- Accueil
- Volume 28 (2024)
- Numéro 1
- Quality of Vanilla spp. from Madagascar: evolution of the compound fingerprints from the green beans to the vanilla pods’ preparation
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Quality of Vanilla spp. from Madagascar: evolution of the compound fingerprints from the green beans to the vanilla pods’ preparation

Document(s) associé(s)
Version PDF originaleRésumé
Qualité des Vanilla spp. de Madagascar : évolution des empreintes chimiques de la gousse verte jusqu’à la gousse préparée
Description du sujet. La vanille est une épice dont le nom vient du mot espagnol vanilla qui signifie petites gousses noires. Elle provient d'une variété d'orchidée, plus précisément de la plante de vanille. Bien que la vanille soit la deuxième épice la plus chère, après le safran, elle reste la plus utilisée dans les industries alimentaires, cosmétiques, des boissons et du tabac. La qualité des gousses de vanille dépend de leur préparation.
Objectifs. Ce travail vise à déterminer quantitativement le taux de cinq composés aromatiques marqueurs de qualité chez cinq espèces de vanille cultivées à Madagascar et à comprendre l'évolution de ces composés au cours de la maturité et de la préparation des gousses.
Méthode. Une analyse par chromatographie liquide à haute performance (HPLC) a été entreprise sur cinq espèces du genre Vanilla, dont Vanilla planifolia Andrews, V. pompona Schiede, V. tsy taitry, V. planifolia var. sterile and V. planifolia var. tsy vaky, pour évaluer la concentration des composés marqueurs, dont la vanilline (VAN), l’acide p-hydroxybenzoïque (p-HBAc), l’acide vanillique (VAc), le guaiacol (GUA) et le p-hydroxybenzaldehyde (p-HB), dans les gousses sélectionnées au cours de leur étape de collecte et de préparation.
Résultats. La VAN (2,63-45,30 µg·ml-1 après 6 mois ; 8,72-122,66 µg·ml-1 après 9 mois ; 26,81-60,69 µg·ml-1 après séchage et 13,35-105,03 µg·ml-1 après conditionnement) était le composant majeur suivi du p-HB (7,59 µg·ml-1 pour la V. pompona de 6 mois ; 5,52-24,47 µg·ml-1 après 9 mois ; 5,40-14,72 µg·ml-1 après séchage et 4,69-24,84 µg·ml-1 après conditionnement) dans chaque espèce de vanille. Généralement, la concentration en vanilline a augmenté du 6è au 9è mois puis a diminué après le processus de séchage et a augmenté à nouveau après le processus de conditionnement. Le gaïacol n'a pas été détecté chez toutes les espèces lors des étapes de collecte et de préparation.
Conclusions. Vanilla planifolia ou V. fragrance reste la vanille avec la plus forte concentration de composés marqueurs importants suggérant qu'elle pourrait avoir la meilleure qualité parmi celles sélectionnées, faisant de cette espèce, celle la plus commercialisée sur le marché international.
Abstract
Description of the subject. Vanilla is a spice whose name comes from the spanish word “vanilla”, meaning little black pods. It comes from a variety of orchids, more specifically the vanilla plant. Although vanilla is the second most expensive spice, next to saffron, it is still the most used in the food, cosmetics, beverage, and tobacco industries. The quality of vanilla pods depends on their preparation.
Objectives. This work aims to quantitatively determine the rate of five aromatic quality marker compounds in five selected vanilla species cultivated in Madagascar and to understand the evolution of these compounds during the maturity and preparation of the pods.
Method. High-Performance Liquid Chromatography (HPLC) analysis was undertaken on five Vanilla species, including Vanilla planifolia Andrews, V. pompona Schiede, V. tsy taitry, V. planifolia var. sterile and V. planifolia var. tsy vaky, to assess the concentration of the marker compounds, including vanillin (VAN), p-hydroxybenzoic acid (p-HBAc), vanillic acid (VAc), guaiacol (GUA), and p-hydroxybenzaldehyde (p-HB), in the selected pods during their stage of collection and preparation.
Results. VAN (2.63-45.30 µg·ml-1 at 6 months old; 8.72-122.66 µg·ml-1 at 9 months old; 26.81-60.69 µg·ml-1 after the drying process and 13.35-105.03 µg·ml-1 after the conditioning process) was the major component followed by the p-HB (7.59 µg·ml-1 for V. pompona at 6 months old; 5.52-24.47 µg·ml-1 at 9 months old; 5.40-14.72 µg·ml-1 after the drying process and 4.69-24.84 µg·ml-1 after the conditioning process) in each vanilla species. Generally, VAN concentration increased from the 6th to the 9th month then decreased after the drying process and increased again after the conditioning process. Guaiacol was not detected in all species during the stages of collection and preparation.
Conclusions. Vanilla planifolia or V. fragrance remains the vanilla with the highest concentration of the important marker compounds suggesting that it could have the best quality among those selected in this study making this species still the most traded in the international market.
Table des matières
Received 9 August 2023, accepted 27 February 2024, available online 5 March 2024.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1Vanilla is a spice whose name comes from the Spanish word “vanilla”, meaning little black pods. It comes from a variety of orchids, more specifically the vanilla plant (Ahmad et al., 2020). Vanilla spp. is a climbing orchid belonging to the orchidaceae family, one of the largest families of flowering plants (Cameron & Molina, 2006). The genus Vanilla includes about 110 vanilla species whereas three species including Vanilla planifolia, V. tahitensis and V. pompona are known to be important for commercial cultivation and for local low-scale vanilla farmers (Odoux, 2010). Vanilla planifolia is the most commercialized vanilla pod, this species is originated from Mexico and introduced in the Indian Ocean (La Reunion, Madagascar, Comoros) in the 19th century (Ranadive, 1994). Vanilla tahithensis is also known as the vanilla of Tahiti and is mostly cultivated in the Pacific Ocean islands, and V. pompona is cultivated in Central America and the Antilles islands (Duval et al., 2006). The fruit of vanilla is a seed capsule, but it is referred to as vanilla bean or vanilla pod. Fresh vanilla pods reach their maturity 8-9 months after pollination and could reach about 15 cm in length. They are odourless and contain flavor components stored as glucosides (Odoux & Brillouet, 2009). They are freshly harvested and processed directly by curing to stop the natural vegetative process inducing the enzymes responsible for the formation of aromatic flavor constituents and preventing microbial growth, thus enabling the long-term preservation of vanilla pods (Odoux, 2010). Curing methods differ from the country of pods origin therefore the curing process has a major influence on the variety, quality and aromatic profile of the pods that are traded (Rao & Ravishankar, 2000).
2Although vanilla is the second most expensive spice, next to saffron, it is still the most used (Ranadive, 2005). Vanilla is a versatile and accepted flavoring thus it is widely used in the food, cosmetics, beverage, and tobacco industries (Korthou & Verpoorte, 2007). Madagascar is the largest vanilla pod producer in the world and possesses the best vanilla pods in terms of quality in the current market with the status “Bourbon” (Grisoni & Nany, 2021). In the market, commercialized pods were firstly categorized by their physical parameters including color (red to black), shape (split or non-split), and length but the fundamental quality criteria were the analytical parameters including moisture and marker compound contents with their ratios (République française, 1988; DGCCRF, 2003; Gassenmeier et al., 2008). The vanilla extract isolated from the vanilla pods includes more than 200 aromatic compounds (De La Cruz Medina et al., 2009). Vanillin (VAN) is the major compound, and the rates of its derivatives depend on the origin of the vanilla pods. Apart from VAN, p-hydroxybenzaldehyde (p-HB), p-hydroxybenzoic acid (p-HBAc) and vanillic acid (Vac) are also three important and major key-flavor compounds in vanilla beans (Ranadive, 1992). According to the Tahitian preparation, which is specifically compared to the bourbon pods preparation, an abnormal fermentation due to a lack of aeration leads to the formation of guaiacol (Brunschwig, 2009). Besides, alteration of VAN during the pod’s preparation could lead to the formation of GUA (Bensaid et al., 2002). At the fruit maturity period, the quality marker compounds are present in their glycosylated forms (Odoux & Brillouet, 2009). During preparation, the markers in glycosylated forms are hydrolyzed under the action of different enzymes including β-glucosidase and peroxidase to give sugars and aglycone forms (Gu et al., 2017). The heat treatment triggers the action of β-glucosidase and the reaction evolves slowly during the drying period while the refining takes place in the wooden box to prevent the marker compounds from evaporating while the pods continue to dry. This work aims to quantitatively determine the rate of these five aromatic quality marker compounds in five selected vanilla species and to understand the evolution of these compounds during the maturity and the preparation of the pods.
2. Methods
2.1. Plant materials and vanilla pods preparation
3The following species of Vanilla were collected at Ambalabe - Antalaha, in the North-East of Madagascar (15°09’44” S; 50°25’06” E; 368 m above the sea level) during the months of April and July 2020 respectively for the 6th and 9th month old pods. The dried and conditioned pods were provided by the Ramanadraibe Export Society during their standardized curing process. All the studied pods here were collected from the same accession including:
4– Vanilla planifolia Andrews or V. fragrance also labelled as Bourbon vanilla, the most planted vanilla species by Malagasy farmers;
5– Vanilla pompona Schiede is native of Central America, northern South America, and the Lesser Antilles. This species is the most used in genetic hybrid with other species due to its resistance to fusaria (Grisoni & Nany, 2021);
6– Vanilla tsy taitry, a species obtained from the back cross of the first hybrid generation (V. planifolia X V. pompona) with V. planifolia (Grisoni & Nany, 2021). The name tsy taitry means not susceptible or resistant to diseases more precisely to Fusarium wilt;
7– Vanilla planifolia var. sterile: this variety is characterized by the fruits of self-pollinated flowers dropping prematurely, its flowers must be pollinated with other vanilla pollen to give normal pods (Grisoni & Nany, 2021);
8– Vanilla planifolia var. tsy vaky: this variety has no dehiscence fiber and does not split. The morphology of the fruits is different from the other varieties.
9There are four stages in the preparation of vanilla pods, viz including killing, sweating, drying, and conditioning (Krushnamurthy et al., 2013). Killing and sweating: mature vanilla pods with 9 months old were warehoused in an aerated place away from sunlight for two days after the collection. The pods were dipped in hot water at 80 °C for 15 min then the pods were packed in vacuum-packed bags. Drying: the pods were dried on a drying grid under the sunlight for 7 h during the day then the hot packaged pods were stored in a covered wooden box during the night. This process was repeated daily for 10 days where the pods reach the required suppleness and fineness with a moisture content below 20%. Conditioning: The pods were stored in wooden box for 6 months. The preparation duration depends on the size of the vanilla pods but not on their variety. Therefore, we selected pods of the similar size for all varieties to eliminate this variable.
10Two hundred grams of pods were finely cut and dried in an oven at 103 °C for 12 h before HPLC analysis.
2.2. Chemicals
11Vanillin (99%), p-hydroxybenzoic acid (98%), vanillic acid (98.2%), guaiacol (99%), and p-hydroxybenzaldehyde (≥ 95%) along with ethanol (96 °C), methanol and formic acid (HPLC grades) were purchased from Sigma-Aldrich (Germany). Distilled water was prepared at the IMRA (Institut Malgache de Recherches Appliquées).
2.3. Moisture content determination
12Approximately, 20 g of finely chopped pods were placed in an oven at 103 °C for 12 h to a complete evaporation of water residues in the raw material as described by Rasoarahona (2017). Moisture content was determined on both fresh pods (6- and 9-months old pods) and Ramanandraibe Export Society pods (dried and cured pods). Three replicates were performed for each sample.
2.4. HPLC analysis of fingerprint compounds
13The dried cut pods were placed in a Soxhlet cartridge and extracted discontinuously with 500 ml of ethanol (96 °C) for 16 h. The ethanolic solution was evaporated to dryness under reduced pressure using a rotary evaporator. The extract obtained was weighed and stored at 4 °C before the preparation of the vanilla sample to be analyzed. Three repetitions of the extraction were carried out for each vanilla sample. Four milligrams of each sample were dissolved in 2 ml of methanol. Each solution was filtered through a 0.2 µm filter (GHP Pall) prior to HPLC injection (10 µl). The compounds were separated using a Surf C-18 Extreme (100 Å, 5 µm, 250 × 4.6 mm) column (ImChem, France) by using HPLC 2695 (Waters, USA) equipped with 2998 Photo Diode Array detector (PDA) from Waters (USA) monitored with an Empower 3 software. A gradient phase consisting of methanol (+ 0.1% HCOOH; pH 3.2 - 3.4) (A) / H2O (+ 0.1% HCOOH) (B) (from 100% B to 40% B) was used as eluent for 20 min with a constant flow rate of 1.2 ml·min-1 at 40 °C as constant column temperature. The acquisition was done at 230, 254, 275 and 280 nm wavelengths. The standard curves were obtained using vanillin (acquisition wavelength 230 nm), p-hydroxybenzoic acid and vanillic acid (254 nm respectively), guaiacol (275 nm), and p-hydroxybenzaldehyde (280 nm) solutions as external standards at different concentrations (Table 1) prepared in methanol. The limit of detection (LOD) and the limit of quantification (LOQ) were calculated from ordinary least-squares regression data. LOD corresponds to the analyte amount for which the area is equal to 3 times the chosen standard deviation, and LOQ corresponds to the analyte amount for which the area is equal to 10 times the chosen standard deviation (Vial & Jardy, 1999). The identification of each biomarker is based on their retention time (Table 1; Figure 1) and UV spectra compared to standard solutions.
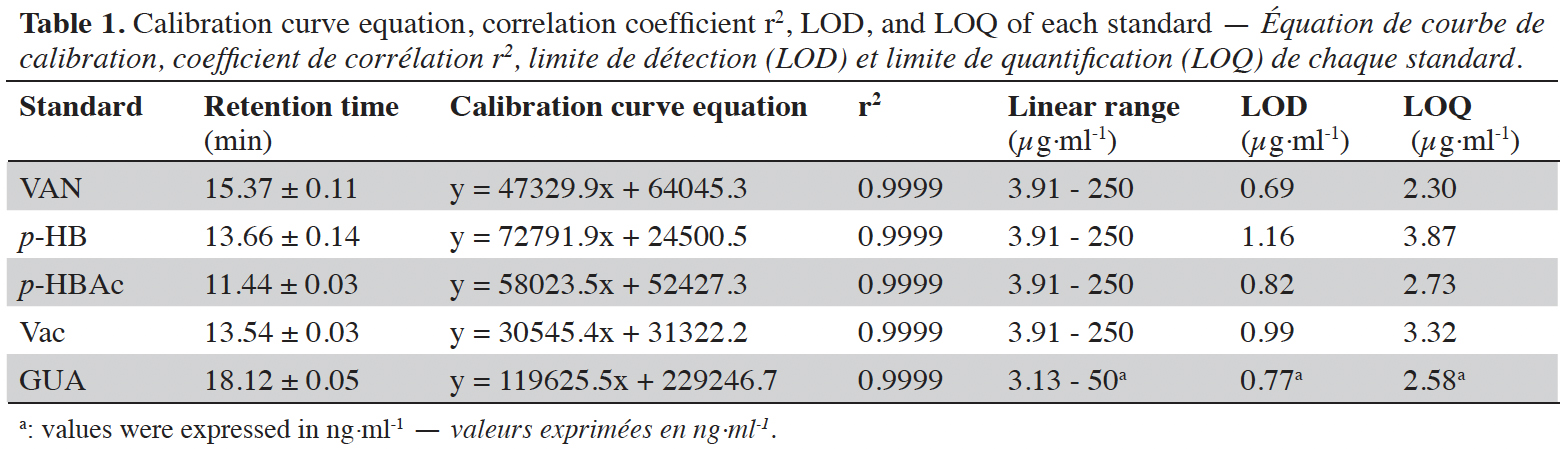
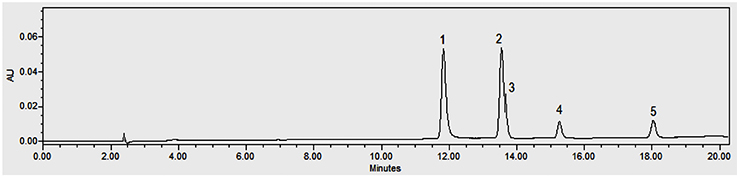
Figure 1. Chromatogram of standards at 254 nm: p-hydroxybenzoic acid (1), vanillic acid (2), p-hydroxybenzaldehyde (3), vanillin (4) and guaiacol (5) — Chromatogramme des standards à 254 nm : acide p-hydroxybenzoïque (1), acide vanillique (2), p-hydroxybenzaldéhyde (3), vanilline (4) et guaiacol (5).
2.5. Statistical analysis
14All the analysis were carried out in triplicate and the results were expressed as mean ± standard deviation (SD). Data following normal distribution were statistically analyzed using Student’s t-test and one-way analysis of variance (ANOVA) followed by Tukey’s HSD (honestly significant difference) multiple range test. All the differences showing a p < 0.05 were accepted as statistically significant (SPSS 20).
3. Results
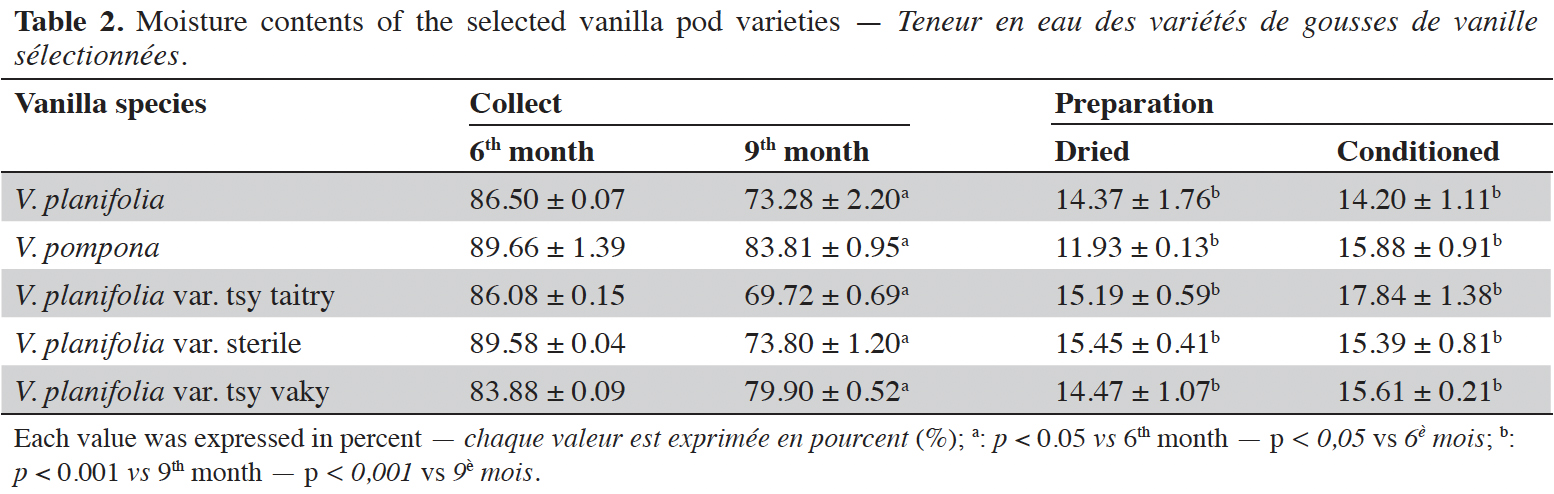
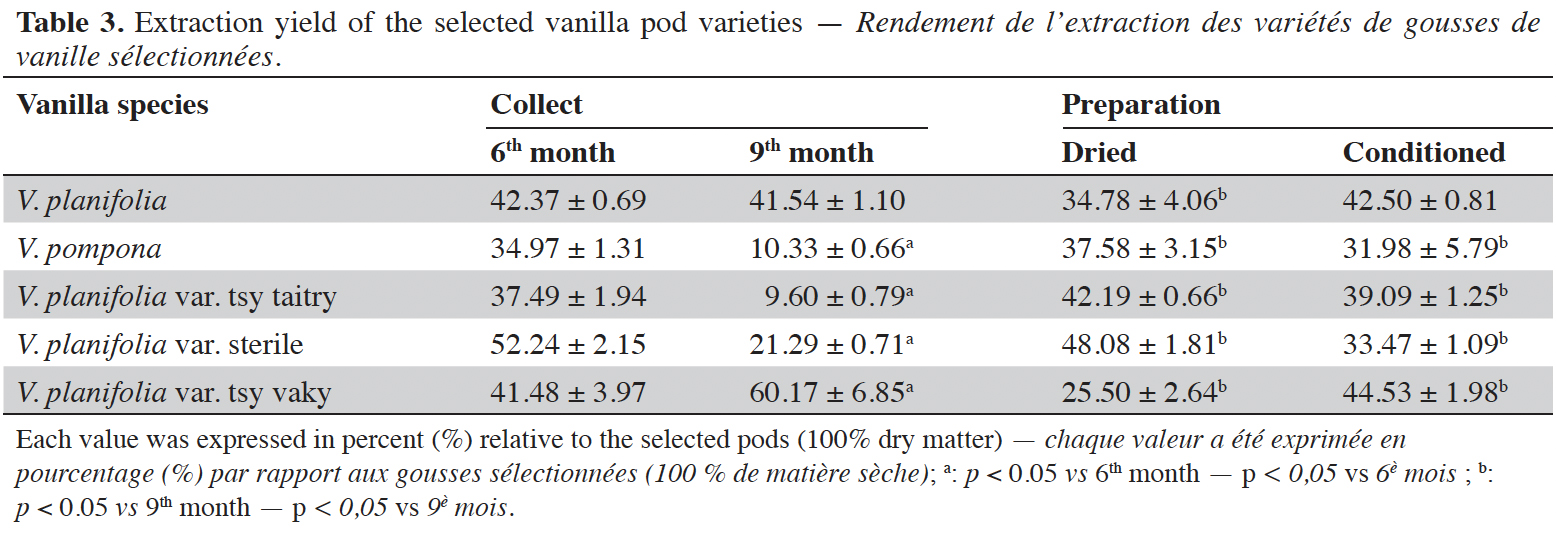
15The moisture content of each vanilla pod variety at the different stages of collection and preparation was reported in table 2. The moisture content decreased slightly from the 6th month to the 9th month (p < 0.05) for each variety and decreased significantly after the drying process (p < 0.001). The yield of the extraction relative to 100% dried pods by the Soxhlet method of each vanilla pod at the different stages of collection and preparation was reported in table 3. The yield decreased significantly for the 9th-month-old pods for the V. pompona and the V. planifolia var. tsy taitry (p < 0.05). HPLC analysis was led to determine the relative quantity of five aromatic compounds in each vanilla species (Figure 1). VAN was, relatively, the major component followed by the p-HB.
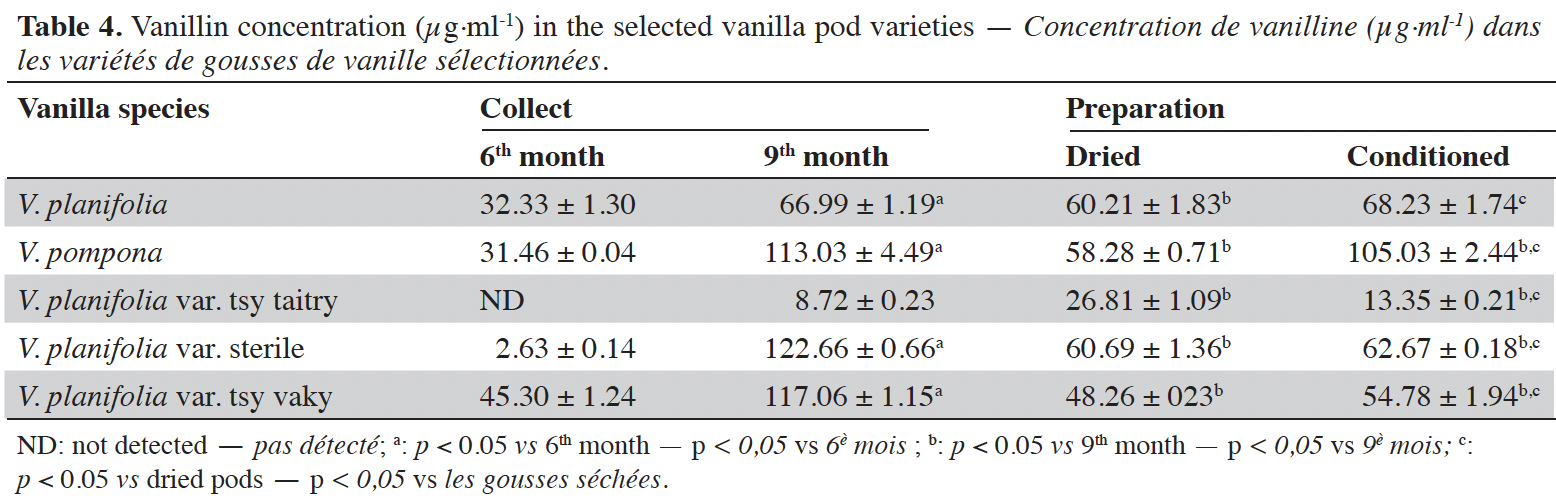
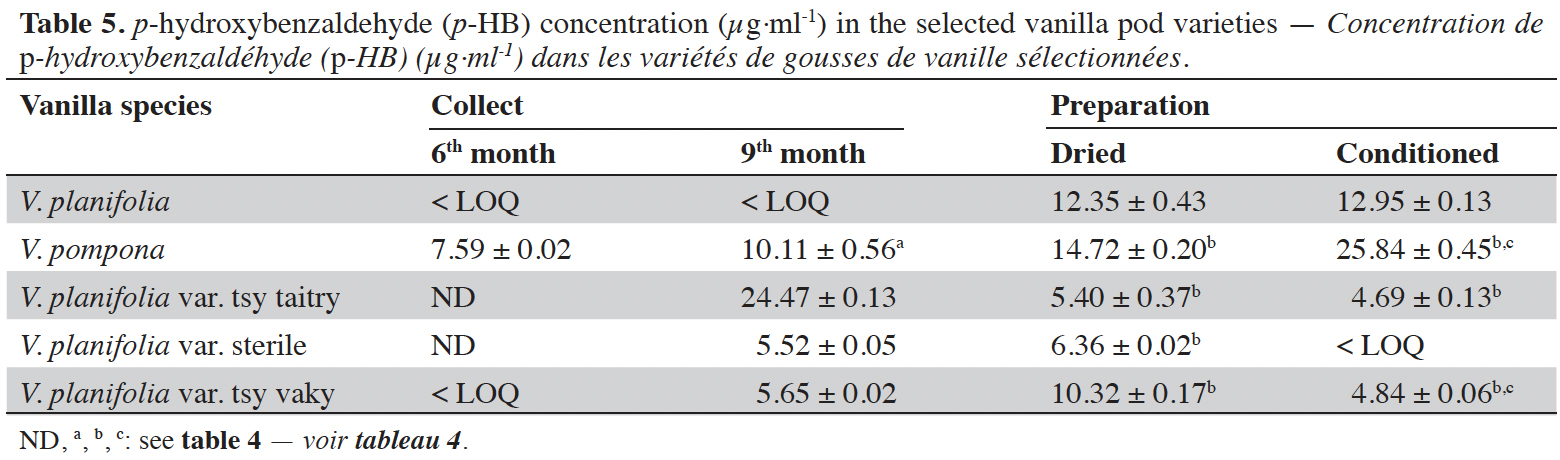
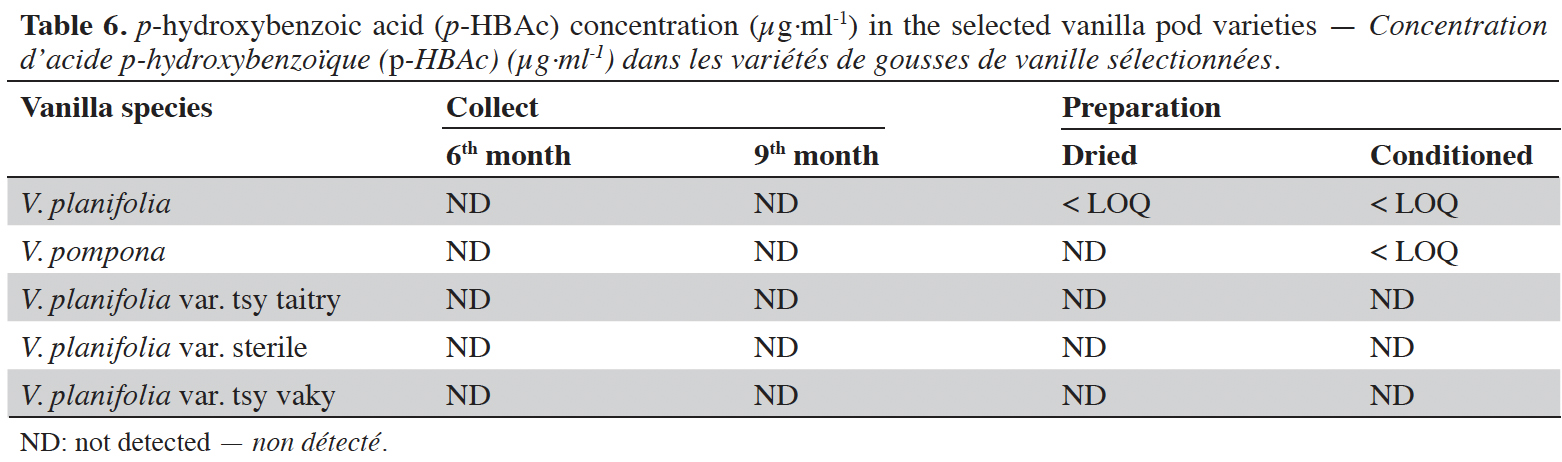
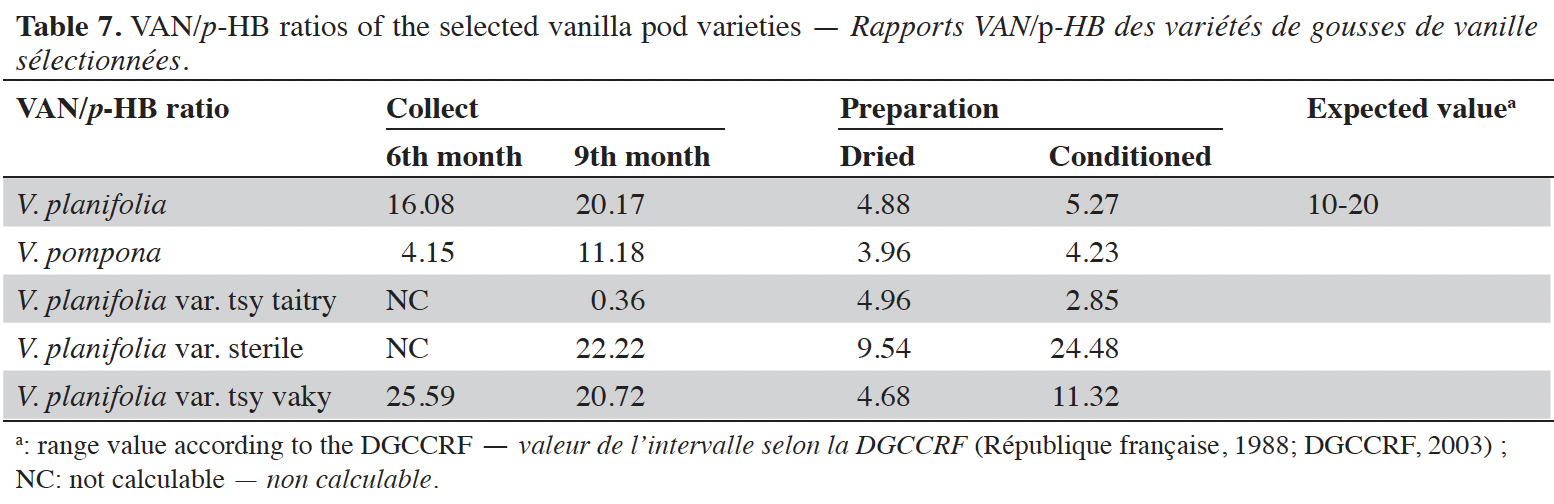
16Vanillin concentrations of each selected vanilla pod at the different stages of collection and preparation were reported in table 4. Generally, VAN concentration increased from the 6th to the 9th month then decreased after the drying process and increased again after the conditioning process. The variety tsy vaky presented the maximum concentration for the 6-months-old pods and the variety sterile showed the maximal concentration for the 9-months-old pods which did not show any significant differences with vanillin concentration in the variety tsy vaky and the species V. pompona. The concentrations of VAN for the dried vanilla pods were similar (p > 0.05) except for the variety tsy taitry with the other species and variety (p < 0.001). After the conditioning process, vanillin concentration increased slightly except for the varieties tsy taitry and sterile. The evolution of vanillin concentration in each vanilla pod appeared similar except for the variety tsy taitry. p-HB concentrations of each selected vanilla pod at the different stages of collection and preparation were reported in table 5. For each selected variety, the content of p-HB increased significantly from the 6th to the 9th months (p < 0.05). The variety tsy taitry showed an exceptional concentration in the 9th month that decreased significantly during the preparation (p < 0.05). However, for the other selected varieties, the evolution of p-HB content in the extracts was similar. p-HBAc concentrations of each selected vanilla pod at the different stages of collection and preparation were reported in table 6. For most of the selected pod varieties, the p-HBAc was not detectable except for V. planifolia and V. pompona after the conditioning process which were detectable but not quantifiable. Vanillic acid (Vac) concentration was only detected in the variety tsy taitry for the 9-months-old pods but was not quantifiable. Guaiacol was not detected in all selected pods at all different periods of collection and preparation. VAN/p-HB ratios of each selected pod variety were reported in table 7. The calculated ratio values ranged from 4.20 to 28.00.
4. Discussion
17This work was conducted to determine the quality of the selected vanilla pods. Fruit maturity is reached after 8-9 months of pollination for all varieties of vanilla. Therefore, the 6-months and 9-months-old pods were selected for each studied variety to be able to know the traceability and the evolution of the aromatic constituents before and at the maturity of the vanilla pods. Since the pollination is carried out by the growers, the collections were done at the 6th and 9th months later. In this work, the moisture content of each selected vanilla pod was under the required in the market (≤ 25-30 %) after the conditioning process (Ranadive, 2018). The lower the moisture content the better the vanilla pods’ quality. Therefore, V. planifolia has the best quality regarding the moisture content parameter (Table 2). Besides, V. planifolia showed a fairly high yield after the conditioning process which is beyond the proposed value (≥ 35%) by the US Food and Drug Analysis regulation (Ranadive, 2018). On the other hand, during the HPLC analysis, the guaiacol which is a fermentation index reflecting bad preparation behavior (Brunschwig, 2009) was not detected at all stages of sampling of vanilla pods showing a good sign related to the quality of all the selected vanilla varieties. Takahashi et al. (2013) stated that guaiacol is considered among the negative phenolic components of vanilla pods therefore the ratio between vanillin and guaiacol could be considered as an index of vanilla quality in the market. Generally, the concentration of each vanilla marker component increased significantly (p < 0.05, respectively, Tables 4-6) from the 6th and 9th month to the preparation, it could be explained by the presence of vanillin and its derivatives in their glycosylated form in the green pods and transformed to glucose with their aromatic aglycone forms after undergoing the β-glucosidase hydrolysis during the preparation process (Silva et al., 2011). The slight decrease in the vanillin content from the drying to the conditioning of the varieties tsy taitry and sterile (Table 4) showed that the conditioning process may be not appropriate for them. However, for the three other selected vanilla pods, the conditioning process was appropriate to reach the good quality of the vanilla pods. Similar remarks have been reported by Odoux (2000) during his research with V. fragrans. Several studies (Funk & Brodelius, 1992; Walton et al., 2003) show that the biosyntheses of vanillic acid and vanillin are similar (from phenylalanine) and differ at the level of the last pathway (from ferulic acid). Vanillic acid is an oxidized form of vanillin and can be obtained from vanillin by oxidation using UV light and heat when the vanillin is in solution (Englis & Manchester, 1949; Mourtzinos et al., 2009). Therefore, the absence of vanillic acid in the pods could be explained, firstly, by the absence of certain enzymes leading to the production of vanillic acid, and secondly, by the resistance from the bark of vanilla beans to the UV light and heat produced by sunlight during the drying process. The VAN/p-HB ratio is a quality indicator of the authenticity of the vanilla pods (Ranadive, 1992). The “Direction Générale de la Concurrence, de la Consommation et de la Répression des Fraudes” (DGCCRF) proposed a range value between 10 and 20 for the qualification of the marketed vanilla pods (République française, 1988; DGCCRF, 2003). The dried V. pompona and V. fragrans ratios which were the most imported vanilla from Madagascar in the international markets fell into the line of DGCCRF showing the high recommendation of the vanilla pods from this country. Those obtained with the varieties tsy vaky and sterile slightly exceed this DGCCRF range. The excess indicates that vanillin content of these varieties could be higher than the other studied varieties or their p-HB content was lower. In any case, the results of this study confirmed the quality of the most imported vanilla pods from Madagascar qualified as vanilla Bourbon which is the V. fragrans and the curing process is well-adapted to the preparation of this species.
5. Conclusions
18This work reports quantitatively the moisture contents, the extraction yields, and the concentration of the main aromatic compounds in five selected varieties of vanilla pods cultivated and produced in Madagascar. The moisture contents and the extraction yields were similar for the selected vanilla varieties. However, VAN, p-HB and p-HBAc concentration varies according to the period of collection and the stages of preparation of vanilla pods. The results showed that V. planifolia or V. fragrance (Bourbon vanilla) remains the most important species and the most qualified in terms of quality among the selected varieties and that the curing process is well-adapted to the preparation of this species. Therefore, it remains the most trade in the international market.
Bibliographie
Ahmad H. et al., 2020. Vanilla. In: Hanif M.A., Nawaz H., Khan M.M. & Byrne H.J., eds. Medicinal plants of South Asia. Amsterdam, The Netherlands: Elsevier, 657-669, doi.org/10.1016/B978-0-08-102659-5.00048-3
Bensaid F.F., Wietzerbin K. & Martin G.J., 2002. Authentication of natural vanilla flavorings: isotopic characterization using degradation of vanillin into guaiacol. J. Agric. Food Chem., 50(22), 6271-6275, doi.org/10.1021/jf020316l
Brunschwig C., 2009. Contribution à la caractérisation phytochimique et sensorielle de la vanille de Tahiti (Vanilla tahitensis). Thèse de doctorat : Université de la Polynésie française, Papeete (Polynésie française).
Cameron K.M. & Molina M.C., 2006. Photosystem II gene sequences of psbB and psbC clarify the phylogenetic position of Vanilla (Vanilloideae, Orchidaceae). Cladistics, 22(3), 239-248, doi.org/10.1111/j.1096-0031.2006.00102.x
De La Cruz Medina J., Jiménes G.C.R. & García H.S., 2009. Vanilla: post-harvest operations. Roma: FAO, http://www.fao.org/fileadmin/user_upload/inpho/docs/Post_Harvest_Compendium_-_Vanilla.pdf, (26/02/2024).
Direction Générale de la Concurrence, de la Consommation et de la Répression des Fraudes (DGCCRF), 2003. Note d’information N° 2003-61, 16th June 2003 regarding vanilla products. Paris : DGCCRF.
Duval M.F. et al., 2006. Diversité génétique des vanilliers dans leurs zones de dispersion secondaire. In: Bureau des Ressources Génétiques, ed. Actes du 6è Colloque national, Les ressources génétiques : des ressources partagées, 2-4 octobre 2006, La Rochelle, France. Paris : BRG, 181-196
Englis D.T. & Manchester M., 1949. Oxidation of vanillin to vanillic acid. Anal. Chem., 21(5), 591-593, doi.org/10.1021/ac60029a018
Funk C. & Brodelius P.E., 1992. Phenylpropanoid metabolism in suspension cultures of Vanilla planifolia Andr. IV. Induction of vanillic acid formation. Plant Physiol., 99(1), 256-262, doi.org/10.1104/pp.99.1.256
Gassenmeier K., Riesen B. & Magyar B., 2008. Commercial quality and analytical parameters of cured vanilla beans (Vanilla planifolia) from different origins from the 2006–2007 crop. Flavour Fragrance J., 23(3), 194-201, doi.org/10.1002/ffj.1874
Grisoni M. & Nany F., 2021. The beautiful hills: half a century of vanilla (Vanilla planifolia Jacks. ex Andrews) breeding in Madagascar. Genet. Resour. Crop Evol., 68(5), 1691-1708, doi.org/10.1007/s10722-021-01119-2
Gu F. et al., 2017. Comparative metabolomics in vanilla pod and vanilla bean revealing the biosynthesis of vanillin during the curing process of vanilla. AMB Express, 7(1), 1-9, doi.org/10.1186/s13568-017-0413-2
Korthou H. & Verpoorte R., 2007. Vanilla. In: Berger R.G., ed. Flavours and fragrances: chemistry, bioprocessing and sustainability. Berlin, Germany: Springer, 203-217.
Krushnamurthy A., Bellur N.S. & Madeneni M.N., 2013. Vanilla - Its science of cultivation, curing, chemistry, and nutraceutical properties. Crit. Rev. Food Sci. Nutr., 53(12), 1250-1276, doi.org/10.1080/10408398.2011.563879
Mourtzinos I., Konteles S., Kalogeropoulos N. & Karathanos V.T., 2009. Thermal oxidation of vanillin affects its antioxidant and antimicrobial properties. Food Chem., 114(3), 791-797, doi.org/10.1016/j.foodchem.2008.10.014
Odoux E., 2000. Changes in vanillin and glucovanillin concentrations during the various stages of the process traditionnally used for curing Vanilla fragrans beans in Réunion. Fruits, 55(2), 119-125.
Odoux E. & Brillouet J.M., 2009. Anatomy, histochemistry and biochemistry of glucovanillin, oleoresin and mucilage accumulation sites in green mature vanilla pod (Vanilla planifolia; Orchidaceae): a comprehensive and critical reexamination. Fruits, 64(4), 221-241, doi.org/10.1051/fruits/2009017
Odoux E., 2010. Vanilla. In: Odoux E. & Grisoni M., eds. Medicinal and aromatic plants – Industrial profiles. London: CRC Press, 1-387.
Ranadive A.S., 1992. Vanillin and related flavor compounds in vanilla extracts made from beans of various global origins. J. Agric. Food Chem., 40(10), 1922-1924, doi.org/10.1021/jf00022a039
Ranadive A.S., 1994. Vanilla: cultivation, curing, chemistry, technology and commercial products. In: Charalambous G., ed. Spices, herbs and edible fungi. Amsterdam, The Netherlands: Elsevier, 517-577.
Ranadive A.S., 2005. Vanilla cultivation. In: Proceedings of the first international congress, Vanilla, 11-13 November 2003, Princeton, NJ, USA. Carol Stream, IL, USA: Allured Publishing Corporation, 25-32.
Ranadive A.S., 2018. Quality control of vanilla beans and extracts. In: Havkin-Frenkel D. & Belanger F.C., eds. Handbook of vanilla science and technology. 2nd ed. John Wiley & Sons Ltd, 237-260, doi.org/10.1002/9781119377320.ch15
Rao S.R. & Ravishankar G.A., 2000. Vanilla flavour: production by conventional and biotechnological routes. J. Sci. Food Agric., 80(3), 289-304, doi.org/10.1002/1097-0010(200002)80:3<289::AID-JSFA543>3.0.CO;2-2
Rasoarahona F.H., 2017. Variabilité de la composition de l’arôme vanille (Vanilla planifolia) : cas des vanilles de Madagascar et de la Réunion. Thèse de doctorat : Université de Antananarivo (Madagascar).
République française, 1988. Note de service N° 5387. Paris : Ministère de l’Économie, des Finances et du Budget, Direction Générale de la Concurrence, de la Consommation et de la Répression de Fraudes, Service de la Consommation, de la Qualité et de la Sécurité, Sous-direction H. Bureau H2.
Silva A.P., Gunata Z., Lepoutre J.P. & Odoux E., 2011. New insight on the genesis and fate of odor-active compounds in vanilla beans (Vanilla planifolia G. Jackson) during traditional curing. Food Res. Int., 44, 2930-2937, doi.org/10.1016/j.foodres.2011.06.048
Takahashi M., Sakamaki S. & Fujita A., 2013. Simultaneous analysis of guaiacol and vanillin in a vanilla extract by using high-performance liquid chromatography with electrochemical detection. Biosci. Biotechnol. Biochem., 77(3), 595-600, doi.org/10.1271/bbb.120835
Vial J. & Jardy A., 1999. Experimental comparison of the different approaches to estimate LOD and LOQ of an HPLC method. Anal. Chem., 71(14), 2672-2677, doi.org/10.1021/ac981179n
Walton N.J., Mayer M.J. & Narbad A., 2003. Vanillin. Phytochemistry, 63(5), 505-515, doi.org/10.1016/S0031-9422(03)00149-3






