- Startpagina tijdschrift
- Volume 19 (2015)
- Numéro 2
- Influence of tropical leaf litter on nitrogen mineralization and community structure of ammonia-oxidizing bacteria
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Influence of tropical leaf litter on nitrogen mineralization and community structure of ammonia-oxidizing bacteria

Nota's van de redactie
Received on April 9, 2014; accepted on February 5, 2015
Résumé
Influence de la qualité des litières tropicales sur la minéralisation de l’azote et la structure des communautés bactériennes nitrifiantes
Description du sujet. Cet article aborde les relations entre la décomposition des litières, la qualité du substrat, la composition des communautés microbiennes AOB et la disponibilité de l’azote. La décomposition de la matière organique peut affecter le cycle biogéochimique du carbone (C) et de l’azote (N) et si la composition de la communauté microbienne change, cela affecte la capacité physiologique de la communauté, ce qui peut avoir des conséquences sur l’écosystème.
Objectifs. L’objectif général de cette étude était de déterminer l’influence de la décomposition des litières de quelques espèces végétales sur la minéralisation de l’azote. Les objectifs spécifiques de ce travail sont de déterminer l’effet de la qualité biochimique de cinq litières tropicales (Faidherbia albida A.Chev., Azadirachta indica A.Juss., Casuarina equisetifolia L., Andropogon gayanus Kunth and Eragrostis tremula Hochst. ex Steud.) sur la minéralisation de l’azote, la nitrification et la structure de la communauté des bactéries nitrifiantes.
Méthode. L’étude de la diversité des communautés bactériennes durant la décomposition des litières dans les sols est effectuée en utilisant la technique DGGE par amplification des gènes des régions 16 rRNA. La structure des communautés AOB a été déterminée sur deux types d’échantillons : en début d’incubation (T0) et en fin d’incubation (T140). Dix échantillons ont été testés et chacun d’eux a produit une bande simple. Ainsi, les modèles de bande de DGGE ont été utilisés pour évaluer la diversité des communautés bactériennes.
Résultats. Les résultats ont montré que la nitrification est affectée par la qualité de la litière. La litière de F. albida a augmenté de manière très significative l’azote minéral du sol après 140 jours d’incubation, alors que les litières de A. gayanus et C. equisetifolia ont immobilisé l’azote dans le sol. Les litières de A. indica et E. tremula n’ont eu aucun effet significatif sur la minéralisation de l'azote. Des composés secondaires de la plante comme les polyphénols sont identifiés comme influençant le cycle des éléments nutritifs en affectant la décomposition de la matière organique, les taux de minéralisation, la disponibilité de l’azote et la formation de l’humus. Une étude au laboratoire nous a permis d’étudier l’influence de six acides phénoliques (férulique, gallique, vanillique, syringique, p-coumarique et p-HBA), généralement trouvés dans les litières, sur la mineralization de l’azote et la production de NH4+ et NO3- dans des sols. Les résultats ont montré que ces acides phénoliques ont considérablement réduit la production de NH4+ et NO3- par rapport au témoin sans apport d’acides. Concernant la communauté bactérienne nitrifiante, un effet qualité litière est noté mais l’effet temps d’incubation est plus marqué. Cependant, dans le cas de la litière de C. equisetifolia, on note une absence d’effet temps d’incubation.
Conclusions. Les résultats ont confirmé que la minéralisation de N variait en fonction du type de litières amendées en conditions contrôlées de laboratoire et que la structure des communautés bactériennes nitrifiantes variait fortement aussi en fonction de la qualité de la litière.
Abstract
Description of the subject. The present study concerns the relationships among leaf litter decomposition, substrate quality, ammonia-oxidizing bacteria (AOB) community composition and nitrogen (N) availability. Decomposition of organic matter affects the biogeochemical cycling of carbon (C) and N. Since the composition of the soil microbial community can alter the physiological capacity of the community, it is timely to study the litter quality effect on N dynamic in ecosystems.
Objectives. The aim of this study was to determine the influence of leaf litter decomposition on N mineralization. The specific objectives of this study were to evaluate the influence of the litter biochemistry of five plants species (Faidherbia albida A.Chev., Azadirachta indica A.Juss., Casuarina equisetifolia L., Andropogon gayanus Kunth and Eragrostis tremula Hochst. ex Steud.) on N mineralization in a tropical ferrous soil (Lixisol), nitrification, and genetic diversity of ammonia-oxidizing bacteria. Denaturing gradient gel electrophoresis (DGGE) of amplified fragments of genes coding for 16S rRNA was used to study the development of bacterial communities during decomposition of leaf litter in soils.
Method. Community structure of AOB was determined at two time periods: day 0 and day 140. Ten strains were tested and each of these strains produced a single band. Thus, DGGE DNA band patterns were used to estimate bacterial diversity. Plant secondary compounds such as polyphenols are purported to influence nutrient cycling by affecting organic matter degradation, mineralization rates, N availability and humus formation. In a laboratory study, we investigated the influence of six phenolic acids (ferulic, gallic, vanillic, syringic, p-coumaric and p-HBA acids) commonly found in the plant residues on N mineralization and NH4+ and NO3- production in soils.
Results. The results showed that litter type did affect soil nitrification. Faidherbia albida litter was associated with increased inorganic N in soil after 140 days of incubation while A. gayanus and C. equisetifolia litter immobilized N. Azadirachta indica and E. tremula amendments had no significant effects in N mineralization. The results show that the addition of six phenolic acids significantly reduced NH4+ and NO3- compared to the control soil but had no significant effect on N mineralization. For the community of ammonium-oxidizing bacteria, a litter quality effect was noted, but the incubation time effect was more pronounced, except for C. equisetifolia litter.
Conclusions. Results confirmed that the N mineralization changed with litter type under controlled conditions and the genetic structure of AOB is highly dependent on litter quality.
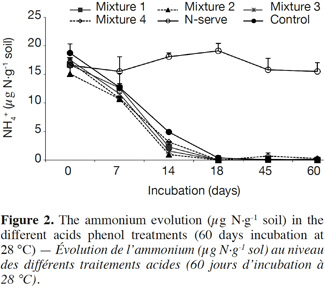
Inhoudstafel
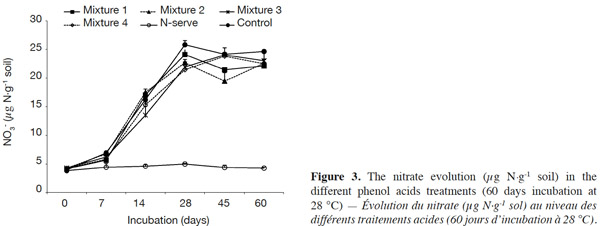
1. Introduction
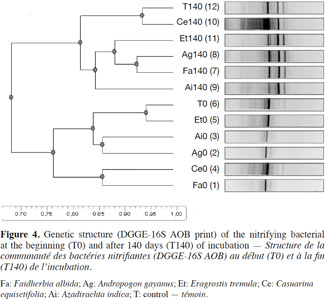
1Low organic inputs are a major limitation to crop productivity in degraded Sahelian soils. Inputs of organic matter are needed to maximize yields and fertilizer and water efficiency in sub-Saharan Africa (Sanchez et al., 1997). The biochemical composition of organic material added to soil can influence rates of decomposition (Cornwell et al., 2008; De-Hui Zeng et al., 2010; Wieder et al., 2013). Modeling kinetics of decay rates has shown that the most important plant parameters that control decomposition are lignin, cellulose, hemicellulose, polyphenols, the lignin/N ratio and/or the C/N ratio of the litter (Mafongoya et al., 2000; Trinsoutrot et al., 2000). A key component that affects microbial communities and the rate of degradation during decomposition is polyphenolic content (Palm et al., 1991). Polyphenols are the most widely distributed class of plant secondary metabolites and several thousand different compounds have been identified. Polyphenols have also been recognized as regulators of soil processes, where it has been suggested that they inhibit nitrification (Rice et al., 1973), as well as decomposition and nutrient recycling (Kuiters, 1990), as a by-product of their antiherbivore activity (Coley et al., 1985). Alternatively, it has been proposed that plant-produced polyphenols could control the pool and the form of nutrients available for plants and/or microbes (Northup et al., 1998).
2Many studies have been published on the evolution of bacterial communities in soil (Muyzer, 1999; Dilly et al., 2004). However, no studies have been done on ammonia-oxidizing bacteria during organic matter decomposition in sandy tropical soils.
3Nitrogen is a limiting factor for agricultural production in ferruginous soil in Senegal (Bationo et al., 1991). Thus, for subsistence farmers in Senegal and throughout the Sahel, organic soil amendments can be a significant source of N after mineralization to inorganic forms (Bernhard-Reversat, 1982).
4Previously we reported that litter addition to soil stimulates in situ N mineralization of a sandy Sahalian soil (Diallo et al., 2005). At the same time there was a negative effect of litter polyphenolic content on N mineralization and onion (Allium cepa) growth (Diallo et al., 2006). These results showed that N mineralization was negatively correlated with litter polyphenolic content (Diallo et al., 2006). This holds potential to improve N efficiency by maintaining N in the soil as a cation (NH4+) which can be bound by cation exchange sites while NO3- can be readily leached out of the rooting zone.
5Chemolithotrophic ammonia oxidation is a key process in the nitrogen cycle because it is the main route of ammonia oxidation in soils in most terrestrial ecosystems. Ammonia, produced by decomposition of organic material (ammonification), is oxidized to nitrite by ammonia-oxidising bacteria in a two-step reaction (Paul et al., 1996). One approach to examine the importance of nitrification in soils has been the use of chemical inhibitors, which disrupts the first step of the nitrification reaction (conversion of ammonia to nitrite). These chemicals, such as nitrapyrin (N-Serve or 2-chloro-6-(trichloromethyl)-pyridine), were primarily developed for agricultural use. Nitrapyrin inhibits the AMO enzyme by killing or interfering with the metabolism of ammonia-oxidizing bacteria. If polyphenolics from plant residue-based soil amendments function similarly to Nitrapyrin then there is a possibility to manipulate organic inputs to suppress nitrification and improve N efficiency; a strategy that could be adopted by subsistence farmers using locally available resources.
6Therefore the objective of this study was to determine the effect of litter chemistry and polyphenolics on N mineralization, nitrification, and the community composition of ammonium-oxidizing bacteria.
2. Materials and methods
2.1. Soil characteristics
7The soil is a leached ferrous tropical soil (Maignien, 1965) classified as a lixisol (FAO, 1998). Soil samples were taken from the 0 to 20 cm depth near the High National School of Agriculture in Thiès, Senegal (14°42’N, 16°57’O). Soil was sampled with a cylindrical Tarier Eijkelkamp (single root auger), which collects soil in a constant and exact volume (754 cm3 for 15 cm thickness). Soil samples were air dried and passed through a 2 mm sieve. A subsample was dried at 105 °C to determine soil water content. The soil had 89.2% sand, 1.8% clay and 8.5% slit, pH 7.86, 5.21 mg·g-1 Corg., and 0.31 mg·g-1 N.
2.2. Litter types
8Faidherbia albida A.Chev. (Mimosaceae), Azadirachta indica A.Juss. (Meliaceae), Andropogon gayanus Kunth (Poaceae), Casuarina equisetifolia L. (Casuarinaceae) and Eragrostis tremula Hochst. ex Steud. (Poaceae) were selected because they are locally available and frequently used in agricultural systems of the Sahel (Diallo et al., 2005). The characterization of the biochemical composition of the litter types (Table 1) has been reported by Diallo et al. (2006).

9The phenolic acid composition of the five litter types was determined following the modified acid extraction procedure of Guissé and Bertru (1987) and quantified by high performance liquid chromatography (HPLC). The main modification of the extraction procedure was the use of HCI 6 N instead of 1 N to optimize extraction, as well as the use of NaCO3 anhydrid to retrieve the second organic stage of litter, which have a demixing phase. After evaporation using a vacuum, the crystallized phenol acids were retrieved by 2 ml of HPLC grade methanol.
2.3. N mineralization
10Litter decomposition. The experiment was a completely randomized design with three replicates and six treatments: five one year old litter types (as described above) and a control. Thirty g of soil were placed in a 60 ml polystyrene bottle that had caps pierced with a small hole for aeration and litter was mixed at the rate of 1% (wt/wt). The soil was weighed twice weekly to determine water loss and its water content was adjusted to soil water potential (g water per g dry soil) with deionized water. The sample was incubated at 28 °C. A vessel containing NaOH (2N) was placed with each experimental unit to trap CO2 produced during the incubation. Samples were destructively collected at: 0, 7, 14, 21, 28, 49, 70, 91 and 140 days. The samples were extracted with 2 M KCl and the extract analyzed for NH4+ and NO3-.
11Incubation of phenolic acids. The experiment had a completely randomized design with three replications and six soil amendment treatments: four mixtures of phenolic acids representative of four plant residues, Nitrapyrine and control. The phenolic acids were selected based on the suite of these compounds commonly found in our plant litter.
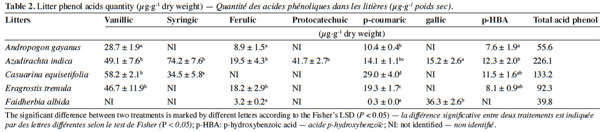
12The phenolic acids were applied at two quantities to simulate residues with low phenolic content (F. albida) or high phenolic acid content for C. equisetifolia (Table 2). The combination of the typical phenolic acids found in F. albida and C. equisetifolia residues are ferulic, gallic, vanillic, syringic, p-coumaric, protocatechuic and p-HBA acids. The rates and specific mix of phenolic acids were developed from the data of table 2 and are reported in table 3. The first (Mixture 1) and second (Mixture 2) combinations represent the suit of acids found in C. equisetifolia and F. albida, respectively. The third combination (Mixture 3) represents C. equisetifolia but at a lower rate for phenolic acids equal to that of F. albida. The fourth combination (Mixture 4) represents F. albida but at a higher content, equal to that of C. equisetifolia.
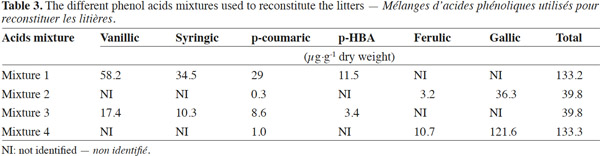
13The phenolic acid combinations were mixed with 30 g of soil as shown in table 3. Ten µg N·g-1 soil as (NH4)2SO4 were added to remove N as a limiting factor. The nitrification inhibitor, Nitropyrin, was added to soil at 100 µg·g-1. The control was soil alone but otherwise treated like the other treatments. The incubation was done at 28 °C for 60 days, maintained at 100% of field capacity (8%) and destructively sampled at days 0, 7, 14, 28, 45, and 60. At each sampling date, NH4+ and NO3- were measured after extracting with 2M KCl and analyzed. The DNA analysis was done at day 0 (T0) and 140 (T140).
2.4. Soil analyses
14Inorganic N analysis. Inorganic N (NH4+ and NO3-) content was determined according to the method of Bremner (1965). Briefly soil samples (20 g equivalent dry weight) were suspended in 75 ml KCl solution (1:3, dry soil/solution, w/v, 2 M KCl final concentration), shaken at 25 °C for 1 h, then filtered with Whatman paper 0.45 µm, and stored at -20 °C. Soil inorganic N was determined by flow injection analysis (Evolution II, Alliance Instrument, France). Total inorganic N was calculated by summing extractable NH4+ and NO3-.
15Ammonia Oxidizers Bacteria (AOB) analysis. Community structure of AOB was determined on two samples: beginning (T0) and end (T140). Total DNA was extracted by adding 0.2 g glass beads (Sigma, 0.1 mm) and 1 ml lysis buffer (0.25 M NaCl, 0.1 M EDTA; pH 8) to 0.5 g soil. The sample was then subjected to bead-beating (Bead-beater, Biospec products) two times for 2 min with an intermittent 2 min heat treatment of 65 °C (Picard et al., 1992). The subsequent steps were carried out as described by Porteous et al. (1997). Briefly, the extract was concentrated with potassium acetate 5 M and PEG 8000 and centrifuged 15 min at 13,000 g. The pellet was dissolved in CTAB 2%, and then extracted with an equal volume of chloroform. DNA was precipitated at -20 °C for 15 min with 0.7 µl isopropanol. After centrifugation at 4 °C for 15 min at 13,000 g, pellets were again dissolved and precipitated with ammonium acetate (2.5 M) and washed with ethanol, air-dried, and then DNA was dissolved in 100 µl 1 x TE (Tris-EDTA).
16Nested polymerase chain reaction (PCR) amplification was performed with eubacterium-specific 16S rDNA primers fD1 and rD1 (Weisburg et al., 1991) followed by ammonia-oxidizing bacteria specific amplification using the CTO primer pair CTO189f-GC and CTO654r (Kowalchuk et al., 1997). The PCR was done using Ready-To-Go Beads (Amersham-Pharmacia, France), 30 ng of template DNA and 1 µM of each primer in 25 µl PCR mixture. The first PCR was performed by using the following thermocycling program: one cycle of 5 min at 94 °C; 35 cycles of 1 min at 94 °C, 45 s at 57 °C, and 1 min 30 s at 72 °C and one cycle of 15 min at 72 °C. Prior to secondary amplification, PCR products were diluted 1:50 and 2 µl of the dilution were used as the template in the following thermocycling regime: 94 °C for 5 min, 30 cycles of 94 °C for 1 min, 55 °C for 45 s, 72 °C for 1 min 30 s and a final cycle of 15 min at 72 °C. The PCR products (3 µl) were examined by electrophoresis in a 0.53 TBE–1% agarose gel (Eurogentec, Belgium), followed by ethidium bromide staining (1 mg·ml-1).
17Denaturing Gradient Gel Electrophoresis (DGGE) analysis. DGGE of PCR products generated by the CTO primer pair was performed by the method described by Muyzer et al. (1993). The fragment used spanned 465 bp of the 16S rDNA gene including the GC clamp. Polyacrylamide gels (8% of a 37.5:1 acrylamide-bisacrylamide mixture in 0.5 TAE buffer), with a gradient of 45 to 70% denaturant (100% denaturant was defined as 7 M urea plus 40% formamide) were made for electrophoresis. Approximately 500 ng of PCR products were loaded per sample. The gel was run in 0.5 x TAE (Tris-Acetate-EDTA) buffer at 60 °C at constant voltage of 150 V for 17 h by using the Ingeny System (Ingeny, The Netherlands). The gel was stained for 30 min with ethidium bromide and washed for 10 min with MilliQ H2O prior to UV transillumination. Banding patterns were visualized on a Dark Reader and were digitized by using a charge-coupled device camera and the Biocapt software program (Vilber Lourmat).
2.5. Statistical analyses
18The data were analyzed by ANOVA (Super Anova software, ABACUS CONCEPTS, BERKELEY, CA) using a Fisher’s LSD test (P < 0.05). For the DGGE profile, the data was analyzed with the Totallab TL 120 Software. This software was used to set up the similarity dendrogram with the Dice coefficient (2% confidence interval) using the UPGMA algorithm method (Unweighted Pair-Group Method Algorithm).
3. Results
19Seven major phenolic acids were found at varying concentrations in the litter types (Table 2). Some acids were common to all litters while others were only found in one litter type. The highest concentrations of phenolic acid concentration were found for A. indica (226.1 μg·g-1), followed by C. equisetifolia (133.2 μg·g-1), E. tremula (92.3 μg·g-1 dry weight), A. gayanus (55.6 μg·g-1 dry weight) and finally F. albida (39.8 μg·g-1 dry weight). Vanillic and p-hydroxybenzoïc (p-HBA) acids were absent from F. albida and so was ferulic acid for C. equisetifolia. Protocatechuic acid was formed at A. indica; syringic acid for C. equisetifolia and gallic acid for A. indica and F. albida.
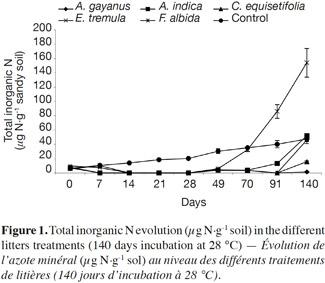
20Litter type. Figure 1 shows total inorganic N levels over the 140 day incubation. Measurable amounts of inorganic N were absent in all soils amended with litter for the first 28 days. However, for the control, inorganic N production increased linearly. After 70 days of decomposition, inorganic N quantity for F. albida (32 µg·g-1 soil) reached the control level (35 µg·g-1 soil). At 140 days, the quantity of inorganic N produced was not significantly higher in soil amended with F. albida (153 µg·g-1 soil) over control soil. The quantity produced was reduced in soil amended with C. equisetifolia litter (15 µg·g-1 soil), and was very low for A. gayanus (1.7 µg·g-1 soil).
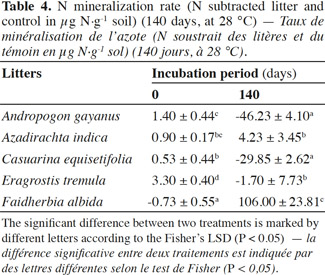
21The net N mineralization as determined by the difference between litter-amended soils and the control soil is shown in table 4. At the beginning of the incubation, there was an increase in N for all the litter treatments except F. albida (-0.73 µg·g-1 soil). The highest rate was for E. tremula (3.3 µg·g-1 soil). After 140 days, there were three major outcomes: a high N mineralization (+106 µg·g-1 soil) for F. albida amendment, initial N mineralization for A. indica (+4.2 µg·g-1 soil) and finally, for C. equisetifolia and A. gayanus treatments, a high immobilizing stage (-29 µg·g-1 soil and -46 µg·g-1 soil, respectively).
22Phenolic acid type. Figure 2 shows the quantities of NH4+ over time where Nitrapyrine treatment remained constant over time. However, in the mixed treatments with phenolic acids, NH4+ was lower compared to the control from 0 to 28 days. After 28 days NH4+ was nearly undetectable.
23There was an increase of the NO3- content for the phenolic acid treatments and the control from 0 to 28 days but NO3- content levels were lower for phenolic acid treatments (Figure 3). In the Nitrapyrine treatment, the NO3- quantity remained low and constant over time.
24Profile of AOB. We compared two profiles of the AOB genetic structure at the beginning and end of the incubation (Figure 4). A few bands of high intensity were noticed at the beginning of the incubation (T0). The analysis of these two sampling dates (Dice coefficient 2%) showed a 95% similarity between the control and the E. tremula treatment. The AOB similarity was 85% between A. indica and A. gayanus, and between C. equisetifolia and F. albida. By day 140, new bands were found. However, C. equisetifolia had a different AOB profile than the other litter treatments. Its similarly rating with the control was 94%, while the A. gayanus profile was more similar to F. albida (92%).
4. Discussion
4.1. Composition in phenol acids of litters
25The analysis of litter showed that phenolic acid content and types varied among the plant litter. Faidherbia albida litter was characterized by a small phenolic acid content compared to A. indica and C. equisetifolia litter types. The grasses (A. gayanus and E. tremula) had the same phenolic acids present (vanillic, ferulic, p-coumaric and p-hydroxybenzoïc). Except for F. albida, the lignin-rich litters (A. indica and C. equisetifolia) had phenolic acid content higher than the grasses. Breman et al. (1995) also found that most of the litter from mature leaves of F. albida had polyphenol concentrations higher than a grass litter (Pennisetum glaucum). Phenolic acid concentrations found in our litter were lower than some concentrations reported by Chapuis-Lardy et al. (2002) and they found some phenolic acids that were not in our litters. This difference was due to different litter types and having used different extractants (water, acid, soda alkaline solution, acetone, etc.) and procedures (intact or ground leaves, duration, extracting temperature and solvent polarity). It is also possible that other phenolic acids were not detectable by our method and therefore could be affecting the outcome of the incubation.
26Our results demonstrated that both the qualitative and the quantitative composition of phenolic compounds depend on the type of the litter. It is essential that in the extracts of litters, the qualitative composition of phenolic acid compounds was changing along with litter type. Phenolic compounds are some of the most abundant and widely distributed groups of substances in the plant kingdom with more than 8,000 phenolic structures currently known (Harbone, 1980). Total phenolics are considered to be biologically active by contributing to allelopathic reactions (Waterman et al., 1994) and by retarding decomposition rates of organic matter (Hättenschwiler et al., 2000).
4.2. N mineralization
27Litter type. There was a relationship between N mineralization rates and litter biochemistry. In the case of A. gayanus and C. equisetifolia amendments there was net N immobilization. For the A. gayanus litter, this immobilization was probably due to the high C/N ratio (Mungai et al., 2006), which can mean N is limiting microbial growth and activity (Bending et al., 1999). It is well established that high C/N ratios of plant residues cause N immobilization as showed for example Bending et al. (1999) and Das et al. (1993) on crop residues amended to soils.
28Low N mineralization of C. equisetifolia litter was likely related to its high polyphenolic content (Schroth, 2003). Studies of Constantinides et al. (1994) on A. indica and C. equisetifolia litters showed that these litters had an inhibiting effect on N mineralization over 16-week lab incubation. The negative effects of the polyphenols were related to the complexation of protein and pectin N by soluble phenolic compounds making N inaccessible to microorganisms for mineralization (Bending et al., 1999).
29Faidherbia albida had high N with a low C/N of 21, and low polyphenolic content which is likely why this residue had net N release over the whole incubation. This is consistent with the results of Seneviratne (2000) for this same residue and other studies on N mineralization on various other residues added to tropical soils (Constantinides et al., 1994; Schwendener et al., 2005). However, it took 70 days before F. albida amended soil released inorganic N. This is likely related to its high lignin that is difficult to break down and release N.
30A significant relationship is found between N mineralization and litter compounds. Increasing N concentrations resulted in plant litter quality (C/N particularly). However, these aboveground differences depended on the litter quality incubated. Finally, the decomposition of the litter residues was only weakly related to the organic N concentration or the C/N ratio of the residues. Thus, very often, the availability of soil inorganic N will, at least in the short-term, control the kinetics of N decomposition. In our study, the possibility of N limitation was avoided by the litter addition to soil so as not to obtain confounding effects of N availability and nature of residue polyphenols on decomposition rate. This explains why the residue N contents did not significantly affect their decomposition.
31Phenolic acids. The various phenolic mixtures added to soils reduced N mineralization and nitrification but did not completely suppress it (Leake et al., 1990). This follows Castells et al. (2004) who amended two siliceous soils and one chalky soil with a high phenolic content (1.73 mg gallic acid and 330 mg total phenols·g-1) litter (Citrus albinus), which only caused a net decrease in N mineralization of 9% over the control.
32In this paper, we have shown that Nitrapyrin, an inhibitor of the action of the AMO enzyme, decreased N mineralization. This reduction related to lower NO3- and suggests that autotrophic ammonia oxidation is the dominant path for nitrate production in this ecosystem. The Nitrapyrine treatment did show that it is possible to inhibit nitrification in the used soil. Similarly, Underhill et al. (1985) found that ethyl xanthane potassium was an effective inhibitor of soil nitrification by suppressing Nitrosomonas europaea and Nitrobacter sp. Rice (1984) showed a complete inhibition on soil nitrification by a phenolic acid. It is therefore possible to obtain nitrification inhibition with the appropriate compounds but the suite of phenolic compounds in our study had very limited effects on nitrification.
33This lack of nitrification inhibition of phenolic compounds and residues high in phenolic acids may be due to various reactions of phenolics with soil. Phenolics could be inactivated by adsorption or complexation by the organic matter (Powell et al., 1986) or due to surface reactions with clay particles and Fe oxides that dominate in our soil (Powell et al., 1986).
34The phenolic acid effects could be related to the particular biochemical properties of the phenolics used in a given substrate by a different experiment. Inderjit et al. (1997) showed that a mixture of phenolic acids caused greater nitrification inhibition than that of a single phenolic acid even though the total concentrations were the same. They use a higher concentration than our experiment. In our study, in both low concentration (Mixtures 2 - 3 to 39.8 µg·g-1) and high concentration (Mixture 1 - 4 to 133.2 µg·g-1) there was no significant difference for nitrification inhibition.
35Another factor is that phenolic could be degraded by a subpopulation of microorganisms. Indeed, Blum et al. (1988) showed that concentrations less than 100 mg phenolic acid per kg are needed before microorganisms stop using these compounds as a substrate. This is an extremely high content of phenolic that would be difficult to achieve using plant residues. For example in our study, A. indica had the highest concentration of phenolic acids for a total of 226 µg·g-1 residue. Using a 1% residue amendment content to soils, which is on the upper end of practical applications for organic amendments to soils, would result in only a 2.26 µg total phenolic acid per g addition to soil.
36It is well established that Nitrapyrine inhibits nitrification (Goring, 1962; Lopez et al., 2003) and undoubtedly this is why NH4+ was maintained for this treatment in our study. Nitrapyrine is a specific inhibitor of oxidizing ammonium bacteria but does not affect heterotrophic bacteria (Sahrawat, 1989). It operates by inactivating the oxidizing cytochrome used for NH4+ oxidation within a cell (Campbell et al., 1965; Somville, 1978). Laboratory studies have shown that the degree of inhibition depends on the types of soil and ammonia-oxidizing bacteria (Lopez et al., 2003). According to Kucharski (1991), the inhibiting effect of the Nitrapyrine is proportional to the concentration and decreases with time of exposure in soil (90% after 30 days and less than 45% after 120 days).
37Community structure of AOB. The DGGE profile based on band intensity changed between two time periods: T0 and T140. Accordingly, the statistical analysis of the similarity thresholds percentages was different between T0 (76%) and T140 (81%). The similarity of 76% at the beginning of incubation for the same soil indicates that litter amendment significantly affected AOB community. The change in AOB diversity between T0 and T140 was similar across all treatments, except for C. equisetifolia. The DNA band patterns obtained from amplified 16S rRNA gene sequences and DGGE indicated that the structure and diversity of bacterial communities changed significantly during 140 days of litter decomposition in soil. The increase in diversity as the activity decreased appeared to reflect the conversion of litter to soil organic matter and the concomitant development of diverse microbial communities adapted to lower availability of nutrients. In the rapidly decomposing litter with higher nutritional value, the amount of bacterial DNA increased rapidly and the bacterial DNA was dominated by fewer organisms, as indicated by the lower number of bands and lower diversity.
38It should be kept in mind that each litter added to soil had an overall effect on the microbial community because of the C inputs and also specific effect on subpopulation due to the unique chemistry of each plant residue. This may partly explain the detection of new bands in the profile by T140. The chemistry (soluble C vs organic C) (Degens et al., 2000) and C/N ratio (Marschner et al., 2003) of plant residues added to soils are major factors in controlling microbial population structure responses. In addition, the inhibiting effect of the litter phenolic compounds on the nitrifying bacteria (Bremner et al., 1993) could partly explain the difference of AOB community in C. equisetifolia over the other litter treatments.
5. Conclusions
39Nitrogen is the most limiting nutrient in a crop production system. When litter amendments are used for N fertilization, they affect N availability, which is important to maintain crop yields. The information obtained from this study was that N mineralization changed with litter type under controlled conditions. Leaf litter amendment additions resulted in significant N mineralization differences. The greatest differences in N mineralization were observed with the F. albida litter treatment seldom stimulated net N mineralization, while the other amendments of A. indica or E. tremula samples had no significant effect on N mineralization. Seventy days were needed before there was net N mineralization for any of the litter amendment. The litters constitute an important source of inorganic N of which the availability in the soil depends on polyphenols composition during the early decomposition stages. The greatest differences were observed also in the phenolic acid treatments. Addition of chemical amendments similar to the phenolic acid content also impacted N mineralization. Results of this study showed that the genetic structure of AOB is highly dependent on litter quality. We had 76% similarity among treatments at the beginning of the incubation for the same soil. These results suggest that the use of leaf litter amendments enhances effects of allelochemical release from plant debris on N mineralization. It also shows that microbial diversity increased during the course of litter decomposition.
Bibliographie
Bationo A. et al., 1991. Fertilizer management strategies for legume-based cropping systems in the West African semi-arid tropics. In: Johansen C., Lee K.K. & Sahrawat K.L., eds. Phosphorous nutrition of grain legumes in the semi-arid tropics. Patancheru, India: ICRISAT, 213-226.
Bending G.D., Turner M.K. & Burns I.G., 1999. Fate of nitrogen from crop residues as affected by biochemical quality and the microbial biomass. Soil Biol. Biochem., 30, 2055-2065.
Bernhard-Reversat F., 1982. Biogeochemical cycle of nitrogen in a semi-arid savanna. Oikos, 38, 321-332.
Blum U. & Shaffer S.R., 1988. Microbial populations and phenolic acids in soil. Soil Biol. Biochem., 20, 793-800.
Breman H. & Kessler J.J., 1995. Woodyplants in agro-ecosystems of semi-arid regions, whith an emphasis on the Sahelian countries. Berlin: Springer Verlag.
Bremner J.M., 1965. Nitrogen availability indexes. In: Black C.A., eds. Methods of soil analysis. Part 2. Madison, WI, USA: American Society of Agronomy, 1324-1345.
Bremner J.M. & Mc Carty G.W., 1993. Inhibition of nitrification in soil by allelochemicals derived from plants and plant residues. Soil Biochem., 8, 181-218.
Campbell N. & Aleem M., 1965. The effect of 2-chloro 6-trichloromethyl pyridine on the chemoautotrophic metabolism of nitrifying bacteria. Antonie Van Leeuwenhoek, 31, 124-136.
Castells E., Penuelas J. & Valentine D.W., 2004. Are phenolic compounds released from the Mediterranean shrub Cistus albidus responsible for changes in N cycling in siliceous and calcareous soils? New Phytol., 162, 187-195.
Chapuis-Lardy L., Contour-Ansel D. & Bernhard-Reversat F., 2002. High-performance liquid chromatography of water-soluble phenols in leaf litter of three Eucalyptus hybrids (Congo). Plant Sci., 163, 217-222.
Coley P.D., Bryant J.P. & Chapin F.S. III, 1985. Resource availability and plant antiherbivore defense. Science, 230, 895-899.
Constantinides M. & Fownes J.H., 1994. Nitrogen mineralization from leaves and litter of tropical plants: relationships to nitrogen, lignin and soluble polyphenol concentration. Soil Biol. Biochem., 26, 49-55.
Cornwell W.K. et al., 2008. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett., 11, 1065-1071.
Das S.K. et al., 1993. Prediction of nitrogen availability in soil after crop residues incorporation. Fertil. Res., 37, 209-215.
Degens B.P., Schipper L.A., Sparling G.P. & Vojvodic-Vukovic M., 2000. Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol. Biochem., 32, 189-196.
De-Hui Z. et al., 2010. Carbon mineralization of tree leaf litter and crop residues from poplar-based agroforestry systems in Northeast China: a laboratory study. Appl. Soil Ecol., 44, 133-137.
Diallo M.D. et al., 2005. In situ effect of some tropical litters on N mineralization. Arid Land Res. Manage., 19, 173-181.
Diallo M.D. et al., 2006. Biological effects of native and exotic plant residues on plant growth, microbial biomass and N availability under controlled conditions. Eur. J. Soil Biol., 42, 238-246.
Dilly O., Bloem J., Vos A. & Munch J.C., 2004. Bacterial diversity in agricultural soils during litter decomposition. Appl. Environ. Microbiol., 70, 468-474.
FAO, 1998. World reference base for soil resources. World soil resources reports. Roma: FAO.
Goring C.A.I., 1962. Control of nitrification by 2-chloro-6-(trichloromethyl) pyridine. Soil Sci., 93, 211-218.
Guissé A. & Bertru G., 1987. Analyse de quelques acides phénoliques dans les extraits de litière par chromatographie liquide (HPLC). Rev. Écol. Biol. Sol, 24, 1-9
Harbone J.B., 1980. Plant phenolics. In: Bell E.A. & Charlwood B.V., eds. Encyclopedia of plant physiology, secondary plant products. Vol. 8. Berlin, Heidelberg, Germany; New York, USA: Springer-Verlag, 329-395.
Hättenschwiler S. & Vitousek P.M., 2000. The role of polyphenols in terrestrial ecosystem nutrient cycling. Tree, 15, 238-243.
Inderjit & Mallik A.U., 1997. Effect of phenolic compound on selected soil properties. For. Ecol. Manage., 92, 11-18.
Kowalchuk G.A. et al., 1997. Analysis of ammonia-oxidizing bacteria of the beta-subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl. Environ. Microbiol., 63, 1489-1497.
Kucharski J., 1991. Effect of nitrification intensity on the yield of winter wheat. Polish J. Soil Sci., 24, 57-63.
Kuiters A.T., 1990. Role of phenolic substances from decomposing forest litter in plant-soil interactions. Acta Bot. Neerl., 27, 329-348.
Leake J.R. & Read D.J., 1990. Proteinase activity in mycorrhizal fungi. II. The effects of mineral and organic nitrogen sources on induction of extracellular proteinase in Hymenoscyphus ericae (Read) Korf and Kernan. New Phytol., 116, 123-129.
Lopez N.I., Austin A.T., Sala O.E. & Mendez B.S., 2003. Controls on nitrification in a water-limited ecosystem: experimental inhibition of ammonia-oxidising bacteria in the Patagonian steppe. Soil Biol. Biochem., 35, 1609-1613.
Mafongoya P.L., Barak P. & Reed J.D., 2000. Carbon, nitrogen and phosphorus mineralization of tree leaves and manure. Biol. Fertil. Soils, 30, 298-305.
Maignien R., 1965. Carte pédologique du Sénégal. Notice explicative en 1/1 000 000. Dakar : ORSTOM.
Marschner P., Kandeler E. & Marschner B., 2003. Structure and fonction of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem., 35, 453-461.
Mungai N.W. & Motavalli P.P., 2006. Litter quality effects on soil carbon and nitrogen dynamics in temperate alley cropping systems. Appl. Soil Ecol., 31, 32-42.
Muyzer G., 1999. DGGE/TGGE: a method for identifying genes from natural ecosystems. Curr. Opin. Microbiol., 2, 317-322.
Muyzer G., Waal E.C.D. & Uitterlinden A.G., 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol., 59, 695-700.
Northup R.R., Dahlgren R.A. & McColl J.G., 1998. Polyphenols as regulators of plant–litter–soil interactions in northern California's pygmy forest: a positive feedback? Biogeochemistry, 42, 189-220.
Palm C.A. & Sanchez P.A., 1991. Nitrogen release from the leaves of some tropical legumes as affected by their lignin and polyphenolic contents. Soil Biol. Biochem., 23, 83-88.
Paul E.A. & Clark F.E., 1996. Soil microbiology and biochemistry. San Diego, CA, USA: Academic Press.
Picard C. et al., 1992. Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl. Environ. Microbiol., 58, 2717-2722.
Porteous L.A., Sidler R.J. & Watrud L.S., 1997. An improved method for purifying DNA from soil for PCR amplification and molecular ecology applications. Mol. Ecol., 6, 787-791.
Powell S.J. & Prosser J.I., 1986. Inhibition of NH4 oxidation by nitrapyrin in soil and liquid culture. Appl. Environ. Microbiol., 52, 782-787.
Rice E.L., 1984. Allelopathy. New York, USA: Academic Press, Inc.
Rice E.L. & Pancholy S.K., 1973. Inhibition of nitrification by climax ecosystems. II. Additional evidence and possible role of tannins. Am. J. Bot., 60, 691-702.
Sahrawat K.L., 1989. Effects of nitrification inhibitors on nitrogen transformations, other than nitrification, in soils. Adv. Agron., 42, 279-309.
Sanchez P.A. et al., 1997. Soil fertility replenishment in Africa: an investment in natural resource capitol. In: Buresh R.J., Sanchez P.A. & Calhoun F., eds. Replenishing soil fertility in Africa. SSSA Special Publication 51. Madison, WI, USA: Soil Science Society of America and American Society of Agronomy, 1-46.
Schroth G., 2003. Decomposition and nutrient supply from biomass. In: Schroth G. & Sinclair F.L., eds. Trees, crops and soil fertility: concepts and research methods. Wallingford, UK: CAB International, 131-150.
Schwendener C.M. et al., 2005. Nitrogen transfer between high- and low-quality leaves on a nutrient-poor Oxisol determined by 15N enrichment. Soil Biol. Biochem., 37, 787-794.
Seneviratne G., 2000. Litter quality and nitrogen release in tropical agriculture: a synthesis. Biol. Fertil. Soils, 31, 60-64.
Somville M., 1978. A method for the measurement of nitrification rates in the water. Water Res., 12, 843-848.
Trinsoutrot I. et al., 2000. Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under no limiting nitrogen conditions. Soil Sci. Soc. Am. J., 64, 918-926.
Underhill S.E. & Prosser J.I., 1985. Inhibition of nitrification by potassium ethyl xanthane in soil and in liquid culture. Soil Biol. Biochem., 17, 229-233.
Waterman P.G. & Mole S., 1994. Analysis of phenolic plant metabolites. Oxford, UK; Boston, USA: Blackwell Scientific.
Weisburg W.G., Barns S.M., Pelletier D.A. & Lane D.J., 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol., 173, 697-703.
Wieder W.R. et al., 2013. Experimental removal and addition of leaf litter inputs reduces nitrate production and loss in a lowland tropical forest. Biogeochemistry, 113, 629-642.






