- Startpagina tijdschrift
- volume 9 (2005)
- numéro 3
- Study of catalase production by an Aspergillus phoenicis mutant strain in date flour extract submerged cultures
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Study of catalase production by an Aspergillus phoenicis mutant strain in date flour extract submerged cultures

Nota's van de redactie
Received 26 April 2004, accepted 11 February 2005
Résumé
Etude de la production de catalase par une souche mutante d’Aspergillus phoenicis en milieu liquide à base d’extrait de farine de dattes. Une souche mutante d’Aspergillus phoenicis (espèce voisine d’Aspergillus niger) a été sélectionnée pour sa capacité à produire de la catalase sur un milieu de culture contenant un extrait soluble de farine de datte. La souche a été cultivée en erlenmeyers de 500 ml et en réacteur de 20 litres. L’activité catalase extracellulaire a atteint 59 U ml-1 dans le surnageant de culture après 48 heures en fermenteur. Cette production est six fois plus élevée que celle obtenue à partir de la souche sauvage. L’examen microscopique de la biomasse en cours de culture montre que l’excrétion de l’enzyme est correlée avec une évolution de la morphologie des pellets de la moisissure. L’apparition d’hyphes ramifiés coïncide avec une excrétion maximale de catalase.
Abstract
The production of extracellular catalase in date flour submerged medium by a selected mutant Aspergillus phoenicis K30 (member of the Aspergillus niger group) was investigated. The strain was tested in 500 ml shake-flasks and in a 20 l bioreactor with date powder as a single carbon source. Extracellular catalase production reached 59 U ml-1 in both cases. This value is much greater than that of a wild-type strain (9.5 U ml-1). Microscopic examination showed that the extracellular catalase production was correlated with the ramified hyphals morphology in the external layer of the pellets.
Inhoudstafel
1. Introduction
1Catalases (H2O2 : H2O2 oxidoreductase, EC 1.11.1.6) are the class of enzymes, which catalyse the decomposition of hydrogen peroxide to oxygen and water. These ubiquitous enzymes have been isolated and purified from different natural sources including animal tissues, plants and micro-organisms. As all aerobic organisms which have evolved specific enzyme systems to neutralise potentially lethal effects of hydrogen peroxide (Halliwell, 1990). Physiologically they act as regulators of H2O2 levels in organelles (Venkateshwaran et al., 1999).
2The Aspergillus niger fungus is the second commercial source of these enzymes.
3Catalases are used in several industrial applications such as food or textile processing to remove hydrogen peroxide that is used for sterilisation or bleaching (Akertek, Tarhan, 1995). Industrially, the extracellular enzymes comparatively to the intracellular enzymes are more advantageous from an economical point of view. However, the catalases located in the cytoplasm and in the peroxisomes are not usually excreted from the cell. Thus there is little information regarding the regulation of catalase synthesis in A. niger and the influence of physiological conditions on the excretion of this enzyme into the medium (Petruccioli et al., 1995; Gromada, Fiedurek, 1997). Despite the obvious commercial interest in A. niger catalases and the desire to obtain mutants of A. niger which produce substantial amount of extracellular catalase (Fiedurek, Gromada, 1997), production of extracellular catalases by filamentous fungi and their mutants in natural media such as date flour extract has not been investigated. The use of this date flour product as a fermentation medium has an economical interest for countries like Algeria.
4The Algerian palm culture resources are estimated today at more than 13 millions palm trees with an immense annual date production of about 400000 tons (Anonymous, 2003). Although this large production comes from different varieties, a large proportion of it is of the dry type i.e. a low grade quality. The dry date pulp contains about 80% of essential sugars such as sucrose, fructose and glucose. In addition to this considerable sugar content, it was proved as a good source of some important minerals. Therefore, the present study was undertaken to investigate the possibility of producing extracellular catalase by an Aspergillus phoenicis mutant grown on date flour soluble extract medium in different conditions.
2. Materials and methods
2.1. Strain
5The A. phoenicis wild-type strain was isolated from foodstuff in the East of Algeria. It was stored at -20°C as a spore suspension in 30% (v/v) glycerol diluted in physiological water. This strain produces small amounts of extracellular catalase on solid medium and in shaken submerged culture (9.5 U.ml-1). It was therefore used in mutagenesis experiments.
2.2. Mutagenesis
6The induction of mutants was carried out as described previously (Witteveen et al., 1990) with some modifications. A suspension of conidia (10 ml, 3 107 conidia ml-1) harvested from a seven days old Potato Dextrose Agar medium (PDA) was shaken vigorously for 1 min followed by filtration to remove mycelial fragments and irradiated in open plastic Petri dishes by UV light (Sylvania G 30W) for 5 to 45 sec. The spores were spread on minimal solid medium (Pontecorvo et al., 1953) plates to select the surviving ones. The colonies which appeared over the background were picked up and tested for extracellular catalase production. To obtain mutants able to grow in the presence of high concentrations of date flour, the irradiated spores were plated on minimal medium supplemented with corn steep 10 g l-1, agar 15 g l-1 and 1% Date Flour Soluble Extract (DFSE). After three days of incubation at 30°C, the largest colonies were inoculated on a similar medium but with increasing concentrations of date flour (gradually up to 6%).
2.3. Selection of mutants
7The selection test of the mutant producing an extracellular catalase activity was carried out as described by Fiedurek, Gromada (1997). In the present study, the proposed medium was supplemented with 0.2% DFSE and the sodium deoxycholate was prepared in 0.1M phosphate buffer pH 7.5. For the visualisation of a catalatic activity area a staining technique was applied (Gregory, Fridovich, 1974). The plates were developed with 0.2 M KI to give colourless zones on a blue background.
2.4. Date Flour Soluble Extract
8To prepare the date flour soluble extract, which forms the basical constituent of the medium in this study, the pulp of a low-grade quality dates (var. MECH-DEGLA) of Phoenix dactylifera (produced in the South of Algeria) was dried for 48 h at 45°C. The dates (40 g) were crushed into a powder and suspended in distilled water. After three hours of agitation, the suspension was centrifuged for one hour at 4500 g. The supernatant was completed to 1000 ml to obtain the final concentration of the DFSE per liter.
2.5. Culture medium
9The culture medium was prepared with one liter of DFSE supplemented by: 10 g corn steep; 2 g Ca(NO3)2 4H2O; 0.5 g KH2PO4; 0.5 g MgSO4 7H2O; 0.5 g KCl; 0.001 g FeSO4 7H2O.
2.6. Shake-flask cultures
10The culture was carried out in 500 ml erlenmeyer flasks filled with 100 ml medium at pH 6.0 and 30°C. The sterilised media were inoculated with a final concentration of 1 × 106 spores ml-1. The conidia were harvested from seven days old PDA culture. The flasks were incubated for 144 h in a rotary shaker operating at 140 rpm (round per minute). Samples were taken every 24 h. At the end of each incubation period, the fungal mycelium was separated from the culture fluid by filtration on Whatman paper n°2. The filtrate was used for pH measurement and for determination of extracellular catalase activity. The mycelium was used for endocellular enzyme activity and dry weight determination.
2.7. Batch bioreactor cultures
11Cultures were carried out in a 20 l stirred Biolafitte bioreactor containing 12 l culture medium equiped with a mechanical foam-breaking system (air volume/liquid volume/minute). The conditions of fermentation were pH 6, temperature 30°C, aeration 1 v/v/m, and agitation 200 rpm. All fermentation parameters were controlled with a Biolafitte regulation unit. A preculture broth (24 h, 120 ml) of Aspergillus K30 grown in a 500 ml shake-flask in the same medium was used for fermentor inoculation. The samples were taken every 6 h. The fungal mycelium was separated from the culture medium by filtration on Whatman paper n°2. The filtrate was used for the determination of extracellular catalase activity and the mycelium was used for endocellular enzyme activity and dry weight determination.
2.8. Microscopy and morphological measurements
12To determine the morphology of tips in hyphal tree and its relation to extracellular catalase production, the mycelia were harvested and stained using the fluorescent labelling technique (Boulos et al., 1999). At several growth stages, the cell morphology was analysed with a Zeiss Axioskop 2 fluorescence microscope (Carl Zeiss, Jena, Germany).
2.9. Analytical procedure
13Extra- and intracellular catalase activities were measured respectively in culture filtrates and in cell free extract as described by Caridis et al. (1991). The cell free extract was prepared by breaking down intermittently the washed mycelium, in 0.1 M phosphate buffer (pH 7.5) in ice-bath (0°C), using the ultra sonic vibration (Sonnifier Main Components Model 250/450, VWR, Leuven, Belgium) during 15 sec. Catalase activity was measured spectrophotometrically by observing the decrease in light absorption at 240 nm during decomposition of H2O2 by the enzyme. The reaction mixture (3 ml) contained 0.1 M phosphate buffer, pH 8.0, 0.03 ml of a suitably diluted enzyme and 0.5 ml of 108.8 mM H2O2 solution. One unit (U) of catalase activity was defined as the amount of enzyme catalysing the decomposition of one mmol of H2O2 min-1 at 25°C and pH 7.5.
3. Results
3.1. Mutant selection
14It was established from agar diffusion method that the extracellular activity produced by the used wild-type strain Aspergillus was not very visible. Among 58 strains selected after exposure to UV-radiation, strain K30 obtained after an exposure time of 30 sec showed the highest extracellular enzymatic activity (Figure 1). The K30 strain grown for three days on Petri dishes has resulted in a clear zone in blue background with a ratio of colourless zone diameter per colony diameter (R) of 1.36 while the same ratio is of about 1.01 for the wild-type. The K30 mutant was also able to grow on date flour medium.
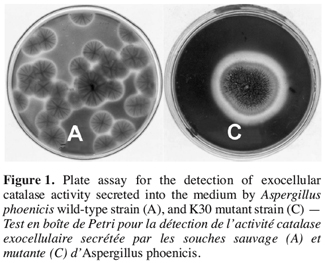
3.2. Catalase production in shake flask cultures with strain K30
15In shake-flasks culture of A. phoenicis K30 strain, intra- and extracellular catalase activities have been detected after 24 h of culture (Table 1). The maximum extracellular catalase activity (59.5 U.ml-1) was obtained after 96 h of growth with cell dry weight of 17.8 g.l-1 (Figure 2). The intracellular activity represented an amount of 40-50 U.ml-1. Interestingly, there was a significant increase in the production of extracellular catalase by strain K30 (59.5 U.ml-1) when compared with that of the wild-type strain (9.5 U.ml-1). The pH decreased during culture time.
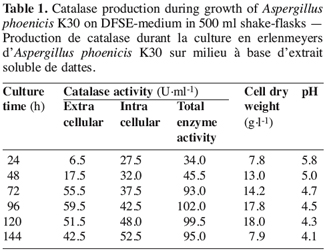
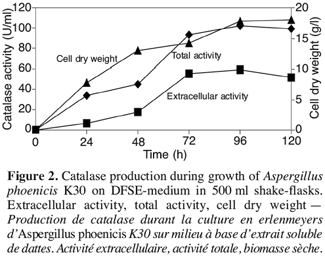
3.3. Catalase production in bioreactor cultures with strain K30
16Figure 3 shows the results of catalase production in a 20 l bioreactor. Extracellular catalase began to be produced into the medium after 12 h of growth. At this time, the cell dry weight started to increase. The best value of total catalase activity (58.5 U.ml-1) was obtained at 90 h of culture, after the production of biomass. The kinetic of enzyme production and amount of enzyme were quite similar in bioreactor and flasks.
3.4. Morphological transformation of fungal biomass during cultures
17The investigation of the fungal morphology in shake-flask cultures revealed a change from a filamentous to pelleted morphology. After 24 h of growth, the flocculent mycelium became progressively more compact (spherical pellets). The microscopical analysis of hairy external layer of pellets showed that, at this step, some ramifications appeared in the apical elongation of hyphae. At this time the extracellular catalase began to be excreted into the medium. The ramifications, the size and density of pellets increased after 48 h of culture. This morphology is correlated with the increase in catalase production. At the end of the fermentation, the pellets had a big size and a low density. Moreover, once the autolysis started the catalase activity decreased.
18As described above, the structural analysis of fungal pellets obtained from 20 l bioreactor culture, showed a varying morphology, from germinated spores after six hours of fermentation (Figure 4A), to loose flocculent mycelium and compact spherical pellets after 20 h of culture period with little increase in the pellet size. During the period of increasing extracellular catalase production, the pellets exhibited a little dense spherical form. It was clear from the fluorescent microscopic examination that the external layer had many ramifications in the apical elongations of hyphae. This morphology increased progressively to give rise to very branched hyphae with more septa at the end of fermentation period (Figure 4B, C, D). During this period, the major part of extracellular and total catalase was produced.
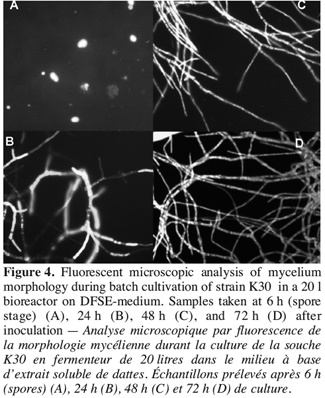
4. Discussion
19The UV rays method has been very effective in inducing mutants from the used Aspergillus phoenicis wild-type strain. The agar diffusion method coupled with stain detection allowed a rapid selection of a single mutant, out of tested strains. This K30 mutant has a real capacity of extracellular catalase secretion on DFSE-medium.
20The production of extracellular catalase by strain K30 in shake-flask cultures was nearly six fold higher than that obtained by the wild-type counterpart. In bioreactor culture, this increased production of extracellular catalase was similar to that in shake flasks, indicating that the selected strain has important potentialities for large scale production. The cell dry weight was quite similar in both cases: 17-22 g l-1, that presents a productivity of 3342- 2659 U g-1 cell dry weight. The fungal biomass presented big pellets in shake-flask cultures and smaller and more dense pellets in bioreactor like results presented by others (Ryoo, Choi, 1999; El-Enshasy et al., 1999).
21In different cultures, the excretion of catalase into the submerged culture began around 20 h post-inoculation and increased as the time progressed. This excretion was preceded by the intracellular catalase activity. There have been many studies on the excretion of protein into the medium by filamentous fungi. The analysis of the glucose oxidase from A. niger, whose excretion kinetics have been reported (Pluschkell et al., 1996), revealed the presence of a signal peptide confirming that the glucose oxidase (GOD) is actively secreted in the culture medium. Wösten et al.,(1999) have also concluded that the glucoamylase was released from hyphal tip and secreted through the wall during apical exocytosis. In contrast, there are only a few reports on the influence of environmental conditions on the excretion of catalase into the medium (Nishikawa et al., 1993; Gromada, Fiedurek, 1997).
22Our comparative study of the results obtained from shake flasks and bioreactor cultures showed that the intra- and extracellular catalase production was related neither to the fungal biomass nor to the size of pellet. However, this production may be directly related to the external layer of the pellet and precisely to the morphology of the hyphae in this region. It has been shown that the ramified morphological form of the hyphae has an important influence on the formation of a range of metabolites by the filamentous fungi (Wongwicharn et al., 1999) and that secretion of proteins is primarily associated with the apical and subapical regions, therefore called active region (Nykanen et al., 1997; Agger et al., 1998).
23Acknowledgment
24The authors gratefully acknowledge financial support from the Commission Universitaire au Développement (CUD), and from the Fonds National de la Recherche Scientifique (FNRS) in Belgium.
Bibliographie
Agger T., Spohr AB., Carlsen M., Nielsen J. (1998). Growth and product formations of Aspergillus oryzae during submerged cultivations: verification of a morphologically structured model using fluorescent probes. Biotechnol. Bioeng. 57, p. 321–329.
Akertek D., Tarhan L. (1995). Characteristics of immobilised catalases and their applications in pasteurisation of milk. Appl. Biochem. Biotechnol. 50, p. 9555–9560.
Anonymous (2003). Données chiffrées N°04, les palmiers dattiers en Algérie. Sous-direction des statistiques agricoles, Ministère de l’Agriculture et de Développement rural, Algeria.
Boulos L., Prevost M., Barbeau B., Coallier J., Desjardins R. (1999). Application of a new rapid staining method of direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 37, p. 77–86.
Caridis KA., Christakopulos P., Macris BJ. (1991). Simultaneous production of glucose oxidase and catalase by Alternaria alternata. Appl. Microb. Biotechnol. 34, p. 794–797.
El-Enshasy H., Hellmuth K., Rinas U. (1999). GpdA-Promoter-Controlled production of glucose oxidase by recombinant. Aspergillus niger using nonglucose carbon sources. Appl. Biochem. Biotechnol. 81, p. 1–11.
Fiedurek J., Gromada A. (1997). Selection of biochemical mutants of Aspergillus niger with enhanced catalase production. Appl. Microbiol. Biotechnol. 47, p. 313–316.
Gregory EM., Fridovich I. (1974). Visualisation of catalase on acrylamide gels. Anal. Biochem. 58, p. 57–62.
Gromada A., Fiedurek J. (1997). Selective isolation of Aspergillus niger mutants with enhanced glucose oxidase production. J. Appl. Microbiol. 82, p. 648–652.
Halliwell B. (1990). How to characterise biological antioxidants. Free Radicals Res. Commun. 9, p. 1–32.
Nishikawa Y., Kawata Y., Nagai J. (1993). Effect of triton X-100 on catalase production by Aspergillus terreus IF06123. J. Ferment. Bioeng. 76, p. 235–236.
Nykanen M., Saarelainen R., Raudaskoski M., Nevalainen KMH., Mikkonen A. (1997). Expression and secretion of barley cysteine protease and peptidase b and cellobiohydrolase1 in Trichoderma reesei. Appl. Environ. Microbiol. 63, p. 4929–4937.
Petruccioli M., Fenice M., Piccioni P., Federici F. (1995). Effect of stirrer speed and buffering agents on the production of glucose oxidase and catalase by Penicillium variabile (P16) in benchtop bioreactor. Enzyme Microb. Technol. 17, p. 336–339.
Pluschkell S., Hellmuth K., Rinas U. (1996). Kinetic of glucose oxidase excretion by recombinant Aspergillus niger. Biotechnol. Bioeng. 51, p. 215–220.
Pontecorvo G., Roper JA., Himmons LM., MacDonald KD., Bufton AWJ. (1953). The genetic of Aspergillus nidulans. Adv. Genet. 5, p. 141–238.
Ryoo D., Choi CS. (1999). Surfaces thermodynamics of pellet formation in Aspergillus niger. Biotechnol. Lett. 21, p. 97–100.
Venkateshwaran G., Somashekar D., Prakash MH., Basappa SC., Richard J. (1999). Production and utilisation of catalase using Saccharomyces cerevisiae. Proc. Biochem. 34, p. 187–191.
Witteveen CFB., de Vandervoort PV., Swart K., Visser J. (1990). Glucose oxidase overproduction and negative mutants of Aspergillus niger. Appl. Microbiol. Biotechnol. 33, p. 683–686.
Wongwicharn A., McNeil B., Harvey LM. (1999). Effect of oxygen enrichment on morphology, growth and heterologous protein production in chemostat cultures of Aspergillus niger B1-D. Biotechnol. Bioeng. 65 (4), p. 416–424.
Wösten HAB., Moukha SM., Sietsma JH., Wessels JGH. (1991). Localisation of growth and secretion of proteins in Aspergillus niger. J. Gen. Microbiol. 137, p. 2017–2023.






