- Home
- Volume 18 (2014)
- numéro 4
- The role of olfaction in wireworms: a review on their foraging behavior and sensory apparatus
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
The role of olfaction in wireworms: a review on their foraging behavior and sensory apparatus

Editor's Notes
Received on April 9, 2014; accepted on October 10, 2014
Résumé
Dévoiler le rôle de l’olfaction chez les larves de taupins : synthèse bibliographique sur leur recherche de nourriture et leur appareil sensoriel.
Introduction. La lutte intégrée contre les larves de taupins (Coléoptères : Elateridae) dépend d’approches à différentes échelles spatiales, du paysage à la rhizosphère. Lorsqu’ils cherchent leur nourriture, ces ravageurs souterrains utilisent des signaux biotiques et abiotiques pour s’orienter vers leur cible. Afin de développer des techniques efficaces utilisables en lutte intégrée, les connaissances relatives à ce processus devraient être améliorées. En particulier, une étape importante consiste à élucider le rôle des composés organiques volatils (COV) émis par les organes souterrains de ces plantes dans l’écologie chimique des larves. De telles informations seraient applicables dans des stratégies de type push-pull et en sélection variétale, développées à l’encontre des taupins.
Littérature. Cet article résume les données disponibles relatives au comportement de recherche de plante-hôte des larves de taupin, ainsi que les variables à prendre en compte lors de la caractérisation du rôle potentiel des COV produits par les plantes et impliqués dans ce comportement. Ces facteurs incluent les gradients de CO2, ainsi que les autres sémiochimiques liés à l’hôte, la température, les humidités relative et réelle du sol, ainsi que l’état physiologique des larves. Les connaissances relatives à l’appareil sensoriel des larves sont également résumées puisque ce dernier est impliqué dans chaque étape d’orientation vers la plante cible.
Conclusion. Des données de base permettant l’étude du rôle des COV dans la recherche de nourriture chez les larves de taupins existent. Les utiliser comme outils en entomologie appliquée devrait résulter en la découverte de sémiochimiques soutenant les interactions trophiques impliquant ces ravageurs. Cependant, la plupart des espèces problématiques ne sont pas décrites complètement, eu égard de ces paramètres. Remplir les inconnues, avec précision, sera nécessaire afin de définir de nouvelles stratégies de lutte intégrée.
Abstract
Introduction. Integrated management of wireworms (Coleoptera: Elateridae) depends upon approaches applied both above- and belowground, and over several spatial scales. While foraging, these soil pests use biotic and abiotic signals to orientate towards target plant organs. Development of efficient techniques for implementation in integrated strategies relies upon improved knowledge of this process. In particular, an important step consists of elucidating the role of volatile organic compounds (VOC), emitted by belowground plant organs, in wireworm chemical ecology. This would have a positive impact on push-pull strategies and varietal selection developed against these insects.
Literature. In this work, we summarized the available data regarding wireworm foraging behavior as well as variables that should be considered when studying the potential role of plant-produced volatile semiochemicals. This includes CO2 gradients and other host-related cues, temperature, relative humidity and soil moisture, and wireworm physiological stage. We also review what is known of the sensory apparatus of wireworms, since this is involved in every step of the foraging process.
Conclusion. Some baseline data for studying VOC related wireworm foraging behavior exists. Using it as a tool in applied entomology should result in discovery of the semiochemicals that underpin trophic interactions involving these pests. However, most of the key pest species are not fully described with regards to the parameters detailed here. Obtaining accurate information to fill the current knowledge gaps will be needed in order to devise new integrated management strategies.
Table of content
1. Introduction
1Multi-trophic interactions involving herbivorous insects and their host plants are mediated by semiochemicals; compounds conveying information, including, notably, the nature or the physiological state of the host (Vet et al., 1992; Kessler et al., 2001). Many insect pests have been studied in this regard in order to develop integrated pest management (IPM) strategies, leading to efficient crop protection without resorting to synthetic pesticides (Cook et al., 2007). To date, the majority of scientific papers on this subject concern aboveground interactions. Indeed, on the Scopus search engine (www.scopus.com, consulted on 24.07.2014), insect-plant interactions are addressed in 10,365 documents, among which 454 discuss semiochemicals (4.4%). Of these, only 143 concern roots (32%), and 115 root pests (25%). However, the latter are attracting increasing attention from the scientific community, and approaches based on semiochemical volatile organic compounds (VOC) in aboveground interactions are now also being applied to soil pests. A recent review outlined the role of VOC for soil insects, and reported compounds with semiochemical properties for a wide variety of pest species (Johnson et al., 2012). The model “maize (Zea mays L.) - Diabrotica virgifera virgifera (LeConte) larvae (Coleoptera: Chysomelidae)” constitutes a very good example of how belowground interactions can be as complex and multi-trophic as aboveground ones (Rasmann et al., 2005; Hiltpold et al., 2008; Robert et al., 2012). Many studies also report on the impact of root pests in aboveground interactions with other pests, as reviewed in Erb et al. (2008).
2The chemical ecology of belowground pests, including the roles of root-emitted VOC in pest-host plant interactions, is still relatively unknown. In particular, data concerning wireworms, belowground pests and larval stages of click beetles (Coleoptera: Elateridae) is scarce (Johnson et al., 2012). Large-scale approaches to achieve wireworm control could benefit from new insights at the individual scale. Since new alternative management strategies against these insects are needed, research on their chemical ecology and foraging behavior, notably under the influence of belowground plant VOC, is also needed (Barsics et al., 2013). This paper summarizes knowledge on:
3– the foraging steps, known compounds, and abiotic parameters involved in wireworm activity;
4– the functions and anatomical locations of sensory appendages in wireworms.
5The information already available constitute a solid base to investigate the role of VOC in wireworm behavior, but important and accurate information are still missing.
2. The foraging behavior of wireworms
2.1. First step: the role of carbon dioxide
6The orientation of wireworms and other belowground pests towards host plants is seen as a three-step sequence influenced by soil nature and structure, abiotic parameters and other soil organisms (Johnson et al., 2012). In wireworms, the feeding phase of each instar only lasts a short time, i.e. 20-29% of the whole development, with the rest being devoted to molting metabolism (Evans et al., 1942; Furlan, 1998; Furlan, 2004). Independent of chemical stimulations, wireworms follow lines of least resistance (Thorpe et al., 1946). This behavior changes in response to encountered gradients of CO2, a very efficient general semiochemical indicating food location (Johnson et al., 2012). Several species in the genus Agriotes Eschscholtz, can locate excised plant roots or CO2 sources (e.g. Klingler, 1957; Doane et al., 1975). The most efficient baits for these species are germinating grains such as wheat, corn and barley (e.g. Parker, 1996; Simmons et al., 1998). These larvae are also strongly attracted to seeds of Medicago sativa L. (alfalfa), Brassica napus L. (rape), Melilotus alba Desv. (sweet clover) and Helianthus annuus L. (sunflower) (Doane et al., 1978). Other wireworm species also respond to germinating grains: wheat and barley strongly attract Limonius canus (LeConte, 1853) (Horton et al., 2002), while rice attracts Melanotus okinawensis (Ôhira, 1982) (Arakaki et al., 2009). In terms of sensitivity, the response threshold of Ctenicera destructor (Brown, 1935) wireworms to CO2 gradients has been reported to be as fine as 1 to 2 ppm over the distance involved in one deflection of the head during klinotactic orientation (Doane et al., 1975), demonstrating the importance of such a signal. Attraction occurs at concentrations of between 360 ppm and 15,000 ppm but depends on the physiological stage: it decreases with proximity to ecdysis (Doane et al., 1975; Doane et al., 1978). In laboratory conditions at between 22 and 25 °C, Agriotes larvae may take up to 70 min to find a CO2 source located 20 cm away (Doane et al., 1978). Repellency may also occur under gradient steepness or overexposure (Doane et al., 1975; Doane et al., 1978).
2.2. Second step: attraction towards host-specific semiochemicals
7The next foraging step for belowground herbivorous insects involves plant-originating substances, notably VOC, allowing host-specific recognition (Johnson et al., 2012). Foraging behavior is impacted by the nature and gradient of concentration of the semiochemical blends wireworms are exposed to (Thorpe et al., 1946; Crombie et al., 1947). A preference for grass species from nutrient-rich grasslands (Lolium perenne L. and Holcus lanatus L.) over species from nutrient-poor grasslands (Festuca rubra L. and Anthoxanthum odoratum L.) has been shown for Agriotes obscurus (L.), with the hypothesis that specific cues other than CO2 underpin this discrimination (Hemerik et al., 2003).
8To classify compounds likely to induce a response in wireworms, one can use two measures: the compound threshold of response and its activity. The first is the lowest concentration needed for a response to occur. The second is defined as minus the logarithm of the threshold, e.g. 9 is the activity of a compound with a threshold of response of 10-9 g·ml-1 (Thorpe et al., 1946; Crombie et al., 1947). A second way to discriminate between compounds is related to induced responses. There is a distinction between compounds eliciting solely orientation responses, mainly involved in the second foraging step, and those eliciting both biting and orientation. In almost all cases, compounds eliciting biting responses have high activities (i.e. with thresholds between 10-9 and 10-11 g·ml-1), while compounds eliciting only orientation are less active (i.e. with thresholds close to 10-3 g·ml-1) (Crombie et al., 1947). These thresholds are 1,000 fold higher than that of CO2 (see above), and suggests that such compounds need to be emitted or produced in higher amounts than biting-eliciting compounds to be used by wireworms, since orientation occurs before biting in the foraging process (Thorpe et al., 1946). It can also indicate at what scale, or from what distance from the target organs, these compounds may influence foraging, provided that their diffusing properties are known.
9To our knowledge, the first molecules highlighted as inducing orientation in wireworms consist of a group of sugars, peptone, triolein and tannic acid each of activity 2-3, and of a group of acids and amides widely distributed in plants, with higher activities (Thorpe et al., 1946). All amides tested by Crombie et al. (1947), as well as urea and guanidine, can attract wireworms. The response is stimulated by chemical structures including -CONH2 or -CNHNH2, but not -CNH2 (indicated by the inactivity of glycine and alanine). Asparagine and glutamine for example, are ubiquitous in plants, reaching high concentrations in certain seedlings as secondary products of seed protein breakdown. Arginine is found in considerable amounts in some seedlings, and is widely distributed at lower concentrations in mature plants (Crombie et al., 1947). This is consistent with constituents of wireworm preferential target plant-organs: mesocotyls, seeds, and seedling (Chaton et al., 2008). Some dicarboxylic acids are also active, notably citric, succinic and malic acids, compounds involved in oxidative respiration in plants. There is not, however, a clear rule for predicting that a given dicarboxylic acid will be active (Crombie et al., 1947). In Agriotes species, there is only one report of VOC inducing wireworm orientation, attributed to 2-pentylfuran (Barsics et al., 2012), a compound listed in the volatile profile of barley roots (Fiers et al., 2013; Gfeller et al., 2013). Finally, regardless of the semiochemical, the sensitivity for orientation increases with starvation duration for seven days (Thorpe et al., 1946). As wireworms approach target organs, the third foraging step starts.
2.3. Final step: biting and retention in the root system
10Most of the compounds capable of inducing biting are members of the three major food groups: carbohydrates, fats and proteins (Thorpe et al., 1946). The most active ones are commonly found in plant sugars, plant juices (e.g. potato, sugar-beet), or, such as starch, in seeds (Thorpe et al., 1946; Chaton et al., 2008). In Agriotes lineatus (L.) and A. obscurus, appetence is equivalent for seed flours of corn, wheat, walnut and sunflower (Chaton et al., 2003). In sugars, the polyhydric alcohol groupings were found to be responsible for induced activity (Thorpe et al., 1946). Triolein is the only triglyceride that induces biting responses even if sodium salts of certain fatty acids are active as well (Thorpe et al., 1946). Active proteins are of animal origin; tested plant proteins are inactive. Partially broken proteins may be active although the parent proteins are not, but no amino acids or mixtures thereof have shown activity (Thorpe et al., 1946). As for orientation, wireworm sensitivity to biting compounds is progressively increased with starvation up to seven days (Thorpe et al., 1946). These properties may extend to other Agriotes species or other genera.
11The biting response is strongly involved in the final step of the foraging process. Unlike compounds causing only orientation, those inducing biting (and therefore also orientation: see above) seem to retain wireworms in the area in which they are detected, with some exceptions. Indeed, although asparagine and related substances are described as orientation compounds, wireworms are extremely sensitive to these substances. They increase the target nature of a root system by causing wireworms to remain in the vicinity of fine roots (Thorpe et al., 1946).
12It was shown that, although inactive individually at certain concentrations, some compounds can induce a response when exposed simultaneously. For example, solutions of 0.5% glucose and 0.126% sucrose are inactive individually, but are active when mixed (Crombie et al., 1947). The following compounds are synergistic: glucose and sucrose for biting and orientation; glucose and peptone, glucose and triolein, peptone and triolein, glucose and tannin for biting (Crombie et al., 1947). It is therefore important to consider as complete and accurate a blend as possible when assessing the effect of substances emitted by plants in the soil on wireworm behavior (De Bruyne et al., 2008). As we have seen above, chemosensory cues encountered before the vicinity of roots and at the root surface can induce acceptance. Conversely, some compounds inhibit activity or repel insects (Johnson et al., 2012), which emphasizes the need to consider the entire chemical environment. For example, the glucose-induced biting response can be inhibited by lead acetate, quinine, allyl-isothiocyanate, and common salt, as well as by acid and alkaline solutions (Crombie et al., 1947). Common salt also inhibits the glucose-induced orientation, but not asparagine-induced orientation (Crombie et al., 1947). Known natural deterrents are chalconine, solanine and glycoalkaloids in potato (Jonasson et al., 1994). However, field experimentations have proved that glycoalkaloid content alone could not be the sole mechanism underpinning wireworm preference among different potato varieties (Johnson et al., 2008). Finally, repulsion due to insecticides has been reported, either with synthetic compounds such as bifenthrin (van Herk et al., 2013a) or in soils treated with the plant derived biopesticide azadirachtin (Cherry et al., 2010), and their effect seems to extend beyond foraging behavior.
2.4. Factors affecting wireworm movement and activity
13In the sections above, we depicted what is known of the chemical background for foraging. Factors other than the compounds themselves will impact the foraging process by affecting wireworm movement or activity. Although not assessed for all of the important wireworm pest species, speed of movement may vary between them. In similar conditions, some species tend to be found further from a given release point than others, as observed for Athous haemorrhoidalis (Fabricius, 1801) over A.obscurus (Hemerik et al., 2003). For certain species, in situ dispersal abilities have been assessed. For Melanotus okinawensis, mark-recapture methods allowed estimating the lifetime natural mean dispersal distance to 105.6 ± 20.1cm (Arakaki et al., 2010). Another in situ method, based on stable isotope signatures of different crops, has revealed that the larvae of A. obscurus will not move laterally in the ground as long as their food supply is sufficient (Schallhart et al., 2011). It has been suggested that horizontal migration will only occur if necessary, i.e. in case of food depletion, because it represents an important energy loss (Sonnemann et al., 2014). Moving rates depend on factors such as temperature (Campbell, 1937; van Herk et al., 2013b), physiological stage, as reported for A. obscurus (van Herk et al., 2013b), and relative humidity, soil moisture and pH (Lees, 1943a; Lees, 1943b; Thorpe et al., 1946). In addition, individual behavior may be modified under high densities of individuals or when food is scarce, which sometimes leads to cannibalism (Furlan, 1998; Hemerik et al., 2003; Traugott et al., 2008).
14Relative humidity (RH) and real water content have slightly different effects on wireworm activity. The optimal RH level for Agriotes wireworms is air saturation; lower RH areas are avoided (Lees, 1943a). In fact, dry air is more intensely avoided when alternatives are closer to saturation. A difference of 7.5% RH (17 °C) suffices to ensure total avoidance of lower RH areas (Lees, 1943a). Moving air or humid air currents have been shown to be attractive to wireworms, although they are not as efficient as carbon dioxide (Doane et al., 1978). Similarly, wireworms migrate rapidly out of dry soil and aggregate in areas with sufficient moisture (Lees, 1943b). From field observations, it is known that wireworms kept in dry soil rapidly die of desiccation. In sandy-loam, the optimal water content ranges from 8% to 20% with an apparent preference for 12-16% (Campbell, 1937). Conversely, water-saturated soil can induce complete discontinuance of activity and sometimes death (Campbell, 1937). Moisture inhibits all muscular activity, including that of mouth-parts. Incidentally, the feeding activity of Agriotes larvae is greater at low moistures compared to higher ones (tested ranges: 10-80%), which is linked to both inactivity in high moistures and the consequent failure to reach food (Lees, 1943b).
15Temperature influences wireworm activity, behavior, and distribution. As for many insects, higher temperatures lead to increased activity and lower ones result in inactivity in wireworms. Extremes lead to death, and larval development rate is strongly related to intermediate temperatures (Campbell, 1937; Furlan, 2004). Throughout their multiyear development cycle, field activity of wireworms is particularly high in spring and fall. With sufficient soil moisture and food, it continues throughout the summer, and decreases in the winter with a temperature-related intensity (Campbell, 1937; Furlan, 2004). One given species will complete its cycle with a different development rate according to latitude. In the winter in Italy, at 41°N compared to 45°N, Agriotes sordidus (Illiger, 1807) larvae continue their development in the food-rich top soil layers instead of burrowing deep into the soil in search of more suitable temperatures (Furlan, 2004). In Southern California (latitudes 34 to 36°N), Limonius californicus (Mannerheim) wireworms never cease their activity entirely (Campbell, 1937). Apparently, in this species, adjustment to the most suitable temperatures will not occur until larvae have been subjected to the changing (lower or higher) temperatures for a month or more (Campbell, 1937). It was shown in rearing conditions that A. sordidus does not grow below 9 °C. In contrast, a constant temperature of 29 °C allows it to complete its lifecycle in four months, starting from the eggs (Furlan, 2004). Developmental rate will also depend on whether or not wireworms previously underwent a period of cold in the winter (Furlan, 2004). Finally, temperature influences the appetence of wireworms; A. obscurus will destroy a higher number of seedlings at 22 °C compared to lower temperatures in similar experimental conditions (van Herk et al., 2013b). As temperature influences wireworms in so many ways, it is one of the key parameters to control during rearing and behavioral assays.
16Gravity has been suggested to exert some influence: when stimulated by an unfavorable, dry environment, the natural movement trend of wireworms is downward, as suggested for L. californicus (Campbell, 1937). However, burrowing Agriotes wireworms do not respond to gravity (Lees, 1943b). Wireworms only occasionally walk on the soil surface, but it does not seem possible to detect any response to odors under such conditions (Thorpe et al., 1946). Physicochemical properties of the substrate also are significant. Movement direction is determined by soil structure (Thorpe et al., 1946) and soil pH can affect wireworm feeding, through changing the activity of compounds, regardless of the nature of the induced response. For example, the pH activity curve for glucose ranges between 6 and 8, while most plant juices have a pH between 5 and 7 (Thorpe et al., 1946).
2.5. Recommendations for behavioral bioassays
17This section details optimal conditions for behavioral assays on wireworms, especially when evaluating the impact of plant VOC.
18Odor stimuli contain three elements of information: identity, intensity, and temporal variation of the latter. Moreover, if an odor is a mixture, its identity can change if the mixture changes (De Bruyne et al., 2008). In behavioral experimentations on wireworms, in a static environment, concentrations of tested blends, as well as ratios between compounds, should therefore be realistic. It requires prior quantification of root VOC emission and/or content and adequate VOC releasing formulations. Also, the devices and substrate must be strictly controlled with regards to known active substances, and insecticide-free plants should be used whenever live material is needed. Considering the importance of carbon dioxide in foraging, its concentration must be at least controlled, and should match soil concentrations. Using these conditions, results should highlight VOC soil concentrations that can impact behavior. In addition, selection of individuals in the feeding phase is possible with baiting techniques, e.g. carbon dioxide sources themselves (Doane et al., 1978).
19In the literature cited, experimentations were performed in a substrate and/or under reduced light conditions. In novel experimentations, these two elements should be consistently combined. As for any other insect, all abiotic parameters should be uniform, both spatially and temporally. But in the particular case of wireworms, which have limited feeding phases, abiotic factors could be more important than tested volatile cues. Olfactometric devices should be designed in a horizontally balanced way. It is not clear whether gravity impacts wireworm movement, but it can modify soil moisture distribution and so indirectly affect behavior. Moreover, moisture should represent less than 20% of the substrate and dry air and soil should be avoided. Temperature regulates wireworm activity in the short and the long term. Although laboratory conditions are often constant, temperature-related life history traits have to be taken into account when planning experimentations with wireworms collected from the field. Rearing conditions should match temperatures needed for activity and remain relevant to the reality of the field. In addition, when assessing the role of VOC, there will be a compromise between wireworm activity and optimum temperatures for chemical diffusion. If gradients disappear due to fast diffusion, attractant or repellent effects may be erased or biased, which would make any conclusions unreliable.
20Uniformity is also important among tested individuals. As much as possible, they should belong to one instar or to a known range of instars, and be in a feeding stage. The cropping history of fields in which the individuals were collected should be known, and areas with no or non-recent history of pesticide use should be preferred for collection. Species-specific foraging speed and interactions with other organisms in the soil community must be accounted for. While foraging speed will determine the experimental conditions suitable to observe behavioral effects, especially timing and temperature, species interactions will shed light on impacts of congeners or conspecifics on foraging behavior. These factors are important for designing management strategies. To our knowledge, nothing is known of the direct or indirect (e.g. through the host plant) effects of other soil organisms on the behavior of wireworms. However, there is evidence for specific aggregation patterns, notably at the field scale, as well as recurrent proximity between given taxa at different spatial scales, such as observed for A. obscurus and non-Agriotes wireworms (Benefer et al., 2010). A further consideration is that of the impact of aboveground organisms on belowground wireworm behavior, mediated through host-plants. However, providing accurate explanations concerning mechanisms controlling such multi-trophic systems involving wireworms necessitates prior investigations, as described.
3. Wireworm chemoreceptors
21Not all substances released by a plant extract necessarily activate chemoreceptors and further induce a specific behavior (Agelopoulos et al., 1999). Electrophysiological assays, including electroantennography (EAG) and single cell recordings, allow discrimination between active compounds and others. This requires accurate description and location of all sensory receptors (Zacharuk et al., 1991). Once determined, relevant blends of active compounds can be tested as to their impact on insect behavior (Agelopoulos et al., 1999). In larvae, nerve impulses induced by signals from the environment can be recorded notably from under-developed antennae, labial or maxillary palps. Some studies link sensilla to feeding-related responses after organ ablation, as shown for carbon dioxide detection in wireworms (Doane et al., 1978). Research increasingly correlates feeding behavior with electrophysiological responses, particularly of sensilla involved in gustation. Similar studies on larval olfaction are more limited (Zacharuk et al., 1991), though a recent study on olfaction in belowground larvae of Melolontha melolontha (Coleoptera: Melolonthidae) combined morphological descriptions from SEM (scanning electron microscopy) and electrophysiological recordings (Eilers et al., 2012); techniques that would be useful for similar investigations in wireworms.
22Larval sensilla are often difficult to categorize. In table 1, we provide descriptions and functions of sensilla types commonly found on immature insects to contextualize the following discussion on knowledge related to wireworm chemosensory receptors. This table is based on sensilla classification followed by Zacharuk et al. (1991) for immature insects. Table 2 gives anatomical locations and functions of sensilla found on wireworms. It is organized based on the work of Zacharuk (1962), who provided details for twelve wireworm species found in different habitats.
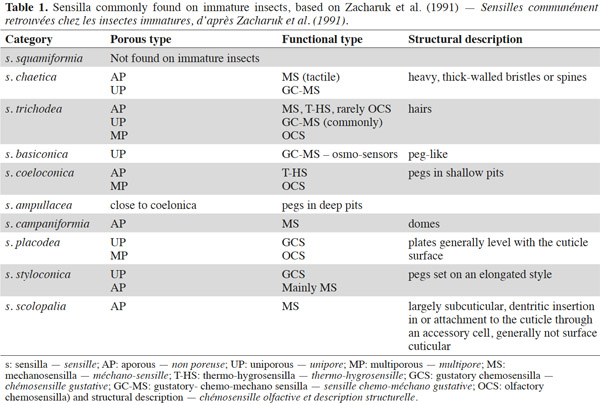
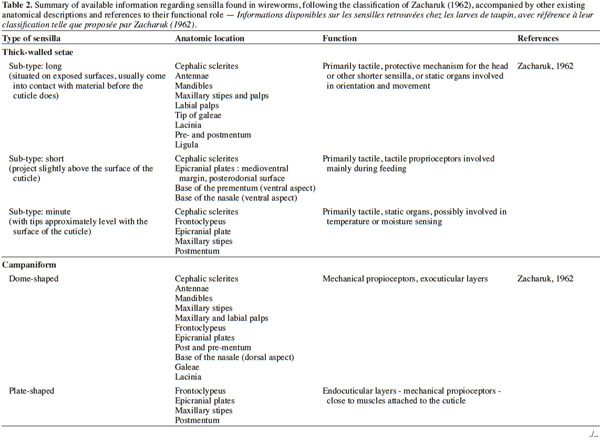
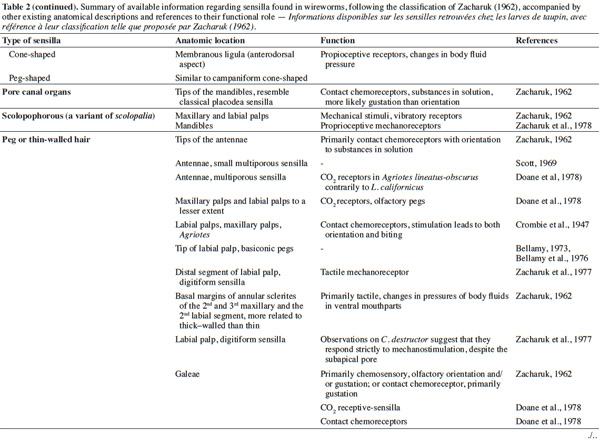
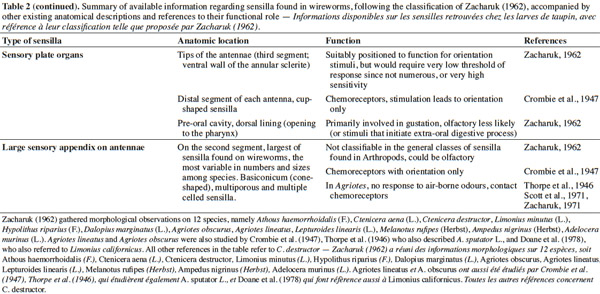
23Multiporous (MP) olfactory sensilla in larvae are not as numerous as in mature insects, but still play an important role (Visser, 1986). Many larvae have large composite MP sensilla on their antennae, generally made of a distinct scape and pedicel, and a one-segmented flagellum (Zacharuk et al., 1991), such as observed on wireworms (Zacharuk, 1962). Ctenicera destructor (Brown) antennae, has two types of MP sensilla. One is a composite basiconicum sensilla, abundantly perforated by a slit-tubule system (Scott et al., 1971), made of 36 neurons grouped by bundles of three. Each of them is ensheathed by an individual inner sheath cell; 12 intermediate and 12 outer sheath cells subtend and enclose a common outer sinus around the neuronal bundles (Scott et al., 1971). The second one is an individual peg-like MP sensilla made of two neurons (Scott, 1969; Scott et al., 1971). Three peg-like sensilla are also found on wireworm labial palps, which contain a total of nine neurons (Bellamy, 1973).
24Many uniporous (UP) peg and hair sensilla have one neuron whose dendrite ends in a tubular body at the base of the sensory cuticle. Those found on antennae are considered to be dually chemo-mechanosensory (Zacharuk, 1985). Ctenicera destructor wears four of these sensilla, with a total of 17 chemosensory and three mechanosensory neurons (Zacharuk, 1971).
25Electrophysiological assays applied on digitiform sensilla (pegs) of C. destructor labial palps suggest that they respond strictly to mechanostimulation, despite the subapical pore (Zacharuk et al., 1977). However, according to Bellamy (1973), the labium possesses a total of 20 such sensilla, provided with 39 chemosensory and 19 mechanosensory neurons. Electrophysiological assays performed on these organs are therefore candidates for mechanical biases. In Agriotes wireworms, known contact chemoreceptors are of two kinds: peg organs, located on labial, maxillary palps and galea, whose stimulation leads to both orientation and biting; and a cup-shaped sensilla on the distal segment of each antenna, whose stimulation leads to orientation only (Crombie et al., 1947). Finally, antennae, maxillary palps, and labial palps notably have the ability to detect variations of relative humidity (Lees, 1943a).
26Only a few compounds are known to stimulate these described sensilla. MP sensilla on the antennae of L. californicus do not appear to be CO2 receptive. CO2 receptors, mainly olfactory pegs, are located on the maxillary palps and on labial palps and to a lesser extent, on labial palps. However, on A. lineatus and A. obscurus larvae, CO2 receptive-sensilla are not restricted to any one of the antennae, maxillary palps, labial palps, or galea, because amputation of these alone does not suppress the orientation response. Contact chemoreceptors found on Agriotes wireworms are stimulated by asparagine and glucose (Crombie et al., 1947). Stimulating molecules reaching receptive chemo-sensilla typically diffuse to the dendritic terminations through a single terminal pore (UP sensilla) or the many pores (MP sensilla) of the chemical conduction system. The form and function of the sidewall pore canal system is modified into a pore tubule system in many MP sensilla (Zacharuk, 1985), clearly distinct in an elaterid larva (Zacharuk, 1971). The sensory tubules are lipoidal; they ensure moisture conservation in the sensillum and channel stimulating molecules through the cuticle to the lymph in the receptor cavity. Dendrites seem to gain contact with signal molecules through the receptor lymph (Zacharuk, 1971). Nothing in the literature deals with the nature of stimulant receptor sites, ion constituents, channels and pumps, or probable enzymes and second messengers in sensilla of wireworms (Zacharuk et al., 1991).
27To conclude, most of the descriptions cited here concern C. destructor wireworms (Zacharuk, 1962; Scott et al., 1971; Zacharuk, 1971; Bellamy, 1973; Zacharuk et al., 1977; Zacharuk, 1985), while table 2 refers to at least 12 different species. According to Zacharuk (1962), there are only a few anatomical differences across species, with variation in number and size being the most important for the large antennal sensory appendix. We have seen above that some of the functions may not be conserved from one appendix to another, or across species, as for CO2 receptive sensilla. It seems useful to proceed to complete and specific descriptions, starting with morphological aspects. The C. destructor background could be upgraded with SEM tools, or transmission electron microscopy (TEM), as suggested by Zacharuk et al. (1991), in order to provide complete sensory maps. This could be done following the morphological approach of Eilers et al. (2012). Where detailed morphology exists, function should be assessed by directly testing rather than by comparison to structure and putative function of similar sensilla in the literature. More collaborative or corroborative studies by morphologists and physiologists would alleviate this deficiency (Zacharuk et al., 1991).
28Furthermore, all aspects of the sensory channel should be studied, starting with the role of each of the neurons in sensilla. Concerning olfaction, the scope and accuracy with which odors can be identified is determined by how many olfactory receptor neurons (ORNs) there are and how they are tuned to different chemicals (De Bruyne et al., 2008). Identifying genes coding for odorant binding proteins in wireworms, through studying complete genomes of close and distant species, would allow the search for common sensory abilities between them. The electrophysiological approach, although far from being complete currently, is complementary to behavioral bioassays, as it indicates where to search for behavioral responses, and reinforces the relevancy of such experimentations. It has been acknowledged that the convergence of genomic, physiological, and ecological data will elucidate the physiological and genetic bases of odor-mediated behaviors (De Bruyne et al., 2008).
4. Conclusions
29The application of semiochemicals in pest management strategies has received increasing interest in recent years (Agelopoulos et al., 1999; Cook et al., 2007). For several belowground pests, including wireworms (Barsics et al., 2013), such approaches still necessitate discovery of compounds involved in their chemical ecology (Johnson et al., 2012).
30For the wireworm species detailed here, existing data on parameters influencing foraging steps, or morphological description and location of their sensilla, are not sufficiently complete or up to date. A model species could be used for complete description. There are sufficient methods in the literature that allow definition of threshold temperatures influencing activity or movement rates, as well as morphological descriptions. It seems that focusing research on the foraging process itself, at the individual scale, necessitates pooling such protocols together to provide researchers with a solid and holistic reference. Consequently, we suggest that the impact of temperature, which seems to have the most variable effect across species and populations from different geographical locations, should be assessed following the work of Furlan (2004) and van Herk et al. (2013b). Concerning the complete morphological descriptions of the sensory arsenal on head appendages, the work of Eilers et al. (2012) constitutes a perfect example of what should be applied to wireworms. Starting with a morphological approach, the achievement of electrophysiological experimentations would be more accurate, and allow a faster highlight of compounds detected by the larvae. There is a wide range of available chemical analysis for profiling plant-produced compounds, which allow investigation of plant-insect interactions, and the number and diversity of those applied belowground is increasing, with an equally increasing list of species subjected to behavioral and electrophysiological experimentations.
31We also insist on the relevancy of assessing behavioral effects induced by blends as realistically as possible. It seems that most phytophagous insects target specific ratios among compounds (Visser, 1986). We have seen here that although inactive individually, some of the compounds induce a behavioral response in wireworms when paired (Crombie et al., 1947), which confirms that assumption in our case. Since most available behavioral assays were performed with only one compound, the realistic semiochemically-induced responses in wireworms have not yet been identified.
32Finally, advances in wireworm chemical ecology, with the long-term purpose of this information being applied in integrated management, rely on both behavioral assays and electrophysiological approaches. These are more than complementary; they are synergistic. Considering the considerable scope of the work to be done, multidisciplinary teams of researchers are necessary. Large-scale population studies, very well investigated (Barsics et al., 2013), combined to approaches at the individual scale, could provide very concrete control measures.
33Acknowledgements
34Our research was funded by the University of Liege - Gembloux Agro-Bio Tech, in the frame of the CURAGx projects. Fanny Barsics is supported by a FRIA grant (Fonds pour la Recherche en Industrie et en Agriculture).
Bibliographie
Agelopoulos N. et al., 1999. Exploiting semiochemicals in insect control. Pestic. Sci., 55(3), 225-235.
Arakaki N., Hokama Y. & Yamamura K., 2009. Efficient bait for sampling the wireworm Melanotus okinawensis (Coleoptera: Elateridae) in a sugarcane field. Appl. Entomol. Zool., 44(4), 561-568.
Arakaki N., Hokama Y. & Yamamura K., 2010. Estimation of the dispersal ability of Melanotus okinawensis (Coleoptera: Elateridae) larvae in soil. Appl. Entomol. Zool., 45(2), 297-302.
Barsics F. et al., 2012. Do root-emitted volatile organic compounds attract wireworms? Commun. Agric. Appl. Biol. Sci., 77(4), 561-565.
BarsicsF., Haubruge É. & Verheggen F.J., 2013. Wireworms' management: an overview of the existing methods, with particular regards to Agriotes spp. (Coleoptera: Elateridae). Insects, 4(1), 117-152.
Bellamy F.W., 1973. Ultrastructure of the labial palp and its associated sensilla of the prairie grain wireworms Ctenicera destructor (Brown) (Elateridae: Coleoptera). PhD thesis: University of Saskatchewan (Canada).
Bellamy F.W. & Zacharuk R.Y., 1976. Structure of the labial palp of a larval elaterid (Coleoptera) and of sinus cells associated with its sensilla. Can. J. Zool., 54(12), 2118-2128.
Benefer C. et al., 2010. The spatial distribution of phytophagous insect larvae in grassland soils. Appl. Soil Ecol., 45(3), 269-274.
Campbell R.E., 1937. Temperature and moisture preferences of wireworms. Ecology, 18(4), 479-489.
Chaton P.F. et al., 2003. Feeding behaviour as a limiting step in insecticide absorption for the wireworm Agriotes sp. (Coleoptera: Elateridae). Pestic. Biochem. Physiol., 77(3), 106-114.
Chaton P.F., Lemperiere G., Tissut M. & Ravanel P., 2008. Biological traits and feeding capacity of Agriotes larvae (Coleoptera: Elateridae): a trial of seed coating to control larval populations with the insecticide fipronil. Pestic. Biochem. Physiol., 90(2), 97-105.
Cherry R. & Nuessly G., 2010. Repellency of the biopesticide, azadirachtin, to wireworms (Coleoptera: Elateridae). Florida Entomol., 93(1), 52-55.
Cook S.M., Khan Z.R. & Pickett J.A., 2007. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol., 52, 375-400.
Crombie A.C. & Darrah J.H., 1947. The chemoreceptors of the wireworm (Agriotes spp.) and the relation of activity to chemical constitution. J. Exp. Biol., 24(1-2), 95-109.
De Bruyne M. & Baker T.C., 2008. Odor detection in insects: volatile codes. J. Chem. Ecol., 34(7), 882-897.
Doane J.F., Lee Y.W., Klingler J. & Westcott N.D., 1975. Orientation response of Ctenicera destructor and other wireworms (Coleoptera: Elateridae) to germinating grain and to carbon dioxide. Can. Entomol., 107(12), 1233-1252.
Doane J.F. & Klingler J., 1978. Location of CO2-receptive sensilla on larvae of wireworms Agriotes lineatus-obscurus and Limonus californicus. Ann. Entomol. Soc. Am., 71(3), 357-363.
Eilers E.J. et al., 2012. Sensing the underground-Ultrastructure and function of sensory organs in root-feeding Melolontha melolontha (Coleoptera: Scarabaeinae) larvae. PLoS ONE, 7(7), e41357.
Erb M., Ton J., Degenhardt J. & Turlings T.C.J., 2008. Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiol., 146(3), 867-874.
Evans A.C. & Gough H.C., 1942. Observations on some factors influencing growth in wireworms of the genus Agriotes Esch. Ann. Appl. Biol., 29, 168-175.
Fiers M., Lognay G., Fauconnier M.-L. & Jijakli M.H., 2013. Volatile compound-mediated interactions between barley and pathogenic fungi in the soil. PLoS ONE, 8(6), e66805
Furlan L., 1998. The biology of Agriotes ustulatus Schaller (Col., Elateridae). II. Larval development, pupation, whole cycle description and practical implications. J. Appl. Entomol., 122(2-3), 71-78.
Furlan L., 2004. The biology of Agriotes sordidus Illiger (Col., Elateridae). J. Appl. Entomol., 128(9-10), 696-706.
Gfeller A. et al., 2013. Characterization of volatile organic compounds emitted by barley (Hordeum vulgare L.) roots and their attractiveness to wireworms. J. Chem. Ecol., 39(8), 1129-1139.
Hemerik L., Gort G. & Brussaard L., 2003. Food preference of wireworms analyzed with multinomial Logit models. J. Insect Behav., 16(5), 647-665.
Hiltpold I. & Turlings T.C.J., 2008. Belowground chemical signaling in maize: when simplicity rhymes with efficiency. J. Chem. Ecol., 34(5), 628-635.
Horton D.R. & Landolt P.J., 2002. Orientation response of Pacific coast wireworm (Coleoptera: Elateridae) to food baits in laboratory and effectiveness of baits in field. Can. Entomol., 134(3), 357-367.
Johnson S.N., Anderson E.A., Dawson G. & Griffiths D.W., 2008. Varietal susceptibility of potatoes to wireworm herbivory. Agric. For. Entomol., 10(2), 167-174.
Johnson S.N. & Nielsen U.N., 2012. Foraging in the dark. Chemically mediated host plant location by belowground insect herbivores. J. Chem. Ecol., 38(6), 604-614.
Jonasson T. & Olsson K., 1994. The influence of glycoalkaloids, chlorogenic acid and sugars on the susceptibility of potato-tubers to wireworms. Potato Res., 37(2), 205-216.
Kessler A. & Baldwin I.T., 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science, 291(5511), 2141-2144.
Klingler J., 1957. Über die Bedeutung des Kohlendioxyds für die Orientierung der Larven von Otiorrhynchus sulcatus F., Melolontha und Agriotes (Col.) im Boden (Vorläufige Mitteilung). Mitt. Schweiz. Ent. Ges., 30, 317-322.
Lees A.D., 1943a. On the behaviour of wireworms of the genus Agriotes Esch. (Coleoptera, Elateridae). I. Reactions to humidity. J. Exp. Biol., 20(1), 43-53.
Lees A.D., 1943b. On the behaviour of wireworms of the genus Agriotes Esch. (Coleoptera, Elateridae). II. Reactions to moisture. J. Exp. Biol., 20(1), 54-60.
Parker W.E., 1996. The development of baiting techniques to detect wireworms (Agriotes spp., Coleoptera: Elateridae) in the field, and the relationship between bait-trap catches and wireworm damage to potato. Crop Prot., 15(6), 521-527.
Rasmann S. et al., 2005. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature, 434(7034), 732-737.
Robert C.A.M. et al., 2012. Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytol., 194(4), 1061-1069.
Schallhart N. et al., 2011. Stable isotope analysis reveals whether soil-living elaterid larvae move between agricultural crops. Soil. Biol. Biochem., 43(7), 1612-1614.
Scott D.A., 1969. Fine structure of sensilla on the antenna of Ctenicera destructor (Brown) (Elateridae: Coleoptera) with special reference to chemoreceptors. PhD: University of Saskatchewan (Canada).
Scott D.A. & Zacharuk R., 1971. Fine structure of the antennal sensory appendix in the larva of Ctenicera destructor (Brown) (Elateridae: Coleoptera). Can. J. Zool., 49(2), 199-210.
Simmons C.L., Pedigo L.P. & Rice M.E., 1998. Evaluation of seven sampling techniques for wireworms (Coleoptera: Elateridae). Environ. Entomol., 27(5), 1062-1068.
Sonnemann I., Grunz S. & Wurst S., 2014. Horizontal migration of click beetle (Agriotes spp.) larvae depends on food availability. Entomol. Exp. Appl., 150(2), 174-178.
Thorpe W.H., Crombie A.C., Hill R. & Darrah J.H., 1946. The behaviour of wireworms in response to chemical stimulation. J. Exp. Biol., 23(3-4), 234-266.
Traugott M., Schallhart N., Kaufmann R. & Juen A., 2008. The feeding ecology of elaterid larvae in Central European arable land: new perspectives based on naturally occurring stable isotopes. Soil Biol. Biochem., 40(2), 342-349.
van Herk W.G., Vernon R.S. & McGinnis S., 2013a. Response of the dusky wireworm, Agriotes obscurus (Coleoptera: Elateridae), to residual levels of bifenthrin in field soil. J. Pest Sci., 86(1), 125-136.
van Herk W.G. & Vernon R., 2013b. Wireworm damage to wheat seedlings: effect of temperature and wireworm state. J. Pest Sci., 86(1), 63-75.
Vet L.E.M. & Dicke M., 1992. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol., 37, 141-172.
Visser J., 1986. Host odor perception in phytophagous insects. Annu. Rev. Entomol., 31(1), 121-144.
Zacharuk R.Y., 1962. Sense organs of the head of larvae of some Elateridae (Coleoptera): their distribution, structure and innervation. J. Morphol., 111(1), 1-33.
Zacharuk R.Y., 1971. Fine structure of peripheral terminations in the porous sensillar cone of larvae of Ctenicera destructor (Brown) (Coleoptera, Elateridae), and probable fixation artifacts. Can. J. Zool., 49(6), 789-799.
Zacharuk R.Y., 1985. Antennae and sensilla. In: Kerkut G.A. & Gilbert L.I., eds. Nervous system: sensory. New York, USA: Pergamon.
Zacharuk R.Y., Albert P. & Bellamy F., 1977. Ultrastructure and function of digitiform sensilla on the labial palp of a larval elaterid (Coleoptera). Can. J. Zool., 55(3), 569-578.
Zacharuk R.Y. & Albert P.J., 1978. Ultrastructure and function of scolopophorous sensilla in the mandible of an elaterid larva (Coleoptera). Can. J. Zool., 56, 246-259.
Zacharuk R.Y. & Shields V.D., 1991. Sensilla of immature insects. Annu. Rev. Entomol., 36(1), 331-354.






