- Portada
- Volume 27 (2023)
- Numéro 2
- Technology of a novel conidia-tablet formulation and packaging type to increase Beauveria bassiana (Hypocreales: Ophiocordycipitaceae) shelf life at room temperature
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Technology of a novel conidia-tablet formulation and packaging type to increase Beauveria bassiana (Hypocreales: Ophiocordycipitaceae) shelf life at room temperature

Documento adjunto(s)
Version PDF originaleRésumé
Technologie d’une nouvelle formulation de tablettes de conidies et d’un nouveau type d’emballage pour augmenter la durée de conservation de Beauveria bassiana (Hypocreales : Ophiocordycipitaceae) à température ambiante
Description du sujet. Cette étude décrit le développement de formulations de mycoinsecticides contenant des conidies sèches du champignon entomopathogène Beauveria bassiana associées à divers excipients inertes sous forme de tablette pour faciliter la dispersion des conidies dans l'eau et maintenir leur viabilité pendant le stockage.
Objectifs. Cette étude visait à évaluer la formation de tablettes de conidies de B. bassiana associées à différents excipients inertes sous compression directe dans une presse hydraulique.
Méthode. Évaluation et validation des propriétés mécaniques et de conservation des tablettes de conidies sous différents types de conditionnement.
Résultats. La formulation de la tablette contenant 30 % de conidies (ingrédient actif) et 70 % d'amidon de maïs (excipient inerte) a montré des résultats satisfaisants en termes de résistance mécanique et de dispersion dans l'eau. Cette tablette présentait une dureté et une friabilité de 173,94 N et 0,34 %, respectivement. Le taux de désintégration de la tablette dans l'eau s'est produit en 2 min, présentant une dispersion rapide de 109 conidies∙ml-1. La tablette stockée dans un pot en polyéthylène avec de la silice polymérisée a montré une viabilité ≥ 80 % sur 180 jours de stockage (25-28 °C).
Conclusions. Le couple formulation-conditionnement mis au point est prometteur compte tenu des paramètres physico-chimiques de la tablette et des données de viabilité. La formulation de type conidies:amidon de maïs offre un rapport cout-bénéfice intéressant pour un procédé de production simple d'un bioinsecticide, outre l'utilisation à faible cout d'adjuvant et de conditionnement (pot en polyéthylène). Considérant que la plupart des produits biopesticides nécessitent un stockage sous réfrigération, ce travail est important puisque la formulation est restée viable pendant 180 jours de stockage dans des conditions de température ambiante.
Abstract
Description of the subject. This study describes development of mycoinsecticide formulations containing dry conidia of the entomopathogenic fungus Beauveria bassiana combined with various inert excipients in tablet form to facilitate conidia dispersion in water and to maintain viability during storage.
Objectives. This study aimed to evaluate the tablet formation of B. bassiana conidia associated with different inert excipient under direct compression in a hydraulic press.
Method. Evaluation and validation of the mechanical and storage properties of conidial tablets in different types of packaging.
Results. The tablet formulation containing 30% conidia (active ingredient) and 70% cornstarch (inert excipient) displayed satisfactory results regarding mechanical resistance and dispersion in water. This tablet exhibited hardness and friability of 173.94 N and 0.34%, respectively. The tablet disintegration rate in water occurred in 2 min, presenting a rapid dispersion of 109 conidia∙ml-1. Tablet stored in polyethylene pot with polymerized silica exhibited viability ≥ 80% over 180 days storage (25-28 °C).
Conclusions. The formulation-packaging binomial is promising, considering the physical-chemical tablet parameters and the viability data. The conidia:cornstarch tablet type formulation provides a relevant cost-benefit ratio of a simple production process of a bioinsecticide, besides the use of low-cost adjuvant and packaging (polyethylene pot). Considering that most biopesticide products require storage under refrigeration, this work becomes important since the formulation remained viable for 180-days storage under room temperature conditions.
Tabla de contenidos
Received 6 October 2022, accepted 15 May 2023, available online 8 June 2023
This article is distributed under the terms and conditions of the CC-BY License (https://creativecommons.org/licenses/by/4.0)
1. Introduction
1Over the last 50 years, the entomopathogenic-fungi exploration with potential in insect pest biological control has gradually gained prominence. Adverse environmental impacts, human health safety risks and also the insect resistance mechanisms development through the use of chemical insecticides are the main reasons for the progressive increase of entomopathogenic-fungi based bioproducts commercialization (Jaronski, 2014; Egbuna et al., 2020; Fiorotti et al., 2022; Kumari et al., 2022; Moustafa et al., 2023).
2Worldwide, entomopathogenic fungi stand out as biocontrol agents, due to their broad action spectrum, their ability to infect insects at different development stages and their tegument penetration capacity. These characteristics show advantages over other groups of microorganisms also applied as biocontrols (Mora et al., 2017; Rajula et al., 2020; Islam et al., 2021). Thereby, Beauveria bassiana, B. brongniartii, Metarhizium anisopliae, and Isaria fumosorosea species have been widely and commonly used to develop the 171 fungal-based bioproducts available in the biological control market (Faria & Wraight, 2007; Maina et al., 2018; Butu et al., 2022).
3Environmental storage conditions impact directly the bioproducts market potential, affecting their shelf life. Although these fungal species are promising biocontrol agents, their large-scale production and commercialization represent a challenge, mainly due to the short survival of the infective propagules when stored at room temperature and above (Batta & Kavallieratos, 2018; Kidanu & Hagos, 2020). Humidity is also an important requirement to improve fungal viability during transport and storage. Therefore, the shelf life can vary depending on fungi species/strains selected, the propagule form, and packaging type, representing a vital role during storage (Maina et al., 2018; Kidanu & Hagos, 2020; Qayyum et al., 2021). For this reason, biopesticides mostly fungal-based produced constitute only 5% of the global crop market (Batista & Singh, 2021; Butu et al., 2022; Jampílek & Kráľová, 2022).
4The development of the entomopathogenic fungi formulations associated with inert excipient depends on several factors, such as viability, storage time, bioproduct handling and application; environmental factors, target pest contacts and interactions (Arnosti et al., 2019; Curkovic et al., 2019; de la Cruz Quiroz et al., 2019). Previous studies have focused their efforts on evaluating the packaging compositions to prolong the shelf life of fungal biopesticides. Furthermore, the excipient formulation and packaging type combinations are important factors for efficient product preservation (de la Cruz Quiroz et al., 2019; Biryol et al., 2022; Jeong et al., 2022).
5The main challenge to commercializing entomopathogenic fungal-based bioinsecticides is the production of an inexpensive and natural formulation (Faria & Wraight, 2007; Mascarin & Jaronski, 2016). Faria & Wraight (2007) presented a list of 171 fungi-based bioproducts, exhibiting an insecticide action of 93.6%. In this context, commercial Beauveria bioproducts are described as dry (mostly wettable powder) and liquid (oil dispersions) formulations containing excipients, fillers, and other additives to obtain a stable preparation. Overall, drying processes are more effective in stabilizing Beauveria propagules since the low water content extends its shelf life. Studies have indicated the efficiency of topical or spray applications of wettable powders and water-dispersible granules in insect and nematode biocontrol (de la Cruz Quiroz et al., 2019).
6Conidia-tablet bioinsecticide formulation development has been limited. Only a few patents describe this technology, WO2009093261A2 (2009), US2015272129A1 (2015) and CN110506755A (2019). Tablets have numerous advantages in the industrial process, such as physical-chemical stability, low-cost production, portion precision, easy handling and storage facility (Armstrong, 2013). Although several steps are required in the tablet production, compression is critical for the effectiveness of a product (Gerhardt, 2010). The mechanical properties of hardness and friability must be quantified as ideal parameters to obtain a homogeneous final product, improve its resistance to handling and transport, and facilitate the active ingredient release (Kumar et al., 2016; Agrawal et al., 2022; Mitra et al., 2022). Based on this, this study evaluated the tablet formation of B. bassiana conidia associated with different inert excipients under direct compression in a hydraulic press to reduce void spaces and, consequently, decrease water adsorption. Therefore, this study aimed to develop a conidium-tablet formulation and evaluate its potential using different types of packaging to estimate the fungal conservation and viability under shelf conditions at room temperature.
2. Materials and methods
2.1. Entomopathogenic fungi
7The entomopathogenic fungus Psi strain was previously isolated from a decomposed Diaphorina citri psyllid. The strain was identified by 18S rRNA region sequencing as a Beauveria bassiana (Hypocreales: Ophiocordycipitaceae) (99% similarity, GenBank number: KM031765.1).
2.2. Dry conidium production
8The Psi strain was cultivated in PDA plates (potato dextrose agar) for 7 days at 26 ± 2 ºC, 12 h photophase. The fungal culture was scraped off and mixed with 10 ml of Tween 80® (0.05%). This suspension (108 spores.ml-1) was inoculated in 200 g of rice (40% moisture) packed in polypropylene bags, and incubated at 26 ± 2 ºC for 15 days. The colonized rice was dried in an acclimatized room (27 ± 2 ºC) for 10 days and separated using vibrating sieve (Analytical Screener AS 300) (Alves, 2006).
2.3. Conidium tablet formulations
9Different compounds were used as inert materials to formulate the conidia-tablets: cornstarch, sodium alginate, microcrystalline cellulose and arabic gum. The process was patented and deposited at the Brazilian National Institute of Industrial Property (Patent number BR1020180123017) (INPI-Brazil). Conidia and inert compound proportions (30:70; 40:60; 50:50 and 40:60%) were calculated respecting the final tablet weight (kg). The mixture was placed in a desiccator coupled to a vacuum pump and monitoring until decrease its moisture and water activity to ± 5% and 0.2, respectively. After mechanical homogenization, the mixture was transferred to 13 mm molds diameter and subjected to the effect of a hydraulic press to obtain 0.5 mg of conidia-tablet. Pressure and time proportions at hydraulic press were 10 kpa/10 min; 5 kpa/5 min and 1 kpa/1 min.
2.4. Conidium tablet disintegration and dispersion
10The dispersion aspect (total dispersion, lumps presence or slow swelling) and disintegration time (min) of a conidium tablet was calculated by adding it to in a Becker containing 100 ml of distilled water at room temperature under magnetic stirrer at 20 rpm, monitoring the time (min). Regarding disintegration, a conidium tablet was mixed with 900 ml of distilled water in tubes with rotating blades at 100 rpm (CIPAC MT-197). After tablet disintegration, aliquots were collected and filtered to estimate conidium release (conidia∙ml-1) by microscopic counting in a Neubauer chamber (×400) (Alves, 2006). Disintegration time and aspect were determinate for all formulations, using shorter disintegration time as selection criterion. Both water dispersion speed and conidium concentration were performed for the selected formulation.
2.5. Conidium tablet mechanical properties assays
11Hardness and friability tests. Hardness and friability assays were determined on the selected formulation. The conidium tablet hardness test was performed in a mechanical durometer (HDT-400) with a precision of 1N. The tablets also were exposed to a friability test apparatus (100 rotations/25 rpm/4 min). Each tablet and its powder residues were weighed. The difference between the initial and final weight indicates the friability, measured as a function of the powder lost percentage (Brazilian Health Regulatory Agency, 2019).
12Packaging selection and storage condition. Two types of packaging were used to evaluate the preservation of the 30/70 formulation conidia-tablets (conidia/inert %):
13– polyester+aluminium+coex nylon 6-poly bag, with desiccator (polymerized silica), vacuum-sealed (PANS bag);
14– polyethylene pot with desiccator (polymerized silica) and airtight closure (PSP pot).
15The conidium tablets also were packed in filter paper and stored under the same condition. The polymerized silica (non-indicator) amount added to the packages was determined according to the methodology described by Redman-Furey et al. (2013). Different packages and control containing the tablets were stored for 365 days in an acclimatized room at 26 ± 2 °C, relative humidity > 70 ± 5% and a 12 h photophase. The experimental design was completely randomized, using four replications per treatment (packages).
2.6. Determination of the packaging preservation potential
16Water Activity (aw) and moisture (%). The water activity (aw) of conidium tablets from each package was evaluated by direct reading using a water activity meter (LabSwitt-aw - Novasina). The moisture (%) was determinate by an analytical infrared balance (AND MX-50). Tablets were weighed before packaging every 30 days throughout the 365 days storage period.
17Viability (%). Conidium tablets were disintegrated in 10 ml of Tween 80® solution (0.05%). Then, serial dilutions were performed, transferring a 150 µl aliquot of this suspension to a Petri dish RODAC® containing PDA medium (potato dextrose agar) and incubating at 26 ± 2 °C, 70% relative humidity, photophase 12 h. After 18 h-incubation, the viability (%) was determined by evaluating proportion between germinated and non-germinated conidia by counting under an optical microscope (×400). This analysis with tablet samples from the different packages was performed monthly during the storage period.
18Statistical analysis. The entire experiment was conducted with four replicates for the variance analysis (ANOVA) and Tukey's test (p < 0.05). Viability results were submitted to regression analysis by linear bivariate model for all storage packaging types. Pearson correlation test and the regression analysis were performed between the moisture (%) and the water activity (aw) concerning the viability (%). ANOVA, Tukey and Pearson correlation tests were performed using the software SPSS v. 23. (IBM, SPSS). Regression analysis was performed in PAST software (Hammer et al., 2001). Moisture (%), water activity (aw) and viability (%) graphics were performed by OriginPro 9 (Origin Corporation, 2012).
3. Results
3.1. Beauveria bassiana conidium tablets formulation
19Inert excipient effect and its mechanical properties. The tablet formulations with different conidia/MCC ratios showed longer disintegration time and a slow swelling capacity in water. Sodium alginate and arabic gum each exhibited a rapid disintegration under the compression effect and showed adhesion to the pressing molds walls (Table 1). The tablet formulation containing conidia and cornstarch at proportion of 30 and 70%, respectively, showed satisfactory results concerning compressibility, aggregation, disintegration, and total dispersion in water. Cornstarch excipient displayed efficient results of the mechanical proprieties, and also in compression, disintegration time and dispersion in water. The 30:70% (conidia:cornstarch) formulation displayed 173,94 N of hardness and 0,34% of friability and presented total water dispersion in 2 min, a dispersion speed of ± 2 min/1 kPa/1 min, releasing 1.3×109 spores∙ml-1 in water (Table 1). Two minutes were the shortest time for the disintegration of all tested formulations.
3.2. Packaging preservation
20Conidium tablets water activity (aw) and moisture (%). Differences in water activity (aw) were observed for the conidium tablets in the two different types of packaging and control through analyzes over the storage period (1 to 12 months) (Figure 1a). Over 12 months period, the aw increased concomitantly with the storage time for each type of packaging. Concerning the tablets packed in PSP pots, this activity variation was lowest (0.388 - 0.545aw) with an increase of 40% after 365-day period (F = 2602.6; d.f. = 24; p < 0.001). In contrast, PANS bag tablets increased in water activity by 78% over 12 months (0.399 - 0.710aw) compared to control after final storage period. The control (tablets packed in filter paper) exhibited an aw of 121% (0.362 - 0.801aw) (12 months) (Figure 1a).
Figure 1. Effect of water activity and moisture content on Beauveria bassiana conidium tablets stored in different types of packages. (a) Water activity (aw) and (b) moisture of conidium tablets packed in different types of packaging. Control: conidium tablets packed in filter paper. Storage during 12 months at 26 ± 2 ºC — Effet de l’activité de l’eau et de la teneur en humidité sur les tablettes de conidies de Beauveria bassiana stockées dans différents types d’emballages. (a) Activité de l’eau (aw) et (b) humidité des tablettes de conidies emballées dans différents types d’emballage. Témoin : tablettes de conidies emballées dans du papier filtre. Stockage pendant 12 mois à 26 ± 2 ºC.
21The increase in tablet moisture was progressive in each packaging type during 12 month-storage (Figure 1b). The PSP pot was more efficient in conidium tablets preservation, exhibiting 2.15% of moisture, the lowest value (F = 5902.1; d.f. = 24; p < 0.001) compared to PANS bags (3.54%) and the control (3.45%) during the storage period (Figure 1b). No significant differences in moisture were observed between PANS bag-packed tablets and the control over 12 months (Figure 1b).
22Conidium tablets viability (%). The conidium tablets packaged into PSP pot showed an 81.50% germination rate at 180 day-storage, corresponding to a 12% loss of viability. Regarding PANS bag-packed tablets compared to the control, the germinations rate was 74.05 and 51.82%, corresponding to viability losses of 18 and 42%, respectively. At 365 days of storage, conidium tablets packed in PSP pots exhibited the highest viability value of 45.35% (F = 442.7; d.f = 24; p = < 0.001) (Figure 2).
Figure 2. Viability (%) of Beauveria bassiana conidium tablets determined by germination rate over its preservation using different types of packaging. Control: conidium tablets packed in filter paper. Storage during 12 months at 26 ± 2 °C — Viabilité (%) des tablettes de conidies de Beauveria bassiana déterminée par le taux de germination au cours de leur conservation en utilisant différents types d’emballage. Témoin : tablettes de conidies emballées dans du papier filtre. Stockage pendant 12 mois à 26 ± 2 °C.
23Pearson's correlation analyses between the packaging types to preserve the tablets indicated negative correlation between water activity (aw) and conidium germination rate (viability %) (ρ = -0.961; p = < 0.001; n = 52). Data also showed negative correlation between moisture (%) and conidium germination rate (viability %) (ρ = -0.987; p = < 0.001; n = 52). These correlations indicate the conidium viability is reduced with increasing water activity and moisture. Overall, the viability of stored conidia decreased over the total storage period with a concomitant increase in the water activity and moisture (Figures 3a and 3b). Notwithstanding, the analyzed parameters referring to the conidium tablets packed into PSP pots displayed the lowest variation over the storage period.
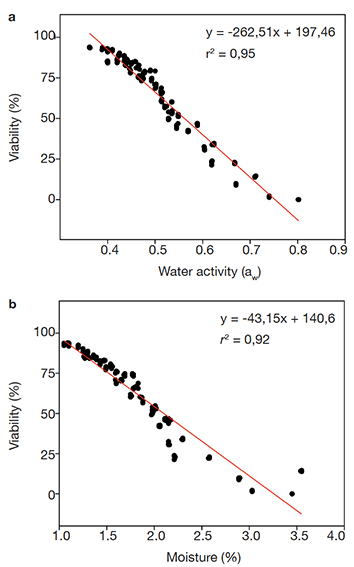
Figure 3. Linear regression analyses between (a) conidium tablet water activity (aw) or (b) conidium tablet moisture (%) and the conidium germination rate (viability %) during 12-month storage at 26 ± 2 ºC — Analyses de régression linéaire entre (a) l’activité de l’eau des tablettes de conidies (aw) ou (b) l’humidité des tablettes de conidies (%) et le taux de germination des conidies (viabilité %) au cours d’un stockage de 12 mois à 26 ± 2 ºC.
4. Discussion
24The sodium alginate and arabic gum used as inert excipient compounds exhibited rapid disintegration under the compression effect and adhesion of the mixture to the pressing molds walls. Therefore, results indicated the infeasibility of these polymers for conidium tested tablet formulations. Regarding microcrystalline cellulose (MCC), tablet formulations showed longer disintegration time and a slow swelling capacity in water (Table 1). MCC is the most used excipient in pharmaceuticals due to its rheological properties in wet mass to obtain tablets with low friability and high density (Chamsai & Sriamornsak, 2013; Cho et al., 2022). Despite these advantages, a limiting factor of this polymer is related to the incomplete tablet disintegration, which has led to the search for other excipients, such as biopolymers (starch and its derivatives), semi-synthetic polymers, and lipids (Dukić-Ott et al., 2009; Chamsai & Sriamornsak, 2013).
25Based on this, the differential of this study was the cornstarch use as the only excipient in the entomopathogenic conidium-tablet composition, which exhibited significant results regarding physicochemical properties and active ingredient dispersion. The experimental data for both hardness and friability correspond to values established by Brazilian pharmacopoeia specifications (Brazilian Health Regulatory Agency, 2019), which delimit ≤ 1.5% for friability and > 30 N for hardness. Although this pharmacopoeia stipulates parameters for health area pill production, this reference was extrapolated to develop conidium tablet formulations. The tablets (30:70%, conidia:cornstarch) displayed 173,94 N of hardness and 0,34% of friability, indicating a satisfactory resistance to avoid mechanical effects during their production, packaging and storage. Furthermore, cornstarch is a low-cost inert excipient with low viscosity, showing moisture resistance (Abotbina et al., 2020; Mitra et al., 2022). Regarding mechanical properties and inert effect, data suggest that this biopolymer may be efficient in the formulation and commercialization of entomopathogenic conidium tablets. In this context, the 30:70 conidium-cornstarch formulation was selected to evaluate its preservation in storage packaging materials.
26The formulation effectiveness depends on the selected fungal strain and its propagule, and the limiting conditions of exposure, such as temperature, humidity and solar radiation (Behle & Jackson, 2014; Islam et al., 2021). Thereby, a limiting factor to commercializing entomopathogenic fungi is the difficulty in maintaining their viability for a long storage period (Silva & Neves, 2016). Shelf environmental conditions are subject to wide variation in temperature and relative humidity, which favor conidial germination with the consequent loss of its infective capacity. Under favorable environmental conditions, conidia can readily germinate within 6 to 12 h (Lopes et al., 2013; Meshram et al., 2022). Relative humidity around 90% and temperature between 25-30 ºC are optimal conditions for fungal germination (Ramanujam et al., 2014). Therefore, the storage control conditions and suitable formulations are important requirements to maintain conidial viability, aiming at enhancing bioproducts performance (Behle & Jackson, 2014; Ayala-Zermeño et al., 2017). Studies have indicated the conidium viability maintenance of B. bassiana and other entomopathogenic fungi at low relative humidity equilibrium during storage (Moore et al., 1996; Derakshan et al., 2008; Sandhu et al., 2008; Lopes & Faria, 2019). In this context, B. bassiana and M. anisopliae conidium formulations exhibited a decrease in their viability in a period below 30 days under storage at 30 ºC and 15.5% humidity (Marques & Alves, 1996). Furthermore, Lopes & Faria (2019) indicated the influence of moisture content on conidia stability, since the reduced moisture condition promotes a very low metabolic state, prolonging fungal viability. At this point, the water activity (aw) is a parameter that provides information on water amount slightly associated with non-aqueous constituents, which is available for metabolic processes such as growth, oxidation and catalytic reactions, unwanted attributes during storage period. The conidia must be with low metabolic activity to remain viable for a longer shelf life (Carareto et al., 2010; Tapia et al., 2020). Our work presents higher conidial viability (%) associated with lower moisture and aw for the 30:70 conidium-cornstarch formulation preserved in polyethylene pot with desiccator (polymerized silica) and airtight closure (PSP pot) over 12 months at room temperature (26 ± 2 ºC). The 180-days period proved to be more adequate for preserving the viability of 81.50%, being this value within the range acceptable for bioproducts commercialization. Conidium viability must maintain an acceptable level above 80% during formulation and storage steps (Faria & Wraight, 2007; Gašić & Tanović, 2013; Butu et al., 2022).
27In this context, different conidial formulations and their correlation with temperature, moisture, water activity and packaging type have been evaluated to extend cell viability over biopesticide shelf life. Despite different available storage methods, each bioproduct has specific characteristics, representing a new challenge for its preservation (Oliveira et al., 2011; Oliveira et al., 2015). Nowadays, the agromarket offers a wide variety of entomopathogenic fungal-based bioproducts, most of which require low storage temperature (Copping, 2004; de la Cruz Quiroz et al., 2019). Different approaches have been applied to prolong conidium shelf life, such as oxygen scavengers, vacuum packaging and nutritional control. Furthermore, storage containers with O2/H2O scavengers or additive incorporation in formulations have been evaluated to improve fungal shelf life for commercial purposes (Oliveira et al., 2015; Mascarin & Jaronski, 2016; Garcia-Riaño et al., 2022). Regarding packaging, the desiccant combination effect of silica and glycerol as oxygen scavengers, which prolonged the Batkoa sp. and Furia sp. dry mycelium viability up to 90 days at 23 °C (Leite et al., 2002; Ayala-Zermeño et al., 2017). Another study evaluated a grainy formulation of B. bassiana conidia on rice storage in three different packages based on coex, metallic polyester and polyethylene. The formulation packed in coex-type packaging showed the highest viability rate over 180 days at 25 ± 2 ºC, exhibiting the lowest moisture (Silva & Neves, 2016). According to de la Cruz Quiroz et al. (2019), most water-dispersible granular formulation storage should be between 0 to 5 °C to maintain its viability beyond 6 months. Furthermore, a study evaluated an alternative modified atmosphere packaging (MAP) system to preserve M. anisopliae-inoculated millet grains, tested different packaging materials (e.g., polypropylene, polyethylene terephthalate, ethylene-vinyl alcohol), gas compositions, and storage temperatures (4-25 °C). The ethylene-vinyl alcohol film displayed satisfactory results by preserving the dry conidium viability at 80.5% with 7.4% moisture for 28 days at 4 °C due to the package modified atmosphere (30% CO2 + 70% N2) (Jeong et al., 2022). In this work, the selected conidium:cornstarch tablet type formulation (30:70%) remained viable at market-desired levels after 180-days period, without the need of refrigeration when preserved in polyethylene pot with desiccator (polymerized silica) and airtight closure (PSP pot). This result is relevant considering that most bioproducts available in the current agromarket require refrigeration to preserve the viability at accept levels. Therefore, the present study presents a simple and less expensive formulation method of a biopesticide production. Furthermore, the conidia tablet can remain viable during a shelf life without being conserved in a fridge or a freezer.
5. Conclusions
28In the present work, the cornstarch-based tablet formulation at 30:70% (conidia:excipient) exhibited satisfactory results concerning mechanical properties and water dispersion. Beauveria bassiana conidium-based tablets stored in PSP pot displayed the smallest value lowest variation in moisture and water activity and, consequently, significant viability, which extended the conidium formulation shelf life. This packaging type maintained 81.50% conidium viability after 180 day-storage at 26 ± 2 ºC, indicated as storage conditions. Conidial viability above 80% and product storage at room temperature are relevant results regarding the bioinsecticide potential effectiveness. Furthermore, this formulation manufacturing process is easy and low-cost compared to other procedures applied to obtain this class of bioproducts.
Acknowledgements
29This research was financially supported by Coordination for the Improvement of Higher Education Personnel (Capes); Foundation for Research and Technological Innovation of the State of Sergipe (FAPITEC/SE); Institute of Technology and Research (ITP) and Sergipe Technological Park (SergipeTec).
Bibliographie
Abotbina W. et al., 2020. Review of corn starch biopolymer. In: Proceedings of the 7th Postgraduate seminar on natural fibre reinforced polymer composites, November 2020, Institute of Tropical Forestry and Forest Product, University Putra Malaysia, Selangor, Malaysia, 37-40.
Agrawal S., Fernandes J., Shaikh F. & Patel V., 2022. Quality aspects in the development of pelletized dosage forms. Helyion, 8, e08956, doi.org/10.1016/j.heliyon.2022.e08956
Alves R.T., 2006. Produção, formulação e aplicação de fungos para o controle de pragas. In: Oliveira-Filho E.C. & Monnerat R.G. (Org.). Fundamentos para a regulação de semioquímicos, inimigos naturais e agentes microbiológicos de controle de pragas. 1st ed. Planaltina, DF, Brazil: Embrapa Cerrados, 239-253.
Armstrong N.A., 2013. Tablet manufacture by direct compression. In: Swarbrick J. (ed.) Encyclopedia of pharmaceutical technology. 4th ed. Boca Raton, FL, USA: CRC Press, 3673-3683, doi.org/10.1201/b19309-20
Arnosti A. et al., 2019. Interactions of adjuvants on adhesion and germination of Isaria fumosorosea on adults of Diaphorina citri. Sci. Agric., 76, 487-493, doi.org/10.1590/1678-992X-2017-0240
Ayala-Zermeño M.A. et al., 2017. Viability, purity, and genetic stability of entomopathogenic fungi species using different preservation methods. Fungal Biol., 121, 920-928, doi.org/10.1016/j.funbio.2017.07.007
Batista B.D. & Singh B.K., 2021. Realities and hopes in the application of microbial tools in agriculture. Microb. Biotechnol., 14, 1258-1268, doi.org/10.1111/1751-7915.13866
Batta Y. & Kavallieratos N.G., 2018. The use of entomopathogenic fungi for the control of stored-grain insects. Int. J. Pest Manage., 64, 77-87, doi.org/10.1080/09670874.2017.1329565
Behle R.W. & Jackson M.A., 2014. Effect of fermentation media on the production, efficacy, and storage stability of Metarhizium brunneum microsclerotia formulated as a prototype granule. J. Econ. Entomol., 107, 582-590, doi.org/10.1603/EC13426
Biryol S., Demirbağ Z., Erdoğan P. & Demir I., 2022. Development of Beauveria bassiana (Ascomycota: Hypocreales) as a mycoinsecticide to control green peach aphid, Myzus persicae (Homoptera: Aphididae) and investigation of its biocontrol potential. J. Asia-Pac. Entomol., 25, 101878, doi.org/10.1016/j.aspen.2022.101878
Brazilian Health Regulatory Agency, 2019. Brazilian Pharmacopoeia, vol. 1. 6th ed., v. 1. Brasilia: Brazilian Health Regulatory Agency.
Butu M., Rodino S. & Butu A., 2022. Biopesticide formulations - current challenges and future perspectives. In: Rakshit A. et al. (eds.). Biopesticides - Advances in bio-inoculant science. Cambridge: Woodhead Publishing, 19-29, doi.org/10.1016/b978-0-12-823355-9.00010-9
Carareto N.D.D., Monteiro Filho E.S., Pessôa Filho P.A. & Meirelles A.J.A., 2010. Water activity of aqueous solutions of ethylene oxide-propylene oxide block copolymers and maltodextrins. Braz. J. Chem. Eng., 27, 173-181, doi.org/10.1590/S0104-66322010000100015
Chamsai B. & Sriamornsak P., 2013. Novel disintegrating microcrystalline cellulose tablets with improved drug dissolution performance. Powder Technol., 233, 278-285, doi.org/10.1016/j.powtec.2012.08.019
Cho B.G. et al., 2022. Adsorption modeling of microcrystalline cellulose for pharmaceutical-based micropollutants. J. Hazard. Mater., 426, 128087, doi.org/10.1016/j.jhazmat.2021.128087
Copping L.G., 2004. The Manual of biocontrol agents. 3rd ed. Alton, UK: BCPC Publications, 702.
Curkovic T. et al., 2019. An agricultural detergent as co-adjuvant for entomopathogenic fungi and chlorpyrifos to control Pseudococcus viburni (Hemiptera: Pseudococcidae). Fla. Entomolog., 102, 101-106, doi.org/10.1653/024.102.0116
de la Cruz Quiroz R.C. et al., 2019. Fungi based biopesticides: shelf life preservation technologies used in commercial products. J. Pest Sci., 92, 1003-1015, doi.org/10.1007/s10340-019-01117-5
Derakshan A., Rabindra R.J., Ramanujam B. & Rahimi M., 2008. Evaluation of different media and methods of cultivation on the production and viability of entomopathogenic fungi, Verticillium lecanii (Zimm.) Viegas. Pak. J. Biol. Sci., 11, 1506-1509, doi.org/10.3923/pjbs.2008.1506.1509
Dukic-Ott A. et al., 2009. Production of tablets via extrusion-spheronisation without the incorporation of microcrystalline cellulose: a critical review. Eur. J. Pharm. Biopharm., 71, 38-46, doi.org/10.1016/j.ejpb.2008.08.005
Egbuna C. et al., 2020. Biopesticides, safety issues and market trends. In: Egbuna C. & Sawicka B. (eds.). Natural remedies for pest, disease and weed control. Cambridge, UK: Academic Press, 43-53, doi.org/10.1016/b978-0-12-819304-4.00004-x
Faria M.R. & Wraight S.P., 2007. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control, 43, 237-256, doi.org/10.1016/j.biocontrol.2007.08.001
Fiorotti J. et al., 2022. The role of complement in the tick cellular immune defense against the entomopathogenic fungus Metarhizium robertsii. Dev. Comp. Immunol., 126, 104234, doi.org/10.1016/j.dci.2021.104234
Garcia-Riaño J.L., Torres-Torres L.A., Santos-Díaz A.M. & Grijalba-Bernal E.P., 2022. In vitro compatibility with soybean agrochemicals and storage stability studies of the Beauveria bassiana biopesticide. Biocatal. Agric. Biotechnol., 39, 102275, doi.org/10.1016/j.bcab.2022.102275
Gašić A. & Tanović B., 2013. Biopesticide formulations, possibility of application and future trends. Pestic. Phytomed., 28, 97-102, doi.org/10.2298/PIF1302097G
Gerhardt A.H., 2010. Fundamentals of tablet compression. J. GXP Compliance, 14, 70-79.
Hammer Ø., Harper D.A.T. & Ryan P.D., 2001. Past: paleontological statistics software package for education and data analysis. Palaeontol. Electron., 4, 9.
Islam W. et al., 2021. Insect-fungal-interactions: a detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathogen., 159, 105122, doi.org/10.1016/j.micpath.2021.105122
Jampílek J. & Kráľová K., 2022. Biopesticides for management of arthropod pests and weeds. In: Rakshit A. et al. (eds.). Biopesticides. Cambridge, UK: Woodhead Publishing, 133-158, doi.org/10.1016/b978-0-12-823355-9.00009-2
Jaronski S.T., 2014. Mass production of entomopathogenic fungi: state of the art. In: Morales-Ramos J.A., Rojas M.G. & Shapiro-Ilan D.I. (eds.). Mass production of beneficial organisms. Cambridge, UK: Academic Press, 357-413, doi.org/10.1016/b978-0-12-391453-8.00011-x
Jeong S.G. et al., 2022. Effect of storage conditions on the shelf-life extension of fungus-colonized substrates based on Metarhizium anisopliae using modified atmosphere packaging. Sci. Rep., 12, 423, doi.org/10.1038/s41598-021-04232-5
Kidanu S. & Hagos L., 2020. Entomopathogenic fungi as a biological pest management option: a review. Int. J. Res. Stud. Agric. Sci., 6, 1-10, doi.org/10.20431/2454-6224.0606001
Kumar D., Singh J., Antil M. & Kumar V., 2016. Quality control of tablets: a review. Int. J. Universal Pharm. Bio Sci., 5, 53-67.
Kumari I. et al., 2022. Microbial biopesticides for sustainable agricultural practices. In: Rakshit A. et al. (eds.). Biopesticides. Cambridge, UK: Woodhead Publishing, 301-317, doi.org/10.1016/b978-0-12-823355-9.00024-9
Leite L.G. et al., 2002. Preservation of Batkoa sp. and Furia sp. (Entomophthorales) dry mycelium with combinations of desiccants and oxygen reducers. Arquivos Inst. Biol., 69, 117-122.
Lopes R.B. & Faria M., 2019. Influence of two formulation types and moisture levels on the storage stability and insecticidal activity of Beauveria bassiana. Biocontrol Sci. Technol., 29, 437-450, doi.org/10.1080/09583157.2019.1566436
Lopes R.B., Martins I., Souza D.A. & Faria M., 2013. Influence of some parameters on the germination assessment of mycopesticides. J. Invertebr. Pathol., 112, 236-242, doi.org/10.1016/j.jip.2012.12.010
Maina U.M., Galadima I.B., Gambo F.M. & Zakaria D., 2018. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud., 6, 27-32.
Marques E.J. & Alves S.B., 1996. Otimização de formulações na preservação de conídios de Beauveria bassiana (Bals.) Vuill. e Metarhizium anisopliae (Metschn.) Sorok. em diferentes condições de armazenamento. Arquivos Inst. Biol., 39, 861-877.
Mascarin G.M. & Jaronski S.T., 2016. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol., 32(11), 177, doi.org/10.1007/s11274-016-2131-3
Meshram S., Bisht S. & Gogoi R., 2022. Current development, application and constraints of biopesticides in plant disease management. In: Rakshit A. et al. (eds.). Biopesticides - Advances in Bio-inoculant Science. Cambridge, UK: Woodhead Publishing, 207-224, doi.org/10.1016/b978-0-12-823355-9.00004-3
Mitra B., Chang J., Hilden J. & Wu S.J., 2022. Deformation potential and tensile strength of tablets of a dry granulated formulation. J. Pharm. Sci., 111, 710-716, doi.org/10.1016/j.xphs.2021.09.033
Moore D., Douro-Kpindou O.K., Jenkins N.E. & Lomer C.J., 1996. Effects of moisture content and temperature on storage of Metarhizium flavoviride conidia. Biocontrol Sci. Technol., 6, 51-61, doi.org/10.1080/09583159650039520
Mora M.A., Castilho A.M.C. & Fraga M.E., 2017. Classification and infection mechanism of entomopathogenic fungi. Arquivos Inst. Biol., 84, e0552015, doi.org/10.1590/1808-1657000552015
Moustafa M.A. et al., 2023. Monitoring resistance and biochemical studies of three Egyptian field strains of Spodoptera littoralis (Lepidoptera: Noctuidae) to six insecticides. Toxics, 11(3), 211, doi.org/10.3390/toxics11030211
Oliveira I., Pereira J.A. & Bento A., 2011. Viability of Beauveria bassiana isolates after storage under several preservation methods. Ann. Microbiol., 61, 339-344, doi.org/10.1007/s13213-010-0147-8
Oliveira D.G.P., Pauli G., Mascarin G.M. & Delalibera I., 2015. A protocol for determination of conidial viability of the fungal entomopathogens Beauveria bassiana and Metarhizium anisopliae from commercial products. J. Microbiol. Methods, 119, 44-52, doi.org/10.1016/j.mimet.2015.09.021
OriginLab Corporation, 2012. OriginPro 9, Version 2012. Northampton, MA, USA: OriginLab Corporation.
Qayyum M.A. et al., 2021. Factors affecting the epizootics of entomopathogenic fungi - A review. J. Bioresour. Manage., 8, 78-85, doi.org/10.35691/JBM.1202.0204
Rajula J., Rahman A. & Krutmuang P., 2020. Entomopathogenic fungi in Southeast Asia and Africa and their possible adoption in biological control. Biol. Control, 151, 104399, doi.org/10.1016/j.biocontrol.2020.104399
Ramanujam B. et al., 2014. Management of insect pests by microorganisms. Proc. Indian Natl. Sci. Acad., 80, 455-471, doi.org/10.16943/ptinsa/2014/v80i2/3
Redman-Furey N., Normand M.D. & Peleg M., 2013. Estimating the needed amount of desiccant, water or moistener to adjust the equilibrium water activity of dry powder mixtures. Trends Food Sci. Technol., 29(2), 135-141, doi.org/10.1016/j.tifs.2012.08.003
Sandhu S.S., Rajak R.C. & Agarwal G.P., 2008. Studies on prolonged storage of Beauveria bassiana conidia: effects of temperature and relative humidity on conidial viability and virulence against chickpea borer, Helicoverpa armigera. Biocontrol Sci. Technol., 3, 47-53, doi.org/10.1080/09583159309355258
Silva R.Z. & Neves P.M.O.J., 2016. Stability of Beauveria bassiana (Bals.) Vuill. conidia stored in different packagings. Arquivos Inst. Biol., 83, e0362014, doi.org/10.1590/1808-1657000362014
Tapia M.S., Alzamora S.M. & Chirife J., 2020. Effects of water activity (aw) on microbial stability as a hurdle in food preservation. In: Barbosa-Cánovas G.V., Fontana Jr. A.J., Schmidt S.J. & Labuza T.P. (eds.). Water activity in foods: fundamentals and applications. Chicago, IL, USA: John Wiley & Sons, 239-271, doi.org/10.1002/9780470376454.ch10






