn D. & Zeyer J., 1999. Origins and fate of fungi and bacteria in the gut of Lumbricus terrestris L. studied by image analysis. FEMS Microbiol. Ecol., 28(3), 235-248.
Shaw C. & Pawluk S., 1986. Faecal microbiology of Octolasion tyrtaeum, Aporrectodea turgida and Lumbricus terrestris and its relation to the carbon budgets of three artificial soils. Pedobiologia, 29(6), 377-389.
Singleton D.R., Hendrix P.F., Coleman D.C. & Whitman W.B., 2003. Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae; Oligochaeta). Soil Biol. Biochem., 35(12), 1547-1555.
Six J., Elliott E. & Paustian K., 2000. Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem., 32(14), 2099-2103.
Six J. et al., 2002. Soil organic matter, biota and aggregation in temperate and tropical soils-effects of no-tillage. Agronomie, 22, 755-775.
Snyder B.A., Boots B. & Hendrix P.F., 2009. Competition between invasive earthworms (Amynthas corticis, Megascolecidae) and native North American millipedes (Pseudopolydesmus erasus, Polydesmidae): effects on carbon cycling and soil structure. Soil Biol. Biochem., 41(7), 1442-1449.
Stockdale E.A., Watson CA., Black H.I.J. & Philipps L., 2006. Do farm management practices alter below-ground biodiversity and ecosystem function? Implications for sustainable land management. JNCC Report No. 364. Peterborough, UK: Joint Nature Conservation Committee.
Suarez E.R. et al., 2004. Effects of exotic earthworms on soil phosphorous cycling in two broadleaf temperate forests. Ecosystems, 7(1), 28-44
Sugumaran P. & Janarthanam B., 2007. Solubilization of potassium containing minerals by bacteria and their effect on plant growth. World J. Agric. Sci., 3(3), 350-355.
Syers J.K. & Springett J.A., 1984. Earthworms and soil fertility. Plant Soil, 76(1-3), 93-104.
Teng S.K., 2012. Evaluation on physical, chemical and biological properties of casts of geophagous earthworm Metaphire tschiliensis tschiliensis. Sci. Res. Essays, 7(10), 1169-1174.
Thakuira D. et al., 2010. Gut wall bacteria of earthworms: a natural selection process. ISME J., 4, 357-366.
Tisdall J.M. & Oades J.M., 1982. Organic matter and water-stable aggregates in soils. J. Soil Sci., 33(2), 141-163.
Tiunov A.V. & Scheu S., 2000. Microbial biomass, biovolume and respiration in Lumbricus terrestris L. cast material of different age. Soil Biol. Biochem., 32(2), 265-275.
Van Eekeren N. et al., 2008. Soil biological quality after 36 years of ley-arable cropping, permanent grassland and permanent arable cropping. Appl. Soil Ecol., 40(3), 432-446.
Wolf D. & Wagner G., 2005. Carbon transformations and soil organic matter formation. In: Sylvia D., Fuhrmann J., Hartel P. & Zuberer D., eds. Principles and applications of soil microbiology. Upper Saddle River, NJ, USA: Pearson Prentice Hall, 285-332.
Wolter C. & Scheu S., 1999. Changes in bacterial numbers and hyphal lengths during the gut passage through Lumbricus terrestris (Lumbricidae, Oligochaeta). Pedobiologia, 43(6), 891-900.
Wolters V. & Joergensen R.G., 1992. Microbial carbon turnover in beech forest soils worked by Aporrectodea caliginosa (Savigny) (Oligochaeta, Lumbricidae). Soil Biol. Biochem., 24(2), 171-177.
Woomer P.L. & Swift M.J., 1994. The biological management of tropical soil fertility. Chichester, UK: John Wiley and Sons.
Wrage N., Velthof G.L., Van Beusichem M.L. & Oenema O., 2001. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem., 33(12-13), 1723-1732.
Zhang B.G. et al., 2000. Changes in microbial biomass C, N, and P and enzyme activities in soil incubated with the earthworms Metaphire guillelmi or Eisenia fetida. Soil Biol. Biochem., 32(14), 2055-2062.
Zhang Q.L. & Hendrix P.F., 1995. Earthworm (Lumbricus rubellus and Aporrectodea caliginosa) effects on carbon flux in soil. Soil Sci. Soc. Am., 59(3), 816-823.






