- Home
- Volume 30 (2026)
- Numéro 1
- Comparative germination and recovery capacity responses of Pergularia tomentosa and Acokanthera oblongifolia under salinity stress: implications for revegetation of arid lands
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Comparative germination and recovery capacity responses of Pergularia tomentosa and Acokanthera oblongifolia under salinity stress: implications for revegetation of arid lands

Attached document(s)
original pdf fileRésumé
Réponses comparatives de germination et de capacité de récupération chez Pergularia tomentosa et Acokanthera oblongifolia sous stress salin : implications pour la revégétalisation des terres arides
Description du sujet. La réhabilitation des terres dégradées est impérative, en particulier dans les régions arides et semi-arides où des défis environnementaux tels que la salinité perturbent la stabilité des écosystèmes. La revégétalisation à l'aide de plantes médicinales et aromatiques constitue une solution économique et écologiquement durable.
Objectifs. Cette étude examine pour la première fois le potentiel de germination et de récupération de Pergularia tomentosa et Acokanthera oblongifolia (Apocynaceae) sous différentes conditions de salinité.
Méthode. Des graines collectées dans la région aride d'Algérie ont été exposées à huit niveaux de salinité allant de 0 à 800 mM NaCl pendant 20 jours. Onze paramètres essentiels liés à la cinétique de germination, la vitesse, la performance, l'émergence des plantules, la vigueur et la récupération de la germination ont été évalués.
Résultats. L’analyse ANOVA à deux facteurs a révélé que la salinité, l’espèce et leur interaction avaient des effets significatifs (p < 0,0001) sur la majorité des traits de germination. Le stress salin a réduit la germination, P. tomentosa montrant une tolérance plus élevée par rapport à A. oblongifolia. Aucune germination n’a été observée sous 300, 400 et 800 mM NaCl. Les deux espèces ont montré une récupération significative (80-90 %), en particulier à des niveaux de salinité modérés (300-400 mM NaCl), suggérant des mécanismes adaptatifs. Malgré une récupération réduite à 800 mM NaCl, P. tomentosa et A. oblongifolia ont maintenu une certaine résilience avec des taux de récupération de 65,5 % et 68,5 %, respectivement.
Conclusions. Ces résultats mettent en évidence le potentiel de ces espèces pour la restauration des terres affectées par la salinité et leur utilisation durable en tant que plantes médicinales. Cette recherche apporte des informations précieuses sur la performance germinative de P. tomentosa et A. oblongifolia sous stress salin, ouvrant ainsi des perspectives prometteuses pour la restauration écologique.
Abstract
Description of the subject. The rehabilitation of degraded lands is imperative, particularly in arid and semi-arid regions where environmental challenges such as salinity disturb ecosystem stability. Revegetation using medicinal and aromatic plants offers an economical and environmentally sustainable solution.
Objectives. This study examines for the first time the germination and recovery potential of Pergularia tomentosa and Acokanthera oblongifolia (Apocynaceae) under varying saline conditions.
Method. Seeds collected from the arid region of Algeria were exposed to eight salinity levels ranging from 0 to 800 mM NaCl for 20 days. Eleven essential germination traits associated with germination kinetics, speed, performance, seedling emergence and vigor, and germination recovery were evaluated.
Results. The two-way ANOVA analysis revealed that salinity, species and their interaction had significant effects (p < 0.0001) on most germination traits. Salinity stress reduced germination, with P. tomentosa exhibiting greater tolerance compared to A. oblongifolia. No germination occurred under 300, 400 and 800 mM NaCl. Both species showed high significant recovery (80-90%), particularly at moderate salinity levels (300-400 mM NaCl), indicating adaptive mechanisms. Despite reduced recovery at 800 mM NaCl, P. tomentosa and A. oblongifolia maintained resilience, with recovery rates of 65.5% and 68.5%, respectively.
Conclusions. These findings highlight the potential of these species for land restoration in saline-affected areas and for sustainable medicinal plant use. This research provides valuable insights into the germination performance of P. tomentosa and A. oblongifolia under salinity stress, offering promising applications in ecological restoration.
Table of content
Received 25 February 2025, accepted 8 October 2025, available online 18 November 2025.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1The restoration of degraded lands has become a major global focus due to the continuous expansion of affected areas. Traditional rehabilitation methods remain effective but often require substantial financial and logistical resources, limiting large-scale application. An alternative approach involves the use of medicinal and aromatic plant species, which offers a cost-effective and environmentally sustainable solution (Gupta et al., 2024). This method enhances soil stability, supports biodiversity, and provides economic advantages through the production of valuable plant resources (García-Caparrós et al., 2021). As a result, incorporating these species into land rehabilitation efforts presents a practical strategy for combating land degradation (Munir et al., 2022). Salinity is a major environmental stress factor that significantly affects seed germination, plant establishment, and ecosystem dynamics, particularly in arid and semi-arid regions (El Sabagh et al., 2020; Tessema et al., 2022). High salinity levels impede seed germination through mechanisms such as osmotic stress, ion toxicity, and metabolic imbalances, which reduce plant recruitment and limit species distribution (Balasubramaniam et al., 2023). However, certain plant species have developed unique adaptive traits that enable them to tolerate and flourish in saline environments, ensuring their survival and ecological success under such conditions (Mann et al., 2023; Mircea et al., 2024).
2Pergularia tomentosa L. and Acokanthera oblongifolia Benth. & Hook.f., both members of the Apocynaceae family, are perennial species native to Africa and the Arabian Peninsula, where they have adapted to arid and semi-arid environments (Dobignard & Chatelain, 2010). Pergularia tomentosa is native to North Africa, particularly found in countries such as Algeria, Tunisia, Egypt, and extending into the Middle East, including the Arabian Peninsula. It thrives in sandy and rocky substrates, reaching up to 1 m in height (Hosseini et al., 2018). Acokanthera oblongifolia, on the other hand, is native to eastern and southern Africa, with its distribution spanning from Ethiopia and Kenya to South Africa. This woody shrub, which can grow up to 2-3 m, is well adapted to saline and nutrient-poor environments (Nasr, 2009; Khamis et al., 2024). In addition to their ecological importance, these species hold significant ethnobotanical and medicinal value in their native regions. Pergularia tomentosa has been traditionally employed for its anti-inflammatory, antimicrobial and wound-healing properties (Farzadinia et al., 2019; Segueni et al., 2023). Its leaves and stems are often prepared as decoctions to address respiratory disorders, skin infections, and digestive ailments (Aldayel, 2024). Similarly, A. oblongifolia is recognized for its high concentration of cardiac glycosides, which have been historically employed to treat heart conditions and as a component of traditional arrow poisons (De Villiers, 1962; Kupicha, 1982; Pecio et al., 2019). Beyond their medicinal value, P. tomentosa and A. oblongifolia hold economic potential as sources of bioactive compounds for pharmaceutical and herbal industries. Cultivating these species in saline-prone regions can provide a sustainable supply of medicinal raw materials while contributing to land restoration and ecosystem stability. The study of their ability to germinate and recover from extreme stress highlights their potential use in revegetation programs, agroforestry, and ecological restoration projects in arid and semi-arid environments.
3Seed germination represents a vital life stage that controls plant establishment and population dynamics, particularly in challenging environments (Reed et al., 2022). In saline habitats, the success of germination depends on intricate interactions between soil moisture and salinity levels (Singh, 2022). Elevated salinity can delay germination, reduce germination rates, and hinder seedling development; however, certain species demonstrate notable recovery once favorable conditions return (Atta et al., 2023). The capacity of P. tomentosa and A. oblongifolia to recover germination after extended exposure to salinity implies the existence of adaptive physiological and biochemical mechanisms, which merit further exploration. The reproductive strategies of these species further enhance their resilience. A thorough understanding of the reproductive and germination strategies is essential for evaluating their ecological adaptability and potential utility in conservation and land restoration initiatives.
4This study investigates the germination behavior of P. tomentosa and A. oblongifolia under varying salinity conditions. Specifically, the research aims to assess the effects of different salinity levels on seed germination percentage, rate and recovery. By combining ecological and pharmacological perspectives, the results may offer critical insights for conservation efforts, the restoration of saline-degraded lands and the sustainable utilization of their medicinal properties.
2. Materials and methods
2.1. Seed collection site
5Seeds of P. tomentosa and A. oblongifolia were collected from their natural habitats in Zeribet El Oued, an arid region of Biskra (SE-Algeria) (Figure 1). Both species were obtained from plants growing in sandy and rocky soils (34°35'N, 6°42'E, 35.7 m elevation). This area has a desert climate characterized by hot, dry summers and mild winters. Summer temperatures often exceed 40 °C, while winters remain relatively mild, with nighttime temperatures dropping to around 5 °C. Rainfall is scarce throughout the year, contributing to arid conditions. The region's soil is predominantly sandy and rocky, with low organic matter and poor water retention, making agriculture challenging. However, the presence of oases and irrigation systems allows for the cultivation of date palms, which thrive in the area's saline and well-drained soils.

Figure 1. Pergularia tomentosa and Acokanthera oblongifolia: leaves, flowers, fruits and seeds – Pergularia tomentosa et Acokanthera oblongifolia : feuilles, fleurs, fruits et graines.
6Soil analysis for the seed collection site (at a depth of 30-40 cm) where the plants of both species were harvested for their seeds, revealed a pH of 7.8, a nitrogen (NO3-) content of 0.128%, a phosphorus (P2O5) concentration of 179.1 mg·kg-1, and a potassium level of 1.34 meq·kg-1.
7The electrical conductivity of 2.67 ms·cm-1 indicates high salinity due to the elevated calcium ion concentration (21.1 meq·100 g-1) compared to magnesium (1.44 meq·100 g-1), potassium (1.23 meq·100 g-1), and sodium (1.98 meq·100 g-1). With a total CaCO3 content of 54.9%, the soil classification is highly calcareous. It has a silty soil mixed with sand in an arid area near the Algerian desert. This combination often characterizes soils in arid regions where silt and sand are prevalent due to weathering and erosion processes.
8Pergularia tomentosa is native to the region and is well-adapted to the harsh climatic conditions of the area, thriving in arid and semi-arid environments. In contrast, A. oblongifolia is native to South Africa and has been the subject of small-scale revegetation efforts in the region since 2019. The seeds of both species were collected in June 2023 and were stored in dry and cool conditions until the germination experiments started. Seed morphological characteristics of both species used in this study are presented in Table 1. The 1,000-seed weight was 10.45 ± 0.56 g for P. tomentosa and 865.25 ± 28.88 g for A. oblongifolia, according to ISTA (2022).
|
Table 1. Seed characteristics of Pergularia tomentosa and Acokanthera oblongifolia (n = 100) – Caractéristiques des graines de Pergularia tomentosa et Acokanthera oblongifolia (n = 100). |
||
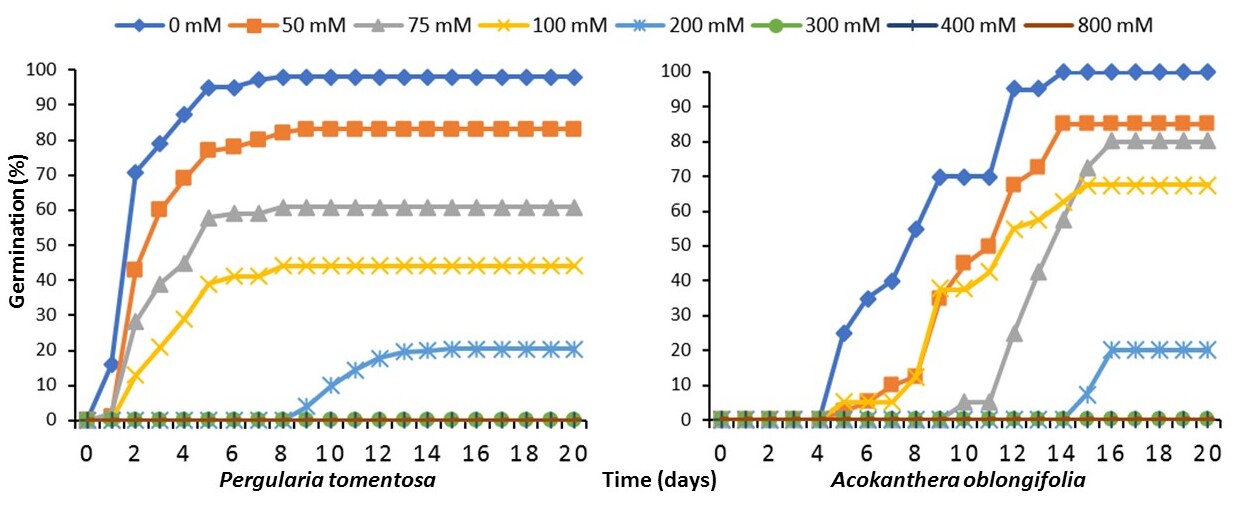
|
Characteristics |
Pergularia tomentosa |
Acokanthera oblongifolia |
|
Length (cm) |
0.76 ± 0.05 |
1.17 ± 0.15 |
|
Width (cm) |
0.56 ± 0.06 |
1.02 ± 0.13 |
|
Thickness (cm) |
0.17 ± 0.01 |
0.58 ± 0.07 |
2.2. Germination experiments
9Seeds of both species (2 species × 8 NaCl concentrations × 4 replicates × 50 seeds) were surface sterilized in a 0.5% sodium hypochlorite solution for 1 min to prevent fungal contamination, followed by thorough rinsing with distilled water and air-drying.
10Germination tests were conducted in plastic containers (25.5 cm L × 16.5 cm W × 7.5 cm H) with two layers of moist filter paper at a temperature of 25 °C (± 2 °C) and under complete darkness to simulate natural soil conditions for 20 days. Each container was moistened with an adequate volume of the test solution, which included distilled water (Control, 0 mM NaCl) and saline solutions of 50, 75, 100, 200, 300, 400 and 800 mM NaCl.
11The range of salinity treatments applied in this study was selected to reflect both ecological relevance and comparative germination thresholds. Ecologically, the target revegetation zone is located near Chott El Melghir (Biskra, NE-Algeria; 34°25′48″N, 6°22′07″E) where soil salinity is extremely high, often exceeding 1,200 mM NaCl in certain depressions. However, in the surrounding zones where vegetation can successfully establish, salinity exhibits greater variability, generally ranging from 50 to 400 mM according to soil depth, distance from saline crusts, and seasonal changes. Thus, the selected concentrations reproduce this natural variability while also simulating moderate to severe salt stress. In parallel, the inclusion of lower concentrations (50-100 mM) allowed assessment of tolerance limits and potential stimulation of germination under mild stress, whereas the higher treatments (200-800 mM) were used to test species-specific thresholds for inhibition, delayed germination, and recovery capacity. The extreme treatment of 800 mM was further included to approximate the upper tolerance margins under laboratory conditions, providing insights relevant for future restoration planning in highly saline soils.
12A completely randomized design was employed for the germination tests, with four replicates of 50 seeds per treatment. Germination was monitored over a 20-day period, with germinated seeds counted and removed every day. Germination was defined as the emergence of radicle from the seed coat. To maintain consistent salt concentrations, small amounts of sterile saline solution (same concentration) was added to maintain consistent levels. This ensured that the salinity levels remained near the target concentrations throughout the experiment.
2.3. Germination parameters
13Based on the results of the quantitative evaluation of seed germination attributes, the following formulas have been used to calculate:
14Final germination percentage (FGP):
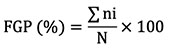
15where ni is the number of germinated seeds on the last day of the test, and N is the total number of seeds incubated per test (Côme, 1970).
16Mean Germination Time (MGT):
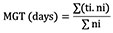
17where ti is the number of days since the beginning of the test, ni is the number of germinated seeds recorded at time t(i), and Σni is the total number of germinated seeds (Orchard, 1977).
18Time to 50% germination (T50):
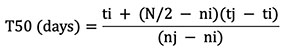
19where N is the final number of seeds emerged ; tᵢ = the earlier time point at which the cumulative count (or percentage) is nᵢ, which is just below N/2 ; tⱼ = the later time point at which the cumulative count (or percentage) is nⱼ, which is just above N/2 ; nj and ni are the cumulative numbers of seeds emerged after adjacent counts during tj and ti, when ni < N/2 > nj (Coolbear et al., 1984).
20Coefficient of the velocity of germination (CVG):
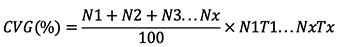
21where N is the frequency of seeds germinating every day and T represents the time from sowing to germination of seed N (Khan et al., 2019).
22Germination index (GI):
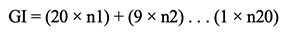
23where n1, n2... n20 is the number of germinated seeds on the first, second and subsequent days until the 20th day and the multipliers (20, 19… etc.) are weights given to the days of the germination (Dastanpoor et al., 2013).
24Mean daily germination (MDG):
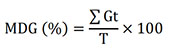
25where Gt is the number of seeds germinated on day t. T is the total number of days in the germination period (Maguire,1962).
26Peak value for germination (PVG):
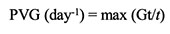
27where Gt is the cumulative number of the maximum of seeds germinated at time t; t is the number of days since incubation began (Bonner, 1986).
28Seedling length (SL): The length of four seedlings from each container/treatment was measured in cm.
29Seedling vigor index (SVI):
30The SVI was calculated in the following formula by Abdul Baki & Anderson (1973):
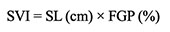
31where SL is the total seedling length and FGP represents the final germination percentage.
32Germination recovery percentage (GRP):
33After 20 days of every salt exposure, seeds that failed to germinate at 300, 400 and 800 mM NaCl were washed with distilled water and then placed in new containers with new filter paper moistened with distilled water, and then monitored for 10 days under the same conditions of temperature for their germination recovery aptitude. The germination recovery percentage was calculated for each treatment as follows:
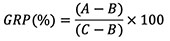
34where A is the number of seeds germinated over the entire experiment, B is the number of seeds germinated in saline solution, and C is the total number of seeds used for processing (Khan & Ungar, 1997).
2.4. Statistical analysis
35A two-way analysis of variance (ANOVA) was performed using SAS software Version 9.0 (SAS Institute Inc., 2002) to test the effects of salinity, species, and their interaction on germination characteristics. Post-hoc comparisons were conducted using Tukey’s Honestly Significant Difference (HSD) test to identify significant differences between treatment means.
3. Results
36The two-way ANOVA analysis revealed that salinity (S), species (SP) and their interaction (S × SP) had significant effects on most germination parameters of P. tomentosa and A. oblongifolia (Table 2). Salinity had a highly significant effect (p < 0.0001) on all germination traits, including final germination percentage (FGP), mean germination time (MGT), coefficient of velocity of germination (CVG), germination index (GI), time to 50% germination (T50), mean daily germination (MDG), peak value for germination (PVG), seedling length (SL), and seedling vigor index (SVI). This highlights the strong impact of increasing NaCl concentrations on seed germination and early seedling growth.
37Species differences were also highly significant for all parameters except for germination recovery percentage (GRP), which showed no significant variation (p > 0.05) between species. This suggests that both species exhibited similar recovery patterns after exposure to high salinity. However, significant species effects were observed for MGT, GI, and T50, indicating that A. oblongifolia exhibited longer germination times compared to P. tomentosa (Table 2).
38The interaction effect (S × SP) was significant for most parameters, including FGP, MGT, CVG, GI, T50, MDG, PVG, SL, and SVI, demonstrating that the response to salinity varied between species. This suggests species-specific tolerance mechanisms under salt stress.
|
Table 2. A two-way ANOVA of the effects of salinity (S), species (SP), and their interaction on germination traits of Pergularia tomentosa and Acokanthera oblongifolia – Analyse ANOVA à deux facteurs des effets de la salinité (S), de l’espèce (SP) et de leur interaction sur les traits de germination de Pergularia tomentosa et Acokanthera oblongifolia. |
||||
|
Dependent variable |
Abbreviation |
S |
SP |
S × SP |
|
Final germination percentage |
FGP |
163.82* |
19.09* |
5.62* |
|
Mean germination time |
MGT |
147.44* |
975.00* |
15.55* |
|
Coefficient of velocity of germination |
CVG |
36.72* |
362.06* |
16.93* |
|
Germination index |
GI |
168.95* |
367.50* |
46.45* |
|
Time to 50% germination |
T50 |
93.40* |
666.13* |
11.93* |
|
Mean daily germination |
MDG |
163.82* |
19.09* |
5.62* |
|
Peak value for germination |
PVG |
77.70* |
143.90* |
24.19* |
|
Seedling length |
SL |
102.34* |
126.58* |
30.18* |
|
Seedling vigor index |
SVI |
341.55* |
10.53* |
23.19* |
|
Germination recovery percentage |
GRP |
40.37* |
0.09ns |
0.42ns |
|
Data represent F-values (significant at * : p < 0.0001 or ns: not significant) – Les données représentent les valeurs de F (significatives à * : p < 0,0001 ou ns : non significatives). |
||||
3.1. Effects on the kinetic of germination
39The germination kinetics of P. tomentosa and A. oblongifolia followed a three-phase pattern consisting of (i) imbibition, (ii) exponential germination, and (iii) a plateau phase, all of which were significantly affected by increasing NaCl concentrations (Figure 2).
40Under non-saline conditions (0 mM NaCl), both species exhibited a rapid water uptake phase (imbibition) within the first 24 h, followed by a steep exponential increase in germination. Pergularia tomentosa reached nearly 100% germination within 6 days, whereas A. oblongifolia took a longer period (10 days) to reach full germination. The plateau phase was observed after maximum germination was reached, indicating the completion of the germination process.
41Figure 2. Cumulative germination percentage of Pergularia tomentosa and Acokanthera oblongifolia seeds during 20 days as influenced by NaCl concentrations (0-800 mM). Seed germination was completely inhibited under 300, 400 and 800 mM NaCl – Pourcentage cumulé de germination des graines de Pergularia tomentosa et Acokanthera oblongifolia sur une période de 20 jours sous l'influence de différentes concentrations de NaCl (0-800 mM). La germination des graines a été complètement inhibée à 300, 400 et 800 mM NaCl.
42At moderate salinity levels (50-100 mM NaCl), all three phases were delayed. The imbibition phase appeared prolonged, suggesting osmotic stress effects on water uptake. The exponential phase was slower compared to control conditions, with P. tomentosa reaching 83% germination at 50 mM and 61% at 75 mM, while A. oblongifolia showed 85% and 80%, respectively. However, at 100 mM NaCl, germination of P. tomentosa was significantly reduced (44%), while A. oblongifolia maintained a higher germination rate (67.5%). The plateau phase occurred later, confirming the inhibitory effects of salinity on the speed of germination.
43At 200 mM NaCl, germination was severely inhibited and the imbibition phase was noticeably extended. The exponential germination stage was weak and delayed beyond 10 days before germination occurred, with P. tomentosa and A. oblongifolia reaching only 20.5% and 20%, respectively. At 300, 400, and 800 mM NaCl, germination was completely inhibited, preventing any transition from imbibition to the exponential and plateau phases (Figure 2).
44Pergularia tomentosa displayed a faster germination response at lower salinity, while A. oblongifolia maintained slightly better germination at higher NaCl concentrations (100 mM). However, both species experienced significant delays in the imbibition and exponential phases and a reduction in the final plateau level under increased salinity, confirming their sensitivity to extreme salt stress (Figure 2).
3.2. Effects on the final germination percentage
45Salinity significantly reduced the final germination percentage in both species, with a progressive decline as NaCl concentration increased (p < 0.0001). Under control conditions, A. oblongifolia showed complete germination (100%), while P. tomentosa had a slightly lower germination rate (98%). Acokanthera oblongifolia maintained higher germination at moderate stress (≈ 67% at 100 mM NaCl) compared to P. tomentosa (≈ 44%), indicating greater tolerance at this level. However, at severe stress (200 mM), germination dropped to around 20% in both species and was completely inhibited at 300 mM NaCl and above (Tables 3 and 4).
|
Table 3. Germination traits of Pergularia tomentosa seeds in 0, 50, 75, 100, 200, 300, 400 and 800 mM NaCl – Traits de germination des graines de Pergularia tomentosa sous 0, 50, 75, 100, 200, 300, 400 et 800 mM NaCl. |
|||||||||
|
NaCl (mM) |
FGP (%) |
MGT (days) |
CVG (%) |
GI |
T50 (days) |
MDG (%) |
PVG (day-1) |
SL (cm) |
SVI |
|
0 |
98a |
3.48c |
29.20a |
15.81a |
2.59c |
4.66a |
23.66a |
9.65a |
946.51a |
|
50 |
83b |
4.07bc |
24.86ab |
11.29b |
3.19c |
3.95b |
15.08b |
9.22a |
767.69b |
|
75 |
61c |
4.25bc |
23.62ab |
8.07c |
3.27bc |
2.90c |
10.83bc |
9.08a |
554.73c |
|
100 |
44d |
4.79b |
21.27b |
5.10d |
4.20b |
2.09d |
6.90c |
6.38b |
281.23d |
|
200 |
20.5e |
11.79a |
8.48c |
0.88e |
11.14a |
0.97e |
1.44d |
6.68b |
137.10e |
|
300 |
0.00f |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
|
400 |
0.00f |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
|
800 |
0.00f |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
|
F-value |
159.79 |
174.92 |
27.28 |
111.92 |
255.91 |
159.79 |
52.56 |
70.80 |
127.03 |
|
p-value |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
|
FGP, MGT, CVG, GI, T50, MDG, PVG, SL, SIV : see table 2 – voir tableau 2 ; Means within a column followed by the same letter are not significantly different at p = 0.05 level (Tukey Test) for the given salinity (n = 4) – Les valeurs dans un colonne suivies par la même lettre ne sont pas significativement différentes à p = 0,05 (test de Tukey) pour la salinité donnée (n = 4). |
|||||||||
|
Table 4. Germination traits of Acokanthera oblongifolia seeds in 0, 50, 75, 100, 200, 300, 400 and 800 mM NaCl – Traits de germination des graines de Acokanthera oblongifolia sous 0, 50, 75, 100, 200, 300, 400 et 800 mM NaCl. |
|||||||||
|
NaCl (mM) |
FGP (%) |
MGT (days) |
CVG (%) |
GI |
T50 (days) |
MDG (%) |
PVG (day-1) |
SL (cm) |
SVI |
|
0 |
100a |
9.45d |
10.73a |
5.82a |
8.76b |
4.76a |
8.01a |
10.62a |
1062.25a |
|
50 |
85ab |
11.43c |
8.94b |
3.89b |
11.24b |
4.04ab |
6.19b |
7.80b |
663.73b |
|
75 |
80b |
14.38b |
6.95c |
2.81c |
14.04a |
3.80b |
4.86b |
5.81c |
464.80c |
|
100 |
67.5b |
11.22cd |
8.79b |
3.18bc |
10.76b |
3.21b |
4.65b |
5.68c |
381.75c |
|
200 |
20c |
16.75a |
5.97c |
0.60d |
16.25a |
0.95c |
1.17c |
5.01c |
101.23d |
|
300 |
0.00d |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
|
400 |
0.00d |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
|
800 |
0.00d |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
|
F-value |
57.38 |
46.35 |
20.67 |
80.04 |
24.43 |
57.38 |
37.90 |
95.16 |
158.99 |
|
p-value |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
|
FGP, MGT, CVG, GI, T50, MDG, PVG, SL, SIV : see table 2 – voir tableau 2 ; Means within a column followed by the same letter are not significantly different at p = 0.05 level (Tukey Test) for the given salinity (n = 4) – Les valeurs dans un colonne suivies par la même lettre ne sont pas significativement différentes à p = 0,05 (test de Tukey) pour la salinité donnée (n = 4). |
|||||||||
3.3. Effects on the mean germination time
46Salinity significantly prolonged mean germination time (MGT) in both species, reflecting delayed seedling emergence under stress. Pergularia tomentosa germinated faster under control conditions (≈ 3.5 days) than A. oblongifolia (≈ 9.5 days), showing an intrinsic advantage in rapid germination. With increasing salinity, both species experienced delays, but A. oblongifolia was more sensitive, reaching over 16 days at 200 mM NaCl compared to less than five days in P. tomentosa. This indicates that although P. tomentosa is generally faster to germinate, both species suffer marked delays as salinity intensifies (Tables 3 and 4).
3.4. Effects on the coefficient of the velocity of germination
47The coefficient of velocity of germination (CVG) decreased progressively with salinity in both species, reflecting slower germination under stress. Under control conditions, P. tomentosa showed a much higher CVG (≈ 29%) than A. oblongifolia (≈ 11%), highlighting its faster germination capacity. With increasing salinity, both species experienced sharp reductions, falling below 10% at 200 mM NaCl. Overall, A. oblongifolia exhibited greater sensitivity to salinity in terms of germination velocity (Tables 3 and 4).
3.5. Effects on the germination index
48The germination index (GI) declined sharply with increasing salinity in both species. Under control conditions, P. tomentosa displayed a higher GI (≈ 16) than A. oblongifolia (≈ 6), indicating stronger seed vigor. At moderate stress (100 mM NaCl), GI values dropped drastically, and at 200 mM they were almost negligible (< 1 in both species), demonstrating the severe inhibitory effect of high salinity on seed performance (Tables 3 and 4).
3.6. Effects on the time to 50% germination
49Salinity significantly prolonged the time to 50% germination (T50) in both species. Under control conditions, P. tomentosa germinated more rapidly (≈ 2.6 days) than A. oblongifolia (≈ 8.8 days). With increasing salinity, both species showed marked delays, reaching over 11 days in P. tomentosa and 16 days in A. oblongifolia at 200 mM NaCl. This indicates that A. oblongifolia is more strongly affected by salinity in terms of germination speed (Tables 3 and 4).
3.7. Effects on the mean daily germination
50Salinity markedly reduced the mean daily germination percentage (MDG) in both species. Under control conditions, values were similar (≈ 4.7% in both) but they declined progressively with increasing NaCl. At moderate stress (100 mM), MDG dropped by more than half, and at 200 mM it fell below 1% in both species. Germination was completely suppressed at 300 mM, confirming the strong inhibitory effect of excessive salinity on daily germination rates (Tables 3 and 4).
3.8. Effects on the peak value of germination
51The peak value of germination (PVG) decreased progressively with salinity in both species. Under control conditions, P. tomentosa exhibited a much higher PVG (≈ 24 day-1) than A. oblongifolia (≈ 8 day-1), indicating a sharper peak in germination activity. With increasing salinity, PVG dropped sharply, falling below 7 day-1 at 100 mM NaCl and reaching nearly negligible levels (< 1.5 day-1) at 200 mM. This confirms the strong inhibitory effect of salinity on the maximum germination rate, with A. oblongifolia showing consistently lower values than P. tomentosa (Tables 3 and 4).
3.9. Effects on the seedling length
52Seedling length decreased significantly with increasing salinity in both species. Under control conditions, seedlings reached around 10 cm but growth declined progressively under stress. At moderate salinity (50 mM), reductions were already visible while at 200 mM lengths, they were almost halved compared to the control (≈ 6.7 cm in P. tomentosa and 5.0 cm in A. oblongifolia). These results indicate substantial inhibition of early seedling growth under high salinity, with A. oblongifolia being more affected (Tables 3 and 4).
3.10. Effects on the seedling vigor index
53The seedling vigor index (SVI) declined progressively with increasing salinity in both species. Under control conditions, values exceeded 900 in P. tomentosa and 1,000 in A. oblongifolia, reflecting strong seedling performance. At moderate stress (100 mM NaCl), SVI dropped to around one-third of the control, and at 200 mM it fell below 140 in both species. These results demonstrate the drastic inhibitory effect of high salinity on seedling vigor (Tables 3 and 4).
3.11. Effects on the germination recovery percentage
54After transferring seeds from high-salinity conditions to distilled water, both species exhibited substantial germination recovery. Following exposure to 300 mM NaCl, germination recovery was 92% in P. tomentosa and 90% in A. oblongifolia. At 400 mM NaCl, recovery rates remained high (81% and 78%, respectively), while at 800 mM NaCl, germination recovery declined to 65.5% in P. tomentosa and 68.5% in A. oblongifolia. These results indicate that both species exhibit strong germination recovery potential after salt stress (Table 5).
|
Table 5. Recovery percentage of germination (GRP) of Pergularia tomentosa and Acokanthera oblongifolia after they were transferred from 300, 400 and 800 mM NaCl to distilled water for 10 day-period – Pourcentage de récupération de la germination (GRP) de Pergularia tomentosa et Acokanthera oblongifolia après leur transfert de 300, 400 et 800 mM NaCl vers de l'eau distillée pendant une période de 10 jours. |
||
|
NaCl (mM) |
Recovery percentage of germination (%) |
|
|
Pergularia tomentosa |
Acokanthera oblongifolia |
|
|
300 |
92 ± 2.82a |
90 ± 5.16a |
|
400 |
81 ± 6.83b |
78 ± 5.16b |
|
800 |
65.5 ± 3.41c |
68.5 ± 3.41b |
|
F-value |
32.07 |
13.27 |
|
p-value |
< 0.0001 |
0.0021 |
|
Means (± Standard Deviation) within a column followed by the same letter are not significantly different at p = 0.05 (Tukey Test) level for the given salinity (n = 4) — Les moyennes (± écart-type) figurant dans une même colonne et suivies de la même lettre ne sont pas significativement différentes au seuil de p = 0,05 (test de Tukey) pour la salinité considérée (n = 4). |
||
4. Discussion
55The rapid spread of land degradation worldwide has amplified the urgency for effective rehabilitation strategies (Gibbs & Salmon, 2015). Traditional methods are costly and complex while using medicinal and aromatic plants offers a viable, eco-friendly alternative. However, salinity remains a significant barrier to plant establishment and growth. Salt stress disrupts physiological processes in plants, altering secondary metabolite production and impacting overall productivity (Arif et al., 2020).
56High salt levels induce hyperionic and hyperosmotic stress, which impairs ionic and osmotic balance, leading to reductions in germination, growth, and plant development (Johnson & Puthur, 2021; Mariyam et al., 2023).
57This study investigated the effects of salinity on seed germination and seedling emergence in Pergularia tomentosa and Acokanthera oblongifolia. Both species exhibited significant declines in germination and seedling growth with increasing salinity, but they also demonstrated remarkable recovery potential following exposure to high salinity levels. These results highlight the germination and growth performance that allow these species to survive under saline environments, offering valuable insights into their resilience and potential applications in land rehabilitation projects. Similar patterns of salinity-induced germination inhibition have been observed in halophytes, where optimal germination typically occurs in freshwater conditions, and salinity acts as a limiting factor (Rahman et al., 2021; Mansouri & Kheloufi, 2024; Kheloufi & Mansouri, 2024). The concept of salinity recovery, defined as the ability of seeds to germinate upon transfer to freshwater after salt exposure, has been widely studied in the context of salt tolerance (Gillard et al., 2021; Balasubramaniam et al., 2023; Zhou et al., 2024).
58In the case of P. tomentosa and A. oblongifolia, salinity significantly reduced germination percentages, particularly at higher NaCl concentrations of 300, 400 and 800 mM. Under non-saline conditions, A. oblongifolia achieved full germination (100%), while P. tomentosa reached 98%, highlighting the adverse effects of salt on water uptake and nutrient availability, as previously reported by Alkharabsheh et al. (2021). Germination rates decrease sharply at salinity levels of 50 mM NaCl and higher, with severe inhibition (20%) at 200 mM NaCl. These findings align with earlier research, which showed that salinity interferes with cellular processes, including metabolic activity and cell division (Colin et al., 2023). Both species showed delayed mean germination time under salinity stress, indicating slower seedling emergence. Pergularia tomentosa had a shorter MGT (3.48 days) compared to A. oblongifolia (9.45 days) in optimal conditions, suggesting faster germination under favorable environments. As salinity levels increased, both species exhibited longer MGT, with A. oblongifolia showing greater sensitivity. An extended mean germination time reflects the additional period required for seeds to mitigate the adverse effects of osmotic and ionic stress during salt exposure. This delay results from reduced water uptake, metabolic adjustments, and the need to activate stress tolerance mechanisms, which decrease the potential of the germination process under saline conditions (Biswas et al., 2023).
59The coefficient of velocity of germination also decreased as salinity increased, indicating slower germination. In the absence of salinity, P. tomentosa exhibited a higher CVG at 29.20%, indicating greater variability in germination rates compared to A. oblongifolia, which had a CVG of 10.73%. This suggests that P. tomentosa tends to germinate more quickly and shows a greater range of germination times under non-saline conditions. However, as salinity increased to 50 mM NaCl, both species experienced a significant reduction in CVG, with A. oblongifolia showing a more substantial decline. This trend supports previous research, such as the study by Bellache et al. (2022), which demonstrated that salt stress can severely impair germination rates, slowing down the process and affecting seedling establishment. The sharper decline in A. oblongifolia’s CVG under saline conditions highlights its increased sensitivity to salt stress, emphasizing the differential responses of plant species to environmental stressors.
60Seedling development exhibited a comparable response in both species, with progressive reductions in seedling length as salinity increased. Under non-saline conditions, A. oblongifolia produced significantly longer seedlings than P. tomentosa; however, exposure to 200 mM NaCl resulted in marked growth inhibition in both species, with seedling length declining by approximately 50%. This reduction is consistent with the negative impact of salinity on cell elongation and division (Johnson & Puthur, 2021). The seedling vigor index also decreased with higher salinity levels, further emphasizing the harmful effects of salt stress on seedling establishment and overall health. This decline suggests that salt exposure impairs early growth, limiting the seedlings' ability to establish strong roots and develop properly (Reed et al., 2022).
61Despite the inhibitory effects of salinity, both species demonstrated notable recovery potential when transferred from saline conditions to distilled water. After exposure to 300 mM NaCl, recovery rates were 92% for P. tomentosa and 90% for A. oblongifolia, with recovery rates remaining high (81% and 78%, respectively) at 400 mM NaCl. These findings suggest that both species possess effective mechanisms for surviving under salinity, such as ion compartmentalization and osmotic adjustment, which allow them to recover from moderate salt stress (Hameed et al., 2021; Maryum et al., 2022). However, recovery rates declined at 800 mM NaCl, with P. tomentosa showing 65.5% recovery and A. oblongifolia showing 68.5%. This indicates that extreme salinity levels may exceed the species' tolerance thresholds, resulting in irreversible damage. This finding aligns with recent studies that reported cellular damage under high salinity conditions (Biswas et al., 2023). Nonetheless, the high degree of resilience demonstrated by both species in response to moderate salinity suggests that they have potential for use in rehabilitation projects on saline soils, similar to other salt-tolerant species within the Apocynaceae family, such as Apocynum lancifolium Russanov (Hamidov et al., 2007), Nerium oleander L. (Trigueros et al., 2012), Periploca visciformis (Vatke) K.Schum. (Brown & Mies, 2012), Aspidosperma pyrifolium Mart. (da Conceição Sabino et al., 2021), Apocynum venetum L. and Apocynum pictum Schrenk (Thevs et al., 2012). These species are widely employed for ornamental purposes and in restoration programs targeting degraded or desertified semi-arid landscapes.
62For halophytes, this pattern underlines their salt-tolerant nature, which serves as an adaptive strategy to reduce competition with other species. This mechanism enables halophytes to thrive, as soil salinity typically fluctuates only for short durations. Many halophyte seeds remain dormant under low water potential, retaining their viability, and can quickly restart germination when exposed to deionized water after experiencing high salinity. This ability to reactivate germination after salt-induced dormancy sets halophytes apart from salt-sensitive plants, which often fail to survive prolonged exposure to seawater salinity. The capacity of halophyte seeds to recover their germination potential is crucial for their success in natural environments, particularly within Mediterranean saline ecosystems (Gairola et al., 2021; Navarro-Torre et al., 2023; Bazihizina et al., 2024).
63In these environments, effective germination strategies are essential for plant survival. Field observations indicate that seed germination typically occurs in the spring or autumn, following seed maturation or in areas with higher soil elevation. During these times, seeds benefit from lower salinity and better water availability. This timing helps avoid germination in flooded areas caused by seawater intrusion, a common challenge in coastal salt marshes. The physiological basis for this behavior is related to the low osmotic potential caused by high salt concentrations, such as NaCl. The low osmotic potential prevents the development of the turgor pressure necessary for cell division and differentiation, which are critical for radicle emergence and successful germination (Obroucheva, 2021). Consequently, seeds remain dormant but metabolically active, awaiting more favorable conditions, such as reduced salt stress (Sano & Marion-Poll, 2021). Proteins and lipids in the seeds are vital to the transition from dormancy to germination. Proteomic studies indicate that high salt concentrations slow the breakdown of reserve proteins into amino acids and fatty acids into carbohydrates. However, these processes restart again quickly once the environmental conditions improve (Bhatla & Lal, 2023).
64This study provides novel insights into the salt tolerance of P. tomentosa and A. oblongifolia under controlled laboratory conditions, highlighting their potential use in revegetation and land restoration programs in saline environments. Nevertheless, we recognize that natural systems involve the simultaneous action of multiple abiotic stresses, including fluctuating salinity, drought, temperature extremes, and soil heterogeneity. Future research should therefore extend these findings through field-based trials and semi-controlled experiments, to validate the ecological relevance of the tolerance patterns observed here and to better inform practical applications in restoration strategies.
65In addition to their intrinsic salt tolerance, the restoration potential of P. tomentosa and A. oblongifolia may be maximized when integrated with other native halophytes. Such multispecies assemblages can enhance vegetation cover, promote soil stabilization, and foster ecosystem resilience under the combined pressures of salinity, drought, and land degradation. This perspective underscores the importance of adopting community-based approaches in restoration ecology, where the use of functionally diverse native species is more effective than monocultures in achieving long-term sustainability of degraded saline landscapes.
5. Conclusions
66This study demonstrates the strong inhibitory effects of salinity on the germination and early seedling growth of P. tomentosa and A. oblongifolia, while also highlighting their remarkable ability to recover once stress is alleviated. Germination was completely inhibited beyond 200 mM NaCl; however, both species exhibited substantial recovery, reaching 80-90% at moderate salinity (300-400 mM NaCl) and maintaining resilience even under extreme conditions, with recovery rates of 65-69% at 800 mM NaCl. The absence of significant interspecific differences in recovery suggests comparable adaptive mechanisms, supporting their potential as promising candidates for the revegetation of saline-degraded lands. These recovery dynamics provide valuable insights for conservation planning, seed priming strategies, and the broader understanding of plant responses to abiotic stress. Future research should focus on unraveling the biochemical and genetic bases of their salt tolerance and developing cultivation strategies under suboptimal conditions. Integrating these species into sustainable agricultural and land management systems could enhance biodiversity conservation, promote ecological restoration, and unlock their medicinal and economic value.
Acknowledgements
67Authors are grateful to the Ministry of Higher Education and Scientific Research, Algeria. The research was conducted as part the PRFU research project D00L02UN050220220001 (University of Batna 2, Algeria), titled Ecophysiological analysis and plant production for the rehabilitation of degraded ecosystems in the Aurès region (Algeria).
Bibliographie
Abdul-Baki A.A. & Anderson J.D., 1973. Vigor determination in soybean seed by multiple criteria 1. Crop Sci., 13(6), 630-633, doi.org/10.2135/cropsci1973.0011183x001300060013x
Aldayel M.F., 2024. Biofabrication of silver nanoparticles using Pergularia tomentosa extract and evaluation of their antibacterial, antioxidant, and cytotoxic properties. Life, 14(12), 1639, doi.org/10.3390/life14121639
Alkharabsheh H.M. et al., 2021. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: a review. Agronomy, 11(11), 2299, doi.org/10.3390/agronomy11112299
Arif Y. et al., 2020. Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol. Biochem., 156, 64-77, doi.org/10.1016/j.plaphy.2020.08.042
Atta K. et al., 2023. Impacts of salinity stress on crop plants: improving salt tolerance through genetic and molecular dissection. Front. Plant Sci., 14, 1241736, doi.org/10.3389/fpls.2023.1241736
Balasubramaniam T., Shen G., Esmaeili N. & Zhang H., 2023. Plants’ response mechanisms to salinity stress. Plants, 12(12), 2253, doi.org/10.3390/plants12122253
Bazihizina N. et al., 2024. The sustainable use of halophytes in salt-affected land: state-of-the-art and next steps in a saltier world. Plants, 13(16), 2322, doi.org/10.3390/plants13162322
Bellache M. et al., 2022. Comparative analysis of tolerance to salt stress and water deficit in two invasive weeds of the genus Erigeron (Asteraceae). Plants, 11(15), 2059, doi.org/10.3390/plants11152059
Bhatla S.C. & Lal M.A., 2023. Seed dormancy and germination. In: Plant physiology, development and metabolism. Singapore: Springer Nature, 625-640, doi.org/10.1007/978-981-99-5736-1_28
Biswas S., Seal P., Majumder B. & Biswas A.K., 2023. Efficacy of seed priming strategies for enhancing salinity tolerance in plants: an overview of the progress and achievements. Plant Stress, 9, 100186, doi.org/10.1016/j.stress.2023.100186
Bonner F.T., 1986. Measurement of seed vigor for loblolly and slash pines. For. Sci., 32(1), 170-178, doi.org/10.1093/forestscience/32.1.170
Brown G. & Mies B.A., 2012. Ecology and adaptive strategies. In: Vegetation ecology of Socotra. Plant and vegetation, vol 7. Dordrecht, The Netherlands: Springer, 93-139, doi.org/10.1007/978-94-007-4141-6_5
Colin L. et al., 2023. The cell biology of primary cell walls during salt stress. Plant Cell, 35(1), 201-217, doi.org/10.1093/plcell/koac292
Côme D., 1970. Les obstacles à la germination. Paris : Masson et Cie, 24-27.
Coolbear P., Francis A. & Grierson D., 1984. The effect of low temperature pre-sowing treatment on the germination performance and membrane integrity of artificially aged tomato seeds. J. Exp. Bot., 35(11), 1609-1617, doi.org/10.1093/jxb/35.11.1609
da Conceição Sabino F. et al., 2021. Morphological characteristics, biomass accumulation and gas exchange of an important species native for restoration in semi-arid Brazilian areas affected by salt and water stress. Plant Stress, 2, 100021, doi.org/10.1016/j.stress.2021.100021
Dastanpoor N. et al., 2013. Effects of hydropriming on seed germination and seedling growth in sage (Salvia officinalis L.). Afr. J. Biotechnol., 12(11), 1223-1228, doi.org/10.5897/AJB12.1941
De Villiers J.P., 1962. The cardiac glycosides of Acokanthera oblongifolia. S. Afr. J. Chem., 15(1), 82-84.
Dobignard A. & Chatelain C., 2010. Index synonymique de la flore d’Afrique du Nord, vol. 1. Genève, Suisse : Éditions des Conservatoire et Jardin botaniques de la Ville de Genève, 455.
El Sabagh A. et al., 2020. Consequences of salinity stress on the quality of crops and its mitigation strategies for sustainable crop production: an outlook of arid and semi-arid regions. Environ. Climate Plant Veg. Growth, 503-533, doi.org/10.1007/978-3-030-49732-3_20
Farzadinia P. et al., 2019. Healing effects of Pergularia tomentosa L., a native medicinal plant in Bushehr province, Iran on burn, in animal model. Pak. J. Pharm. Sci., 32(1), 21-28.
Gairola S., Shabana H.A., Al Ketbi A. & Mahmoud T., 2021. Seed germination behavior of halophytes distributed in arid Arabian deserts: a review of current research. In: Grigore M.N., ed. Handbook of halophytes. Cham, Suisse: Springer, 1421-1437, doi.org/10.1007/978-3-030-57635-6_45
García-Caparrós P., Llanderal A. & Lao M.T., 2021. Halophytes as an option for the restoration of degraded areas and landscaping. In: Grigore M.N., ed. Handbook of halophytes. Cham, Suisse: Springer, 2795-2810, doi.org/10.1007/978-3-030-57635-6_116
Gibbs H.K. & Salmon J.M., 2015. Mapping the world's degraded lands. Appl. Geogr., 57, 12-21, doi.org/10.1016/j.apgeog.2014.11.024
Gillard M.B. et al., 2021. High aqueous salinity does not preclude germination of invasive Iris pseudacorus from estuarine populations. Ecosphere, 12(5), e03486, doi.org/10.1002/ecs2.3486
Gupta S.R., Dagar J.C. & Sharma H.R., 2024. Halophytes and agroforestry in the restoration of salt-affected landscapes in changed environment. J. Soil Salinity Water Qual., 16(2), 152-165, doi.org/10.56093/jsswq.v16i2.156303
Hameed A. et al., 2021. Effects of salinity stress on chloroplast structure and function. Cells, 10(8), doi.org/10.3390/cells10082023
Hamidov A. et al., 2007. Apocynum lancifolium and Chenopodium album - potential species to remediate saline soils. WSEAS Trans. Environ. Dev., 3(7), 123-128.
Hosseini S.H. et al., 2018. Modeling potential habitats for Pergularia tomentosa using maximum entropy model and effect of environmental variables on its quantitative characteristics in arid rangelands, southeastern Iran. J. Ecol. Environ., 42, 1-13, doi.org/10.1186/s41610-018-0083-2
ISTA (International Seed Testing Association), 2022. International rules for seed testing. Bassersdorf, Switzerland: ISTA, doi.org/10.15258/istarules.2015.i
Johnson R. & Puthur J.T., 2021. Seed priming as a cost-effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem., 162, 247-257, doi.org/10.1016/j.plaphy.2021.02.034
Khamis W. et al., 2024. Phytochemical composition and insecticidal activity of Acokanthera oblongifolia (Hochst.) Benth & Hook.f. ex B.D.Jacks. extract on life span and biological aspects of Spodoptera littoralis (Biosd.). Open Agric., 9(1), 20220394, doi.org/10.1515/biol-2022-0962
Khan M.A. & Ungar I.A., 1997. Effects of thermoperiod on recovery of seed germination of halophytes from saline conditions. Am. J. Bot., 84(2), 279-283, doi.org/10.2307/2446089
Khan M.N. et al., 2019. Eco-taxonomic study of family Poaceae (Gramineae). RADS J. Biol. Res. Appl. Sci., 10, 63-75, doi.org/10.37962/jbas.v10i2.191
Kheloufi A. & Mansouri L.M., 2024. Salinity tolerance in Atriplex halimus L.: differential effects of soluble salts on seed germination and recovery. Acta Universitatis Sapientiae Agric. Environ., 16, 48-63, doi.org/10.47745/ausae-2024-0005
Kupicha F.K., 1982. Studies on African Apocynaceae: the genus Acokanthera. Kew Bull., 37, 41-67, doi.org/10.2307/4114719
Maguire J.D., 1962. Speed of germination-aid in selection aid in evolution for seedling emergence and vigor. Crop Sci., 2(2), 176-177, doi.org/10.2135/cropsci1962.0011183x000200020033x
Mann A. et al., 2023. Halophytes as new model plant species for salt tolerance strategies. Front. Plant Sci., 14, 1137211, doi.org/10.3389/fpls.2023.1137211
Mansouri L.M. & Kheloufi A., 2024. Salinity effects on germination of Portulaca oleracea L.: a multipurpose halophyte from arid rangelands. J. Appl. Res. Med. Aromatic Plants, 41, 100549, doi.org/10.1016/j.jarmap.2024.100549
Mariyam S. et al., 2023. Review on nitric oxide at the forefront of rapid systemic signaling in mitigation of salinity stress in plants: crosstalk with calcium and hydrogen peroxide. Plant Sci., 336, 111835, doi.org/10.1016/j.plantsci.2023.111835
Maryum Z. et al., 2022. An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cotton. Front. Plant Sci., 13, 907937, doi.org/10.3389/fpls.2022.907937
Mircea D.M. et al., 2024. Abiotic stress tolerance and invasive potential of ornamental plants in the Mediterranean area: implications for sustainable landscaping. Agronomy, 15(1), 52, doi.org/10.3390/agronomy15010052
Munir N., Hasnain M., Roessner U. & Abideen Z., 2022. Strategies in improving plant salinity resistance and use of salinity resistant plants for economic sustainability. Crit. Rev. Environ. Sci. Technol., 52(12), 2150-2196, doi.org/10.1080/10643389.2021.1877033
Nasr A.A.M., 2009. Response of Acokanthera spectabilis plants to saline water irrigation. J. Prod. Dev., 14(3), 583-595, doi.org/10.21608/jpd.2009.44715
Navarro-Torre S. et al., 2023. Sustainable agricultural management of saline soils in arid and semi-arid Mediterranean regions through halophytes, microbial and soil-based technologies. Environ. Exp. Bot., 212, 105397, doi.org/10.1016/j.envexpbot.2023.105397
Nóbrega J.S., Lopes K.P., Gomes C.D.L., Sá J.M.D., Oliveira O.H.D. & Paiva F.J.D.S. 2021. Seed quality and vigor of germination of Moringa oleifera Lam. in saline stress. Brazilian Archives of Biology and Technology 64, e21210106. https://doi.org/10.1590/1678-4324-2021210106
Obroucheva N.V., 2021. Germination program in non-dormant seeds: programming, saving and implementation. Russ. J. Plant Physiol., 68(6), 1003-1017, doi.org/10.1134/s1021443721060145
Orchard T., 1977. Estimating the parameters of plant seedling emergence. Seed Sci. Technol., 5, 61-69.
Pecio Ł. et al., 2019. Cytotoxic cardenolides from the leaves of Acokanthera oblongifolia. Planta Medica, 85(11-12), 965-972, doi.org/10.1055/a-0958-2566
Rahman M.M. et al., 2021. Adaptive mechanisms of halophytes and their potential in improving salinity tolerance in plants. Int. J. Mol. Sci., 22(19), 10733, doi.org/10.3390/ijms221910733
Reed R.C., Bradford K.J. & Khanday I., 2022. Seed germination and vigor: ensuring crop sustainability in a changing climate. Heredity, 128(6), 450-459, doi.org/10.1038/s41437-022-00497-2
Sano N. & Marion-Poll A., 2021. ABA metabolism and homeostasis in seed dormancy and germination. Int. J. Mol. Sci., 22(10), 5069, doi.org/10.3390/ijms22105069
SAS Institute Inc., 2002. User installation guide for the SAS® system version 9 for Microsoft® Windows®. Cary, NC, USA: SAS Institute Inc.
Segueni K., Chouikh A. & Tlili M.L., 2023. Phytochemical profile, antioxidant and anti-inflammatory activities of crude latex (Pergularia tomentosa L.) in Algerian Saharan. Notulae Scientia Biologicae, 15(4), 11772, doi.org/10.55779/nsb15411772
Singh A., 2022. Soil salinity: a global threat to sustainable development. Soil Use Manage., 38(1), 39-67, doi.org/10.1111/sum.12772
Tessema N., Yadeta D., Kebede A. & Ayele G.T., 2022. Soil and irrigation water salinity, and its consequences for agriculture in Ethiopia: a systematic review. Agriculture, 13(1), 109, doi.org/10.3390/agriculture13010109
Thevs N. et al., 2012. Apocynum venetum L. and Apocynum pictum Schrenk (Apocynaceae) as multi-functional and multi-service plant species in Central Asia: a review on biology, ecology, and utilization. J. Appl. Bot. Food Qual., 85(2), 159.
Trigueros D., Mingorance M.D. & Oliva S.R., 2012. Evaluation of the ability of Nerium oleander L. to remediate Pb-contaminated soils. J. Geochem. Explor., 114, 126-133, doi.org/10.1016/j.gexplo.2012.01.005
Zhou H. et al., 2024. Insights into plant salt stress signaling and tolerance. J. Genet. Genomics, 51(1), 16-34, doi.org/10.1016/j.jgg.2023.08.007






