- Home
- Volume 24 (2020)
- Numéro 3
- Relationship between protein markers and the sensory/physicochemical parameters of ovine meat during refrigerated storage
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Relationship between protein markers and the sensory/physicochemical parameters of ovine meat during refrigerated storage

Editor's Notes
Received 3 August 2017, accepted 4 May 2020, available online 8 June 2020
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
Résumé
Relation entre marqueurs protéiques et paramètres sensoriels et physico-chimiques de la viande ovine au cours de la réfrigération
Description du sujet. La commercialisation de la viande implique une période de stockage, en général sous réfrigération et en emballage sous vide. Au cours de cette étape, de nombreuses réactions biochimiques peuvent modifier les propriétés sensorielles et physico-chimiques de la viande.
Objectifs. Les objectifs de cette étude étaient d’utiliser une analyse multifactorielle pour corréler les changements physico-chimiques, sensoriels et ceux liés à la protéolyse ayant lieu dans la viande ovine au cours de la réfrigération. Pendant le stockage, les quantités relatives des protéines desmine, vinculine et myosine ont diminué au cours du temps.
Méthode. Les muscles longissimus lumborum ont été prélevés 6 h post mortem. Une évaluation sensorielle et physico-chimique ainsi qu'une électrophorèse 2D et un western blot ont été réalisés. Les concentrations protéiques relatives et leur relation avec les paramètres physico-chimiques et sensoriels ont été analysés par analyse factorielle multiple.
Résultats. L’analyse multifactorielle a montré que la force de cisaillement de Warner-Bratzler était hautement corrélée à la concentration relative de desmine, à la concentration relative de vinculine et à l’activité protéasique acide. Les activités des protéases acides et neutres étaient positivement corrélées à la quantité de myosine au jour 3, mais négativement corrélées à la tendreté manuelle et à la quantité de myosine aux jours 5 et 14.
Conclusions. Par conséquent, la desmine, la myosine et la vinculine, corrélées aux paramètres sensoriels et physico-chimiques pendant la réfrigération, pourraient constituer des marqueurs pertinents de la texture de viande ovine.
Abstract
Description of the subject. Commercialization requires the storage of meat for a period of time according to the distribution and supply chain, usually under refrigeration and vacuum packaging. During this stage, many biochemical reactions can modify the sensory and physicochemical properties of meat. Proteolysis is one of the most important of these reactions as proteins are the building blocks of muscle, and their degradation affects tenderness.
Objectives. The objectives of this study were to use multiple factorial analyses to correlate the physicochemical, sensory, and proteolytic changes in ovine meat during refrigeration.
Method. Each loin was separated and randomly assigned to day 3, 5, or 14. Sensory panel evaluated the meat for appearance, odor and texture parameters. Warner-Bratzler shear force and texture profile analysis were evaluated. Similarly, protease activity was evaluated. Samples for 2D-electrophoresis and western blotting were collected at days 3, 5, and 14 post mortem. The results were analyzed by multiple factorial analyses.
Results. The relative protein levels of desmin, vinculin, and myosin were found to decrease with time. Multiple factor analysis showed that the Warner-Bratzler shear force (WBSF) was highly correlated with the relative desmin concentration, the relative vinculin concentration, and acidic protease activity. The activities of acidic and neutral proteases were positively correlated with myosin level on day 3, but were negatively correlated with manual tenderness and myosin level on days 5 and 14.
Conclusions. Therefore, desmin, myosin and vinculin, which correlate with sensory and physicochemical parameters during refrigerated storage, may prove useful as markers for tenderness.
Table of content
1. Introduction
1In recent years, ovine meat production has grown at about 0.8% annually in world (FAO, 2019). It is estimated that 15.301 million tons of meat will be consumed worldwide in 2020 and projections show a rise up to 17 million tons for 2028 (OECD, 2020). Among the different sources for meat production, the ovine production has added benefits since they can eat a variety of low-quality crops and are able to live in moderate to harsh environments.
2A wide variety of physicochemical and sensory changes during the meat storage influences the final meat characteristics, such as appearance, odor and texture, which in turn affects their purchasing behavior. Meat storage, under refrigeration, reduces the shortening of meat as well as improves its odor and tenderness. This is due to the intensive degradation of the myofibrillar and sarcoplasmic proteins during the storage, which ultimately contributes to the quality of meat. Proteomics have great potential to enhance knowledge on the biochemical processes underlying the conversion of muscle into meat, by identifying biomarkers specific to meat quality, immediately after slaughter, which can be used to select the best-quality carcasses (Pospiech et al., 2007).
3The relationship between proteolysis and tenderness has been previously studied including degradation of desmin in pig biceps femoris muscle that explained 38% of the variation in sensory tenderness. However, the same behavior was not observed, using pig longissimus dorsi muscle (Wheeler et al., 2000). Moreover, Tomisaka et al. (2010) reported that the level of labeled desmin decreased by 51% in 12 to 96 h post mortem in chicken gizzard. Similarly, another study in bovine longissimus thoracis muscles observed desmin by products 76 h post mortem to day 56 of storage (Huff-Lonergan et al., 1996). Likewise, Anderson et al. (2012) have identified potential markers, like myosin light chain-1, actin and myomesin-2, in muscles that differ in tenderness. Furthermore, Gagaoua et al. (2017) analyzed the relationship between protein biomarkers and meat quality in PDO Maine-Anjou bovines. They observed that myosin light chain played an important role in meat quality by regulating the activities of glycolytic enzymes. In addition, Kiran et al. (2016) identified proteins, namely uroplakin-1b, aspartate aminotransferase, myosin-IIIa, glycogen phosphorylase, cytosolic carboxypeptidase 3 and phosphatidylinositol transfer protein β isoform, having positive correlation with tenderness in buffalo meat. Likewise, heat shock, metabolic, structural, oxidative resistant and proteolytic proteins were reported as potential biomarkers for tenderness in beef (Picard et al., 2014).
4There are numerous reports regarding the proteomics of cattle, pork, poultry meat, but there are few proteomic studies on ovine meat. Although, sensory attributes have been studied in ovine (Revilla et al., 2009; Muela et al., 2012; Jandasek et al., 2014), there are few studies studying the relationship between the changes in proteins and physicochemical parameters occurring during storage (Starkey et al., 2016).
5The objectives of the present study were to use multiple factorial analysis in an effort to correlate the physicochemical parameters, sensory parameters, and proteolytic changes in ovine meat during refrigerated storage. Likewise, the biomarkers were associated to sensory and physicochemical characteristics.
2. Materials and methods
2.1. Animals and sampling
6Twenty-seven 1-year-old Pelibuey lambs were slaughtered in a commercial abattoir in Mexico City. The longissimus lumborum muscles were dissected after 6 h post mortem. One loin/animal was separated and randomly assigned to day 3, 5, or 14.
7The loins were vacuum packaged at −700 mbar in Cryovac LB-50 bags (Sealed Air de Mexico SRL CV, Mexico) using a Multivac vacuum (model D-8941; Koch, Kansas City, MO, USA) and stored at 2 ± 1 °C for 14 days without light.
2.2. Sensory evaluation
8The panel was recruited and selected from an initial group of 30 participants (22 women, 8 men) ranging from 20 to 26 years old. The process included a questionnaire about eating habits, age, allergies and availability. The selection was based on the volunteers’ ability to differentiate basic tastes, their discriminative ability (ASTM 1981) and olfactory capacity (Severiano-Pérez et al., 2012). Participants that presented a threshold equal to or lower than that of the whole group were chosen. In addition, participants who obtained a high answer percentage (greater or equal to 60%) in the triangulated food test, odor identification and odor discrimination tests were also included. Therefore, 15 participants (4 men and 11 women) integrated the panel.
9Then, the panelists evaluated raw ovine meat during the training sessions. The test consisted of generating and selecting descriptive terms over two sessions. Afterwards, panelists defined and selected the intensity of attributes using a 9-point numeric scale, on which 1 signified the minimum intensity perception and 9 signified the maximum over four sessions (Table 1; ISO 13299-2016). The panelists were trained over ten sessions with a scheduled between 10 a.m. and 2 p.m until the group variation coefficient (VC) was 30%. The panelists were ready to assess the samples of longissimus muscles for appearance, odor and texture parameters indicated in table 1 (Stone et al., 1974; Civille et Liska, 1975; Martínez-Arellano et al., 2013). Trained panelists evaluated three raw samples after 15 min blooming period (18 ± 2 °C) using a randomized three-digit code (FIZZ v2.3) (Biosystemes 2007, Couternon, France), over six sessions scheduled between 10 a.m. and 2 p.m. The samples were placed in porcelain dishes.
2.3. Physicochemical analysis
10Warner-Bratzler shear force (WBSF) was evaluated and texture profile analysis (TPA) was performed on raw meat using a texture analyzer (TA-XT2-Plus and the Texture Exponent 32, 2009 software; Texture Technology Corp., New York, NY, USA). The WBSF used the following parameters: sample size, 1 cm3; room temperature 18 ± 2 °C; the steel blade perpendicular to the fiber; test speed, 1 mm·s-1; and down stroke distance, 30 mm. For TPA, the following parameters were used: 1 cm3 sample size; cylindrical 50 mm diameter probe of ebonite PMS/50; compression, 30%; test speed, 1 mm·s-1; room temperature 18 ± 2 °C. Determinations were carried out in quadruplicate.
11Acidic protease activity was evaluated by using 1% (w/v) hemoglobin as the substrate in universal buffer, pH 3 (Dublán-García et al., 2006), and neutral protease activity was evaluated by using 1% (w/v) casein as the substrate in 20 mM·l-1 phosphate buffer, pH 7, containing 0.9% (w/v) NaCl (Yamaguchi et al., 1982).
2.4. Two-dimensional (2D) electrophoresis of myofibrillar proteins
12Sample preparation. Raw meat (1 g) was homogenized with 8 mL of 20 mM Tris-HCl buffer, pH 7.6, containing 2 mM EDTA, 4 mM MgCl2, 0.2 mM PMSF, and 0.2 mM trypsin inhibitor (Begonya et al., 2010). The sample was then pelleted, rinsed five times in the above-described buffer, and solubilized in 7 M urea, 2 M thiourea, 2% CHAPS, and 1% DTT. Samples for 2D electrophoresis and western blotting (WB) were collected at days 3, 5, and 14 post mortem. The protein concentration of each sample was determined in triplicate using a modified Bradford assay (Bio-Rad Protein Assay; Bio-Rad, Richmond, CA, USA).
132D electrophoresis. IPG 3-10 strips (Ready Strip™ IPG Strips, Bio-Rad) were used for the 2D electrophoresis of myofibrillar proteins. The protein sample was solubilized to 0.5 µg·µL-1 in rehydration buffer (8 M urea, 2 M thiourea, 2% CHAPS, 50 mM DTT, 1.5 µl ampholite, pH 3-10 [100X, Bio-Rad], 0.002% bromophenol blue). Each IPG strip was passively rehydrated with the before mentioned solution for 23 h at 20 °C, and isoelectric focusing (the first dimension of electrophoresis) was run on a Protean® IEF Cell (Bio-Rad). The IPG strip was incubated with 800 µl of buffer containing 6 M urea, 0.375 M Tris-HCl, pH 8.8, 2% SDS, and 50 mM DTT, followed by incubation in the same solution containing 150 mM of iodoacetamide instead of DTT. For the second dimension of electrophoresis, the IPG strip was incubated for 15 min in a test tube containing running buffer, pH 8.3 (Laemmli, 1970), and SDS-PAGE was carried out on 12% T gels using a Mini Protean III system (Bio-Rad) at 150 V and 18 ± 1 °C. Broad-range molecular weight markers were used (205-6.5 kDa; Sigma). The gels were stained with 0.5% potassium and aluminum sulfate, 10% absolute ethanol, 0.022% Coomassie blue G-250, and 5.5% orthophosphoric acid, and images were digitalized with a Gel-Doc™ XR (Molecular Imager, Imaging Systems; Bio-Rad).
2.5. Western blotting of myofibrillar proteins
14Resolved proteins were transferred to a membrane (Sequi-Blot PVDF, 0.2 µm; Bio-Rad) in transfer buffer, pH 8.3 (25 mM Tris, 192 mM glycine, 0.05% SDS, 20% methanol), for 12 h at 30 V and 15 ºC (Criterion™ Blotter; Bio-Rad). The membranes were blocked twice in 5% non-fat milk dissolved in TBST (pH 7.5, 150 mM NaCl, 20 mM Tris-HCl, 0.1% Tween-20) for 30 min each time, and then rinsed three times in TBST for 5 min each time. Each blocked membrane was incubated for 1.5 h at 37 °C with monoclonal anti-desmin (DE-U-10; Sigma Aldrich) diluted 1:200 in TBST containing 0.5% non-fat milk. The membranes were washed for 10 min with TBST containing 0.5% non-fat milk, rinsed twice with TBST for 5 min each time, and incubated with IgG-HRP sc-2005 anti-goat (1:10,000; Santa Cruz Biotechnology, Dallas, TX, USA), monoclonal anti-vinculin (1:1,000, MAB3574; Millipore, Billerica, MA, USA), or monoclonal anti-myosin (1:200, MY-32; Sigma Aldrich) for 1 h at 20 °C. Following incubation with the appropriate secondary antibody, the membranes were rinsed with TBST for 10 min and then with TBS (pH 7.5, 150 mM NaCl, 20 mM Tris-HCl) for 5 min. The protein spots were visualized using an Immobilon Western Chemiluminescent HRP kit (Millipore) with incubation for 10 min, and exposed to film (Logic 1500 Kodak; Imaging System).
2.6. Statistical analysis
15The relative protein concentrations obtained by western blot analysis and their relationships with the physicochemical and sensory parameters of raw and grilled lamb meat were analyzed by multiple factorial analysis using the XLSTAT software 2012, Addinsoft, v10.0, New York, USA.
3. Results
3.1. Analysis of sensory and physicochemical parameters
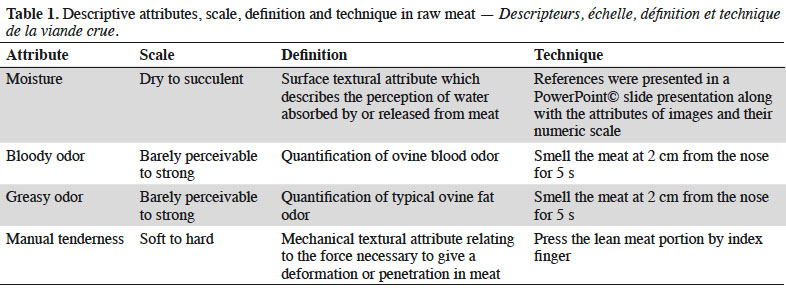
16Results for sensory and physicochemical parameters are presented in table 2, showing that moisture had the maximum value on day 14 as well as the bloody and greasy odor muscle. Manual tenderness did not significantly differ during storage, but the texture parameters, WBSF and TPA, reached their maximum hardness values on day 3. WBSF declined from 2.6 kg on day 3 to 1.9 kg over the following days (p ≤ 0.001). In addition an increment in the acidic protease activity was observed at day 3 (5.38 UI·mg-1 protein).
3.2. 2D electrophoresis and blotting of myofibrillar proteins
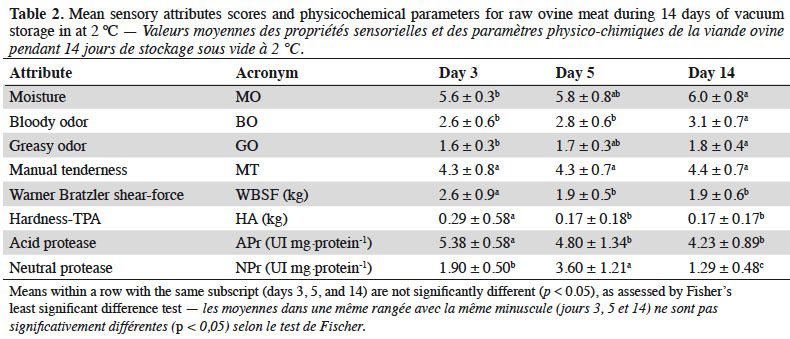
17Figure 1 shows the electrophoresis and blotting of myofibrillar proteins, where a signal corresponding to intact desmin (Figure 1a) was highly intense on day 3, while those of its degradation products (three spots) were first seen on day 14. The relative concentration of intact desmin decreased to 20-40% of the day 3 level by day 5, and from there on to 16% of the initial level on day 14. In addition, the relative expression level for vinculin, compared to the level seen on day 3, decreased to 38% and 24% on days 5 and 14, respectively (Figure 1b). Finally, myosin heavy chain (MHC) was observed as one spot on day 3 and two spots thereafter. On day 14, the relative protein concentrations of the spots were reduced to 3% and 9%, respectively, on day 5 (Figure 1c).
3.3. Multiple factor analysis
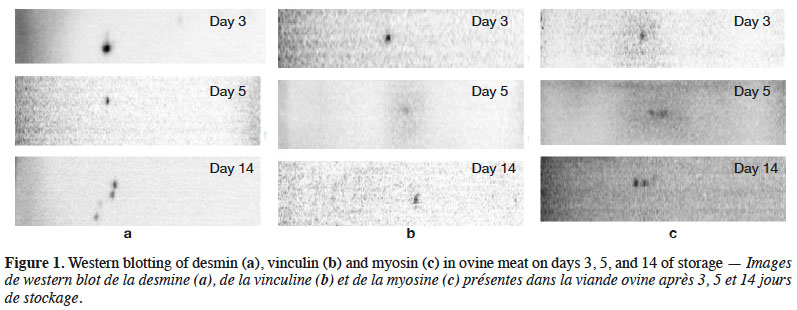
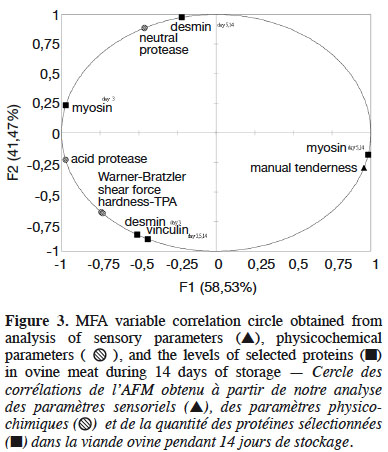
18Multiple factor analysis was used to correlate the relative protein concentrations (desmin, vinculin, myosin), physicochemical parameters (protease activity, WBSF, hardness-TPA), and sensory parameters (manual tenderness). Three groups of variables were used: the protein variables included the relative protein concentrations for desmin, vinculin, and myosin; the physicochemical variables included protease activity, WBSF, and hardness-TPA; and the sensory variable consisted of manual tenderness. The first axis explained 58.53% and the second 47.47% of total variance (Figure 2). The Rv coefficients were generated and considered significant as values where higher than 0.6, according to Cartier et al. (2006). The Rv values were 0.6 and 0.88, respectively, for the protein-sensory parameters and protein-physicochemical parameters, while that for the physicochemical-sensory parameters was 0.6.
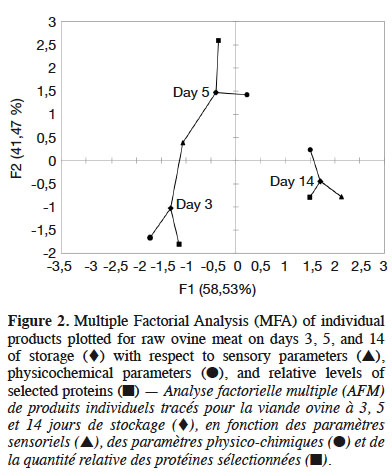
19Figure 2 shows that the physicochemical parameters were followed by the protein parameters described on day 3 in the first dimension, whereas this order was reversed on day 5. Finally, day 14 was described by the protein parameters, followed by the sensory and then physicochemical parameters. As shown in figure 2, the sensory parameters were overall less descriptive than the other parameters. While, figure 3 shows a correlation circle comparing the protein, physicochemical and sensory parameters. The vectors showed that WBSF was correlated with the relative desmin concentration on day 3 concentration (r = 0.95). In addition, the shear force was correlated with the relative vinculin concentrations on days 3, 5 and 14 (r = 0.93), hardness-TPA (r = 1), and acidic protease activity (r = 0.875). Manual tenderness was positively correlated with myosin on days 5 and 14 (r = 0.9), and myosin on day 3 was positively correlated with the neutral (r = 0.65) and acidic (r = 0.89) activities. But the relative myosin concentration on day 3 was negatively correlated with manual tenderness (r = -0.98), and the relative myosin concentrations on days 5 and 14 were negatively related to acidic protease activity (r = -0.91).
4. Discussion
4.1. Analysis of sensory and physicochemical parameters
20Among the sensory parameters, the increase in moisture and variation in odor may be related to protein denaturation and the release of water to the meat surface, which reflects more light. Similarly, Kosowska et al. (2017) reported that non-ripened beef had a weak, plain aroma, whereas the ripening process increased and intensified its flavor; for example, the ripening for up to 14 days increased the greasy taste. Those changes may be related to the presence of reactive compounds (acids, alcohol, aldehydes or ketones) originated by transformation in fatty tissue (phospholipid). On the other side, some authors have proposed that neutral proteases are the main factors responsible for enzymatic activity in meat, while others have suggested that there is a multi-enzymatic process involving neutral proteases, acid proteases, proteasomes and caspases that may explain the results associated to texture (Herrera-Mendez et al., 2006).
4.2. 2D electrophoresis and blotting of myofibrillar proteins
21As the texture of meat is also an important attribute for the consumer, the hydrolysis levels of desmin, myosin and vinculin proteins by endogenous proteases were tested. As shown in figure 1, variations in desmin, vinculin and myosin where evident during the study. These findings are consistent with other authors, for instance Kristensen & Purslow (2001), who reported that desmin immune-labeling declined to 29% of the initial value in pork by day 10 of storage, and Iwanowska et al. (2010), who observed a gradual diminution of the relative desmin concentration over time in bull’s meat, with degradation products increased on days 2 and 4 of storage. While, vinculin reduction was also reported by Kristensen & Purslow (2001) showing that the relative vinculin concentration in pork decreased in 37% of its initial value after 10 days of storage. In relation to myosin, a study from Lametsch et al. (2003) indicated that only the globular portion of myosin is reduced during the first 48 h post mortem in pork, and that the resulting degradation products were correlated with meat tenderness. However, other authors observed that neither myosin nor actin were broken down post mortem in bovines (Huff-Lonergan et al., 1996).
4.3. Multiple factor analysis
22Multiple factor analysis was used to correlate the relative protein concentrations, physicochemical parameters and sensory parameters. This technique analyzes the similarity among groups of observations explained by different sets of variables with comparable or opposing scales; the influence of each variable is balanced, multiple groups of data are compared, and per-attribute correlation patterns are generated (Abdi & Valentin, 2007). Significant Rv coefficients with 0.6 value were observed for the protein-sensory parameters and protein-physicochemical parameters. These findings agree with those of Muroya et al. (2010), who reported that some cytoskeletal proteins, including troponin-T and desmin, are related to meat quality characteristics of pork. Wheeler & Koohmaraie (1999) found that desmin is a good indicator of post mortem proteolysis and tenderness in lamb. Hwang et al. (2005) reported that the labeling density of desmin in pork decreased to 46% of the initial value, but its relationship with WBSF was weak (r = 0.46). The latter finding likely reflects the inherent limitation of proteomic analysis, wherein proteins and/or their intermediary degradation products may co-migrate. Finally, actin and myosin being the most abundant proteins in meat, and their post mortem degradation affects the meat structure yielding a softer meat texture. As reported by other authors (D’Alessandro et al., 2012), there is no universal protein marker for meat, but markers need to be selected by the type of meat.
5. Conclusions
23Ovine meat had the highest value of moisture, greasy and blood odor and the lowest value of WBSF hardness, neutral and acid protease activities on day 14. Manual tenderness was not significantly affected during storage. The relative levels of immunoblotted desmin, myosin and vinculin had correlation coefficients (Rv) with the physicochemical and sensory parameters greater than 0.6, indicating that these proteins could be potential markers for predicting the texture quality of ovine meat. In the future, an electronic sensor (microarray technology), capable of testing an array of specific proteins, might be used to quickly analyze and classify carcasses on the line during the slaughter process. In addition, sarcoplasmic proteome could be studied (triosephosphate isomerase, HSP20, etc.) to be linked with sensory and physicochemical parameters.
Bibliographie
Abdi H. & Valentin D., 2007. Multiple correspondence analysis. In: Salkind N.J., ed. Encyclopedia of measurement and statistics. Thousand Oaks, CA, USA: SAGE Publications Inc., 651-657.
Anderson M.J., Lonergan S.M. & Huff-Lonergan E., 2012. Myosin light chain 1 release from myofibrillar fraction during post mortem aging is a potential indicator of proteolysis and tenderness of beef. Meat Sci., 90, 345-351, doi.org/10.1016/j.meatsci.2011.07.021
ASTM, 1981. Guidelines for the selection and training of sensory panel members, STP758. West Conshohocken, PA, USA: ASTM International, 1-12, doi.org/10.1520/stp41626s
Begonya M., Kerry J.P. & Mullen A.M., 2010. High pressure induced changes on sarcoplasmic protein fraction and quality indicators. Meat Sci., 85, 115-120, doi.org/10.1016/j.meatsci.2009.12.014
Cartier R. et al., 2006. Sorting procedures as an alternative to quantitative descriptive analysis to obtain a product sensory map. Food Qual. Preference, 17(7), 562-571, doi.org/10.1016/j.foodqual.2006.03.020
Civille G.V. & Liska I.H., 1975. Modifications and applications to foods of the general foods sensory texture profile technique. J. Texture Stud., 6, 19-31, doi.org/10.1111/j.1745-4603.1975.tb01115.x
D'Alessandro A. et al., 2012. Love me tender: an omics window on the bovine meat tenderness network. J. Proteomics, 75, 4360-4380, doi.org/10.1016/j.jprot.2012.02.013
Dublán-García O., Cruz-Camarillo R., Guerrero-Legarreta I. & Ponce-Alquicira E., 2006. Effect of refrigerated storage on proteolytic activity and physicochemical and microstructural properties of giant squid mantle muscle. J. Muscle Foods, 17, 291-310, doi.org/10.1111/j.1745-4573.2006.00051.x
FAO, 2019. Meat market review, March. Roma: FAO.
Gagaoua M. et al., 2017. Associations among protein biomarkers and pH and color traits in longissimus thoracis and rectus abdominis muscles in protected designation of origin Maine-Anjou cull cows. J. Agric. Food Chem., 65, 3569-3580, doi.org/10.1021/acs.jafc.7b00434.s001
Herrera-Mendez C.H., Becila S., Boudjellal A. & Ouali A., 2006. Meat aging: reconsideration of the current concept. Trends Food Sci. Technol., 17, 394-405, doi.org/10.1016/j.tifs.2006.01.011
Huff-Lonergan E. et al., 1996. Proteolysis of specific muscle structural proteins by mu-calpain at low pH and temperature is similar to degradation in post mortem bovine muscle. J. Anim. Sci., 74, 993-1008, doi.org/10.2527/1996.745993x
Hwang I.H. et al., 2005. Assessment of post mortem proteolysis by gel-based proteome analysis and its relationship to meat quality traits in pig longissimus. Meat Sci., 69, 79-91, doi.org/10.1016/j.meatsci.2004.06.019
ISO 13299, 2016. Sensory analysis: methodology – general guidance for establishing a sensory profile. Geneva, Switzerland : ISO.
Iwanowska A. et al., 2010. Changes in proteins and tenderness of meat from young bulls of four breeds at three ages over 10 days of cold storage. Anim. Sci. Papers Rep., 28(1), 13-25.
Jandasek J., Milerski M. & Lichovnikova M., 2014. Effect of sire breed on physico-chemical and sensory characteristics of lamb meat. Meat Sci., 96(1), 88-93, doi.org/10.1016/j.meatsci.2013.06.011
Kiran M. et al., 2016. Understanding tenderness variability and ageing changes in buffalo meat: biochemical, ultrastructural and proteome characterization. Animal, 10(6), 1007-1015, doi.org/10.1017/s1751731115002931
Kosowska M.A., Majcher M. & Fortuna T., 2017. Volatile compounds in meat and meat products. Food Sci. Technol., 37(1), 1-7, doi.org/10.1590/1678-457x.08416
Kristensen L. & Purslow P., 2001. The effect of ageing on the water-holding capacity of pork: role of cytoskeletal proteins. Meat Sci., 58(1), 17-23, doi.org/10.1016/s0309-1740(00)00125-x
Laemmli U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature, 227, 680-685, doi.org/10.1038/227680a0
Lametsch R. et al., 2003. Post mortem proteome changes of porcine muscle related to tenderness. J. Agric. Food Chem., 51(24), 6992-6997.
Martínez-Arellano I., Severiano-Pérez P., Fernández F. J. & Ponce-Alquicira E., 2013. Changes in the physicochemical and sensory characteristics in raw and grilled ovine meat. J. Sci. Food Agric., 93, 1743-1750, doi.org/10.1002/jsfa.5964
Muela E. et al., 2012. Effect of freezing method and frozen storage duration on lamb sensory quality. Meat Sci., 90, 209-215, doi.org/10.1016/j.meatsci.2011.07.003
Muroya S., Ertbjerg P., Pomponio L. & Christensen M., 2010. Desmin and troponin T are degraded faster in type IIb muscle fibers than in type I fibers during post mortem aging of porcine muscle. Meat Sci., 86(3), 764-769, doi.org/10.1016/j.meatsci.2010.06.019
OECD, 2020. Meat consumption (indicator), doi.org/10.1787/fa290fd0-en
Picard B. et al., 2014. Inverse relationships between biomarkers and beef tenderness according to contractile and metabolic properties of the muscle. J. Agric. Food Chem., 62, 9808-9818, doi.org/10.1021/jf501528s
Pospiech E. et al., 2007. Proteins of meat as a potential indicator of its quality-a review. Pol. J. Food Nutr. Sci., 57(1), 11-16.
Revilla I. et al., 2009. Comparison of the sensory characteristics of suckling lamb meat: organic against conventional production. Czech J. Food Sci., 27, 267-270, doi.org/10.17221/949-cjfs
Severiano-Pérez P., Cadena-Aguilar A.A., Vargas-Chanes D. & Guevara-Guzmán R., 2012. Questionnaire on Mexicans' familiarity with odor names. J. Sensory Stud., 27, 277-285, doi.org/10.1111/j.1745-459x.2012.00390.x
Starkey C.P. et al., 2016. Do sarcomere length, collagen content, pH, intramuscular fat and desmin degradation explain variation in the tenderness of three ovine muscles? Meat Sci., 113, 51-58, doi.org/10.1016/j.meatsci.2015.11.013
Stone H. et al., 1974. Sensory evaluation by quantitative descriptive analysis. Food Technol., 28, 24-33.
Tomisaka Y. et al., 2010. Changes in water-holding capacity and textural properties of chicken gizzard stored at 4 °C. J. Anim. Sci., 81, 362-368, doi.org/10.1111/j.1740-0929.2010.00739.x
Wheeler T.L. & Koohmaraie M., 1999. The extent of proteolysis is independent of sarcomere length in lamb longissimus and psoas major. J. Anim. Sci., 77, 2444-2451, doi.org/10.2527/1999.7792444x
Wheeler T.L., Shackelford S.D. & Koohmaraie M., 2000. Variation in proteolysis sarcomere length, collagen content and tenderness among major pork muscles. J. Anim. Sci., 78(4), 958-965, doi.org/10.2527/2000.784958x
Yamaguchi T., Yashita Y., Takeda I. & Kiso H., 1982. Proteolytic enzymes in green asparagus, kiwi fruit and miut: occurrence and partial characterization. Agric. Biol. Chem., 46(8), 1983-1986.






