- Portada
- Volume 19 (2015)
- numéro 3
- An analysis of potential resistance of the phytophagous mite, Tetranychus urticae Koch (Acari: Tetranychidae) to four botanical pesticides
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
An analysis of potential resistance of the phytophagous mite, Tetranychus urticae Koch (Acari: Tetranychidae) to four botanical pesticides

Notes de la rédaction
Received on September 30, 2014; accepted on March 10, 2015
Résumé
Analyse de la résistance à quatre extraits de plantes chez l'acarien phytophage Tetranychus urticae Koch (Acari : Tetranychidae)
Description du sujet. Le développement des stratégies de lutte intégrée est en croissance depuis que de nombreux problèmes sont apparus suite à l’utilisation abusive et non raisonnée des pesticides de synthèse. Les extraits de plantes sont de plus en plus considérés, au sein des stratégies de lutte intégrée, comme des approches alternatives ou complémentaires aux traitements insecticides. Beaucoup d'huiles végétales essentielles montrent un large spectre d'activité contre les ravageurs.
Objectifs. Notre objectif est de tester si l’acarien Tetranychus urticae arrive à développer une résistance vis-à-vis des extraits naturels de plantes.
Méthode. Nous avons analysé l’émergence de la résistance de quatre extraits végétaux : Deverra scoparia Coss. & Durieu (Araliales : Apiaceae), Santolina africana Jord. & Fourr. (Asterales : Asteraceae), Hertia cheirifolia (L.) Kuntze (Asterales : Asteraceae) et Allium sativum L. (Asparagales : Alliaceae) sur T. urticae, l'un des ravageurs les plus importants dans le monde, durant 20 générations successives.
Résultats. Le traitement par S. africana ne montre aucune résistance, tandis qu'un faible développement de la résistance a été observé avec H. cheirifolia, A. sativum et D. scoparia.
Conclusions. Ce travail a démontré l'efficacité de ces extraits contre T. urticae. De plus, le fait qu’ils induisent peu de résistance confirme leur valeur en tant qu’outil efficace pouvant être utilisé dans une lutte intégrée contre les ravageurs.
Abstract
Description of the subject. Synthetic acaricides have been widely used to manage Tetranychus urticae. Due to the excessive use of biocide and the associated problems of pesticide resistance and environmental pollution, there is an increasing demand for sustainable, environmentally-friendly control methods. Among the current alternative strategies aimed at decreasing the pest populations, the pesticides based on plant extracts are currently one of the most promising methods. Essential oils with acaricidal properties have been categorized as green pesticides because they are biodegradable and predominantly non-toxic to vertebrates.
Objectives. With an aim to reduce the use of synthetic pesticides, they represent a promising approach for eco-chemical control of mites.
Method. The aim of the present work was to analyze the risk of resistance emergence of T. urticae to repeated treatments with four plant extracts: Deverra scoparia Coss. & Durieu (Araliales: Apiaceae), Hertia cheirifolia (L.) Kuntze (Asterales: Ateraceae), Santolina africana Jord. & Fourr. (Asterales: Asteraceae) essential oils and garlic distillate Allium sativum L. (Asparagales: Alliaceae) after 20 generations.
Results. Repeated treatments with S. africana essential oil during 20 generations did not provoke an emergence of resistance while a low development of resistance was observed with H. cheirifolia, A. sativum and D. scoparia extracts.
Conclusions. The efficacy of these extracts against the two spotted spider mite and their low development of resistance make them a promising use for pest management.
Tabla de contenidos
1. Introduction
1Tetranychus urticae Koch (Acari: Tetranychidae), the two spotted spider mite is a highly polyphagous pest that can devastate many crops (Migeon et al., 2007) of over 900 species of plant-hosts such as citrus, avocado, beans, cotton, apples, pears, plums and many other horticultural and ornamentals crops (Helle et al., 1985). It is especially dominant in intensive, high-yield cropping systems. It affects yields by feeding on leaves thereby reducing the area of photosynthetic activity (Helle et al., 1985; Rabbinge, 1985). When severe infestations, it causes leaf abscission. Spider mites are thus a major concern for growers worldwide in terms of damage and control cost and are therefore considered as one of the most important pests (Helle et al., 1985). Synthetic acaricides are usually applied by farmers to reduce losses. However, the extensive use of theses substances had led to the development of resistance. Tetranychus urticae’ pesticide-resistant populations have been reported in more than 40 countries in both greenhouses and open field crops (Georghiou et al., 1991; Stumpf et al., 2001; Van Leeuwen et al., 2007; Van Leeuwen et al., 2010). The rapid development of resistance in T. urticae is favored by its high reproductive potential, extremely short life cycle and arrhenotokous mating system (Helle et al., 1985; Van Leeuwen et al., 2005): for commercial acaricides, such as organophosphates, dicofol, organotins, failures in the chemical control of spider mites caused by resistance have been reported only a few years after their introduction (Jeppson et al., 1975; Cranham et al., 1985; Van Leeuwen et al., 2006). Another problem is the possible cross-resistance between active matters that put the availability of pesticides under further pressure (Van Pottelberge et al., 2009). Among current alternative strategies aiming at decreasing the use of chemical pesticides, ecochemical control based on plant-insect relationships is one of the most promising methods. Indeed, higher plants produce different bioactive secondary metabolites involved in their natural resistance to pathogens, insects and mites. Essential oil extract contains many of these compounds particularly secondary metabolites that are frequently concentrated in the leaves, bark, or fruit of aromatic plants (Isman et al., 2001; Kumar et al., 2011). Plant resistance to insect pests can result from the secretion of these essential oils (Regnault-Roger, 1997). For example, the resistance of Scotch pine Pinus sylvestris (conifers) to flat bugs Aradus betulae (Aradidae) was related to a high content of essential oil in the primary bark (Smelyanets et al., 1973). Another example is Chrysanthemum cinerariaefolium (Compositae) and Chrysanthemum coccineum that contain pyrethrin, a potent insecticide with a rapid knock down action (Ryan et al., 1988). The pulegone found in mint oils repels the German cockroach Blatella germanica (L.) (Praveena et al., 2011). These examples show that plant products may provide eco-chemical and biorational strategy to protect cereals, pulses and other agricultural commodities from losses by pests (Dubey, 2010; Attia et al., 2013).
2However, the repeated application of essential oils for pest control could also potentially induce the acquisition of resistance. It is therefore necessary to test that possibility even if it is less likely that mites will evolve resistance to essential oils as they are a mixture of different active compounds. This low acquisition of resistance linked to the complexity of essential oil composition was already shown in insects (Liu et al., 2004) but has never been explored in mites. The purpose of this study is to analyze the emergence of the resistance of a Tetranychus urticae population to repeated applications of Deverra scoparia Coss. & Durieu, Santolina africana Jord. & Fourr., Hertia cheirifolia (L.) Kuntze essential oils and of a garlic extract, Allium sativum L. This selection of plants is based on previous works (Attia et al., 2011a; Attia et al., 2011b; Attia et al., 2011c; Attia et al., 2012) where we examined the effect of different concentrations of S. africana, H. cheirifolia, D. scoparia essential oils and garlic extract A. sativum against T. urticae. We showed that they caused significant T. urticae mortality after 24 h at low concentrations, with LC50 values of 2.35 mg·l-1 for S. africana, 3.43mg·l-1 for H. cheirifolia, 1.79 mg·l-1 for D. scoparia and 7.49 mg·l-1 for A. sativum (Attia et al., 2011a; Attia et al., 2011b; Attia et al., 2011c; Attia et al., 2012). In addition, fecundity decreased after treatments with sublethal concentrations of extracts corresponding for D. scoparia to 0.07, 0.09, 0.29 mg·l-1, H. cheirifolia and S. africana oils to 0.064, 0.08, 0.26 mg·l-1 respectively and for the garlic distillate, A. sativum, to 0.36 and 0.74 mg·l-1 (Attia et al., 2011b; Attia et al., 2011c; Attia et al., 2012). In the present study, emergence of resistance experiments was performed on 20 generations. Our study is the first to evaluate the emergence of resistance to some natural biopesticides of one of the most important pest in the world, T. urticae.
2. Materials and methods
2.1. Collection and maintenance of mites
3Tetranychus urticae were collected from infested plants in citrus orchards in Tunisia and transferred to a climate controlled room (26 °C, 50–60% RH, 16:8 [L:D]) in a laboratory at the Biodiversity Research Centre, UCL, Louvain-la-Neuve (Belgium) for more than 5 years without any contact with pesticides before the experiments. The population was reared on bean leaves (Phaseolus vulgaris) placed on moistened cotton in Petri dishes (Overmeer, 1985). Only young adult (24h old) females were chosen for this bioassay.
2.2. Plant and essential oil extraction
4Plants used for this study were collected locally in Tunisia, and were free of any pre-harvest chemical treatments (organic products such as Spirodiclofen and Fenbutatin oxide). The plants were freshly harvested and sorted for uniformity and absence of defects before being stored at -2 °C until the analysis and the tests.
5Extracts of A. sativum and D. scoparia, S. africana and H. cheirifolia oils were obtained from 3 kg of the aerial parts of the plants by hydrodistillation for 3 h using a Clevenger-type apparatus. Resulting oils were diluted 1:100 in absolute ethanol.
6The essential oil yield of the plants used in the current study was 0.5% of the dry weight of S. africana, 0.7% of H. cheirifolia and 0.5% of D. scoparia.
2.3. Toxicity bioassay
7A group of 2,000 virgin young adult females T. urticae (24 h) were randomly selected and then transferred to 40 fresh bean leaf discs (diameter = 35 mm) mounted with the adaxial side uppermost on moistened cotton in Petri dishes (90 x 15 mm), meaning 50 T. urticae by leaf disc. A Potter spray tower producing a uniform deposit was used to spray individuals of T. urticae after settling time on the leaf discs at a 1.4 bar pressure. Plants extracts were diluted in ethanol to obtain a homogenous spray film at 20 ± 2 °C. After this first selection (or treatments with extracts), the surviving T. urticae were transferred back to untreated bean plants and the population was allowed to increase during one generation. The second selection (or treatment) was conducted after two generations: once again, a group of 2,000 young adult (24 h) females T. urticae were randomly selected in the population coming from the surviving individuals of the last treatment. The tested T. urticae were sprayed in the similar conditions as described before. In whole, 20 successive selections (10 months) were done each separated by two generations of T. urticae.
8Tetranychus urticae were treated with extracts of A. sativum, D. scoparia, S. africana and H. cheirifolia at the LC90 of 13.5, 5.07, 3.79 and 3.2 mg·l-1 of ethanol respectively to test the induction of resistance. These values were calculated from previous works (Attia et al., 2011b; Attia et al., 2011c; Attia et al., 2012) using concentrations that killed 90% of T. urticae, for each kind of extract respectively.
9One control treatment was done and without any plant extracts but ethanol at the same concentration.
2.4. Statistical analyses
10For each treatment, we calculated the number of surviving individuals on the 40 x 50 sampled every two generations. Our hypothesis was that, if there was no resistance, the number of surviving T. urticae should not differ after each selection, meaning that the number of surviving T. urticae induced by the first selection (that we called Ns1) should be similar to the number of surviving T. urticae induced by the second selection (Ns2) and so on.
11We calculated all the differences between all the Ns: Ns1-Ns2 (called Ns1-2), Ns1-Ns3,… Ns1-Ns20, Ns2-Ns3, Ns2-Ns4,… Ns2-Ns20,… Ns3-Ns4, … Ns19-Ns20. If there was no resistance, the median of all these differences should not statistically differ from 0. If there is resistance, the Ns should increase selection after selection and the median of the differences should be lower than 0. Our hypothesis was that: Ho: mediate of Ns-all = 0 vs HA: mediate of Ns-all ≠ 0.
12As the calculated differences (called Ns-all) were not normally distributed, we used a Wilkson Singed Rank test to compare the median of each treatment with the value “0”. To compare all the treatments between them, we used a Kruskall-Wallis test. Tests were performed using Graph Pad Prism version 5.01 for Windows® (Graph Pad Software, San Diego, USA). All tests were applied under two-tailed hypotheses and the significance level p was set at 0.05.
3. Results
3.1. Effects of the treatments on the resistance
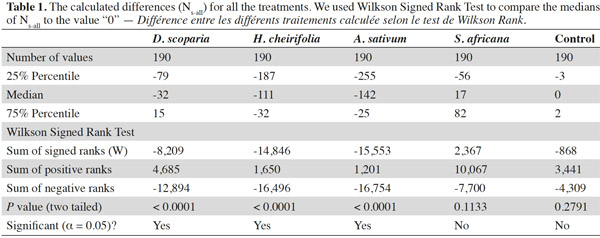
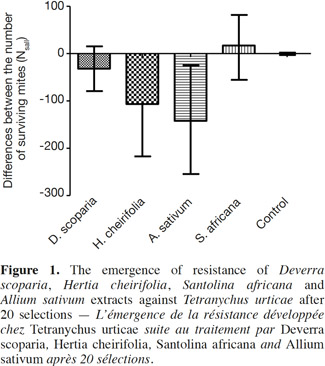
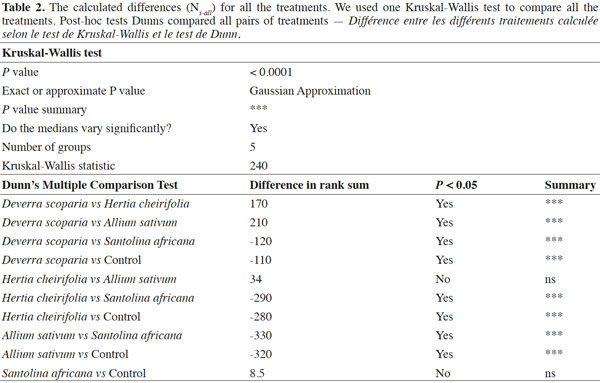
13The median of the calculated differences (Ns-all) for each treatment was compared to the value 0 (Table 1, Figure 1): two treatments, the control and S. africana, were not statistically different from “0”. Thus, these two treatments did not induce any resistance in T. urticae for this lap of time. The medians of Ns-all of the three other treatments, H. cheirifolia, D. scoparia and A. sativum were statistically different from “0”, thus indicating the induction of resistance (Table 1, Figure 1).
3.2. Differences between the five treatments
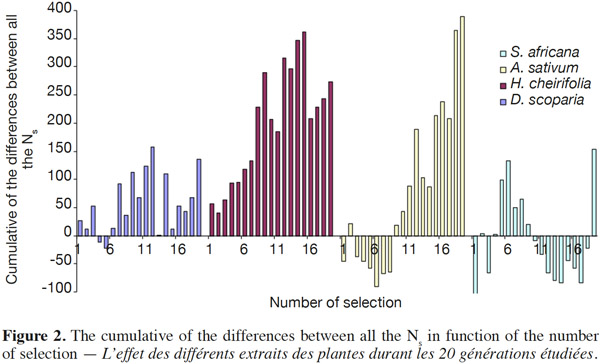
14Concerning the four plant extracts, there was a significant difference between all the treatments. The post-hoc tests showed no statistical differences between the treatment with S. africana oil and the control treatment (Table 2) nor between H. cheirifolia vs A. sativum extracts. The other post-hoc tests showed statistical differences between all other pairs of treatments. In comparison of the cumulative of the differences between all the Ns in function of the number of selection, no resistance was observed towards S. africana (0%); however a little resistance developed with D. scoparia oil (about 10%), H. cheirifolia oil (30%) and A. sativum extract (50%) (Figures 1 and 2).
4. Discussion
15Our results showed that T. urticae can develop a resistance towards D. scoparia, H. cheirifolia and A. sativum extracts within 10 months and 20 selections, but quite limited. However no resistance was observed in the case of S. africana essential oil. The resistance depends on the mode of application and may suggest that the essential oil has to be first in contact with the cells (Rafii et al., 2007). In the case of S. africana, it is possible that T. urticae does not have the ability to detoxify some of the chemicals contained in the oil that have perhaps different mechanisms of action.
16The mode of action of the essential oils is not yet well known but some authors suggest it may also be due to suffocation, by blocking the spiracles of pests (Chaubey, 2008). Indeed, it has been shown with petroleum-derived oils (Beattie et al., 1995) that heavy saturated oil fractions can penetrate a long distance into the tracheae. This fraction forms physical barriers to gaseous exchanges and provokes suffocation. With this hypothesis, the efficiency of essential oils should depend on their physical more than on their chemical characteristics.
17However, we think that suffocation is not the mode of action of essential oils as most of their compounds are light, unsaturated and volatile ones. Moreover, it is known that suffocation is an extremely slow process. During our observations, T. urticae were killed 24 h after treatments with essential oils. Therefore, it is likely that other factors than suffocation are the prime cause of mortality.
18Differences in chemical composition of oils may probably explain why plant extract differ in resistance induction (Attia et al., 2011b; Attia et al., 2011c; Attia et al., 2012).
19In previous works, we observed that S. africana, H. cheirifolia and A. sativum are especially rich in oxygenated compounds with 94.83%, 78.99%, and 36.8% respectively while D. scoparia extract is rich in hydrogenated compounds with 92.96%. The most abundant components in the Santolina oil were terpinen-4-ol (54.96%), α-terpineol (14%) and borneol (8.37%). In fact, compounds as limonene, trans-chrysanthenol, borneol, carvenone, cis-jasmone and guaiol are specific to S. africana oil (Attia et al., 2013). The results of Attia et al. (2012) showed that the presence of all these constituents was necessary to equal the toxicity of the natural oil of S. africana against T. urticae (Attia et al., 2012). Moreover, complexity of the mixture should increase the time needed for resistance development compared to a pesticide based on a single active ingredient. This phenomenon was observed in the case of the green peach aphids, Myzus persicae, that can develop resistance to azadirachtin, the major ingredient of neem, Azadirachta indica A.Juss (insecticide) but can not develop resistance to a refined neem seed extract containing the same absolute amount of azadirachtin but with many other constituents present (Feng et al., 1995). Ryan et al. (1988) showed that six terpinoids corresponding to pulegone, gossypol, citral, linalool, bornyl acetate and cineole may act as plant defense compounds and may inhibit the enzyme acethylcholinesterase. The insecticide effect of Cedrus deodora (Pinaceae) extracts to the pulse beetle Callosobruchus analis (Coleoptera: Bruchiadae) and to the house fly Musca domestica (Diptera: Muscidea) was related to a high content of terpens compounds diversified (Agarwal et al., 1988).
20We still are, however, lacking information on the exact way of action of essential oil compounds, whereas it is well-known for synthetic pesticides (Dekeyser, 2005; Isman et al., 2006; Van Leeuwen et al., 2007; Van Nieuwenhuyse et al., 2009). The rapid toxicity from essential oils or their monoterpenes in insects and other arthropods points to a neurotoxic mode of action (Isman et al., 2006). In fact, the octopaminergic nervous system is considered to be the site of action of some compounds of essential oils in the control of the American cockroach, Periplaneta Americana (Enan, 2001), and fruit fly (Enan, 2005). Symptoms are hyperactivity followed by hypertension of the legs and abdomen culminating and immobilization (Enan, 2001). The same author pointed that many essential oil constituents such as monoterpenoids poison insects by blocking octopamine receptors (Enan, 2001; Enan, 2005). In fact, octopamine, synthetized from tyramine, is a biogenic monoamine structurally related to noradrenaline that acts as a neurohormone, a neuromodulator and may be a neurotransmitter in Arthropods. It is present in relatively high concentrations in neuronal systems as well as in non-neuronal tissues of most invertebrate species including Arthropods (Roeder, 1999).
21The toxic effects of essential oils might be attributed to their major components, as well as other minor and/or trace compounds. The toxicity could vary with both the compounds of the plant extract and the sensitivity of the mite. It has been suggested that volatile oils, either inhaled or applied to the cuticle, act by means of their lipophilic fraction reacting with the lipid parts of the cell membranes, and as a result, modify the activity of the calcium ion channels (Buchbauer et al., 1994). At certain levels of dosage, the volatile oils saturate the membranes and show effects similar to those of local anaesthetics. They can interact with the cell membranes by means of their physio-chemical properties and molecular shapes, and can influence their enzymes, carriers, ion channels and receptors. The interaction of various components in the essential oil might be another important factor for the difficulty of resistance-induction. Future experiments are necessary to establish if similar process are involved in T. urticae.
22Regarding the resistance against synthetic insecticides, three main metabolic enzyme groups are known to be involved: esterases, glutathione S-transferases (GST) and mono-oxygenases (Van Leeuwen et al., 2010). Dekeyser (2005) pointed out that several acaricides have been identified as mitochondrial respiration inhibitors; these include active ingredients as pyridazine, pyrazole, quinazoline, naphthoquinone, pyrrole, thiourea and pyrimidine chemistries. Others are growth inhibitors, such as benzoylphenylureas, tetrazines, tetronic acids and oxazolines (Dekeyser, 2005). Only tetronic acids like spirodiclofen, spiromesifen work via inhibition of lipid synthesis. Benzoylphenylurea’s and etoxazole work via inhibition of chitin synthase (Van Leeuwen et al., 2010). Others have unknown mode of action. Among growth inhibitors insecticides, spirodiclofen differs from others in its biochemical mechanism as it interferes with lipid biosynthesis, blocking the enzyme acetyl-CoA carboxylase that allows T. urticae to form important fatty acids (Dekeyser, 2005; Bretschneider et al., 2007). Finally, insecticides have been identified as complex III inhibitor such as Bifenzate, Halfenprox, Lubrocythrinate, and Milbemectin. Their mode of action is reported by Dekeyser (2005): in fact they could block the nervous signal or induce repetitive discharge and prolonge the opening of sodium ion channels, or, finally, act as a GABA agonist (Dekeyser, 2005) By analogy, future experiments should be focused on the identification of the mode and site of action of the plants extracts tested here and the discovery of compounds affecting developmental processes in T. urticae.
23For recurrent applications, S. africana essential oil seems to be the best candidate in our study. Santolina africana oil may be used as alternative strategy against T. urticae to the heavy use of classical insecticides.
Bibliographie
Agarwal R.A. & Singh D.K., 1988. Harmful gastropods and their control. Acta Hydrochim. Hydrobiol., 16, 113-138.
Attia S. et al., 2011a. Acaricidal activity of 31 essential oils extracted from plants collected in Tunisia. J. Essent. Oil Res., 24, 279-288.
Attia S. et al., 2011b. Efficient concentration of garlic distillate (Allium sativum) for the control of Tetranychus urticae (Tetranychidae). J. Appl. Entomol., 136, 302-312.
Attia S. et al., 2011c. Chemical composition and acaricidal properties of Deverra scoparia essential oil (Araliales: Apiaceae) and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae). J. Econ. Entomol., 104, 1220-1228.
Attia S. et al., 2012. Acaricidal activities of Santolina africana and Hertia cheirifolia essential oils against the two spotted spider mite (Tetranychus urticae). Pest Manage. Sci., 68, 1069-1076.
Attia S. et al., 2013. A review of the major approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special references to natural pesticides. J. Pest Sci., 86, 361-386.
Beattie G.A.C. et al., 1995. Evaluation of petroleum spray oils and polysaccharides for control of Phyllocnistis citrella Stainton (Lepidoptera: Gracillaridae). J. Aust. Entomol. Soc., 34, 349-353.
Bretschneider T. & Fischer R.N., 2007. Inhibitors of lipid synthesis (acetyl-CoA-carboxylase inhibitors). In: Modern crop protection compounds. Weinheim, Germany: Wiley, 909-925.
Buchbauer G. & Jirovetz L., 1994. Aromatherapy – Use of fragrances and essential oils as medicaments. Flavour Fragrance J., 9, 217-222
Chaubey M.K., 2008. Fumigant toxicity of essential oils from some common species against pulse beetle Callosobruchus chinensis (Coleoptera: Bruchidae). J. Oleo Sci., 57, 171-179.
Cranham J.E. & Helle W., 1985. Pesticide resistance in Tetranychidae. In: Helle H. & Sabelis M.W., eds. Spider mites: their biology, natural enemies and control. World crop pests. Vol 1B. Amsterdam, The Netherlands: Elsevier, 405-421.
Dekeyser M.A., 2005. Acaricide mode of action. Pest Manage. Sci., 61, 103-110.
Dubey N.K., 2010. Natural products in plant pest management. 1st ed. Wallingford, UK: CABI.
Enan E., 2001. Insecticidal activity of essential oils: octopaminergic sites of action. Comp. Biochem. Physiol. C: Toxicol. Pharmacol., 130, 325-337.
Enan E., 2005. Molecular and pharmacological analysis of an octopamine receptor from American cockroach and fruit fly in response to plant essential oils. Arch. Insect. Biochem. Physiol., 59, 161-171.
Feng R. & Isman M.B., 1995. Selection for resistance to azadirachtin in the green peach aphid Myzus persicae. Experientia, 51, 831-833.
Georghiou G.P. & Lagunes-Tejada A., 1991. The occurrence of resistance to pesticides in arthropods. An index of cases reported through 1989. Roma: FAO.
Helle H. & Sabelis M.W., 1985. Spider mites: their biology, natural enemies and control. World crop pests. Amsterdam, The Netherlands: Elsevier.
Isman M.B., Wan A.J. & Passreiter C.M., 2001. Insecticidal activity of essential oils to the tobacco cutworm Spodoptera lituta. Fitoterapia, 72, 65-68.
Isman M.B. & Machial C.M., 2006. Chapter 2. Pesticides based on plant essential oils: from traditional practice to commercialization. In: Rai M. & Carpinella M., eds. Naturally occurring bioactive compounds. Vol. 3. Elsevier, 29-44.
Jeppson L.R., Keifer H.H. & Baker E.W., 1975. Mites injurious to economic plants. London: University of California Press.
Kumar P., Mishra S., Malik A. & Satya S., 2011. Insecticidal properties of Mentha species: a review. Ind. Crop. Prod., 34, 802-817.
Liu B. & Şengonca C., 2004. Biotechnological development of GCSC-BtA as a new type of biocide. Gottingen, Germany: Cuvillier Verlag.
Migeon A. & Dorkeld F., 2007. Spider mites web: a comprehensive database for the Tetranychidae, http://www.montpellier.inra.fr/CBGP/spmweb, (09/06/15).
Overmeer W.P.J., 1985. Alternative prey and other food resources. In: Helle H. & Sabelis M.W., eds. Spider mites: their biology, natural ennemies and control. World crop pests. Amsterdam, The Netherlands: Elsevier, 131-139.
Praveena A. & Sanjayan K.P., 2011. Inhibition of acethylcholinesterase in three insects of economic importance by linalool, a monoterpene phytochemical. In: Dunston P. Ambrose, ed. Insect pest management. A current scenario. Palayamkottai, India: Entomology Research Unit, St. Xavier’s College, 340-345.
Rabbinge R., 1985. Aspects of damage assessment. In: Helle H. & Sabelis M.W., eds. Spider mites: their biology, natural enemies and control. World crop pests. Vol. 1B. Amsterdam, The Netherlands: Elsevier, 261-272.
Rafii F. & Shahverdi A.R., 2007. Comparison of essential oils from three plants for enhancement of antimicrobial activity of nitrofurantoin against enterobacteria. Chemotherapy, 53, 21-25.
Regnault-Roger C., 1997. The potential of botanical essential oils for insect pest control. Integr. Pest Manage. Rev., 2, 25-34.
Roeder T., 1999. Octopamine in invertebrates. Progress Neurobiol., 59, 533-561.
Ryan M.F. & Byrne O., 1988. Plant insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol., 14, 1965-1975.
Smelyanets V.P. & Khursin L.A., 1973. Significance of individual terpenoids in the mechanism of population distribution of pests on Scotch pine stands. Zashchita Rasteni (Kiev), 17, 33-44.
Stumpf N. et al., 2001. Resistance to organophosphates and biochemical genotyping of acetylcholinesterases in Tetranychus urticae (Acari: Tetranychidae). Pestic. Biochem. Physiol., 69, 131-142.
Van Leeuwen T., Van Pottelberge S. & Tirry L., 2005. Comparative acaricide susceptibility and detoxifying enzyme activities in field-collected resistant and susceptible strains of Tetranychus urticae. Pest Manage. Sci., 61, 499-507.
Van Leeuwen T., Van Pottelberge S. & Tirry L., 2006. Biochemical analysis of a chlorfenapyr-selected resistant strain of Tetranychus urticae Koch. Pest Manage. Sci., 62, 425-433.
Van Leeuwen T., Van Pottelberge S., Nauen R. & Tirry L., 2007. Organophosphate insecticides and acaricides antagonise bifenazate toxicity through esterase inhibition in Tetranychus urticae. Pest Manage. Sci., 63, 1172-1177.
Van Leeuwen T. et al., 2010. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem. Mol. Biol., 40, 563-572.
Van Nieuwenhuyse P. et al., 2009. Mutations in the mitochondrial cytochrome b of Tetranychus urticae Koch (Acari: Tetranychidae) confer cross-resistance between bifenazate and acequinocyl. Pest Manage. Sci., 65, 404-412.
Van Pottelberge S., Van Leeuwen T., Nauen R. & Tirry L., 2009. Resistance mechanisms to mitochondrial electron transport inhibitors in a field-collected strain of Tetranychus urticae Koch (Acari: Tetranychidae). Bull. Entomol. Res., 99, 23-31.






