- Accueil
- Volume 26 (2022)
- Numéro 4
- Quality enhancement of fruits conserved with essential oils prolonging storage
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Quality enhancement of fruits conserved with essential oils prolonging storage

Document(s) associé(s)
Version PDF originaleRésumé
Amélioration de la qualité des fruits conservés avec des huiles essentielles prolongeant le stockage
Description du sujet. Les framboises et les fraises sont très périssables à cause du développement fongique après récolte. De nombreuses études ont démontré que les huiles essentielles peuvent être des biopesticides prometteurs pour maintenir la qualité des fruits frais pendant le stockage post-récolte.
Objectifs. Ce travail visait à évaluer le potentiel des huiles essentielles (HEs) d’Ismelia carinata et de Cladanthus arabicus (Asteraceae) à prolonger la durée de conservation des framboises et des fraises.
Méthode. La composition chimique des huiles essentielles d’I. carinata et de C. arabicus a été analysée par CG/SM. Les effets antifongiques in vitro des HEs ont été testés contre Botrytis cinerea par les méthodes de dilution en gélose et de diffusion sur disque. Les HEs d’I. carinata et de C. arabicus ont été utilisées comme agents bioactifs pour préserver la qualité des baies pendant 15 jours en chambre froide. Le pH, l’acidité titrable, les solides solubles totaux et l’indice de maturité des baies traitées avec 400 mg·l-1 d’HE d’I. carinata ont également été analysés.
Résultats. L’analyse par CG/SM a révélé que les principaux composés d’I. carinata étaient le tau-cadinol (65,93 %) et le tau-muurolol (24,65 %) et ceux de C. arabicus étaient le β-pinène (23,58 %) et le tau-cadinol (9,54 %). Les deux HE testées ont montré les meilleurs pourcentages d'inhibition contre B. cinerea en utilisant la méthode de dilution en gélose. L’HE d’I. carinata était un agent de conservation efficace pour les framboises et les fraises pendant le stockage à 4 °C. L’indice de maturité des deux baies était significatif par rapport aux témoins non traités (p ≤ 0,05).
Conclusions. L’HE d’I. carinata est prometteuse pour l’amélioration de la qualité des fruits en chambre froide, avec un bénéfice ultérieur pour les chaines d’approvisionnement en fruits.
Abstract
Description of the subject. Raspberries and strawberries are highly perishable due to postharvest fungal development. Numerous studies have shown that essential oils can be promising biopesticides for maintaining the quality of fresh fruit during postharvest storage.
Objectives. This work aimed to evaluate the potential of Ismelia carinata and Cladanthus arabicus (Asteraceae) essential oils (EOs) to extend the shelf life of raspberries and strawberries.
Method. The chemical composition of EOs from I. carinata and C. arabicus was analyzed using GC/MS. In vitro antifungal effects of EOs using agar dilution and disk diffusion methods were tested against Botrytis cinerea. The I. carinata and C. arabicus EOs were used as bioactive agents to preserve the quality of berries during 15 days in cold storage. Also, pH, titratable acidity, total soluble solids, and maturity index of berries treated with 400 mg·l-1 of I. carinata EO are analyzed.
Results. The GC/MS analysis revealed that the major compounds were tau-cadinol (65.93%) and tau-muurolol (24.65%) for I. carinata, β-pinene (23.58%), and tau-cadinol (9.54%) for C. arabicus. Both EOs tested showed best inhibition percentages against B.cinerea using the agar dilution method. Ismelia carinata EO was an efficient preservative agent for raspberries and strawberries during storage at 4 °C. The maturity index of both berries was significant compared to untreated controls (p ≤ 0.05).
Conclusions. Ismelia carinata EO holds promise for extending fruit quality in cold storage, with subsequent benefit to fruit marketing supply chains.
Table des matières
Received 17 September 2021, accepted 6 October 2022, available online 27 October 2022.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1It is estimated that approximately one-third of horticultural products are lost after harvest reaching 1.3 billion tons of food worldwide, costing the global economy around 750 billion US dollars (Gastavsson et al., 2011; Prusky, 2011). Strawberries and raspberries are among the most rapidly perishable fruits after harvest because of their susceptibility to mechanical injury, physiological deterioration, water loss, and postharvest decay. A postharvest attack is the factor causing the most drastic economic losses, especially in the fruit marketing chain (Prusky, 2011). Botrytis cinerea, the cause of gray mold, is one of the most common postharvest decays of fresh fruit and vegetables (Romanazzi & Feliziani, 2014). Based on scientific and economic importance, this fungus ranked second into the world Top 10 fungal plant pathogens list (Dean et al., 2012). Research has shown that B. cinerea is one of the most prevalent fungi on strawberries and raspberries, reducing their shelf life after harvest (Tournas & Katsoudas, 2005; Farzaneh et al., 2015). Therefore, handling these berries from harvest to consumer represents a real challenge.
2To increase the marketability of fresh berries, the use of postharvest technologies of cold storage and modified atmosphere packaging results in maintaining the postharvest quality of raspberry and strawberry fruit for 10-12 days. However, the application of an efficient treatment significantly reducing the postharvest incidence can imply a considerable change in fruit sweetness and palatability (Agar & Streif, 1997; Zheng et al., 2007).
3Essential oils (EOs) are plant extracts composed of aromatic and lipophilic volatile compounds that plants synthesize as secondary metabolites to protect themselves from abiotic and biotic stress (Bakkali et al., 2008). Research has focused on their use as potential biopesticides to sustain fresh fruit quality during postharvest periods. Many EO treatments showed promising results in controlling gray mold of table grapes (Abdollahi et al., 2012) and apples (Lopez- Reyes et al., 2010), and their effectiveness against several other postharvest decays (Suradeep & Proshanta, 2018). Also, consumers accept EOs more readily because aromatic plants have wide use in general culinary practices (Sivakumar & Bautista-Baños, 2014; Sarkhosh et al., 2017).
4Cladanthus arabicus Cass. and Ismelia carinata Schousb. are Anthemideae species from the Compositae family (Benabid, 2000). Cladanthus arabicus is an annual plant with many erected stems and yellow flowers, growing in southern Spain and North Africa (Tutin et al., 1964). Previous studies have investigated the chemical composition of C. arabicus EO and some of its biological activities (El Hanbali et al., 2005; Aghraz et al., 2017). Ismelia carinata is a spring-flowering plant native to Morocco (El Oualidi et al., 2012). This bushy plant has fleshy-light green and lacinate leaves and its flowers are characterized by variable colors that form different concentric rings. Ismelia carinata is also known as I. versicolor Cass. or Chrysanthemum carinatum Schousb. and it represents the only species of the Ismelia genus (Turland, 2004). These species of plants are naturalized outside their native geographical sites (Buist, 1846; Soreng & Cope, 1991).
5The purpose of this study is to examine the chemical composition of EOs of two Anthemideae species and to evaluate their in vitro antifungal effect against Botrytis cinerea using two different screening assays. Also, the EOs will be tested for their ability to preserve fresh raspberries and strawberries and assessed in terms of consequences on fruit sweetness after storage.
2. Materials and methods
2.1. Plant materials and EOs extraction
6Aerial parts of two plants were collected between March and May 2017, from wild populations in different regions of Morocco: Ismelia carinata in Tiznit, Tifnit (geographic coordinates 30°12’0” N, 9°37’48” W) and Cladanthus arabicus in the Agadir region (geographic coordinates 30°25’12” N, 9°35’53” W). Plants were identified at the Scientific Institute of Rabat (Morocco). A voucher specimen of each plant was deposited (C. arabicus No. CA-17 and I. carinata No. IC-17) in the herbarium of the Laboratory of Biotechnology at the National School of Applied Sciences, Ibn Zohr University, Agadir, Morocco. The plant materials were air-dried at shade and ambient temperature (25 °C) for about 4 weeks. Essential oils were obtained by hydrodistillation of the air-dried plants for 3 h using a Clevenger type apparatus as recommended by European Pharmacopoeia (Council of Europe, 1997). Essential oils were dried over anhydrous sodium and kept in the dark at 4 °C until use.
2.2. Gas chromatography-mass spectrometry analysis
7The chemical composition of essential oils was investigated by gas chromatography/mass spectrometry (GC/MS) using a Shimadzu system. A VB5 capillary column was used (30 m x 0.25 mm inner diameter, 0.25 µm film thickness), and Helium was the carrier gas at 1 ml·min-1. The GC oven temperature was conserved at 50 °C for 1 min and programmed to 280 °C at a rate of 10 °C·min-1, then kept constant at 280 °C for 2 min. The split flow was used; the injector temperature was 250 °C. The mass spectrometer was operating at ionization energy of 70 eV and 300 °C. The mass spectrometer range was from 20 to 350 m/z, and the interface was at 300 °C. A library search was composed using the “Wiley GC/MS Library” Nist147 database LIB (Adams, 2007).
2.3. Fungal culture of Botrytis cinerea
8Botrytis cinerea was isolated directly from naturally decayed strawberries in three replicates using potato dextrose agar medium (PDA), amended with gentamycin (10 mg·l-1) to stop the growth of bacteria. Plates were incubated in the dark for five days at 25 ± 2 °C. The isolate collected was repeatedly purified before its identification. A pure culture of B. cinerea was maintained on PDA and stored at 4 °C.
2.4. In vitro antifungal assays
9The agar dilution (Chebli et al., 2003) and disk diffusion (Znini et al., 2013) methods were adopted to evaluate the in vitro antifungal effect of I. carinata and C. arabicus EOs against mycelial growth of B. cinerea. Six concentrations of I. carinata and C. arabicus EOs, 0.125, 0.25, 0.5, 1, 2, and 4 µl·ml-1 were tested in both antifungal assays.
10In the agar dilution method, a stock solution of EOs was prepared by dissolving pure EOs in a sterile solution of Tween 80 (2 ml·l-1). To obtain the desired concentrations, appropriate amounts of a stock solution were mixed with autoclaved potato dextrose agar medium (PDA) maintained at 45 °C just before pouring into Petri dishes. A mycelial plug, 6 mm in diameter, was excised from a pure culture of B. cinerea and placed at the center of each Petri dish. Controls consisted of un-amended PDA. The plates were sealed with parafilm and incubated at 25 °C.
11In the disk diffusion assay, Petri dishes were filled with sterile PDA medium and then inoculated with mycelial plugs, cut from a pure culture of the fungus. After solidification, Petri dishes were inverted, and according to each concentration, pure EOs were deposited in sterile filter paper disks, which then were placed on the inner surface of Petri dish lids. The plates were sealed with parafilm and incubated upside-down at 25 °C. The control consisted of sterile distilled water-soaked filter paper.
12Tests were stopped when control Petri dishes were covered by mycelial culture. Three replicates were used for each concentration. The percentage inhibition of fungal growth was determined on the growth in the test plate compared to that of the control plate according to the equation:
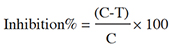
13where C was the diameter of fungal growth on the control, and T was the diameter on the test plate.
2.5. Evaluation of EOs on the shelf life of raspberries and strawberries
14The experiment was performed using Vu et al. (2011) method with slight modifications. Two concentrations (200 and 400 mg·l-1) of the EOs from I. carinata (IC200; IC400) and C. arabicus (CA200; CA400) were prepared as aqueous emulsions using a sterile solution of Tween 80 (4 ml·l-1) and distilled water under vigorous stirring. Fresh raspberry and strawberry fruits were harvested from orchards using standard culture practices in the Agadir region. The berries were commercially mature and showed no spoilage signs.
15The EO emulsions were sprayed onto fresh fruits at room temperature. The amounts sprayed were equal between treatments. After spraying, fresh fruits were allowed to dry for 15 min, and then were stored at 4 °C. Visual decay of raspberries and strawberries was evaluated for 15 days, and the contamination levels (percentage of berries showing decay signs) were observed daily during storage. Treatments and controls were performed in three replicates. To sum up, the experimental design was as follows: two concentrations of each essential oil (four modalities) are tested separately on strawberries and raspberries (two types of fruits) and three replicates of each concentration were performed. This makes that: [(4 modalities x 3 replications) x 2 (types of fruits)] + 3 replicates of the strawberry control + 3 replicates of the raspberry control = 30 trays. Each replicate involved ten raspberries and six strawberries tested separately.
2.6. Physical and chemical qualities of berries treated with 400 mg·l-1 of I. carinata EO
16After 15 days of cold storage, each replicate of berries treated with 400 mg·l-1 of I. carinata EO was subjected to physical and chemical quality analyses. The physical and chemical measurements were realized using the method of Gunness et al. (2009). In equal weights, deionized water and berries were mixed for 30 s and the pH of the puree was measured with a calibrated pH meter. The puree was centrifuged at 4,400 rpm for 30 min, and the supernatant was refined in glass fiber. A digital refractometer was used to gauge the refractive index of berries clear juice, and the reading was taken in Brix degrees (°Bx) that give the total percentage of solids content (TSS) in fruit. Titratable acidity (TA) was measured by diluting 5 g of clear juice in 100 ml distilled water and then titrating to pH 8.1 with a solution of NaOH (4 g of NaOH·l-1 of distilled water). Citric acid is the main organic acid found in raspberry and strawberry fruit (Spanos, 1986; Watson et al., 2002); TA was expressed in g·l-1 of citric acid equivalent in juice and was calculated using the formula:
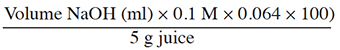
17Also, the maturity index was calculated (TSS/TA). The physical and chemical qualities of controls were taken after 12 days of storage of untreated berries.
2.7. Statistical analysis
18All data were subjected to analysis of variance (ANOVA) using SPSS version 16.0 software. Means comparison was evaluated using Newman Keuls tests. The results were expressed as mean values ± standard deviation with different letters, which are statistically significant at 5% level probability.
3. Results
3.1. Chemical composition of EOs
19The average yield of I. carinata and C. arabicus EOs were 0.1 and 0.44% (w/w), respectively. Qualitative and quantitative analyses of the chemical composition of tested EOs are presented in table 1.
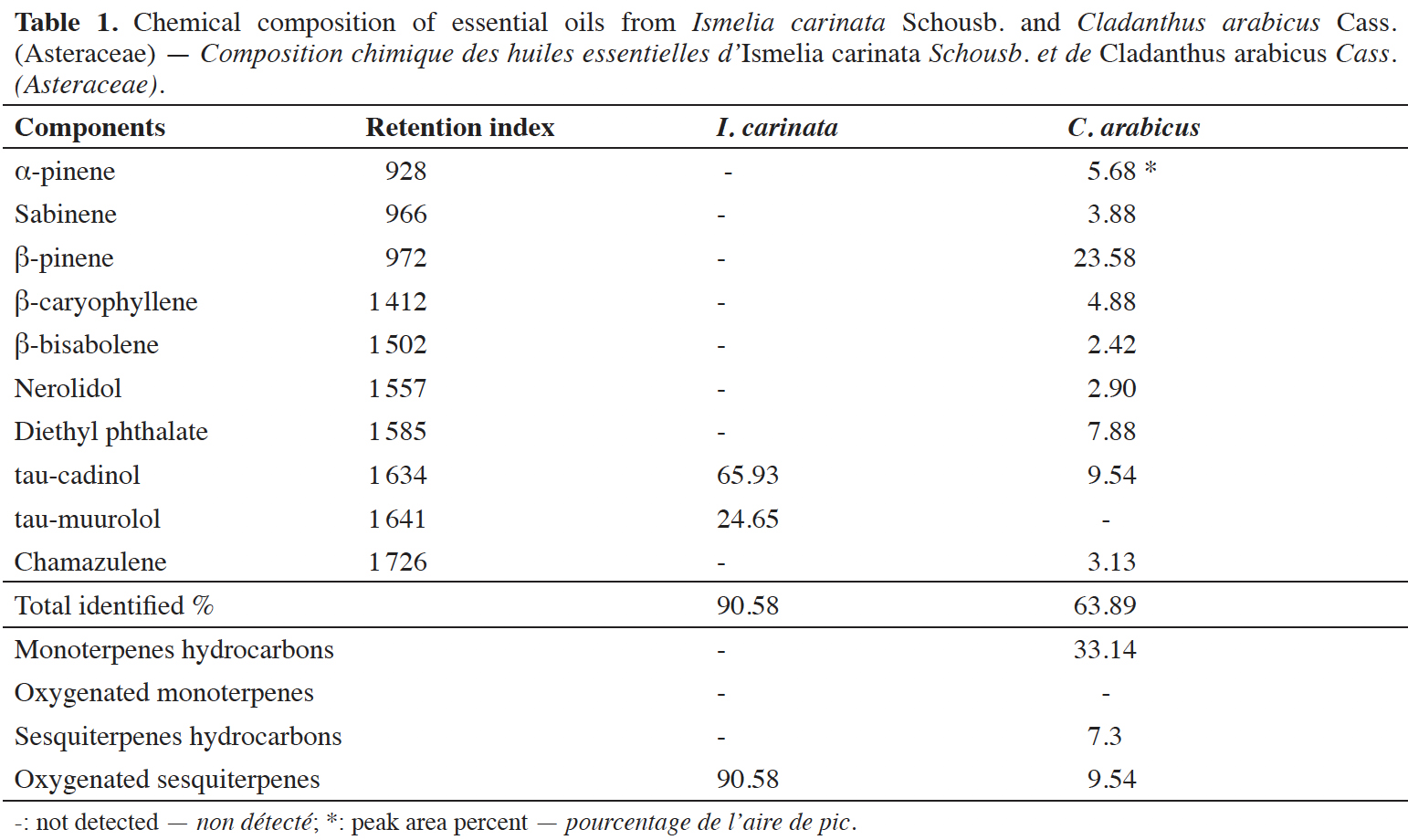
20Ismelia carinata EO was dominated by the oxygenated sesquiterpenes, representing 90.58% of the total oil, which were tau-cadinol (65.93%) and tau-muurolol (24.65%). In C. arabicus EO, nine constituents were identified accounting for 63.89% of the total oil. It was dominated by hydrocarbon fraction (40.44%) followed by oxygenated sesquiterpenes (12.44%). Among which the major compounds were β-pinene (23.58%), tau-cadinol (9.54%), diethyl phthalate (7.88%), α-pinene (5.68%) and β-caryophyllene (4.88%).
3.2. In vitro antifungal assays
21The inhibition percentages obtained in assays of antifungal activity at different concentrations of I. carinata and C. arabicus EOs using agar dilution and disk diffusion techniques are summarized in table 2.
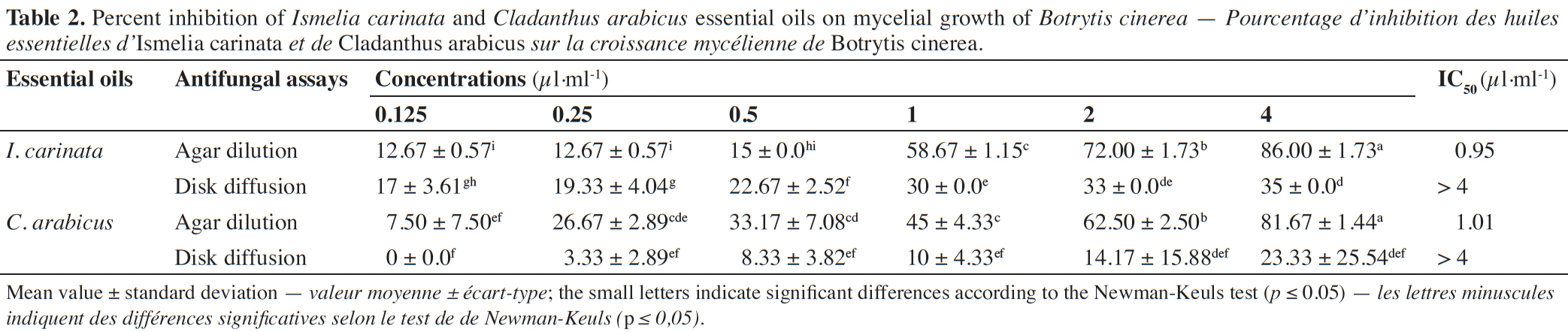
22In both techniques, the results showed that the inhibition of the mycelial growth of B. cinerea was significantly influenced by the EOs concentration (p ≤ 0.05). In the agar dilution method, the EOs exhibited antifungal effect from 12.33 ± 0.57 to 86 ± 1.73% and from 1 ± 3.61 to 81.67 ± 1.44% for I. carinata and C. arabicus, respectively. In disk diffusion, at the highest concentration, the inhibition percentages were 35 ± 0.0% and 23.33 ± 25.54% for I. carinata and C. arabicus EOs, respectively. In the agar dilution technique, the IC50 values were 0.95 µl·ml-1 and 1.27 µl·ml-1 for I. carinata and C. arabicus EOs, respectively. However, in the disk diffusion method, the IC50 values were > 4 µl·ml-1. These results show that the inhibitory effect in the agar dilution technique was more prominent against B. cinerea than in the disk diffusion assay for the two EOs studied (Figure 1).
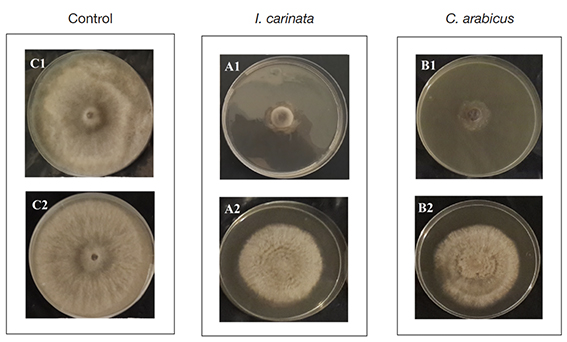
Figure 1. Antifungal effect of 4 µl.ml-1 of Ismelia carinata and Cladanthus arabicus essential oils in agar dilution (A1; B1) and disk diffusion (A2; B2) assays on mycelial growth of Botrytis cinerea compared to the control (C1; C2) — Effet antifongique de 4 µl.ml-1 d’huile essentielle d’Ismelia carinata et de Cladanthus arabicus avec la dilution en gélose (A1; B1) et la diffusion sur disque (A2; B2) sur la croissance mycélienne de Botrytis cinerea par rapport au contrôle (C1; C2).
3.3. Evaluation of EOs on the shelf life of raspberries and strawberries
23The decay rates daily measured of EOs treated berries and their controls (untreated) during 15 days at cold storage are presented in figure 2 (raspberry) and figure 3 (strawberry).
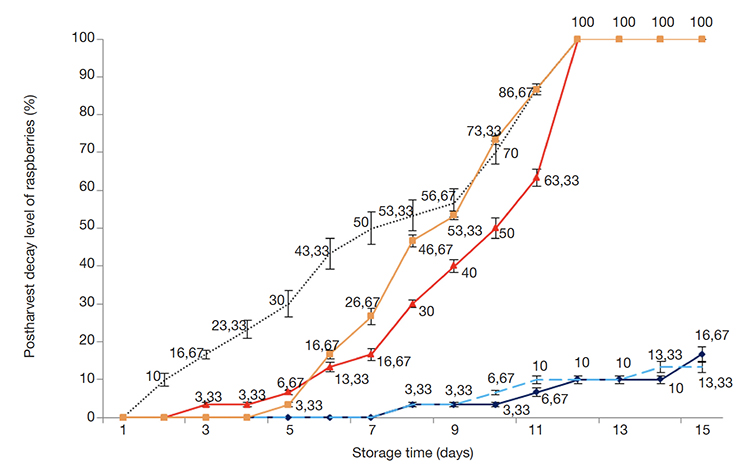
Figure 2. The daily evolution of the decay rate of raspberries treated with Ismelia carinata (IC) and Cladanthus arabicus (CA) essential oils during 15 days at 4 °C with IC200 mg.l-1 (--), IC400 mg.l-1 (–.–), CA200 mg.l-1 (--), CA400 mg.l-1 (--). The sample of untreated raspberries was used as control (···). Mean of three replicates (mean [number] ± SD [vertical bar]) — L’évolution journalière du taux de pourriture des framboises traitées aux huiles essentielles d’Ismelia carinata (IC) et de Cladanthus arabicus (CA) pendant 15 jours à 4 °C avec IC200 mg.l-1 (--), IC400 mg.l-1 (–.–), CA200 mg.l-1 (--), CA400 mg.l-1 (--). L’échantillon de framboises non traitées a servi de témoin (···). Moyenne de trois répétitions (moyenne [nombre)] ± écart-type [barre verticale]).
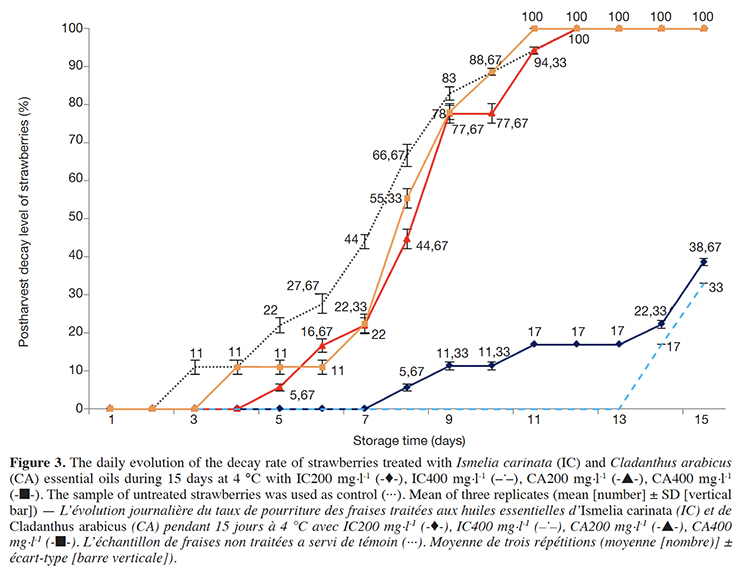
Figure 3. The daily evolution of the decay rate of strawberries treated with Ismelia carinata (IC) and Cladanthus arabicus (CA) essential oils during 15 days at 4 °C with IC200 mg.l-1 (--), IC400 mg.l-1 (–.–), CA200 mg.l-1 (--), CA400 mg.l-1 (--). The sample of untreated strawberries was used as control (···). Mean of three replicates (mean [number] ± SD [vertical bar]) — L’évolution journalière du taux de pourriture des fraises traitées aux huiles essentielles d’Ismelia carinata (IC) et de Cladanthus arabicus (CA) pendant 15 jours à 4 °C avec IC200 mg.l-1 (--), IC400 mg.l-1 (–.–), CA200 mg.l-1 (--), CA400 mg.l-1 (--). L’échantillon de fraises non traitées a servi de témoin (···). Moyenne de trois répétitions (moyenne [nombre)] ± écart-type [barre verticale]).
24For raspberries (Figure 2), from day 1 to 7 the decay rates (%) of treated raspberries with the two EOs were less than the control (50 ± 4.36%) (p ≤ 0.05). During the eighth and ninth days, the decay levels of CA200 (40 ± 1.73%) and CA400 (53.33 ± 1.16%) treated raspberries were more rapidly bordered than that of the control (56.67 ± 3.8%). On day 12, CA200 and CA400 treated raspberry fruits, and control samples were completely infected (100%). At the end-storage day (day 15), the decay rates of IC200 and IC400 treated raspberries were 16.67% and 13.33%, respectively.
25For strawberries (Figure 3), from day 1 to day 7 the decay rates (%) of treated strawberries were lower than that of the control (p ≤ 0.05). From day 8 to 12, the decay rates of CA200 and CA400 treated strawberries were statistically equal to that of untreated strawberry fruits and they were all completely (100%) infected on day 12, while no decayed strawberry fruits were obtained when I. carinata EO was applied at 400 mg·l-1 and for IC200 treated strawberries, only 17% of decay rate was obtained. On the end-storage day, the decay rates of IC200 and IC400 treated strawberries were 38.67% and 33%, respectively.
26For both treated berries no significant difference was observed between 200 and 400 mg·l-1 of I. carinata EO (p ≤ 0.05). The pictures of treated berries with I. carinata EO and controls (untreated berries) are presented in figure 4. It can be observed that untreated berries (A1; A2) are largely postharvest decayed at day 12, while IC200 and IC400 treated berries (B1; B2; C1; C2) maintain good red color and aspect. Furthermore, the berries treated with IC400 (D1; D2) showed good appearance even at day 18.
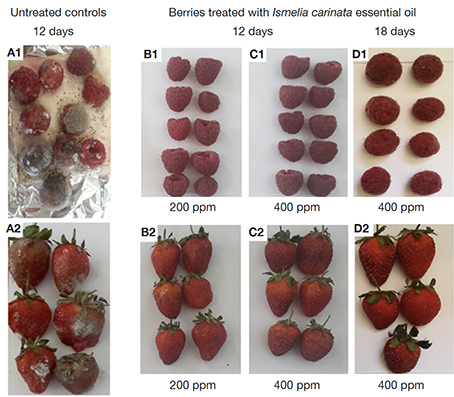
Figure 4. Pictures of berries treated with Ismelia carinata essential oil (B1, B2, C1, C2, D1, D2) and untreated controls (A1, A2) — Photographies des baies traitées (B1, B2, C1, C2, D1, D2) à l’huile essentielle d’Ismelia carinata et des témoins non traités (A1, A2).
3.4. Physical and chemical qualities of berries treated with 400 mg·l-1of I. carinata EO
27The results of physical and chemical analyses of berries treated with 400 mg·l-1 of I. carinata EO after storage for 15 days at 4 °C compared to the control (untreated berries) are presented in table 3a (raspberries) and table 3b (strawberries).
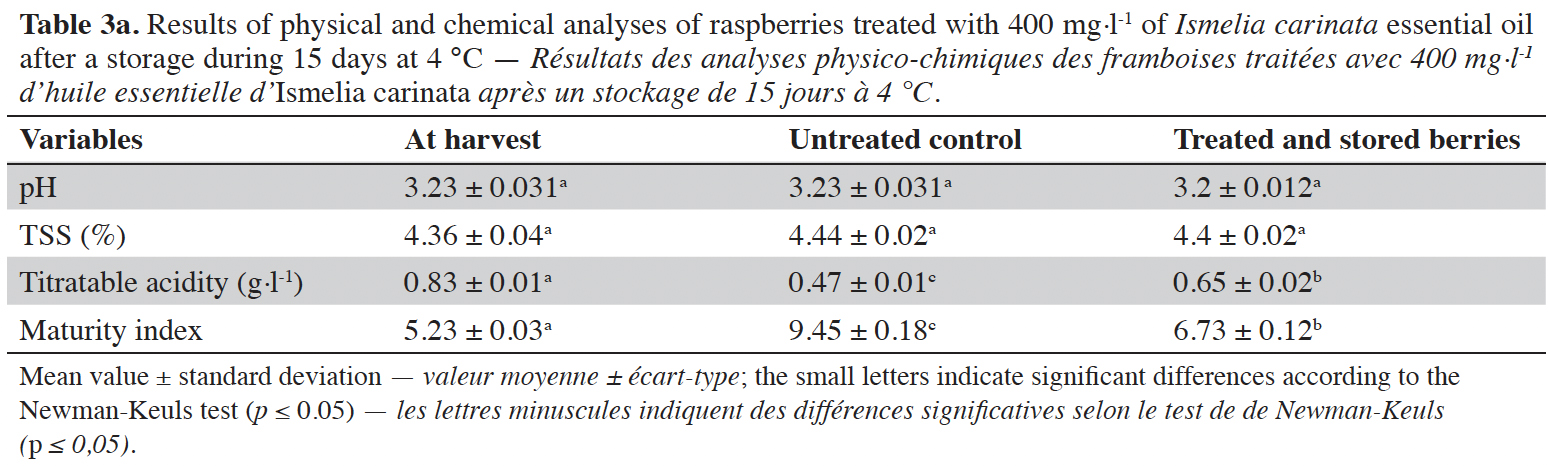
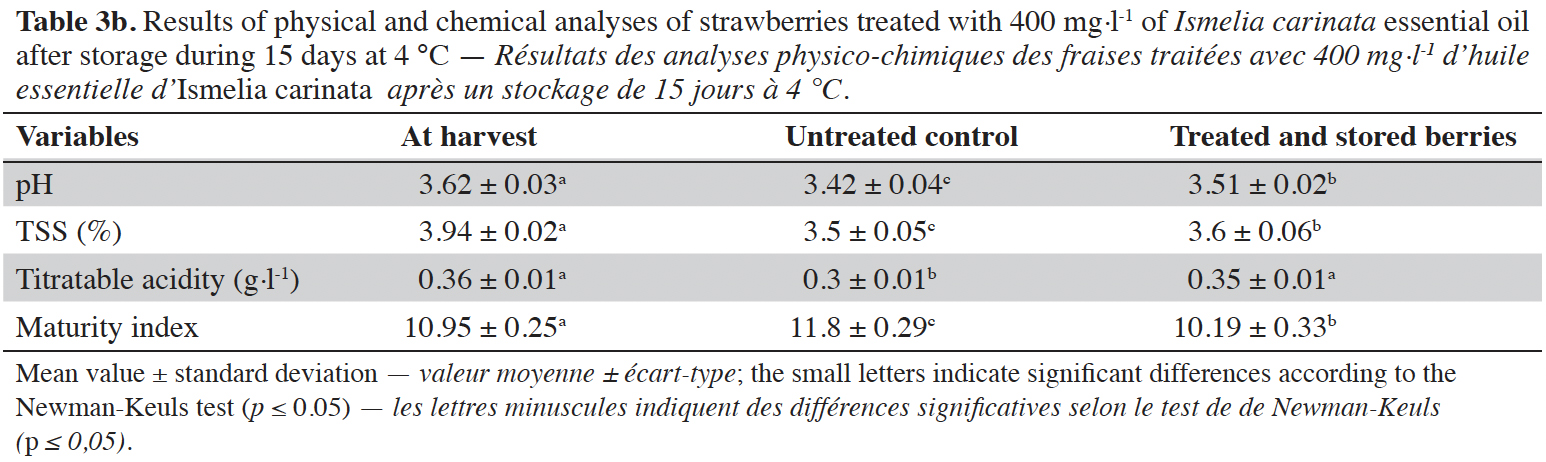
28For raspberries, pH and total soluble solids did not differ whereas titrable acidity and maturity index were significantly different between control and treated samples ( p≤ 0.05). For strawberries, the difference between control and treated samples is significant in all quality factors (p ≤ 0.05). On both berries, the EO treatment maintained high titratable acidity compared to the control, which reduced the change in maturity index of treated berries compared to the controls.
4. Discussion
29To the best of our knowledge, it is the first report of the chemical composition of the I. carinata EO. The EO of I. carinata contained two oxygenated sesquiterpenes as main compounds. This phytochemical profile is different from that of the other species belonging to the Chrysanthemum genus to which I. carinata was earlier grouped (Hosni et al., 2013). These results support a previous study indicating the chemotaxonomic difference of I. carinata, based on flavonoids analysis (Williams et al., 2001).
30The results of the chemical composition of C. arabicus EO showed qualitative and quantitative dissimilarities compared to previous studies using GC/MS to ascertain the composition of C. arabicus EO collected during the flowering stage in Ouarzazate and Marrakech Moroccan regions (El Hanbali et al., 2005; Aghraz et al., 2017). El Hanbali et al. (2005) have identified 36 constituents while Aghraz et al. (2017) have investigated 67 compounds. These two studies cited sabinene, β-pinene, α-pinene, myrcene as major compounds but in different fractions. The current work and the prior studies found α and β-pinene among the major constituents and highly dominance of monoterpenes hydrocarbons in the chemical composition of C. arabicus EO. Diethyl phthalate in the chemical composition of C. arabicus is considered an environmental pollutant. The mechanism of production of this compound by plants is not yet confirmed to conclude that it is a biogenic compound (Romeh, 2013). It is suggested that the plant may possess an ability to accumulate phthalate diethyl from its environment (Saeidnia & Abdollahi, 2013).
31The variations recorded in the chemical composition of C. arabicus EO, are postulated to be due to various factors related to geographical settings, plant genotype, seasonality, and physiological harvesting age (Shanjani et al., 2010; Aboukhalid et al., 2016; Angioni et al., 2006).
32In the in vitro antifungal tests, the agar dilution method presented the best inhibiting effect of EOs against Botrytis cinerea. It has been proposed that Tween 80 enables better distribution of components through the agar. Also, Alvarez-Castellanos et al. (2001) tested the fungicide effect of Chrysanthemum coronarium EO. They concluded that the volatile activity of C. coronarium EO was less persistent against fast-growing fungi as B. cinerea.
33Previous works have tested the fungicidal activity of EOs from the Asteraceae family species against B. cinerea. The reported activities depend on EO composition and especially on the main antifungal component. Liu et al. (2016) demonstrated that the vapors of Solidago canadensis L. EO rich in α–pinene (59.5%) inhibited by 42% the mycelial growth of the fungus. It has been indicated that 1.85 µl·ml-1 of Tagetes patula EO composed of nearly 50% of monoterpenes ketones, reduced by 47.4% the mycelial growth in liquid phases (Romagnoli et al., 2005). Rahman et al. (2010) showed that Erigeron ramosus EO containing 38.5% of sesquiterpenes hydrocarbons was not able to produce any growth inhibition at 1 µl·ml-1. As a result of the current study, it is suggested that liquid phases of the EOs tested have a great in vitro antifungal potential against B. cinerea. Also, the antifungal effects of I. carinata and C. arabicus EOs are related to their main components, which were reported as effective antifungal agents against phytopathogenic fungi (Hammer et al., 2003; Chang et al., 2008). Inhibitory potential of EOs depends on plant taxonomy and the types of chemical molecules in EOs (Pinto et al., 2006; Bajpai et al., 2013). Chang et al. (2008) noted that sesquiterpenes exhibited stronger antifungal effect than monoterpenes. Also, EOs have been suggested to act on cell membrane integrity of pathogens (Bajpai et al., 2013; Liu et al., 2016). Further, the minor components of EOs may interact in synergy with other active compounds linked to cellular signaling and transduction mechanisms (Znini et al., 2013).
34In the berry test, the efficiency of C. arabicus EO has not been visualized, while I. carinata EO minimized the postharvest decay of raspberries and strawberries during 15 days of storage at 4 °C. Many mechanisms of essential oils and their components to deactivate fungi were described (Freiesleben & Jager, 2014). The difference of mode of action between the main compounds of the two EOs could explain the in vivo efficiency difference (Andrade et al., 2018; dos Santos et al., 2021). The components of C. arabicus EO may be more volatile than those of I. carinata EO, as Hay & Waterman (1993) noted that sesquiterpenes are less volatile than other terpenes. This may afford for EO long-term stability under in vivo conditions. It could also be suggested that some components in C. arabicus EO had detrimental effects on sensitive berries and counteracted the fungicidal effect of other major constituents. For example, diethyl phthalate representing 7.88% of the total oil in the investigated chemical composition of C. arabicus EO should be used in limited doses to avoid its toxicological effects (Api, 2001). It is likely that a solubility problem of C. arabicus EO occurred (Demirci et al., 2008) or that the in vivo results were retained from the interactions of phenolic compounds with the food matrix (Yooussef et al., 2016). Previous works reported similar results on Lavandula angustifolia and Origanum compactum EOs, whose antifungal effect under in vivo conditions was not as strong as that in vitro (Hadian et al., 2008; Vu et al., 2011). Further, Lopez-Reyes et al. (2010) concluded that the choice of EOs for delaying postharvest decay should take into consideration the characteristics of the fruit and feasible storage time.
35Other studies have shown the efficiency of EOs, and/or their components against raspberry and strawberry postharvest decays. Allyl isothiocyanate (5 µl·l-1) and methyl jasmonate (22.4 µl·l-1) preserved fresh raspberries without fungal rot deterioration during a storage of 10 days at 10 °C (Wang, 2003; Chanjirakul et al., 2006). Carvacrol and methyl cinnamate - enriched films inhibited by 57% the postharvest decay in treated strawberries than the control (Peretto et al., 2014). Also, strawberries exposed to EOs vapors of Eucalyptus globules and Cinnamomum zeylanicum showed 57 to 63% less spoilage than unexposed fruits (Tzortzakis, 2007). Alikhani & Daraei Garmakhany (2012) noted that microencapsulated Rosmarinus officinalis and Thymus vulgaris EOs were able to reduce the decay development on strawberry fruits stored at 5 °C for 9 days. Liu et al. (2016) reported the effectiveness of Solidago canadensis EO vapor to maintain the apparent quality of fresh strawberries stored at 20 °C. Although appearing as a costly option, the high commercial value of treated strawberries and raspberries would compensate for their postharvest treatment with EOs.
36As raspberries and strawberries are non-climacteric, slight changes in TSS and organic acids occurred. However, it was reported that several factors such as cultivar, storage, and fruit maturity at picking could incur fruit quality changes during the postharvest life of these berries (Shin et al., 2008). In the current study, it was observed that the postharvest treatment with 400 mg·l-1 of I. carinata EO reduced changes in physical and chemical qualities of berries compared to the control.
37The results of this work are consistent with previous works carried out on raspberries and strawberries treated with EOs on postharvest and revealing changes in fruit quality (Wang, 2003; Alikhani & Daraei Garmakhany, 2012).
5. Conclusions
38In this paper, the EOs of I. carinata and C. arabicus were evaluated for their chemical compositions and in vitro antifungal potential using two different screening assays. Furthermore, the study assessed the efficiency of EOs to preserve visual acceptance and fruit sweetness of raspberries and strawberries during cold storage. Ismelia carinata and C. arabicus EOs showed the best in vitro antifungal effect in liquid phases. Ismelia carinata EO minimized postharvest decays of both berries. The results of the current study suggest that I. carinata EO is a promising alternative in preserving fruit quality during fresh raspberries and strawberries storage and transit. Further work on other quality properties after storage of treated raspberries and strawberries is required. Cladanthus arabicus EO should be tested on other fruits as a postharvest treatment to clarify its antifungal potential under in vivo conditions.
Bibliographie
Abdollahi A. et al., 2012. Evaluation of essential oils for maintaining postharvest quality of Thompson seedless table grape. Nat. Prod. Res., 26, 77-83, doi.org/10.1080/14786419.2010.541887
Aboukhalid K. et al., 2016. Chemical polymorphism of Origanum compactum grown in all natural habitats in Morocco. Chem. Biodivers., 13(9), 1126-1139, doi.org/10.1002/cbdv.201500511
Adams R.P., 2007. Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Carol Stream, IL, USA: Allured Publishing Corporation.
Agar I.T. & Streif J., 1996. Effect of high CO2 and controlled atmosphere (CA) storage on the fruit quality of raspberry. Eur. J. Hortic. Sci., 61(6), 261-267,
Aghraz A. et al., 2017. Chemical composition, in vitro antioxidant, antimicrobial and insecticidal activities of essential oil from Cladanthus arabicus. J. Essent. Oil Bear. Plants, 20(3), 601-609, doi.org/10.1080/0972060X.2017.1331143
Alikhani M. & Daraei Garmakhany A., 2012. Effect of microencapsulated essential oils on storage life and quality of strawberry (Fragaria ananassa cv. Camarosa). Qual. Assur. Saf. Crops Foods, 4(2), 106-112, doi.org/10.1111/j.1757-837X.2012.00128.x
Alvarez-Castellanos P.P., Bishop C.D. & Pascual-Villalobos M.J., 2001. Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthemum coronarium) against agricultural pathogens. Phytochemistry, 57(1), 99-102, doi.org/10.1016/S0031-9422(00)00461-1
Andrade A.C.M. et al., 2018. Antifungal activity, mode of action, docking prediction and anti-biofilm effects of (+)-β-pinene enantiomers against Candida spp. Curr. Top. Med. Chem., 18(29), 2481-2490, doi.org/10.2174/1568026618666181115103104
Angioni A. et al., 2006. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem., 54(12), 4364-4370, doi.org/10.1021/jf0603329
Api A.M., 2001. Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food Chem. Toxicol., 39(2), 97-108, doi.org/10.1016/S0278-6915(00)00124-1
Bajpai V.K., Sharma A. & Baek K.H., 2013. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control, 32(2), 582-590, doi.org/10.1016/j.foodcont.2013.01.032
Bakkali F., Averbeck S., Averbeck D. & Idaomar M., 2008. Biological effects of essential oils–a review. Food Chem. Toxicol., 46(2), 446-475, doi.org/10.1016/j.fct.2007.09.106
Benabid A., 2000. Flore et écosystèmes du Maroc : évaluation et préservation de la biodiversité. Paris : Ibis Press.
Buist R., 1846. Catalogue of horticultural and agricultural seed and implements. Philadelphia, USA: Collins.
Chang H.T. et al., 2008. Antifungal activity of essential oil and its constituents from Calocedrus macrolepis var. formosana Florin leaf against plant pathogenic fungi. Bioresour. Technol., 99(14), 6266-6270, doi.org/10.1016/j.biortech.2007.12.005
Chanjirakul K., Wang S.Y., Wang C.Y. & Siriphanich J., 2006. Effect of natural volatile compounds on antioxidant capacity and antioxidant enzymes in raspberries. Postharvest Biol. Technol., 40(2), 106-115, doi.org/10.1016/j.postharvbio.2006.01.004
Chebli B., Achouri M., Hassani Idrissi L.M. & Hmamouchi M., 2003. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol., 89(1), 165-169, doi.org/10.1016/S0378-8741(03)00275-7
Council of Europe, 1997. European Pharmacopoeia. 3rd ed. Strasbourg, France: Council of Europe.
Dean R. et al., 2012. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol., 13, 414-430.
Demirci F. et al., 2008. Antibacterial activity of two Phlomis essential oils against food pathogens. Food Control, 19(12), 1159-1164, doi.org/10.1016/j.foodcont.2008.01.001
dos Santos A.L. et al., 2021. (-)-T-Cadinol—a sesquiterpene isolated from Casearia sylvestris (Salicaceae) — displayed in vitro activity and causes hyperpolarization of the membrane potential of Trypanosoma cruzi. Front. Pharmacol., 12, article 734127, doi: 10.3389/fphar.2021.734127
El Hanbali F. et al., 2005. Composition and antibacterial activity of essential oils of Cladanthus arabicus Cass.(Asteraceae). J. Essent. Oil Bear. Plants, 8(2), 213-217, doi.org/10.1080/0972060X.2005.10643448
El Oualidi J. et al., 2012. Checklist des endémiques et spécimens types de la flore vasculaire de l’Afrique du Nord. Rabat : Université Mohammed V-Agdal.
Farzaneh M. et al., 2015. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol. Technol., 109, 145-151, doi.org/10.1016/j.postharvbio.2015.06.014
Freisesleben S.H. & Jager A.K., 2014. Correlation between plant secondary metabolites and their antifungal mechanism—a review. Med. Aromat. Plants, 3, 1-6, doi.org/10.4172/2167-0412.1000154
Gastavsson J., Cederberg C. & Sonesson U., 2011. Global food losses and food waste. Roma: FAO.
Gunness P. et al., 2009. Sensory analysis of individual strawberry fruit and comparison with instrumental analysis. Postharvest Biol. Technol., 52(2), 164-172, doi.org/10.1016/j.postharvbio.2008.11.006
Hadian J. et al., 2008. Antifungal potency of some essential oils in control of postharvest decay of strawberry caused by Botrytis cinerea, Rhizopus stolonifer and Aspergillus niger. J. Essent. Oil Bear. Plants, 11(5), 553-562, doi.org/10.1080/0972060X.2008.10643666
Hammer K.A., Carson C.F. & Riley T.V., 2003. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol., 95(4), 853-860, doi.org/10.1046/j.1365-2672.2003.02059.x
Hay R.K.M. & Waterman P.G., 1993. Volatile oil crops: their biology, biochemistry and production. New York, USA: John Wiley.
Hosni K., Hassen I., Sebei H. & Casabianca H., 2013. Secondary metabolites from Chrysanthemum coronarium (Garland) flowerheads: chemical composition and biological activities. Ind. Crops Prod., 44, 263-271, doi.org/10.1016/j.indcrop.2012.11.033
Liu S. et al., 2016. Solidago canadensis L. essential oil vapor effectively inhibits Botrytis cinerea growth and preserves postharvest quality of strawberry as a food model system. Front. Microbiol., 7, 1179, doi.org/10.3389/fmicb.2016.01179
Lopez-Reyes J.G., Spadaro D., Gullino M.L. & Garibaldi A., 2010. Efficacy of plant essential oils on postharvest control of rot caused by fungi on four cultivars of apples in vivo. Flavour Fragrance J., 25(3), 171-177, doi.org/10.1002/ffj.1989
Peretto G. et al., 2014. Increasing strawberry shelf-life with carvacrol and methyl cinnamate antimicrobial vapors released from edible films. Postharvest Biol. Technol., 89, 11-18, doi.org/10.1016/j.postharvbio.2013.11.003
Pinto E. et al., 2006. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol., 55(10), 1367-1373, doi.org/10.1099/jmm.0.46443-0
Prusky D., 2011. Reduction of the incidence of postharvest quality losses, and future prospects. Food Secur., 3(4), 463-474, doi.org/10.1007/s12571-011-0147-y
Rahman A., Hossain M.A. & Kang S.C., 2010. Control of phytopathogenic fungi by the essential oil and methanolic extracts of Erigeron ramosus (Walt.) BSP. Eur. J. Plant Pathol., 128(2), 211-219, doi.org/10.1007/s10658-010-9645-6
Romagnoli C. et al., 2005. Chemical characterization and antifungal activity of essential oil of capitula from wild Indian Tagetes patula L. Protoplasma, 225(1-2), 57-65, doi.org/10.1007/s00709-005-0084-8
Romanazzi G. & Feliziani E., 2014. Botrytis cinerea. In: Bautista-Baños S. (ed.). Postharvest decay: control strategies. Elsevier, 131-146.
Romeh A.A., 2013. Diethyl phthalate and dioctyl phthalate in Plantago major L. Afr. J. Agric. Res., 8(32), 4360-4364.
Saeidnia S. & Abdollahi M., 2013. Are medicinal plants polluted with phthalates? DARU J. Pharm. Sci., 21(1), 43, doi.org/10.1186/2008-2231-21-43
Sarkhosh A. et al., 2017. Postharvest management of anthracnose in avocado (Persea americana Mill.) fruit with plant-extracted oils. Food Packag. Shelf Life, 12, 16-22, doi.org/10.1016/j.fpsl.2017.02.001
Shanjani P.S., Mirza M., Calagari M. & Adams R.P., 2010. Effects drying and harvest season on the essential oil composition from foliage and berries of Juniperus excelsa. Ind. Crops Prod., 32(2), 83-87, doi.org/10.1016/j.indcrop.2010.03.003
Shin Y. et al., 2008. Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruit. Postharvest Biol. Technol., 49(2), 201-209, doi.org/10.1016/j.postharvbio.2008.02.008
Sivakumar D. & Bautista-Baños S., 2014. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot., 64, 27-37, doi.org/10.1016/j.cropro.2014.05.012
Soreng R.J. & Cope E.A., 1991. On the taxonomy of cultivated species of the Chrysanthemum genus-complex (Anthemideae: Compositae). Baileya, 23(3), 145-165.
Spanos G.A., 1986. Anthocyanin pigment, nonvolatile acid and sugar composition of red raspberry juice. Phd thesis: Oregon State University, Corvallis (USA).
Suradeep B. & Proshanta G., 2018. A review on antifungal activity and mode of action of essential oils and their delivery as nano-sized oil droplets in food system. J. Food Sci. Technol., 55(12), 4701-4710.
Tournas V.H. & Katsoudas E., 2005. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int. J. Food Microbiol., 105, 11-17.
Turland N.J., 2004. (1647) Proposal to conserve the name Chrysanthemum coronarium (Compositae) with a conserved type. Taxon, 53(4), 1072-1074, doi.org/10.2307/4135582
Tutin T.G. et al., 1964. Flora Europea. Cambridge, UK: Cambridge University Press.
Tzortzakis N.G., 2007. Maintaining postharvest quality of fresh produce with volatile compounds. Innovative Food Sci. Emerg. Technol., 8(1), 111-116, doi.org/10.1016/j.ifset.2006.08.001
Vu K.D. et al., 2011. Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries. Food Res. Int., 44(1), 198-203, doi.org/10.1016/j.foodres.2010.10.037
Wang C.Y., 2003. Maintaining postharvest quality of raspberries with natural volatile compounds. Int. J. Food Sci. Technol., 38(8), 869-875, doi.org/10.1046/j.0950-5423.2003.00758.x
Watson R. et al., 2002. Influence of harvest date and light integral on the development of strawberry flavour compounds. J. Exp. Bot., 53(377), 2121-2129, doi.org/10.1093/jxb/erf088
Williams C.A., Greenham J. & Harborne J.B., 2001. The role of lipophilic and polar flavonoids in the classification of temperate members of the Anthemideae. Biochem. Syst. Ecol., 29(9), 929-945, doi.org/10.1016/S0305-1978(01)00039-4
Yooussef M.M., Pham Q., Achar P.N. & Sreenivasa M.Y., 2016. Antifungal activity of essential oils on Aspergillus parasiticus isolated from peanuts. J. Plant Prot. Res., 56(2), 139-142, doi.org/10.1515/jppr-2016-0021
Zheng Y., Wang S.Y., Wang C.Y. & Zheng W., 2007. Changes in strawberry phenolics, anthocyanins, and antioxidant capacity in response to high oxygen treatments. LWT Food Sci. Technol., 40(1), 49-57, doi.org/10.1016/j.lwt.2005.08.013
Znini M. et al., 2013. Essential oil composition and antifungal activity of Pulicaria mauritanica Coss., against postharvest phytopathogenic fungi in apples. LWT Food Sci. Technol., 54(2), 564-569, doi.org/10.1016/j.lwt.2013.05.030






