- Home
- volume 10 (2006)
- numéro 2
- Optimization of a reliable, fast, cheap and sensitive silver staining method to detect SSR markers in polyacrylamide gels
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Optimization of a reliable, fast, cheap and sensitive silver staining method to detect SSR markers in polyacrylamide gels

Editor's Notes
Received 30 March 2004, accepted 4 January 2006
Résumé
Développement d’une méthode fiable, rapide, économique et sensible de coloration au nitrate d’argent pour la détection des marqueurs SSR sur gels de polyacrylamide. Une méthode fiable, rapide, économique et sensible de coloration au nitrate d’argent pour la détection des acides nucléiques sur gels de polyacrylamide a été développée à partir de deux procédures standards. Les principales différences entre les trois méthodes comparées sont : (i) le prétraitement au formaldéhyde pendant l’imprégnation au nitrate d’argent, (ii) l’addition d’hydroxyde de sodium à la place du thiosulfate de sodium et du carbonate de sodium durant le développement, (iii) l’élimination de l’étape stop ou l’ajout d’éthanol absolu avec de l’acide acétique dans la solution stop et (iv) la durée des différentes réactions à chaque étape. Toutes les méthodes ont permis la détection du même polymorphisme pour une répétition de séquences simples avec différents génotypes de cotonnier mais d’importantes différences concernant le contraste, la couleur de fond du gel et la durée de conservation ont été observées. Deux méthodes ont donné une sensibilité supérieure. La méthode améliorée est sensible, rapide (20 min) et permet la réutilisation de toutes les solutions utilisées dans la procédure de coloration de cinq à sept fois, ce qui la rend très économique.
Abstract
A reliable, fast, cheap and sensitive silver staining method to detect nucleic acids in polyacrylamide gels was developed from two standard stain procedures. The main differences between the three methods concerned (i) the preexposure with formaldehyde during silver nitrate impregnation, (ii) the addition of sodium thiosulfate and sodium carbonate instead of sodium hydroxide during development; (iii) the removal of the stop reaction or the inclusion of absolute ethanol with acetic acid in the stop solution and (iv) the duration of the different reaction steps. All methods allowed the detection of similar polymorphisms for single sequence repeats with different cotton genotypes but important differences regarding the contrast, background and conservation duration of the gels were observed. Two methods gave superior sensitivity. The improved method was sensitive, fast (20 min), gave lower backgrounds, produced gels with long-term conservation ability, and allowed a reutilization of all the solutions used in the staining procedure from five to seven times, making it quite cheap.
1. Introduction
1Radioactive or fluorescent detection approaches of DNA molecular markers are expensive, time-consuming and require special facilities that render them impracticable in most tropical countries where sophisticated infrastructure are lacking. On the opposite, silver staining is a relatively rapid, inexpensive alternative to these techniques. It was originally described for ultrasensitive detection of polypeptides separated by polyacrylamide gel electrophoresis (Merril et al., 1981) and later modified for nucleic acid detection with increased sensitivity due to various small adaptations of the original silver staining methods (Sommerville, Wang, 1981; Beidler et al., 1982, Goldman, Merril, 1982; Blum et al., 1987; Bassam et al., 1991; Sanguinetti et al., 1994). The last detection methods of nucleic acids using silver staining can be considered as sensitive as autoradiography and fluorescence labelling techniques (Christensen et al., 1999).
2Compared to the photochemical silver staining procedure devised by Merril et al. (1981) for polypeptide detection, Bassam et al. (1991) enhanced the sensitivity (as low as 1 pg.mm-2) by including a gel preexposure with formaldehyde during silver nitrate impregnation and by lowering the concentration of silver nitrate. Backgrounds were reduced by inclusion of sodium thiosulfate and by eliminating an oxidation pre-treatment with potassium dichromate and nitric acid. This staining method is very sensitive and is often considered as a standard for non radioactive DNA detection. Its main constraints concern (i) its long duration; (ii) a relatively poor contrast of the DNA bands; (iii) the need to maintain the temperature at 10° C for the development and stop solutions; and (iv) the rather low rate of reuse of the staining solutions. The objective of this study was to develop a new method of silver staining that is as sensitive as the one of Bassam et al. (1991) but much simpler, faster and cheaper using interspecific hybrid of cotton as a model system. A quick staining procedure developed from the method of Sanguinetti et al. (1994) was modified in our laboratory in order to improve its level of sensitivity and the conservation ability of the gels after staining. The new staining procedures were compared to the standard method of Bassam et al. (1991) and the conditions for detection of SSRs were optimised using denaturing polyacrylamide gels bound to glass plates.
2. Materials and Methods
3Total genomic DNA was extracted from fresh young leaves of 12 greenhouse-grown cotton genotypes (Gossypium hirsutum L. cultivars, wild species and interspecific hybrid) using a modified procedure (Benbouza et al. submitted) adapted from Vroh Bi et al. (1996).
4Amplification of genomic DNA was done according to the protocol of Liu et al. (2000). PCR reactions were performed in 10 µl volume containing 10 ng of cotton DNA template, 10 x PCR Buffer (100 mM Tris-HCL, pH 9 and 500 mM KCl, 15 mM MgCl2) (Amersham Pharmacia Biotech, Inc), 2-3.5 mM MgCl2, 0.2 mM dNTPs, 0.15 µM of each single primer (Invitrogen), 0.4 U of AmpliTaq (Amersham Pharmacia Biotech, Inc).
5The amplification profile consisted of an initial period of DNA denaturation and AmpliTaq activation at 95° C for 12 min, followed by 40 cycles of 93° C (step 1) for 15 s, 55° C (step 2) for 30 s, and 72° C (step 3) for 1 min. The ramp time was 42 s to step 1, 36 s to step 2, and 40 s to step 3. After 40 cycles, the extension temperature of 72° C was held for 6 min.
6The polyacrylamide electrophoresis gels were prepared as follows. Wash a 33.2 x 38.2 cm glass plate with 5 ml of absolute ethanol using paper towel. Let dry for 2 min and repeat the operation again. Treat the glass with 15 µl of g-methacryloxy-propyl-trimethoxysilane (Bind Silane, M-6514, Sigma) in 4 ml of absolute ethanol and 1.25 ml of 10 % acidic acid. Let dry 5 min, and then polish using Kimwipes paper. Then, remove the excess using Kinwipe paper moistened with absolute ethanol.
7Treat a larger glass plate (33.2 x 41.6 cm) 5 ml of absolute ethanol using Kimwipes paper. Let dry for 2 min, repeat the operation again and polish with Kimwipes paper.
8Prepare a 40 % acrylamide solution with 38 g acrylamide and 2 g N, N’-methylene bisacrylamide in 100 ml distilled water.
9Prepare a urea : acrylamide solution with 75 ml of 40 % acrylamide solution, 50 ml 10x TBE (1M Trizma, 89 mM boric acid, 20 mM Na2 EDTA ) and 210 g urea. Add distilled water to a final volume of 500 ml, filter and stock at 4° C in a brown bottle.
10Prepare gels (6 % polyacrylamide; 8M urea) by mixing 60 ml of the urea: acrylamide solution in 10x TBE with 250 µl of ammonium persulfate and 50 µl of TEMED (Sigma). Apply the gel solution to the assembled gel plates (1.5 mm thick) using S2 Sequencing gel electrophoresis apparatus (Invitrogen). Allow the gel to polymerize during 30 min.
11The amplification products were denatured for 2 min at 92° C in the thermocycler and placed on ice before being applied to the gel in 5 µl containing an equal volume of stop solution (10 mM NaOH, 95 % Formamide, 0.05 % Bromophenol Blue, 0.05 % Xylene Cyanol). The running conditions were 1680 V, 40 mA, 80 W for 75 min, after a pre-run of the gels for 1 h. The electrophoresis running buffer (TBE 1x) contained 10 mM Trizma, 8.9 mM boric acid, 2 mM Na2 EDTA.
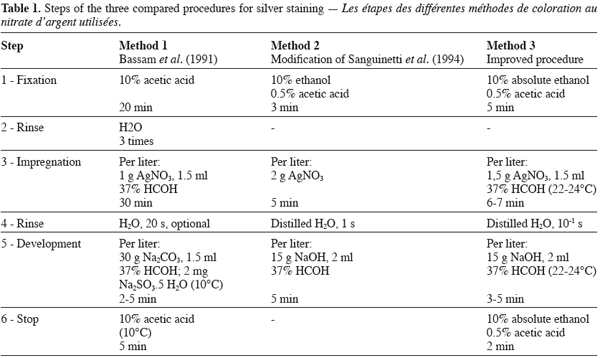
12Gels were silver stained using three methods according to the steps described in table 1. The protocol of Bassam et al. (1991) (Method 1) was followed exactly as recommended. The method derived from the procedure of Sanguinetti et al. (1994) (Nachit, Elouafi, personnal communication) (Method 2) reduces drastically the time devoted to the different steps, by adding a very short rinse step (1 s) in distilled water after the impregnation of the gel with silver nitrate, by using NaOH as reagent to increase the pH of the development solution which also includes a higher content of formaldehyde and by suppressing the stop reaction. The improved staining method we developed (Method 3) is a combination of the different steps proposed by Bassam et al. (1991) and Sanguinetti et al. (1994). It consisted in the following steps: after electrophoresis, gels were washed in 2000 ml cold (10-12° C) fixing solution (10 % absolute ethanol, 0.5 % acetic acid) for 5 min. Washed gels were soaked for 6‑7 min at room temperature (22-24° C) in a 2000 ml solution of 0.15 % AgNO3, 2 ml 37% HCOH. Gels were rinsed quickly (10-15 s) once with 2000 ml distilled H2O. They were then developed by soaking them at room temperature (22-24° C) in a 2000 ml developing solution (1.5 % NaOH, 3 ml 37 % HCOH) until the bands appear with a sufficient intensity (3-5 min). When the desired intensity was achieved development was stopped by impregnating the gel in a 2000 ml stop solution (10 % absolute ethanol, 0.5 % acetic acid) for 2 min. All steps were done in plastic containers. The gel plates were agitated in a shaker throughout the staining process. All solutions were prepared using ultra pure distilled water (12 MΩ.cm). The fix stop, developer and silver nitrate solutions were prepared in advance but formaldehyde was added just before use. Impregnation with silver nitrate was performed under room lighting. We added systematically 1 ml of formaldehyde during gels development after the first use of silver nitrate solution. Gels were dried at room temperature and DNA bands were viewed directly with aid of a white light box, then photographed or scanned.
13We used absolute ethanol and sodium hydroxide from Merck Eurolab. Acetic acid was from Sigma. Formaldehyde and silver nitrate were ACS reagent from Sigma and Aldrich respectively.
3. Results and discussion
14DNA from twelve cotton genotypes (diploid species, tetraploid cultivars and interspecific tetraploid hybrids) was amplified by PCR using SSR primer pairs BNL1673 and BNL3792 on polyacrylamide gel. These primer pairs were developed at Brookhaven National Laboratory. Clone sequences used for primer definition are available at http://ukcrop.net/perl/ace/search/cottonDB. Samples of the same DNA amplication reactions were applied to three identical 6 % polyacrylamide gels bound to glass plates. The three silver staining methods were used for detection of SSR polymorphism. The methods of Bassam et al. (1991) and the improved procedure disclosed identical banding patterns with identical alleles (Figure 1) while some bands were not visible for the method inspired from Sanguinetti et al. (1994). The methods also differed regarding the contrast, the conservation period of the gels and the number of possible reuse of the different solutions involved in the staining procedure.
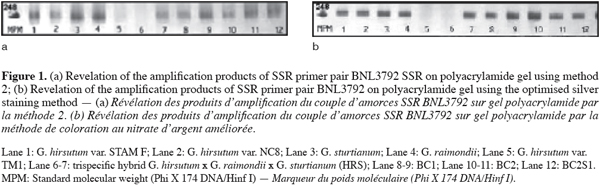
15Method 1 was long (63 min) and exhibited a relatively poor image contrast when applied in our laboratory, yielding grey-black bands on a light grey background. It was however as sensitive as the improved method we developed allowing the detection of the smallest fragments of the standard molecular weight (Phi X 174 DNA/Hinf I) (48 bp). The fixing solution could be reused three times and the stopping solutions five times while the silver nitrate solution could be reused only three times without loss of sensitivity. Method 2 was very fast (12 min) without need of controlling the temperature during the development step but it gave the lowest sensitivity producing a strong yellow background with discrete bands that were not visible for the smallest DNA fragments. The different solutions used in the staining process could be reused from three (coloration and development) to five times (fixation). The major problem with this method was the very rapid degradation (appearance of cracks) of the gels bound on glass plates a few hours after the end of the developing step.
16The optimised method was fast (20 min) and at least as sensitive as the method of Bassam et al. (1991) with a better contrast. All the steps can be carried out at room temperature. The polyacrylamide gels stained using this procedure could be stored up to 12 months and all the solutions used in the different staining steps could be reused at least five times, with a maximum of seven times for the silver nitrate solution, without loss of sensitivity. Preparation and handling of the solutions were the same for all methods. The silver nitrate and developing solutions were stored at room temperature while the fixing solution was stored at 4 ° C.
17The presence of absolute ethanol at a rate of 10 % in the fixing solution permits elimination of molecules that interfere with silver staining such as electrophoresis buffer, urea, denaturants and detergents. It also allowed a drastic reduction of the concentration of acetic acid (from 10 % to 0.5 %) necessary to render the macromolecules insoluble in the gel and prevent them from diffusing out of the matrix during the subsequent staining steps.
18A concentration of 1.5 g.l-1 AgNO3 gives optimal sensitivity in relatively short time of impregnation (6-7 min). The presence of formaldehyde in the silver impregnation solution improves both sensitivity and intensity of the detected bands. Washing the gels briefly following this step removes the excess of silver from their surface.
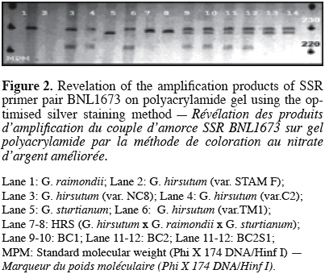
19Replacing sodium carbonate by sodium hydroxide permits to establish alkaline conditions (pH 13) for silver ion reduction to metallic silver by formaldehyde. A high formaldehyde concentration (3 ml 37 % HCOH per liter) in the development solution combined with a development at room temperature (22-24 ° C) apparently increased band intensity (Figure 2) and therefore also the sensitivity of the method and it decreased development time. The reduction of silver by formaldehyde is temperature-dependent. It is enhanced by increasing temperature; conversely, decreasing the temperature of the development solution below the 18‑25 ° C range (Blum et al., 1987) increases developing time. Bands appeared dark brown-black on an uniformly pale yellow background; over development resulted in an orange brown background with low contrast bands. For some amplified primers, minor shadow bands arising from PCR slippage were commonly visible generally two bases but sometimes three or more, below the main band. As for the fixing step, the addition of absolute ethanol permits to decrease the concentration of acetic acid to 0.5 % in the solution that stops the reduction of Ag+ ions to metallic silver.
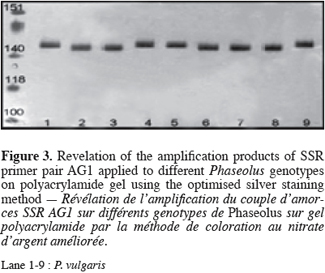
20We have used the improved method presented here routinely in our laboratory for analyses of SSRs in various tropical crops (Figure 3) (Phaseolus polyanthus Greenm, P. coccineus L., P. vulgaris L., Oryza sativa L.) (Silué et al., 2004). It gives satisfying results and is simple and cheap without the risks associated to radioisotope use. Its short duration permits the staining of a large number of gels per day.
21Acknowledgments
22The authors thank very much Drs M. Nachit and I. Elouafi from ICARDA (Syria) for the informations and advises.
Bibliographie
Bassam BJ., Anollés GC., Gresshoff PM. (1991). Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal. Biochem. 196, p. 80–83.
Beidler JL., Hilliard PR., Rill RL. (1982). Ultrasensitive staining of nucleic acids with silver. Anal. Biochem. 126, p. 374–380.
Benbouza H., Baudoin JP., Mergeai G. (2006). Amélioration de la méthode d’extraction d’ADN au CTAB appliquée aux feuilles de cotonnier. Biotechnol. Agr. Soc. Environ., 10 (2), p. 73—76.
Blum H., Beier H., Gross HJ. (1987). Improved silver staining of plant-proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8, p. 93–99.
Christensen M., Sunde L., Bolund L., Orntoft TF. (1999). Comparison of three methods of microsatellites detection. Scand. J. Clin. Lab. Invest. 59, p. 167–178.
Nachit, Elouafi Personnal communication.
Goldman D., Merril CR. (1982). Silver staining of DNA in polyacrylamide gels: linearity and effect of fragment size. Electrophoresis 3, p. 24–26.
Liu S., Saha S., Stelly D., Burr B., Cantrell RG. (2000). Chromosomal assignment of microsatellites loci in Cotton. Amer. Genet. Assoc. 91, p. 326–332.
Merill CR., Goldman D., Sedman SA, Ebert MH. (1981). Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science 211, p. 1437–1438.
Sanguinetti CJ., Dias Neto E., Simpson AJG. (1994). Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques 17, p. 915–919.
Silué S., Jacquemin JM., Toussaint A., Baudoin JP. (2004). étude des gènes impliqués dans l’embryogenèse de Phaseolus. In: Gumedzoe Y.M.D (éd.). Biotechnologies Végétales, Biodiversité et Biosécurité : Défis et Enjeux. IXe Journées Scientifiques du Réseau BIOVEG, AUF. p 73–76.
Sommerville LL., Wang K. (1981). The ultrasensitive silver protein stain also detects nanograms of nucleic acids. Biochem. Biophys. Res. Commun. 120, p. 530–548.
Vroh Bi I., Harvengt L., Chandelier A., Mergeai G., Du Jardin P. (1996). Improved RAPD amplification of recalcitrant plant DNA by the use of activated charcoal during DNA extraction. Plant Breed. 115, p. 205–206.
To cite this article
About: Halima Benbouza
Unité de Phytotechnie tropicale et d’Horticulture. Faculté universitaire des Sciences agronomiques. Passage des Déportés, 2, B-5030 Gembloux (Belgique). E-mail : mergeai.g@fsagx.ac.be
About: Jean-Marie Jacquemin
Département de Biotechnologie, Centre wallon de Recherches agronomiques. Rue de Liroux, 9. B-5030 Gembloux (Belgique).
About: Jean-Pierre Baudoin
Unité de Phytotechnie tropicale et d’Horticulture. Faculté universitaire des Sciences agronomiques. Passage des Déportés, 2, B-5030 Gembloux (Belgique).
About: Guy Mergeai
Unité de Phytotechnie tropicale et d’Horticulture. Faculté universitaire des Sciences agronomiques. Passage des Déportés, 2, B-5030 Gembloux (Belgique).






