- Portada
- volume 10 (2006)
- numéro 2
- Investigations on Cationic Exchange Capacity and Unused Bed Zone according to operational conditions in a Fixed Bed Reactor for water lead removal by a natural zeolite
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Investigations on Cationic Exchange Capacity and Unused Bed Zone according to operational conditions in a Fixed Bed Reactor for water lead removal by a natural zeolite

Notes de la rédaction
Received 21 February 2005, accepted 11 January 2006
Résumé
Etude de la capacité d’échange cationique d’une zéolite naturelle et de la zone non utilisée d’un réacteur à lit fixe en fonction des conditions opératoires pour l’élimination du plomb dans l’eau. Dans cette étude l’attention a été portée sur la variation de la Capacité d’Echange Cationique (CEC) et de la Zone du Lit non Utilisée (UBZ), en fonction des paramètres opératoires (longueur du lit : L, diamètre de la colonne : D, granulométrie : d et le débit : Q) dans un réacteur à lit fixe (FBR). Les expérimentations sont réalisées pour l’étude du plomb sur de la clinoptilolite de Nouvelle Zélande à la température de 25 ± 1 ° C. Les résultats montrent une constante CEC opératoire de 1,00 ± 0,015 meq.g-1 qui est indépendante des paramètres cités ci-dessus. La performance des opérations exprimée par UBZ, montre un optimum pour le rapport L/D (longueur du lit sur diamètre de la colonne) fixé à 18,1 pour Q = 2,9 BV.h-1 (volume de lit par heure) et une granulométrie d = 0,38 mm (le rapport granulométrie sur diamètre colonne d / D à 0,057). Cette performance maximale est atteinte pour la plus faible valeur de UBZ qui est de 5,6 % de la CEC. Les interprétations déduites des résultats en fonction de UBZ montrent que la diminution de la granulométrie augmente la performance du processus d’échange ionique, le même résultat est observé avec la diminution du débit. Néanmoins, l’augmentation du rapport L / D ne semble pas accroître les performances de l’échange ionique.
Abstract
In this study, attention has been focused on the behaviour of the Cationic Exchange Capacity (CEC) and the Unused Bed Zone (UBZ), according to the operating parameters (bed length: L, column diameter: D, particle diameter: d and flow rate: Q) in a Fixed Bed Reactor (FBR). The investigations are performed for a single-component study of lead on New Zealand clinoptilolite at 25 ± 1°C. The results show a constant operating CEC of 1.00 ± 0.015 meq.g-1 which is independent of the operational parameters listed above. The performance of the operations expressed as UBZ, shows an optimum for the ratio L/D (bed length and column diameter) fixed at 18.1, for Q = 2.9 BV.h-1 (bed volume per hour) and particle diameter d = 0.38 mm (the ratio particle diameter on column diameter d/D at 0.057). This maximum performance is reached with the lowest UBZ value of 5.6% of the operating CEC. Overall interpretation drawn from the results according to UBZ shows that decreasing the particles size improves the performance of ion exchange process; as well as decreasing the flow rate. Nevertheless, the increase of L/D to a certain extent does not improve ion exchange performances.
Tabla de contenidos
1. Introduction
1Problems of environmental pollution by heavy metals from industrial activity represent a serious threat to human health and have been widely described (Keane, 1998; Bliefert, Perraud, 2001; Alvarez-Ayuso et al., 2003; Inglezakis, Grigoropoulou, 2003). Serious threats for Haitian community (Port-au-Prince) have been reported due to the presence of toxic heavy metals such as lead in waste waters (Carré, 1997) and even in drinking water (Angerville et al., 2004). Due to the high toxicity related to lead, the United States Environmental Protection Agency (US EPA) since early 2004 has lowered the contamination level in drinking water from 50 to 15 µg.l-1 (X1, 2005). The lead presence in Port-au-Prince urban environment results from activities such as paint manufacturing wastes waters, battery repairing shops, metallic gates handicrafts, welding activities, vehicle body and motor repair shops and many other activities not cited here. Since that cation exchange on zeolites is an effective and economic way to treat industrial heavy metals contaminated wastes (Keane, 1998; Rodríguez-Iznaga et al., 2002; Perić et al., 2003) investigations focussing on practical use of such process have been undertaken. The general aim of this study is to investigate the behaviour of lead retention by natural clinoptilolite zeolite. However it has been highlighted that recent data were scarce in that field especially for clinoptilolite in Fixed Bed Reactors (FBR) (Inglezakis, Grigoropoulou, 2004). Cation Exchange Capacity (CEC) given for a material in a commercial purpose is different from the one obtained under given applied conditions by an investigator. The first one may be defined as theoretical as it has been evaluated by chemical methods or spectrometric techniques (X ray diffraction, NMR, …). It refers to the total number of sites available on the material for cation exchange expressed in equivalents per gram. Some authors called it the Total Exchange Capacity (TEC) (Inglezakis et al., 2002). Since all these ionogenic groups on the material may not really be available for exchange, (cation hydrated radii too large to fit into a channel (Helfferich, 1995), some of the ionogenic groups located at inaccessible sites of the material structure (Pabalan, 1994)), the CEC obtained by an operator is expected to be different. Inglezakis and his co-workers (2002), have defined this CEC as the Maximum Exchange Level (MEL). In this study we will be referring to CEC as the operating exchange capacity, the one obtained by an operator for a given cation. Some authors report that operating CEC is strongly dependent on the operating conditions such as flow rate (Q), particle diameter (d) or column geometry (L/D) (Hlaway et al., 1982; Inglezakis, Grigoropoulou, 2003). On the opposite, Lieu and Weber (1981), Helfferich (1995) agree with the basic theory which has stated that the operating CEC for a specific ion exchange system is independent of the experimental conditions applied to the system. The study is designed to investigate the operating CEC according to the operating parameters applied in a fixed bed reactor such as the flow rate, the bed characteristics and the particle diameter of the material involved. At the same time for a better understanding of the FBR process, we will investigate the Unused Bed Zone (UBZ) which is a critical performance parameter bound to operations in FBR (Lau et al., 2003).
2. Theoretical
2Operation in fixed bed columns have been widely described elsewhere (Pansini, 1996). The operation in fixed bed columns leads to the breakthrough curves (Figure 1). This type of curve is the plotting of the cumulated effluent volume (V) against concentration (C). From the breakthrough curves will be drawn the parameters that will permit an interpretation of the operation (Lau et al., 2003).
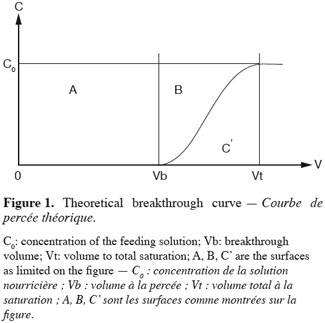
3The Work Exchange Capacity (WEC) which is represented on the figure 1 by the area A. This area expresses the total amount of pollutants trapped over the material before breakthrough occurs. The later happens when a concentration (C) corresponding to a percentage of the initial concentration (C0) in the feed reservoir is detected in the effluent. This percentage of the initial concentration of the pollutant acceptable in the effluent marks the limit of the WEC and is set in advance by the operator. The WEC expressed by mass unit of the material is given by the equation 1:
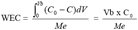
4(1)
5in which Me is the mass of the material used.
6The operating CEC as defined before (Figure 1) corresponds to the sum of the A and B areas which represent the amount of cation kept over the material until complete saturation. The CEC expressed by mass unit of the material is given by the equation 2:
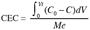
7(2)
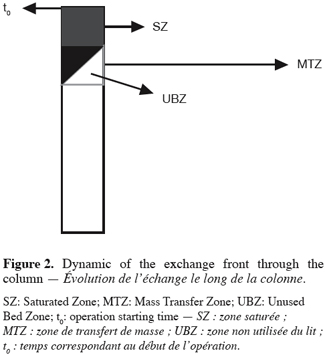
8according to Nastro and Colella (1983), when t > t0, (t0 being the time corresponding to the launch of the operation) a front is constituted and moves along the column toward the end. This front keeps a constant shape during its curse and is made of three parts. This situation is illustrated in the figure 2.
9The Saturated Zone is the exhausted area of the material where exchange has already taken place and equilibrium exists between the solid and the liquid phase.
10The Mass Transfer Zone is the area where exchange is taking place and where exhaustion and equilibrium have not yet been achieved.
11The Unused Bed Zone is the part of the MTZ which has not yet undergone exchange.
12The concept of UBZ is of practical meaning. As seen in figure 2, breakthrough occurs when the front reaches the end of the column. Practically the operator decides to stop the operation at the breakthrough point which is related to a fraction of the initial allowable concentration in the effluent. So, a remaining part of the material is systematically left unused. This fraction is fixed according to the environmental guidelines. This unused part corresponds to the UBZ and is represented by the B area. The UBZ and the MTZ are the main surfaces which when expressed as a ratio of the total surface representing the CEC may well evaluate the FBR performance. The MTZ and the UBZ as defined are dimensionless and are given by the equations 3 and 4:
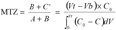
13(3)
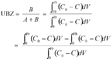
14(4)
15In the case of symmetrical breakthrough curves, the B and C’ areas are identical and then equation 3 becomes:
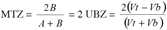
16(5)
3. Materials and methods
3.1. Chemicals
17The metal ion studied was Pb2+ from a 10-3mol.l-1 solution of Pb(NO3)2, (207.2 mg.l-1 of Pb2+). A solution of 0.5 mol.l-1 NaNO3, was used as pre-treatment solution. All chemicals used were of analytical grade from Merck Eurolab (Darmstadt).
3.2. Sorbent material
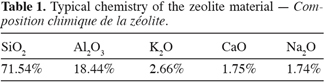
18The sorbent material used in this study was obtained from the « NZ Natural Zeolite, Zeotec Water » from New Zealand. According to the specifications, (X2, 2005) the mineral content was 75% pure clinoptilolite and mordenite with 25% impurities such as Opal C1 and smectite. The specific gravity is 0.65 g.cm-3. The chemical composition of the material is summarized in table 1.
19The material is crushed and sieved to three different particles sizes: 0.65 mm (500 µm < F < 800 µm); 0.38 mm (250 µm < F < 500 µm); 0.20 mm (150 µm < F < 250 µm), it is then washed to eliminate dust, dried at 103°C ± 2°C for 16 h, vacuumed in distilled water to eliminate gas molecules from the pores and packed on the columns.
3.3. Columns
20Glass columns of 0.66 cm internal diameter equipped with adjustable plungers for length control have been used within this study. Three different column lengths 5.0 cm, 8.5 cm and 12.0 cm have been set.
3.4. Pre-treatment
21Near homo ionic Na form of the material was obtained in situ by passing through the column 50 BV (BV: Bed Volume) of the 0.5 mol.l-1 NaNO3 solution at the flow rate of ± 7 BV.h-1. The column was then rinsed in situ with 50 BV of fresh distilled water at the same flow rate.
3.5. Analytical
22The effluent was automatically collected with a LKB Helirac 212 fraction collector in 20 ml glass tubes. The samples were acidified with 1 ml of HCl 2M and analyzed for Pb2+ content by a Metrohm Herissau E 502 polarograph (Switzerland). 6 ml of the acidified samples (0.1M HCl) were placed in the cell and analyzed in differential pulse mode (50 mV pulse, with linear potential variation of 3 mV. sec-1) from - 0.2 to - 0.6 V. The lead peak maximum appears at - 0.38 V (vs Ag/AgCl, saturated KCl). The LOQ was established at 10-8 M in pulse mode.
3.6. Experimental
23The columns were operated in the up-flow mode in order to assure a complete wetting of the material. The feed was delivered to the column by mean of a Waters 515 HPLC pump with a variation of ± 0.1 ml.min-1. The exchange temperature was kept at 25 ± 1°C. The flow rates were taken between 2 and 50 BV.h-1 according to Inglezakis and Grigoropoulou (2003), who recommended between 5 and 15 BV.h-1. Three different bed lengths (L = 5.0 cm; 8.5 cm and 12.0 cm) for a fixed column diameter (D = 0.66 cm) allowed to be roughly in agreement with the general chromatographic principles which require a L/D ratio between ten and twenty. Finally three particles sizes (d = 0.20 mm, 0.38 mm and 0.65 mm) were selected in order to fit the recommendations of Inczédy (1966): D/d must be taken superior to ten. The table 2 lists the technical parameters and the experimentations carried out in this study. These runs are the result of a combination between the parameters and do not strictly follow a rigorous statistical rule. Nevertheless the variability of the experiments is of 6.85% (Fanfan et al., 2005).
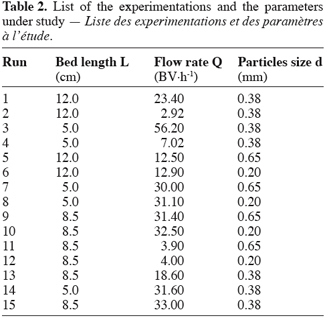
4. Results
24The experimental UBZ and corresponding CEC from the breakthrough curves are summarized in table 3. Practically, for the purpose of the study the breakthrough point has been fixed at 1% (2 mg.l-1) of the initial concentration.
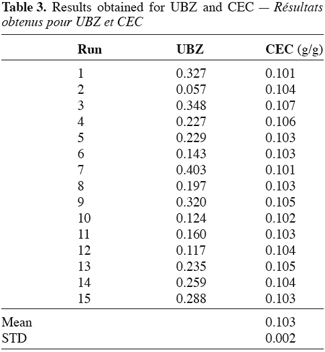
5. Discussion
25As can be seen, it is obvious from the table 3 that the operating CEC of a zeolite operated in fixed bed column, for a given cation does not depend on the operational parameters such as flow rate, column geometry or particles diameters. This finding is in line with Lieu, Weber (1981) and Helfferich (1995) results. For fifteen runs achieved, with different values for the operation parameters the CEC of this material does not vary significantly. The operating capacity of this clinoptilolite has been evaluated to 0.103 ± 0.002 g of lead per gram of material or 1.00 ± 0.015 meq.g-1. The present mean result is in agreement with those found by several authors such as Leppert (1990), for lead retention on clinoptilolite. The explanation could be formulated as follows: the number of sites available on a given mass of the material for a given cation remains the same and all of them will be occupied before complete saturation occurred despite flow rate, particles sizes or bed geometry. This finding according to clinoptilolite and lead is different from others which results seem to prove the reverse (Hashimoto et al., 1977; Inglezakis et al., 2002; Inglezakis, Grigoropoulou, 2003). The reverse is also obtained for ammonia (McLaren, Farquhar, 1973; Jörgensen et al., 1976). Operating CEC could have been affected by the operating conditions especially where enough care hasn’t been taken in order to avoid preferential path ways into the material. Indeed channelling causes an incomplete wetting of the zeolite particles (Hlaway et al., 1982; Guangsheng et al., 1988). Incomplete wetting drives to partial use of the material then to a misevaluation of the real mass used and consequently to different values for the operating capacity. Another case where the CEC could have changed is, if the pre-treatment procedure differs from an experiment to another. Klieve and Semmens (1980) have reported that a clinoptilolite sample treated with sodium hydroxide has a higher CEC.
26As reported before, the UBZ is a good parameter for performance evaluation in a fixed bed reactor. As a matter of fact it can be stated that the lower the UBZ the better the operation. For a given operating CEC a low UBZ drives to a more important WEC and a sharper profile of the breakthrough curve. Figures 3, 4 and 5 give the breakthrough curves for the experimentations with the same amount of material but different operating parameters.
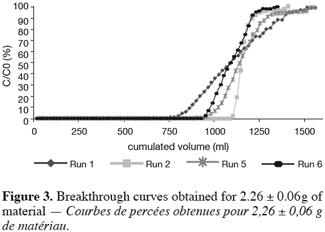
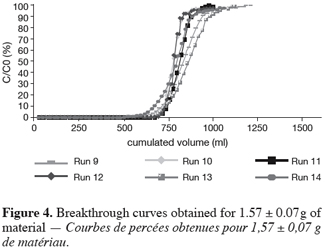
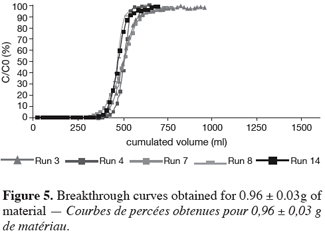
27The fifteen reported curves may be divided into three categories. All the parameters calculated from those curves are fully comparable because they have been reported to the unit mass of material. In every set of curves, the sharper ones are the ones for which better operational conditions have been achieved, leading to a very low UBZ. As shown on figure 3 the run 2 has led to the lowest UBZ (5.6% of the operating CEC). The operational conditions drawn from table 2 were L/D = 18.1 (for L = 12.0 cm and D = 0.66 cm), Q = 2.9 BV.h-1 and particles size d = 0.38 mm (the d/D ratio at 0.057). We can also notice run 1 with the biggest UBZ of 32.7% of the operating CEC. The flow rate for this run is Q = 23.4 BV.h-1 for the same particle to column diameter ratio of 0.057. This observation demonstrates clearly that increasing the flow rate induces a decrease in the operation performance as UBZ. The following figures 6, 7 and 8 illustrate the variation of UBZ according to the three different operational parameters. This has to be considered as roughly informative as no statistical considerations have been made.
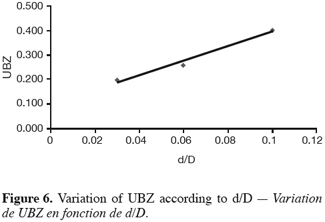
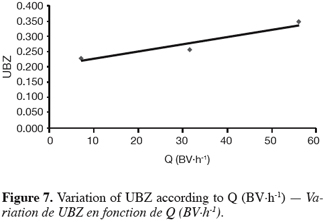
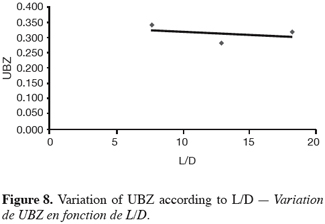
28The results presented in figure 6 (indicating a diminution of UBZ according to the diminution of particles size) are in accordance with the theory: decreasing the particles size improves the performance of the ion exchange process (Hashimoto et al., 1977; Helfferich 1995; Jun-Boum et al., 2002; Inglezakis, Grigoroupoulou, 2004). Decreasing the flow rate improves the performance of ion exchange process; this statement has also been verified by our results (Figure 7) as for Inglezakis and Grigoroupoulou (2004). On the other hand, the increase of the L/D ratio to a certain extent (Figure 8) does not seem improving the ion exchange performances.
6. Conclusion
29The results gathered herein show that the operating capacity of a given material for a given cation operated in fixed bed reactor is constant regardless the operational conditions applied such as the flow rate, the L/D ratio and the d/D ratio. The performance parameter UBZ was also investigated according to the operating conditions and this study shows that it varies with the operational conditions. Although few literature data are related to the use of UBZ as performance parameter in FBR, the results are still comparable to those obtained with the breakthrough volumes, because a diminution of the UBZ leads to an increase of the breakthrough volume. This observation is of critical importance for further scaling up of the process. This paper presents some promising results and trends in understanding the retention behaviour of lead on clinoptilolite zeolite. In a complimentary approach the ongoing study will be extended to other heavy metal cations alone or in combinations and finally transposed to real samples of polluted water.
30Acknowledgements
31The authors would like to acknowledge Doctor Inglezakis VJ., for his scientific support and help, the CUD (Coopération Universitaire au Développement) and the AUF (Agence Universitaire de la Francophonie) for their financial support and the Department of Statistics of Gembloux University.
Bibliographie
Alvarez-Ayuso E., Garcia-Sanchez A., Querol X. (2003). Purification of metal electroplating waste waters using zeolites. Water Res. 37 (20), p. 4855–4862.
Angerville R., Rony FJ., Balthazard K., Emmanuel E. (2004). Assessment of human exposures to lead in drinking water. XXIX Congrès Interaméricain du Génie Sanitaire et des Sciences de l’Environnement, San Juan, Porto-Rico.
Bliefert C., Perraud R. (2001). Chimie de l’environnement air, eau, sols, déchets. Bruxelles : De Boeck Université, 477 p.
Carré JC. (1997). Étude de l’impact des peintures laques et vernis sur l’environnement et la santé. Haïti : Ministère de l’Environnement & l’OPS/OMS, 36 p.
Fanfan P., Mabon N., Carletti I., Claustriaux JJ, Thonart P., Lognay G., Copin A., Barthelemy JP (2005). New model for performance prediction in fixed bed reactors based on the approach of the unused bed zone. Water Res. 39, p. 4004–4010.
Guangsheng Z., Xingzheng L., Guangju L., Quanchang Z. (1988). Removal of copper from electroplating effluents (potch water) using clinoptilolite. In: Kallo D., Sherry HS. Occurences, Properties and Utilization of Natural Zeolites. Budapest : Akademiai Kiado, p. 529–539.
Hashimoto K., Miura K., Tsukano M. (1977). Experimental verification of design methods for liquid phase fixed bed absorbers. J. Chem. Eng. Jpn 10, p. 27–34.
Helfferich F. (1995). Ion Exchange. New York, Dover, p. 421.
Hlaway J., Vigh G., Olaszi V., Inczedy J. (1982). Investigations on natural Hungarian zeolite for ammonia removal. Water Res. 16, p. 417–420.
Inczédy J.(1966). Analytical applications of ion exchangers. Budapest : Akademiai Kiado, p. 1–80.
Inglezakis VJ., Grigoropoulou H. (2004). Effects of operating conditions on the removal of heavy metals by zeolite in fixed bed reactors. J. Hazardous Mater. B112, p. 37–43.
Inglezakis VJ., Grigoropoulou H. (2003). Modeling of Ion Exchange of Pb2+ in Fixed Beds of Clinoptilolite. Microporous and Mesoporous Mater. 61 (1-3), p. 273–282.
Inglezakis VJ., Loizidou MD., Grigoropoulou H. (2002). Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res. 36, p. 2784–2792.
Jörgensen SE., Libor O., Graber KL., Barkacs K. (1976). Ammonia removal by use of clinoptilolite. Water Res. 10, p. 213.
Jun-Boum P., Seung-Hak L., Jae-Won L., Chae-Young L. (2002). Lab scale experiments for permeable reactive barriers against contaminated groundwater with ammonium and heavy metals using clinoptilolite. J. Hazardous Mater. 95 (1-2), p. 65–79.
Keane MA. (1998). The removal of copper and nickel from aqueous solution using Y zeolite ion exchanger. Colloids and surfaces A. Physicochem. Eng. Aspects 138, p. 11–20.
Klieve JR., Semmens MJ. (1980). An evaluation of pretreated natural zeolites for ammonium removal. Water Res. 14, p. 161–168.
Lau A., Crittenden BD., Field RW. (2004). Enhancement of Liquid Phase Adsorption Column Performance By Means of Oscilatory Flow: an experimental study. Sep. Purif. Technol. 35 (2), p. 113–124.
Leppert D. (1990). Heavy metal sorption with clinoptilolite zeolite : alternatives for treating contaminated soil and water. Mining Eng. 42 (6), p. 604–608.
Lieu KT., Weber WJ. (1981). J. WPCF 53, p. 1541.
McLaren JR., Farquhar GJ. (1973). Factors affecting ammonia removal by clinoptilolite. J. Environ. Eng. Div. EE4, p. 429–446.
Nastro A., Colella C. (1983). Column ion exchange data for ammonium removal water by phillipsite tuff. Ing. Chim. Ital. 19 (5-6), p. 41–45.
Pabalan RT. (1994) Thermodynamics of ion exchange between clinoptilolite and aqueous solutions of Na+/K+ and Na+/Ca2+. Geoch. Cosmochim. Acta 58, p. 4573–4590.
Pansini M. (1996). Natural Zeolites as Cation Exchangers for Environmental Protection. Mineral Deposita 31, p. 563–575.
Perić J., Trgo M., Vukojević Medvidović N. (2003). Removal of zinc, copper and lad by natural zeolite - a comparison of adsorption isotherms. Water Res. Artic. Press.
Rodríguez-Iznaga I., Gómez A., Rodríguez-Fuentes G., Benítez-Aguilar A. (2002). Natural clinoptilolite as an exchanger of Ni2+ and NH4+ ions under hydrothermal conditions and high ammonia concentration. Microporous Mesoporous Mater. 53 (1-3), p. 71–80.
X1, US Environmental Protection Agency (2005). http://www.epa.gov/dclead. Consulted on 18/08/05.
X2, NZ Natural Zeolite Zeotec Water (2005). http://www.zeolite.co.nz/what.html. Consulted on 11/02/05.
Para citar este artículo
Acerca de: Pierre Naïder Fanfan
Department of Analytical Chemistry. Gembloux Agricultural University (FUSAGx). Passage des Déportés, 2. B-5030 Gembloux (Belgique). E-mail : fanfan.p@fsagx.ac.be
Acerca de: Nicolas Mabon
Department of Analytical Chemistry. Gembloux Agricultural University (FUSAGx). Passage des Déportés, 2. B-5030 Gembloux (Belgique).
Acerca de: Philippe Thonart
Walloon Centre of Industrial Biology (CWBI). Passage des Déportés, 2. B-5030 Gembloux (Belgique).
Acerca de: Georges Lognay
Department of Analytical Chemistry. Gembloux Agricultural University (FUSAGx). Passage des Déportés, 2. B-5030 Gembloux (Belgique).
Acerca de: Alfred Copin
Department of Analytical Chemistry. Gembloux Agricultural University (FUSAGx). Passage des Déportés, 2. B-5030 Gembloux (Belgique).
Acerca de: Jean-Paul Barthélemy
Department of Analytical Chemistry. Gembloux Agricultural University (FUSAGx). Passage des Déportés, 2. B-5030 Gembloux (Belgique).






