- Accueil
- Volume 29 (2025)
- Numéro 2
- Pheromones in the integrated management of Arecaceae-defoliating pests. A review
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Pheromones in the integrated management of Arecaceae-defoliating pests. A review

Document(s) associé(s)
Version PDF originaleRésumé
Phéromones dans la gestion intégrée des ravageurs défoliateurs d’Arecaceae (synthèse bibliographique)
Introduction. La culture des Arecaceae est sujette à une infestation par des insectes défoliateurs, ce qui nécessite des méthodes de surveillance efficaces. Les substances sémiochimiques, principalement les phéromones sexuelles, sont désormais largement utilisées. Cette revue de littérature vise à répertorier les principales espèces de ravageurs défoliateurs des Arecaceae et leurs substances sémiochimiques associées, afin d’identifier l’ensemble des recherches sur l’utilisation des phéromones pour contrôler ces ravageurs et de discuter des orientations futures pour leur surveillance et leur contrôle dans la culture des Arecaceae.
Littérature. Les principaux insectes défoliateurs sont les lépidoptères, les coléoptères et les orthoptères. L’application de phéromones pour leur contrôle est une pratique établie pour huit genres de Lepidoptera, avec des études publiées sur l’utilisation de phéromones sexuelles synthétiques utilisées principalement en Asie du Sud-Est et du Sud. Ces phéromones comprennent principalement les esters saturés et insaturés, les alcools, les aldéhydes, les terpénoïdes et les hydrocarbures polyinsaturés. Les phéromones des insectes défoliateurs généralistes sont également abordées, de même que les sauterelles grégères et la nécessité d’une recherche continue sur le développement et l’utilisation des phéromones dans les régions productrices.
Conclusions. Les phéromones sont des outils importants pour la surveillance et le contrôle des ravageurs d’Arecaceae. Cependant, il est important d’étudier les espèces qui n’ont pas encore fait l’objet de recherche. Pour une meilleure efficacité de leur utilisation, il est nécessaire d’améliorer et d’adapter les techniques de terrain aux conditions environnementales et de développer des technologies de suivi et de contrôle pour toutes les régions productrices d’Arecaceae.
Abstract
Introduction. The cultivation of Arecaceae is subject to infestation by defoliating insects, which requires efficient monitoring methods. Semiochemicals, mainly sex pheromones, are now widely employed. The present literature review aims to list the main defoliating pest species of Arecaceae and their associated semiochemicals to identify all research efforts on the use of pheromones to control these pests, and to discuss further research directions for their monitoring and control in Arecaceae cultivation.
Literature. The main defoliating insects are Lepidoptera, Coleoptera and Orthoptera. Pheromone application for their control is an established practice for eight genera of Lepidoptera, with published studies on the use of synthetic sex pheromones employed mainly in Southeast and South Asia. These pheromones mainly include saturated and unsaturated esters, alcohols, aldehydes, terpenoids and polyunsaturated hydrocarbons. The generalist defoliator insects’ pheromones are also discussed as well as gregarious grasshoppers and the need for continuous research on developing and using pheromones in producing regions.
Conclusions. Pheromones are important tools for monitoring and controlling Arecaceae pests; however, it is important to study species that have not yet been researched. For better efficiency in their use, it is necessary to improve and adapt field techniques to environmental conditions and to develop monitoring and control technologies for all Arecaceae-producing regions.
Table des matières
* These authors have equally contributed to this work.
Received 16 February 2024, accepted 28 January 2025, available online 17 March 2025.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1The Arecaceae family has a wide geographic distribution in tropical regions, including species commonly known as palm trees. Arecaceae display great productivity and a variety of uses, being of great nutritional, medicinal, sociocultural and economic importance (Zambrana et al., 2007; Souza & Lima, 2019). Among the factors limiting their cultivation is the incidence of insect pests in cultivation regions, and these pests cause significant losses throughout cultivation, delays in vegetative development, loss of productivity and significant economic losses, especially in monoculture systems (Ferreira, 2008; Gitau et al., 2009). Insect pests associated with Arecaceae cause injuries to various parts of the plant; the feeding behavior of these pests makes it possible to classify them as defoliators, borers, suckers and moths, in addition to the presence of mites of agricultural relevance (Ferreira & Lins, 2006). Defoliators generally cause injuries to the leaves, reducing the photosynthetic area, and can even cause total defoliation, delaying plant development, reducing production and causing premature fruit fall. This type of pest is often associated with Arecaceae of agricultural importance, such as Cocos nucifera L., Elaeis guineensis Jacq., Euterpe oleracea Mart. and Phoenix dactylifera L., in addition to the many ornamental palm trees and species present in native vegetation (Mohan et al., 2010; Brandão et al., 2017; Ghnimi et al., 2017; Almeida et al., 2019).
2The adoption of pest management programs in Arecaceae crops advocates the development of sustainable techniques for surveying, monitoring and controlling insect populations in the field (Faleiro et al., 2016). Semiochemicals play an essential role in these crops given the specificity of these substances. The use of pheromones in these crops is carried out through traps to monitor and control insect pests, thereby reducing their populations and reproductive success (Ceruti, 2007).
3The present literature review aims to list the main defoliating pest species of Arecaceae and their associated semiochemicals to identify all research efforts on the use of pheromones to control these pests, and to discuss further research directions for their monitoring and control in Arecaceae cultivation.
2. Literature
2.1. Methods
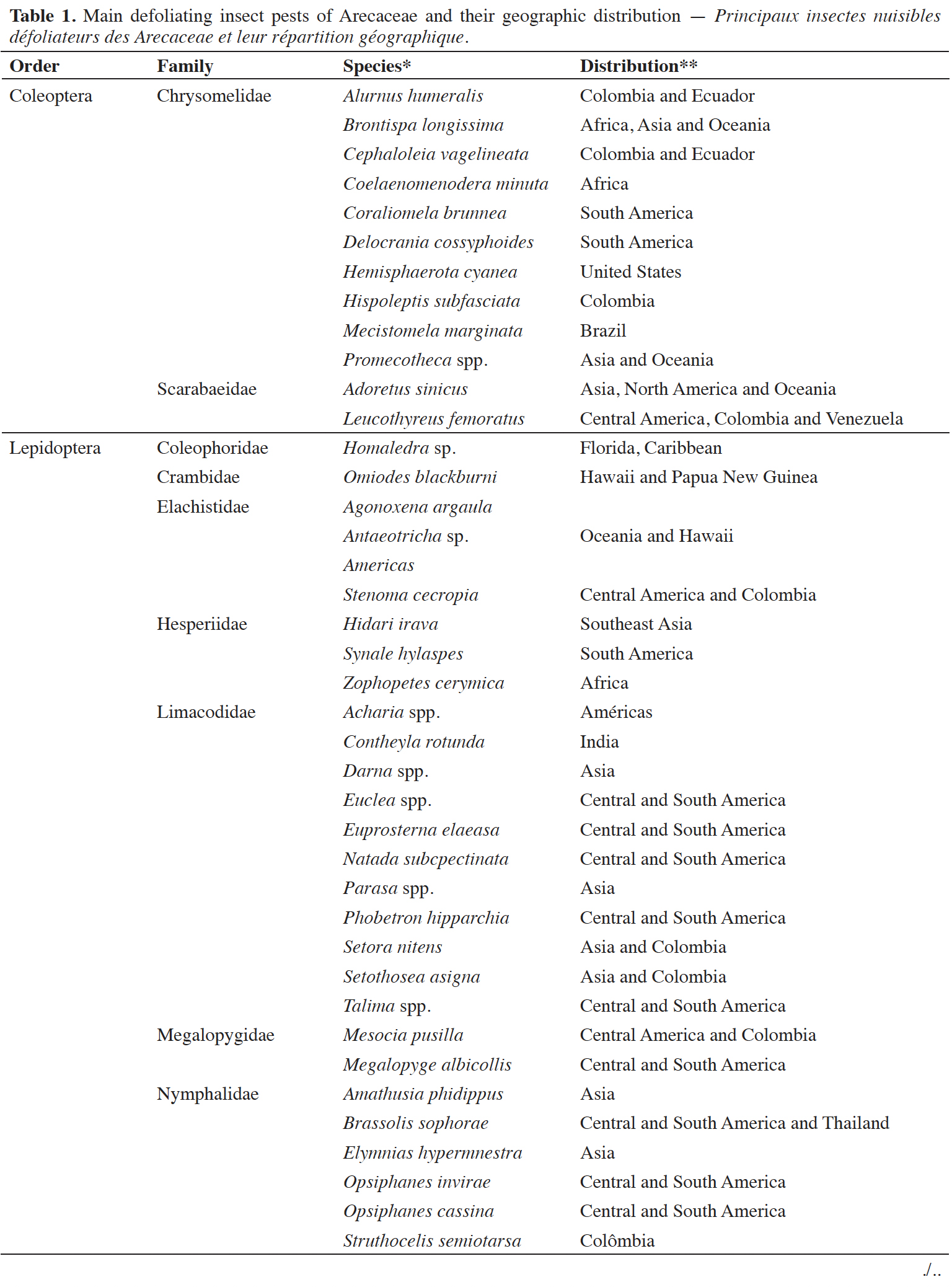
4This work was carried out using a qualitative approach with the objective of an exploratory study and a bibliographic review. To list the pests described as defoliating Arecaceae, surveys by Ferreira et al. (1998), Howard et al. (2001), Martínez et al. (2009) and Oliveira et al. (2018b) were considered. The geographic distribution of these pests was indicated based on consultations on the CABI and GBIF Secretariat platforms.
5To identify the research efforts conducted in the field of chemical ecology of Arecaceae defoliators, indexes in the Web of Science, Scopus and Scielo databases were used, with descriptors aligned with the objective of the work. The following descriptors and combinations using Boolean operators were used: Acharia OR Adoretus OR Agonoxena OR Alurnus OR Amathusia OR Antaeotricha OR Artona OR Atta OR Aularches OR Automeris OR Brassolis OR Brontispa OR Cephaloleia OR Coelaenomenodera OR Contheyla OR Coraliomela OR Darna OR Delocrania OR Dirphia OR Elymnias OR Euclea OR Euprosterna OR Hemisphaerota OR Hidari OR Hispoleptis OR Homaledra OR Homophylotis OR Leucothyreus OR Mahasena OR Mecistomela OR Megalopyge OR Mesocia OR Metisa OR Microtylopteryx OR Natada OR Oiketicus OR Omiodes OR Opisina OR Opsiphanes OR Parasa OR Phobetron OR Promecotheca OR Segestidea OR Setora OR Setothosea OR Sexava OR Stenoma OR Struthocelis OR Synale OR Talima OR Tropidacris OR Zophopetes) AND (‘chemical ecology’ OR semiochemicals OR pheromone OR kairomone OR allomone OR synomone OR ‘volatile compounds’ OR VOC OR HIPV.
6The search was carried out on September 12, 2023, using the descriptors, and 115 works were identified considering the characteristics of the research and the inclusion criteria (scientific articles) after initial filtering in the databases themselves. In the State of the Art through Systematic Review (StArt) tool (Zamboni et al., 2010), duplicate works were identified (n = 54) and analyzed per research topic. Works that did not present a description of compounds related to genera of Arecaceae-defoliating insects were excluded (n = 27). Thus, 34 studies were selected.
2.2. Arecaceae-defoliating pests
7Insects considered defoliators feed on the leaf blade, causing partial or total plant defoliation. Furthermore, some insects can scrape the leaf’s epidermal tissues or undermine the internal tissues between the upper and lower epidermis, causing drying and loss of leaves (Ferreira et al., 1998). These pests generally cause delays in plant development, reduce production, and cause premature fruit fall.
8The main defoliators of Arecaceae are insects from the orders Lepidoptera, Coleoptera and Orthoptera (Table 1). The most diversity of species that attack palm trees is associated with the order Lepidoptera in different regions. They are critical pests, as in high infestations, they can cause complete plant defoliation and irreversible damage (Lemos & Boari, 2010). The second order is Coleoptera, mainly including insects from the Chrysomelidae family. Some Orthoptera species stand out as defoliators in Africa, Asia, and Oceania. Insects from the orders Phasmatodea (stick insects) and Hymenoptera (leaf-cutter ants) are also associated with cultivation regions but have little relevance (Howard et al., 2001). Lepidoptera are critical defoliators of palm trees across the planet and are characterized by their immature phase as phytophagous caterpillars. Opisina arenosella Walker, 1864 (Lepidoptera: Oecophoridae) and Artona catoxantha Hamps, 1892 (Lepidoptera: Zyganeidae) in Asia, Agonoxena argaula Meyrick, 1921 (Lepidoptera: Agonoxenidae) in Oceania, Zophopetes cerymica Hewitson, 1867 (Lepidoptera: Hesperiidae) in tropical Africa, and Brassolis sophorae Linnaeus, 1758 (Lepidoptera: Nymphalidae) in the Americas are among the main species that stand out, in addition to several species of stinging caterpillars from the Limacodidae and Saturniidae families (Table 1).
9The nutritional value and quantity of Arecaceae leaves consumed can directly influence the life cycle of defoliating insects, which may be longer or shorter depending on the host species. The oil palm leaf has a high content of indigestible cellulose fibres and a low nutritional value; therefore, a limited number of insects have evolved as defoliators of this palm tree (Howard et al., 2001). The larval stages of these insects generally develop slowly (Rodrigues et al., 2006). Furthermore, the abundance of caterpillars is lower in young leaves than in older leaves, possibly influenced by nutritional variation. It has also been observed that younger leaves may present a more significant number of secondary metabolites directly involved in plant defence against herbivory (Hartmann, 1996).
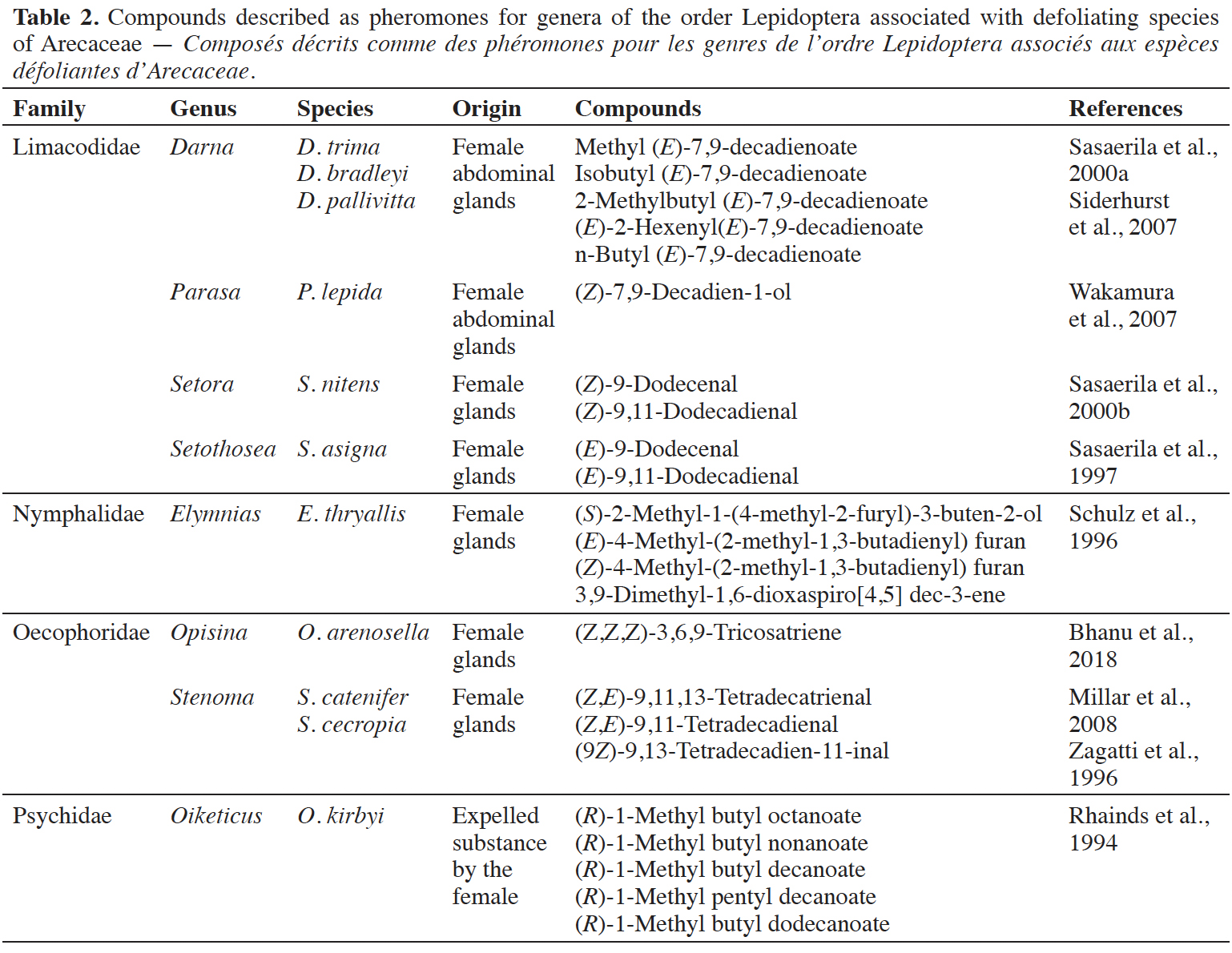
10The abundance of Opsiphanes invirae Hübner, 1818 (Lepidoptera: Nymphalidae) caterpillars varies depending on the leaf region sampled, where the most significant number of caterpillars was observed on leaflets 51–101, which corresponds to the intermediate part of the leaves (Oliveira et al., 2018a). The most significant number of O. cassina Felder (1862) caterpillars were observed on leaflets 41–80 due to the lower tannin and high nitrogen levels in this leaf region (González et al., 2011). Injuries caused by immature Nymphalidae species are characterized by feeding from the edges to the center of the leaves, regardless of the host and with partial or total consumption of the leaflets (Figure 1a). In oil palm, O. cassina can consume an average of 356.51 cm² of leaves in the field during its larval phase and is characterized by leaf feeding, leaving only the central vein of the leaflets (González et al., 2012). For the species B. sophorae, partial or total defoliation has been observed, with the caterpillars consuming 500-600 cm² of leaf area (between 2 and 2.5 leaflets). Brassolis isthmia Bates, 1864 (Lepidoptera: Nymphalidae) presented an average consumption of 820.62 cm2 during the larval phase (Lemos & Boari, 2010; Mexzón, 2011). In the Limacodidae family, a high consumption rate for the caterpillar Acharia fusca Stoll, 1999 (Lepidoptera: Limacodidae), which consumes around 402.31 cm² of Elaeis guineensis leaf area (Martínez et al., 2014), has been observed in the laboratory. In high infestations, these species can cause complete plant defoliation in a few days (Figure 1b), demonstrating the importance of monitoring for decision making in managing these pests.

Figure 1. Defoliation caused by the attack of Nymphalidae species caterpillar on Arecaceae in Brazil — Défoliation causée par l’attaque de la chenille d’espèces de Nymphalidae sur les Arecaceae au Brésil.
a. Cultivation of Euterpe oleracea in Tailândia, Pará, Brazil — Culture d’Euterpe oleracea à Tailândia, Pará, Brésil. b. Total defoliation of Veitchia merrillii ornamental in Rio Largo, Alagoas, Brazil — Défoliation totale de Veitchia merrillii ornementale à Rio Largo, Alagoas, Brésil.
11In the order Coleoptera, attack by defoliating insects is not restricted to the immature phase; in some species, the adults feed on leaves, such as Leucothyreus femoratus Burmeister, 1844 (Coleoptera: Scarabaeidae), a significant pest in the regions of Central America, Colombia and Venezuela, where they are rhizophagous in the larval stage and feed from the edge of the leaflets as adults, consuming an average of 13 mm2 per day (Martínez et al., 2000; Martínez et al., 2013). In South America, Coraliomela spp. exhibits this behavior, with the adults feeding on the parenchyma, drawing a straight line parallel to the central vein of the coconut leaflets. However, the most significant damage is caused by the larva, which feeds by making holes in the leaves, reducing the leaf area of the young palm plant and causing delays in plant development (Ferreira et al., 1998; Manca et al., 2014).
12In the Orthoptera order, species from the Tettigoniidae family are considered the main defoliating pests of oil palm crops in Papua New Guinea (Oceania), with species of the genus Sexava reported as the most critical, where the combined attack of overlapping generation cycles is more harmful and causes a reduction in the photosynthetic surface area of the leaflets and, consequently, a decrease in fruit production in the affected palm trees (Ero et al., 2013). With generalist habits, the species Aularches miliaris Linnaeus, 1758 (Orthoptera: Pyrgomorphidae) is associated with attacks in coconut and oil palm areas in Southeast and South Asia (Mathew et al., 2021).
13In some regions, important Arecaceae-defoliating species are still poorly studied with respect to their behavior and the development of techniques that can contribute to integrated management of these pests. In Colombia, a campaign is being developed to monitor, prevent and intervene in the integrated management of defoliating pests in oil palm crops that implements an action plan based on the diagnosis for species that predominate in the region (Martínez et al., 2009). However, there is a lack of action related to technological advances for implementing management programs for these pests, making cultivation unfeasible. As occurs in the development and use of pheromones, despite advances, the accessibility of these products to potential users is compromised by a deficiency in technology transfer to producers (Moreira et al., 2005; Goulart et al., 2015).
2.3. Pheromones of Arecaceae-defoliating pests
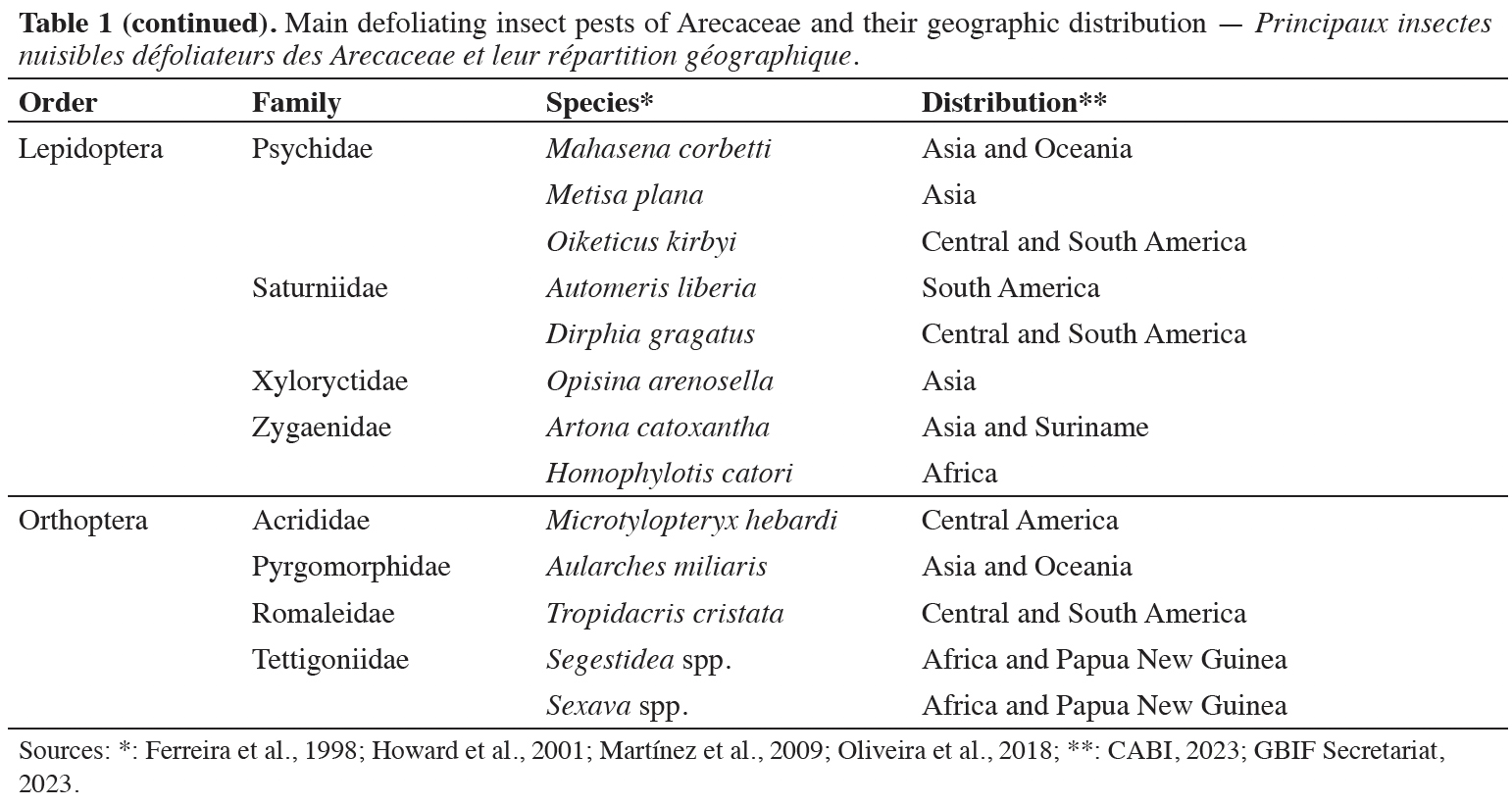
14Using pheromones to control Arecaceae insect pests is an established practice for monitoring and control and contributes to integrated pest management in several producing regions. Among the insects considered defoliators in the order Lepidoptera, studies on eight genera have been published, including the description of the organic chemical compounds used as sex pheromones, most of which were extracted from glands present in females (Table 2).
15Pheromones are chemicals produced by specialized glands that affect insect behavior, development and physiology. They are classified according to their effects; they can have a preparatory effect (more extended action) or a triggering effect (immediate action) (Nordlund & Lewis, 1976; Ferreira & Zarbin, 1998). The presence of abdominal scent glands is a common characteristic of lepidopterans, mainly female moths. In this respect, moths differ from butterfly species, which have release structures in androconia on the wings, tufts of scales, or specialized bristles at the end of the abdomen of males. These structures can release compounds with short- and long-range action as pheromones, and the structure is often similar to plant secondary metabolites or fatty acid derivatives (Ganai et al., 2017).
16Some species observed in the survey were not associated with Arecaceae pests (Table 2). However, this evidence can help to guide studies on chemical ecology with species of the same genus, about sexual behavior, odorous compound release structures, types of compounds and forms of use in the field. The following stinging caterpillars were among the species considered defoliating pests of Arecaceae observed in the surveys: Darna trima Moore, 1859; D. bradleyi Holloway, 1986; D. pallivitta Moore, 1877; Setothosea asigna van Eecke, 1929; Setora nitens Walker, 1855 and Parasa lepida (Cramer, 1779) (Lepidoptera: Limacodidae). Field studies have used synthetic sex pheromones identified from female glands (Sasaerila et al., 1997, 2000a, 2000b; Siderhurst et al., 2007) in the capture of males using delta-type traps to monitor or control these pests in commercial oil palm plantations in Southeast Asia (Sasaerila et al., 2007).
17For species of the genus Darna, the decadienoates 2-methylbutyl (E)-7,9-decadienoate and (E)-2-hexenyl (E)-7,9-decadienoate in combination have been shown to be essential and synergistic components of pheromones for attracting D. trima males. For D. bradleyi, the compound isobutyl (E)-7,9-decadienoate individually attracts males but the addition of methyl (E)-7,9-decadienoate in a ratio of 1:10 significantly increased the attractiveness of the female bait (Sasaerila et al., 2000a).
18In field tests with D. pallivitta, males were attracted to bait containing n-butyl (E)-7,9-decadienoate, which outperformed attractive baits with virgin moths (Siderhurst et al., 2007; Jang et al., 2009). The use of the pheromone showed the potential to control D. pallivitta through the interruption of mating using traps and by the interruption of communication through a species carrying the pheromone (Bactrocera cucurbitae [Coquillett, 1899] [Diptera: Tephritidae]) to disrupt the everyday mating communications of the target pest (Siderhurst et al., 2015).
19In the search for the sex pheromone of S. assigna, seven active compounds were identified through gas chromatography and electroantennography analysis. However, only two have demonstrated efficiency in field capture experiments, the aldehydes (E)-9-dodecenal and (E)-9,11-dodecadienal in a 1:1 ratio but not alone (Sasaerila et al., 1997). In studies with S. nitens using mass spectrometry and electroantennography analyses, the components of the pheromone of this species were the isomers corresponding to the aldehydes identified as components of the S. assigna pheromone (Z)-9-dodecenal and (Z)-9,11-dodecadienal, and they were efficient in a 1:1 ratio, as verified through a field experiment carried out with synthetic compounds in Malaysia (Sasaerila et al., 2000b).
20These sympatric and co-seasonal species in oil palm plantations remain reproductively isolated through specificity in their intra- and interspecific communication signal, which affects their daily periodicity and micro-location for chemical signalling (Sasaerila et al., 2000c). However, the application of these described sex pheromones in managing stinging caterpillars is poorly documented. The stinging caterpillar, P. lepida, a defoliating pest of coconut trees in southwest Asia, has also been found on coffee, mango and cocoa plantations (Sawada et al., 2008). The sex pheromone of this pest was extracted from the glands of virgin females, and the identified compound (Z)-7,9-decadien-1-ol (Z7,9-10:OH) showed efficiency in capturing males in the field with sticky traps using rubber septa as a release during 12 days of exposure (Wakamura et al., 2007; Islam et al., 2009).
21In coconut cultivation in India, the sex pheromone (Z,Z,Z)-3,6,9-tricosatriene can be used to capture the masses of Opisina arenosella Walker, 1864 (Lepidoptera: Oecophoridae), a critical defoliating species. The use of white ‘pinwheel’ traps placed in the middle of the palm tree canopy make it an efficient technique to control this pest (Muniyappa et al., 2018). This sex pheromone was identified in extracts from the glands of one-day-old females (Bhanu et al., 2018). Females of O. arenosella are attracted to kairomones (linalool, acetophenone and limonene) found in coconut leaves and larval excreta; in oviposition preference tests, these volatiles have been used to enhance the semiochemical-based management method for this pest (Kumara et al., 2015; Shameer et al., 2017).
22The aldehyde compounds (Z,E)-9,11,13-tetradecatrienal and (Z,E)-9,11-tetradecadienal are considered components of the sex pheromone of Stenoma cecropia Meyrick, 1916 (Lepidoptera: Elachistidae) and have been identified in extracts from female glands and tested using electroantennography, laboratory olfactometry and trap experiments in an oil palm cultivation area in Colombia. Attraction to males was observed only with the use of a blend consisting of the two aldehydes identified in proportions of 83% for (Z,E)-9,11,13:Ald and (Z,E)-9,11,14:Ald and 17% for (Z,E)-9,11–14:Ald, but lower capture rates were observed compared to those obtained using virgin females as bait (Zagatti et al., 1996).
23An important pest of avocado fruit, Stenoma catenifer Walsingham, 1912 also produces an unsaturated aldehyde compound, (Z)-9,13-tetradecadien-11-inal, as a pheromone, and it attracted more males in field tests compared to caged virgin female moths, demonstrating the frequent relationship that occurs with congeneric species in the identification of compounds used as pheromones (Millar et al., 2008; Hoddle et al., 2009). In commercial avocado orchards, parameters for using the pheromone were observed, such as the type of bait, height, trap density and bait effectiveness time, in which grey or white rubber septa are the best releasers in avocado traps. Type Pherocon IC wings (Trécé Inc., Adair, OK, USA), at a height between 4 and 6 m with a distribution of three traps per hectare, captured the most males per trap in 15 days (Hoddle et al., 2011; Cruz-López et al., 2020; Velázquez-Martínez et al., 2023). Males were captured using the sex pheromone of S. catenifer throughout the year, with population peaks in June–August and December–January, with the additional influence of food abundance, climate (precipitation and temperature) and altitude. Furthermore, this pheromone attracted the species Antaeotricha nictitans Zeller, 1854 (Lepidoptera: Elachistidae) of unknown hosts (Castillo et al., 2012; Vázquez et al., 2017; Velázquez-Martínez et al., 2022), and this genus is considered a defoliating pest of Arecaceae (Table 1).
24The basket bug, Oiketicus kirbyi Guilding, 1827 (Lepidoptera: Psychidae), a pest associated with several commercial crops, is also reported to defoliate palm trees (Oliveira et al., 2018b). Females release the sex pheromone to attract males to the lower portion of the baskets. This pheromone has been identified as a mixture of five chiral esters, (R)-1-methylbutyl octanoate, (R)-1-methylbutyl nonanoate, (R)-1-methylpentyl decanoate, (R)-1-methylbutyl and (R)-1-methylbutyl decanoate, the latter being the majority compound. In field tests in oil palm plantations, the mixture with 1,000 μg of the main compound and 100 μg of the other compounds in different combinations attracted males, demonstrating their potential utility in the management of O. kirbyi populations (Rhainds et al., 1994).
25Evidence of sex pheromones has also been reported for Metisa plano Walker, 1883 (Lepidoptera: Psychidae), a critical defoliator in oil palm plantations in Malaysia (Abdullah et al., 2012). The use of receptive females in sticky traps resulted in the mass capture of moths in the field, reducing leaf damage by 35–45% in the capture blocks tested. The use of traps with females and aerial spraying of Bacillus thuringiensis (Bt) reduced the caterpillar population by 94% in the first cycle of the caterpillar population (Kamarudin et al., 2010; Ahmad et al., 2017). Furthermore, false pheromone trails generated by the increase in the number of females of the same species per palm tree can interrupt the natural mating of this pest in oil palm crops (Rhainds, 2018).
26Regarding the genus Elymnias, species with pheromones described in the literature are not pests associated with Arecaceae (Elymnias thryallis Kirsch, 1876 [Lepidoptera: Satyridae]), which produces elymniafuran ((S)-2-methyl-1-(4-methyl-2-furyl)-3-buten-2-ol), formulated as small amounts of (E)- and (Z)-4-methyl-(2-methyl-1,3-butadienyl) furan and 3,9-dimethyl-1,6-dioxaspiro [4,5] dec-3-ene (Schulz et al., 1996). Observations from these studies can contribute to research on the species E. hypermnestra, which is a critical defoliator found in oil palm plantations in South Asia (Table 1).
27Lepidoptera pheromones are varied and classified according to their general chemical structure during biosynthesis (Ando & Yamamoto, 2020). Around 75% of these compounds are classified as type I pheromones, which are biosynthesized de novo via general saturated fatty acids comprising the classes alcohols, aldehydes and acetates, mono- or diunsaturated and containing 10–18 carbon atoms in their chain. Type II pheromones represent approximately 15% of known lepidopteran pheromones and have been identified only from some limited groups of compounds, such as polyunsaturated hydrocarbons and their epoxides (Allison & Cardé, 2016; Ando & Yamamoto, 2020; Jurenka, 2021).
28In addition to these, there are also type III pheromones, branched hydrocarbons, type 0 pheromones, secondary alcohols and short-chain ketones (7–9 carbon atoms), which are considered a more ancestral type of Lepidoptera pheromone. Other types include terpenes and their derivatives, methyl ketones, secondary alcohols with methyl groups as branches and unsaturated ketones (Allison & Cardé, 2016).
29The presence of saturated and unsaturated esters has been observed in the chemical classification of pheromones from insect pests of genera of the order Lepidoptera that are associated with Arecaceae defoliators (Figure 2a). These compounds as well as alcohols and aldehydes (Figure 2b) are considered type I pheromones and are quite common in Lepidoptera species (Jurenka, 2021). This type of pheromone, generally derived from fatty acids, originates from palmitic and stearic acids, which, in turn, are produced in the pheromone gland in the female abdomen (Allison & Cardé, 2016). Type I compounds with the presence of saturated and unsaturated esters have been identified as pheromone components for species of the genera Darna and Oiketicus (Figure 2a), and alcohols and aldehydes have been identified in the genera Setora, Setothosea, Stenoma and Parasa (Figure 2b). Other pheromones found and described for these Lepidoptera species include terpenoids and polyunsaturated hydrocarbons (Figure 3).
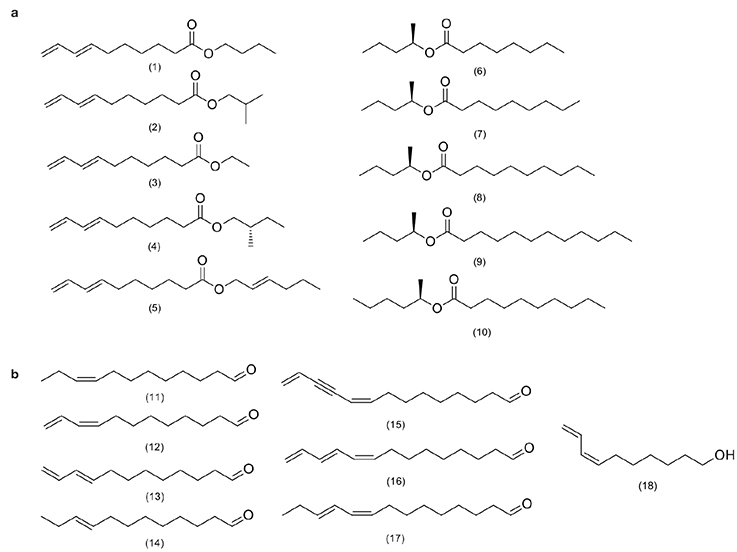
Figure 2. Type I pheromones of Lepidoptera order insect pests associated as defoliators of Arecaceae — Les phéromones de type I de l’ordre des insectes ravageurs Lepidoptera, associées aux défoliateurs d’Arecaceae.
a. Saturated and unsaturated esters — Esters saturés et insaturés; b. Alcohols and aldehydes — Alcools et aldéhydes; (1) butyl (E)-7,9-deca-dienoate; (2) isobutyl (E)-7,9-decadienoate; (3) ethyl (E)-7,9-decadienoate; (4) (S,E)-2-methylbutyl 7,9-decadienoate; (5) (E)(E)-2-hexenyl 7,9-decadienoate; (6) methylbutyl octanoate; (7) methylbutyl nonanoate; (8) methylbutyl decanoate; (9) methylbutyl dodecanoate; (10) methylpentyl decanoate; (11) (Z)-9-dodecenal; (12) (Z)-9,11-dodecadienal; (13) (E)-9,11-dodecadienal; (14) (E)-9-dodecenal; (15) (Z)-9,13-tetradecadien-11-ynal; (16) (Z,E)-9,11,13-tetradecatrienal; (17) (Z,E)-9,11-tetradecadienal; (18) (Z)-7,9-decadien-1-ol.
30The compound (Z,Z,Z)-3,6,9-tricosatriene, described as a pheromone component from species of the genus Opisina, is considered a type II pheromone. This type of pheromone is derived from linoleic or linolenic acids obtained from the insect’s diet, maintaining the pattern of two or three Z double bonds separated by a methylene group (CH2) (Ando & Yamamoto, 2020; Jurenka, 2021). Pheromones of the genus Elymnias include terpenoids (Figure 3). Among lepidopteran species of Arecaceae pests, eight species of the Limacodidae family that produce pheromones have been reported in the literature, with studies concentrated in Southeast Asia, Central and South America, and Africa. The pheromones belong to the classes of unsaturated alcohols, esters and aldehydes with chains containing 8–20 carbons. Polyunsaturated alkenes have also been reported, and all identified compounds share the same biosynthetic pathway (Reis et al., 2023).
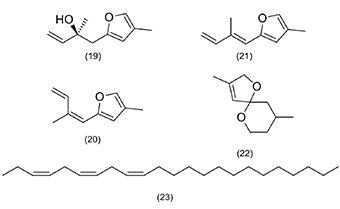
Figure 3. Terpenoid class pheromones and polyunsaturated hydrocarbon of Lepidoptera order insect pests associated as defoliators of Arecaceae — Phéromones de classe terpénoïde et hydrocarbures polyinsaturés de l’ordre des insectes ravageurs lépidoptères, associées aux défoliateurs d’Arecaceae.
(19) (S)-2-methyl-1-(4-methylfuran-2-yl)but-3-en-2-ol; (20) (Z)-4-methyl-2-(2-methylbuta-1,3-dien-1-yl)furan; (21) (E)-4-methyl-2-(2-methylbuta-1,3-dien-1-yl)furan; (22) 3,9-dimethyl-1,6-dioxaspiro[4.5]dec-3-ene; (23) (Z, Z, Z)-3,6,9-tricosatriene.
31For the order Coleoptera, there are descriptions of active compounds identified for the genus Brontispa Sharp, 1904 (Coleoptera: Chrysomelidae), characterized as allelochemicals emitted by the coconut tree. The mixture of leaf compounds β-myrcene, (-)-limonene and E-2-hexen-1-ol, demonstrated attractiveness for males and females and the induction of oviposition, which is important in modulating insect behavior (Fang et al., 2011). Evidence regarding the use of pheromones by B. longissima (Gestro, 1885) is associated with the presence of proteins involved in the reception and detection of odorants in antennal transcriptomes (Bin et al., 2017). Furthermore, the contact sex pheromone of this pest consists of one or more polar compounds, which are likely saturated hydrocarbons present in the elytra of females (Kawazu et al., 2011).
32Indications for chemical recognition in the form of sex pheromones have also been observed among adults of Leucothyreus marginaticollis Blanchard, 1843 (Coleoptera: Scarabaeidae) during mating behavior (Ferreira & Rodrigues, 2017).
33Studies on chemical ecology have not been performed with genera of the order Orthoptera that are Arecaceae defoliators (Table 1). However, as some species of grasshopper present gregarious, migratory behavior, are generalist defoliators and considered insect pests of great importance worldwide, most chemoreception and chemical ecology studies for this order have focused on species with these characteristics, such as Locusta migratoria (Linnaeus, 1758) and species of Schistocerca spp. (Orthoptera: Acrididae). These pests preferentially occupy open areas and cause clouds of locusts that migrate and attack large areas of plantations, including coconut and oil palm crops in regions of Asia, Africa and some parts of South America (Howard et al., 2001).
34Aggregation pheromones, such as common compounds found in body parts and faecal droppings that are emitted by species of Schistocerca spp. and L. migratoria, anisole, veratrole, benzaldehyde, 4-vinylanisole, guaiacol, phenol and phenyl acetonitrile, are an essential tool for managing Orthoptera species (Figure 4) (Torto et al., 1994; Mahamat et al., 2000; Wei et al., 2017). Volatiles originating from excrement are guaiacol and phenol and are components of the aggregation pheromone of S. gregariae Forskål, 1775 (Torto et al., 1996; Dillon et al., 2002).
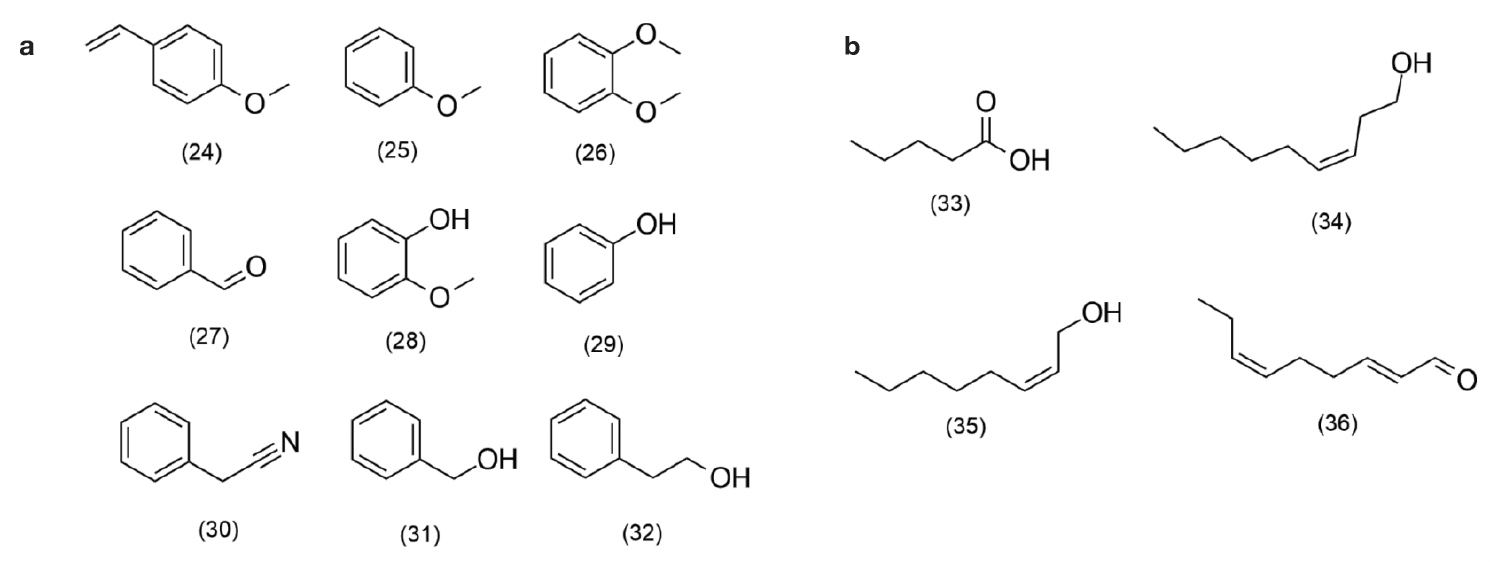
Figure 4. Pheromones described for species of Schistocerca spp. and Locusta migratoria: 4-vinylanisole (24), anisole (25), veratrol (26), benzaldehyde (27), guaiacol (28), phenol (29), phenylacetonitrile (30), benzyl alcohol (31), 2-phenylethanol (32), pentanoic acid (33), (Z)-3-nonen-1-ol (34), (Z)-2-octen-1-ol (35), (E,Z)-2,6-nonadienal (36) — Phéromones décrites pour les espèces de Schistocerca spp. et Locusta migratoria : 4-vinylanisole (24), anisole (25), veratrol (26), benzaldéhyde (27), guaiacol (28), phénol (29), phénylacetonitrile (30), alcool benzylique (31), 2-phényléthanol (32), acide pentanoïque (33), (Z)-3-en-nonol-1-ol (34), (Z)-2-octen-1-ol (35), (E,Z)-2,6-nonadienal (36).
35Phenylacetonitrile (PAN), which is emitted by sexually mature males, is involved in sexual behavior as a repellent during mating and inhibits competition from other males (Seidelmann & Ferenz, 2022). PAN, as an aposematic compound, is also a component of the locust defense mechanism, signaling toxicity to predators (Wei et al., 2019). This compound, together with (Z)-2-octen-1-ol and (Z)-3-nonen-1-ol, is released explicitly by males in an agglomeration of S. piceifrons Walker, 1870, and although they do not show any attractive or repellent effect, these compounds are related to female mating partner evaluation pheromones (Stahr et al., 2016).
36(Z)-3-nonen-1-ol produced by gregarious males of S. americana Drury, 1773 is involved in sexual behavior as an anti-aphrodisiac and has the effect of accelerating maturation, enabling the synchronization of sexual development (Stahr et al., 2013). In S. cancellata Serville, 1838, volatile male-specific benzyl alcohol and 2-phenylethanol are emitted almost exclusively from the abdomen, with an intensity influenced by sexual competition between males (Seidelmann & Stahr, 2023). Released by the glands of adult S. gregaria females, pentanoic acid has been shown to stimulate pre-mating behavior in males (Njagi & Torto, 2002).
37The only pheromone for the solitary grasshopper is (E,Z)-2,6-nonadienal, which is released by solitary S. gregaria females and known to induce behavioral attraction to males (Ochieng & Hansson, 1999).
38The volatile compounds emitted in the fecal excrement of L. migratoria are the same as those described for species of the genus Schistocerca. However, only 4-vinylanisole has the action of attracting solitary and gregarious grasshoppers, regardless of age or sex, and experimental populations and wild animals in the field (Wei et al., 2017; Guo et al., 2020). These compounds, although common between species, present differences in emission dynamics, given that relative concentrations in pheromone mixtures provide specific signals for each species, sex and developmental stage (Nakano et al., 2022).
39In the intra- and interspecific aggregation of these species, a smaller pattern of stage and sex differentiation has been observed for the aggregation pheromone of L. migratoria, in which adults respond to the nymphs’ pheromone, and the pheromone is emitted by both males and females. In contrast, there is no aggregative response between stages in S. gregaria, and pheromone production is specific to males (Niassy et al., 1999).
40The compounds observed for Orthoptera species are simple aromatics (Figure 4) and used as pheromones in communication and as defense compounds (Brückner et al., 2020). Within the order Orthoptera, fatty acid derivatives, such as alcohols and aldehydes, which are common compounds for insect pest species of the order Lepidoptera, have also been observed (Figure 4).
41The use of these compounds in traps for monitoring and controlling species in the field has rarely been discussed in the literature, being restricted to experiments to verify attraction under reduced sampling conditions. Research that contributes to the development of management strategies using the pheromones described is needed to enable progress in the prevention and control of locust outbreaks.
42Many of the pheromones studied require field validation, and a very small number are available on the market, such as the O. arenosella pheromone used for monitoring and control in India. This highlights the need for chemical synthesis of identified pheromones and further field studies to improve formulations, release strategies and trap types, which are fundamental variables for pheromone application as a tool for pest monitoring and control. In addition, for substances already in use, continuous studies are necessary to maintain the efficiency of these products in the field.
43Around 80% of insect species that are considered Arecaceae pests have not yet been studied for the identification of pheromones. This may be related to the expansion of monocultures, where insects that are not important can become pest insects, thus demonstrating an open field of research for the development of studies aiming at monitoring and control.
3. Conclusions
44The studies evaluated in this survey focused on defoliating insects from crops distributed in Southeast and South Asia, which have the highest production of oil palm and coconut in the world. However, other tropical regions that produce Arecaceae crops, such as Central and South America, also have critical species of defoliating caterpillars, such as those from the family Nymphalidae (B. sophorae and O. invirae Hübner, 1818) and Saturniidae (Automeris liberia Cramer, 1780) (Calvache et al., 2010). These species are the subject of studies for developing pheromones to contribute to integrated pest management. Evidence of pheromone use was observed for A. liberia with the characterization of sensilla involved in recognizing pheromone molecules in males and females (Silva et al., 2019).
45These contributions to the literature are necessary given the diversity of insect pests and forms of cultivation, even for insects that are not considered main pests, considering that climate change can affect the growth and distribution of pest species, the expansion of their geographic distribution, and the increase in their survival and number of generations. Thus, strategies should focus on intensive monitoring of these insect populations, as the leading management practice for dealing with changes in pest status (Skendžić et al., 2021; Yasin & Rehman, 2021).
46Semiochemicals are essential tools for monitoring these pests; however, to ensure practical use in the field, it is necessary to improve existing techniques, with a particular emphasis on environmental factors, such as relative humidity, temperature and air speed, which can have an impact on how pheromones are used under these conditions (Andrew & Hill, 2017). Additionally, active substances should be studied in insects for which they have not yet been identified.
Funding sources
47This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001. This study was funded by INCT Semioquímicos na Agricultura (CNPq Proc. 465511/2014-7 and Fapesp Proc. 2014/50871-0).
Bibliographie
Abdullah F. et al., 2012. Response of the male bagworm moth (Metisa plana Walker, Lepidoptera: Psychidae) towards female bagworm pheromone lure in wind tunnel bioassays. Asia Life Sci., 21, 375-389.
Ahmad M.N. et al., 2017. Efficacy of pheromone trapping and aerial spraying of Bacillus thuringiensis (Bt) for controlling bagworm, Metisa plana Walker (Lepidoptera: Psychidae) in Yong Peng, Johor, Malaysia. J. Oil Palm Res., 29, 55-65, doi.org/10.21894/jopr.2017.2901.06
Allison J.D. & Cardé R.T., eds, 2016. Pheromone communication in moths: evolution, behavior, and application. Berkeley, CA, USA: University of California Press.
Almeida U.O. et al., 2019. Registro de ocorrência de Herminodes sp. em açaizeiro (Euterpe oleracea Mart.) no estado do Acre. South Am. J. Basic Educ. Tech. Technol., 6, 541-545.
Ando T. & Yamamoto M., 2020. Semiochemicals containing lepidopteran sex pheromones: wonderland for a natural product chemist. J. Pestic. Sci., 45, 191-205, doi.org/10.1584/jpestics.d20-046
Andrew N.R. & Hill S.J., 2017. Effect of climate change on insect pest management. In: Coll M. & Wajnberg E., eds. Environmental pest management: challenges for agronomists, ecologists, economists and policymakers. Cambridge, UK: Cambridge University Press, 195-223.
Bhanu K.R.M. et al., 2018. Identification and field evaluation of female sex pheromone of leaf-eating caterpillar, Opisina arenosella (Lepidoptera: Oecophoridae). Int. J. Trop. Insect Sci., 38, 274-282, doi.org/10.1017/s1742758418000243
Bin S.Y. et al., 2017. Antennal and abdominal transcriptomes reveal chemosensory gene families in the coconut hispine beetle, Brontispa longissima. Sci. Rep., 7, 2809, doi.org/10.1038/s41598-017-03263-1
Brandão A.D.S. et al., 2017. Spatial and temporal distribution of Opsiphanes invirae (Lepidoptera: Nymphalidae) in oil palm, Pará State, Brazil. Rev. Bras. Cienc. Agrar., 12, 464-469, doi.org/10.5039/agraria.v12i4a5479
Brückner A., Kaltenpoth M. & Heethoff M., 2020. De novo biosynthesis of simple aromatic compounds by an arthropod (Archegozetes longisetosus). Proc. R. Soc. B., 287, 20201429, doi.org/10.1098/rspb.2020.1429
CABI, 2023. Cabi compendium : invasive species, https://www.cabi.org/isc/, (19/08/2023).
Calvache G., Franco B. & Pedro N., 2010. Manual de plagas de la palma de aceite en Colombia. Bogota : Centro de Investigación en Palma de Aceite, Cenipalma.
Castillo A., Cruz-Lopez L. & Gómez J., 2012. Moth species captured with the sex pheromone of Stenoma catenifer (Lepidoptera: Elachistidae) in avocado plantations of southern Mexico. Fla. Entomolog., 95, 1111-1116.
Ceruti F.C., 2007. Interações entre feromônios de insetos e semioquímicos de plantas. Rev. Acad. Ciênc. Anim., 5, 73-82, doi.org/10.7213/cienciaanimal.v5i1.9606
Cruz-López L., Vázquez M.A & Castillo A., 2020. Effectiveness of the sex pheromone for monitoring Stenoma catenifer (Lepidoptera: Elachistidae) in avocado orchards. Neotrop. Entomol., 49, 332-336, doi.org/10.1007/s13744-019-00749-7
Dillon R.J., Vennard C.T. & Charnley A.K., 2002. A note: gut bacteria produce components of a locust cohesion pheromone. J. Appl. Microbiol., 92, 759-763, doi.org/10.1046/j.1365-2672.2002.01581.x
Ero M.M., Manjobie T. & Dewhurst C.F., 2013. Oil palm foliage damage by Segestes decoratus Redtenbacher (Orthoptea: Tettigoniidae: Mecopodinae) in West New Britain, Papua New Guinea. Planter, 89, 481-490, doi.org/10.56333/tp.2013.004
Faleiro J.R. et al., 2016. Integrated Pest Management (IPM) of palm pests. In: Abrol D.P., ed. Integrated Pest Management in the tropics. New Delhi, India: New India Publishing Agency, 439-497.
Fang Y., Sun J. & Zhang Z., 2011. Response of Brontispa longissima to coconut palm (Cocos nucifera) leaf volatiles. Physiol. Entomol., 36, 321-326, doi.org/10.1111/j.1365-3032.2011.00799.x
Ferreira J.M.S., 2008. Manejo integrado de pragas do coqueiro. Ciênc. Agríc., 8, 21-29.
Ferreira J.M.S. et al., 1998. Pragas do coqueiro. In: Ferreira J.M.S., Warwick D.R.N. & Siqueira L.A., eds. A cultura do coqueiro no Brasil. Brasília: Embrapa, 189-267.
Ferreira J.M.S. & Lins P.M.P., 2006. Pragas do Coqueiro. In: Ferreira J.M.S. & Fontes H.R., eds. Produção integrada de coco: identificação de pragas, doenças e desordens nutricionais e fisiológicas. Aracaju, Brazil : Embrapa Tabuleiros Costeiros, 11-68.
Ferreira J.T.B. & Zarbin P.H.G., 1998. Amor ao primeiro odor. Quim. Nova Escola, 7, 3-6.
Ferreira K.R. & Rodrigues S.R., 2017. The mating behavior of Leucothyreus marginaticollis Blanchard, 1843 (Coleoptera: Scarabaeidae: Rutelinae). Biota Neotrop., 17, e20170330, doi.org/10.1590/1676-0611-bn-2017-0330
Ganai M.A., Khan Z.H. & Dar M.A., 2017. Pheromones in lepidopteran insects: types, production, reception and its application. J. Pharmacogn. Phytochem., 6, 2552-2558.
GBIF Secretariat, 2023. GBIF Backbone Taxonomy, https://doi.org/10.15468/39omei, (19/08/2023).
Ghnimi S., Umer S., Karim A. & Kamal-Eldin A., 2017. Date fruit (Phoenix dactylifera L.): an underutilized food seeking industrial valorization. NFS J., 6, 1-10, doi.org/10.1016/j.nfs.2016.12.001
Gitau C.W. et al., 2009. Insect pests and insect-vectored diseases of palms. Aust. J. Entomol., 48, 328-342, doi.org/10.1111/j.1440-6055.2009.00724.x
González G.R. et al., 2011. Metodología para muestrear las fases inmaduras del defoliador Opsiphanes cassina Felder (1862) (Lepidoptera: Nymphalidae) en palma aceitera. Rev. Cient. UDO Agric., 11, 104-108.
González G.R. et al., 2012. Aspectos bioecológicos del defoliador de la palma aceitera, Opsiphanes cassina Felder (Lepidoptera: Nymphalidae). Rev. Cient. UDO Agric., 12, 617-626.
Goulart H.F., Lima M.R.F., Morais R.K. & Bernardo V.B., 2015. Feromônios: uma alternativa verde para o manejo integrado de pragas. Rev. Virtual Quim., 7, 1205-1224.
Guo X. et al., 2020. 4-Vinylanisole is an aggregation pheromone in locusts. Nature, 584, 584-588, doi.org/10.1038/s41586-020-2610-4
Hartmann T., 1996. Diversity and variability of plant secondary metabolism: a mechanistic view. Entomol. Exp. Appl., 80, 177-188, doi.org/10.1111/j.1570-7458.1996.tb00914.x
Hoddle M.S. et al., 2009. Synthesis and field evaluation of the sex pheromone of Stenoma catenifer (Lepidoptera: Elachistidae). J. Econ. Entomol., 102, 1460-1467, doi.org/10.1603/029.102.0409
Hoddle M.S. et al., 2011. Field optimization of the sex pheromone of Stenoma catenifer (Lepidoptera: Elachistidae): evaluation of lure types, trap height, male flight distances, and number of traps needed per avocado orchard for detection. Bull. Entomol. Res., 101, 145-152, doi.org/10.1017/s0007485310000301
Howard F.W., Moore D., Giblin-Davis R.M. & Abad R.G., 2001. Insects on palms. New York, NY, USA: CABI Publishing, 33-108.
Islam M.A. et al., 2009. Instrumental analysis of terminal-conjugated dienes for reexamination of the sex pheromone secreted by a nettle moth, Parasa lepida lepida. Biosci. Biotechnol. Biochem., 73, 1156-1162, doi.org/10.1271/bbb.90047
Jang E.B., Siderhurst M.S., Conant P. & Siderhurst L.A., 2009. Phenology and population radiation of the nettle caterpillar, Darna pallivitta (Moore) (Lepidoptera: Limacodidae) in Hawai’i. Chemoecology, 19, 7-12, doi.org/10.1007/s00049-009-0002-1
Jurenka R.A., 2021. Lepidoptera: female sex pheromone biosynthesis and its hormonal regulation. In: Blomquist G.J. & Vogt R.G., eds. Insect pheromone biochemistry and molecular biology. Academic Press, 13-88.
Kamarudin N., Ahmad S.N., Arshad O. & Wahid M.B., 2010. Pheromone mass trapping of bagworm moths, Metisa plana Walker (Lepidoptera: Psychidae), for its control in mature oil palms in Perak, Malaysia. J. Asia-Pac. Entomol., 13, 101-106, doi.org/10.1016/j.aspen.2009.11.003
Kawazu K., Ichiki R.T., Dang D.T. & Nakamura S., 2011. Mating sequence and evidence for the existence of a female contact sex pheromone in Brontispa longissima (Coleoptera: Chrysomelidae). Jpn. Agric. Res. Q., 45, 99-106, doi.org/10.6090/jarq.45.99
Kumara A.D.N.T., Chakravarthy A.K. & Subaharan K., 2015. Role of kairomones in ovipositional preference of coconut black-headed caterpillar Opisina arenosella Walker (Lepidoptera: Oecophoridae). Mysore J. Agric. Sci., 49, 314-317.
Lemos W.P. & Boari A.J., 2010. Manejo de pragas e doenças para a cultura de palma de óleo na Amazônia. In: Freitas P.L. & Teixeira W.G., eds. Zoneamento agroecológico, produção e manejo para a cultura da palma de óleo na Amazônia. Rio de Janeiro, Brazil: Embrapa Solos, 145-152.
Mahamat H., Hassanali A. & Odongo H., 2000. The role of different components of the pheromone emission of mature males of the desert locust, Schistocerca gregaria (Forskål) (Orthoptera: Acrididae) in accelerating maturation of immature adults. Int. J. Trop. Insect Sci., 20, 1-5, doi.org/10.1017/s174275840001777x
Manca S.J.T., Defagó M.T. & Salvo A., 2014. Insectos fitófagos asociados a palmeras en la ciudad de Córdoba, Argentina. Rev. Soc. Entomol., 73, 145-154.
Martínez L.C., Torre J.A.A., Calvache H.H. & Vilianueva A., 2000. Biología de Leucothyreus sp. (Coleoptera: Scarabaeidae) defoliador de palma de aceite (Elaeis guineensis Jacq.), en San Vicente de Chucurí (Santander). Palmas, 21, 212-220.
Martínez L.C., Hurtado R.E., Torres L.A. & Romero V.R., 2009. Avances de la campaña regional para el manejo de la información de insectos defoliadores en la zona central. Palmas, 30, 51-61.
Martínez L.C., Plata-Rueda A., Zanuncio J.C. & Serrao J.E., 2013. Leucothyreus femoratus (Coleoptera: Scarabaeidae): feeding and behavioral activities as an oil palm defoliator. Fla. Entomol., 96, 55-63, doi.org/10.1653/024.096.0107
Martínez L.C., Plata-Rueda A., Serrao J.E. & Zanuncio J.C., 2014. Life history traits and damage potential of an invasive pest Acharia fusca (Lepidoptera: Limacodidae) on oil palm. Ann. Entomol. Soc. Am., 107, 1086-1093, doi.org/10.1603/an13102
Mathew M. et al., 2021. Outbreak of spotted coffee locust, Aularches miliaris Linnaeus (Orthoptera: Pyrgomorphidae) in Kerala. Pest Manage. Hortic. Ecosyst., 27, 98-102, doi.org/10.5958/0974-4541.2021.00019.9
Mexzón R.G., 2011. Brassolis isthmia (Lepidoptera: Nymphalidae) in peach palm and coconut palm in Costa Rica. Agron. Mesoam., 22, 149-155, doi.org/10.15517/am.v22i1.11834
Millar J.G. et al., 2008. (9Z)-9, 13-Tetradecadien-11-ynal, the sex pheromone of the avocado seed moth, Stenoma catenifer. Tetrahedron Lett., 49, 4820-4823, doi.org/10.1016/j.tetlet.2008.06.019
Mohan C., Nair C.R., Nampoothiri C.K. & Rajan P., 2010. Leaf-eating caterpillar (Opisina arenosella)-induced yield loss in coconut palm. Int. J. Trop. Insect Sci., 30, 132-137, doi.org/10.1017/s174275841000024x
Moreira M.A.B., Zarbin P.H.G. & Coracini M.D.A., 2005. Feromônios associados aos coleópteros-praga de produtos armazenados. Quim. Nova, 28, 472-477, doi.org/10.1590/s0100-40422005000300019
Muniyappa C. et al., 2018. Factors affecting catch of the black-headed caterpillar, Opisina arenosella Walker in sex pheromone-baited traps and evidence for population suppression by mass trapping. Orient. Insects, 52, 143-158, doi.org/10.1080/00305316.2017.1381652
Nakano M., Morgan-Richards M., Trewick S.A. & Clavijo-McCormick A., 2022. Chemical ecology and olfaction in short-horned grasshoppers (Orthoptera: Acrididae). J. Chem. Ecol., 48, 121-140, doi.org/10.1007/s10886-021-01333-3
Niassy A. et al., 1999. Intra-and interspecific aggregation responses of Locusta migratoria migratorioides and Schistocerca gregaria and a comparison of their pheromone emissions. J. Chem. Ecol., 25, 1029-1042.
Njagi P.G.N. & Torto B., 2002. Evidence for a compound in Comstock-Kellog glands modulating premating behavior in male desert locust, Schistocerca gregaria. J. Chem. Ecol., 28, 1065-1074.
Nordlund D.A. & Lewis W.J., 1976. Terminology of chemical releasing stimuli in intraspecific and interspecific interactions. J. Chem. Ecol., 2, 211-220.
Ochieng S.A. & Hansson B.S., 1999. Responses of olfactory receptor neurones to behaviourally important odours in the gregarious and solitarious desert locust, Schistocerca gregaria. Physiol. Entomol., 24, 28-36, doi.org/10.1046/j.1365-3032.1999.00107.x
Oliveira T.A. et al., 2018a. Validation of the sampling methodology for Opsiphanes invirae caterpillars on oil palm plantations in the Brazilian Amazon. Ciênc. Rural, 48, 1-4, doi.org/10.1590/0103-8478cr20170742
Oliveira T.A., Lemos W.D.P., Tinôco R.S. & Martins I.C.F., 2018b. Lepidópteros desfolhadores de palma-de-óleo no Estado do Pará. Belém, Brazil: Embrapa Amazônia Oriental, 13-48.
Reis A.C. et al., 2023. Lepidopteran pheromones as potential tools for pest insect control in the Arecaceae family. Res. Soc. Dev., 12, e1612239362, doi.org/10.33448/rsd-v12i2.39362
Rhainds M., 2018. Natural mating disruption in a protogynous bagworm (Lepidoptera: Psychidae). Ecol. Entomol., 43, 543-546, doi.org/10.1111/een.12526
Rhainds M. et al., 1994. Chiral esters: sex pheromone of the bagworm, Oiketicus kirbyi (Lepidoptera: Psychidae). J. Chem. Ecol., 20, 3083-3096, doi.org/10.1007/bf02033712
Rodrigues M.R.L. et al., 2006. Avaliação do estado nutricional do dendezeiro: análise foliar. Manaus, Brazil: Embrapa, 1-12.
Sasaerila Y. et al., 1997. Identification of sex pheromone components of nettle caterpillar, Setothosea asigna. J. Chem. Ecol., 23, 2187-2196, doi.org/10.1023/b:joec.0000006438.03728.1a
Sasaerila Y. et al., 2000a. Decadienoates: sex pheromone components of nettle caterpillars Darna trima and D. bradleyi. J. Chem. Ecol., 26, 1969-1981.
Sasaerila Y. et al., 2000b. Sex pheromone components of nettle caterpillar, Setora nitens. J. Chem. Ecol., 26, 1983-1990.
Sasaerila Y., Gries G., Gries R. & Chor Boo T., 2000c. Specificity of communication channels in four limacodid moths: Darna bradleyi, Darna trima, Setothosea asigna, and Setora nitens (Lepidoptera: Limacodidae). Chemoecology, 10, 193-199, doi.org/10.1007/pl00001822
Sawada H. et al., 2008. Population dynamics of an invasive grub moth, Parasa lepida (Cramer) that damages ornamental trees: the seasonal and annual fluctuations of the cocoon density. Jpn. J. Appl. Entomol. Zool., 19, 115-124.
Schulz S., Steffensky M. & Roisin Y., 1996. Identification and synthesis of elymniafuran, a new monoterpene from the butterfly Elymnias thryallis. Liebigs Ann., 1996, 941-946, doi.org/10.1002/jlac.199619960612
Seidelmann K. & Ferenz H.J., 2022. Courtship inhibition pheromone in desert locusts, Schistocerca gregaria. J. Insect Physiol., 48, 991-996, doi.org/10.1016/s0022-1910(02)00178-6
Seidelmann K. & Stahr C., 2023. Gregarious mature male-specific volatiles and the semivolatile cuticular hydrocarbon fraction of the South American locust, Schistocerca cancellata. Chemoecology, 33, 63-70, doi.org/10.1007/s00049-023-00385-z
Shameer K.S., McCormick A.C., Subaharan K. & Nasser M., 2017. Volatile organic compounds in healthy and Opisina arenosella Walker (Lepidoptera: Oecophoridae) infested leaves of coconut palms. Entomon, 42, 121-132.
Siderhurst M.S., Jang E.B., Hara A.H. & Conant P., 2007. n‐Butyl (E)‐7, 9‐decadienoate: sex pheromone component of the nettle caterpillar, Darna pallivitta. Entomol. Exp. Appl., 125, 63-69, doi.org/10.1111/j.1570-7458.2007.00593.x
Siderhurst M.S. et al., 2015. Disruption of Darna pallivitta (Lepidoptera: Limacodidae) by conventional and mobile pheromone deployment. J. Insect Sci., 15, 67, doi.org/10.1093/jisesa/iev052
Silva K.B. et al., 2019. Morphology and distribution of antennal sensilla of Automeris liberia (Lepidoptera: Saturniidae). Micron, 123, 102682, doi.org/10.1016/j.micron.2019.102682
Skendžić S. et al., 2021. The impact of climate change on agricultural insect pests. Insects, 12, 440, doi.org/10.3390/insects12050440
Souza F.G. & Lima R.A.A., 2019. Importância da família Arecaceae para a região Norte. Educamazônia, 23, 100-110.
Stahr C., Svatoš A. & Seidelmann K., 2013. Chemical identification, emission pattern and function of male-specific pheromones released by a rarely swarming locust, Schistocerca americana. J. Chem. Ecol., 39, 15-27, doi.org/10.1007/s10886-012-0233-4
Stahr C. & Seidelmann K., 2016. Individual pheromone signature in males: a prerequisite for pheromone-mediated mate assessment in the central American locust, Schistocerca piceifrons. J. Chem. Ecol., 42, 1304-1313, doi.org/10.1007/s10886-016-0793-9
Torto B. et al., 1994. Aggregation pheromone system of adult gregarious desert locust Schistocerca gregaria (Forskal). J. Chem. Ecol., 20, 1749-1762, doi.org/10.1007/bf02059896
Torto B., Njagi P.G., Hassanali A. & Amiani H., 1996. Aggregation pheromone system of the nymphal gregarious desert locust, Schistocerca gregaria (Forskål). J. Chem. Ecol., 22, 2273-2281, doi.org/10.1007/bf02029546
Vázquez M.A., Cruz-López L., Gómez J. & Castillo A., 2017. Annual capture of two elachistidae moth species using Stenoma catenifer sex pheromone in criollo avocado (Persea americana) at Chiapas, Mexico. Southwest. Entomol., 42, 91-101, doi.org/10.3958/059.042.0106
Velázquez-Martínez G.C. et al., 2022. Population dynamics of Stenoma catenifer Walsingham (Lepidoptera: Depressariidae) on Hass avocado orchards in México. J. Asia-Pac. Entomol., 25, 101866, doi.org/10.1016/j.aspen.2021.101866
Velázquez-Martínez G.C. et al., 2023. Captures of Stenoma catenifer (Lepidoptera: Depressariidae) are influenced by pheromone trap density in Hass avocado orchards. Fla. Entomol., 105, 267-274, doi.org/10.1653/024.105.0401
Wakamura S. et al., 2007. Sex pheromone of the blue-striped nettle grub moth Parasa lepida (Cramer) (Lepidoptera: Limacodidae): identification and field attraction. Appl. Entomol. Zool., 42, 347-352, doi.org/10.1303/aez.2007.347
Wei J. et al., 2017. Composition and emission dynamics of migratory locust volatiles in response to changes in developmental stages and population density. Insect Sci., 24, 60-72, doi.org/10.1111/1744-7917.12396
Wei J. et al., 2019. Phenylacetonitrile in locusts facilitates an antipredator defence by acting as an olfactory aposematic signal and cyanide precursor. Sci. Adv., 5, eaav5495, doi.org/10.1126/sciadv.aav5495
Yasin M. & Rehman A., 2021. Impact of climate change on pests and disease incidence on agricultural crops: a global prospective. Academia Lett., 2, doi.org/10.20935/al3667
Zagatti P. et al., 1996. Sex pheromone of Stenoma cecropia Meyrick (Lepidoptera: Elachistidae). J. Chem. Ecol., 22, 1103-1121, doi.org/10.1007/bf02027948
Zamboni A.B., Thommazo A.D., Hernandes E.C.M. & Fabbri S.C.P.F., 2010. StArt uma ferramenta computacional de apoio à revisão sistemática. In: Conferência Brasileira de Software: Teoria e Prática, 27 September-1 October 2010, Universidade Federal da Bahia, Salvadore, Brazil, 91-96.
Zambrana N.Y.P. et al., 2007. Diversity of palm uses in the western Amazon. Biodivers. Conserv., 16, 2771-2787, doi.org/10.1007/s10531-007-9218-y






